The Improved Antineoplastic Activity of Thermophilic L-Asparaginase Tli10209 via Site-Directed Mutagenesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Strains and Vectors
2.3. Experimental Methods
2.3.1. Simulation and Analysis of the Tertiary Structure of L-Asparaginase
2.3.2. Construction of a Recombinant Plasmid of Site-Mutated L-Asparaginase
2.3.3. Expression and Purification of Proteins
2.3.4. Determination of L-Asparaginase Enzyme Activity
2.3.5. Characterization of L-Asparaginase Tli10209 and Mutants
2.3.6. MD Simulation
2.3.7. Synthesis of Gold Nanorods and Immobilization of Enzymes
2.3.8. Cell Proliferation Inhibition Assay
3. Results
3.1. The Selection of an Improved Active Site-Mutation of L-Asparaginase
3.2. Construction, Expression and Purification of Single Mutants of Tli10209
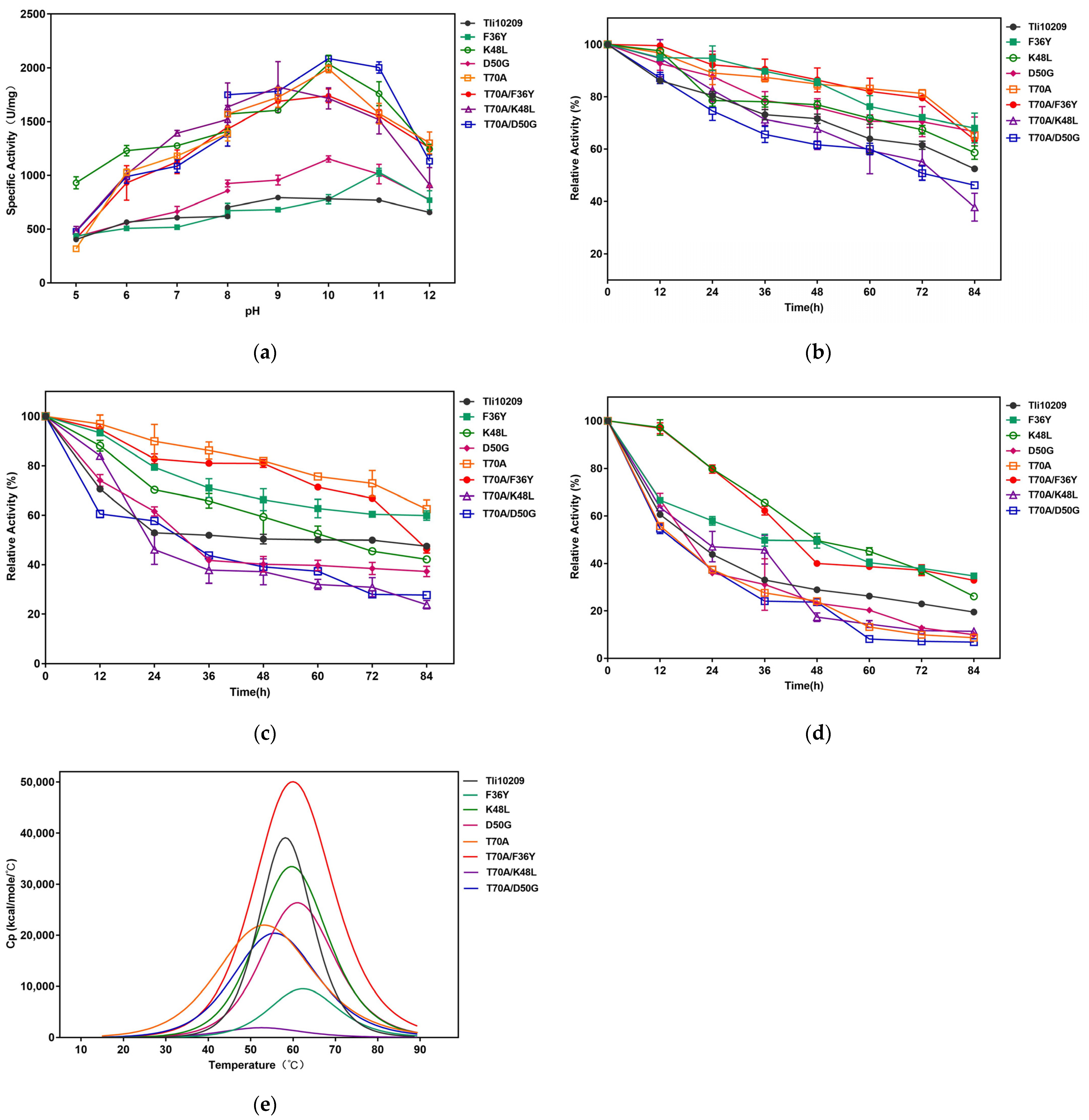
3.3. Determination of Mutants’ Specific Activity
3.4. Kinetic Analysis of Mutant Enzymes
3.5. Characterization of Enzyme
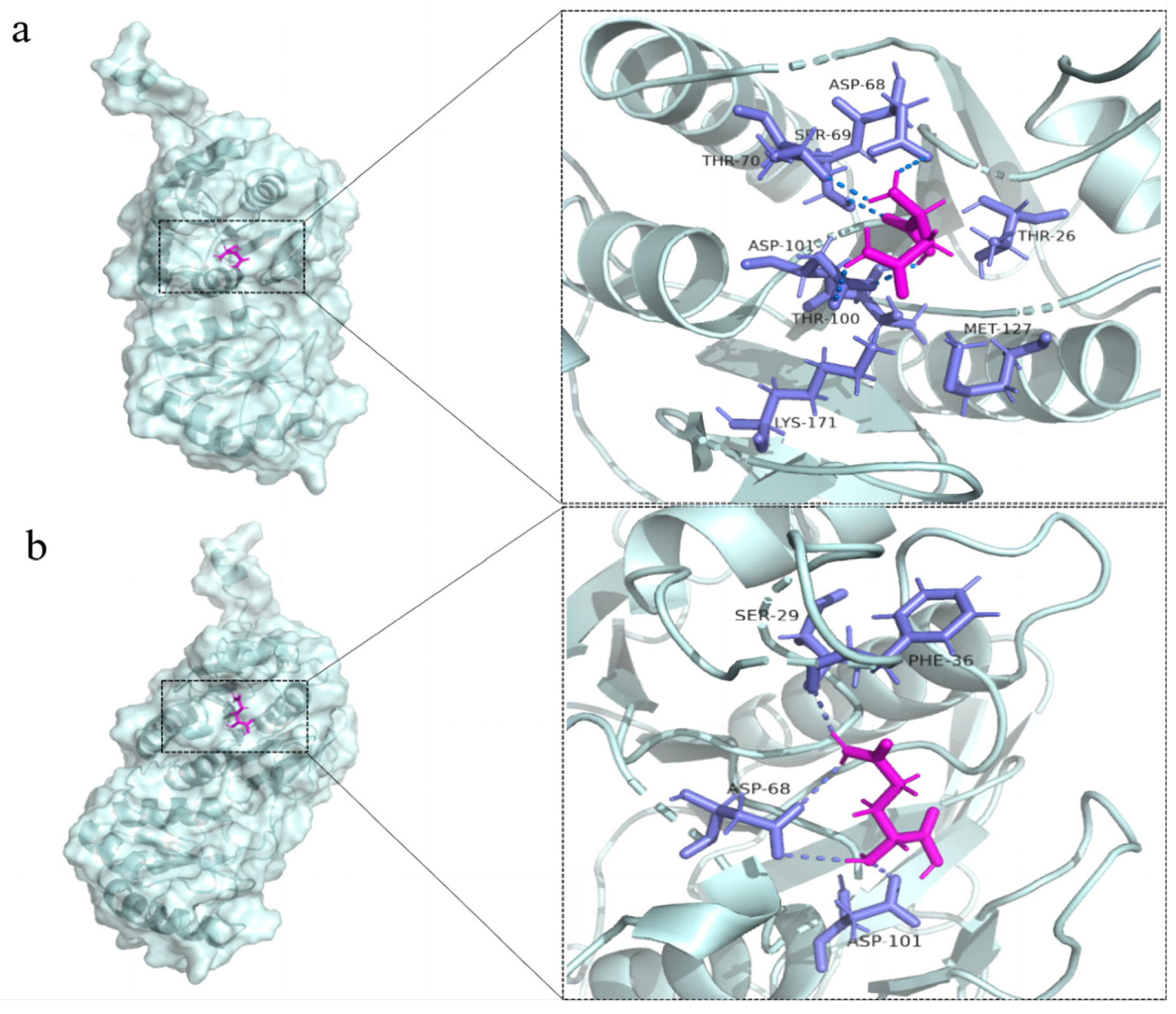
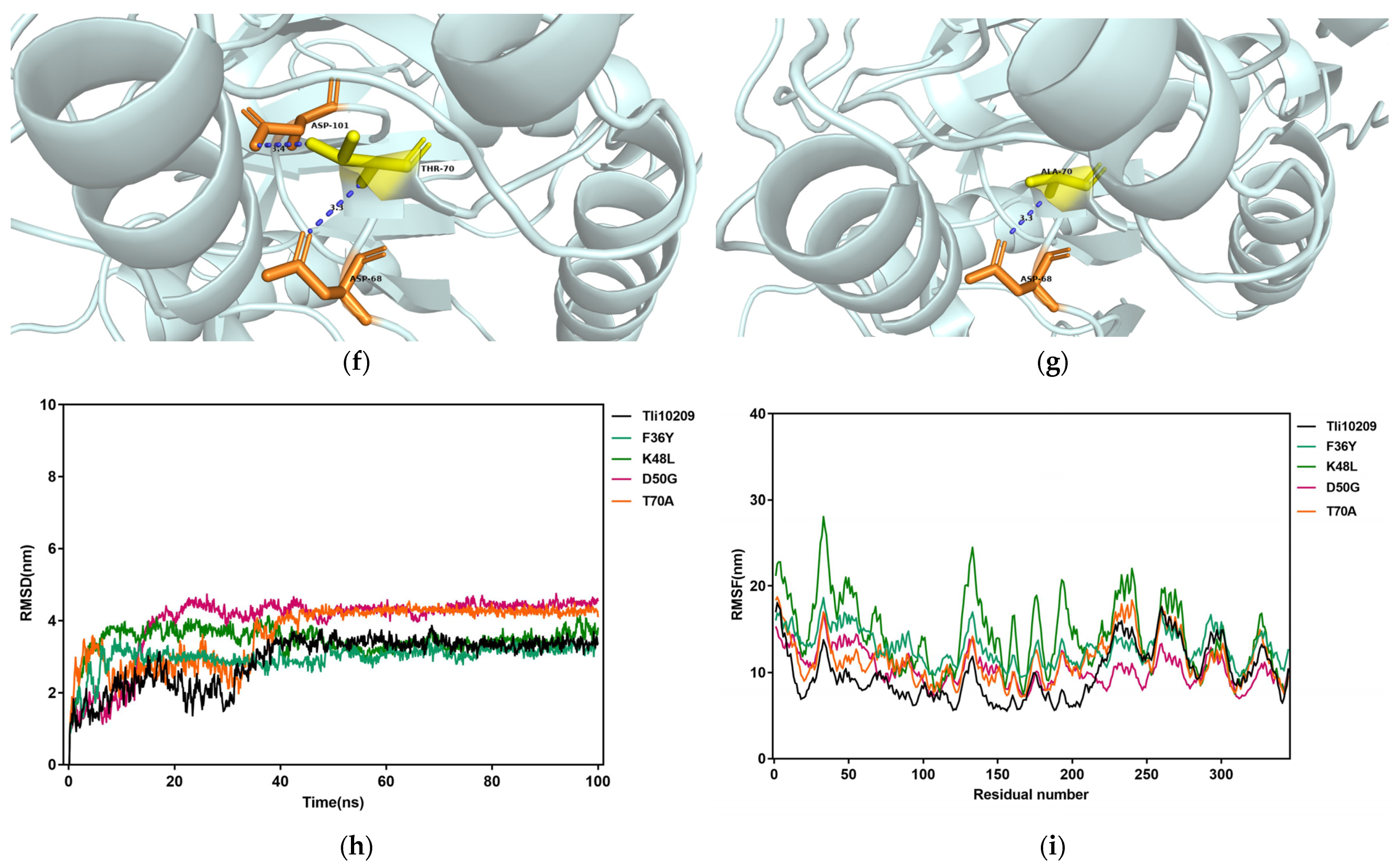
3.6. Structural Simulation and Analysis of L-Asparaginase
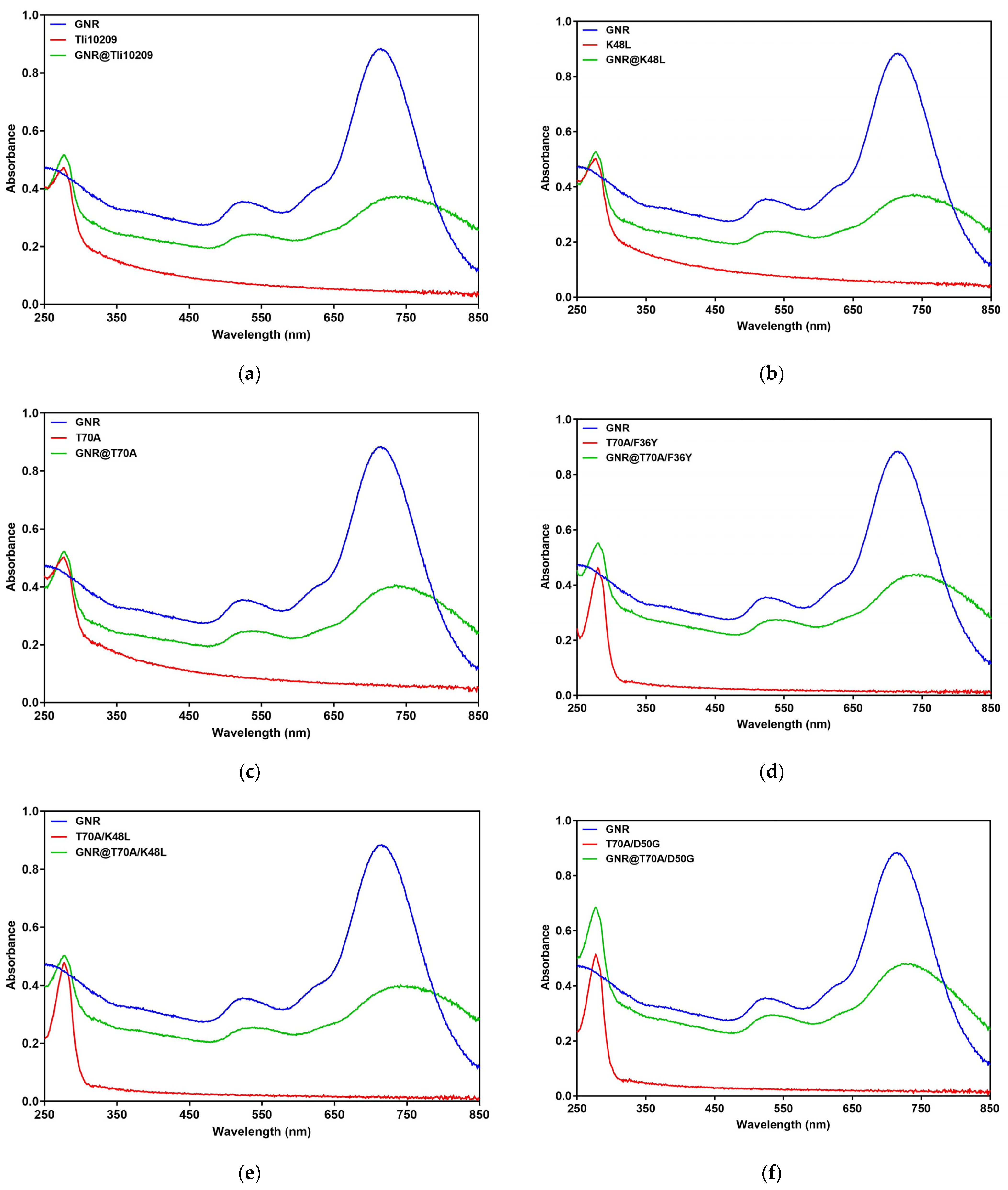
3.7. Synthesis and Enzymatic Immobilization of Gold Nanorods
