Can We Explain Thousands of Molecularly Identified Mouse Neuronal Types? From Knowing to Understanding
Abstract
:1. Introduction
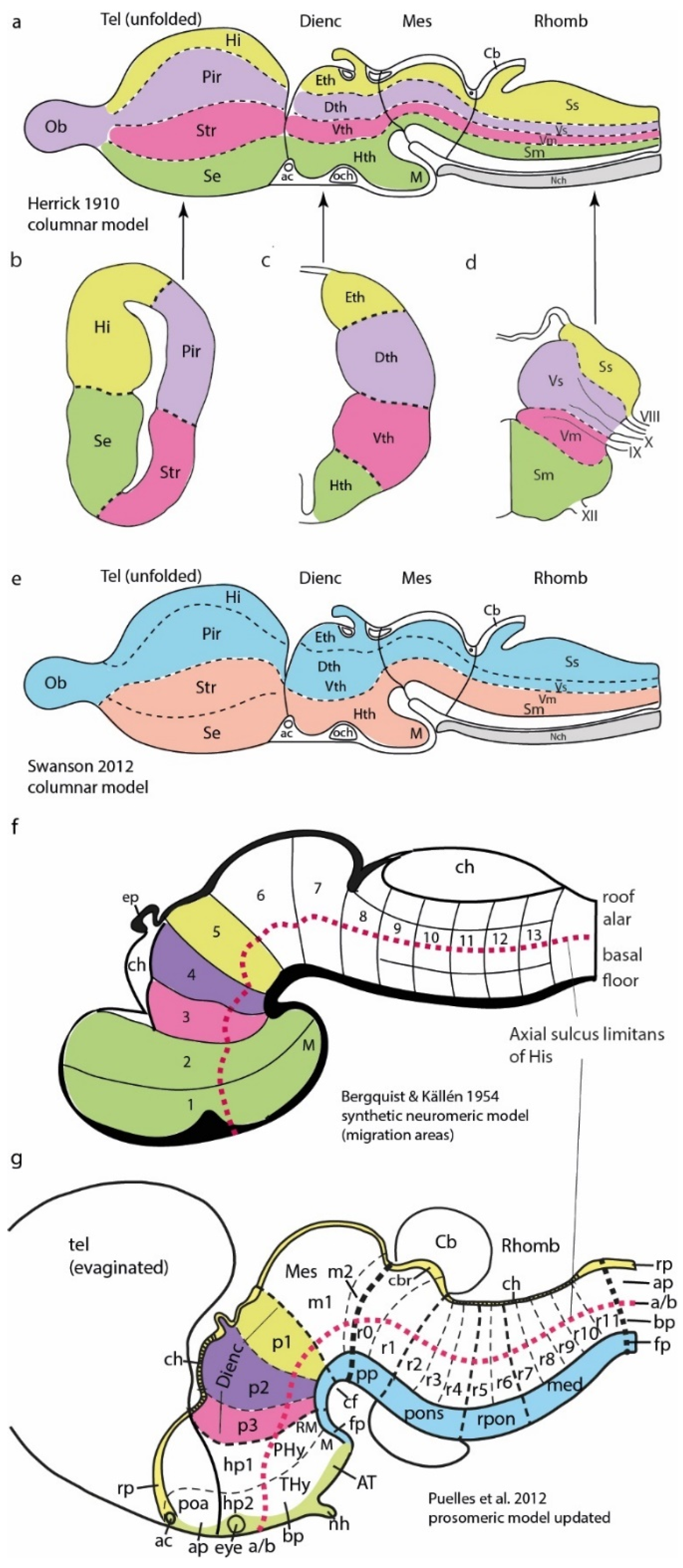
2. Alternative Models
3. Longitudinal Zones of His
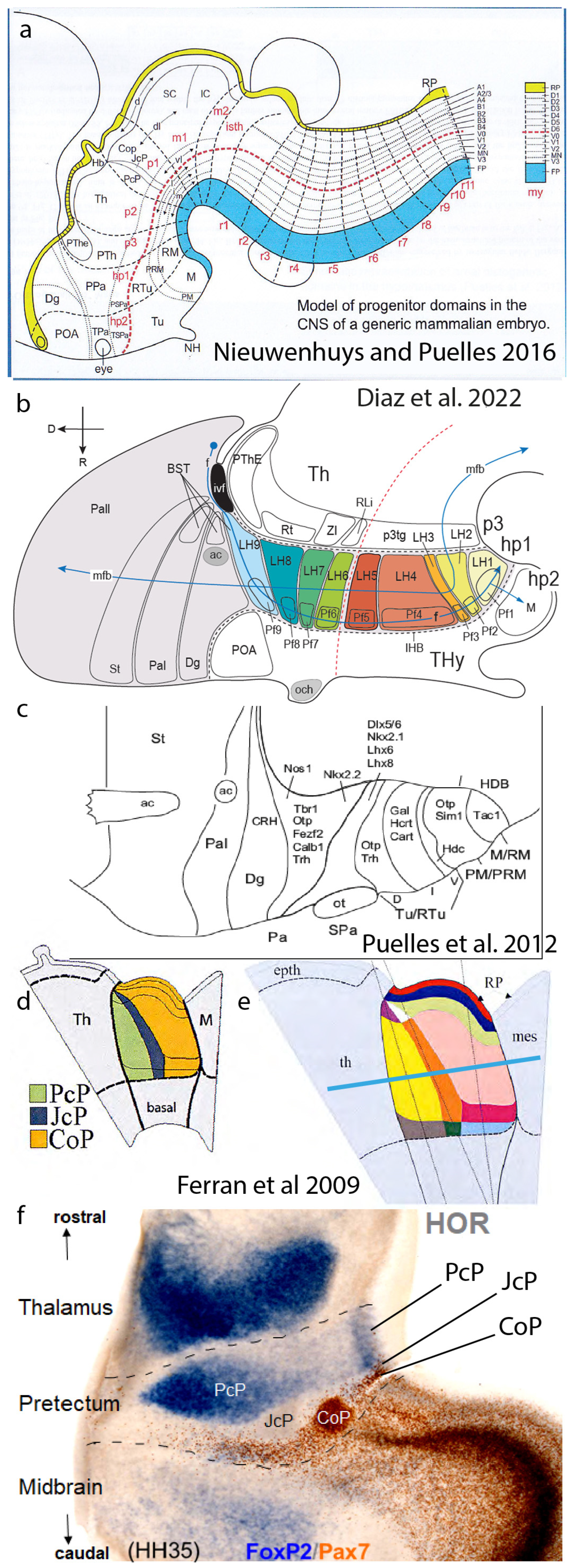
4. The Synthetic Neuromeric Models, Leading to the Prosomeric Model
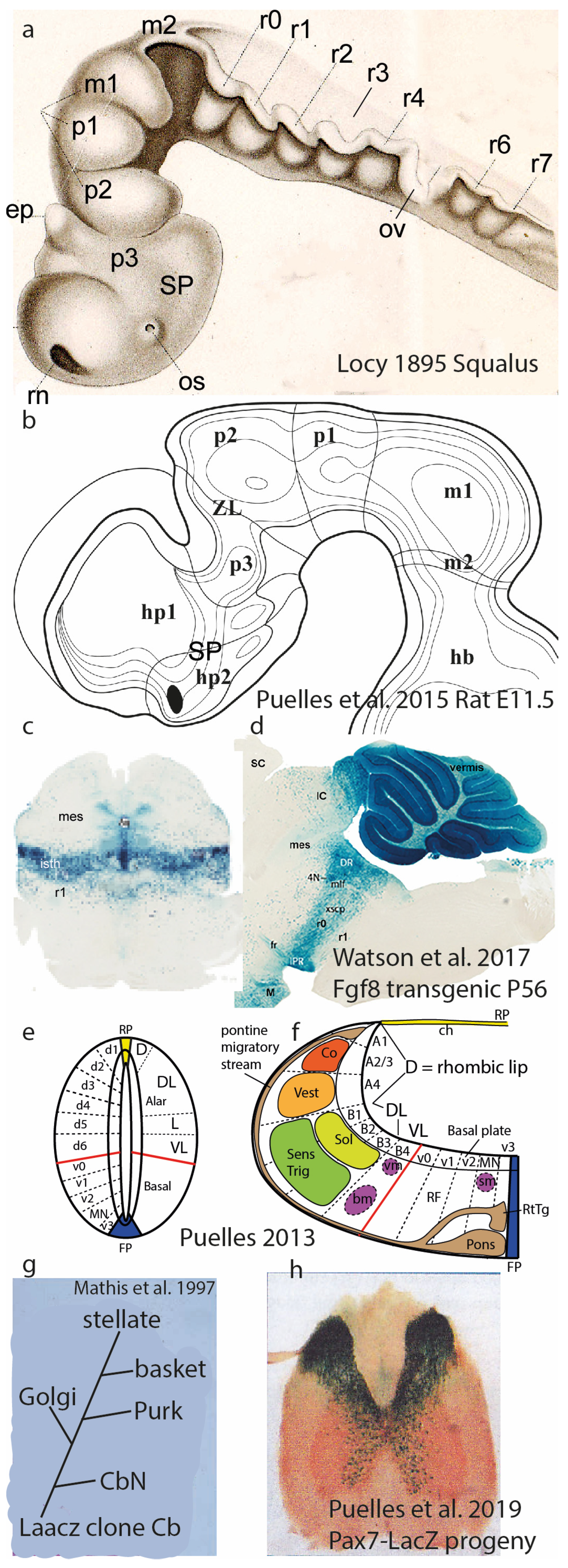
5. Dorsoventral Microzones
6. Anteroposterior Microzones
7. Areal Stratification and Clonal Typological Variation
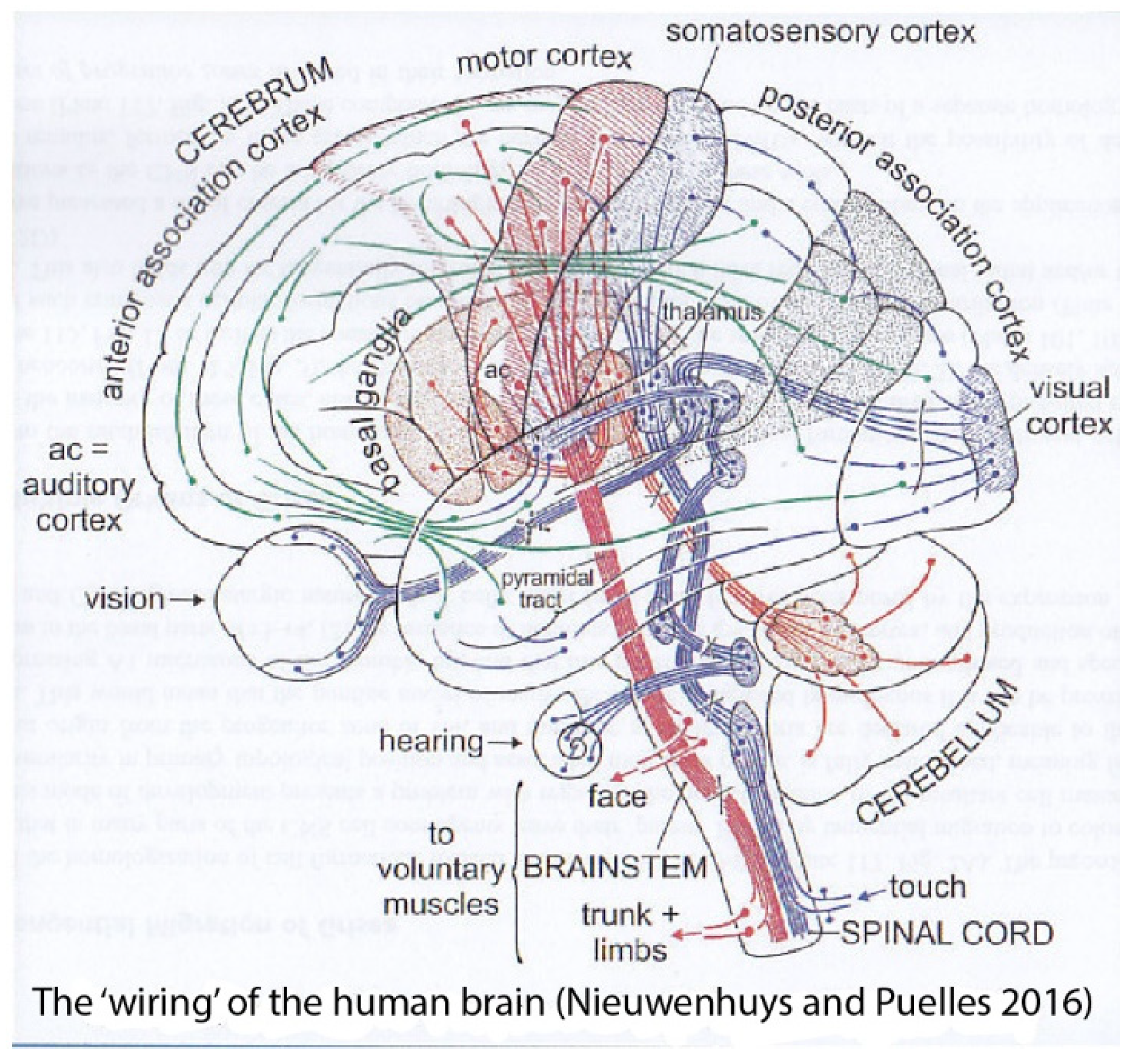
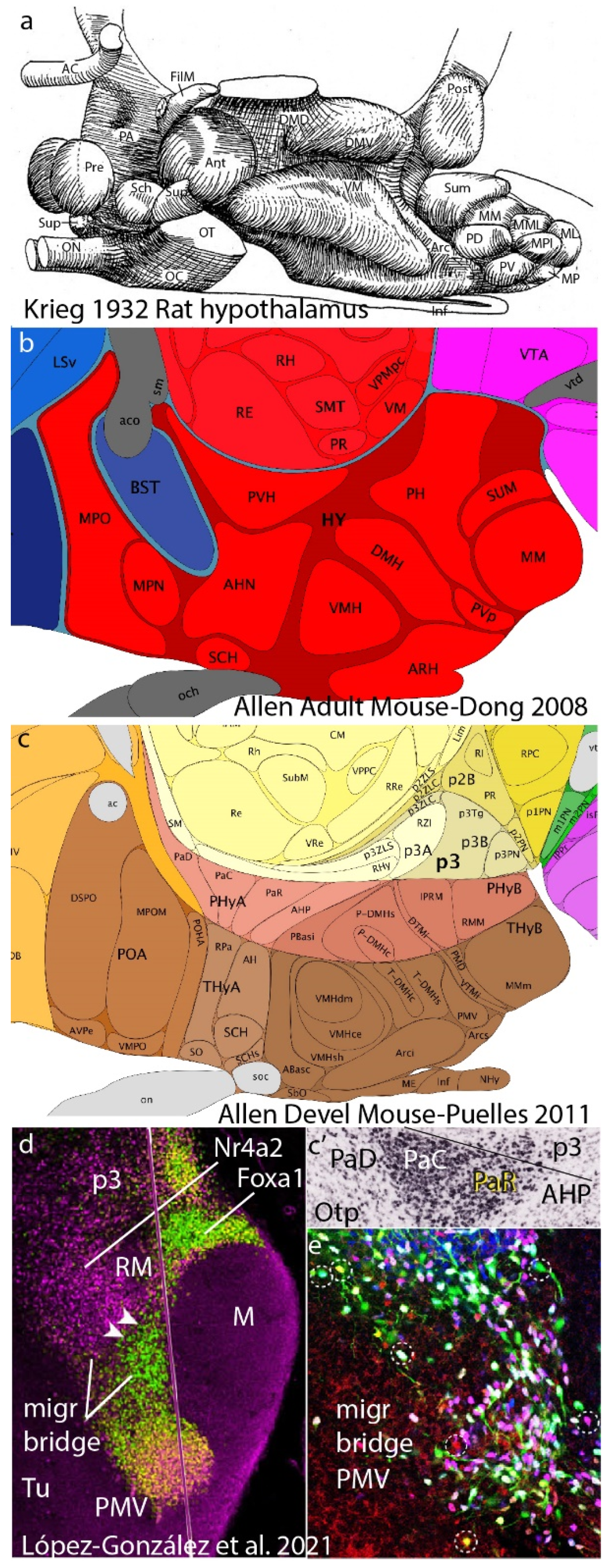
8. Recapitulation and Tangential Migrations
9. Further Considerations
10. Conclusions
Funding
Conflicts of Interest
References
- Herrick, C.J. The morphology of the forebrain in Amphibia and Reptilia. J. Comp. Neurol. Psychol. 1910, 20, 413–547. [Google Scholar] [CrossRef]
- Swanson, L.W. Brain Maps: Structure of the Rat Brain, 3rd ed.; Elsevier Academic Press: San Diego, CA, USA, 2004. [Google Scholar]
- Swanson, L.W. Brain Architecture: Understanding the Basic Plan, 2nd ed.; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Dong, H.W. The Allen Reference Atlas: A Digital Color Brain Atlas of C57BL/6J Male Mouse; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Bergquist, H.; Källén, B. Notes on the early histogenesis and morphogenesis of the central nervous system in vertebrates. J. Comp. Neurol. 1954, 100, 627–660. [Google Scholar] [CrossRef] [PubMed]
- His, W. Über das frontale Ende des Gehirnrohres. Arch. Anat. EntwGesch 1893, 1893, 157–171. [Google Scholar]
