Abstract
Human health is now inextricably linked to lifestyle choices, which can either protect or predispose people to serious illnesses. The Mediterranean diet, characterized by the consumption of various medicinal plants and their byproducts, plays a significant role in protecting against ailments such as oxidative stress, cancer, and diabetes. To uncover the secrets of this natural treasure, this review seeks to consolidate diverse data concerning the pharmacology, toxicology, phytochemistry, and botany of Olea europaea L. (O. europaea). Its aim is to explore the potential therapeutic applications and propose avenues for future research. Through web literature searches (using Google Scholar, PubMed, Web of Science, and Scopus), all information currently available on O. europaea was acquired. Worldwide, ethnomedical usage of O. europaea has been reported, indicating its effectiveness in treating a range of illnesses. Phytochemical studies have identified a range of compounds, including flavanones, iridoids, secoiridoids, flavonoids, triterpenes, biophenols, benzoic acid derivatives, among others. These components exhibit diverse pharmacological activities both in vitro and in vivo, such as antidiabetic, antibacterial, antifungal, antioxidant, anticancer, and wound-healing properties. O. europaea serves as a valuable source of conventional medicine for treating various conditions. The findings from pharmacological and phytochemical investigations presented in this review enhance our understanding of its therapeutic potential and support its potential future use in modern medicine.
1. Introduction
In ancient times, humans and plants shared a close relationship that encompassed various uses such as food, medicine, and other applications. The olive tree, or Olea europaea L., is an evergreen member of the Oleaceae family. The shrub is known for its fruit and is primarily grown in Mediterranean regions [1]. Currently, 98% of olive products originate from the Mediterranean basin, playing a significant role in the region’s economy. Beyond their economic value, olive trees offer nutritional and medicinal benefits [2]. Notably, it is the sole species within its genus utilized as a food source [3]. They are typically found along the coastlines of the Mediterranean basin’s eastern region, southeastern Europe, northern Iran near the Caspian Sea’s southern end, western Asia, and northern Africa [4]. One important aspect of the Mediterranean diet is its abundant consumption of phytonutrients, including natural phenols and vitamins [5]. Scientists are keenly interested in these compounds due to their ability to activate various signaling pathways involved in regulating metabolism, DNA repair, protein balance, antioxidant defenses, and combating numerous diseases [6]. The main products from the olive, such as leaves, fruit, seeds, olive oil, and olive pomace, possess a diverse chemical makeup. They can be classified according to their fundamental molecular qualities, such as lignans, secoiridoids, flavonoids, and simple phenols and acids [7]; these include oleuropein, flavonols such as rutin and catechin, substituted phenols (vanillic acid, tyrosol, vanillin hydroxytyrosol, and caffeic acid), as well as a range of flavones (diosmetin, diosmetin-7-glucoside, luteolin, and apigenin-7-glucoside) [8]. Also, olive leaves contain a variety of chemicals, including related secoiridoids and oleuropein, which is the main chemical [9]. Among these compounds, oleuropein is commonly cited as the predominant one. Flavonoids may also be present in significant quantities [10]. Chemically, olive tree extracts display an array of pharmacological properties; these encompass anti-obesity, antidiabetic, antimicrobial, anti-inflammatory, anticancer, and immunosuppressive effects. These extracts are efficient in combating both Gram-positive and Gram-negative bacteria [11,12], as well as fungal strains [13,14]. Biological investigations have shown that olive extracts effectively inhibit enzymes involved in the glucose metabolism, proving its antidiabetic efficacy [15,16,17] and potent antioxidant effects [18,19]. Moreover, it displays potent cytotoxic activity, reducing the viability of tumor cells [20,21]. Importantly, investigations have found no toxic effects associated with olive extract consumption [22]. Several factors can influence the qualitative and quantitative phenolic content of olive leaves, such as harvesting time, drying conditions, growing location, extraction process, and cultivar. This review aims to summarize the key studies on the rich and diverse chemical composition of O. europaea extracts. It will discuss findings from both in vivo and in vitro investigations of its biological activities, including anticancer, antidiabetic, antimicrobial, and antioxidant effects.
2. Investigative Methodology
The data collection of the botanical characterization and classification, distribution, phytochemistry, ethnobotany, and pharmacology of Olea europaea utilized various scientific search platforms, such as Web of Science, Google Scholar, PubMed, and Scopus. Only English-language articles published between 2010 and 2024 were included, along with some additional information from the 1990s. In this review, the collected data were methodically arranged, categorized, examined, and condensed by each relevant field. A bibliometric analysis was carried out utilizing multiple search terms linked to Olea europaea, such as Olea europaea vegetable oil, olive leaf essential oils, Olea europaea extracts, biological effects of O. europaea, in vivo and in vitro studies, chemical composition, and the genus Olea. For plant chemistry data, ChemDraw Pro 17.0 software was used to construct chemical structures and the PubChem database was used to confirm the IUPAC designations of the chemical compounds discovered.
3. Results and Discussion
3.1. Taxonomy, Botany, Morphology, and Ecology Description of Olive
The olive tree, scientifically known as Olea europaea L., places it within the kingdom of green plants, with a more specific categorization in the subkingdom Tracheobionta and the superdivision Spermatophyta. It belongs to the division Magnoliopsida and the subclass Asteridae. Its order can be either Scrophulariales or Lamiales, the Oleaceae family. The genus is Olea, and the species is europaea [23]. Morphologically, the olive tree typically reaches heights of up to 10 m. Its stem exhibits a considerable diameter and often has a twisted or bent appearance. The leaves are smooth, tapering, lanceolate, short, ovate, thin, leathery, and oblong. They have a silvery whitish hue. The petiole measures 1 to 3 cm in width and 5 to 10 cm in length. Abundant small hermaphrodite flowers are present, with a milky white hue. The calyx is brief and bears four little teeth, while the corolla measures 1 to 2 mm in length. The fruit is ovoid and tiny, turning blackish-violet when ripe, typically measuring 1 to 2.5 cm in length. Cultivated kinds tend to have larger fruit compared to wild plants, and the bark’s color is often light gray [24]. The olive tree is adaptable to a range of climates. It thrives at an optimal temperature of 40 °C, with growth occurring between 15 °C to 20 °C. However, high-altitude areas are unsuitable due to the risk of frost. Olive cultivation is feasible even in arid and calcareous soils. Nevertheless, sandy loam soils with appropriate levels of phosphorus, nitrogen, and potassium are the most conducive for olive growth. Olive trees are known for their resilience to drought conditions, and they excel in soils with a pH level lower than 8.5. Additionally, these plants can flourish in soils with elevated boron content without experiencing toxicity issues [25].
3.2. Geographical Location of Olea europaea L., in the World
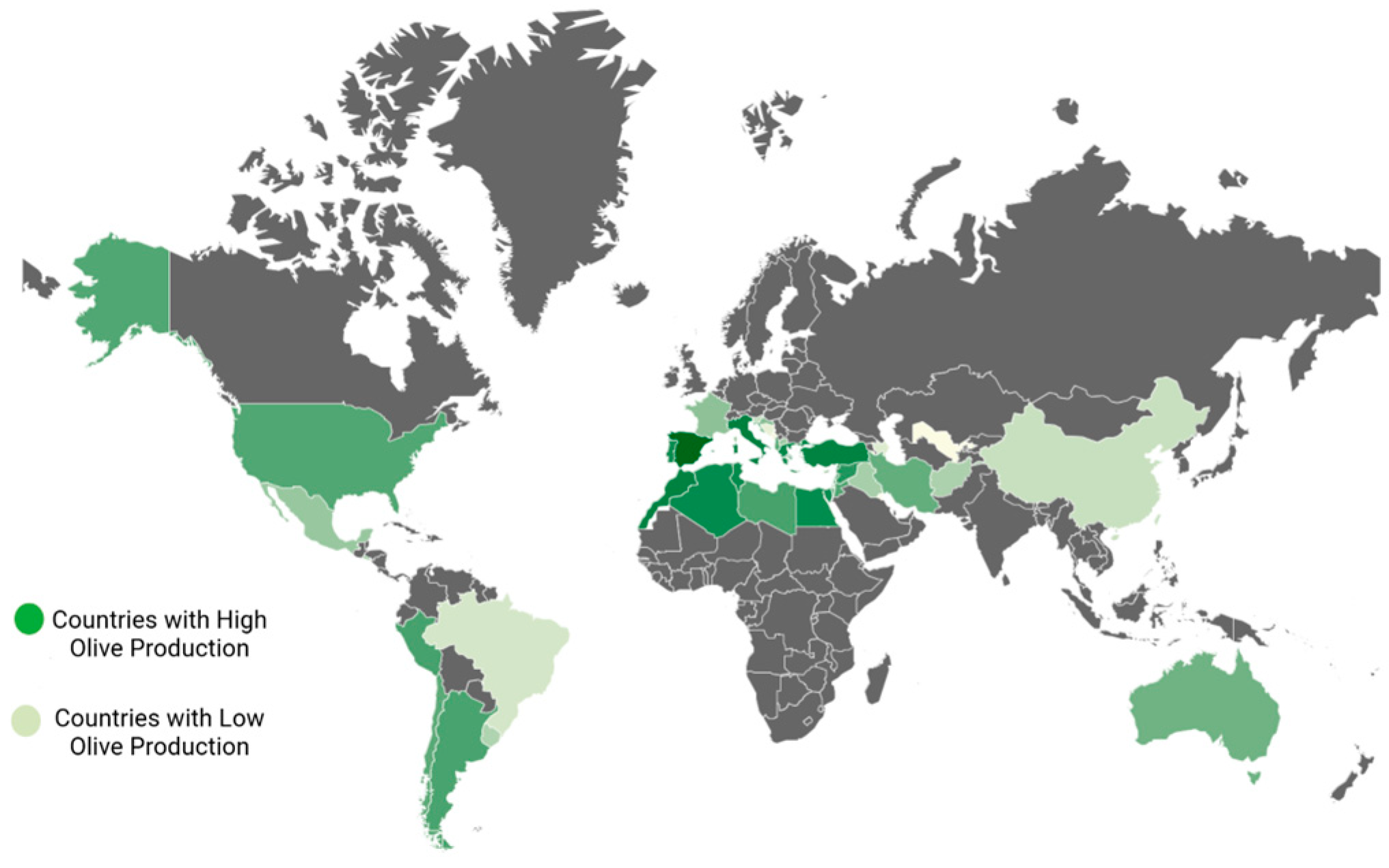
The Mediterranean climate, characterized by warm, arid summers and cool, wet winters, is one that olive trees can withstand. The olive tree is a thermophilic species, well-suited to endure both drought and salinity challenges [26]. It thrives in a variety of soil types, though it exhibits a preference for sandy loam soils that have a moderate depth [3]. Olives are cultivated in the following regions across the globe (Figure 1): in Asia, you can find olive cultivation (in countries such as China, India, Iran, Syria, Pakistan, Jordan, Palestine, Israel, Iraq, Lebanon, and Turkey); in Oceania, olive trees are grown (in New Zealand and Australia); in the Americas, olives thrive (in the United States, Mexico, Uruguay, Chile, Peru, and Argentina); in Europe, olive cultivation is prominent (in Greece, Italy, Spain, Cyprus, Montenegro, France, Portugal, Croatia, and Albania); Lastly, in Africa, you will find olive trees flourishing (in Morocco, South Africa, Tunisia, Egypt, Libya and Algeria) [27,28].
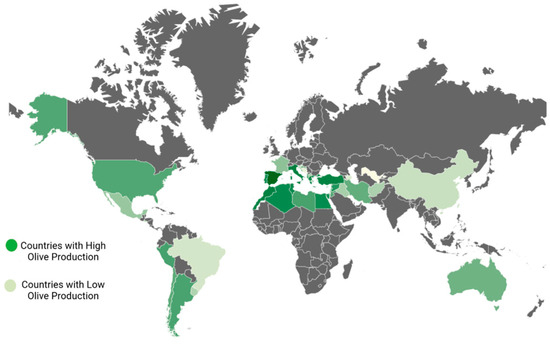
Figure 1.
Worldwide geographical locations of olive cultivation.
3.3. Worldwide Naming of Olive Varieties
The cultivated O. europaea worldwide exhibits various distinct varieties, and the nomenclature of these varieties often evolves from one country to another, reflecting traditional specifications and local practices, as presented in Table 1.

Table 1.
Charting the worldwide dispersion of olive varieties.
3.4. Phytochemistry Characteristics
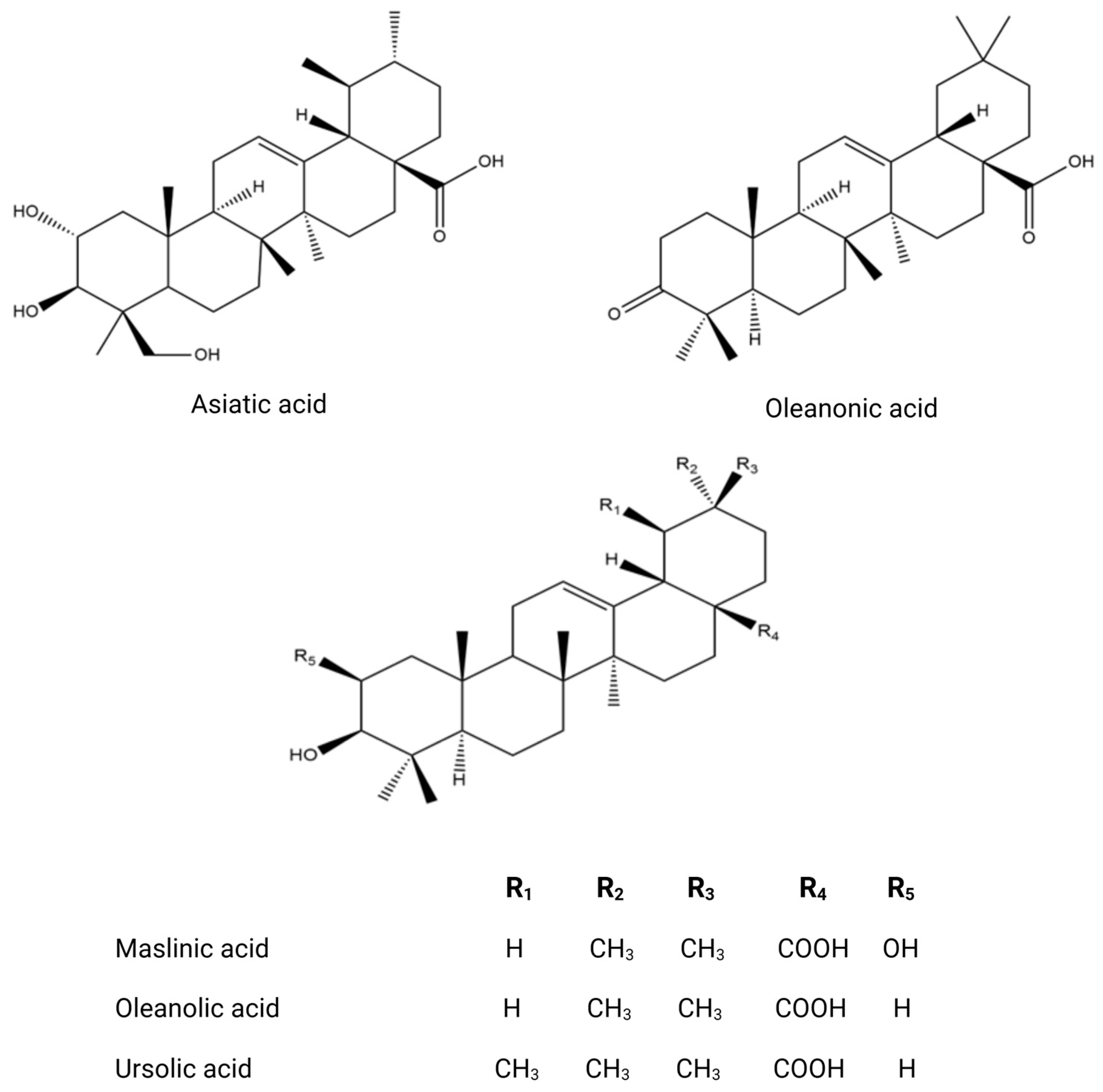
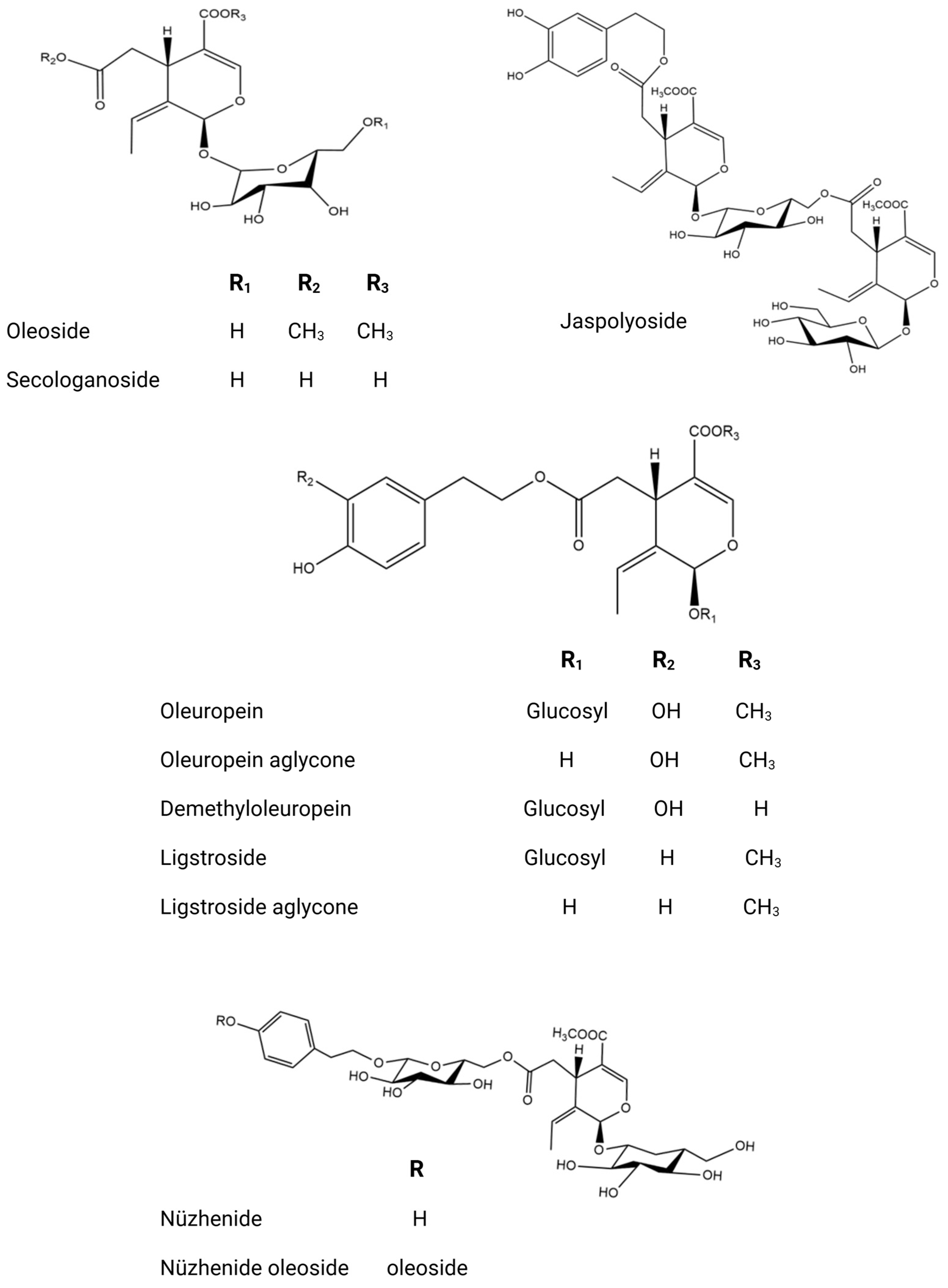
The phytochemical research on O. europaea composition has revealed distinct profiles of secondary metabolites across various parts of the tree (Table 2). This species exhibits a wide range of biochemical complexity, encompassing triterpenoids, simple phenolics, iridoids, secoiridoids, and flavonoids, as illustrated in Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6 [49]. Furthermore, the abundance of lipids, proteins, and minerals in its approximate composition highlights its potential as a great source of nutrition for human consumption.

