Insights into the Role of Glutathione Peroxidase 3 in Non-Neoplastic Diseases
Abstract
:1. Introduction
2. GPXs
3. Basic Information on GPX3
3.1. Discovery and Nomenclature of GPX3
3.2. Expression and Distribution of GPX3 in Cells
3.3. Regulation of GPX3 Expression
3.4. GPX3 Function
4. Research Progress in GPX3 and Non-Neoplastic Diseases
4.1. GPX3 and Kidney Diseases
4.1.1. AKI
4.1.2. CKD
4.2. GPX3 and Cardiovascular Diseases (CVDs)
4.2.1. Atherosclerosis
4.2.2. Acute Coronary Syndrome (ACS)
4.2.3. Pressure Overload Induced Cardiac Remodeling
4.2.4. Heart Failure (HF)
4.2.5. Hypertension
4.2.6. Atrial Fibrillation (AF)
4.3. GPX3 and Respiratory Diseases
4.3.1. Pulmonary Artery Hypertension (PAH)
4.3.2. Asthma
4.3.3. COPD
4.3.4. Idiopathic Pulmonary Fibrosis (IPF)
4.3.5. Other Respiratory Diseases
4.4. GPX3 and Metabolic Disorders
4.4.1. Obesity
4.4.2. Diabetes Mellitus (DM)
4.4.3. Metabolic Syndrome
4.5. GPX3 in Digestive System Diseases
4.5.1. IBD
4.5.2. Hepatic IR Injury
4.6. GPX3 and Neurological Disorders
4.6.1. Amyotrophic Lateral Sclerosis (ALS)
4.6.2. Parkinson’s Disease (PD)
4.6.3. Cerebrovascular Diseases
4.7. GPX3 and Bone and Joint Diseases
Kashin-Beck Disease (KBD)
4.8. GPX3 and Other Diseases
5. Conclusions
- (1)
- Variability in GPX3 expression: GPX3 expression varies across different tissues and in response to diverse oxidative stress conditions (Table 2). These disparities likely stem from differences in experimental animal models, tissue-specific locations, distinct cellular phenotypes responsive to oxidative stimuli, diverse pathophysiological mechanisms underlying diseases or tissue-specific regulatory pathways. For example, heightened GPX3 expression may result from the activation of antioxidant systems in response to oxidative damage [165], whereas diminished GPX3 expression often correlates with gene methylation and microRNA-mediated regulation [40,42,43].
- (2)
- Diagnostic potential of GPX3: As an extracellular protein, GPX3 is readily detectable in blood, making it a promising biomarker for diagnosing diseases, assessing disease progression, and informing therapeutic decisions, for conditions such as cancer, IBD, inflammatory diseases, and ALS. Nevertheless, the limitation of using GPX3 as a biomarker is its credibility. Current studies are limited to animal models and small numbers of clinical samples, and GPX3 expression can be influenced by medications, diet and lifestyle. Expanding GPX3 testing in larger cohorts and diverse biospecimen repositories could validate its reliability as a biomarker.
- (3)
- Therapeutic targeting of GPX3: Targeting GPX3 has shown promise in preclinical models (Table 3). Developing treatments to enhance GPX3 expression could extend to other diseases characterized by low GPX3 levels. However, there are currently no suitable medicines or agonists that can directly induce GPX3 expression, and many researchers have constructed overexpression plasmids to increase GPX3 expression in cells [50]. Further research is needed to develop new drugs based on stimulators or inhibitors that affect GPX3 expression, the enzyme itself and the involved receptors, and signaling pathways.
Author Contributions
Funding
Conflicts of Interest
References
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, Z.; Min, W. Mitochondria, Oxidative Stress and Innate Immunity. Front. Physiol. 2018, 9, 1487. [Google Scholar] [CrossRef] [PubMed]
- Climent, M.; Viggiani, G.; Chen, Y.-W.; Coulis, G.; Castaldi, A. MicroRNA and ROS Crosstalk in Cardiac and Pulmonary Diseases. Int. J. Mol. Sci. 2020, 21, 4370. [Google Scholar] [CrossRef] [PubMed]
- Cheung, E.C.; Vousden, K.H. The role of ROS in tumour development and progression. Nat. Rev. Cancer 2022, 22, 280–297. [Google Scholar] [CrossRef] [PubMed]
- Sosa, V.; Moliné, T.; Somoza, R.; Paciucci, R.; Kondoh, H.; Lleonart, M.E. Oxidative stress and cancer: An overview. Ageing Res. Rev. 2013, 12, 376–390. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pan, Y.; Liu, Y.; Disasa, D.; Akira, M.; Xiang, L.; Qi, J.J.A. A New Geniposidic Acid Derivative Exerts Antiaging Effects through Antioxidative Stress and Autophagy Induction. Antioxidants 2021, 10, 987. [Google Scholar] [CrossRef] [PubMed]
- Tapeinos, C.; Pandit, A. Physical, Chemical, and Biological Structures based on ROS-Sensitive Moieties that are Able to Respond to Oxidative Microenvironments. Adv. Mater. 2016, 28, 5553–5585. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defense against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S. The Mitochondrial Basis of Aging and Age-Related Disorders. Genes 2017, 8, 398. [Google Scholar] [CrossRef] [PubMed]
- Toppo, S.; Vanin, S.; Bosello, V.; Tosatto, S.C. Evolutionary and structural insights into the multifaceted glutathione peroxidase (Gpx) superfamily. Antioxid. Redox Signal. 2008, 10, 1501–1514. [Google Scholar] [CrossRef]
- Brigelius-Flohe, R.; Flohe, L. Regulatory Phenomena in the Glutathione Peroxidase Superfamily. Antioxid. Redox Signal. 2020, 33, 498–516. [Google Scholar] [CrossRef]
- Mariotti, M.; Ridge, P.G.; Zhang, Y.; Lobanov, A.V.; Pringle, T.H.; Guigo, R.; Hatfield, D.L.; Gladyshev, V.N. Composition and evolution of the vertebrate and mammalian selenoproteomes. PLoS ONE 2012, 7, e33066. [Google Scholar] [CrossRef] [PubMed]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione peroxidase-1 in health and disease: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011, 15, 1957–1997. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R.; Kipp, A.P. Physiological functions of GPx2 and its role in inflammation-triggered carcinogenesis. Ann. N. Y. Acad. Sci. 2012, 1259, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Avissar, N.; Ornt, D.B.; Yagil, Y.; Horowitz, S.; Watkins, R.H.; Kerl, E.A.; Takahashi, K.; Palmer, I.S.; Cohen, H.J. Human kidney proximal tubules are the main source of plasma glutathione peroxidase. Am. J. Physiol. 1994, 266, C367–C375. [Google Scholar] [CrossRef]
- Olson, G.E.; Whitin, J.C.; Hill, K.E.; Winfrey, V.P.; Motley, A.K.; Austin, L.M.; Deal, J.; Cohen, H.J.; Burk, R.F. Extracellular glutathione peroxidase (Gpx3) binds specifically to basement membranes of mouse renal cortex tubule cells. Am. J. Physiol. Ren. Physiol. 2010, 298, F1244–F1253. [Google Scholar] [CrossRef] [PubMed]
- Savaskan, N.E.; Ufer, C.; Kühn, H.; Borchert, A. Molecular biology of glutathione peroxidase 4: From genomic structure to developmental expression and neural function. Biol. Chem. 2007, 388, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.; Robson, A.; Houghton, B.C.; Jepson, C.A.; Ford, W.C.; Frayne, J. Epididymal specific, selenium-independent GPX5 protects cells from oxidative stress-induced lipid peroxidation and DNA mutation. Hum. Reprod. 2013, 28, 2332–2342. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R. Glutathione peroxidases and redox-regulated transcription factors. Biol. Chem. 2006, 387, 1329–1335. [Google Scholar] [CrossRef]
- Hanouskova, B.; Vavrova, G.; Ambroz, M.; Bousova, I.; Karlsen, T.A.; Skalova, L.; Matouskova, P. MicroRNAs mediated regulation of glutathione peroxidase 7 expression and its changes during adipogenesis. Biochim. Biophys. Acta Gene Regul. Mech. 2021, 1864, 194734. [Google Scholar] [CrossRef]
- Khatib, A.; Solaimuthu, B.; Ben Yosef, M.; Abu Rmaileh, A.; Tanna, M.; Oren, G.; Schlesinger Frisch, M.; Axelrod, J.H.; Lichtenstein, M.; Shaul, Y.D. The glutathione peroxidase 8 (GPX8)/IL-6/STAT3 axis is essential in maintaining an aggressive breast cancer phenotype. Proc. Natl. Acad. Sci. USA 2020, 117, 21420–21431. [Google Scholar] [CrossRef] [PubMed]
- Herbette, S.; Roeckel-Drevet, P.; Drevet, J.R. Seleno-independent glutathione peroxidases. More than simple antioxidant scavengers. FEBS J. 2007, 274, 2163–2180. [Google Scholar] [CrossRef] [PubMed]
- Nirgude, S.; Choudhary, B. Insights into the role of GPX3, a highly efficient plasma antioxidant, in cancer. Biochem. Pharm. 2021, 184, 114365. [Google Scholar] [CrossRef]
- Chang, C.; Worley, B.L.; Phaeton, R.; Hempel, N. Extracellular Glutathione Peroxidase GPx3 and Its Role in Cancer. Cancers 2020, 12, 2197. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Chen, J.; Yang, W.; Xu, M.; Zhou, J.; Tan, J.; Huang, T. GPX3 expression was down-regulated but positively correlated with poor outcome in human cancers. Front. Oncol. 2023, 13, 990551. [Google Scholar] [CrossRef] [PubMed]