- His, W. Die Entwickelung des Menschlichen Gehirns Während der Ersten Monate; S. Hirzel: Leipzig, Germany, 1904. [Google Scholar]
- Puelles, L.; Martinez-de-la-Torre, M.; Bardet, S.; Rubenstein, J.L.R. Hypothalamus. In The Mouse Nervous System; Watson, C., Paxinos, G., Puelles, L., Eds.; Academic Press; Elsevier: New York, NY, USA, 2012; pp. 221–312. [Google Scholar]
- Kuhlenbeck, H. The Central Nervous System of Vertebrates; Part II Overall Morphologic Pattern; Karger: Basel, Switzerland, 1973; Volume 3. [Google Scholar]
- Masland, R.H. Neuronal cell types. Curr. Evol. 2004, 14, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Krieg, W.J.S. The hypothalamus of the albino rat. J. Comp. Neurol. 1932, 5, 19–89. [Google Scholar] [CrossRef]
- Ramon y Cajal, S. Histologie du Système Nerveux de l’Homme et des Vertebrés; Vol. I. (trad. L. Azoulay); Consejo Superior de Investigaciones Científicas/Instituto Ramón y Cajal: Madrid, Spain, 1909; (reprinted in 1955). [Google Scholar]
- Ramon y Cajal, S. Histologie du Système Nerveux de l’Homme et des Vertebrés; Vol. II. (trad. L. Azoulay); Consejo Superior de Investigaciones Científicas/Instituto Ramón y Cajal: Madrid, Spain, 1911; (reprinted in 1955). [Google Scholar]
- Puelles, L. Functional implications of the prosomeric brain model. Biomolecules 2024, 14, 331. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuys, R.; Puelles, L. Towards a New Neuromorphology; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 978-3-319-25692-4. [Google Scholar]
- Sánchez-Arrones, L.; Ferrán, J.L.; Rodríguez-Gallardo, L.; Puelles, L. Incipient forebrain boundaries traced by differential gene expression and fate mapping in the chick neural plate. Dev. Biol. 2009, 335, 43–65. [Google Scholar] [CrossRef] [PubMed]
