Quercetin Protects Blood–Brain Barrier Integrity via the PI3K/Akt/Erk Signaling Pathway in a Mouse Model of Meningitis Induced by Glaesserella parasuis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Drug
2.2. Experimental Animals and Cells
2.3. G. parasuis Infection and Quercetin Pretreatment
2.4. RNA and Protein Extraction
2.5. Transcriptome Sequencing and Data Analysis
2.6. Cell Viability Assays
2.7. Adhesion and Invasion Test of GPS
2.8. Hematoxylin and Eosin (H&E) Staining and Immunofluorescence Microscopy
2.9. The qRT-PCR Analysis
2.10. Western Blotting
2.11. Statistical Analysis
3. Results
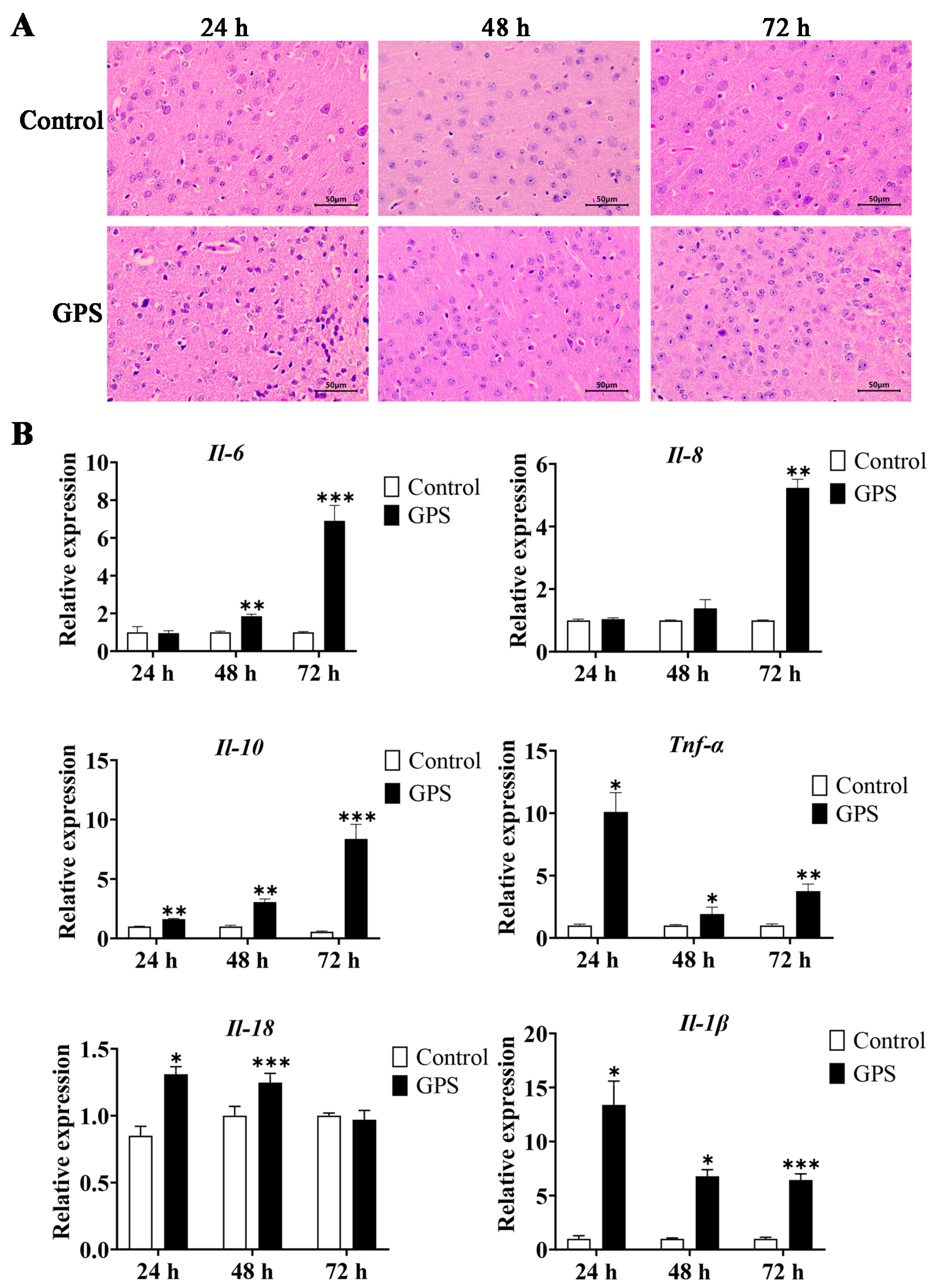
3.1. GPS-Induced Meningitis Traits in Mice
3.2. Transcriptome Changes and Significant Pathways in the Cerebrum of GPS-Infected Mice
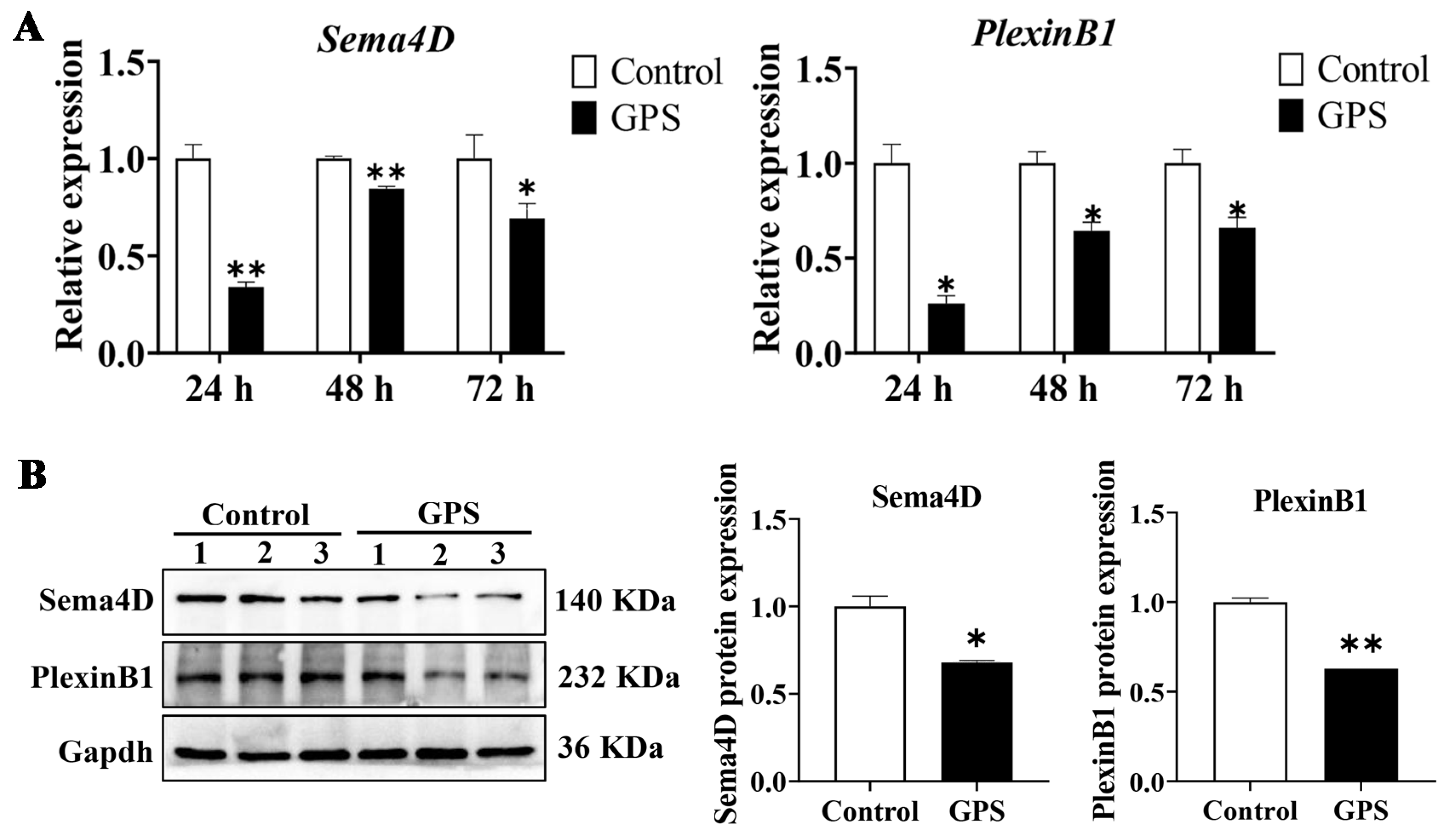
3.3. GPS Infection Decreased the Expression of Angiogenetic Genes
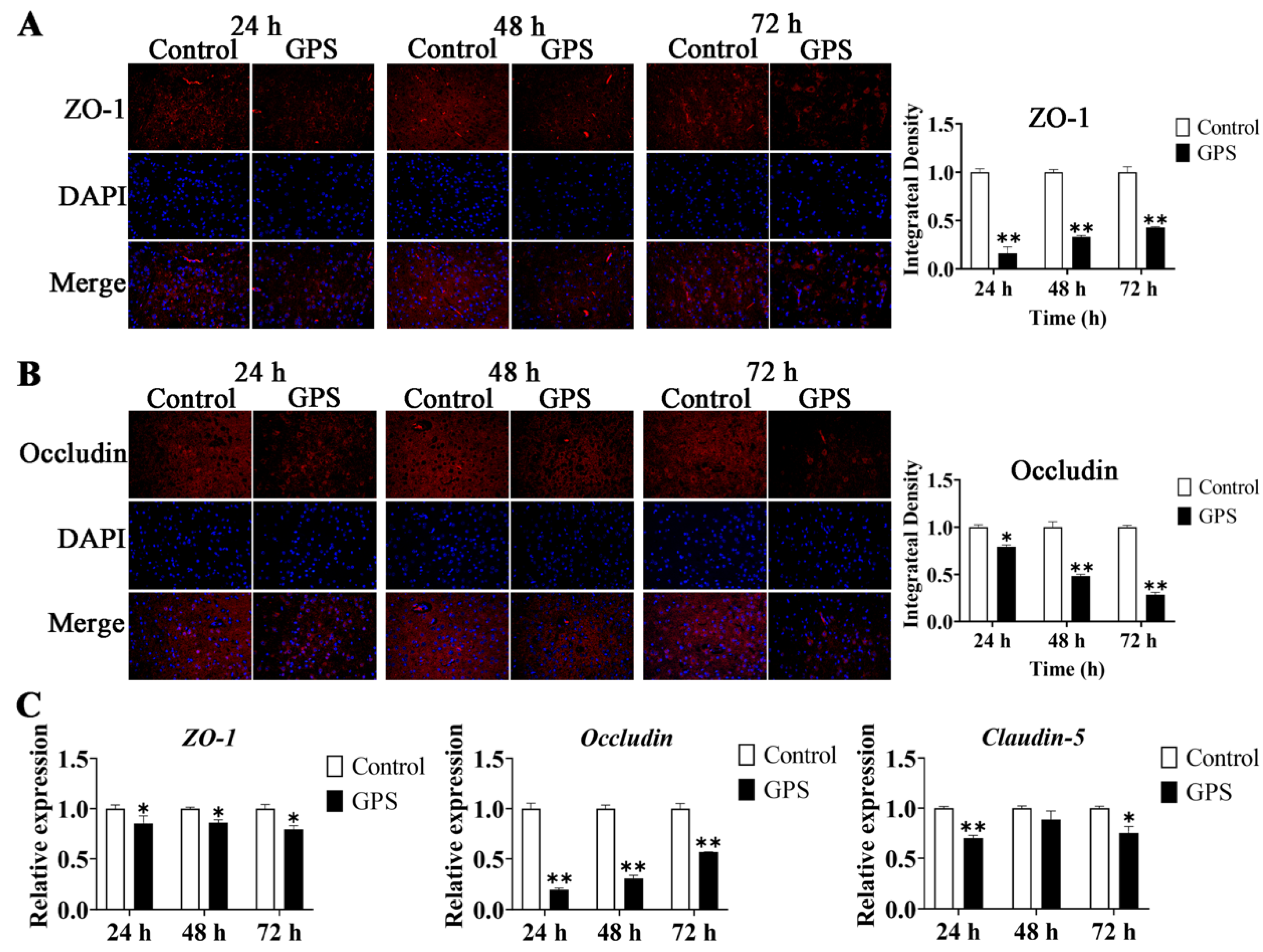
3.4. GPS Infection Disrupted the TJ of the Mouse Cerebrum In Vivo
3.5. GPS Infection Promoted the Expression of BBB-Permeability Marker Genes
3.6. GPS Infection Suppressed the Activation of PI3K/Akt/Erk Pathways In Vivo
3.7. GPS Infection Model in Mouse Brain Microvascular Endothelial Cells (bEnd.3)
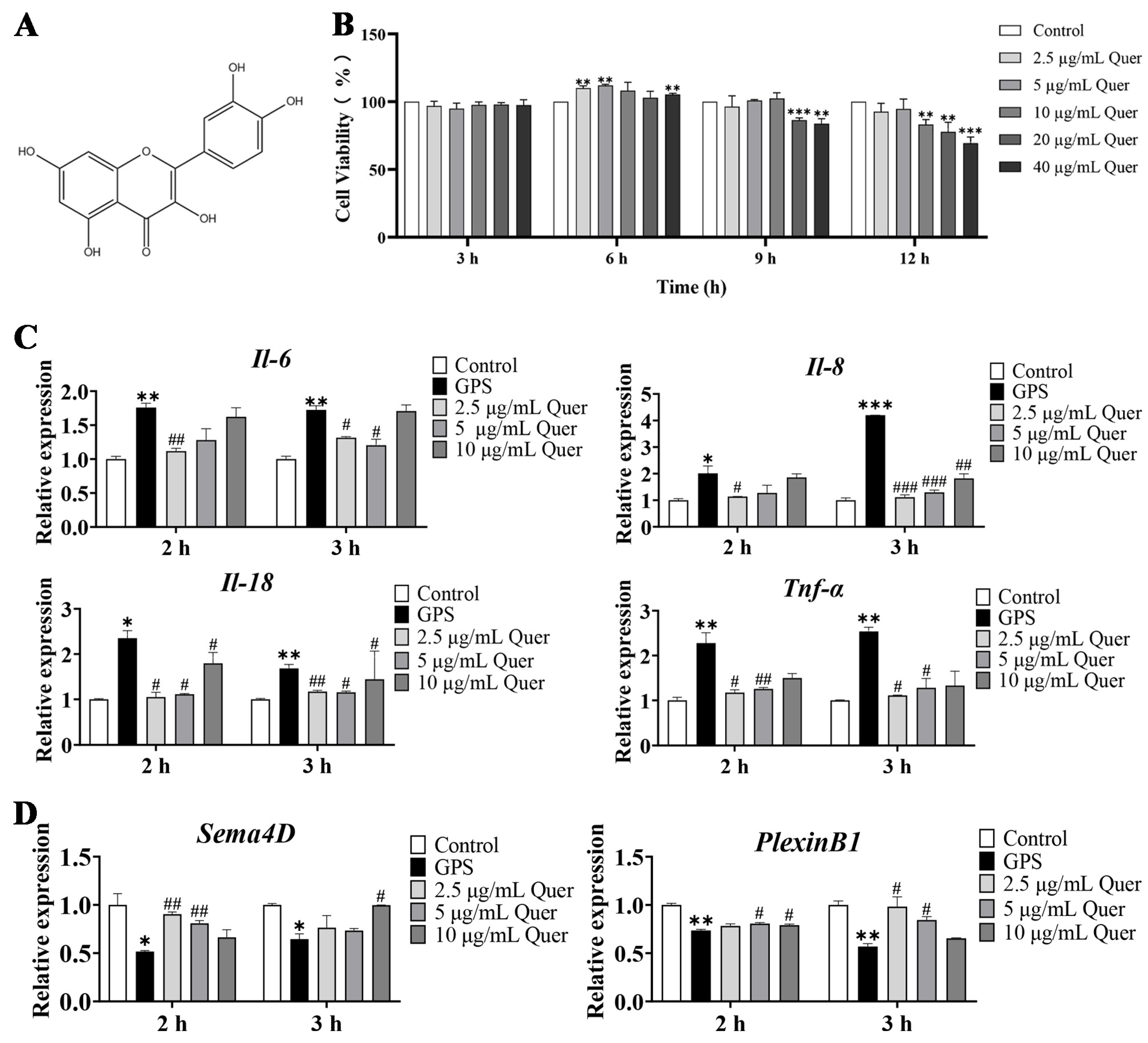
3.8. Structure and Cytotoxicity of Quercetin
3.9. Quercetin Suppressed GPS-Induced Inflammatory Responses in bEnd.3 Cells
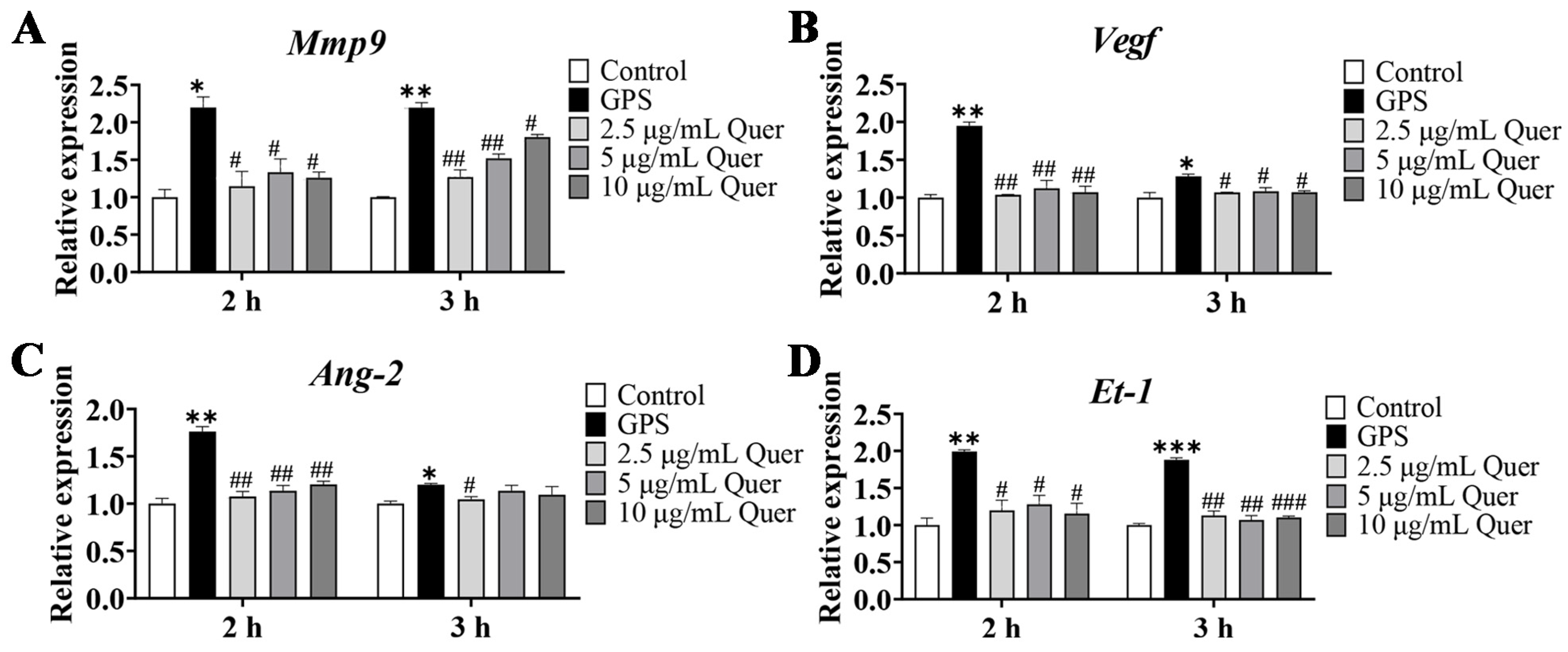
3.10. Quercetin Increased the Expression of Angiogenetic Genes in bEnd.3 Cells
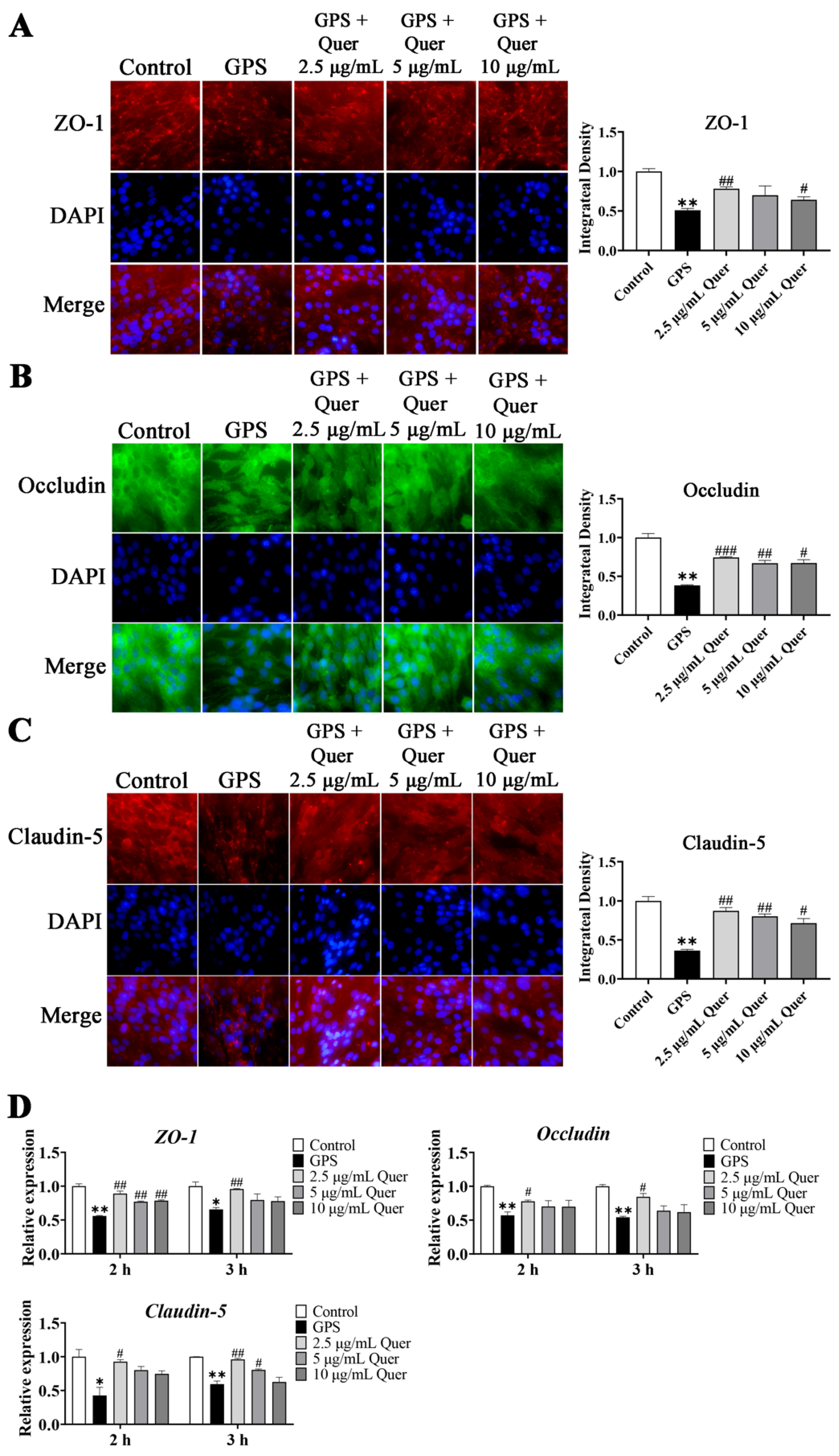
3.11. GPS-Induced TJ Disruption In Vitro
3.12. Quercetin Suppressed GPS-Induced Upregulation of BBB-Permeability Marker Genes
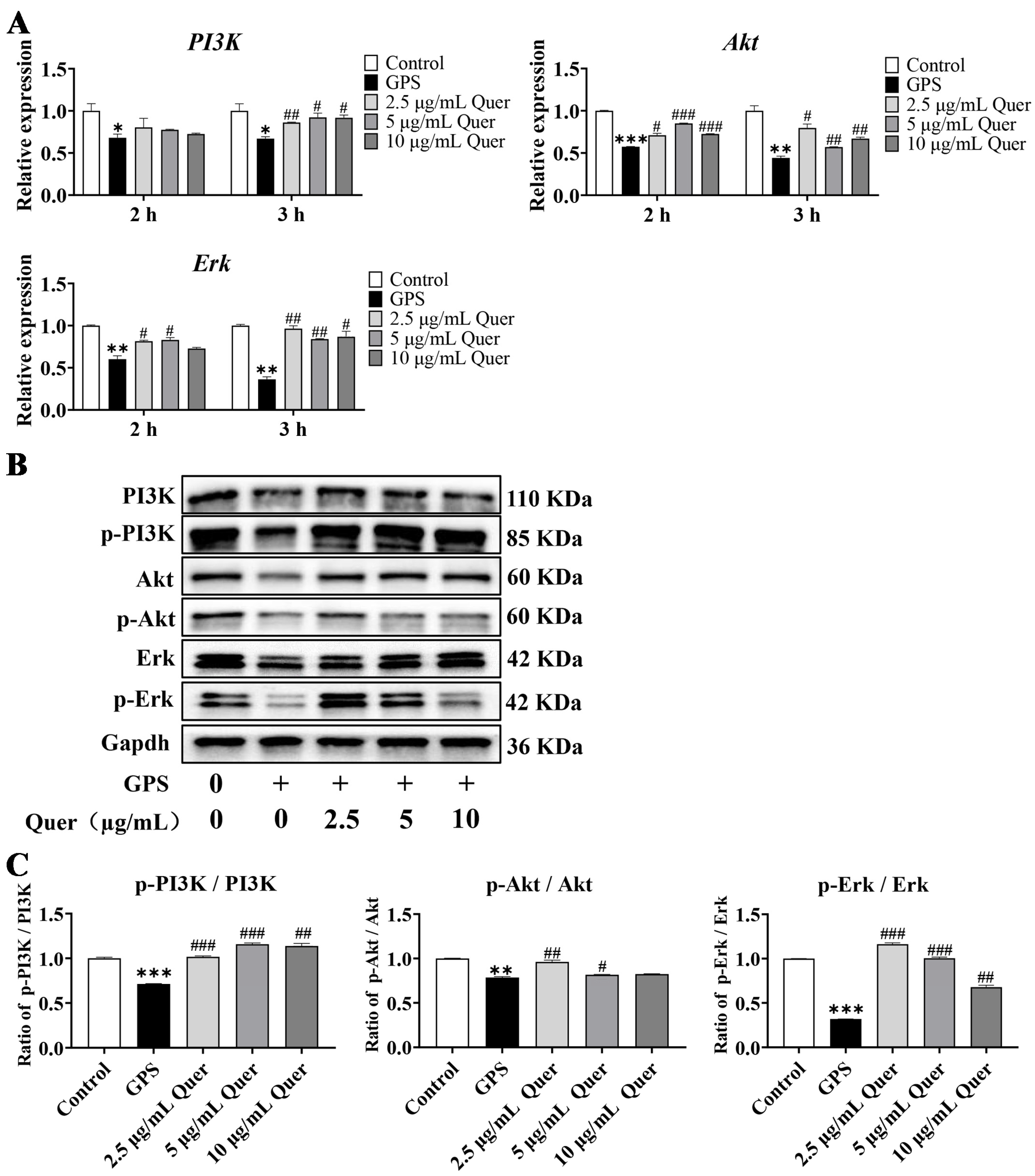
3.13. Quercetin Reactivated GPS-Induced Suppression of the PI3K/Akt/Erk Pathway In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turni, C.; Wu, Y.; Omaleki, L.; Giang, N.; Blackall, P.J.; Christensen, H. Glaesserella australis sp. nov., isolated from the lungs of pigs. Int. J. Syst. Evol. Microbiol. 2020, 70, 3686–3692. [Google Scholar] [CrossRef]
- Guo, L.; Cheng, H.; Fu, S.; Liu, J.; Zhang, Y.; Qiu, Y.; Chen, H. Methylome and Transcriptome-Based Integration Analysis Identified Molecular Signatures Associated with Meningitis Induced by Glaesserella parasuis. Front. Immunol. 2022, 13, 840399. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.B.; Gong, Q.L.; Zhao, Q.; Li, X.Y.; Zhang, X.X. Prevalence of Haemophilus parasuis “Glaesserella parasuis” in pigs in China: A systematic review and meta-analysis. Prev. Vet. Med. 2020, 182, 105083. [Google Scholar] [CrossRef] [PubMed]