Table 2.
Differences in the chemical composition of O. europaea in various plant parts.
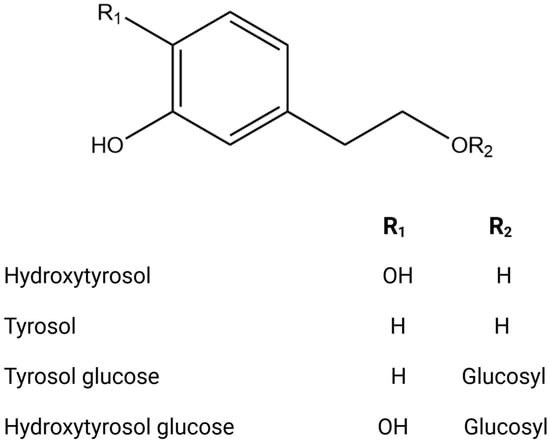
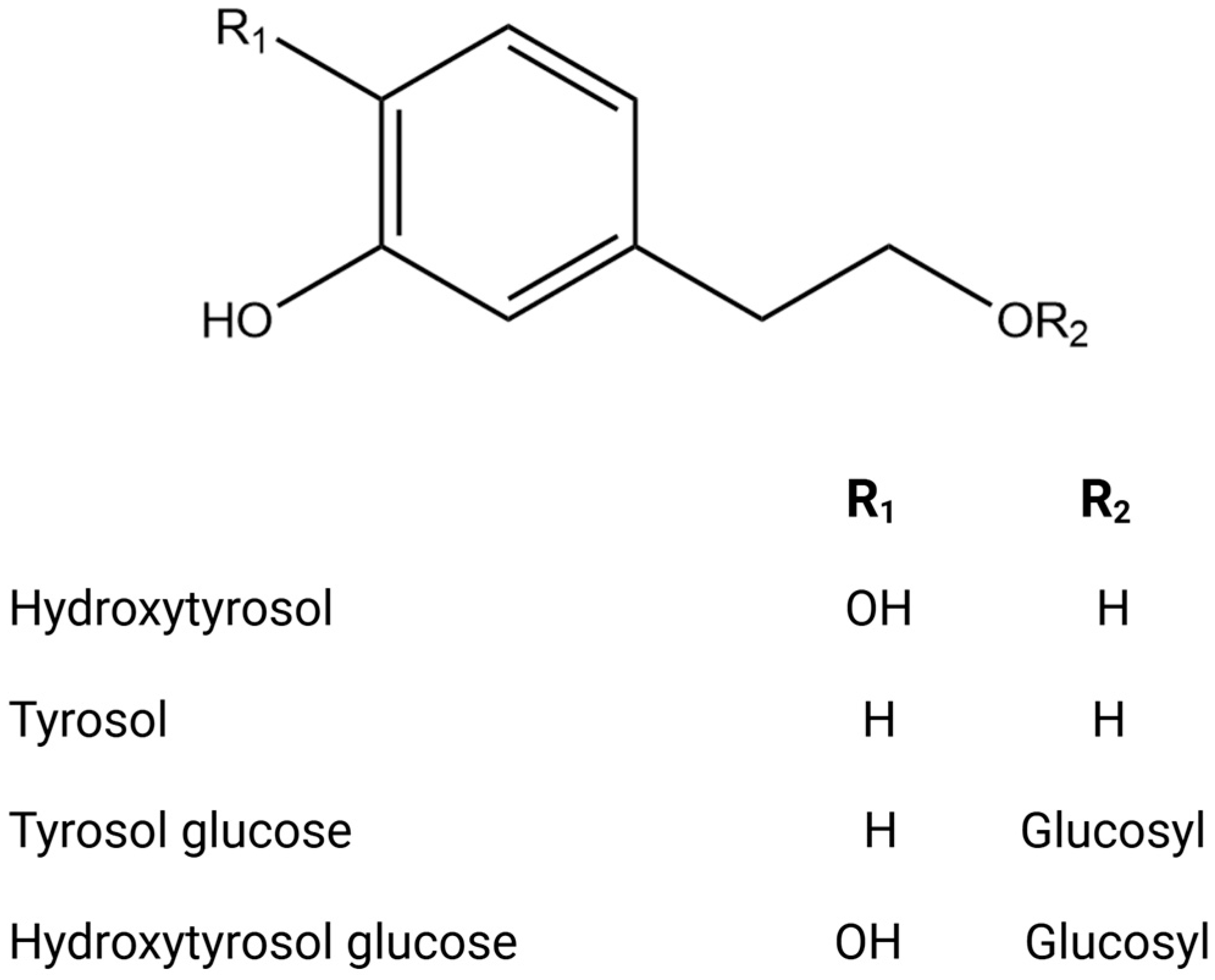
Figure 2.
Chemical structures of key phenolic alcohols.
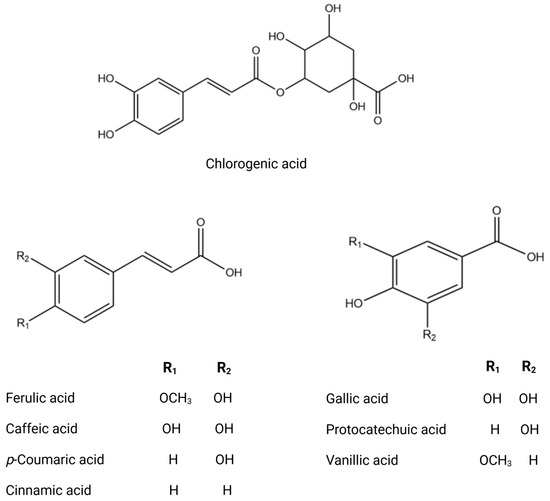
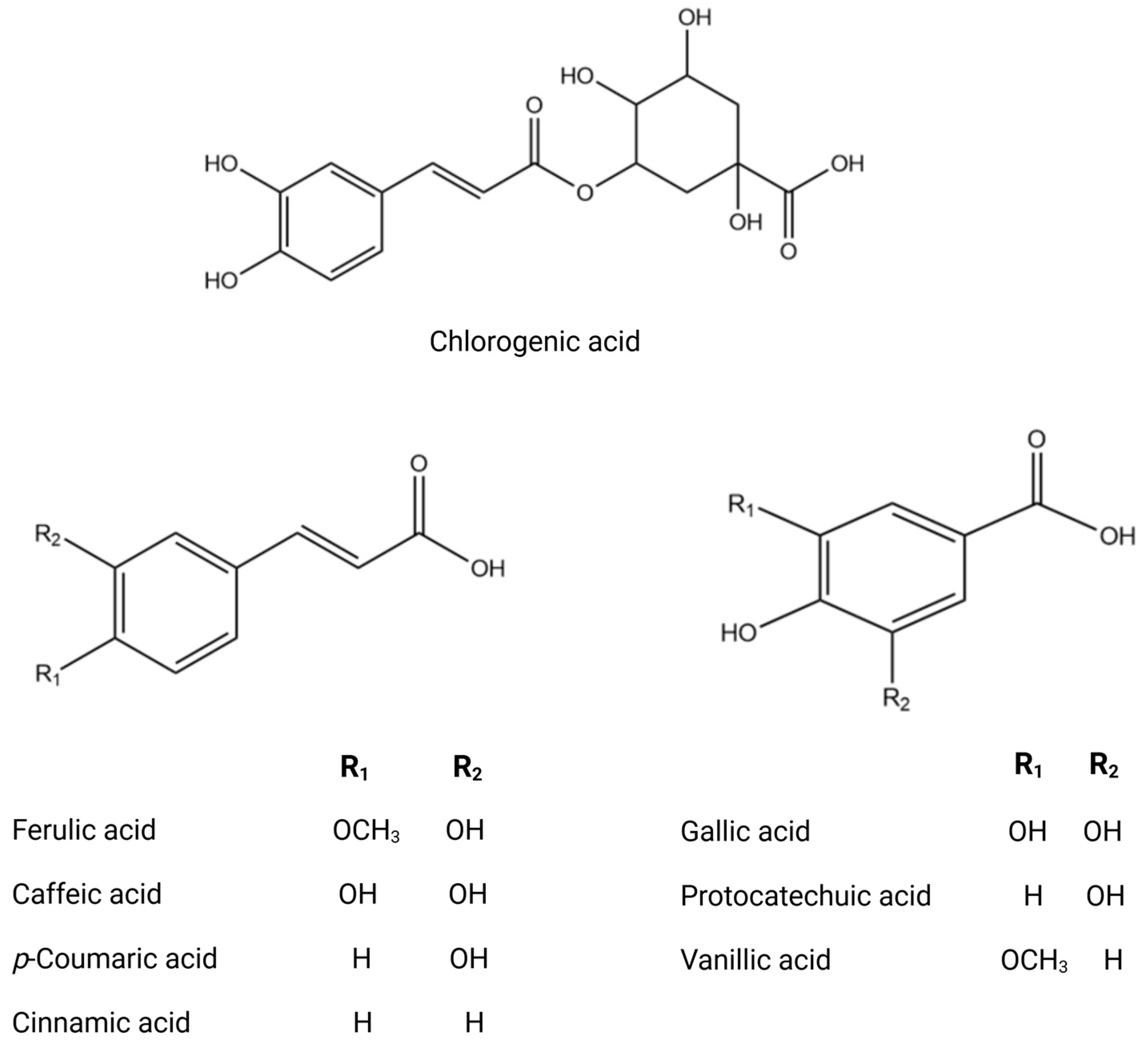
Figure 3.
Chemical structures of key phenolic acids.
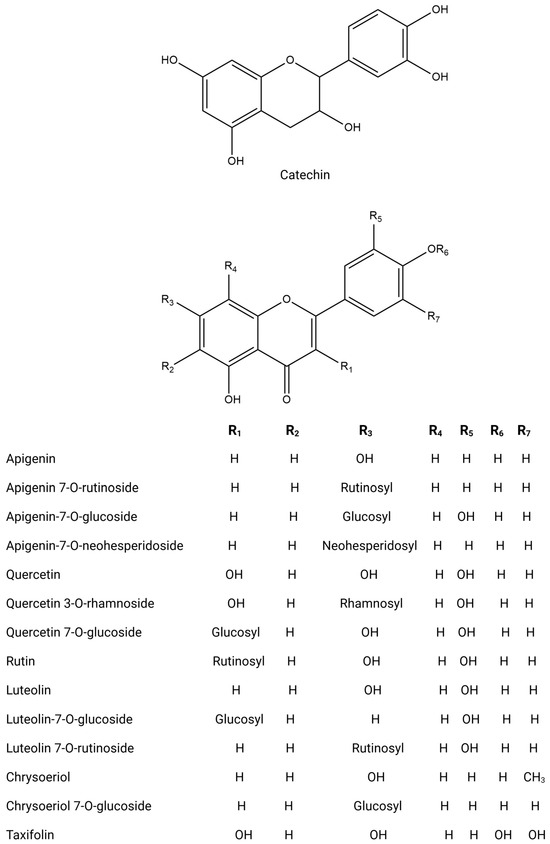
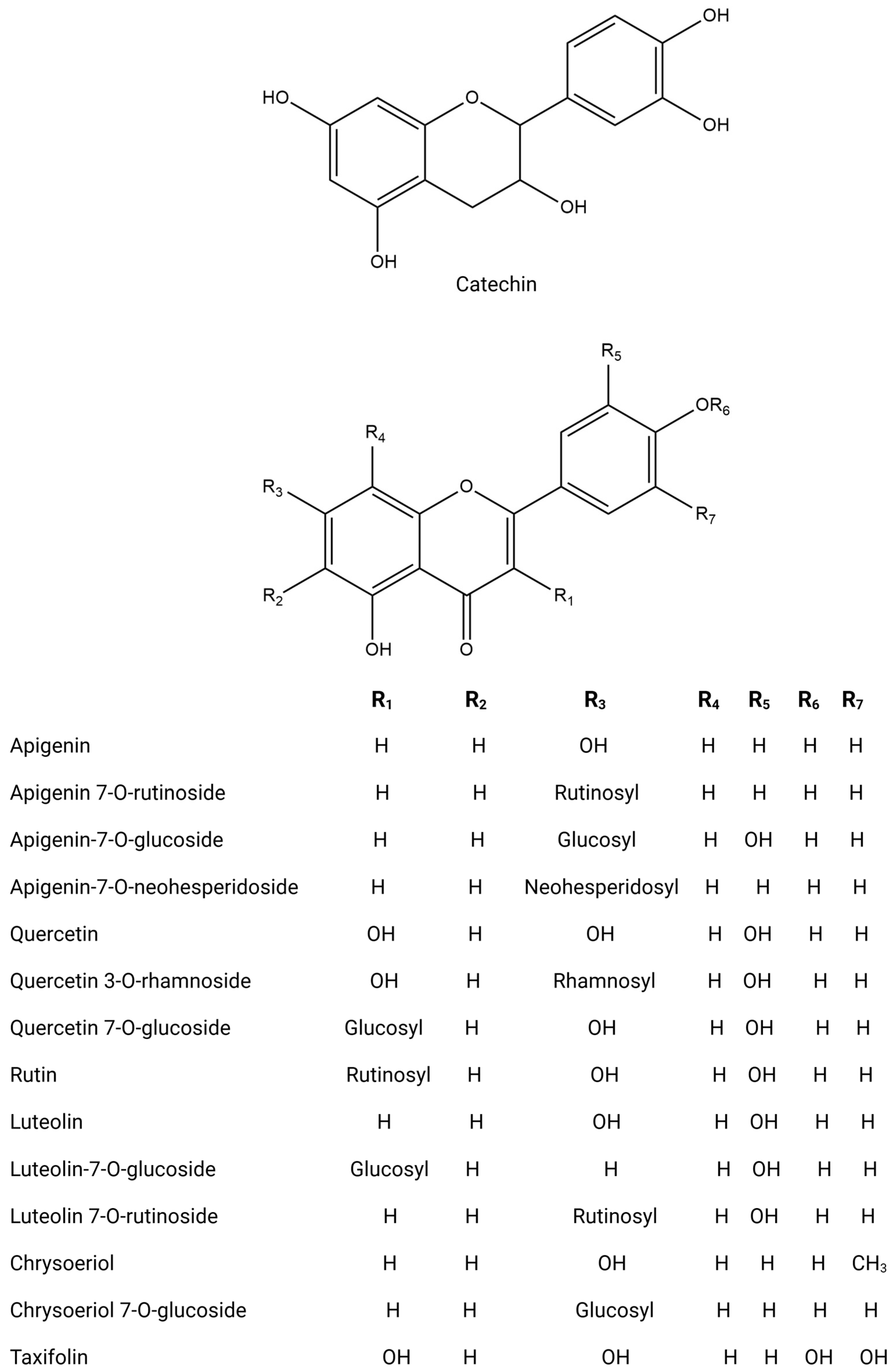
Figure 4.
Chemical structures of key flavonoids.
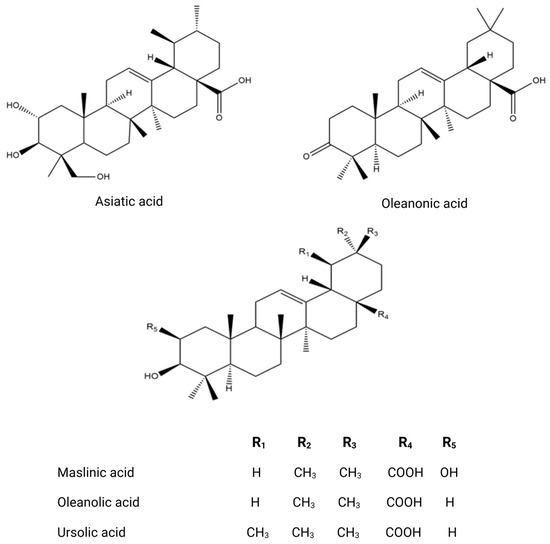
Figure 5.
Chemical structures of key triterpenoids.

Figure 6.
Comprehensive chemical structures of key secoiridoids.
Olive trees have a wide variety of bioactive chemicals in all their sections. Being a part of the Oleaceae family, O. europaea is distinguished by a particular subclass of phenolic chemicals called secoiridoids [60]. The ripening period of olives depends on geographic origin and the cultivation methods, which affect the concentration and quantity of secoiridoid distribution inside the olive tissues [76]. As a component of secondary metabolism, the production of oleuropein in Oleaceae involves a branching reaction inside the route of mevalonic acid, which eventually results in the generation of oleosides [77]. The stems and branches exhibit a high concentration of secondary metabolites, including triterpenoids like erythrodiol and maslinic acid, as well as phenolic substances like taxifolin, comselogoside, and oleuropein [52]. Numerous studies, including those by Talhaoui et al., (2014) and Cecchi et al., (2015) show support for this [9,78].