- Acampora, D.; Boyl, P.P.; Martinez-Barrera, J.P.; Annino, A.; Signore, M.; Simaone, A. Otx genes in evolution: Are they involved in instructing the vertebrate brain morphology? J. Anat. 2001, 199, 53–62. [Google Scholar] [CrossRef] [PubMed]
- García-Calero, E.; Fernández-Garre, P.; Martínez, S.; Puelles, L. Early mamillary pouch specification in the course of prechordal ventralization of the forebrain tegmentum. Dev. Biol. 2008, 320, 366–377. [Google Scholar] [CrossRef]
- Amat, J.A.; Martínez-de-la-Torre, M.; Trujillo, C.M.; Fernández, B.; Puelles, L. Neurogenetic heterochrony in chick, lizard and rat mapped with wholemount AChE and the prosomeric model. Brain Behav. Evol. 2022, 97, 48–82. [Google Scholar] [CrossRef]
- Ju, M.J.; Aroca, P.; Puelles, L.; Redies, C. Molecular profiling indicates avian branchiomotor nuclei invade the hindbrain alar plate. Neuroscience 2004, 128, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Puelles, L.; Tvrdik, P.; Martínez-de-la-Torre, M. The postmigratory alar topography of visceral cranial nerve efferents challenges the classical model of hindbrain columns. Anat. Rec. 2019, 302, 485–504. [Google Scholar] [CrossRef] [PubMed]
- Orr, H. Contribution to the embryology of the lizard. J. Morphol. 1887, 1, 311–372. [Google Scholar] [CrossRef]
- McClure, C.F.W. The segmentation of the primitive vertebrate brain. J. Morphol. 1890, 4, 35–56. [Google Scholar] [CrossRef]