- Scherrer, S.; Rademacher, F.; Stephan, R.; Peterhans, S. Identification of Glaesserella parasuis and Differentiation of Its 15 Serovars Using High-Resolution Melting Assays. Pathogens 2022, 11, 752. [Google Scholar] [CrossRef] [PubMed]
- Costa-Hurtado, M.; Barba-Vidal, E.; Maldonado, J.; Aragon, V. Update on Glasser’s disease: How to control the disease under restrictive use of antimicrobials. Vet. Microbiol. 2020, 242, 108595. [Google Scholar] [CrossRef]
- Putz, K.; Hayani, K.; Zar, F.A. Meningitis. Prim. Care 2013, 40, 707–726. [Google Scholar] [CrossRef]
- Le Guennec, L.; Coureuil, M.; Nassif, X.; Bourdoulous, S. Strategies used by bacterial pathogens to cross the blood-brain barrier. Cell. Microbiol. 2020, 22, e13132. [Google Scholar] [CrossRef]
- Yang, R.C.; Wang, J.D.; Wang, F.; Zhang, H.P.; Tan, C.; Chen, H.C.; Wang, X.R. Blood-Brain Barrier Integrity Damage in Bacterial Meningitis: The Underlying Link, Mechanisms, and Therapeutic Targets. Int. J. Mol. Sci. 2023, 24, 2852. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Stamatovic, S.M.; Johnson, A.M.; Keep, R.F.; Andjelkovic, A.V. Junctional proteins of the blood-brain barrier: New insights into function and dysfunction. Tissue Barriers 2016, 4, e1154641. [Google Scholar] [CrossRef]
- Bhat, A.A.; Uppada, S.; Achkar, I.W.; Hashem, S.; Yadav, S.K.; Shanmugakonar, M.; Al-Naemi, H.A.; Haris, M.; Uddin, S. Tight Junction Proteins and Signaling Pathways in Cancer and Inflammation: A Functional Crosstalk. Front. Physiol. 2019, 9, 1942. [Google Scholar] [CrossRef]
- Kunze, R.; Marti, H.H. Angioneurins—Key regulators of blood-brain barrier integrity during hypoxic and ischemic brain injury. Prog. Neurobiol. 2019, 178, 101611. [Google Scholar]
- Michinaga, S.; Kimura, A.; Hatanaka, S.; Minami, S.; Asano, A.; Ikushima, Y.; Matsui, S.; Toriyama, Y.; Fujii, M.; Koyama, Y. Delayed Administration of BQ788, an ETB Antagonist, after Experimental Traumatic Brain Injury Promotes Recovery of Blood-Brain Barrier Function and a Reduction of Cerebral Edema in Mice. J. Neurotrauma 2018, 35, 1481–1494. [Google Scholar] [CrossRef] [PubMed]