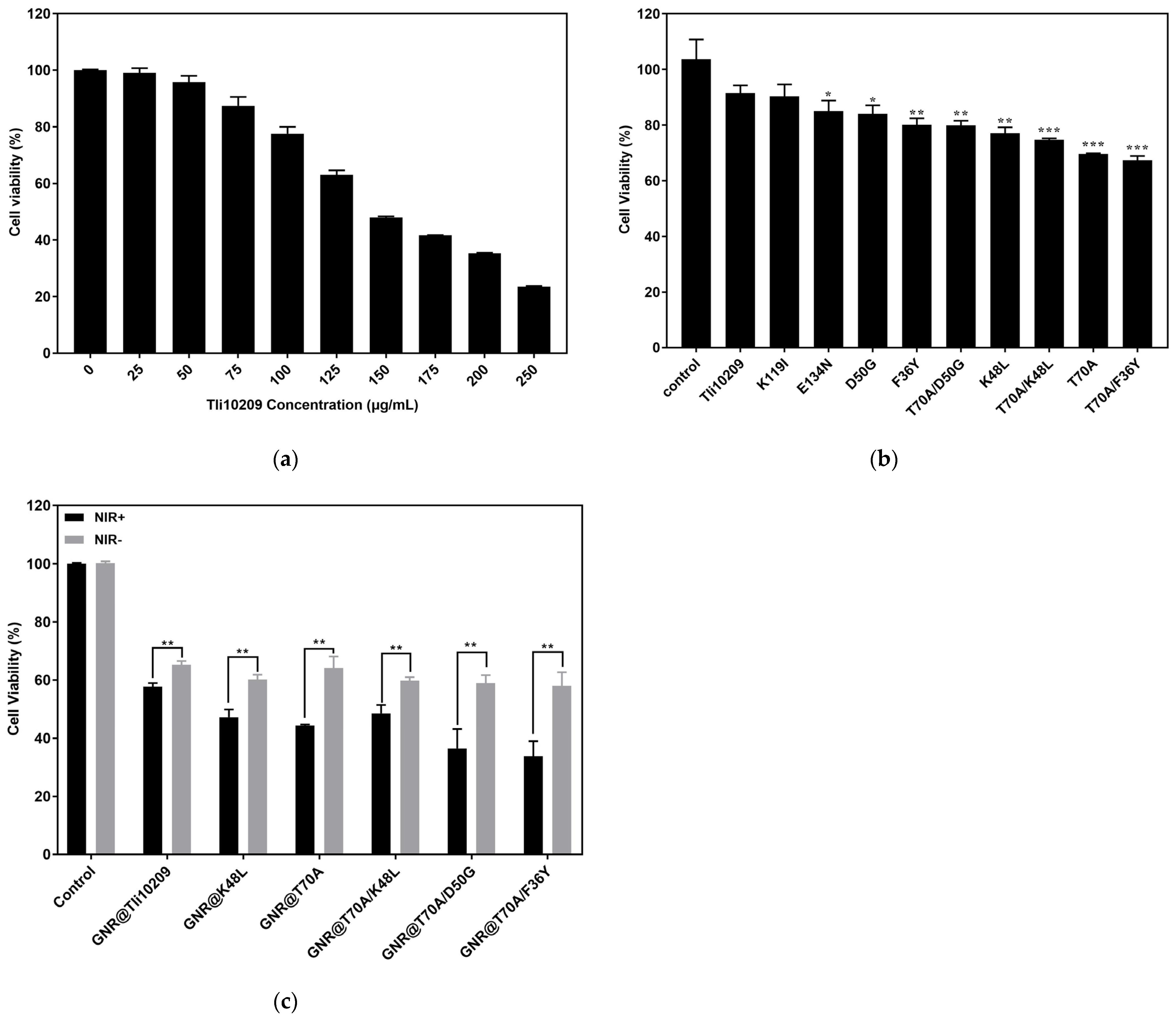
3.8. Cell Proliferation Inhibition Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tennant, D.A.; Durán, R.V.; Gottlieb, E. Targeting metabolic transformation for cancer therapy. Nat. Rev. Cancer 2010, 10, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Lieu, E.L.; Nguyen, T.; Rhyne, S.; Kim, J. Amino acids in cancer. Exp. Mol. Med. 2020, 52, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Pokrovsky, V.S.; Abo Qoura, L.; Morozova, E.; Bunik, V.I. Predictive markers for efficiency of the amino-acid deprivation therapies in cancer. Front. Med. 2022, 9, 1035356. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Mishra, A.; Gautam, P.; Feroz, Z.; Sivakumar, V.; Likos, E.M.; Shukla, G.C.; Kumar, M. Metabolic Pathways, Enzymes, and Metabolites: Opportunities in Cancer Therapy. Cancers 2022, 14, 5268. [Google Scholar] [CrossRef]
- Butler, M.; van der Meer, L.T.; van Leeuwen, F.N. Amino Acid Depletion Therapies: Starving Cancer Cells to Death. Trends Endocrinol. Metab. 2021, 32, 367–381. [Google Scholar] [CrossRef] [PubMed]