- Locy, W.A.C. Contributions to the structure and development of the vertebrate head. J. Morphol. 1895, 11, 497–594. [Google Scholar] [CrossRef]
- Von Kupffer, C. Die Morphogenesis des Centralnervensystems. In Handbuch der vergleichenden und experimentellen Entwicklungslehre der Wirbeltiere; Hertwig, O., Ed.; Bd.2, Teil 3; Gustav Fisher: Jena, Germany, 1906. [Google Scholar]
- Vaage, S. The segmentation of the primitive neural tube in chick embryos (Gallus domesticus). A morphological, histochemical and autoradiographical investigation. Adv. Anat. Embryol. Cell Biol. 1969, 41, 1–88. [Google Scholar]
- Puelles, L. Developmental studies of avian brain organization. Int. J. Dev. Biol. 2018, 62, 207–224. [Google Scholar] [CrossRef]
- Puelles, L.; Fernández, B.; Martinez-de-la-Torre, M. Neuromeric Landmarks in the Rat Midbrain, Diencephalon and Hypothalamus, Compared with Acetylcholinesterase Histochemistry. In The Rat Nervous System, 4th ed.; Paxinos, G., Ed.; Academic Press; Elsevier: New York, NY, USA, 2015; pp. 25–43. [Google Scholar]
- Watson, C.; Shimogori, T.; Puelles, L. Mouse FGF8-Cre lineage analysis defines the territory of the postnatal mammalian isthmus. J. Comp. Neurol. 2017, 525, 2782–2799. [Google Scholar] [CrossRef]
- Mathis, L.; Bonnerot, C.; Puelles, L.; Nicolas, J.-F. Retrospective clonal analysis of the cerebellum using genetic laacZ/lacZ mouse mutants. Development 1997, 124, 4089–4104. [Google Scholar] [CrossRef]
- Bulfone, A.; Puelles, L.; Porteus, M.H.; Frohman, M.A.; Martin, G.R.; Rubenstein, J.L.R. Spatially restricted expression of Dlx-1, Dlx-2 (Tes-1), Gbx-2 and Wnt-3 in the embryonic day 12.5 mouse forebrain defines potential transverse and longitudinal segmental boundaries. J. Neurosci. 1993, 13, 3155–3172. [Google Scholar] [CrossRef]