- Hauffe, R.; Stein, V.; Chudoba, C.; Flore, T.; Rath, M.; Ritter, K.; Schell, M.; Wardelmann, K.; Deubel, S.; Kopp, J.F.; et al. GPx3 dysregulation impacts adipose tissue insulin receptor expression and sensitivity. J. Clin. Investig. 2020, 5, 136283. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; He, M.; Tang, X.; Huang, J.; Li, J.; Hong, X.; Fu, H.; Liu, Y. Proteomic landscape of the extracellular matrix in the fibrotic kidney. Kidney Int. 2023, 103, 1063–1076. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Qin, Y.; Cheng, Z.; Cheng, X.; Wang, R.; Luo, X.; Zhao, Y.; Zhang, D.; Li, G. Gpx3 and Egr1 Are Involved in Regulating the Differentiation Fate of Cardiac Fibroblasts under Pressure Overload. Oxid. Med. Cell. Longev. 2022, 2022, 3235250. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohe, R.; Maiorino, M. Glutathione peroxidases. Biochim. Biophys. Acta 2013, 1830, 3289–3303. [Google Scholar] [CrossRef] [PubMed]
- Maddipati, K.R.; Marnett, L.J. Characterization of the major hydroperoxide-reducing activity of human plasma. Purification and properties of a selenium-dependent glutathione peroxidase. J. Biol. Chem. 1987, 262, 17398–17403. [Google Scholar] [CrossRef]
- Katzer, D.; Pauli, L.; Mueller, A.; Reutter, H.; Reinsberg, J.; Fimmers, R.; Bartmann, P.; Bagci, S. Melatonin Concentrations and Antioxidative Capacity of Human Breast Milk According to Gestational Age and the Time of Day. J. Hum. Lact. 2016, 32, NP105–NP110. [Google Scholar] [CrossRef]
- Burk, R.F.; Olson, G.E.; Winfrey, V.P.; Hill, K.E.; Yin, D. Glutathione peroxidase-3 produced by the kidney binds to a population of basement membranes in the gastrointestinal tract and in other tissues. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G32–G38. [Google Scholar] [CrossRef]
- Peng, D.F.; Hu, T.L.; Schneider, B.G.; Chen, Z.; Xu, Z.K.; El-Rifai, W. Silencing of glutathione peroxidase 3 through DNA hypermethylation is associated with lymph node metastasis in gastric carcinomas. PLoS ONE 2012, 7, e46214. [Google Scholar] [CrossRef]
- Yoshimura, S.; Suemizu, H.; Taniguchi, Y.; Arimori, K.; Kawabe, N.; Moriuchi, T. The human plasma glutathione peroxidase-encoding gene: Organization, sequence and localization to chromosome 5q32. Gene 1994, 145, 293–297. [Google Scholar] [CrossRef]
- Bierl, C.; Voetsch, B.; Jin, R.C.; Handy, D.E.; Loscalzo, J. Determinants of Human Plasma Glutathione Peroxidase (GPx-3) Expression. J. Biol. Chem. 2004, 279, 26839–26845. [Google Scholar] [CrossRef]
- Kensler, T.W.; Wakabayashi, N.; Biswal, S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 89–116. [Google Scholar] [CrossRef]
- Westphal, K.; Stangl, V.; Fahling, M.; Dreger, H.; Weller, A.; Baumann, G.; Stangl, K.; Meiners, S. Human-specific induction of glutathione peroxidase-3 by proteasome inhibition in cardiovascular cells. Free Radic. Biol. Med. 2009, 47, 1652–1660. [Google Scholar] [CrossRef]
- Padhy, G.; Sethy, N.K.; Ganju, L.; Bhargava, K. Abundance of plasma antioxidant proteins confers tolerance to acute hypobaric hypoxia exposure. High Alt. Med. Biol. 2013, 14, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.S.; Kim, M.; Youn, B.-S.; Lee, N.S.; Park, J.W.; Lee, I.K.; Lee, Y.S.; Kim, J.B.; Cho, Y.M.; Lee, H.K.; et al. Glutathione Peroxidase 3 Mediates the Antioxidant Effect of Peroxisome Proliferator-Activated Receptor γ in Human Skeletal Muscle Cells. Mol. Cell. Biol. 2009, 29, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Rao, X.; House, M.G.; Nephew, K.P.; Cullen, K.J.; Guo, Z. GPx3 promoter hypermethylation is a frequent event in human cancer and is associated with tumorigenesis and chemotherapy response. Cancer Lett. 2011, 309, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Pan, W.; Xiao, X.; Zhou, X.; Gu, W.; Liu, Y.; Zhao, Y.; Li, L.; Zheng, C.; Liu, J.; et al. MicroRNA-483-5p accentuates cisplatin-induced acute kidney injury by targeting GPX3. Lab. Investig. A J. Tech. Methods Pathol. 2022, 102, 589–601. [Google Scholar] [CrossRef]
- Choi, J.Y.; An, B.C.; Jung, I.J.; Kim, J.H.; Lee, S.W. MiR-921 directly downregulates GPx3 in A549 lung cancer cells. Gene 2019, 700, 163–167. [Google Scholar] [CrossRef]
- Liu, Q.; Bai, W.; Huang, F.; Tang, J.; Lin, X. Downregulation of microRNA-196a inhibits stem cell self-renewal ability and stemness in non-small-cell lung cancer through upregulating GPX3 expression. Int. J. Biochem. Cell Biol. 2019, 115, 105571. [Google Scholar] [CrossRef]
- Schaub, J.A.; Hamidi, H.; Subramanian, L.; Kretzler, M. Systems Biology and Kidney Disease. Clin. J. Am. Soc. Nephrol. 2020, 15, 695–703. [Google Scholar] [CrossRef]
- Bao, Y.W.; Yuan, Y.; Chen, J.H.; Lin, W.Q. Kidney disease models: Tools to identify mechanisms and potential therapeutic targets. Zool. Res. 2018, 39, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Daenen, K.; Andries, A.; Mekahli, D.; Van Schepdael, A.; Jouret, F.; Bammens, B. Oxidative stress in chronic kidney disease. Pediatr. Nephrol. 2019, 34, 975–991. [Google Scholar] [CrossRef]
- Levey, A.S.; James, M.T. Acute Kidney Injury. Ann. Intern. Med. 2017, 167, ITC66–ITC80. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Tian, X.; Yu, C.; Luo, J.; Zhang, J.; Hua, Y.; Wei, G. GPX3 and GSTT1 as biomarkers related to oxidative stress during renal ischemia reperfusion injuries and their relationship with immune infiltration. Front. Immunol. 2023, 14, 1136146. [Google Scholar] [CrossRef] [PubMed]
- Basile, D.P.; Leonard, E.C.; Beal, A.G.; Schleuter, D.; Friedrich, J. Persistent oxidative stress following renal ischemia-reperfusion injury increases ANG II hemodynamic and fibrotic activity. Am. J. Physiol. Ren. Physiol. 2012, 302, F1494–F1502. [Google Scholar] [CrossRef]
- Wu, X.; Tang, S.; Dai, Q.; Yi, B.; Yang, S.; Sun, J.; Zhong, Y.; Lin, W.; Liu, J.; Liu, Y.; et al. Vitamin D-vitamin D receptor alleviates oxidative stress in ischemic acute kidney injury via upregulating glutathione peroxidase 3. FASEB J. 2023, 37, e22738. [Google Scholar] [CrossRef]
- Wu, Y.; Shi, H.; Xu, Y.; Wen, R.; Gong, M.; Hong, G.; Xu, S. Selenoprotein Gene mRNA Expression Evaluation During Renal Ischemia-Reperfusion Injury in Rats and Ebselen Intervention Effects. Biol. Trace Elem. Res. 2023, 201, 1792–1805. [Google Scholar] [CrossRef]
- Revesz, C.; Kaucsar, T.; Godo, M.; Bocskai, K.; Krenacs, T.; Mocsai, A.; Szenasi, G.; Hamar, P. Neutrophils and NADPH Oxidases Are Major Contributors to Mild but Not Severe Ischemic Acute Kidney Injury in Mice. Int. J. Mol. Sci. 2024, 25, 2948. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, Y.; Hao, G.; Wu, Y.E.; Yin, R.; Zheng, Y.; Zhao, W. Recombinant Klotho alleviates vancomycin-induced acute kidney injury by upregulating anti-oxidative capacity via JAK2/STAT3/GPx3 axis. Toxicology 2023, 499, 153657. [Google Scholar] [CrossRef]
- Zou, Z.; Ren, T.; Li, Y.; Zeng, Q.; Wang, X.; Teng, J.; Xu, J.; Jia, P.; Ding, X. The Association Between Serum Glutathione Peroxidase-3 Concentration and Risk of Acute Kidney Injury After Cardiac Surgery: A Nested Case-Control Study. Am. J. Cardiol. 2023, 209, 29–35. [Google Scholar] [CrossRef]
- Drawz, P.; Rahman, M. Chronic kidney disease. Ann. Intern. Med. 2015, 162, ITC1–ITC16. [Google Scholar] [CrossRef]
- Srivastava, A.; Tomar, B.; Sharma, D.; Rath, S.K. Mitochondrial dysfunction and oxidative stress: Role in chronic kidney disease. Life Sci. 2023, 319, 121432. [Google Scholar] [CrossRef]
- Li, L.; Lu, M.; Peng, Y.; Huang, J.; Tang, X.; Chen, J.; Li, J.; Hong, X.; He, M.; Fu, H.; et al. Oxidatively stressed extracellular microenvironment drives fibroblast activation and kidney fibrosis. Redox Biol. 2023, 67, 102868. [Google Scholar] [CrossRef]
- Pang, P.; Abbott, M.; Abdi, M.; Fucci, Q.A.; Chauhan, N.; Mistri, M.; Proctor, B.; Chin, M.; Wang, B.; Yin, W.; et al. Pre-clinical model of severe glutathione peroxidase-3 deficiency and chronic kidney disease results in coronary artery thrombosis and depressed left ventricular function. Nephrol. Dial. Transpl. 2018, 33, 923–934. [Google Scholar] [CrossRef]