- El-Fakharany, E.; Orabi, H.; Abdelkhalek, E.; Sidkey, N. Purification and biotechnological applications of L-asparaginase from newly isolated Bacillus halotolerans OHEM18 as antitumor and antioxidant agent. J. Biomol. Struct. Dyn. 2022, 40, 3837–3849. [Google Scholar] [CrossRef]
- Mazloum-Ravasan, S.; Madadi, E.; Mohammadi, A.; Mansoori, B.; Amini, M.; Mokhtarzadeh, A.; Baradaran, B.; Darvishi, F. Yarrowia lipolytica L-asparaginase inhibits the growth and migration of lung (A549) and breast (MCF7) cancer cells. Int. J. Biol. Macromol. 2021, 170, 406–414. [Google Scholar] [CrossRef]
- Lorenzi, P.L.; Reinhold, W.C.; Rudelius, M.; Gunsior, M.; Shankavaram, U.; Bussey, K.J.; Scherf, U.; Eichler, G.S.; Martin, S.E.; Chin, K.; et al. Asparagine synthetase as a causal, predictive biomarker for L-asparaginase activity in ovarian cancer cells. Mol. Cancer Ther. 2006, 5, 2613–2623. [Google Scholar] [CrossRef] [PubMed]
- DeBerardinis, R.J.; Mancuso, A.; Daikhin, E.; Nissim, I.; Yudkoff, M.; Wehrli, S.; Thompson, C.B. Beyond aerobic glycolysis: Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. USA 2007, 104, 19345–19350. [Google Scholar] [CrossRef]
- Michalska, K.; Jaskolski, M. Structural aspects of L-asparaginases, their friends and relations. Acta Biochim. Pol. 2006, 53, 627–640. [Google Scholar] [CrossRef]