- Puelles, L.; Rubenstein, J.L.R. Expression patterns of homeobox and other putative regulatory genes in the embryonic mouse forebrain suggest a neuromeric organization. Trends Neurosci. 1993, 16, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, J.L.R.; Martínez, S.; Shimamura, K.; Puelles, L. The embryonic vertebrate forebrain: The prosomeric model. Science 1994, 266, 578–580. [Google Scholar] [CrossRef] [PubMed]
- Puelles, L.; Rubenstein, J.L.R. Forebrain gene expression domains and the evolving prosomeric model. Trends Neurosci. 2003, 26, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Puelles, L.; Rubenstein, J.L. A new scenario of hypothalamic organization: Rationale of new hypotheses introduced in the updated prosomeric model. Front. Neuroanat. 2015, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Roca, L.; Corral-San-Miguel, R.; Aroca, P.; Puelles, L.; Marín, F. Crypto-rhombomeres of the mouse medulla oblongata, defined by molecular and morphological features. Brain Struct. Funct. 2016, 221, 815–838. [Google Scholar] [CrossRef] [PubMed]
- Puelles, L. Plan of the Developing Vertebrate Nervous System Relating Embryology to the Adult Nervous System (Prosomere Model, Overview of Brain Organization). In Comprehensive Developmental Neuroscience: Patterning and Cell Type Specification in the Developing CNS and PNS; Rubenstein, J.L.R., Rakic, P., Eds.; Academic Press: Amsterdam, The Netherlands, 2013; pp. 187–209. [Google Scholar]