- Zitouni, K.; Steyn, M.; Lyka, E.; Kelly, F.J.; Cook, P.; Ster, I.C.; Earle, K.A. Derepression of glomerular filtration, renal blood flow and antioxidant defence in patients with type 2 diabetes at high-risk of cardiorenal disease. Free Radic. Biol. Med. 2020, 161, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Mensah, G.A.; Roth, G.A.; Fuster, V. The Global Burden of Cardiovascular Diseases and Risk Factors: 2020 and Beyond. J. Am. Coll. Cardiol. 2019, 74, 2529–2532. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, H.; Kinugawa, S.; Matsushima, S. Oxidative stress and heart failure. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H2181–H2190. [Google Scholar] [CrossRef] [PubMed]
- Ramachandra, C.J.A.; Cong, S.; Chan, X.; Yap, E.P.; Yu, F.; Hausenloy, D.J. Oxidative stress in cardiac hypertrophy: From molecular mechanisms to novel therapeutic targets. Free Radic. Biol. Med. 2021, 166, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Shih, C.M.; Tsai, L.W.; Dubey, R.; Gupta, D.; Chakraborty, T.; Sharma, N.; Singh, A.V.; Swarup, V.; Singh, H.N. Transcriptomic Profiling Unravels Novel Deregulated Gene Signatures Associated with Acute Myocardial Infarction: A Bioinformatics Approach. Genes 2022, 13, 2321. [Google Scholar] [CrossRef] [PubMed]
- Buijsse, B.; Lee, D.H.; Steffen, L.; Erickson, R.R.; Luepker, R.V.; Jacobs, D.R., Jr.; Holtzman, J.L. Low serum glutathione peroxidase activity is associated with increased cardiovascular mortality in individuals with low HDLc’s. PLoS ONE 2012, 7, e38901. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Yang, J.; Cai, J.; Liu, Q.; Zhang, Z. Selenoprotein Gpx3 knockdown induces myocardial damage through Ca(2+) leaks in chickens. Metallomics 2020, 12, 1713–1728. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Yang, J.; Cai, J.; Liu, Q.; Zhang, J.M.; Zhang, Z. Effect of Gpx3 gene silencing by siRNA on apoptosis and autophagy in chicken cardiomyocytes. J. Cell. Physiol. 2019, 234, 7828–7838. [Google Scholar] [CrossRef] [PubMed]
- Batty, M.; Bennett, M.R.; Yu, E. The Role of Oxidative Stress in Atherosclerosis. Cells 2022, 11, 3843. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef]
- Dogru-Abbasoglu, S.; Kanbagli, O.; Bulur, H.; Babalik, E.; Oztürk, S.; Aykaç-Toker, G.; Uysal, M. Lipid peroxides and antioxidant status in serum of patients with angiographically defined coronary atherosclerosis. Clin. Biochem. 1999, 32, 671–672. [Google Scholar] [CrossRef]
- Jin, R.C.; Mahoney, C.E.; Coleman Anderson, L.; Ottaviano, F.; Croce, K.; Leopold, J.A.; Zhang, Y.Y.; Tang, S.S.; Handy, D.E.; Loscalzo, J. Glutathione peroxidase-3 deficiency promotes platelet-dependent thrombosis in vivo. Circulation 2011, 123, 1963–1973. [Google Scholar] [CrossRef]
- Wolin, M.S. Plasma glutathione peroxidase activity is potentially a key regulator of vascular disease-associated thrombosis. Circulation 2011, 123, 1923–1924. [Google Scholar] [CrossRef] [PubMed]
- Bergmark, B.A.; Mathenge, N.; Merlini, P.A.; Lawrence-Wright, M.B.; Giugliano, R.P. Acute coronary syndromes. Lancet 2022, 399, 1347–1358. [Google Scholar] [CrossRef] [PubMed]
- Lubrano, V.; Pingitore, A.; Traghella, I.; Storti, S.; Parri, S.; Berti, S.; Ndreu, R.; Andrenelli, A.; Palmieri, C.; Iervasi, G.; et al. Emerging Biomarkers of Oxidative Stress in Acute and Stable Coronary Artery Disease: Levels and Determinants. Antioxidants 2019, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Vassalle, C.; Bianchi, S.; Battaglia, D.; Landi, P.; Bianchi, F.; Carpeggiani, C. Elevated levels of oxidative stress as a prognostic predictor of major adverse cardiovascular events in patients with coronary artery disease. J. Atheroscler. Thromb. 2012, 19, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Holley, A.; Pitman, J.; Miller, J.; Harding, S.; Larsen, P. Glutathione peroxidase activity and expression levels are significantly increased in acute coronary syndromes. J. Investig. Med. 2017, 65, 919–925. [Google Scholar] [CrossRef]
- Chen, Q.M. Nrf2 for protection against oxidant generation and mitochondrial damage in cardiac injury. Free Radic. Biol. Med. 2022, 179, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.J.; Efird, J.T.; Davies, S.W.; O’Neal, W.T.; Darden, T.M.; Thayne, K.A.; Katunga, L.A.; Kindell, L.C.; Ferguson, T.B.; Anderson, C.A.; et al. Monoamine oxidase is a major determinant of redox balance in human atrial myocardium and is associated with postoperative atrial fibrillation. J. Am. Heart Assoc. 2014, 3, e000713. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Hou, H.T.; Chen, H.X.; Liu, X.C.; Wang, J.; Yang, Q.; He, G.W. Preoperative plasma biomarkers associated with atrial fibrillation after coronary artery bypass surgery. J. Thorac. Cardiovasc. Surg. 2021, 162, 851–863.e3. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yang, T.; Jiang, Q.; Sun, J.; Gu, L.; Wang, S.; Li, Y.; Chen, B.; Zhao, D.; Sun, R.; et al. Integrated transcriptomic and proteomic analysis reveals potential targets for heart regeneration. Biomol. Biomed. 2023, 23, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Nwabuo, C.C.; Vasan, R.S. Pathophysiology of Hypertensive Heart Disease: Beyond Left Ventricular Hypertrophy. Curr. Hypertens. Rep. 2020, 22, 11. [Google Scholar] [CrossRef]
- Varasteh, Z.; Weber, W.A.; Rischpler, C. Nuclear Molecular Imaging of Cardiac Remodeling after Myocardial Infarction. Pharmaceuticals 2022, 15, 183. [Google Scholar] [CrossRef] [PubMed]
- Debonnaire, P.; Delgado, V.; Bax, J.J. Potential role of fibrosis imaging in severe valvular heart disease. Heart 2015, 101, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Schiattarella, G.G.; Hill, J.A. Metabolic control and oxidative stress in pathological cardiac remodelling. Eur. Heart J. 2017, 38, 1399–1401. [Google Scholar] [CrossRef]
- Covington, T.A.; Pilz, P.M.; Mulhern, R.M.; Ngoy, S.; Loscalzo, A.; Liu, J.; Fisch, S.; Grune, J. GPx3 deficiency exacerbates maladaptive right ventricular remodeling in experimental pulmonary artery banding. Am. J. Physiol. Lung Cell. Mol. Physiol. 2023, 324, L550–L556. [Google Scholar] [CrossRef] [PubMed]
- Aimo, A.; Castiglione, V.; Borrelli, C.; Saccaro, L.F.; Franzini, M.; Masi, S.; Emdin, M.; Giannoni, A. Oxidative stress and inflammation in the evolution of heart failure: From pathophysiology to therapeutic strategies. Eur. J. Prev. Cardiol. 2020, 27, 494–510. [Google Scholar] [CrossRef] [PubMed]
- Munzel, T.; Gori, T.; Keaney, J.F., Jr.; Maack, C.; Daiber, A. Pathophysiological role of oxidative stress in systolic and diastolic heart failure and its therapeutic implications. Eur. Heart J. 2015, 36, 2555–2564. [Google Scholar] [CrossRef]
- Cong, W.; Ruan, D.; Xuan, Y.; Niu, C.; Tao, Y.; Wang, Y.; Zhan, K.; Cai, L.; Jin, L.; Tan, Y. Cardiac-specific overexpression of catalase prevents diabetes-induced pathological changes by inhibiting NF-kappaB signaling activation in the heart. J. Mol. Cell. Cardiol. 2015, 89, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Porter, M.; Pearson, D.J.; Suarez-Mendez, V.J.; Blann, A.D. Plasma, platelet and erythrocyte glutathione peroxidases as risk factors in ischaemic heart disease in man. Clin. Sci. 1992, 83, 343–345. [Google Scholar] [CrossRef]
- Lu, Y.; An, L.; Taylor, M.R.G.; Chen, Q.M. Nrf2 signaling in heart failure: Expression of Nrf2, Keap1, antioxidant, and detoxification genes in dilated or ischemic cardiomyopathy. Physiol. Genom. 2022, 54, 115–127. [Google Scholar] [CrossRef]
- Choi, M.H.; Oh, S.; Choi, J.Y.; Kim, J.H.; Lee, S.W. A statistical learning framework for predicting left ventricular ejection fraction based on glutathione peroxidase-3 level in ischemic heart disease. Comput. Biol. Med. 2022, 149, 105929. [Google Scholar] [CrossRef]
- Franco, C.; Sciatti, E.; Favero, G.; Bonomini, F.; Vizzardi, E.; Rezzani, R. Essential Hypertension and Oxidative Stress: Novel Future Perspectives. Int. J. Mol. Sci. 2022, 23, 14489. [Google Scholar] [CrossRef]
- Hao, Y.; Wu, B.G.; Shi, J.; Chen, Y.L.; Sun, Z.Q.; Zheng, L.Q.; Zhang, X.G.; Geng, N.; Li, T.J.; Li, H.; et al. Association of tag SNPs of GPx-3 with essential hypertension in rural Han Chinese in Fuxin, Liaoning, China. Chin. Med. J. 2011, 124, 2113–2116. [Google Scholar]
- Iwai, N.; Kajimoto, K.; Kokubo, Y.; Tomoike, H. Extensive genetic analysis of 10 candidate genes for hypertension in Japanese. Hypertension 2006, 48, 901–907. [Google Scholar] [CrossRef]