- Van Trimpont, M.; Peeters, E.; De Visser, Y.; Schalk, A.M.; Mondelaers, V.; De Moerloose, B.; Lavie, A.; Lammens, T.; Goossens, S.; Van Vlierberghe, P. Novel Insights on the Use of L-Asparaginase as an Efficient and Safe Anti-Cancer Therapy. Cancers 2022, 14, 902. [Google Scholar] [CrossRef]
- Laterza, O.F.; Gerhardt, G.; Sokoll, L.J. Measurement of plasma ammonia is affected in patients receiving asparaginase therapy. Clin. Chem. 2003, 49, 1710–1711. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zhang, L.; Huang, M.; Wang, F.; Xie, G.; Huo, R.; Gao, R. Selective enhanced cytotoxicity of amino acid deprivation for cancer therapy using thermozyme functionalized nanocatalyst. J. Nanobiotechnol. 2024, 22, 53. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, H.; Sengupta, S.; Purohit, V.; Kotagere, A.; Moulik, N.R.; Prasad, M.; Dhamne, C.; Narula, G.; Banavali, S.; Gota, V. A comparison of asparaginase activity in generic formulations of E. coli derived L-asparaginase: In-vitro study and retrospective analysis of asparaginase monitoring in pediatric patients with leukemia. Br. J. Clin. Pharmacol. 