- D’Orléans-Juste, P.; Ndunge, O.B.A.; Desbiens, L.; Tanowitz, H.B.; Desruisseaux, M.S. Endothelins in inflammatory neurological diseases. Pharmacol. Ther. 2019, 194, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Szabo, K.; Mitrea, L.; Calinoiu, L.F.; Teleky, B.E.; Martau, G.A.; Plamada, D.; Pascuta, M.S.; Nemes, S.A.; Varvara, R.A.; Vodnar, D.C. Natural Polyphenol Recovery from Apple-, Cereal-, and Tomato-Processing By-Products and Related Health-Promoting Properties. Molecules 2022, 27, 7977. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Razavi, B.M.; Banach, M.; Hosseinzadeh, H. Quercetin and metabolic syndrome: A review. Phytother. Res. 2021, 35, 5352–5364. [Google Scholar] [PubMed]
- Di Petrillo, A.; Orrù, G.; Fais, A.; Fantini, M.C. Quercetin and its derivates as antiviral potentials: A comprehensive review. Phytother. Res. 2022, 36, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Arif, Y.; Bajguz, A.; Hayat, S. The role of quercetin in plants. Plant Physiol. Biochem. 2021, 166, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, S.R.; Ebrahimzadeh, M.A. Quercetin derivatives: Drug design, development, and biological activities, a review. Eur. J. Med. Chem. 2022, 229, 114068. [Google Scholar] [CrossRef]
- Biswas, P.; Dey, D.; Biswas, P.K.; Rahaman, T.I.; Saha, S.; Parvez, A.; Khan, D.A.; Lily, N.J.; Saha, K.; Sohel, M.; et al. A Comprehensive Analysis and Anti-Cancer Activities of Quercetin in ROS-Mediated Cancer and Cancer Stem Cells. Int. J. Mol. Sci. 2022, 23, 11746. [Google Scholar] [CrossRef]
- Reyes-Farias, M.; Carrasco-Pozo, C. The Anti-Cancer Effect of Quercetin: Molecular Implications in Cancer Metabolism. Int. J. Mol. Sci. 2019, 20, 3177. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.P.; Li, H.W.; Wang, Z.Q.; Zhou, Q.; Chen, S.P.; Yang, B.; Yin, D.; He, H.; He, M. Quercetin protects the vascular endothelium against iron overload damages ROS/ADMA/DDAHII/eNOS/NO pathway. Eur. J. Pharmacol. 2020, 868, 172885. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, H.M.; Yang, Y.B.; Chen, B. Quercetin regulates vascular endothelium function in chronic renal failure via modulation of Eph/Cav-1 signaling. Drug Dev. Res. 2022, 83, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Jafari-Garageshlaghi, F.; Hashtarkhani, F.; Soraya, H.; Malekinejad, H. Quercetin Protected from Aluminum Phosphide-induced Acute and Subacute Cardio- and Hepatotoxicity in Rats. Curr. Pharm. Des. 2022, 28, 3513–3524. [Google Scholar] [PubMed]