- Puelles, L. Role of Secondary Organizers in the Evolution of Forebrain Development in Vertebrates. In Handbook of Evolutionary Neuroscience; Shepherd, S.V., Ed.; Blackwell-Wiley: Chichester, UK, 2017; pp. 350–387. [Google Scholar]
- Nieuwenhuys, R. Principles of current vertebrate neuromorphology. Brain Behav. Evol. 2017, 90, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Flames, N.; Pla, R.; Gelman, D.M.; Rubenstein, J.L.R.; Puelles, L.; Marín, O. Delineation of multiple subpallial progenitor domains by the combinatorial expression of transcriptional codes. J. Neurosci. 2007, 27, 9682–9695. [Google Scholar] [CrossRef]
- Diaz, C.; Rubenstein, J.; Puelles, L. Dorsoventral arrangement of lateral hypothalamus populations in the mouse hypothalamus: A prosomeric genoarchitectonic analysis. Mol. Neurobiol. 2022, 60, 687–731. [Google Scholar] [CrossRef] [PubMed]
- Puelles, E.; Martinez-de-la-Torre, M.; Watson, C.; Puelles, L. Midbrain. In The Mouse Nervous System; Watson, C., Paxinos, G., Puelles, L., Eds.; Academic Press; Elsevier: New York, NY, USA, 2012; pp. 337–359. [Google Scholar]
- Puelles, L.; Diaz, C.; Stühmer, T.; Ferran, J.L.; Martínez-de la Torre, M.; Rubenstein, J.L.R. LacZ-reporter mapping of Dlx5/6 expression and genoarchitectural analysis of the postnatal mouse prethalamus. J. Comp. Neurol. 2021, 529, 367–420. [Google Scholar] [CrossRef]
- Ferran, J.L.; Sánchez-Arrones, L.; Bardet, S.M.; Sandoval, J.; Martínez-de-la-Torre, M.; Puelles, L. Early pretectal gene expression pattern shows a conserved anteroposterior tripartition in mouse and chicken. Brain Res. Bull. 2008, 75, 295–298. [Google Scholar] [CrossRef]