- Decharatchakul, N.; Settasatian, C.; Settasatian, N.; Komanasin, N.; Kukongviriyapan, U.; Intharapetch, P.; Senthong, V.; Sawanyawisuth, K. Association of combined genetic variations in SOD3, GPX3, PON1, and GSTT1 with hypertension and severity of coronary artery disease. Heart Vessel. 2020, 35, 918–929. [Google Scholar] [CrossRef]
- Berillo, O.; Huo, K.G.; Richer, C.; Fraulob-Aquino, J.C.; Briet, M.; Lipman, M.L.; Sinnett, D.; Paradis, P.; Schiffrin, E.L. Distinct transcriptomic profile of small arteries of hypertensive patients with chronic kidney disease identified miR-338-3p targeting GPX3 and PTPRS. J. Hypertens. 2022, 40, 1394–1405. [Google Scholar] [CrossRef]
- Gao, P.; Gao, X.; Xie, B.; Tse, G.; Liu, T. Aging and atrial fibrillation: A vicious circle. Int. J. Cardiol. 2024, 395, 131445. [Google Scholar] [CrossRef]
- Menezes Junior, A.D.S.; Franca, E.S.A.L.G.; Oliveira, J.M.; Silva, D.M.D. Developing Pharmacological Therapies for Atrial Fibrillation Targeting Mitochondrial Dysfunction and Oxidative Stress: A Scoping Review. Int. J. Mol. Sci. 2023, 25, 535. [Google Scholar] [CrossRef]
- Pastori, D.; Pignatelli, P.; Farcomeni, A.; Menichelli, D.; Nocella, C.; Carnevale, R.; Violi, F. Aging-Related Decline of Glutathione Peroxidase 3 and Risk of Cardiovascular Events in Patients With Atrial Fibrillation. J. Am. Heart Assoc. 2016, 5, e003682. [Google Scholar] [CrossRef]
- Pastori, D.; Carnevale, R.; Menichelli, D.; Nocella, C.; Bartimoccia, S.; Novo, M.; Leo, I.; Violi, F.; Pignatelli, P. Is There an Interplay Between Adherence to Mediterranean Diet, Antioxidant Status, and Vascular Disease in Atrial Fibrillation Patients? Antioxid. Redox Signal. 2016, 25, 751–755. [Google Scholar] [CrossRef]
- Menichelli, D.; Carnevale, R.; Nocella, C.; Cammisotto, V.; Castellani, V.; Bartimoccia, S.; Frati, G.; Pignatelli, P.; Pastori, D. Circulating Lipopolysaccharides and Impaired Antioxidant Status in Patients With Atrial Fibrillation. Data From the ATHERO-AF Study. Front. Cardiovasc. Med. 2021, 8, 779503. [Google Scholar] [CrossRef]
- Otoupalova, E.; Smith, S.; Cheng, G.; Thannickal, V.J. Oxidative Stress in Pulmonary Fibrosis. Compr. Physiol. 2020, 10, 509–547. [Google Scholar] [CrossRef]
- Dua, K.; Malyla, V.; Singhvi, G.; Wadhwa, R.; Krishna, R.V.; Shukla, S.D.; Shastri, M.D.; Chellappan, D.K.; Maurya, P.K.; Satija, S.; et al. Increasing complexity and interactions of oxidative stress in chronic respiratory diseases: An emerging need for novel drug delivery systems. Chem. Biol. Interact. 2019, 299, 168–178. [Google Scholar] [CrossRef]
- Schamberger, A.C.; Schiller, H.B.; Fernandez, I.E.; Sterclova, M.; Heinzelmann, K.; Hennen, E.; Hatz, R.; Behr, J.; Vasakova, M.; Mann, M.; et al. Glutathione peroxidase 3 localizes to the epithelial lining fluid and the extracellular matrix in interstitial lung disease. Sci. Rep. 2016, 6, 29952. [Google Scholar] [CrossRef]
- Yamada, Y.; Limmon, G.V.; Zheng, D.; Li, N.; Li, L.; Yin, L.; Chow, V.T.; Chen, J.; Engelward, B.P. Major shifts in the spatio-temporal distribution of lung antioxidant enzymes during influenza pneumonia. PLoS ONE 2012, 7, e31494. [Google Scholar] [CrossRef]
- De Jesus Perez, V.A. Molecular pathogenesis and current pathology of pulmonary hypertension. Heart Fail. Rev. 2016, 21, 239–257. [Google Scholar] [CrossRef]
- Rabinovitch, M. Molecular pathogenesis of pulmonary arterial hypertension. J. Clin. Investig. 2012, 122, 4306–4313. [Google Scholar] [CrossRef]
- Redout, E.M.; van der Toorn, A.; Zuidwijk, M.J.; van de Kolk, C.W.; van Echteld, C.J.; Musters, R.J.; van Hardeveld, C.; Paulus, W.J.; Simonides, W.S. Antioxidant treatment attenuates pulmonary arterial hypertension-induced heart failure. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H1038–H1047. [Google Scholar] [CrossRef]
- He, Y.Y.; Xie, X.M.; Zhang, H.D.; Ye, J.; Gencer, S.; van der Vorst, E.P.C.; Doring, Y.; Weber, C.; Pang, X.B.; Jing, Z.C.; et al. Identification of Hypoxia Induced Metabolism Associated Genes in Pulmonary Hypertension. Front. Pharm. 2021, 12, 753727. [Google Scholar] [CrossRef]
- Sun, Q.; Hackler, J.; Hilger, J.; Gluschke, H.; Muric, A.; Simmons, S.; Schomburg, L.; Siegert, E. Selenium and Copper as Biomarkers for Pulmonary Arterial Hypertension in Systemic Sclerosis. Nutrients 2020, 12, 1894. [Google Scholar] [CrossRef]
- Liu, K.; Hua, S.; Song, L. PM2.5 Exposure and Asthma Development: The Key Role of Oxidative Stress. Oxid. Med. Cell. Longev. 2022, 2022, 3618806. [Google Scholar] [CrossRef]
- Michaeloudes, C.; Abubakar-Waziri, H.; Lakhdar, R.; Raby, K.; Dixey, P.; Adcock, I.M.; Mumby, S.; Bhavsar, P.K.; Chung, K.F. Molecular mechanisms of oxidative stress in asthma. Mol. Asp. Med. 2022, 85, 101026. [Google Scholar] [CrossRef]
- Al-Afaleg, N.O.; Al-Senaidy, A.; El-Ansary, A. Oxidative stress and antioxidant status in Saudi asthmatic patients. Clin. Biochem. 2011, 44, 612–617. [Google Scholar] [CrossRef]
- Ahmad, A.; Shameem, M.; Husain, Q. Relation of oxidant-antioxidant imbalance with disease progression in patients with asthma. Ann. Thorac. Med. 2012, 7, 226–232. [Google Scholar] [CrossRef]
- Laprise, C.; Sladek, R.; Ponton, A.; Bernier, M.C.; Hudson, T.J.; Laviolette, M. Functional classes of bronchial mucosa genes that are differentially expressed in asthma. BMC Genom. 2004, 5, 21. [Google Scholar] [CrossRef]
- Iorio, A.; Velocci, M.; Graziano, M.E.; Piacentini, S.; Polimanti, R.; Manfellotto, D.; Fuciarelli, M. GPX1*Pro198Leu AND GPX3 rs2070593 as genetic risk markers for Italian asthmatic patients. Clin. Exp. Pharm. Physiol. 2016, 43, 277–279. [Google Scholar] [CrossRef]
- Wiegman, C.H.; Li, F.; Ryffel, B.; Togbe, D.; Chung, K.F. Oxidative Stress in Ozone-Induced Chronic Lung Inflammation and Emphysema: A Facet of Chronic Obstructive Pulmonary Disease. Front. Immunol. 2020, 11, 1957. [Google Scholar] [CrossRef]
- Ahmad, A.; Shameem, M.; Husain, Q. Altered oxidant-antioxidant levels in the disease prognosis of chronic obstructive pulmonary disease. Int. J. Tuberc. Lung Dis. 2013, 17, 1104–1109. [Google Scholar] [CrossRef]
- Penailillo, L.; Miranda-Fuentes, C.; Gutierrez, S.; Garcia-Vicencio, S.; Jannas-Vela, S.; Acevedo, C.C.; Penailillo, R.S. Systemic Inflammation but not Oxidative Stress Is Associated with Physical Performance in Moderate Chronic Obstructive Pulmonary Disease. Adv. Exp. Med. Biol. 2024, 1450, 121–130. [Google Scholar] [CrossRef]
- Reddy, A.T.; Lakshmi, S.P.; Banno, A.; Reddy, R.C. Role of GPx3 in PPAR gamma-induced protection against COPD-associated oxidative stress. Free Radic. Biol. Med. 2018, 126, 350–357. [Google Scholar] [CrossRef]
- Zinellu, E.; Zinellu, A.; Pau, M.C.; Piras, B.; Fois, A.G.; Mellino, S.; Carru, C.; Mangoni, A.A.; Pirina, P. Glutathione Peroxidase in Stable Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-analysis. Antioxidants 2021, 10, 1745. [Google Scholar] [CrossRef]
- Olker, C.; Siese, A.; Stumpf, S.; Muller, B.; Gemsa, D.; Garn, H. Impaired superoxide radical production by bronchoalveolar lavage cells from NO(2)-exposed rats. Free Radic. Biol. Med. 2004, 37, 977–987. [Google Scholar] [CrossRef]
- Makena, P.; Kikalova, T.; Prasad, G.L.; Baxter, S.A. Oxidative Stress and Lung Fibrosis: Towards an Adverse Outcome Pathway. Int. J. Mol. Sci. 2023, 24, 12490. [Google Scholar] [CrossRef]
- Liu, J.; Wu, Z.; Liu, Y.; Zhan, Z.; Yang, L.; Wang, C.; Jiang, Q.; Ran, H.; Li, P.; Wang, Z. ROS-responsive liposomes as an inhaled drug delivery nanoplatform for idiopathic pulmonary fibrosis treatment via Nrf2 signaling. J. Nanobiotechnol. 2022, 20, 213. [Google Scholar] [CrossRef]
- Alaaeldin, R.; Mohyeldin, R.H.; Bekhit, A.A.; Gomaa, W.; Zhao, Q.L.; Fathy, M. Vincamine Ameliorates Epithelial-Mesenchymal Transition in Bleomycin-Induced Pulmonary Fibrosis in Rats; Targeting TGF-beta/MAPK/Snai1 Pathway. Molecules 2023, 28, 4665. [Google Scholar] [CrossRef]
- Yousefi-Manesh, H.; Noori, T.; Asgardoon, M.H.; Derakhshan, M.H.; Tavangar, S.M.; Sheibani, M.; Shirooie, S.; Dehpour, A.R. Protective effect of dapsone against bleomycin-induced lung fibrosis in rat. Exp. Mol. Pathol. 2022, 124, 104737. [Google Scholar] [CrossRef]
- Zeng, Y.; Huang, J.; Guo, R.; Cao, S.; Yang, H.; Ouyang, W. Identification and validation of metabolism-related hub genes in idiopathic pulmonary fibrosis. Front. Genet. 2023, 14, 1058582. [Google Scholar] [CrossRef]