- Ge, J.; Shelby, S.L.; Wang, Y.J.; Morse, P.D.; Coffey, K.; Li, J.L.; Geng, T.Y.; Huang, Y. Cardioprotective properties of quercetin in fescue toxicosis-induced cardiotoxicity via heart-gut axis in lambs. J. Hazard. Mater. 2023, 458, 131843. [Google Scholar] [CrossRef] [PubMed]
- Chiang, M.C.; Tsai, T.Y.; Wang, C.J. The Potential Benefits of Quercetin for Brain Health: A Review of Anti-Inflammatory and Neuroprotective Mechanisms. Int. J. Mol. Sci. 2023, 24, 6382. [Google Scholar] [CrossRef] [PubMed]
- Jain, J.; Hasan, W.; Biswas, P.; Yadav, R.S.; Jat, D. Neuroprotective effect of quercetin against rotenone-induced neuroinflammation and alterations in mice behavior. J. Biochem. Mol. Toxicol. 2022, 36, e23165. [Google Scholar] [CrossRef]
- Acikara, O.B.; Karatoprak, G.S.; Yücel, C.; Akkol, E.K.; Sobarzo-Sánchez, E.; Khayatkashani, M.; Kamal, M.A.; Kashani, H.R.K. A Critical Analysis of Quercetin as the Attractive Target for the Treatment of Parkinson’s Disease. CNS Neurol. Disord. Drug Targets 2022, 21, 795–817. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Y.; Liu, Z.; Wang, J. Quercetin effectively improves LPS-induced intestinal inflammation, pyroptosis, and disruption of the barrier function through the TLR4/NF-κB/NLRP3 signaling pathway in vivo and in vitro. Food Nutr. Res. 2022, 66, 8948. [Google Scholar] [CrossRef]
- Fu, S.; Zhang, M.; Ou, J.; Liu, H.; Tan, C.; Liu, J.; Chen, H.; Bei, W. Construction and immune effect of Haemophilus parasuis DNA vaccine encoding glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in mice. Vaccine 2012, 30, 6839–6844. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [PubMed]
- Fu, S.; Xu, L.; Li, S.; Qiu, Y.; Liu, Y.; Wu, Z.; Ye, C.; Hou, Y.; Hu, C.A. Baicalin suppresses NLRP3 inflammasome and nuclear factor-kappa B (NF-κB) signaling during Haemophilus parasuis infection. Vet. Res. 2016, 47, 80. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhang, H.; Gui, G.; Luo, J.; Liu, S. Macleaya cordata extract modulates inflammation via inhibition of the NF-κB and MAPK signaling pathways in porcine alveolar macrophages induced by Glaesserella parasuis. Can. J. Vet. Res. 2022, 86, 254–260. [Google Scholar] [PubMed]
- Yang, Z.; Tang, X.; Wang, K.; Dai, K.; Chang, Y.F.; Du, S.; Zhao, Q.; Huang, X.; Wu, R.; Yan, Q.; et al. Metal Ion Periplasmic-Binding Protein YfeA of Glaesserella parasuis Induces the Secretion of Pro-Inflammatory Cytokines of Macrophages via MAPK and NF-κB Signaling through TLR2 and TLR4. Int. J. Mol. Sci. 2022, 23, 9627. [Google Scholar] [CrossRef] [PubMed]