The fruit of the olive tree exhibits a significant diversity in both primary and secondary metabolites. Its vegetable oil is enriched with a variety of fatty acid compounds, predominantly polyunsaturated fatty acids, along with tocopherols and carotenoids, which play crucial roles in protecting against oxidative stress [54]. Additionally, olive oil is distinguished by the existence of volatile compounds such as isoprene, (E)-Hex-2-enal and α-copaene and other volatile molecules [79], as well as phenolic compounds including hydroxytyrosol, p-coumaric acid, quercetin, and luteolin. Numerous studies have highlighted the diverse chemical composition of olive oil [80,81]. The olive fruit possesses a valuable phenolic composition, characterized by flavonoids, secoiridoids, coumarins, phenolic acids, and triterpenoids, as evidenced by research [62,82,83]. Olive tree seeds are extremely interesting due to the wide variety of primary metabolites they contain, including proteins, fatty acids, and minerals [84]. One notable defining feature of olive seed oil is the presence of polar lipids, which include 94 lipid species and 10 polar lipid classes in their chemical makeup. Phospholipids, glycolipids, acylsterolglycosides, and sphingolipids are some of these classes [85]. When compared to saturated fats, the fraction of polyunsaturated fatty acids in these polar lipids is much larger [86]. Numerous investigations into the polar lipidome of olive seeds have identified notable variations across olive tree cultivars, which are ascribed to the existence or non-existence of certain lipid species. Polar lipid composition has been established and has attracted interest from the cosmetics and culinary sectors because of its significant nutritional significance [87]. Moreover, one fascinating finding about the seed’s phenolic compounds is that they belong to the subclass of secoiridoids, which is defined by the existence of nüzhenide and its derivatives (Figure 6). These compounds, which are exclusive to olive seeds, have strong biological activity and show great promise for improving human health [88]. The constituents of olive leaves, as reported from the essential oil extracted via hydrodistillation, exhibit an interesting chemical composition comprising oxygenated monoterpenes, oxygenated sesquiterpenes, and monoterpene hydrocarbons [61,89]. As secondary metabolites, sesquiterpenes have drawn significant attention in bio-pharmacology, particularly as potential natural anticancer agents [90]. An olive leaf extract contains a wide variety of phenolic chemicals, including phenolic acids (gallic acid, protocatechuic acid, vanillic acid, and salicylic acid); flavonoids (rutin, diosmetin, and apigenin); lignans (pinoresinol and syringaresinol); triterpenoids, secoiridoids, and derivatives (oleuropein, loganoside, and ligstroside) [91,92,93,94,95]. In light of the fact that olive leaves contain numerous bioactive molecules with varied biological functions, they have gained significant attention as potent by-products of the olive tree [96,97]. The phytochemical composition of the olive tree is highly diverse, resulting in various biological activities. Its bioactive molecules can act as antidiabetic, anti-inflammatory, antifungal, antibacterial, antioxidant, and anticancer agents [98,99,100,101,102].
3.5. Biological Properties
3.5.1. Antibacterial Activity
Infections with bacterial pathogens have a significant impact on human pathology. Serious conditions can arise, such as Helicobacter pylori contributing to intestinal issues and Mycobacterium tuberculosis leading to respiratory tract diseases. Additionally, Propionibacterium acnes, and Staphylococcus epidermidis colonize the skin, resulting in similar opportunistic pathologies [103]. Numerous studies have showcased the antibacterial properties of olive extracts from the leaf, fruit, and seed. There are numerous screening results presented in Table 3 that demonstrate the evaluation of these properties using various methods, such as clinical trials, in vivo and in vitro testing.
The olive tree seed is characterized by specific sub-phenolic secoiridoids, notably nüzhenide and its derivatives [52]. Kadir et al. [104] investigated the amounts of flavonoid and phenolic chemicals, as well as the antibacterial, antioxidant, and antithrombotic capabilities of extracts produced from the seed, fruit, and leaf of the Halhali olive tree, which is planted in Hatay, Turkey’s Arsuz area. Their findings revealed that seed extracts exhibited antimicrobial activity, with minimum inhibitory concentration values ranging from 100 to 200 mg/mL against Klebsiella pneumoniae, Pseudomonas aeruginosa, Staphylococcus aureus, Enterococcus faecalis, and Escherichia coli [104]. The olive fruit possesses an intriguing chemical composition, as confirmed by numerous studies highlighting its rich bioactive composition [82]. Hanene et al. (2014) investigated a methanolic extract from olive oleaster fruit pulp, which demonstrated strong effectiveness against Gram-positive and Gram-negative bacteria, indicating non-selective antibacterial activity [105]. Guo et al. (2019) discovered that olive oil polyphenol extract (OOPE) inhibited the development of L. monocytogenes, with 1.25 mg/mL as the minimal inhibitory concentration (MIC). When compared to untreated cells, OOPE-treated cells showed a substantial drop (p < 0.05) in intracellular ATP concentrations, DNA levels or bacterial protein [102]. Other studies have also confirmed the antibacterial effects of olive oil [106,107]. It was suggested by Wang et al., (2017) that phenolic compounds affected the minor groove of genomic DNA, changing its secondary structure and morphology, in addition to increasing permeability of the cell membrane and inducing morphological changes in the cells [108]. Furthermore, the literature highlights the strong antibacterial activity demonstrated by olive leaf extract in numerous studies. Abdullah et al. [109] found that the heated olive leaf aqueous extract exhibited excellent antibacterial activity in opposition to other selected strains. Concentrations of the extract ranged from 0.5% to 6%, resulting in inhibition zone diameters ranging from 1 mm to 10.3 mm. Notably, Staphylococcus aureus and Streptococcus mutans demonstrated the greatest sensitivity to the extract. To elucidate the mechanism of action involving a mixture of polyphenols with absorption and bioavailability, agarose gel electrophoresis analysis of plasmid DNA isolated from kanamycin- and ampicillin-resistant bacterial strains was performed. The analysis aimed to determine if the olive leaf extract, rich in polyphenols, could interact with the plasmid DNA of the resistant bacterial strains. This interaction could potentially lead to the cleavage or alteration of the plasmid DNA, affecting the bacteria’s resistance mechanisms [109]. Alsulaymani et al. (2021) found that the extracts exhibited significant antibacterial activity against Escherichia coli, with inhibition zone measurements ranging from 8 mm to 15 mm [110]. De la Ossa et al. (2021) found that 100 mg/mL of olive leaf extract (OLE) showed antibacterial action; however, it was unable to completely inactivate the germs. Plain OLE decreased L. innocua and S. aureus growth, whereas E. coli growth was unaffected [111]. Himour et al. [112] noted that the variety of the olive tree and the choice of solvent in the extraction process significantly influenced the extraction of secondary compounds, consequently impacting extraction activity [112].
Furthermore, Rosa et al. [113] reported that microwave-assisted extraction (MAE) was more effective than ultrasonic-assisted extraction (UAE), inhibiting E. coli growth by 100% vs. 80%, respectively, at a dose of 50 mg/mL. Additionally, the inhibition effect of the UAE extract reached almost 92% at 75 mg/mL. The extraction process directly impacts the composition of the bioactive molecule extract, potentially altering its biological activity [113,114]. Vural et al. [115] found that the essential oil extracted from olive leaves displayed a highly intriguing volatile composition, showcasing potent antibacterial activity [115].

Table 3.
A synopsis of research on olive tree extracts’ antibacterial activity.
Table 3.
A synopsis of research on olive tree extracts’ antibacterial activity.
| Part Used | Extract | Strains | Method | Key Results | Reference |
|---|---|---|---|---|---|
| Seed | Vegetal extract | GP Staphylococcus aureus Enterococcus faecalis Listeria innocua Listeria monocytogenes Bacillus cereus Streptococcus mutans Bacillus subtilis Staphylococcus epidermidis Clostridium sporogenes Bacillus subtilis subsp. Spizizenii Bacillus subtilis Streptococcus sobrinus Streptococcus ralis Staphylococcus epedermidis, Propionibacterium acnes Lactobacillus plantarum GN Escherichia coli Salmonella typhimurium Aeromonas hydrophila Agrobacterium tumefaciens Pseudomonas aeruginosa Acinetobacter baumannii Shigella. sonnei Proteus mirabilis Citrobacter freundii Salmonella. enteritidis Pseudomonas fluorescens Brochotrix thermosphacta Pseudomonas fragi Pseudomonas putida Salmonella enterica Enterobacter cloacae Klebsiella pneumoniae Salmonella Enteritidis Pseudomonas vulgaris Morganella Morganii Haemophilus influenzae Yersinia enterolitica Salmonella enterica subsp. heindelberg | Disc diffusion method Minimum inhibitory concentration Minimum bactericidal concentration Broth micro-dilution assay Live/dead bacterial staining assay Bacterial inhibition assays Bacterial motility assays | MIC = 100–200 µg/mL | [104] |
| Fruit | Vegetable oil | Ø = 5–18 mm | [81,116] | ||
| Vegetal extract | Ø = 13–18.5 mg/mL MIC = 12.5–25 mg/mL MBC = 25–50 mg/mL | [102,105] | |||
| Leaves | Essential oil | Ø = 9–29 mm MIC = 0.625–5 mg/mL | [6,96,115,117] | ||
| Vegetal extract | Ø = 1–20 mm MIC = 0.60–25 mg/mL MBC = 0.70–12.5 mg/mL | [109,110,118,119,120,121,122,123,124,125,126,127] |
Ø = zone of inhibition; MIC = minimum inhibition concentration; MBC = minimum bactericidal concentration; GN = Gram negative; GP = Gram positive.
3.5.2. Antifungal Activity
Microbial infections have emerged as a significant global concern, with the Candida albicans species being one of the most prevalent fungal strains found on human mucosal membranes [126]. This yeast can attach to epithelial tissues, resulting in superficial infections commonly referred to as candidiasis. Under certain conditions, candidiasis has become a major contributor to morbidity and mortality among immunocompromised individuals worldwide [128]. A primary challenge lies in the multidrug-resistant nature of both bacterial and fungal strains [129,130]. Consequently, researchers are increasingly turning to phytotherapy using medicinal plants, which possess antimicrobial properties due to their chemical compounds. However, the diversity of extraction techniques for bioactive molecules poses a critical challenge to their efficacy against fungal infections. O. europaea, a medicinal plant, is known for its potent microbial-infectious molecules due to its phenolic composition, particularly secoiridoids [131]. Several studies have investigated the biological activity of various portions of the olive tree, as shown in Table 4. Olive leaves, in particular, are characterized by their phenolic composition, which exhibits potent antifungal properties [132]. Vural et al. [115] assessed the antimicrobial effects of dried leaf essential oil towards microbial strains, and discovered considerable antifungal activity against Candida albicans [115]. Similarly, other researchers observed intriguing effects of leaf essential oil against fungal infections [133]. However, several factors can affect the effectiveness of these extracts, such as the variety of olive cultivar, the extraction process, the solvent used, and the by-product harvesting time. Alimosazade et al. [134] examined olive extracts from four cultivars against various fungal strains. The minimum inhibitory concentration (MIC) varied due to differences in total phenolic content, indicating the impact of cultivars on biological activity efficacy [134]. Bawadekji et al. [135] performed analyses on the phytochemical composition and antimicrobial properties of olive leaf extracts obtained using water-immiscible solvents, observing significant inhibition of Aspergillus niger and Candida albicans growth [135]. This study emphasized the impact of extraction solvent on antifungal activity. Overall, olive leaf extracts exhibited substantial antifungal activity attributed to their rich phenolic compound content, particularly hydroxytyrosol, oleuropein, and secoiridoid derivatives, consistent with findings from several studies [136,137,138]. The olive fruit is known for its rich array of bioactive molecules that possess antimicrobial properties [139]. Naje et al. [140] investigated the O. europaea fatty oil’s possible antifungal action against clinical isolates of fungal strains, yielding promising results as presented in (Table 4) [140]. Similarly, Janakat et al. [141] reported a favorable MIC of olive fruit oil against fungal growth [141]. Additionally, Umai et al. [142] produced silver nanoparticles from olive fruit extract as an environmentally sound approach to demonstrate effective MIC values against tested fungal strains [142]. Suwan et al. [143] also highlighted the noticeable activity of silver nanoparticles synthesized from olive extract against fungal species [143]. Biofilm-associated infections, accounting for up to 80% of total human infections, pose significant challenges to the medical community and protect microbial cells from the host immune response [144]. Slobodnikova et al. [14] revealed the significant activity of phenolic compounds from olive fruit against biofilms formed by bacteria and fungi species [14]. Furthermore, nanoparticle-based treatments derived from olive extracts exhibited powerful antibiofilm activity [145]. Several research works have examined the chemical makeup of olive seeds and their antifungal activity, with olive seed extracts demonstrating activity against various fungal strains [146]. The bioactive compounds of the olive tree are strongly correlated with the efficacy of our extracts against fungi.

Table 4.
Antifungal activity of various parts of O. europaea: summary of research findings.
3.5.3. Antioxidant Activity
Reactive oxygen and nitrogen species (RONS) are chemically active free radicals and non-radical substances produced during normal cellular processes and mitochondrial respiration, including ozone (O3), hydrogen peroxide (H2O2), hypochlorous acid (HOCl), peroxynitrite ion (ONOO), and singlet oxygen (O2) and oxygen radicals like hydroxyl (HO), superoxide anion (O2), peroxyl (ROO), hydroperoxyl (HO2), and alkoxyl (RO). Correspondingly, reactive nitrogen species (RNS) comprise nitric oxide radical (NO), nitrogen dioxide radical (NO2), peroxynitrite ion (ONOO), and certain nitrogen oxides [151,152]. To manage these reactive species, the body employs endogenous enzymes like catalase, glutathione peroxidase, and superoxide dismutase [153]. Yet, under conditions of increased stress or physiological imbalance, the generation of RONS might exceed the capabilities of the body’s internal antioxidant mechanisms, resulting in oxidative stress. The structure or functionality of biological macromolecules such as proteins, lipids, and DNA may change as a result of this stress [154,155]. Interestingly, the oxidation of macromolecules in cells is believed to be a significant factor in the development of various age-related and degenerative diseases [156,157,158,159]. Considerable research has focused on exploring natural sources of bioactive molecules found in medicinal plants to address the imbalance of ROS within the human body. Among these efforts, there has been extensive investigation into the effects of the chemical composition of O. europaea in combating oxidative stress. Table 5 summarizes the findings from this research.

Table 5.
Antioxidant activity of various parts of O. europaea: summary of research findings.
Kadir et al. [96] conducted a comprehensive exploration of the potential for pharmaceuticals of extracts from the Halhali olive’s leaf, fruit, and seeds using various methodologies. Their investigation revealed significant antioxidant activity in the extracts. Specifically, with 317.24 μg GAE, the seed extract had the highest phenolic content and demonstrated the strongest reducing potential for Fe3+/Fe2+. Additionally, it displayed notable radical scavenging activity against DPPH, with an IC50 value of 5.25 µg/mL [96]. Celik et al. [99] reported that aqueous and ethanolic olive seed extracts exhibited significant antioxidant activity against DPPH, ABTS and H2O2 radicals at a concentration of 30 µg/mL. The high phenolic and flavonoid content of both extracts may account for this strong activity [99]. Notably, Bouarroudj et al. [99] reported that oleaster oil exhibited the richest phenolic composition among all samples studied. They identified two new molecules, namely eriodictyol and naringenin. In addition, the antioxidant potential of olive oil against DPPH and ABTS radicals showed significant effects, with 80% inhibition of free radicals in both tests. The phenolic composition and amount of tocopherol in olive oil are responsible for its greater inhibitory capacity [160]. Fernández-Poyatos et al. [167] studied the antioxidant capacity and phenolic content of table olives and the effect of in vitro simulated digestion. They stated that the extract from table olive fruit contained a significant amount of phenolic compounds, comprising thirty different compounds. The in vitro digestion assay revealed a reduction of over 50% in total phenolic content, thereby impacting antioxidant potency. Both antioxidant assays (ABTS and DPPH) showed a substantial decrease in activity. The digested assay offers an initial insight into the potential of these compounds to traverse the digestive system. The bioavailability of these molecules is key to optimizing their protective effects [167]. Furthermore, Tamasi et al. [168] aimed to investigate the O. europaea products’ chemical composition and antioxidant capabilities; they found that olive fruit extract exhibited a high level of total phenolic content (TPC), with quantitative variations influenced by factors like olive variety, harvest time, soil, and climatic conditions. The antioxidant assays (DPPH and ABTS) showed correlated results with TPC, indicating that higher TPC correlated with increased antioxidant potential [168,169]. Moreover, numerous studies have underscored the significant antioxidant role of olive leaves. Elnahas et al., (2021) found that Egyptian olive leaf extracts exhibited 87.55% scavenging activity against DPPH free radicals at a concentration of 50 mg/mL [119]. Blasi et al. [170] examined the seasonal variations in the antioxidant components of O. europaea leaf extracts from different Italian cultivars. They observed that seasonal variation significantly influenced the antioxidant potential of the olive leaf extract and the TPC. Moreover, the predominant phenolic content and components are at the origin of the extract’s antioxidant capacity [171]. These findings suggest that a higher TPC in a season does not necessarily correlate with the highest antioxidant capacity. For instance, DPPH inhibition ranged from 55% to 86%, and ABTS ranged from 53.6 to 88.4 mg TE/g [170]. Farag et al. [7] conducted phytochemical screening and assessed the crude juices’ antioxidant content from various medicinal plants. They observed that the antioxidant capacity of the olive leaf extract had a positive effect compared to the standard (std) used. In the DPPH assay, the IC50 was 42.5 µg/mL, while the std/IC50 was 21 µg/mL. For metal chelating activity, the EDTA/IC50 was 155 µg/mL, and the std/IC50 was 37.95 µg/mL. Raw olive leaf juice is ideally suited to practical use as a dietary supplement to delay fat oxidation [7]. Lins et al., found that olive leaf extract inhibited DPPH (EC50 = 13.8 ± 0.8 mg/mL) and ABTS (EC50 = 16.1 ± 1.2 mg/mL), and FRAP analysis produced a value of 281.8 ± 22.8 mg TE/g dry weight. The extract effectively scavenged HOCl (EC50 = 714.1 ± 31.4 mg/mL), NO (EC50 = 48.4 ± 6.8 mg/mL), and O2 (EC50 = 52.6 ± 2.1 mg/mL). In addition, it prevented erythrocytes from undergoing the action of peroxyl radicals that produce hemolysis (EC50 = 11.5 ± 1.5 mg/mL), TBARS formation (EC50 = 38.0 ± 11.7 mg/mL) and hemoglobin oxidation (EC50 = 186.3 ± 29.7 mg/mL). These findings indicate that olive leaf extract is a remarkable, natural antioxidant source, exhibiting potent antioxidant activity against a range of reactive species and protecting human erythrocytes against free radical damage [172]. Furthermore, Ayoub et al. [173] conducted a phytochemical screening and assessed the antioxidant activity and inhibitory capacity of O. europaea and Ficus carica leaves. They observed that olive leaf extract exhibited robust antioxidant potency in scavenging free radicals, with a relationship between antioxidant activity and secoiridoids and flavonoid contents (Figure 7). The IC50 value of the O. europaea extract was determined to be 170.134 ± 0.06 μg/mL, indicating its efficacy in inhibiting DPPH free radicals and mitigating oxidative stress [173]. Lfitat et al. [163] conducted in vitro antioxidant studies to confirm the antioxidant potency of olive leaves and their presence in secondary metabolites, which aid in metal chelation and free radical scavenging [163]. Monteleone et al. also confirmed that olive leaf extract (OLE) displayed significant antioxidant capacity. At peak levels of phenolic and flavonoid content, the extract demonstrated the most potent inhibitory effect on oxidation, with a DPPH = 2.85 ± 0.01 TEAC mM for 90% inhibition [18,174]. Ribas et al. assessed the nutritional composition, bioactive components, antioxidant properties, and color characteristics of leaves sourced from eight different olive cultivars in Brazil. They found that the olive leaf extract (OLE) from these varieties exhibited the greatest level of antioxidant capability, with ABTS = 78.15% and DPPH = 93.56%. Several factors have been identified that influence the content of secondary metabolites and antioxidant activity, including olive cultivar, date of collection, drying conditions, climatic conditions and extraction method [175,176,177]. Furthermore, Pennisi et al. [165] sought to assess the antiviral and antioxidant properties of leaf extracts obtained from O. europaea vars. sativa and sylvestris. Researchers discovered that exposure to olive leaf extracts prevents lipid peroxidation in human HeLa cells and boosts the levels of antioxidant enzymes including catalase, superoxide dismutase, and glutathione peroxidase, which in turn lessens the oxidation of free radicals [165].