- Chien, L.H.; Deng, J.S.; Jiang, W.P.; Chou, Y.N.; Lin, J.G.; Huang, G.J. Evaluation of lung protection of Sanghuangporus sanghuang through TLR4/NF-kappaB/MAPK, keap1/Nrf2/HO-1, CaMKK/AMPK/Sirt1, and TGF-beta/SMAD3 signaling pathways mediating apoptosis and autophagy. Biomed. Pharm. 2023, 165, 115080. [Google Scholar] [CrossRef]
- Kim, K.K.; Whitin, J.C.; Sukhova, N.M.; Cohen, H.J. Increase in extracellular glutatione peroxidase in plasma and lungs of mice exposed to hyperoxia. Pediatr. Res. 1999, 46, 715–721. [Google Scholar] [CrossRef]
- He, J.; Wang, B.; Chen, M.; Song, L.; Li, H. Machine learning-based metabolism-related genes signature, single-cell RNA sequencing, and experimental validation in hypersensitivity pneumonitis. Medicine 2023, 102, e34940. [Google Scholar] [CrossRef]
- Markovic, M.; Ranin, J.; Bukumiric, Z.; Jerotic, D.; Savic-Radojevic, A.; Pljesa-Ercegovac, M.; Djukic, T.; Ercegovac, M.; Asanin, M.; Milosevic, I.; et al. GPX3 Variant Genotype Affects the Risk of Developing Severe Forms of COVID-19. Int. J. Mol. Sci. 2023, 24, 16151. [Google Scholar] [CrossRef]
- Clemente-Suarez, V.J.; Martin-Rodriguez, A.; Redondo-Florez, L.; Lopez-Mora, C.; Yanez-Sepulveda, R.; Tornero-Aguilera, J.F. New Insights and Potential Therapeutic Interventions in Metabolic Diseases. Int. J. Mol. Sci. 2023, 24, 10672. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity: Implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes. Res. Clin. Pract. 2013, 7, e330–e341. [Google Scholar] [CrossRef] [PubMed]
- Pesta, D.; Roden, M. The Janus Head of Oxidative Stress in Metabolic Diseases and During Physical Exercise. Curr. Diabetes Rep. 2017, 17, 41. [Google Scholar] [CrossRef] [PubMed]
- Caruso, A.; Gelsomino, L.; Panza, S.; Accattatis, F.M.; Naimo, G.D.; Barone, I.; Giordano, C.; Catalano, S.; Ando, S. Leptin: A Heavyweight Player in Obesity-Related Cancers. Biomolecules 2023, 13, 1084. [Google Scholar] [CrossRef] [PubMed]
- Bluher, M. Adipose tissue dysfunction contributes to obesity related metabolic diseases. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Okubo, K.; Shimomura, I.; Mizuno, K.; Matsuzawa, Y.; Matsubara, K. Analysis of an expression profile of genes in the human adipose tissue. Gene 1997, 190, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Baez-Duarte, B.G.; Zamora-Ginez, I.; Mendoza-Carrera, F.; Ruiz-Vivanco, G.; Torres-Rasgado, E.; Gonzalez-Mejia, M.E.; Garcia-Zapien, A.; Flores-Martinez, S.E.; Perez-Fuentes, R. Serum levels of glutathione peroxidase 3 in overweight and obese subjects from central Mexico. Arch. Med. Res. 2012, 43, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Costa-Urrutia, P.; Flores-Buendia, A.M.; Ascencio-Montiel, I.; Solares-Tlapechco, J.; Medina-Campos, O.N.; Pedraza-Chaverri, J.; Granados, J.; Jimenez-Osorio, A.S.; Rodriguez-Arellano, M.E. Antioxidant Enzymes Haplotypes and Polymorphisms Associated with Obesity in Mexican Children. Antioxidants 2023, 13, 1084. [Google Scholar] [CrossRef] [PubMed]
- Langhardt, J.; Flehmig, G.; Kloting, N.; Lehmann, S.; Ebert, T.; Kern, M.; Schon, M.R.; Gartner, D.; Lohmann, T.; Dressler, M.; et al. Effects of Weight Loss on Glutathione Peroxidase 3 Serum Concentrations and Adipose Tissue Expression in Human Obesity. Obes. Facts 2018, 11, 475–490. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, A.Y.; Choi, J.W.; Kim, M.; Yasue, S.; Son, H.J.; Masuzaki, H.; Park, K.S.; Kim, J.B. Dysregulation of adipose glutathione peroxidase 3 in obesity contributes to local and systemic oxidative stress. Mol. Endocrinol. 2008, 22, 2176–2189. [Google Scholar] [CrossRef]
- Ighodaro, O.M. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed. Pharm. 2018, 108, 656–662. [Google Scholar] [CrossRef]
- An, Y.; Xu, B.T.; Wan, S.R.; Ma, X.M.; Long, Y.; Xu, Y.; Jiang, Z.Z. The role of oxidative stress in diabetes mellitus-induced vascular endothelial dysfunction. Cardiovasc. Diabetol. 2023, 22, 237. [Google Scholar] [CrossRef] [PubMed]
- Park, P.J.; Kong, S.W.; Tebaldi, T.; Lai, W.R.; Kasif, S.; Kohane, I.S. Integration of heterogeneous expression data sets extends the role of the retinol pathway in diabetes and insulin resistance. Bioinformatics 2009, 25, 3121–3127. [Google Scholar] [CrossRef] [PubMed]
- Iwata, K.; Nishinaka, T.; Matsuno, K.; Yabe-Nishimura, C. Increased gene expression of glutathione peroxidase-3 in diabetic mouse heart. Biol. Pharm. Bull. 2006, 29, 1042–1045. [Google Scholar] [CrossRef] [PubMed]
- Ling, P.; Shan, W.; Zhai, G.; Qiu, C.; Liu, Y.; Xu, Y.; Yang, X. Association between glutathione peroxidase-3 activity and carotid atherosclerosis in patients with type 2 diabetes mellitus. Brain Behav. 2020, 10, e01773. [Google Scholar] [CrossRef] [PubMed]
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc. Diabetol. 2018, 17, 83. [Google Scholar] [CrossRef] [PubMed]
- Demircan, K.; Jensen, R.C.; Chillon, T.S.; Jensen, T.K.; Sun, Q.; Bonnema, S.J.; Hackler, J.; Korevaar, T.I.M.; Glintborg, D.; Schomburg, L.; et al. Serum selenium, selenoprotein P, and glutathione peroxidase 3 during early and late pregnancy in association with gestational diabetes mellitus: Prospective Odense Child Cohort. Am. J. Clin. Nutr. 2023, 118, 1224–1234. [Google Scholar] [CrossRef] [PubMed]
- Asayama, K.; Nakane, T.; Dobashi, K.; Kodera, K.; Hayashibe, H.; Uchida, N.; Nakazawa, S. Effect of obesity and troglitazone on expression of two glutathione peroxidases: Cellular and extracellular types in serum, kidney and adipose tissue. Free Radic. Res. 2001, 34, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.K.; Hevener, A.L.; Barnard, R.J. Metabolic syndrome and insulin resistance: Underlying causes and modification by exercise training. Compr. Physiol. 2013, 3, 1–58. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Farrokhi, F.R.; Butler, A.E.; Sahebkar, A. Insulin resistance: Review of the underlying molecular mechanisms. J. Cell. Physiol. 2019, 234, 8152–8161. [Google Scholar] [CrossRef]
- Grattagliano, I.; Palmieri, V.O.; Portincasa, P.; Moschetta, A.; Palasciano, G. Oxidative stress-induced risk factors associated with the metabolic syndrome: A unifying hypothesis. J. Nutr. Biochem. 2008, 19, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef] [PubMed]
- Pugh, M.E.; Newman, J.H.; Williams, D.B.; Brittain, E.; Robbins, I.M.; Hemnes, A.R. Hemodynamic improvement of pulmonary arterial hypertension after bariatric surgery: Potential role for metabolic regulation. Diabetes Care 2013, 36, e32–e33. [Google Scholar] [CrossRef] [PubMed]
- Baez-Duarte, B.G.; Mendoza-Carrera, F.; Garcia-Zapien, A.; Flores-Martinez, S.E.; Sanchez-Corona, J.; Zamora-Ginez, I.; Torres-Rasgado, E.; Leon-Chavez, B.A.; Perez-Fuentes, R.; Multidisciplinary Research Group on Diabetes of the Instituto Mexicano del Seguro Social. Glutathione peroxidase 3 serum levels and GPX3 gene polymorphisms in subjects with metabolic syndrome. Arch. Med. Res. 2014, 45, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Qiang, R.; Li, Y.; Dai, X.; Lv, W. NLRP3 inflammasome in digestive diseases: From mechanism to therapy. Front. Immunol. 2022, 13, 978190. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Guo, F.; Jin, Y.; Ma, Y. Applications of human organoids in the personalized treatment for digestive diseases. Signal Transduct. Target. 2022, 7, 336. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and Nutritional Antioxidants in Human Diseases. Front. Physiol. 2018, 9, 477. [Google Scholar] [CrossRef] [PubMed]
- Tham, D.M.; Whitin, J.C.; Kim, K.K.; Zhu, S.X.; Cohen, H.J. Expression of extracellular glutathione peroxidase in human and mouse gastrointestinal tract. Am. J. Physiol. 1998, 275, G1463–G1471. [Google Scholar] [CrossRef]
- Hodson, R. Inflammatory bowel disease. Nature 2016, 540, S97. [Google Scholar] [CrossRef]
- Iborra, M.; Moret, I.; Rausell, F.; Bastida, G.; Aguas, M.; Cerrillo, E.; Nos, P.; Beltran, B. Role of oxidative stress and antioxidant enzymes in Crohn’s disease. Biochem. Soc. Trans. 2011, 39, 1102–1106. [Google Scholar] [CrossRef]
- Sahoo, D.K.; Heilmann, R.M.; Paital, B.; Patel, A.; Yadav, V.K.; Wong, D.; Jergens, A.E. Oxidative stress, hormones, and effects of natural antioxidants on intestinal inflammation in inflammatory bowel disease. Front. Endocrinol. 2023, 14, 1217165. [Google Scholar] [CrossRef]