- Dai, K.; Ma, X.; Yang, Z.; Chang, Y.F.; Cao, S.; Zhao, Q.; Huang, X.; Wu, R.; Huang, Y.; Yan, Q.; et al. Polyamine Transport Protein PotD Protects Mice against Haemophilus parasuis and Elevates the Secretion of Pro-Inflammatory Cytokines of Macrophage via JNK-MAPK and NF-κB Signal Pathways through TLR4. Vaccines 2019, 7, 216. [Google Scholar] [CrossRef]
- Sul, O.J.; Ra, S.W. Quercetin Prevents LPS-Induced Oxidative Stress and Inflammation by Modulating NOX2/ROS/NF-kB in Lung Epithelial Cells. Molecules 2021, 26, 6949. [Google Scholar] [CrossRef]
- Jia, X.; Gu, M.; Dai, J.; Wang, J.; Zhang, Y.; Pang, Z. Quercetin attenuates Pseudomonas aeruginosa-induced acute lung inflammation by inhibiting PI3K/AKT/NF-κB signaling pathway. Inflammopharmacology 2024, 32, 1059–1076. [Google Scholar] [CrossRef]
- Gong, X.; Huang, Y.; Ma, Q.; Jiang, M.; Zhan, K.; Zhao, G. Quercetin Alleviates Lipopolysaccharide-Induced Cell Damage and Inflammation via Regulation of the TLR4/NF-κB Pathway in Bovine Intestinal Epithelial Cells. Curr. Issues Mol. Biol. 2022, 44, 5234–5246. [Google Scholar] [CrossRef]
- Tu, X.K.; Yang, W.Z.; Chen, J.P.; Chen, Y.; Chen, Q.; Chen, P.P.; Shi, S.S. Repetitive ischemic preconditioning attenuates inflammatory reaction and brain damage after focal cerebral ischemia in rats: Involvement of PI3K/Akt and ERK1/2 signaling pathway. J. Mol. Neurosci. 2015, 55, 912–922. [Google Scholar] [CrossRef]
- He, X.; Song, X.; Cao, H.; Zhou, Q.; Zhang, J.; Yue, H.; Zhang, B. Glaesserella parasuis induces IL-17 production might through PKC-ERK/MAPK and IκB/NF-κB signaling pathways. Vet. Microbiol. 2022, 273, 109521. [Google Scholar] [CrossRef]
- Li, J.; Liu, S.; Dong, Q.; Fu, Y.; Sun, Y.; Luo, R.; Tian, X.; Guo, L.; Liu, W.; Qiu, Y.; et al. PD-1/PD-L1 axis induced host immunosuppression via PI3K/Akt/mTOR signalling pathway in piglets infected by Glaesserella Parasuis. BMC Vet. Res. 2024, 20, 141. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, H.; Wu, Z.; Yu, X.; Yin, Y.; Qian, S.; Wang, Z.; Huang, J.; Wang, W.; Liu, T.; et al. Quercitrin Rapidly Alleviated Depression-like Behaviors in Lipopolysaccharide-Treated Mice: The Involvement of PI3K/AKT/NF-κB Signaling Suppression and CREB/BDNF Signaling Restoration in the Hippocampus. ACS Chem. Neurosci. 2021, 12, 3387–3396. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.L.; Zhao, C.H.; Yao, X.L.; Zhang, H. Quercetin attenuates high fructose feeding-induced atherosclerosis by suppressing inflammation and apoptosis via ROS-regulated PI3K/AKT signaling pathway. Biomed. Pharmacother. 