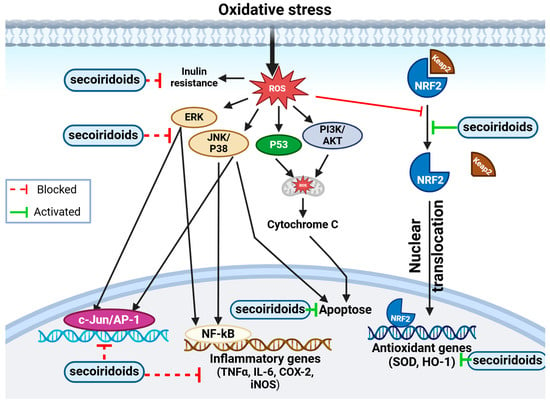
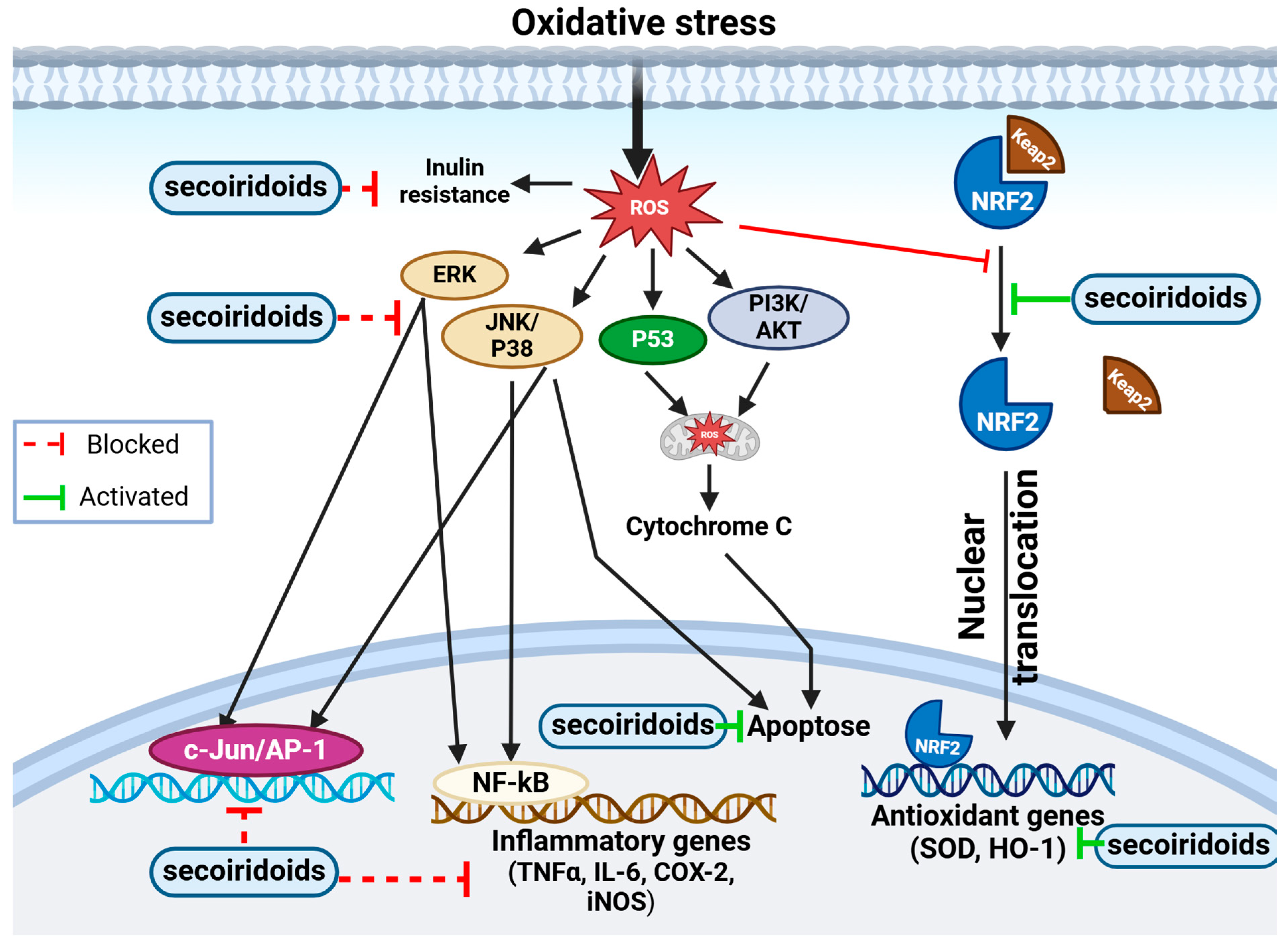
Figure 7.
Figure illustrating the molecular representation of the antioxidant mechanism of secoiridoids. This potent antioxidant has the potential to mitigate intracellular ROS levels. ROS, known to induce inflammation via NF-kB and AP-1 activation, trigger various cellular signaling processes leading to the production of inflammatory mediators. Secoiridoids effectively block these mediators. Additionally, secoiridoids might regulate cellular signaling by impacting signal transduction pathways. They demonstrate protective properties by activating the PI3-kinase/Akt pathway, stimulating MAPK proteins (ERK, JNK, and P38), and facilitating Nrf2 translocation into the nucleus. Furthermore, secoiridoids elevate the expression of MnSOD and HO-1, providing defense against oxidative stress through the Nrf2 pathway.
3.5.4. Antidiabetic Activity
Diabetes mellitus (DM) and its treatment represent considerable social, economic, and healthcare challenges on a global scale. DM is a metabolic disorder characterized by chronic hyperglycemia, along with imbalances in lipid, protein, secretion, and insulin action [178]. Projections suggest that by 2025, over 300 million people will be affected by diabetes [179]. Type I diabetes arises from defects in insulin secretion due to inherited and/or acquired deficiencies in pancreatic insulin production [180]. On the other hand, type II diabetes results from insulin ineffectiveness caused by insulin resistance in the liver and peripheral tissues [181]. As type II diabetes advances, there is a decrease in cell mass and function, disrupted insulin signaling, changes in lipid metabolism, low-grade inflammation, and heightened oxidative stress [182]. Diabetes pathogenesis and complications are greatly influenced by oxidative stress. It induces injury in pancreatic cells and enzymes, increases lipid peroxidation, and leads to insulin resistance in cells [15]. There has been strong evidence that O. europaea may be used as an ethnomedicine in the treatment of diabetes, as illustrated in Table 6 and Table 7. Notably, Perri et al. [183] found that O. europaea bud extracts inhibited pancreatic lipase and α-amylase activity in several Italian cultivars. The study found significant suppression of digestive enzymes, with IC50 values of 33.21 ± 0.23 µg/mL for α-amylase and 1.27 ± 0.04 mg/mL for lipase inhibition [183]. O. europaea subsp. cuspidata (Indian olive) seed extracts were studied by Akhtar et al., (2022) for their antioxidant activities and potential as antidiabetic agents. At a concentration of 1.6 mg/mL, in vitro tests revealed an 82.10% inhibition of α-Amylase. Rats were used in in vivo tests to generate diabetes with a single intraperitoneal quantity of alloxan monohydrate (150 mg/kg). Three days following the injection of alloxan, rats’ serum FBG levels were assessed; those rats whose FBG levels were 200 mg/dL were classified as diabetic. Rats without diabetes were given normal saline orally in Group 1, while diabetic rats were given glimepiride (0.2 mg/kg) orally in Group 2, which served as the standard control. Rats with diabetes who were given normal saline alone made-up Group 3, the negative control. The application of MEOE resulted in a dose-dependent recovery of the Langerhans islets, reduced kidney blood vessel inflammation and thrombosis, and lessened glomerulus and tubule necrosis caused by alloxan [184]. Furthermore, olives are rich in biophenol compounds, providing them with the ability to combat various biological diseases. Zakari et al. [185] worked on the hypoglycemic effect of olive oil on alloxan-induced diabetic albino rats. Their study revealed that olive oil demonstrated a hypoglycemic effect on alloxan-induced diabetic Albino rats at a lower dose (150 mg/kg/b.w.) [185]. Figueiredo-González and colleagues [186] examined the potential antidiabetic effects of phenolic-rich extracts derived from autochthonous extra virgin olive oils in Galicia towards the inhibition of α-amylase and α-glucosidase. They discovered that EVOO had a stronger antidiabetic impact on α-glucosidase (IC50 = 0.06 ± 0.008 mg/mL) than it did on α-amylase [186]. Additionally, Abdelkarim et al. [187] studied the effects of olive leaf powder (OLP) on circulating adipokines and insulin secretion in rats with streptozotocin-induced diabetes. Four groups (n = 10) of 40 male albino rats from Wistar weighing 200–225 g each were created: normal healthy rats were included in Group I; diabetic control rats were included in Group II, diabetic rats, fed a balanced diet along with standard antidiabetic medication (metformin, 600 mg/b.w.), were included in Group III, and diabetic rats were included in Group IV, given a diet plus 2.0% OLP. After analyzing their data, they concluded that OLP significantly improved blood glucose regulation. It did this by lowering serum levels of glucose, low-density lipoprotein, triglycerides, and total cholesterol, elevating levels of high-density lipoprotein; decreasing levels of atherogenic index and atherogenic coefficient; raising serum adiponectin concentration; and lowering serum leptin concentration [187]. Al-Shudiefat et al. [188] sought to investigate the mechanism behind this phenomenon by assessing proteins involved in glucose metabolism, including adenosine monophosphate-activated protein kinase (AMPK α2), glucose transporter 4 (Glut4), and Akt substrate of 160 kDa (AS160), in an effort to understand the mechanism behind this behavior. Their findings revealed that treatments with OLE at 1% and 3% effectively reduced blood sugar levels and regulated biochemical parameters such as LDL, HDL, TG, and insulin secretion. They observed that OLP inhibits AS160, resulting in decreased blood glucose levels [188]. Chigurupati et al. [189] found that OLE exhibited a significant α-amylase inhibitory effect, with an IC50 of 0.037 µg/mL, demonstrating competitive kinetic inhibition [189]. Additionally, Laaboudi et al. [190] sought to investigate the hypolipidemic and hypoglycemic properties of olive tree extract phenol on streptozotocin-induced diabetic rats. They discovered that taking olive extract orally led to a decrease in blood glucose levels in the diabetic rat group, which were significantly lower at 4 weeks compared to control diabetic rats. Furthermore, the extract regulated the biochemical parameters to approximate those of normal rat models [190].

Table 6.
Antidiabetic activities of various parts of O. europaea: summary of in vitro research findings.
Table 6.
Antidiabetic activities of various parts of O. europaea: summary of in vitro research findings.
| Part Used | Extract | Method In Vitro | Key Results | Reference |
|---|---|---|---|---|
| Buds | Hydroalcoholic extract | Pancreatic lipase activity inhibition (PLI) The inhibition of α-amylase (IAM) The inhibition of α-glucosidase Glucose uptake using the yeast cells assay Determination of surface GLUT4myc L6-GLUT4myc cell line | IC50 = 1.27 ± 0.04 mg/mL IC50 = 0.1269 ± 0.023 mg/mL | [183] |
| Seed | Aqueous extract | IC50 = 0.3194 mg/mL | [184] | |
| Fruit | Ethyl acetate extracts | IC50 = 0.00531 ± 0.003 mg/mL IC50 = 0.0547 ± 0.001 mg/mL | [191] | |
| Extra virgin olive oil | IC50 = 0.06 ± 0.008 mg/mL | [186] | ||
| Leaves | Hydroalcoholic extract | IC50 = 150 µM | [101] | |
| Ethanolic extract | IC50 = 0.037 mg/mL | [189] | ||
| Aqueous extract | IC50 = 0.014 ± 0.041 µg/mL | [17] | ||
| IC50 = 0.2 mg/mL | [192] | |||
| Methanolic extract | IC50 = 43.47 µM EC50 = 47.12 µM | [193] |
Mansour et al. [17] showed that administering OLE alone or combined with metformin normalized blood glucose, glycated hemoglobin, lipid profiles, and liver enzyme levels. Histological analysis indicated that OLE, either alone or combined with metformin, effectively restored liver, kidney, and pancreatic tissues [17]. Mechchate et al. [16] explored the in vivo antidiabetic effects and the in silico mode of action of flavonoids found in the leaves the Oleaster using LC/MS-MS analysis. They found that the flavonoid extracted from olive leaves, in two concentrations (25–50 mg/kg/b.w.), effectively managed diabetes induced by alloxan in in vivo experiments. It demonstrated a strong regulation of biochemical parameters, with this effect being even more pronounced when coupled with an antidiabetic drug [16]. Rauf et al. [193] investigated the antidiabetic effect of Ferruginan using the yeast cell glucose uptake assay. At the highest tested dose (100 μM), Ferruginan showed a maximum reduction in inflammation of 71.82% and improved absorption up to 74.96% of glucose by yeast cells. Additionally, Ferruginan dose dependently inhibited α-amylase, showing inhibition of up to 75.45% at the same concentration [193]. Therefore, the extract from olive leaves has the potential to exhibit inhibitory effects on α-amylase and α-glucosidase, similar to acarbose, due to the presence of flavonoids, secoiridoids and other bioactive compounds [194].

Table 7.
Antidiabetic activities of various parts of O. europaea: summary of in vivo research findings.
Table 7.
Antidiabetic activities of various parts of O. europaea: summary of in vivo research findings.