- Ferrán, J.L.; Dutra de Oliveira, E.; Sánchez-Arrones, L.; Sandoval, J.E.; Martínez-de-la-Torre, M.; Puelles, L. Genoarchitectonic profile of developing nuclear groups in the chicken pretectum. J. Comp. Neurol. 2009, 517, 405–451. [Google Scholar] [CrossRef]
- Puelles, L.; Hidalgo-Sánchez, M. The midbrain preisthmus: A poorly known effect of the isthmic organizer. Int. J. Mol. Sci. 2023, 24, 9769. [Google Scholar] [CrossRef]
- Puelles, L.; Martinez, S. Patterning of the Diencephalon. In Comprehensive Developmental Neuroscience: Patterning and Cell Type Specification in the Developing CNS and PNS; Rubenstein, J.L.R., Rakic, P., Eds.; Academic Press: Amsterdam, The Netherlands, 2013; pp. 151–172. [Google Scholar]
- Nieuwenhuys, R.; Broere, C.A.J.; Cerliani, L. A new myeloarchitectonic map of the human neocortex based on data from the Vogt-Vogt school. Brain Struct. Funct. 2015, 220, 2551–2573. [Google Scholar] [CrossRef]
- Garcia-Calero, E.; Martínez-de-la-Torre, M.; Puelles, L. A radial histogenetic model of the mouse pallial amygdala. Brain Struct. Funct. 2020, 225, 1921–1956. [Google Scholar] [CrossRef] [PubMed]
- López-González, L.; Alonso, A.; García-Calero, E.; de Puelles, E.; Puelles, L. Tangential intrahypothalamic migration of the mouse ventral premamillary nucleus depends on Fgf8 signaling. Front. Cell Dev. Biol. 2021, 9, 676121. [Google Scholar] [CrossRef]