2020, 86, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.; Wang, C.; Chen, C.; Zhang, R.; Liu, H.; Lu, W.; Jiang, L.; Du, M. Identification of a novel ACE-inhibitory peptide from casein and evaluation of the inhibitory mechanisms. Food Chem. 2018, 256, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Seeliger, D.; de Groot, B.L. Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J. Comput.-Aided Mol. Des. 2010, 24, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Imada, A.; Igarasi, S.; Nakahama, K.; Isono, M. Asparaginase and glutaminase activities of micro-organisms. J. Gen. Microbiol. 1973, 76, 85–99. [Google Scholar] [CrossRef]
- Case, D.A.; Cheatham III, T.E.; Darden, T.; Gohlke, H.; Luo, R.; Merz, K.M., Jr.; Onufriev, A.; Simmerling, C.; Wang, B.; Woods, R.J. The Amber biomolecular simulation programs. J. Comput. Chem. 2005, 26, 1668–1688. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1998, 79, 926–935. [Google Scholar] [CrossRef]
- Berendsen, H.J.; Postma, J.V.; Van Gunsteren, W.F.; DiNola, A.R.H.J.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Chen, H.; Shao, L.; Li, Q.; Wang, J. Gold nanorods and their plasmonic properties. Chem. Soc. Rev. 2013, 42, 2679–2724. [Google Scholar] [CrossRef] [PubMed]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and Growth Mechanism of Gold Nanorods (NRs) Using Seed-Mediated Growth Method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]
- Zou, T.; Lum, C.T.; Lok, C.-N.; Zhang, J.-J.; Che, C.-M. Chemical biology of anticancer gold(iii) and gold(i) complexes. Chem. Soc. Rev. 2015, 44, 8786–8801. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.K.; Lorenzi, P.L.; Anishkin, A.; Purwaha, P.; Rogers, D.M.; Sukharev, S.; Rempe, S.B.; Weinstein, J.N. The glutaminase activity of L-asparaginase is not required for anticancer activity against ASNS-negative cells. Blood 2014, 123, 3596–3606. [Google Scholar] [CrossRef] [PubMed]
- Aghaeepoor, M.; Akbarzadeh, A.; Mirzaie, S.; Hadian, A.; Jamshidi Aval, S.; Dehnavi, E. Selective reduction in glutaminase activity of l-Asparaginase by asparagine 248 to serine mutation: A combined computational and experimental effort in blood cancer treatment. Int. J. Biol. Macromol. 2018, 120 Pt B, 2448–2457. [Google Scholar] [CrossRef]