2017, 85, 658–671. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Lin, W.; Ba, X.; Huang, Y.; Chen, Z.; Han, L.; Qin, K.; Huang, Y.; Tu, S. Quercetin-mediated SIRT1 activation attenuates collagen-induced mice arthritis. J. Ethnopharmacol. 2021, 279, 114213. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Si, E. MicroRNA-25 aggravates Aβ1-42-induced hippocampal neuron injury in Alzheimer’s disease by downregulating KLF2 via the Nrf2 signaling pathway in a mouse model. J. Cell. Biochem. 2019, 120, 15891–15905. [Google Scholar] [PubMed]
- Guo, P.; Liu, L.; Yang, X.; Li, M.; Zhao, Q.; Wu, H. Irisin improves BBB dysfunction in SAP rats by inhibiting MMP-9 via the ERK/NF-κB signaling pathway. Cell Signal. 2022, 93, 110300. [Google Scholar] [PubMed]
- You, M.; Miao, Z.; Pan, Y.; Hu, F. Trans-10-hydroxy-2-decenoic acid alleviates LPS-induced blood-brain barrier dysfunction by activating the AMPK/PI3K/AKT pathway. Eur. J. Pharmacol. 2019, 865, 172736. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, X.; Zhang, Z.; Zhang, J.; Xu, J.; Qiu, Y.; Ye, C.; Fu, S.; Wu, Z.; Hu, C.A. Baicalin Protects Vascular Tight Junctions in Piglets During Glaesserella parasuis Infection. Front. Vet. Sci. 2021, 8, 671936. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Zhang, J.; Wang, Q.; Tang, J.; Li, Y.; Zhou, H.; Lin, H.; Ma, Z.; Fan, H. Porcine circovirus type 2 and Glaesserella parasuis serotype 4 co-infection activates Snail1 to disrupt the intercellular junctions and facilitate bacteria translocation across the tracheal epithelium. Vet. Microbiol. 2024, 288, 109954. [Google Scholar] [CrossRef]
- Beken, B.; Serttas, R.; Yazicioglu, M.; Turkekul, K.; Erdogan, S. Quercetin Improves Inflammation, Oxidative Stress, and Impaired Wound Healing in Atopic Dermatitis Model of Human Keratinocytes. Pediatr. Allergy Immunol. Pulmonol. 2020, 33, 69–79. [Google Scholar] [CrossRef]
- McCarty, J.H. MMP9 Clears the Way for Metastatic Cell Penetration Across the Blood-Brain Barrier. Cancer Res. 2023, 83, 1167–1169. [Google Scholar] [PubMed]