- Lorente-Cánovas, B.; Marín, F.; Corral-San-Miguel, R.; Hidalgo-Sánchez, M.; Ferran, J.L.; Puelles, L.; Aroca, P. Multiple origins, migratory paths and molecular profiles of cells populating the avian interpeduncular nucleus. Dev. Biol. 2012, 361, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Marín, F.; Puelles, L. Morphological fate of rhombomeres in quail/chick chimeras: A segmental analysis of hindbrain nuclei. Eur. J. Neurosci. 1995, 7, 1714–1738. [Google Scholar] [CrossRef] [PubMed]
- Cambronero, F.; Puelles, L. Rostrocaudal nuclear relationships in the avian medulla oblongata: Fate-map with quail-chick chimeras. J. Comp. Neurol. 2000, 427, 522–545. [Google Scholar] [CrossRef]
- Marín, F.; Aroca, P.; Puelles, L. Hox gene colinear expression in the avian medulla oblongata is correlated with pseudorhombomeric domains. Dev. Biol. 2008, 323, 230–247. [Google Scholar] [CrossRef]
- García-Guillén, I.M.; Martínez-de-la-Torre, M.; Puelles, L.; Aroca, P.; Marín, F. Molecular segmentation of the sensory trigeminal nucleus in the adult mouse brain. Front. Neuroanat. 2021, 15, 785840. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puelles, L.; Nieuwenhuys, R. Can We Explain Thousands of Molecularly Identified Mouse Neuronal Types? From Knowing to Understanding. Biomolecules 2024, 14, 708. https://doi.org/10.3390/biom14060708
Puelles L, Nieuwenhuys R. Can We Explain Thousands of Molecularly Identified Mouse Neuronal Types? From Knowing to Understanding. Biomolecules. 2024; 14(6):708. https://doi.org/10.3390/biom14060708
Chicago/Turabian StylePuelles, Luis, and Rudolf Nieuwenhuys. 2024. "Can We Explain Thousands of Molecularly Identified Mouse Neuronal Types? From Knowing to Understanding" Biomolecules 14, no. 6: 708. https://doi.org/10.3390/biom14060708