- Derst, C.; Henseling, J.; Röhm, K.H. Engineering the substrate specificity of Escherichia coli asparaginase. II. Selective reduction of glutaminase activity by amino acid replacements at position 248. Protein Sci. 2000, 9, 2009–2017. [Google Scholar] [CrossRef]
- Bendl, J.; Stourac, J.; Sebestova, E.; Vavra, O.; Musil, M.; Brezovsky, J.; Damborsky, J. HotSpot Wizard 2.0: Automated design of site-specific mutations and smart libraries in protein engineering. Nucleic Acids Res. 2016, 44, W479–W487. [Google Scholar] [CrossRef]
- Maggi, M.; Mittelman, S.D.; Parmentier, J.H.; Colombo, G.; Meli, M.; Whitmire, J.M.; Merrell, D.S.; Whitelegge, J.; Scotti, C. A protease-resistant Escherichia coli asparaginase with outstanding stability and enhanced anti-leukaemic activity in vitro. Sci. Rep. 2017, 7, 14479. [Google Scholar] [CrossRef] [PubMed]
- Weder, N.; Probst, B.; Sévery, L.; Fernández-Terán, R.J.; Beckord, J.; Blacque, O.; Tilley, S.D.; Hamm, P.; Osterwalder, J.; Alberto, R. Mechanistic insights into photocatalysis and over two days of stable H2 generation in electrocatalysis by a molecular cobalt catalyst immobilized on TiO2. Catal. Sci. Technol. 2020, 10, 2549–2560. [Google Scholar] [CrossRef]




| Title 1 | Km (mM) | kcat (s−1) | Vmax (μmol·min−1·mL−1) | kcat/Km (mM−1·s−1) |