| Part Used | Extract | Dose | Method In Vivo | Key Results | Reference |
|---|---|---|---|---|---|
| Seed | Methanolic extract | 750 mg/kg body weight | Alloxan-induced diabetes in Wistar albino rats Swiss albino mice (aged 3–4 weeks) of both sex Wistar albino male rats (streptozotocin) Sprague–Dawley male rats (streptozotocin) | The treatment of seed MeOH extracts was seen to have a significant hypoglycemic impact and to have reversed weight loss in diabetic rats. | [184] |
| Fruit | Vegetable oil | 150 mg/kg body weight | The blood sugar levels of the group 2 alloxan-induced diabetic rats exhibited a gradual decline, eventually stabilizing within the normal range of 4.9–5.5 mg/dl. | [185] | |
| Leaves | 2%/kg body weight | Low-density lipoprotein, total cholesterol, triglycerides, and serum glucose levels were all lowered. High-density lipoprotein levels rose as a result. It reduced atherogenic index and atherogenic coefficient. | [187] | ||
| Aqueous extract | 200–400 mg/kg body weight | The application of both low and high doses of olive leaf extract effectively ameliorated the identified physiological, molecular, and histopathological changes. | [195] | ||
| 1–3%/kg body weight | Lowered blood glucose levels, regulated biochemical parameters including LDL, HDL, TG, and insulin secretion. OLP functions by inhibiting AS160, thereby causing a reduction in blood glucose levels. | [188] | |||
| 100 mg/kg body weight | Oral treatment with olive extract controlled biochemical parameters to mimic normal rat models and contributed to a considerable reduction in blood glucose levels in the diabetic rat group after 4 weeks when compared with control diabetic rats. | [190] | |||
| Methanolic extract | 25 and 50 mg/kg body weight | It demonstrated a strong regulation of biochemical parameters, and this effect was even more pronounced when coupled with an antidiabetic drug. | [196] | ||
| 25 mg/kg body weight | The flavonoid extracted from olive leaves had a positive effect on the blood glucose level, showing a significant reduction of 49.59% compared to the normal control group. | [16] |
3.5.5. Anticancer Activity
Cancer, a multifaceted chronic degenerative ailment, involves a multistep transformation where normal cells become malignant, displaying aberrant proliferation and diminished apoptosis [197]. Advances in cancer molecular understanding have uncovered key targets for anticancer drug development and treatment strategies [198]. Biophenols have emerged as agents capable of modulating cell growth at different stages of cancer development by either triggering apoptosis or restraining proliferation through a variety of mechanisms [199,200]. Constituents of O. europaea exhibit potent anticancer properties against various types of cancer [201]. The summarized results of various studies are reported in Table 8. Additionally, Xie et al. [50] reported that the seed extract from the Frantoio olive cultivar demonstrated the least cytotoxicity on colorectal cells, as evidenced by its high IC50 value. Triterpenoids, notably ursolic acid found in the seed extract, have been shown to inhibit proliferation in colorectal cancer cells (HCT-116). This study evaluated the cytotoxic effects of the seed extract on HCT-116 colorectal cells, providing valuable insights into its potential for cancer therapy [50]. Carpi et al. [202] aimed to investigate the in vitro antimelanoma activity of OA and its mechanism of action. Their findings revealed that oleacein (OA) inhibited cell growth in melanoma cells with an IC50 in the low micromolar range, showing time- and concentration-dependent effects. In melanoma cells, it caused DNA fragmentation, G1/S phase arrest, and downregulation of genes producing proliferative and antiapoptotic proteins. The overt antigrowth activity of OA in melanoma cells (501 Mel) occurs at low micromolar concentrations, suggesting specific targeting mechanisms that are hyper-reactive in cancer [202]. Additionally, Maalej et al. [203] sought to examine the phytochemical composition of ethanolic extracts from olive fruits of three distinct cultivars (OFE) and explored their antioxidant properties and potential anticancer effects. Their study revealed that after 48 h of cultivation, flow cytometry analysis demonstrated that OFE caused cell cycle arrest in the S-phase in both Caco-2 cells and HepG2. The IC50 of OFE was higher in HepG2 cells compared to Caco-2 cells, indicating that the olive fruit extract was more sensitive against the Caco-2 cell line than the HepG2 cell line [83]. Quero et al. [204] investigated the hydroethanolic extract of olive pomace, revealing its diverse polyphenolic composition. Their study showed that the extract significantly altered the mitochondrial membrane during a 72 h cell culture, which resulted in cell cycle arrest in the G1/S stages and death. In the Caco-2 cell line, this mechanism involves the activation of the proteins caspase 3 and p53 [204]. Albogami and Hassan [205] investigated the anticancer properties of an olive leaf extract in vitro using colorectal (HT29) and prostate (PC3) cancer cell lines. In both cell lines, the aqueous olive leaf (AOL) extracts stopped cells in the S phase. Moreover, they decreased cell migration and displayed multiple apoptotic characteristics, such as nuclear fragmentation, microtubule protrusions, apoptotic bodies, and blebbing. Additionally, the AOL extract induced DNA fragmentation in both cell lines [205]. Uğuz et al. [206] showed that when tested against the HL-60 leukemic cell line, olive leaf extract (OLE) had strong cytotoxicity. Remarkably, the removal of chlorophyll pigments significantly diminished the cytotoxic effect in both cases. This suggests that chlorophylls play a crucial role in the tumor-killing ability of such plant-derived extracts [206]. Notably, Shah et al. [207] investigated the Olea ferruginaea bark ethyl acetate extract, resulting in the identification of two previously known chemicals, ferruginan (2) and cycloolivil (3), as well as a new compound, ferruginan A (1). These substances were examined in tests using the MCF-7 breast cancer cell line. Compounds 1–3 showed modest cytotoxicity (IC50 = 8.03–12.01 μg/mL) in comparison to the standard (IC50 = 4.41 μg/mL) against MCF-7 cells, whereas the ethyl acetate fraction had high cytotoxic activity (79.31% inhibition at 250 μg/mL). This demonstrates that the separated chemicals have a modest anticancer effect [207]. Samet et al. [208] investigated the effects of olive leaf extract (OLE) on human chronic myelogenous leukemia K562 cells, focusing on apoptosis induction and monocyte/macrophage differentiation. After 72 h of treatment with 150 μg/mL of OLE, cell proliferation was inhibited to 17% compared to the control cells. However, the mechanisms underlying its differentiation-inducing properties remain poorly understood [208]. Moreover, using four cancer cell lines (breast, colon, hepatocellular, and cervical carcinoma cells), Morsy and Abdel-Aziz [209] examined the anticancer properties of the methanolic extract of olive leaves. The results were compared with those of the standard drug vinblastine. The findings demonstrated the cytotoxic activity of olive leaf extract against various cancer cell lines. The IC50 values of the methanolic extract from olive leaves against HCT, MCF-7, HELA, and HEPG-2 cancer cell lines were determined as 81.6, 43, 21.5, and 77.9 mg GAE/L, respectively. These findings highlight the strong impact of the olive leaf extract on the HEPG-2 cancer cell line, meeting the cytotoxicity criterion for crude extracts established by the US National Cancer Institute, which is an IC50 < 30 mg/L [209].

Table 8.
Cytotoxicity activity of various parts of O. europaea: summary of research findings.
3.5.6. Toxicology Investigation
Wistar rats were used in the examination of the acute and subacute oral toxicities of the ethanolic extract of olive leaves (EEO) by histopathological analysis and the assessment of biochemical and hematological markers. Acute toxicity was evaluated through a single oral gavage of 2000 mg/kg of EEO in male and female rats. Subacute toxicity was assessed by administering different doses (100, 200, and 400 mg/kg) of EEO to male and female rats for 28 days via oral gavage. Animals were divided into four groups of ten (five males and five females), and their body weights were recorded during the treatment period. Liver and kidney samples were histopathologically analyzed post-euthanasia, involving routine processing, paraffin embedding, sectioning, and staining with hematoxylin and eosin. Hematological and biochemical parameters were measured, and histological analysis was performed by a trained histologist [22]. During the acute oral administration test, administering a dose of 2000 mg/kg of EEO did not lead to any mortality or signs of toxicity throughout the treatment period. Notably, there were no notable variations in body weight between male and female subjects, and there were no observable alterations in behavior among the exposed animals. Although certain hematological parameters, such as HGB, CHCM, HCT, RBC, MCV, and PLT, differed significantly from the control group (both males and females), they remained within the normal range. Long-term exposure to EEO at doses of 100, 200, and 400 mg/kg was tested for subacute toxicity, and the results showed no differences between the treatment and control groups in the measured hematological parameters (HGB, CHCM, HCT, RBC, MCV, PLT, and WBC). Nevertheless, the blood concentration of BUN notably rose in male subjects exposed to EEO at doses of 100 and 400 mg/kg compared to the control group. Importantly, histological analysis of liver and kidney tissues revealed no abnormalities following treatment with EEO in rats for both experiments [22]. Another study aimed to investigate the toxicological safety assessment of an extract of O. europaea leaves (Bonolive™). The results of the study showed no evidence of mutagenicity in an in vitro mammalian chromosomal aberration test and a bacterial reverse mutation test. Moreover, in an in vivo mouse micronucleus test, no genotoxic effects were found, even at doses that reached the maximal dosage of 2000 mg/kg/bw/d. In a 90 d repeated oral toxicity assessment, Bonolive™ did not induce mortality or adverse effects in Crl:(WI) BR Wistar rats at doses of 360, 600, and 1000 mg/kg/bw/d. The highest dose tested, 1000 mg/kg bw/d, was determined as the no-observed adverse-effect level for both male and female rats in the 90 d study [216]. Additional randomized controlled human clinical studies along with comprehensive toxicity evaluations are required to identify any health effects and ensure safety.
4. Conclusions and Prospects
In conclusion, this review outlines the usage of O. europaea in ethnobotany, phytochemistry, pharmacology, and toxicological studies. Found extensively across various countries, this plant is utilized by diverse populations for treating specific ailments. Ethnobotanical surveys demonstrate a wide range of uses influenced by the plant part utilized, targeted disease, and geographical region. Phytochemical analysis revealed that O. europaea contains secoiridoids and various chemical compounds from classes like terpenoids, flavonoids, and phenolic acids. Pharmacological assays have scientifically validated the traditional uses of O. europaea, correlating ethnopharmacological applications with its biological activities and secondary metabolite content. Additionally, both in vitro and in vivo studies on O. europaea essential oils, vegetable oils, and extracts have revealed various effects, including antibacterial, antifungal, antioxidant, antidiabetic, and anticancer activities. Toxicological evidence in animal models did not reveal significant toxicity. The published data generally show consistent and satisfactory results, but knowledge remains limited, particularly in clinical trials. Through more preclinical and clinical research into illnesses associated with reactive oxygen species (ROS), more study is needed to clarify the biochemical and biological activities, as well as the pharmacokinetics and pharmacodynamics, of secoiridoids from olive trees.
Author Contributions
Conceptualization, A.B. and I.C.; methodology, A.B. and H.E.; software, H.E.; formal analysis, H.E.; investigation, H.E., O.A. and C.E.K.; resources, H.E.; data curation, H.E. and A.B.; writing—original draft preparation, H.E., O.A. and C.E.K.; writing—review and editing, I.C., M.G. and D.N.; visualization, I.C. and M.G.; supervision, A.B.; project administration, A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data used are cited within the manuscript.
Acknowledgments
The authors thank the Partnership for Research and Innovation in the Mediterranean Area (PRIMA) for supporting the VALONSTONE project.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bracci, T.; Busconi, M.; Fogher, C.; Sebastiani, L. Molecular studies in olive (Olea europaea L.): Overview on DNA markers applications and recent advances in genome analysis. Plant Cell Rep. 2011, 30, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, R.; Anwar, F.; Alkharfy, K.M.; Gilani, A.-H.; Saari, N. Valuable Nutrients and Functional Bioactives in Different Parts of Olive (Olea europaea L.)—A Review. Int. J. Mol. Sci. 2012, 13, 3291–3340. [Google Scholar] [CrossRef] [PubMed]
- Spennemann, D.H.R.; Allen, L.R. Feral olives (Olea europaea) as future woody weeds in Australia: A review. Aust. J. Exp. Agric. 2000, 40, 889–901. [Google Scholar] [CrossRef]
- Waisel, Y.; Geller-Bernstein, C.; Keynan, N.; Arad, G. Antigenicity of the pollen proteins of various cultivars of Olea europaea. Allergy 1996, 51, 819–821. [Google Scholar] [CrossRef] [PubMed]
- Zighed, A.; Derradji, L.; Hadef, Y.; Zighed, A.; Derradji, L.; Hadef, Y. Determination of polyphenolic components by High Performance Liquid Chromatography (HPLC) and evaluation of the antioxidant activity of leaves of Olea europaea L. var. sylvestris (Miller) Lehr. GSC Biol. Pharm. Sci. 2022, 20, 095–099. [Google Scholar] [CrossRef]
- Boukhebti, H.; Chaker, A.; Lograda, T.; Messaoud, R. Pharmacology & Toxicology. Int. J. Pharmacol. Toxicol. 2015, 5, 42–46. [Google Scholar]
- Farag, R.S.; Abdel-Latif, M.S.; Abd El Baky, H.H.; Tawfeek, L.S. Phytochemical screening and antioxidant activity of some medicinal plants’ crude juices. Biotechnol. Rep. 2020, 28, e00536. [Google Scholar] [CrossRef] [PubMed]
- Hannachi, H.; Elfalleh, W.; Laajel, M.; Ennajeh, I.; Mechlouch, R.F.; Nagaz, K. Chemical Profiles and Antioxidant Activities of Leaf, Pulp, and Stone of Cultivated and Wild Olive Trees (Olea europaea L.). Int. J. Fruit Sci. 2020, 20, 350–370. [Google Scholar] [CrossRef]
- Talhaoui, N.; Gómez-Caravaca, A.M.; León, L.; De la Rosa, R.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Determination of phenolic compounds of ‘Sikitita’ olive leaves by HPLC-DAD-TOF-MS. Comparison with its parents ‘Arbequina’ and ‘Picual’ olive leaves. LWT—Food Sci. Technol. 2014, 58, 28–34. [Google Scholar] [CrossRef]
- Ben-Amor, I.; Musarra-Pizzo, M.; Smeriglio, A.; D’Arrigo, M.; Pennisi, R.; Attia, H.; Gargouri, B.; Trombetta, D.; Mandalari, G.; Sciortino, M.T. Phytochemical Characterization of Olea europaea Leaf Extracts and Assessment of Their Anti-Microbial and Anti-HSV-1 Activity. Viruses 2021, 13, 1085. [Google Scholar] [CrossRef]
- Qabaha, K.; AL-Rimawi, F.; Qasem, A.; Naser, S.A. Oleuropein Is Responsible for the Major Anti-Inflammatory Effects of Olive Leaf Extract. J. Med. Food 2018, 21, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Ramata-Stunda, A.; Petriņa, Z.; Valkovska, V.; Borodušķis, M.; Gibnere, L.; Gurkovska, E.; Nikolajeva, V. Synergistic Effect of Polyphenol-Rich Complex of Plant and Green Propolis Extracts with Antibiotics against Respiratory Infections Causing Bacteria. Antibiotics 2022, 11, 160. [Google Scholar] [CrossRef] [PubMed]
- Shialy, Z.; Zarrin, M.; Sadeghi Nejad, B.; Yusef Naanaie, S. In vitro antifungal properties of Pistacia atlantica and olive extracts on different fungal species. Curr. Med. Mycol. 2015, 1, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Slobodníková, L.; Fialová, S.; Rendeková, K.; Kováč, J.; Mučaji, P. Antibiofilm Activity of Plant Polyphenols. Molecules 2016, 21, 1717. [Google Scholar] [CrossRef] [PubMed]
- Sheweita, S.A.; Mashaly, S.; Newairy, A.A.; Abdou, H.M.; Eweda, S.M. Changes in Oxidative Stress and Antioxidant Enzyme Activities in Streptozotocin-Induced Diabetes Mellitus in Rats: Role of Alhagi maurorum Extracts. Oxid. Med. Cell. Longev. 2016, 2016, e5264064. [Google Scholar] [CrossRef] [PubMed]
- Mechchate, H.; Es-Safi, I.; Bourhia, M.; Kyrylchuk, A.; El Moussaoui, A.; Conte, R.; Ullah, R.; Ezzeldin, E.; Mostafa, G.A.; Grafov, A.; et al. In-Vivo Antidiabetic Activity and In-Silico Mode of Action of LC/MS-MS Identified Flavonoids in Oleaster Leaves. Molecules 2020, 25, 5073. [Google Scholar] [CrossRef] [PubMed]
- Mansour, H.M.M.; Zeitoun, A.A.; Abd-Rabou, H.S.; El Enshasy, H.A.; Dailin, D.J.; Zeitoun, M.A.A.; El-Sohaimy, S.A. Antioxidant and Anti-Diabetic Properties of Olive (Olea europaea) Leaf Extracts: In Vitro and In Vivo Evaluation. Antioxidants 2023, 12, 1275. [Google Scholar] [CrossRef] [PubMed]
- Qidwai, A.; Pandey, M.; Kumar, R.; Dikshit, A. Comprehensive evaluation of pharmacological properties of Olea europaea L. for Cosmeceuticals prospects. Clin. Phytoscience 2017, 3, 12. [Google Scholar] [CrossRef]
- Tunç, Y.; Yaman, M.; Keçe, Y.M.; Yilmaz, K.U.; Yildiz, E.; Güneş, A. Characterization of Olive (Olea Europaea L.) Cultivars; Colour Properties, Biochemical Contents, Antioxidant Activity and Nutrient Contents. Genet. Resour. Crop Evol. 2024. [Google Scholar] [CrossRef]
- Wang, B.; Qu, J.; Luo, S.; Feng, S.; Li, T.; Yuan, M.; Huang, Y.; Liao, J.; Yang, R.; Ding, C. Optimization of Ultrasound-Assisted Extraction of Flavonoids from Olive (Olea europaea) Leaves, and Evaluation of Their Antioxidant and Anticancer Activities. Molecules 2018, 23, 2513. [Google Scholar] [CrossRef]
- Widyaningrum, N.; Hussaana, A.; Puspitasari, N. Invitro Antioxidant and Cytotoxic Potential of Ficus carica L. and Olea europeae L. against Cervical Cancer. Sains Med. 2020, 11, 14. [Google Scholar] [CrossRef]
- Guex, C.G.; Reginato, F.Z.; Figueredo, K.C.; da Silva, A.R.H.; Pires, F.B.; Jesus, R.d.S.; Lhamas, C.L.; Lopes, G.H.H.; Bauermann, L.d.F. Safety assessment of ethanolic extract of Olea europaea L. leaves after acute and subacute administration to Wistar rats. Regul. Toxicol. Pharmacol. 2018, 95, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Besnard, G.; Henry, P.; Wille, L.; Cooke, D.; Chapuis, E. On the origin of the invasive olives (Olea europaea L., Oleaceae). Heredity 2007, 99, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, M.A.; Khan, A.; Hanif, M.; Farooq, U.; Perveen, S. Traditional Uses, Phytochemistry, and Pharmacology of Olea europaea (Olive). Evid. Based Complement. Altern. Med. 2015, 2015, e541591. [Google Scholar] [CrossRef] [PubMed]
- Tsantili, E.; Evangelou, E.; Kiritsakis, A. Botanical characteristics of olive trees: Cultivation and growth conditions—Defense mechanisms to various stressors and effects on olive growth and functional compounds. In Olives and Olive Oil as Functional Foods, 1st ed.; Shahidi, F., Kiritsakis, A., Eds.; Wiley: Hoboken, NJ, USA, 2017; pp. 13–33. [Google Scholar] [CrossRef]
- Muzzalupo, I. Olive Germplasm: Italian Catalogue of Olive Varieties; BoD—Books on Demand: Norderstedt, Germany, 2012. [Google Scholar]
- Therios, I.N. Olives; CABI: Wallingford, UK, 2009. [Google Scholar]
- The Olive Tree. International Olive Council. Available online: https://www.internationaloliveoil.org/olive-world/olive-tree/ (accessed on 7 June 2024).