- Yang, Y.; Thompson, J.F.; Taheri, S.; Salayandia, V.M.; McAvoy, T.A.; Hill, J.W.; Yang, Y.; Estrada, E.Y.; Rosenberg, G.A. Early inhibition of MMP activity in ischemic rat brain promotes expression of tight junction proteins and angiogenesis during recovery. J. Cereb. Blood Flow. Metab. 2013, 33, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, D.A.; Jin, K. Vascular endothelial growth factors (VEGFs) and stroke. Cell. Mol. Life Sci. 2013, 70, 1753–1761. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, C.; Yang, S.; Zhang, Z.; Bai, X.; Tang, H.; Huang, J. Cepharanthine maintains integrity of the blood-brain barrier (BBB) in stroke via the VEGF/VEGFR2/ZO-1 signaling pathway. Aging 2024, 16, 5905–5915. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.; Xin, C.; Usman, M.; Zhu, Y.; Lu, H. Effect of Bevacizumab on traumatic penumbra brain edema in rats at different time points. Tissue Barriers 2023, 12, 2292463. [Google Scholar] [CrossRef] [PubMed]
- Eklund, L.; Saharinen, P. Angiopoietin signaling in the vasculature. Exp. Cell Res. 2013, 319, 1271–1280. [Google Scholar] [CrossRef] [PubMed]
- Thurston, G.; Rudge, J.S.; Ioffe, E.; Zhou, H.; Ross, L.; Croll, S.D.; Glazer, N.; Holash, J.; McDonald, D.M.; Yancopoulos, G.D. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat. Med. 2000, 6, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Gurnik, S.; Devraj, K.; Macas, J.; Yamaji, M.; Starke, J.; Scholz, A.; Sommer, K.; Di Tacchio, M.; Vutukuri, R.; Beck, H.; et al. Angiopoietin-2-induced blood-brain barrier compromise and increased stroke size are rescued by VE-PTP-dependent restoration of Tie2 signaling. Acta Neuropathol. 2016, 131, 753–773. [Google Scholar] [CrossRef]
- Pancotto, T.E.; Rossmeisl, J.H., Jr.; Huckle, W.R.; Inzana, K.D.; Zimmerman, K.L. Evaluation of endothelin-1 and MMPs-2, -9, -14 in cerebrospinal fluid as indirect indicators of blood-brain barrier dysfunction in chronic canine hypothyroidism. Res. Vet. Sci. 2016, 105, 115–120. [Google Scholar]




| Gene | Nucleotide Sequences (5′–3′) | Tm (°C) | Length (bp) | |
|---|---|---|---|---|
| Il-1β | Forward | TGCCACCTTTTGACAGTGATG | 59.04 | 138 |
| Reverse | TGATGTGCTGCTGCGAGATT | 60.39 | ||
| Il-6 | Forward | GCCCACCAAGAACGATAGTC | 56.90 | 104 |
| Reverse | GTCGTTGTCACCAGCATCAG | 56.60 | ||
| Il-8 | Forward | GGCTTTGCGTTGATTCTGG | 58.90 | 217 |
| Reverse | CGGTGTCCTGATTATCGTCCT | 59.00 | ||
| Il-10 | Forward | GCTGGACAACATACTGCTAACC | 58.60 | 162 |
| Reverse | TCACCCAGGGAATTCAAATG | 59.30 | ||
| Il-18 | Forward | GGACACTTTCTTGCTTGCCA | 60.00 | 166 |
| Reverse | CAGCCTCGGGTATTCTGTTATG | 59.70 | ||
| Tnf-α | Forward | GCCCCCAGTCTGTATCCTTCTA | 58.60 | 209 |
| Reverse | TTCGGAAAGCCCATTTGAGT | 59.30 | ||
| Sema4D | Forward | CTGCCTCTTTTCCTACAACTGCT | 60.00 | 212 |