|---|---|---|---|---|
| Tli10209 | 6.78 | 2304.55 | 359.3 | 339.90 |
| F36Y | 7.00 | 2502.10 | 390.1 | 357.34 |
| K48L | 9.62 | 5077.32 | 791.6 | 527.84 |
| D50G | 9.03 | 3887.53 | 606.1 | 430.70 |
| T70A | 7.90 | 4145.37 | 646.3 | 524.73 |
| T70A/F36Y | 6.28 | 2926.71 | 456.3 | 465.74 |
| T70A/K48L | 6.65 | 2762.51 | 430.7 | 415.29 |
| T70A/D50G | 2.68 | 2047.35 | 319.2 | 763.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Ding, S.; Tang, X.; Gao, R.; Huo, R.; Xie, G. The Improved Antineoplastic Activity of Thermophilic L-Asparaginase Tli10209 via Site-Directed Mutagenesis. Biomolecules 2024, 14, 686. https://doi.org/10.3390/biom14060686
Zhang L, Ding S, Tang X, Gao R, Huo R, Xie G. The Improved Antineoplastic Activity of Thermophilic L-Asparaginase Tli10209 via Site-Directed Mutagenesis. Biomolecules. 2024; 14(6):686. https://doi.org/10.3390/biom14060686
Chicago/Turabian StyleZhang, Lijuan, Simeng Ding, Xiuhui Tang, Renjun Gao, Rui Huo, and Guiqiu Xie. 2024. "The Improved Antineoplastic Activity of Thermophilic L-Asparaginase Tli10209 via Site-Directed Mutagenesis" Biomolecules 14, no. 6: 686. https://doi.org/10.3390/biom14060686