- Gixhari, B.; Hodaj, B.; Gjeloshi, A.; Ismaili, H. Olive in the story and art in Albania. In Proceedings of the International Conference “Adriatic Olive Grove: Risk Prevention, Sustainability, Learning”, Corfu, Greece, 19–20 June 2014. [Google Scholar]
- Haddad, B.; Gristina, A.S.; Mercati, F.; Saadi, A.E.; Aiter, N.; Martorana, A.; Sharaf, A.; Carimi, F. Molecular Analysis of the Official Algerian Olive Collection Highlighted a Hotspot of Biodiversity in the Central Mediterranean Basin. Genes 2020, 11, 303. [Google Scholar] [CrossRef]
- Trentacoste, E.R.; Banco, A.P.; Piccoli, P.N.; Monasterio, R.P. Olive oil characterization of cv. ‘Arauco’ harvested at different times in areas with early frost in Mendoza, Argentina. J. Sci. Food Agric. 2020, 100, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Contreras, R.C.; Aguayo, F.; Guerra, A.; Tapia, F.; Porcile, V. Genetic characterization of centennial olive trees from northern Chile: The case of extra virgin olive oil from huasco in the process of designation of origin. Chil. J. Agric. Anim. Sci. 2018, 34, 126–139. [Google Scholar] [CrossRef]
- Emmanouilidou, M.G.; Kyriacou, M.C.; Trujillo, I. Characterization and Identification of Indigenous Olive Germplasm from Cyprus Using Morphological and Simple Sequence Repeat Markers. HortScience 2018, 53, 1306–1313. [Google Scholar] [CrossRef]
- Klepo, T.; Benčić, Đ.; Liber, Z.; Belaj, A.; Strikić, F.; Kević, N.; Šatović, Z. Revealing the Diversity and Complex Relationships of Croatian Olive Germplasm. Int. J. Mol. Sci. 2024, 25, 3170. [Google Scholar] [CrossRef]
- Mohamed, A.A.H.; Nagaty, M.A.; El-Baghdady, M.M.S.; Radwan, K.H. Morphological and molecular characterization of some olive (Olea europaea) cultivars in El-Arish, Egypt. J. Biosci. Appl. Res. 2017, 3, 237–251. [Google Scholar] [CrossRef]
- Khadari, B.; El Bakkali, A.; Essalouh, L.; Tollon, C.; Pinatel, C.; Besnard, G. Cultivated Olive Diversification at Local and Regional Scales: Evidence from the Genetic Characterization of French Genetic Resources. Front. Plant Sci. 2019, 10, 1593. [Google Scholar] [CrossRef]
- Roubos, K.; Moustakas, M.; Aravanopoulos, F.A. Molecular identification of Greek olive (Olea europaea) cultivars based on microsatellite loci. Genet. Mol. Res. 2010, 9, 1865–1876. [Google Scholar] [CrossRef]
- Goldental-Cohen, S.; Biton, I.; Many, Y.; Ben-Sason, S.; Zemach, H.; Avidan, B.; Ben-Ari, G. Green Olive Browning Differ Between Cultivars. Front. Plant Sci. 2019, 10, 1260. [Google Scholar] [CrossRef]
- Cicatelli, A.; Fortunati, T.; De Feis, I.; Castiglione, S. Oil composition and genetic biodiversity of ancient and new olive (Olea europea L.) varieties and accessions of southern Italy. Plant Sci. 2013, 210, 82–92. [Google Scholar] [CrossRef]
- Al Ganideh, S.F.; Good, L.K. Nothing Tastes as Local: Jordanians’ Perceptions of Buying Domestic Olive Oil. J. Food Prod. Mark. 2016, 22, 168–190. [Google Scholar] [CrossRef]
- El Riachy, M.; Moubarak, P.; Al Hawi, G.; Geha, M.; Mushantaf, W.; Estephan, N.; Skaff, W. Fatty Acid and Phenolic Profiles of Virgin Olive Oils from Local and European Varieties Planted in Lebanon. Plants 2023, 12, 2681. [Google Scholar] [CrossRef]
- Bajoub, A.; Medina-Rodríguez, S.; Olmo-García, L.; Ajal, E.A.; Monasterio, R.P.; Hanine, H.; Fernández-Gutiérrez, A.; Carrasco-Pancorbo, A. In-Depth Two-Year Study of Phenolic Profile Variability among Olive Oils from Autochthonous and Mediterranean Varieties in Morocco, as Revealed by a LC-MS Chemometric Profiling Approach. Int. J. Mol. Sci. 2017, 18, 52. [Google Scholar] [CrossRef]
- Abuamsha, R.; Abueid, M.; Hajjeh, H.; Salman, M. Evaluation of the Incidence and Severity of Olive Leaf Spot caused by Spilocaea oleagina in different olive cultivars in Palestine. J. Agric. Environ. Int. Dev. JAEID 2013, 107, 201–212. [Google Scholar] [CrossRef]
- Seabra, R.M.; Andrade, P.B.; Valentão, P.; Faria, M.; Paice, A.G.; Oliveira, M.B.P.P. Chapter 20—Phenolic Profiles of Portuguese Olives: Cultivar and Geographics. In Olives and Olive Oil in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Eds.; Academic Press: San Diego, CA, USA, 2010; pp. 177–186. [Google Scholar] [CrossRef]
- Arenas-Castro, S.; Gonçalves, J.F.; Moreno, M.; Villar, R. Projected climate changes are expected to decrease the suitability and production of olive varieties in southern Spain. Sci. Total Environ. 2020, 709, 136161. [Google Scholar] [CrossRef]
- Fabbri, A.; Baldoni, L.; Caruso, T.; Famiani, F. The Olive: Botany and Production; CABI: Wallingford, UK, 2023. [Google Scholar]
- Saddoud Debbabi, O.; Rahmani Mnasri, S.; Ben Amar, F.; Ben Naceur, M.; Montemurro, C.; Miazzi, M.M. Applications of Microsatellite Markers for the Characterization of Olive Genetic Resources of Tunisia. Genes 2021, 12, 286. [Google Scholar] [CrossRef]
- Dıraman, H. Characterization by chemometry of the most important domestic and foreign olive cultivars from the National Olive Collection Orchard of Turkey. Grasas Aceites 2010, 61, 341–351. [Google Scholar] [CrossRef][Green Version]
- Jerman, T.; Trebše, P.; Mozetič Vodopivec, B. Ultrasound-assisted solid liquid extraction (USLE) of olive fruit (Olea europaea) phenolic compounds. Food Chem. 2010, 123, 175–182. [Google Scholar] [CrossRef]
- Xie, P.; Cecchi, L.; Bellumori, M.; Balli, D.; Giovannelli, L.; Huang, L.; Mulinacci, N. Phenolic Compounds and Triterpenes in Different Olive Tissues and Olive Oil By-Products, and Cytotoxicity on Human Colorectal Cancer Cells: The Case of Frantoio, Moraiolo and Leccino Cultivars (Olea europaea L.). Foods 2021, 10, 2823. [Google Scholar] [CrossRef]
- Ammar, S.; Contreras, M.d.M.; Gargouri, B.; Segura-Carretero, A.; Bouaziz, M. RP-HPLC-DAD-ESI-QTOF-MS based metabolic profiling of the potential Olea europaea by-product “wood” and its comparison with leaf counterpart. Phytochem. Anal. 2017, 28, 217–229. [Google Scholar] [CrossRef]
- Khlif, I.; Jellali, K.; Michel, T.; Halabalaki, M.; Skaltsounis, A.L.; Allouche, N. Characteristics, Phytochemical Analysis and Biological Activities of Extracts from Tunisian Chetoui Olea europaea Variety. J. Chem. 2015, 2015, e418731. [Google Scholar] [CrossRef]
- Alves, E.; Rey, F.; Melo, T.; Barros, M.P.; Domingues, P.; Domingues, R. Bioprospecting Bioactive Polar Lipids from Olive (Olea europaea cv. Galega vulgar) Fruit Seeds: LC-HR-MS/MS Fingerprinting and Sub-Geographic Comparison. Foods 2022, 11, 951. [Google Scholar] [CrossRef]
- Bouarroudj, K.; Tamendjari, A.; Larbat, R. Quality, composition and antioxidant activity of Algerian wild olive (Olea europaea L. subsp. Oleaster) oil. Ind. Crops Prod. 2016, 83, 484–491. [Google Scholar] [CrossRef]
- Angelis, A.; Antoniadi, L.; Stathopoulos, P.; Halabalaki, M.; Skaltsounis, L.A. Oleocanthalic and Oleaceinic acids: New compounds from Extra Virgin Olive Oil (EVOO). Phytochem. Lett. 2018, 26, 190–194. [Google Scholar] [CrossRef]
- Pérez, A.G.; León, L.; Pascual, M.; de la Rosa, R.; Belaj, A.; Sanz, C. Analysis of Olive (Olea europaea L.) Genetic Resources in Relation to the Content of Vitamin E in Virgin Olive Oil. Antioxidants 2019, 8, 242. [Google Scholar] [CrossRef]
- Cecchi, L.; Piazzini, V.; D’Ambrosio, M.; Luceri, C.; Rocco, F.; Innocenti, M.; Vanti, G.; Mulinacci, N.; Bergonzi, M.C. Formulation of a Phenol-Rich Extract from Unripe Olives (Olea europaea L.) in Microemulsion to Improve Its Solubility and Intestinal Permeability. Molecules 2020, 25, 3198. [Google Scholar] [CrossRef]
- Fernández-Poyatos, M.d.P.; Ruiz-Medina, A.; Llorent-Martínez, E.J. Phytochemical profile and mineral content of Royal variety olive fruits. Influence of the ripening stage. J. Food Compos. Anal. 2021, 95, 103671. [Google Scholar] [CrossRef]
- Xie, P.; Huang, L.; Zhang, C.; Zhang, Y. Phenolic compositions, and antioxidant performance of olive leaf and fruit (Olea europaea L.) extracts and their structure–activity relationships. J. Funct. Foods 2015, 16, 460–471. [Google Scholar] [CrossRef]
- Jurišić Grubešić, R.; Nazlić, M.; Miletić, T.; Vuko, E.; Vuletić, N.; Ljubenkov, I.; Dunkić, V. Antioxidant Capacity of Free Volatile Compounds from Olea europaea L. cv. Oblica Leaves Depending on the Vegetation Stage. Antioxidants 2021, 10, 1832. [Google Scholar] [CrossRef]
- Tlili, A.; Bouziane, M.; Flamini, G.; Hadj Mahammed, M. Volatiles Variation of Two Major Cultivars of Olea europaea L. Cultivated in Mediterranean and Arid Regions of Algeria. Rec. Nat. Prod. 2021, 16, 34–45. [Google Scholar] [CrossRef]
- Brahmi, F.; Mechri, B.; Dhibi, M.; Hammami, M. Variations in phenolic compounds and antiradical scavenging activity of Olea europaea leaves and fruits extracts collected in two different seasons. Ind. Crops Prod. 2013, 49, 256–264. [Google Scholar] [CrossRef]
- Benincasa, C.; Romano, E.; Pellegrino, M.; Perri, E. Characterization of Phenolic Profiles of Italian Single Cultivar Olive Leaves (Olea europaea L.) by Mass Spectrometry. Mass Spectrom. Purif. Tech. 2018, 4, 124. [Google Scholar] [CrossRef]
- Aljeddani, G.S. Evaluation of Phytochemical, Antimicrobial, and Antioxidant Properties of Wild versus Cultivated Olive Leaves. Nat. Sci. 2022, 14, 448–461. [Google Scholar] [CrossRef]
- Charisiadis, P.; Kontogianni, V.G.; Tsiafoulis, C.G.; Tzakos, A.G.; Gerothanassis, I.P. Determination of Polyphenolic Phytochemicals using Highly Deshielded –OH 1H-NMR Signals. Phytochem. Anal. 2016, 28, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Cukrov, M.; Žurga, P.; Germek, V.M.; Brkljača, M.; Ban, D.; Lukić, I.; Ban, S.G.; Pasković, I. Effect of Olive (Olea europaea L.) Variety on Leaf Biophenolic Profile. Agric. Conspec. Sci. 2021, 86, 277–282. [Google Scholar]
- Dini, I.; Graziani, G.; Fedele, F.L.; Sicari, A.; Vinale, F.; Castaldo, L.; Ritieni, A. Effects of Trichoderma Biostimulation on the Phenolic Profile of Extra-Virgin Olive Oil and Olive Oil By-Products. Antioxidants 2020, 9, 284. [Google Scholar] [CrossRef]
- Edziri, H.; Jaziri, R.; Chehab, H.; Verschaeve, L.; Flamini, G.; Boujnah, D.; Hammami, M.; Aouni, M.; Mastouri, M. A comparative study on chemical composition, antibiofilm and biological activities of leaves extracts of four Tunisian olive cultivars. Heliyon 2019, 5, e01604. [Google Scholar] [CrossRef] [PubMed]
- Essafi, H.; Trabelsi, N.; Benincasa, C.; Tamaalli, A.; Perri, E.; Zarrouk, M. Phytochemical profile, antioxidant and antiproliferative activities of olive leaf extracts from autochthonous Tunisian cultivars. Acta Aliment. 2019, 48, 384–390. [Google Scholar] [CrossRef]
- Orak, H.H.; Karamać, M.; Amarowicz, R.; Orak, A.; Penkacik, K. Genotype-Related Differences in the Phenolic Compound Profile and Antioxidant Activity of Extracts from Olive (Olea europaea L.) Leaves. Molecules 2019, 24, 1130. [Google Scholar] [CrossRef] [PubMed]
- Majumder, D.; Debnath, M.; Libin Kumar, K.V.; Nath, P.; Debnath, R.; Sarkar, C.; Prasad, G.B.K.S.; Verma, Y.K.; Maiti, D. Metabolic profiling and investigations on crude extract of Olea europaea L. leaves as a potential therapeutic agent against skin cancer. J. Funct. Foods 2019, 58, 266–274. [Google Scholar] [CrossRef]
- Nicolì, F.; Negro, C.; Vergine, M.; Aprile, A.; Nutricati, E.; Sabella, E.; Miceli, A.; Luvisi, A.; De Bellis, L. Evaluation of Phytochemical and Antioxidant Properties of 15 Italian Olea europaea L. Cultivar Leaves. Molecules 2019, 24, 1998. [Google Scholar] [CrossRef] [PubMed]
- Omar, S.H.; Kerr, P.G.; Scott, C.J.; Hamlin, A.S.; Obied, H.K. Olive (Olea europaea L.) Biophenols: A Nutriceutical against Oxidative Stress in SH-SY5Y Cells. Molecules 2017, 22, 1858. [Google Scholar] [CrossRef]
- Gagour, J.; Hallouch, O.; Asbbane, A.; Bijla, L.; Laknifli, A.; Lee, L.-H.; Zengin, G.; Bouyahya, A.; Sakar, E.H.; Gharby, S. A Review of Recent Progresses on Olive Oil Chemical Profiling, Extraction Technology, Shelf-Life, and Quality Control. Chem. Biodivers. 2024, 21, e202301697. [Google Scholar] [CrossRef] [PubMed]
- Sarikurkcu, C.; Locatelli, M.; Tartaglia, A.; Ferrone, V.; Juszczak, A.M.; Ozer, M.S.; Tepe, B.; Tomczyk, M. Enzyme and Biological Activities of the Water Extracts from the Plants Aesculus hippocastanum, Olea europaea and Hypericum perforatum That Are Used as Folk Remedies in Turkey. Molecules 2020, 25, 1202. [Google Scholar] [CrossRef]
- Termentzi, A.; Halabalaki, M.; Skaltsounis, A.L. 6—From Drupes to Olive Oil: An Exploration of Olive Key Metabolites. In Olive and Olive Oil Bioactive Constituents; Boskou, D., Ed.; AOCS Press: Champaign, IL, USA, 2015; pp. 147–177. [Google Scholar] [CrossRef]
- Damtoft, S.; Franzyk, H.; Jensen, S.R. Excelsioside, a secoiridoid glucoside from Fraxinus excelsior. Phytochemistry 1992, 31, 4197–4201. [Google Scholar] [CrossRef]
- Cecchi, L.; Migliorini, M.; Cherubini, C.; Innocenti, M.; Mulinacci, N. Whole Lyophilized Olives as Sources of Unexpectedly High Amounts of Secoiridoids: The Case of Three Tuscan Cultivars. J. Agric. Food Chem. 2015, 63, 1175–1185. [Google Scholar] [CrossRef]
- Šarolić, M.; Gugić, M.; Tuberoso, C.I.G.; Jerković, I.; Šuste, M.; Marijanović, Z.; Kuś, P.M. Volatile Profile, Phytochemicals and Antioxidant Activity of Virgin Olive Oils from Croatian Autochthonous Varieties Mašnjača and Krvavica in Comparison with Italian Variety Leccino. Molecules 2014, 19, 881–895. [Google Scholar] [CrossRef]
- Waqar Ahmad, N.A.; Afridi, M.S.; Rahman, H.; Adnan, M.; Ullah, N.; Muhammad, U.; Ilyas, M.; Khan, H. Phytochemical profile, antimicrobial potential and GC-MS analysis of wild variety of Olea europaea (Olive) cultivated in Pakistan. Pure Appl. Biol. PAB 2017, 6, 337–345. [Google Scholar]
- Nazzaro, F.; Fratianni, F.; Cozzolino, R.; Martignetti, A.; Malorni, L.; De Feo, V.; Cruz, A.G.; d’Acierno, A. Antibacterial Activity of Three Extra Virgin Olive Oils of the Campania Region, Southern Italy, Related to Their Polyphenol Content and Composition. Microorganisms 2019, 7, 321. [Google Scholar] [CrossRef]
- Alagna, F.; Geu-Flores, F.; Kries, H.; Panara, F.; Baldoni, L.; O’Connor, S.E.; Osbourn, A. Identification and Characterization of the Iridoid Synthase Involved in Oleuropein Biosynthesis in Olive (Olea europaea) Fruits*. J. Biol. Chem. 2016, 291, 5542–5554. [Google Scholar] [CrossRef]
- Maalej, A.; Bouallagui, Z.; Hadrich, F.; Isoda, H.; Sayadi, S. Assessment of Olea europaea L. fruit extracts: Phytochemical characterization and anticancer pathway investigation. Biomed. Pharmacother. 2017, 90, 179–186. [Google Scholar] [CrossRef]
- Hannachi, H.; Elfalleh, W.; Marzouk, S. Oil, protein, antioxidants and free radical scavenging activity of stone from wild olive trees (Olea europaea L.). Pak. J. Pharm. Sci. 2013, 26, 503–510. [Google Scholar] [PubMed]
- Montealegre, C.; Sánchez-Hernández, L.; Crego, A.L.; Marina, M.L. Determination and Characterization of Glycerophospholipids in Olive Fruit and Oil by Nonaqueous Capillary Electrophoresis with Electrospray-Mass Spectrometric Detection. J. Agric. Food Chem. 2013, 61, 1823–1832. [Google Scholar] [CrossRef]