- Thomas, A.G.; Miller, V.; Shenkin, A.; Fell, G.S.; Taylor, F. Selenium and glutathione peroxidase status in paediatric health and gastrointestinal disease. J. Pediatr. Gastroenterol. Nutr. 1994, 19, 213–219. [Google Scholar] [CrossRef]
- Hoffenberg, E.J.; Deutsch, J.; Smith, S.; Sokol, R.J. Circulating antioxidant concentrations in children with inflammatory bowel disease. Am. J. Clin. Nutr. 1997, 65, 1482–1488. [Google Scholar] [CrossRef] [PubMed]
- Tham, D.M.; Whitin, J.C.; Cohen, H.J. Increased expression of extracellular glutathione peroxidase in mice with dextran sodium sulfate-induced experimental colitis. Pediatr. Res. 2002, 51, 641–646. [Google Scholar] [CrossRef]
- Guo, H.; Guo, H.; Xie, Y.; Chen, Y.; Lu, C.; Yang, Z.; Zhu, Y.; Ouyang, Y.; Zhang, Y.; Wang, X. Mo3Se4 nanoparticle with ROS scavenging and multi-enzyme activity for the treatment of DSS-induced colitis in mice. Redox Biol. 2022, 56, 102441. [Google Scholar] [CrossRef] [PubMed]
- Deris Zayeri, Z.; Parsi, A.; Shahrabi, S.; Kargar, M.; Davari, N.; Saki, N. Epigenetic and metabolic reprogramming in inflammatory bowel diseases: Diagnostic and prognostic biomarkers in colorectal cancer. Cancer Cell Int. 2023, 23, 264. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Castro, M.B.; Cornide-Petronio, M.E.; Gracia-Sancho, J.; Peralta, C. Inflammasome-Mediated Inflammation in Liver Ischemia-Reperfusion Injury. Cells 2019, 8, 1131. [Google Scholar] [CrossRef]
- Liu, J.; Man, K. Mechanistic Insight and Clinical Implications of Ischemia/Reperfusion Injury Post Liver Transplantation. Cell. Mol. Gastroenterol. Hepatol. 2023, 15, 1463–1474. [Google Scholar] [CrossRef]
- Abu-Amara, M.; Yang, S.Y.; Tapuria, N.; Fuller, B.; Davidson, B.; Seifalian, A. Liver ischemia/reperfusion injury: Processes in inflammatory networks--a review. Liver Transpl. 2010, 16, 1016–1032. [Google Scholar] [CrossRef]
- Qi, X.; Ng, K.T.; Lian, Q.; Li, C.X.; Geng, W.; Ling, C.C.; Yeung, W.H.; Ma, Y.Y.; Liu, X.B.; Liu, H.; et al. Glutathione Peroxidase 3 Delivered by hiPSC-MSCs Ameliorated Hepatic IR Injury via Inhibition of Hepatic Senescence. Theranostics 2018, 8, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.E.; Wen, Z.; Song, Y.; Wang, H. Paeoniflorin attenuates hepatic ischemia/reperfusion injury via anti-oxidative, anti-inflammatory and anti-apoptotic pathways. Exp. Med. 2016, 11, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Nong, K.; Wang, W.; Niu, X.; Hu, B.; Ma, C.; Bai, Y.; Wu, B.; Wang, Y.; Ai, K. Hepatoprotective effect of exosomes from human-induced pluripotent stem cell—Derived mesenchymal stromal cells against hepatic ischemia-reperfusion injury in rats. Cytotherapy 2016, 18, 1548–1559. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.G.; Ji, D.F.; Zhong, S.; Shi, L.G.; Hu, G.Y.; Chen, S. Saponins from Panax japonicus protect against alcohol-induced hepatic injury in mice by up-regulating the expression of GPX3, SOD1 and SOD3. Alcohol. Alcohol. 2010, 45, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Barascu, A.; Le Chalony, C.; Pennarun, G.; Genet, D.; Zaarour, N.; Bertrand, P. Oxydative stress alters nuclear shape through lamins dysregulation: A route to senescence. Nucleus 2012, 3, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Comakli, S.; Ozdemir, S.; Guloglu, M. Chrysin attenuates paclitaxel-induced hepatorenal toxicity in rats by suppressing oxidative damage, inflammation, and apoptosis. Life Sci. 2023, 332, 122096. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Dai, L.; Li, D. Mitophagy in neurological disorders. J. Neuroinflamm. 2021, 18, 297. [Google Scholar] [CrossRef] [PubMed]
- Soheili, M.; Alinaghipour, A.; Salami, M. Good bacteria, oxidative stress and neurological disorders: Possible therapeutical considerations. Life Sci. 2022, 301, 120605. [Google Scholar] [CrossRef] [PubMed]
- Korczowska-Lacka, I.; Hurla, M.; Banaszek, N.; Kobylarek, D.; Szymanowicz, O.; Kozubski, W.; Dorszewska, J. Selected Biomarkers of Oxidative Stress and Energy Metabolism Disorders in Neurological Diseases. Mol. Neurobiol. 2023, 60, 4132–4149. [Google Scholar] [CrossRef]
- Wainberg, M.; Andrews, S.J.; Tripathy, S.J. Shared genetic risk loci between Alzheimer’s disease and related dementias, Parkinson’s disease, and amyotrophic lateral sclerosis. Alzheimers Res. 2023, 15, 113. [Google Scholar] [CrossRef]
- Longinetti, E.; Fang, F. Epidemiology of amyotrophic lateral sclerosis: An update of recent literature. Curr. Opin. Neurol. 2019, 32, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Tripolszki, K.; Danis, J.; Padhi, A.K.; Gomes, J.; Bozo, R.; Nagy, Z.F.; Nagy, D.; Klivenyi, P.; Engelhardt, J.I.; Szell, M. Angiogenin mutations in Hungarian patients with amyotrophic lateral sclerosis: Clinical, genetic, computational, and functional analyses. Brain Behav. 2019, 9, e01293. [Google Scholar] [CrossRef] [PubMed]
- Masrori, P.; Van Damme, P. Amyotrophic lateral sclerosis: A clinical review. Eur. J. Neurol. 2020, 27, 1918–1929. [Google Scholar] [CrossRef] [PubMed]
- Restuadi, R.; Steyn, F.J.; Kabashi, E.; Ngo, S.T.; Cheng, F.F.; Nabais, M.F.; Thompson, M.J.; Qi, T.; Wu, Y.; Henders, A.K.; et al. Functional characterisation of the amyotrophic lateral sclerosis risk locus GPX3/TNIP1. Genome Med. 2022, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Benyamin, B.; He, J.; Zhao, Q.; Gratten, J.; Garton, F.; Leo, P.J.; Liu, Z.; Mangelsdorf, M.; Al-Chalabi, A.; Anderson, L.; et al. Cross-ethnic meta-analysis identifies association of the GPX3-TNIP1 locus with amyotrophic lateral sclerosis. Nat. Commun. 2017, 8, 611. [Google Scholar] [CrossRef] [PubMed]
- Moumen, R.; Nouvelot, A.; Duval, D.; Lechevalier, B.; Viader, F. Plasma superoxide dismutase and glutathione peroxidase activity in sporadic amyotrophic lateral sclerosis. J. Neurol. Sci. 1997, 151, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Shimazawa, M.; Takata, M.; Kaneko, H.; Tsuruma, K.; Ikeda, T.; Warita, H.; Aoki, M.; Yamada, M.; Takahashi, H.; et al. ITIH4 and Gpx3 are potential biomarkers for amyotrophic lateral sclerosis. J. Neurol. 2013, 260, 1782–1797. [Google Scholar] [CrossRef]
- Duan, Q.Q.; Wang, H.; Su, W.M.; Gu, X.J.; Shen, X.F.; Jiang, Z.; Ren, Y.L.; Cao, B.; Li, G.B.; Wang, Y.; et al. TBK1, a prioritized drug repurposing target for amyotrophic lateral sclerosis: Evidence from druggable genome Mendelian randomization and pharmacological verification in vitro. BMC Med. 2024, 22, 96. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, E.; Garrido, A.; Scholz, S.W.; Poewe, W. Challenges in the diagnosis of Parkinson’s disease. Lancet Neurol. 2021, 20, 385–397. [Google Scholar] [CrossRef]
- Percario, S.; da Silva Barbosa, A.; Varela, E.L.P.; Gomes, A.R.Q.; Ferreira, M.E.S.; de Nazare Araujo Moreira, T.; Dolabela, M.F. Oxidative Stress in Parkinson’s Disease: Potential Benefits of Antioxidant Supplementation. Oxid. Med. Cell. Longev. 2020, 2020, 2360872. [Google Scholar] [CrossRef]
- Duke, D.C.; Moran, L.B.; Pearce, R.K.; Graeber, M.B. The medial and lateral substantia nigra in Parkinson’s disease: mRNA profiles associated with higher brain tissue vulnerability. Neurogenetics 2007, 8, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Wu, Q.; Sun, S.; Bi, G.; Guo, L. Identification of potential diagnostic biomarkers for Parkinson’s disease. FEBS Open Bio 2019, 9, 1460–1468. [Google Scholar] [CrossRef] [PubMed]
- Perrelli, A.; Fatehbasharzad, P.; Benedetti, V.; Ferraris, C.; Fontanella, M.; De Luca, E.; Moglianetti, M.; Battaglia, L.; Retta, S.F. Towards precision nanomedicine for cerebrovascular diseases with emphasis on Cerebral Cavernous Malformation (CCM). Expert Opin. Drug Deliv. 2021, 18, 849–876. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, W.; Wang, X.; Liu, Y.; Wei, H. Nanozyme-Enabled Treatment of Cardio- and Cerebrovascular Diseases. Small 2023, 19, e2204809. [Google Scholar] [CrossRef] [PubMed]
- Freedman, J.E.; Loscalzo, J.; Benoit, S.E.; Valeri, C.R.; Barnard, M.R.; Michelson, A.D. Decreased platelet inhibition by nitric oxide in two brothers with a history of arterial thrombosis. J. Clin. Investig. 1996, 97, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Kenet, G.; Freedman, J.; Shenkman, B.; Regina, E.; Brok-Simoni, F.; Holzman, F.; Vavva, F.; Brand, N.; Michelson, A.; Trolliet, M.; et al. Plasma glutathione peroxidase deficiency and platelet insensitivity to nitric oxide in children with familial stroke. Arter. Thromb. Vasc. Biol. 1999, 19, 2017–2023. [Google Scholar] [CrossRef] [PubMed]