| Reverse | GCTCCGTTTCATAGCCCGTA | 60.40 | ||
| PlexinB1 | Forward | CCCCTTCTAGAGCTTTCCGTG | 60.80 | 121 |
| Reverse | GCAGCCTGGATAAGACCGTAA | 59.60 | ||
| PI3K | Forward | GAAGATGATGAGGATTTGCCC | 58.50 | 174 |
| Reverse | CTTGACTTCGCCGTCTACCAC | 59.80 | ||
| Akt | Forward | CGGTTCTTTGCCAACATCGT | 59.41 | 156 |
| Reverse | TAGGAGAACTTGATCAGGCGG | 59.24 | ||
| Erk | Forward | CACCCATACCTGGAGCAGTA | 58.50 | 221 |
| Reverse | TACACCATCTCTCCCTTGCTAT | 58.07 | ||
| ZO-1 | Forward | CAAAGGGAAAACCCGAAAC | 57.20 | 184 |
| Reverse | GATACTGAGTTGCCTTCACCCT | 57.70 | ||
| Occludin | Forward | CCCCTCTTTCCTTAGGCGAC | 59.82 | 168 |
| Reverse | TTCAAAAGGCCTCACGGACA | 59.82 | ||
| Claudin-5 | Forward | GGGTGAGCATTCAGTCTTTAGC | 58.50 | 174 |
| Reverse | ACAGCCCCTTCCAAGTCGT | 59.10 | ||
| Vegf | Forward | GCACTGGACCCTGGCTTTAC | 60.96 | 144 |
| Reverse | GTCTCAATCGGACGGCAGTA | 59.55 | ||
| Mmp9 | Forward | TCTAGGCCCAGAGGTAACCC | 60.03 | 141 |
| Reverse | TTATCCACGCGAATGACGCT | 60.18 | ||
| Ang-1 | Forward | CTGAAGAGTTGACACAGGGCT | 59.93 | 119 |
| Reverse | ATCTGGGCCATCTCCGACTT | 60.69 | ||
| Ang-2 | Forward | CCGCGGGCAAAATAAGTAGC | 59.97 | 126 |
| Reverse | CACATGCGTCAAACCACCAG | 60.04 | ||
| Et-1 | Forward | GGCCCAAAGTACCATGCAGA | 60.32 | 137 |
| Reverse | TGCTATTGCTGATGGCCTCC | 60.18 | ||
| Gapdh | Forward | AAGCCCATCACCATCTTCCA | 60.00 | 88 |
| Reverse | CACCAGTAGACTCCACGACA | 60.00 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, P.; Yang, Y.; Yang, L.; Qian, Y.; Liang, M.; Chen, H.; Zhang, J.; Qiu, Y.; Guo, L.; Fu, S. Quercetin Protects Blood–Brain Barrier Integrity via the PI3K/Akt/Erk Signaling Pathway in a Mouse Model of Meningitis Induced by Glaesserella parasuis. Biomolecules 2024, 14, 696. https://doi.org/10.3390/biom14060696
Sun P, Yang Y, Yang L, Qian Y, Liang M, Chen H, Zhang J, Qiu Y, Guo L, Fu S. Quercetin Protects Blood–Brain Barrier Integrity via the PI3K/Akt/Erk Signaling Pathway in a Mouse Model of Meningitis Induced by Glaesserella parasuis. Biomolecules. 2024; 14(6):696. https://doi.org/10.3390/biom14060696
Chicago/Turabian StyleSun, Peiyan, Yaqiong Yang, Linrong Yang, Yuanzhuo Qian, Mingxia Liang, Hongbo Chen, Jing Zhang, Yinsheng Qiu, Ling Guo, and Shulin Fu. 2024. "Quercetin Protects Blood–Brain Barrier Integrity via the PI3K/Akt/Erk Signaling Pathway in a Mouse Model of Meningitis Induced by Glaesserella parasuis" Biomolecules 14, no. 6: 696. https://doi.org/10.3390/biom14060696