- He, M.; Ding, N.-Z. Plant Unsaturated Fatty Acids: Multiple Roles in Stress Response. Front. Plant Sci. 2020, 11, 562785. [Google Scholar] [CrossRef]
- Alves, E.; Melo, T.; Rey, F.; Moreira, A.S.P.; Domingues, P.; Domingues, M.R. Polar lipid profiling of olive oils as a useful tool in helping to decipher their unique fingerprint. LWT 2016, 74, 371–377. [Google Scholar] [CrossRef]
- Silva, S.; Gomes, L.; Leitão, F.; Bronze, M.; Coelho, A.V.; Boas, L.V. Secoiridoids in olive seed: Characterization of nüzhenide and 11-methyl oleosides by liquid chromatography with diode array and mass spectrometry. Grasas Aceites 2010, 61, 157–164. [Google Scholar] [CrossRef]
- Cano-Lamadrid, M.; Lipan, L.; Hernández, F.; Martínez, J.J.; Legua, P.; Carbonell-Barrachina, Á.A.; Melgarejo, P. Quality Parameters, Volatile Composition, and Sensory Profiles of Highly Endangered Spanish Citrus Fruits. J. Food Qual. 2018, 2018, e3475461. [Google Scholar] [CrossRef]
- Abu-Izneid, T.; Rauf, A.; Shariati, M.A.; Khalil, A.A.; Imran, M.; Rebezov, M.; Uddin, M.d.S.; Mahomoodally, M.F.; Rengasamy, K.R.R. Sesquiterpenes and their derivatives-natural anticancer compounds: An update. Pharmacol. Res. 2020, 161, 105165. [Google Scholar] [CrossRef]
- Belščak-Cvitanović, A.; Durgo, K.; Bušić, A.; Franekić, J.; Komes, D. Phytochemical Attributes of Four Conventionally Extracted Medicinal Plants and Cytotoxic Evaluation of Their Extracts on Human Laryngeal Carcinoma (HEp2) Cells. J. Med. Food 2014, 17, 206–217. [Google Scholar] [CrossRef]
- El-Darier, S.; Abdel-Rahman, A.; Saad, T. Differential Responses of Hordeum vulgare L. to Allelochemicals of Some Olea europaea L. Cultivars. Catrina Int. J. Environ. Sci. 2018, 17, 91–101. [Google Scholar] [CrossRef]
- Badawy, M.; Faraj, H. Chemical Composition and Activity of Bark and Leaf Extracts of Pinus Halepensis and Olea europaea Grown in Al-Jabel Al-Akhdar Region, Libya against some Plant Phytopathogens. J. Appl. Biotechnol. Bioeng. 2019, 3, 331–342. [Google Scholar] [CrossRef]
- Dobrinčić, A.; Repajić, M.; Garofulić, I.E.; Tuđen, L.; Dragović-Uzelac, V.; Levaj, B. Comparison of Different Extraction Methods for the Recovery of Olive Leaves Polyphenols. Processes 2020, 8, 1008. [Google Scholar] [CrossRef]
- Mechri, B.; Tekaya, M.; Hammami, M.; Chehab, H. Effects of drought stress on phenolic accumulation in greenhouse-grown olive trees (Olea europaea). Biochem. Syst. Ecol. 2020, 92, 104112. [Google Scholar] [CrossRef]
- Šikić Pogačar, M.; Klančnik, A.; Bucar, F.; Langerholc, T.; Smole Možina, S. Anti-adhesion activity of thyme (Thymus vulgaris L.) extract, thyme post-distillation waste, and olive (Olea europea L.) leaf extract against Campylobacter jejuni on polystyrene and intestine epithelial cells. J. Sci. Food Agric. 2016, 96, 2723–2730. [Google Scholar] [CrossRef]
- Rashed, S.A.; Saad, T.I.; El-Darier, S.M. Potential aptitude of four olive cultivars as anticancer and antioxidant agents: Oleuropein content. Rend. Lincei Sci. Fis. Nat. 2022, 33, 195–203. [Google Scholar] [CrossRef]
- Ghosia, L.; Khan, A.; Tila, H.; Hussain, A.; Khan, A. Evaluation of Plants Extracts for Proximate Chemical Composition, Antimicrobial and Antifungal Activities. Am.-Eurasian J. Agric. Environ. Sci. 2014, 14, 964–970. [Google Scholar]
- Celik, H.; Nadaroglu, H.; Senol, M. Evaluation of antioxidant, antiradicalic and antimicrobial activities of olive pits (Olea europaea L.). Bulg. J. Agric. Sci. 2014, 20, 1392–1400. [Google Scholar]
- Coccia, A.; Bastianelli, D.; Mosca, L.; Monticolo, R.; Panuccio, I.; Carbone, A.; Calogero, A.; Lendaro, E. Extra Virgin Olive Oil Phenols Suppress Migration and Invasion of T24 Human Bladder Cancer Cells through Modulation of Matrix Metalloproteinase-2. Nutr. Cancer 2014, 66, 946–954. [Google Scholar] [CrossRef]
- Hadrich, F.; Bouallagui, Z.; Junkyu, H.; Isoda, H.; Sayadi, S. The α-Glucosidase and α-Amylase Enzyme Inhibitory of Hydroxytyrosol and Oleuropein. J. Oleo Sci. 2015, 64, 835–843. [Google Scholar] [CrossRef]
- Guo, L.; Sun, Q.; Gong, S.; Bi, X.; Jiang, W.; Xue, W.; Fei, P. Antimicrobial Activity and Action Approach of the Olive Oil Polyphenol Extract against Listeria monocytogenes. Front. Microbiol. 2019, 10, 1586. [Google Scholar] [CrossRef]
- Lood, R.; Waldetoft, K.W.; Nordenfelt, P. Localization-triggered bacterial pathogenesis. Future Microbiol. 2015, 10, 1659–1668. [Google Scholar] [CrossRef]
- Kadir, B.; Zehra, K.F.; Abdullah, A.M.; Selami, G.; Yakup, Y. Antioxidant and antithrombotic properties of fruit, leaf, and seed extracts of the halhali olive (Olea europaea L.) native to the hatay region in turkey. Foods Raw Mater. 2023, 11, 84–93. [Google Scholar]
- Hanene, G.; Aouadhi, C.; Hamrouni, S.; Mnif, W. Antibacterial, antifungal and antioxidant activities of tunisian Olea europaea ssp. oleaster fruit pulp and its essential fatty acids. Int. J. Pharm. Pharm. Sci 2014, 7, 52–55. [Google Scholar]
- Chen, M.; Zhao, Z.; Meng, H.; Yu, S. The antibiotic activity and mechanisms of sugar beet (Beta vulgaris) molasses polyphenols against selected food-borne pathogens. LWT—Food Sci. Technol. 2017, 82, 354–360. [Google Scholar] [CrossRef]
- Dias, M.I.; Barros, L.; Barreira, J.C.M.; Alves, M.J.; Barracosa, P.; Ferreira, I.C.F.R. Phenolic profile and bioactivity of cardoon (Cynara cardunculus L.) inflorescence parts: Selecting the best genotype for food applications. Food Chem. 2018, 268, 196–202. [Google Scholar] [CrossRef]
- Wang, L.-H.; Zhang, Z.-H.; Zeng, X.-A.; Gong, D.-M.; Wang, M.-S. Combination of microbiological, spectroscopic and molecular docking techniques to study the antibacterial mechanism of thymol against Staphylococcus aureus: Membrane damage and genomic DNA binding. Anal. Bioanal. Chem. 2017, 409, 1615–1625. [Google Scholar] [CrossRef]
- Abdullah, B.A.; Saeed, H.S.M.; Al-Taii, N.A. Antimicrobial activity and evaluation of genetic effects of olive leaves using molecular techniques. J. Entomol. Zool. Stud. 2017, 6, 1493–1495. [Google Scholar]
- Alsulaymani, F.A.; Elmhdwi, M.; Gaber, S. In Vitro Antioxidant and Antibacterial Activity of Olive Leave Extract. J. Pharm. Appl. Chem. 2021, 7, 41–47. [Google Scholar] [CrossRef]
- De la Ossa, J.G.; El Kadri, H.; Gutierrez-Merino, J.; Wantock, T.; Harle, T.; Seggiani, M.; Danti, S.; Di Stefano, R.; Velliou, E. Combined Antimicrobial Effect of Bio-Waste Olive Leaf Extract and Remote Cold Atmospheric Plasma Effluent. Molecules 2021, 26, 1890. [Google Scholar] [CrossRef]
- Himour, S.; Yahia, A.; Belattar, H. Oleuropein and Antibacterial Activities of Olea europaea L. Leaf Extract. Eur. Sci. J. ESJ 2017, 13, 342. [Google Scholar] [CrossRef]
- Da Rosa, G.S.; Martiny, T.R.; Dotto, G.L.; Vanga, S.K.; Parrine, D.; Gariepy, Y.; Lefsrud, M.; Raghavan, V. Eco-friendly extraction for the recovery of bioactive compounds from Brazilian olive leaves. Sustain. Mater. Technol. 2021, 28, e00276. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Rabii, N.S.; Garbaj, A.M.; Abolghait, S.K. Antibacterial Effect of Olive (Olea europaea L.) Leaves Extract in Raw Peeled Undeveined Shrimp (Penaeus semisulcatus). Int. J. Vet. Sci. Med. 2014, 2, 53–56. [Google Scholar] [CrossRef]
- Vural, N.; Akay, M.A. Chemical compounds, antioxidant properties and antimicrobial activity of olive leaves derived volatile oil in West Anatolia. J. Turk. Chem. Soc. Sect. Chem. 2021, 8, 511–518. [Google Scholar] [CrossRef]
- El Yamani, M.; Sakar, E.H.; Boussakouran, A.; Benali, T.; Rharrabti, Y. Antibacterial and antioxidant potentials of phenolic extracts from olive mill wastewater and their use to enhance the stability of olive oil. Riv. Ital. Delle Sostanze Grasse 2020, 97, 31–42. [Google Scholar]
- Guo, L.; Gong, S.; Wang, Y.; Sun, Q.; Duo, K.; Fei, P. Antibacterial Activity of Olive Oil Polyphenol Extract against Salmonella Typhimurium and Staphylococcus aureus: Possible Mechanisms. Foodborne Pathog. Dis. 2020, 17, 396–403. [Google Scholar] [CrossRef]
- Ahmad, W.; Khan, H.; Safeer, M.; Khan, S.; Junaid, M.; Mehmood, A.; Siddiqueafridi, M.; Rafi, A.; Khan, A.; Ayaz, U.; et al. Comparative antimicrobial activities of wild and cultivated varieties of Olea europaea different leaves extracts in Pakistan. Bull. Env. Pharmacol. Life Sci. 2020, 9, 73–82. [Google Scholar]
- Elnahas, R.A.; Elwakil, B.H.; Elshewemi, S.S.; Olama, Z.A. Egyptian Olea europaea leaves bioactive extract: Antibacterial and wound healing activity in normal and diabetic rats. J. Tradit. Complement. Med. 2021, 11, 427–434. [Google Scholar] [CrossRef]
- Benali, T.; Habbadi, K.; Khabbach, A.; Marmouzi, I.; Zengin, G.; Bouyahya, A.; Chamkhi, I.; Chtibi, H.; Aanniz, T.; Achbani, E.H.; et al. GC–MS Analysis, Antioxidant and Antimicrobial Activities of Achillea odorata Subsp. Pectinata and Ruta montana Essential Oils and Their Potential Use as Food Preservatives. Foods 2020, 9, 668. [Google Scholar] [CrossRef]
- Testa, B.; Lombardi, S.J.; Macciola, E.; Succi, M.; Tremonte, P.; Iorizzo, M. Efficacy of olive leaf extract (Olea europaea L. cv Gentile di Larino) in marinated anchovies (Engraulis encrasicolus, L.) process. Heliyon 2019, 5, e01727. [Google Scholar] [CrossRef]
- Dada, E.O. Antibacterial Activities of Olea europaea Leaf Extract on Some Bacteria Isolated from a Refused Dump Site in Akure, Nigeria. J. Biol. 2013, 1, 118–124. [Google Scholar]
- Ghomari, O.; Sounni, F.; Massaoudi, Y.; Ghanam, J.; Drissi Kaitouni, L.B.; Merzouki, M.; Benlemlih, M. Phenolic profile (HPLC-UV) of olive leaves according to extraction procedure and assessment of antibacterial activity. Biotechnol. Rep. 2019, 23, e00347. [Google Scholar] [CrossRef]
- Golestannejad, Z.; Khozeimeh, F.; Abtahi, R.; Zarei, Z.; Sadeghalbanaei, L.; Sadeghian, R. Inhibitory effects of ethanolic, methanolic, and hydroalcoholic extracts of olive (Olea europaea) leaf on growth, acid production, and adhesion of Streptococcus mutans. Dent. Res. J. 2020, 17, 179–185. [Google Scholar]
- Karygianni, L.; Cecere, M.; Skaltsounis, A.L.; Argyropoulou, A.; Hellwig, E.; Aligiannis, N.; Wittmer, A.; Al-Ahmad, A. High-Level Antimicrobial Efficacy of Representative Mediterranean Natural Plant Extracts against Oral Microorganisms. BioMed Res. Int. 2014, 2014, e839019. [Google Scholar] [CrossRef]
- Lass-Flörl, C.; Kanj, S.S.; Govender, N.P.; Thompson, G.R.; Ostrosky- Zeichner, L.; Govrins, M.A. Invasive Candidiasis. Nat. Rev. Dis. Primers 2024, 10, 1–18. [Google Scholar] [CrossRef]
- Noor, S. Synergistic Effect of the Methanolic Extract of Lemongrass and Some Antibiotics to Treat Urinary Tract Bacteria. J. Biosci. Med. 2016, 4, 48–58. [Google Scholar] [CrossRef][Green Version]
- Bodey, G.P.; Mardani, M.; Hanna, H.A.; Boktour, M.; Abbas, J.; Girgawy, E.; Hachem, R.Y.; Kontoyiannis, D.P.; Raad, I.I. The epidemiology of Candida glabrata and Candida albicans fungemia in immunocompromised patients with cancer. Am. J. Med. 2002, 112, 380–385. [Google Scholar] [CrossRef]
- Brandt, C.; Makarewicz, O.; Fischer, T.; Stein, C.; Pfeifer, Y.; Werner, G.; Pletz, M.W. The bigger picture: The history of antibiotics and antimicrobial resistance displayed by scientometric data. Int. J. Antimicrob. Agents 2014, 44, 424–430. [Google Scholar] [CrossRef]
- Llor, C.; Bjerrum, L. Antimicrobial resistance: Risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf. 2014, 5, 229–241. [Google Scholar] [CrossRef]
- Khalil, M.M.H.; Ismail, E.H.; El-Baghdady, K.Z.; Mohamed, D. Green synthesis of silver nanoparticles using olive leaf extract and its antibacterial activity. Arab. J. Chem. 2014, 7, 1131–1139. [Google Scholar] [CrossRef]
- Brahmi, F.; Flamini, G.; Issaoui, M.; Dhibi, M.; Dabbou, S.; Mastouri, M.; Hammami, M. Chemical composition and biological activities of volatile fractions from three Tunisian cultivars of olive leaves. Med. Chem. Res. 2012, 21, 2863–2872. [Google Scholar] [CrossRef]
- Upadhyay, R.K. Evaluation of antibacterial and antifungal activities of olive (Olea europaea) essential oil. Int. J. Green Pharm. IJGP 2014, 8, 180. [Google Scholar]
- Alimosazadeh, P.; Khan, M.S.A.; Sanei, S.J. Phytochemical and anticandidal efficacy of Olea europaea leaf extract from different cultivars and seasonal variation. J. Herbmed. Pharmacol. 2023, 12, 505–511. [Google Scholar] [CrossRef]
- Bawadekji, A.; Imran, M.; Randahawa, M.A. Antimicrobial Effects of the Water Immiscible Solvent Extracts of Olive Tree Leaves. J. Pure Appl. Microbiol. 2019, 13, 2189–2194. [Google Scholar] [CrossRef]
- Nasrollahi, Z.; Abolhasannezhad, M. Evaluation of the antifungal activity of olive leaf aqueous extracts against Candida albicans PTCC-5027. Curr. Med. Mycol. 2015, 1, 37–39. [Google Scholar] [CrossRef]
- Omar, H.S.; Abd El-Rahman, S.N.; AlGhannam, S.M.; Reyad, N.E.-H.A.; Sedeek, M.S. Antifungal Evaluation and Molecular Docking Studies of Olea europaea Leaf Extract, Thymus vulgaris and Boswellia carteri Essential Oil as Prospective Fungal Inhibitor Candidates. Molecules 2021, 26, 6118. [Google Scholar] [CrossRef]
- Wang, B.; Shen, S.; Qu, J.; Xu, Z.; Feng, S.; Chen, T.; Ding, C. Optimizing Total Phenolic and Oleuropein of Chinese Olive (Olea europaea) Leaves for Enhancement of the Phenols Content and Antioxidant Activity. Agronomy 2021, 11, 686. [Google Scholar] [CrossRef]
- Ilias, F.; Kholkhal, W.; Gaouar, N.; Bekhechi, C.; Bekkara, F. Antibacterial and antifungal Activities of olive (Olea europaea L.) from Algeria. J. Microbiol. Biotech Res. 2011, 1, 69–73. [Google Scholar]
- Najee, H.B.; Alkurjia, D.; Almahdawy, O.; Kamerzan, C.; Marutescu, L.; Gheorghe, I.; Popa, M.; Chifiriuc, M.C.; Lazăr, V. Antimicrobial Activity of Olea europaea Fatty Oil against Multi-Drug Resistant and Biofilm Forming Microorganisms. Not. Sci. Biol. 2018, 10, 498–502. [Google Scholar] [CrossRef][Green Version]
- Janakat, S.; Al-Nabulsi, A.A.R.; Allehdan, S.; Olaimat, A.N.; Holley, R.A. Antimicrobial activity of amurca (olive oil lees) extract against selected foodborne pathogens. Food Sci. Technol. 2015, 35, 259–265. [Google Scholar] [CrossRef]
- Umai, D.; Vikranth, A.; Meenambiga, S.S. A study on the green synthesis of silver nanoparticles from Olea europaea and its activity against oral pathogens. Mater. Today Proc. 2021, 44, 3647–3651. [Google Scholar] [CrossRef]
- Suwan, T.; Khongkhunthian, S.; Okonogi, S. Green synthesis and inhibitory effects against oral pathogens of silver nanoparticles mediated by rice extracts. Drug Discov. Ther. 2018, 12, 189–196. [Google Scholar] [CrossRef]
- Fleming, D.; Rumbaugh, K.P. Approaches to Dispersing Medical Biofilms. Microorganisms 2017, 5, 15. [Google Scholar] [CrossRef]
- Mohanta, Y.K.; Biswas, K.; Jena, S.K.; Hashem, A.; Abd_Allah, E.F.; Mohanta, T.K. Anti-biofilm and Antibacterial Activities of Silver Nanoparticles Synthesized by the Reducing Activity of Phytoconstituents Present in the Indian Medicinal Plants. Front. Microbiol. 2020, 11, 1143. [Google Scholar] [CrossRef]