- Voetsch, B.; Jin, R.C.; Bierl, C.; Benke, K.S.; Kenet, G.; Simioni, P.; Ottaviano, F.; Damasceno, B.P.; Annichino-Bizacchi, J.M.; Handy, D.E.; et al. Promoter polymorphisms in the plasma glutathione peroxidase (GPx-3) gene: A novel risk factor for arterial ischemic stroke among young adults and children. Stroke 2007, 38, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Voetsch, B.; Jin, R.C.; Bierl, C.; Deus-Silva, L.; Camargo, E.C.S.; Annichino-Bizacchi, J.M.; Handy, D.E.; Loscalzo, J. Role of Promoter Polymorphisms in the Plasma Glutathione Peroxidase (GPx-3) Gene as a Risk Factor for Cerebral Venous Thrombosis. Stroke 2008, 39, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Akhter, M.S.; Biswas, A.; Rashid, H.; Devi, L.; Behari, M.; Saxena, R. Screening of the GPX3 gene identifies the “T” allele of the SNP -861A/T as a risk for ischemic stroke in young Asian Indians. J. Stroke Cereb. Dis. 2014, 23, 2060–2068. [Google Scholar] [CrossRef]
- Nowak-Gottl, U.; Fiedler, B.; Huge, A.; Niederstadt, T.; Thedieck, S.; Seehafer, T.; Stoll, M. Plasma glutathione peroxidase in pediatric stroke families. J. Thromb. Haemost. 2011, 9, 33–38. [Google Scholar] [CrossRef]
- Loscalzo, J. Nitric oxide insufficiency, platelet activation, and arterial thrombosis. Circ. Res. 2001, 88, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Riegger, J.; Schoppa, A.; Ruths, L.; Haffner-Luntzer, M.; Ignatius, A. Oxidative stress as a key modulator of cell fate decision in osteoarthritis and osteoporosis: A narrative review. Cell. Mol. Biol. Lett. 2023, 28, 76. [Google Scholar] [CrossRef] [PubMed]
- Marchev, A.S.; Dimitrova, P.A.; Burns, A.J.; Kostov, R.V.; Dinkova-Kostova, A.T.; Georgiev, M.I. Oxidative stress and chronic inflammation in osteoarthritis: Can NRF2 counteract these partners in crime? Ann. N. Y. Acad. Sci. 2017, 1401, 114–135. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yu, J.; Liu, H.; Liu, Y.; Liu, N.; Cao, Y.; Zhang, X.; Sun, D. Endemic Kashin-Beck disease: A food-sourced osteoarthropathy. Semin. Arthritis Rheum. 2020, 50, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, D.; Yang, X.; Zhang, D.; Li, Q.; Wang, C.; Yang, X.; Guo, H.; Xiong, Y. CpG methylation of the GPX3 promoter in patients with Kashin-Beck Disease potentially promotes chondrocyte apoptosis. J. Trace Elem. Med. Biol. 2022, 71, 126943. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Yang, X.; Sun, W.; Li, Z.; Ren, H.; Li, B.; Zhang, R.; Zhang, D.; Shi, Z.; Liu, J.; et al. The study of GPX3 methylation in patients with Kashin-Beck Disease and its mechanism in chondrocyte apoptosis. Bone 2018, 117, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Foger-Samwald, U.; Vekszler, G.; Horz-Schuch, E.; Salem, S.; Wipperich, M.; Ritschl, P.; Mousavi, M.; Pietschmann, P. Molecular mechanisms of osteoporotic hip fractures in elderly women. Exp. Gerontol. 2016, 73, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Chiellini, C.; Casini, A.; Cochet, O.; Gabbiani, C.; Ailhaud, G.; Dani, C.; Messori, L.; Amri, E.Z. The influence of auranofin, a clinically established antiarthritic gold drug, on bone metabolism: Analysis of its effects on human multipotent adipose-derived stem cells, taken as a model. Chem. Biodivers. 2008, 5, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Manzanares, W.; Biestro, A.; Galusso, F.; Torre, M.H.; Manay, N.; Pittini, G.; Facchin, G.; Hardy, G. Serum selenium and glutathione peroxidase-3 activity: Biomarkers of systemic inflammation in the critically ill? Intensive Care Med. 2009, 35, 882–889. [Google Scholar] [CrossRef]
- Kim, U.; Kim, C.Y.; Lee, J.M.; Ryu, B.; Kim, J.; Bang, J.; Ahn, N.; Park, J.H. Loss of glutathione peroxidase 3 induces ROS and contributes to prostatic hyperplasia in Nkx3.1 knockout mice. Andrology 2020, 8, 1486–1493. [Google Scholar] [CrossRef]

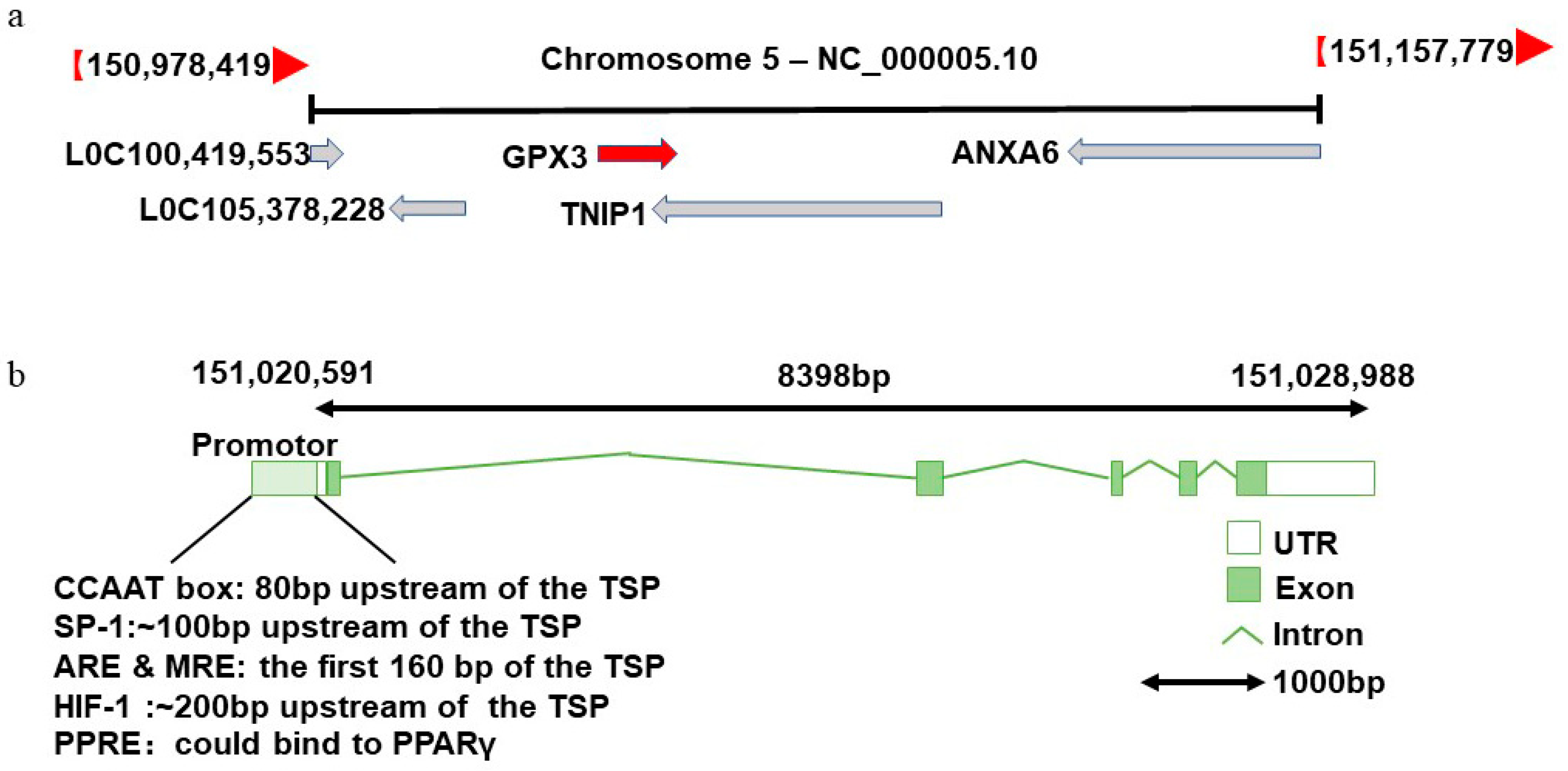
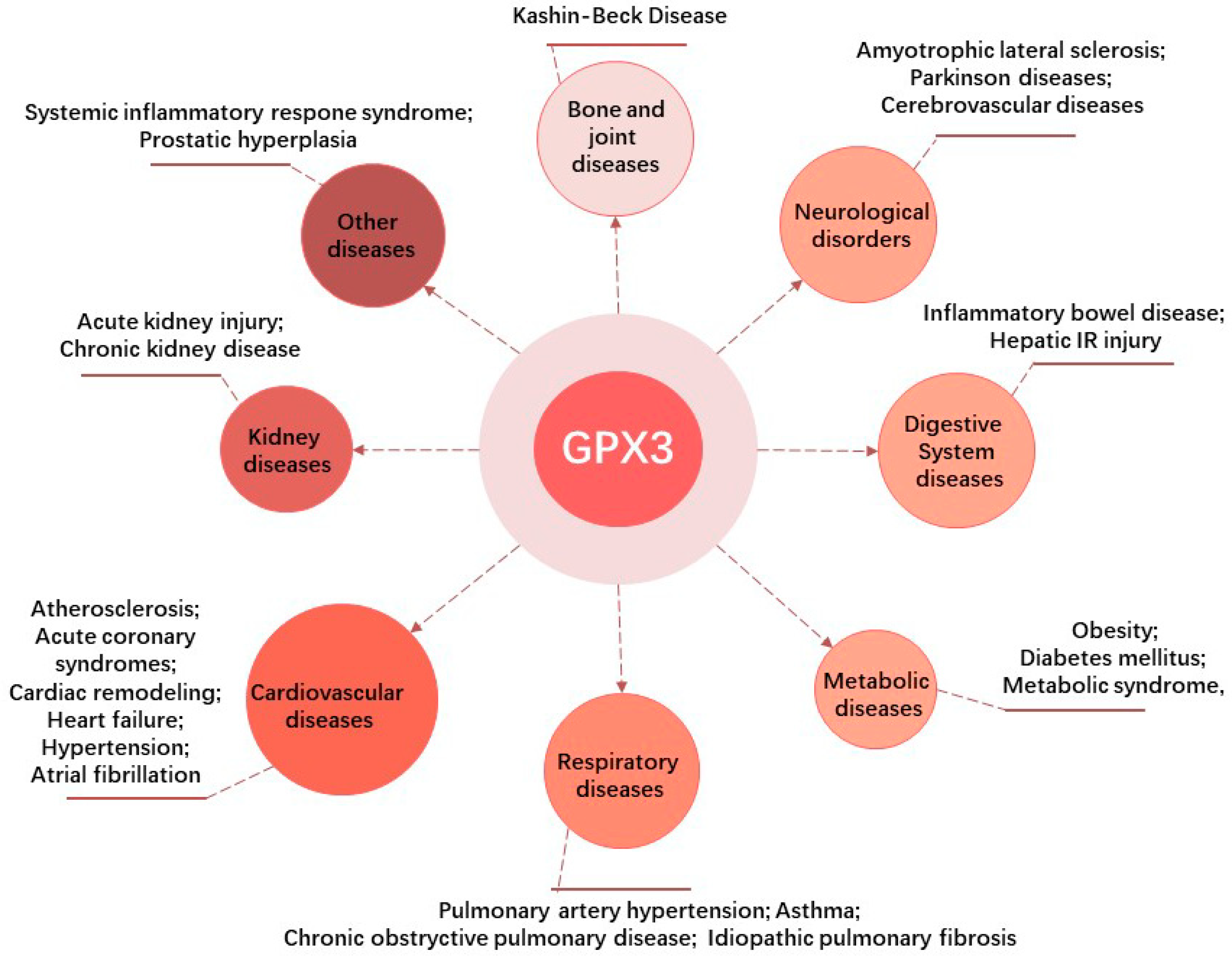

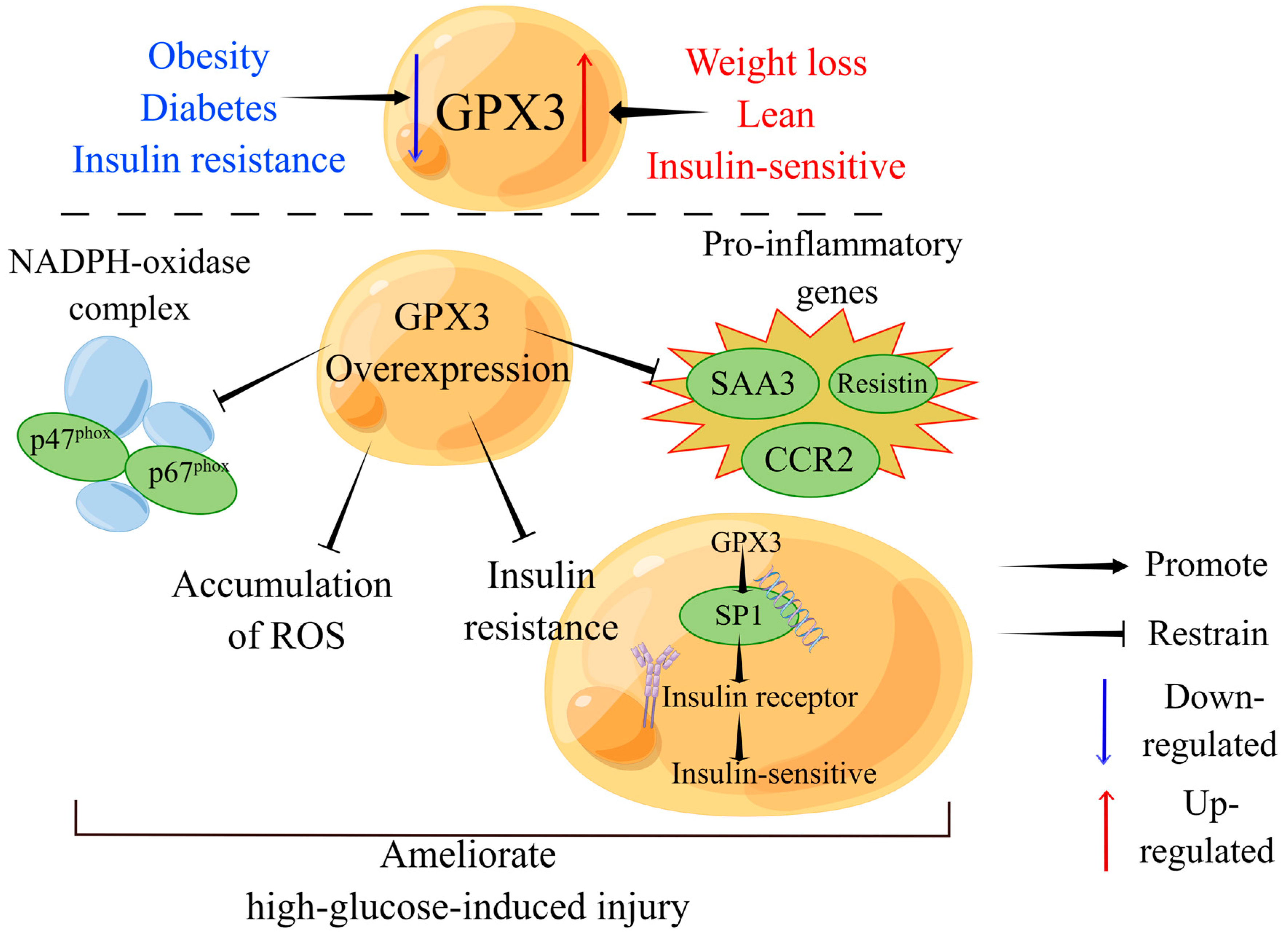
| GPX Type | Peroxidatic Residue | Gene Location | Tissue Distribution | Cellular Localization | Reference |
|---|---|---|---|---|---|
| GPX1 | Sec | 3p21.31 | Ubiquitously expressed; high in kidneys, liver, erythrocytes | Cytosol, peroxisome, mitochondrion | [13] |
| GPX2 | Sec | 14q23.3 | Intestinal and pulmonary epithelium, | Cytosol | [14] |
| GPX3 | Sec | 5q33.1 | Plasma, kidneys, adipose tissue, extracellular body fluids | Extracellular space | [15,16] |
| GPX4 | Sec | 19p13.3 | Fat, testis, spermatozoa | Cytosol, nucleus, mitochondrion | [17] |
| GPX5 | Cys | 6p22.1 | Testis, prostate, epididymis, spermatozoa | Extracellular | [18] |
| GPX6 | Sec | 6p22.1 | Embryos, olfactory epithelium | Extracellular | [19] |
| GPX7 | Cys | 1p32.3 | Placenta, thyroid, urinary bladder | Extracellular, endoplasmic reticulum | [20] |
| GPX8 | Cys | 5q11.2 | Placenta, endometrium, ovary | Endoplasmic reticulum | [21] |
| Disease | Expression of GPX3 in Disease | Pathway Regulated | Roles of GPX3 | Reference |
|---|---|---|---|---|
| AKI (IR induced) | Down in renal tissue | Oxidative stress; Apoptosis and immune responses | As a biomarker related to oxidative stress during renal IR injuries; Related to immune infiltration | [48] |
| AKI (IR induced) | Down in renal tissue | Oxidative stress | Positive correlation between GPX3 levels and the severity of renal injury | [49] |
| AKI (IR induced) | Down in renal tissue | Oxidative stress; Apoptosis | Loss of renal GPX3 may promote renal oxidative stress injury | [50] |
| AKI (IR induced) | Down in renal tissue | Oxidative stress | Protects proximal tubular epithelial cell membrane from oxidative damage in renal IR injury | [51] |
| AKI (IR or vancomycin induced) | Down in renal tissue | - | - | [52,53] |
| AKI (cardiac surgery associated) | Down in the serum | - | GPX3 ratio has predictive value for cardiac surgery associated AKI | [54] |
| CKD | Down in renal tubules | NOX4 upregulation in the extracellular microenvironment leads to fibroblast activation | Loss of GPX3 mediates fibroblast activation via an oxidatively stressed extracellular microenvironment | [27] |
| CKD | Down in the kidney | Triggers NOX4 expression, oxidative stress and fibroblasts proliferation and activation | Orchestrates an oxidatively stressed extracellular microenvironment | [57] |
| CKD | Down in the kidney | - | GPX3 deficiency contributes to kidney disease-induced cardiac disease | [58] |
| Atherosclerosis | - | - | GPX3 deficiency promotes platelet-dependent thrombosis and enhances arterial thrombotic risk | [70] |
| ACS patients | Elevated in plasma (activity, protein, mRNA) | - | Higher levels of GPX3 associated with improved outcomes | [75] |
| AMI patients | Upregulated in blood | - | GPX3 may help develop therapeutic strategies for acute MI management | [63] |
| POAF following CABG | Increased in plasma | - | GPX3 may serve as biomarkers to predict POAF | [78] |
| MI (Mouse model) | Upregulated in myocardial tissue | - | GPX3 as a potential target for heart regeneration therapy | [79] |
| Cardiac remodeling | - | Inhibited oxidative stress | GPX3 promotes differentiation of cardiac fibroblasts into a protective state, attenuating myocardial fibrosis | [28] |
| HF (DCM and ICM) | Decreased in hearts | - | Higher serum GPX3 levels (≥5.314 μg/mL) closely related to reduced LVEF (<50%) | [89,90] |
| Hypertensive patients with CKD | Upregulated in gluteal subcutaneous small arteries | - | GPX3 could represent novel therapeutic targets for hypertension and vascular damage in CKD | [95] |
| Aging | Decline in serum | - | GPX’s decline increases the risk of cardiovascular events in individuals with atrial fibrillation | [98] |
| PAH | Lower in lung tissues | GPX3 is a hypoxia-induced metabolism-associated hub gene | Provides insight into the molecular mechanisms of hypoxic PAH and potential therapeutic targets | [108] |
| Systemic sclerosis related PAH | Activity is reduced in serum | - | - | [109] |
| Asthma | Downregulated in bronchial biopsies tissue | - | - | [114] |
| COPD (Patients and CSE-treated cells) | Reduced in human bronchial epithelial cells. | CSE reduces GPX3 expression by downregulating PPARγ expression or activity | GPX3 as a PPARγ transcriptional target, provides valuable information for developing more effective therapeutics for COPD. | [119] |
| COPD patients | No significantly difference in serum/plasma GPX3 | - | - | [120] |
| COPD (Rats exposed to NO2) | Increased mRNA and activity in bronchoalveolar lavage fluid | - | - | [121] |
| IPF patients | Reduced in lung tissues | Oxidative stress | Influence the fibrotic phenotype | [126,127] |
| IPF patients | Upregulated in lung homogenates | - | - | [103] |
| IPF (Bleomycin- induced lung fibrosis) | Upregulated in mouse bronchoalveolar lavage fluid | - | - | [103] |
| Obesity | Elevated in the serum in central Mexico | Serum GPX3 concentration positively correlates with body weight and inversely with insulin sensitivity | [137] | |
| Obesity | No difference in serum concentrations, lower in subcutaneous adipose tissue. | - | GPX3 higher in lean and insulin-sensitive individuals compared to obese and insulin-resistant counterparts | [139] |
| Obesity | Downregulated in plasma and adipose tissue | - | - | [140] |
| Diabetes mellitus (type 2 patients) | Lower mRNA in adipose tissue | - | - | [139] |
| Diabetes mellitus (mice) | Upregulated in hearts | Oxidative stress | Plays an important role in protecting cardiomyocytes | [144] |
| Metabolic syndrome patients | elevated in serum | - | Elevated GPX3 levels correlated with low insulin sensitivity and cardiovascular risk | [154] |
| IBD (children) | Increased activity in plasma | - | - | [163,164] |
| IBD mouse (dextran sodium sulfate-fed) | Increased plasma activity | Inflammatory | GPX3 protein associated with increased GPX3 mRNA levels in the kidney | [165] |
| ALS | No difference in plasma concentration; Lower GPX3 activity | - | GPX3 as a lead ALS risk gene, GPX3-TNIP1 locus associated with ALS | [184,185,186] |
| ALS (Rats with mutant SOD1H46R) | Higher in serum in pre-symptomatic stage, decreased as disease progressed | - | - | [187] |
| Parkinson’s disease | Upregulated in blood | - | GPX3 as a potential biomarker for the disease | [192] |
| Cerebral thrombotic disorder patients | Decreased activity in blood | High ROS accumulation and rapid inactivation of nitric oxide | Exogenous GPX3 supplementation in plasma defends against thrombotic disorder | [195] |
| Kashin-Beck Disease | Reduced mRNA in chondrocytes | Elevated methylation of CpGs reduces antioxidant function and promotes chondrocyte apoptosis, | Reduced GPX3 accelerates KBD development | [206] |
| Hip fracture patients | Increased in bone samples | - | Increased GPX3 suggests increased antioxidative activity in bone samples. | [208] |
| Multiple organ failure and systemic inflammatory response patients | Decreased activity in serum | - | Early decrease of Se and GPX3 associated with multiple organ failure and systemic inflammatory response in ICU | [209] |
| Disease | Therapies Based on GPX3 | Effect | Involved Pathways | Therapeutic Target | Potential Disease Biomarkers | Reference |
|---|---|---|---|---|---|---|
| AKI (IR induced) | GPX3-overexpression plasmid; vitamin Dvitamin D receptor; VDR agonist paricalcitol | Protects kidneys from oxidative stress injury. | Oxidative stress; Apoptosis | Maintaining renal GPX3 could be a strategy for AKI | Loss of renal GPX3 may promote renal oxidative stress injury | [50] |
| AKI (IR induced) | Apocynin; Neutrophil deficiency | Attenuated IR-induced renal functional impairment | Antioxidant | Enhancing GPX3 could be a strategic antioxidant defense against renal IR injury | - | [52] |
| AKI (Cisplatin induced) | Overexpression of GPX3 by virus injection | Prevents cisplatin induced AKI | Inhibiting oxidative stress and apoptosis of tubular cells | - | - | [41] |
| AKI (Vancomycin induced) | Recombinant Klotho | Alleviates vancomycin induced AKI | Upregulating anti-oxidative capacity via -JAK2/STAT3/GPX3 axis | - | - | [53] |
| AKI (Cardiac surgery associated) | - | - | - | - | GPX3 ratio as an early diagnostic marker for AKI | [54] |
| CKD | Overexpression of exogenous GPX3 | Alleviates kidney fibrosis | Inhibits NADPH oxidase 2 and p38 mitogen activated protein kinase | - | - | [27] |
| CKD patients | High GPX3 activity with usual care | Sustained increase in renal function | Derepresses renal blood flow and a rise in eGFR | - | Inverse relationship between GPX3 activity and rate of eGFR decline | [59] |
| Atherosclerosis | - | - | - | - | GPX3 may impair endothelial function and result in atherosclerosis | [70,71] |
| MI (Mouse model of heart regeneration) | - | - | - | GPX3 as a target for heart regeneration therapy | - | [79] |
| Hypertension | - | - | - | - | GPX3 rs3828599-GG associated with hypertension incidence | [94] |
| Hypertensive patients with CKD | miR-338-3p | Target GPX3 | - | GPX3 for managing hypertension and vascular damage in CKD | - | [95] |
| Aging | - | - | - | - | Decreased GPX3 activity may predict cardiovascular complications | [98] |
| AF | Mediterranean diet | Reduces vascular events rate | Modulates antioxidant activity of GPX3, | Nutritional strategy to prevent vascular events | - | [99] |
| Asthma | Two allelic mutations in GPX3 rs2070593 | Prevents asthma development | - | GPX3 rs2070593 is as a protective locus for asthma | - | [115] |
| Hypersensitivity pneumonitis | - | - | - | GPX3 as a diagnostic biomarker with hypersensitivity pneumonitis | - | [129] |
| COVID-19 | GPX3 rs8177412 variant genotype | Associated with severe COVID-19 | - | GPX3 might be a complementing otheras a diagnostic tool for COVID-19 course prediction | - | [130] |
| Obesity | GPX3 rs922429 | Protects against obesity classified by body fat percentage | - | - | - | [138] |
| T2DM | - | - | - | - | Lower GPX3 activity may predict carotid atherosclerosis in T2DM | [145,146] |
| Gestational DM | - | - | - | - | Low GPX3 activity associated with higher risk of gestational DM | [147] |
| Metabolic syndrome | Selenite | Enhances insulin receptor expression and adipocyte differentiation | Induces GPX3 expression; activates SP1 | - | - | [26] |
| Metabolic syndrome | Overexpression of GPX3 in adipocytes | Suppresses pro-inflammatory gene and insulin resistance | Suppresses p47 and p67 subunits of NADPH-oxidase complex | - | - | [140] |
| IBD (sodium dextran sulfate induced) | Polyethylene glycol-modified Mo3Se4 nano flakes with multiple antioxidant enzymatic activities, including GPX3 | Reduces disease activity index scores and reverses SS-induced IBD | Nrf2-keap1 antioxidant pathway | - | - | [166] |
| Hepatic IR injury | hiPSC-MSCs delivering GPX3; Recombinant GPX3 | Ameliorates hepatic IR injury | Inhibits hepatic senescence and apoptosis; promotes liver regeneration | - | - | [171] |
| Hepatic IR injury | Paeoniflorin | Attenuates hepatic IR injury | Enhances hepatic GPX3 activity | Modulating micro-environment; Enhancing GPX3 supplementation might be a strategy for liver IR injury. | - | [172,173] |
| Hepatic injury (alcohol induced) | Up-regulation of GPX3 | Protects against alcohol induced hepatic injury | - | - | - | [174] |
| ALS (Rats with mutant SOD1H46R) | - | - | - | Detects disease presence and stage progression | Potential serum biomarker for ALS | [187,188] |
| Cerebral thrombotic disorder | Exogenous GPX3 supplementation in plasma | Defends against thrombotic disorder | Restores nitric oxide-mediated platelet inhibition | - | Targeting GPX3 for thrombotic disorders | [195,201] |
| Kashin-Beck Disease | Selenium supplementation | Exerts protective effect | Reverses methylation status of GPX3, increases GPX3 expression, inhibits PI3K/Akt/c-fos pathway | - | - | [206] |
| Systemic inflammatory response syndrome | - | - | - | - | Decreased GPX3 activity as a predictor for this syndrome. | [209] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, N.; Liao, H.; Lin, Z.; Tang, Q. Insights into the Role of Glutathione Peroxidase 3 in Non-Neoplastic Diseases. Biomolecules 2024, 14, 689. https://doi.org/10.3390/biom14060689
Zhang N, Liao H, Lin Z, Tang Q. Insights into the Role of Glutathione Peroxidase 3 in Non-Neoplastic Diseases. Biomolecules. 2024; 14(6):689. https://doi.org/10.3390/biom14060689
Chicago/Turabian StyleZhang, Nan, Haihan Liao, Zheng Lin, and Qizhu Tang. 2024. "Insights into the Role of Glutathione Peroxidase 3 in Non-Neoplastic Diseases" Biomolecules 14, no. 6: 689. https://doi.org/10.3390/biom14060689