- Hussain, M.A.; Khan, M.Q.; Ali, I.; Dar, M.E.U.I.; Habib, T. 08. Antifungal potential of different parts of Olea europaea and Olea cuspidata growing in Azad Jammu and Kashmir. Pure Appl. Biol. PAB 2021, 4, 204–216. [Google Scholar] [CrossRef]
- Di Pietro, M.; Filardo, S.; Mattioli, R.; Francioso, A.; Raponi, G.; Mosca, L.; Sessa, R. Extra Virgin Olive Oil-Based Green Formulations with Promising Antimicrobial Activity against Drug-Resistant Isolates. Front. Pharmacol. 2022, 13, 885735. [Google Scholar] [CrossRef]
- Kleef, F.; Salman, M. Antifungal Effect of Ambrosia artemisiifolia L. Extract and Chemical Fungicide against Spilocaea oleagina Causing Olive Leaf Spot. Arab. J. Sci. Eng. 2022, 47, 113–117. [Google Scholar] [CrossRef]
- Zorić, N.; Kopjar, N.; Kraljić, K.; Oršolić, N.; Tomić, S.; Kosalec, I. Olive leaf extract activity against Candida albicans and C. dubliniensis—The in vitro viability study. Acta Pharm. 2016, 66, 411–431. [Google Scholar] [CrossRef]
- Laaboudi, W. Eco-extraction of phenolic compounds from Moroccan olive fruits and leaves and their potential use as antimicrobial agents. Eur. J. Sci. Res. 2015, 132, 255–265. [Google Scholar]
- Halliwell, B. Oxygen and nitrogen are pro-carcinogens. Damage to DNA by reactive oxygen, chlorine and nitrogen species: Measurement, mechanism and the effects of nutrition. Mutat. Res. 1999, 443, 37–52. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Dec, K.; Kałduńska, J.; Kawczuga, D.; Kochman, J.; Janda, K. Reactive oxygen species—Sources, functions, oxidative damage. Pol. Merkur. Lek. Organ Pol. Tow. Lek. 2020, 48, 124–127. [Google Scholar]
- Anjaneyulu, E.; Reddy, P.S.; Sunita, M.S.; Kishor, P.B.K.; Meriga, B. Salt tolerance and activity of antioxidative enzymes of transgenic finger millet overexpressing a vacuolar H+-pyrophosphatase gene (SbVPPase) from Sorghum bicolor. J. Plant Physiol. 2014, 171, 789–798. [Google Scholar] [CrossRef]
- Hahn, A.; Zuryn, S. Mitochondrial Genome (mtDNA) Mutations that Generate Reactive Oxygen Species. Antioxidants 2019, 8, 392. [Google Scholar] [CrossRef]
- Yadav, D.K.; Kumar, S.; Choi, E.-H.; Chaudhary, S.; Kim, M.-H. Molecular dynamic simulations of oxidized skin lipid bilayer and permeability of reactive oxygen species. Sci. Rep. 2019, 9, 4496. [Google Scholar] [CrossRef]
- Höhn, A.; Tramutola, A.; Cascella, R. Proteostasis Failure in Neurodegenerative Diseases: Focus on Oxidative Stress. Oxid. Med. Cell. Longev. 2020, 2020, 5497046. [Google Scholar] [CrossRef]
- Kao, Y.-C.; Ho, P.-C.; Tu, Y.-K.; Jou, I.-M.; Tsai, K.-J. Lipids and Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 1505. [Google Scholar] [CrossRef]
- Raghav, K.; Bailey, A.M.; Loree, J.M.; Kopetz, S.; Holla, V.; Yap, T.A.; Wang, F.; Chen, K.; Salgia, R.; Hong, D. Untying the gordion knot of targeting MET in cancer. Cancer Treat. Rev. 2018, 66, 95–103. [Google Scholar] [CrossRef]
- Cadenas, S. Mitochondrial uncoupling, ROS generation and cardioprotection. Biochim. Biophys. Acta Bioenerg. 2018, 1859, 940–950. [Google Scholar] [CrossRef]
- Zaïri, A.; Nouir, S.; Zarrouk, A.; Haddad, H.; Khélifa, A.; Achour, L. Phytochemical Profile, Cytotoxic, Antioxidant, and Allelopathic Potentials of Aqueous Leaf Extracts of Olea europaea. Food Sci. Nutr. 2020, 8, 4805–4813. [Google Scholar] [CrossRef]
- Dağdelen, A. Identifying Antioxidant and Antimicrobial Activities of the Phenolic Extracts and Mineral Contents of Virgin Olive Oils (Olea europaea L. cv. Edincik Su) from Different Regions in Turkey. J. Chem. 2016, 2016, e9589763. [Google Scholar] [CrossRef]
- Yuan, J.-J.; Wang, C.-Z.; Ye, J.-Z.; Tao, R.; Zhang, Y.-S. Enzymatic Hydrolysis of Oleuropein from Olea europea (Olive) Leaf Extract and Antioxidant Activities. Molecules 2015, 20, 2903–2921. [Google Scholar] [CrossRef]
- Lfitat, A.; Zejli, H.; Bousselham, A.; Atki, Y.E.; Lyoussi, B.; Gourch, A.; Abdellaoui, A. Comparative Evaluation of Argania spinosa and Olea europaea Leaf Phenolic Compounds and their Antioxidant Activity. Botanica 2020, 26, 76–87. [Google Scholar] [CrossRef]
- De Melo, M.P. Antioxidant actions of olive leaf extract (Olea europaea L.) on reactive species scavengers. J. Anal. Pharm. Res. 2020, 9, 68–71. [Google Scholar] [CrossRef]
- Pennisi, R.; Ben Amor, I.; Gargouri, B.; Attia, H.; Zaabi, R.; Chira, A.B.; Saoudi, M.; Piperno, A.; Trischitta, P.; Tamburello, M.P.; et al. Analysis of Antioxidant and Antiviral Effects of Olive (Olea europaea L.) Leaf Extracts and Pure Compound Using Cancer Cell Model. Biomolecules 2023, 13, 238. [Google Scholar] [CrossRef]
- Zun-Qiu, W.; Gui-Zhou, Y.; Qing-Ping, Z.; You-Jun, J.; Kai-Yu, T.; Hua-Ping, C.; Ze-Shen, Y.; Qian-Ming, H. Purification, Dynamic Changes and Antioxidant Activities of Oleuropein in Olive (Olea europaea L.) Leaves. J. Food Biochem. 2015, 39, 566–574. [Google Scholar] [CrossRef]
- Fernández-Poyatos, M.P.; Ruiz-Medina, A.; Llorent-Martínez, E.J. Phytochemical profile, mineral content, and antioxidant activity of Olea europaea L. cv. Cornezuelo table olives. Influence of in vitro simulated gastrointestinal digestion. Food Chem. 2019, 297, 124933. [Google Scholar] [CrossRef]
- Tamasi, G.; Baratto, M.C.; Bonechi, C.; Byelyakova, A.; Pardini, A.; Donati, A.; Leone, G.; Consumi, M.; Lamponi, S.; Magnani, A.; et al. Chemical characterization and antioxidant properties of products and by-products from Olea europaea L. Food Sci. Nutr. 2019, 7, 2907–2920. [Google Scholar] [CrossRef]
- Borjan, D.; Leitgeb, M.; Knez, Ž.; Hrnčič, M.K. Microbiological and Antioxidant Activity of Phenolic Compounds in Olive Leaf Extract. Molecules 2020, 25, 5946. [Google Scholar] [CrossRef]
- Blasi, F.; Urbani, E.; Simonetti, M.; Chiesi, C.; Cossignani, L. Seasonal variations in antioxidant compounds of Olea europaea leaves collected from different Italian cultivars. J. Appl. Bot. Food Qual. 2016, 89, 202–207. [Google Scholar] [CrossRef]
- Calvo, M.M.; Martín-Diana, A.B.; Rico, D.; López-Caballero, M.E.; Martínez-Álvarez, O. Antioxidant, Antihypertensive, Hypoglycaemic and Nootropic Activity of a Polyphenolic Extract from the Halophyte Ice Plant (Mesembryanthemum crystallinum). Foods 2022, 11, 1581. [Google Scholar] [CrossRef]
- Lins, P.G.; Marina Piccoli Pugine, S.; Scatolini, A.M.; De Melo, M.P. In vitro antioxidant activity of olive leaf extract (Olea europaea L.) and its protective effect on oxidative damage in human erythrocytes. Heliyon 2018, 4, e00805. [Google Scholar] [CrossRef]
- Ayoub, L.; Hassan, F.; Hamid, S.; Abdelhamid, Z.; Souad, A. Phytochemical screening, antioxidant activity and inhibitory potential of Ficus carica and Olea europaea leaves. Bioinformation 2019, 15, 226–232. [Google Scholar] [CrossRef]
- Monteleone, J.I.; Sperlinga, E.; Siracusa, L.; Spagna, G.; Parafati, L.; Todaro, A.; Palmeri, R. Water as a Solvent of Election for Obtaining Oleuropein-Rich Extracts from Olive (Olea europaea) Leaves. Agronomy 2021, 11, 465. [Google Scholar] [CrossRef]
- Zhao, H.; Avena-Bustillos, R.J.; Wang, S.C. Extraction, Purification and In Vitro Antioxidant Activity Evaluation of Phenolic Compounds in California Olive Pomace. Foods 2022, 11, 174. [Google Scholar] [CrossRef]
- Ribas, J.C.R.; Lazzari, A.; Gonzalez, L.B.F.; da Silva, C.M.; Adamuchio, L.G.; Cuquel, F.L.; Sakurada, R.; Pintro, P.T.M. Bioactive compounds and antioxidant activity of leaves from olive trees grown in Paraná, Brazil. Pesqui. Agropecuária Bras. 2023, 58, e03025. [Google Scholar] [CrossRef]
- Romero-Márquez, J.M.; Navarro-Hortal, M.D.; Forbes-Hernández, T.Y.; Varela-López, A.; Puentes, J.G.; Pino-García, R.D.; Sánchez-González, C.; Elio, I.; Battino, M.; García, R.; et al. Exploring the Antioxidant, Neuroprotective, and Anti-Inflammatory Potential of Olive Leaf Extracts from Spain, Portugal, Greece, and Italy. Antioxidants 2023, 12, 1538. [Google Scholar] [CrossRef]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- Park, M.-K.; Jung, U.; Roh, C. Fucoidan from Marine Brown Algae Inhibits Lipid Accumulation. Mar. Drugs 2011, 9, 1359–1367. [Google Scholar] [CrossRef]
- Thomas, N.J.; Jones, S.E.; Weedon, M.N.; Shields, B.M.; Oram, R.A.; Hattersley, A.T. Frequency and phenotype of type 1 diabetes in the first six decades of life: A cross-sectional, genetically stratified survival analysis from UK Biobank. Lancet Diabetes Endocrinol. 2018, 6, 122–129. [Google Scholar] [CrossRef]
- Priscilla, D.H.; Jayakumar, M.; Thirumurugan, K. Flavanone naringenin: An effective antihyperglycemic and antihyperlipidemic nutraceutical agent on high fat diet fed streptozotocin induced type 2 diabetic rats. J. Funct. Foods 2015, 14, 363–373. [Google Scholar] [CrossRef]
- Sundaram, R.; Shanthi, P.; Sachdanandam, P. Effect of tangeretin, a polymethoxylated flavone on glucose metabolism in streptozotocin-induced diabetic rats. Phytomedicine 2014, 21, 793–799. [Google Scholar] [CrossRef]
- Perri, M.R.; Marrelli, M.; Statti, G.; Conforti, F. Olea europaea bud extracts: Inhibitory effects on pancreatic lipase and α-amylase activities of different cultivars from Calabria region (Italy). Plant Biosyst.—Int. J. Deal. Asp. Plant Biol. 2022, 156, 338–344. [Google Scholar] [CrossRef]
- Akhtar, M.F.; Ashraf, K.M.; Saleem, A.; Sharif, A.; Zubair, H.M.; Anwar, F. Antidiabetic Potential and Antioxidant Activity of Olea europaea subsp. Cuspidata (Indian Olive) Seed Extracts. Evid. Based Complement. Altern. Med. 2022, 2022, e5164985. [Google Scholar] [CrossRef]
- Zakari, A.; Umar, H.; Auwal, I. Hypoglycemic Effect of Olive Oil (Olea europea) on Alloxan-Induced Diabetic Albino Rats. Niger. J. Chem. Res. 2023, 28, 051–058. [Google Scholar] [CrossRef]
- Figueiredo-González, M.; Reboredo-Rodríguez, P.; González-Barreiro, C.; Carrasco-Pancorbo, A.; Cancho-Grande, B.; Simal-Gándara, J. The involvement of phenolic-rich extracts from Galician autochthonous extra-virgin olive oils against the α-glucosidase and α-amylase inhibition. Food Res. Int. 2019, 116, 447–454. [Google Scholar] [CrossRef]
- Abdelkarem, H.M.; El-Sherif, M.A.; Gomma, S.B.; Kassem, S.S.; Abdelkader, M.M. Olive Leaf Powder Modulate Insulin Production and Circulating Adipokines in Streptozotocin Induced Diabetic Rats. J. Diet. Suppl. 2022, 19, 550–565. [Google Scholar] [CrossRef]
- Al-Shudiefat, A.A.-R.; Alturk, H.; Al-Ameer, H.J.; Zihlif, M.; Alenazy, M. Olive Leaf Extract of Olea europaea Reduces Blood Glucose Level through Inhibition of AS160 in Diabetic Rats. Appl. Sci. 2023, 13, 5939. [Google Scholar] [CrossRef]
- Chigurupati, S.; Alharbi, F.S.; Almahmoud, S.; Aldubayan, M.; Almoshari, Y.; Vijayabalan, S.; Bhatia, S.; Chinnam, S.; Venugopal, V. Molecular docking of phenolic compounds and screening of antioxidant and antidiabetic potential of Olea europaea L. Ethanolic leaves extract. Arab. J. Chem. 2021, 14, 103422. [Google Scholar] [CrossRef]
- Laaboudi, W.; Ghanam, J.; Ghoumari, O.; Sounni, F.; Merzouki, M.; Benlemlih, M. Hypoglycemic and hypolipidemic effects of phenolic olive tree extract in streptozotocin diabetic rats. Int. J. Pharm. Pharm. Sci. 2016, 8, 287. [Google Scholar] [CrossRef]
- Dekdouk, N.; Malafronte, N.; Russo, D.; Faraone, I.; De Tommasi, N.; Ameddah, S.; Severino, L.; Milella, L. Phenolic Compounds from Olea europaea L. Possess Antioxidant Activity and Inhibit Carbohydrate Metabolizing Enzymes In Vitro. Evid. Based Complement. Altern. Med. 2015, 2015, e684925. [Google Scholar] [CrossRef]
- Palmeri, R.; Parafati, L.; Trippa, D.; Siracusa, L.; Arena, E.; Restuccia, C.; Fallico, B. Addition of Olive Leaf Extract (OLE) for Producing Fortified Fresh Pasteurized Milk with an Extended Shelf Life. Antioxidants 2019, 8, 255. [Google Scholar] [CrossRef]
- Rauf, A.; Rashid, U.; Shah, Z.A.; Rehman, G.; Bashir, K.; Jamil, J.; Iftikhar; Rahman, A.; Alsahammari, A.; Alharbi, M.; et al. Anti-Inflammatory and Anti-Diabetic Activity of Ferruginan, a Natural Compound from Olea ferruginea. Processes 2023, 11, 545. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, J.; Xin, X.; Zhu, S.; Niu, E.; Wu, Q.; Li, T.; Liu, D. Changes in Phytochemical Profiles and Biological Activity of Olive Leaves Treated by Two Drying Methods. Front. Nutr. 2022, 9, 854680. [Google Scholar] [CrossRef]
- Al-Attar, A.M.; Alsalmi, F.A. Effect of Olea europaea leaves extract on streptozotocin induced diabetes in male albino rats. Saudi J. Biol. Sci. 2019, 26, 118–128. [Google Scholar] [CrossRef]
- Mechchate, H.; Ouedrhiri, W.; Es-safi, I.; Amaghnouje, A.; Jawhari, F.Z.; Bousta, D. Optimization of a New Antihyperglycemic Formulation Using a Mixture of Linum usitatissimum L., Coriandrum sativum L., and Olea europaea var. sylvestris Flavonoids: A Mixture Design Approach. Biologics 2021, 1, 154–163. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T.; et al. Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives. Signal Transduct. Target. Ther. 2021, 6, 201. [Google Scholar] [CrossRef]
- Quirantes-Piné, R.; Zurek, G.; Barrajón-Catalán, E.; Bäßmann, C.; Micol, V.; Segura-Carretero, A.; Fernández-Gutiérrez, A. A metabolite-profiling approach to assess the uptake and metabolism of phenolic compounds from olive leaves in SKBR3 cells by HPLC–ESI-QTOF-MS. J. Pharm. Biomed. Anal. 2013, 72, 121–126. [Google Scholar] [CrossRef]
- Ruzzolini, J.; Peppicelli, S.; Andreucci, E.; Bianchini, F.; Scardigli, A.; Romani, A.; La Marca, G.; Nediani, C.; Calorini, L. Oleuropein, the Main Polyphenol of Olea europaea Leaf Extract, Has an Anti-Cancer Effect on Human BRAF Melanoma Cells and Potentiates the Cytotoxicity of Current Chemotherapies. Nutrients 2018, 10, 1950. [Google Scholar] [CrossRef]
- Casaburi, I.; Puoci, F.; Chimento, A.; Sirianni, R.; Ruggiero, C.; Avena, P.; Pezzi, V. Potential of olive oil phenols as chemopreventive and therapeutic agents against cancer: A review of in vitro studies. Mol. Nutr. Food Res. 2013, 57, 71–83. [Google Scholar] [CrossRef]
- Carpi, S.; Polini, B.; Manera, C.; Digiacomo, M.; Salsano, J.E.; Macchia, M.; Scoditti, E.; Nieri, P. miRNA Modulation and Antitumor Activity by the Extra-Virgin Olive Oil Polyphenol Oleacein in Human Melanoma Cells. Front. Pharmacol. 2020, 11, 574317. [Google Scholar] [CrossRef]
- Boss, A.; Bishop, K.S.; Marlow, G.; Barnett, M.P.G.; Ferguson, L.R. Evidence to Support the Anti-Cancer Effect of Olive Leaf Extract and Future Directions. Nutrients 2016, 8, 513. [Google Scholar] [CrossRef]
- Quero, J.; Ballesteros, L.F.; Ferreira-Santos, P.; Velderrain-Rodriguez, G.R.; Rocha, C.M.R.; Pereira, R.N.; Teixeira, J.A.; Martin-Belloso, O.; Osada, J.; Rodríguez-Yoldi, M.J. Unveiling the Antioxidant Therapeutic Functionality of Sustainable Olive Pomace Active Ingredients. Antioxidants 2022, 11, 828. [Google Scholar] [CrossRef]
- Albogami, S.; Hassan, A.M. Assessment of the Efficacy of Olive Leaf (Olea europaea L.) Extracts in the Treatment of Colorectal Cancer and Prostate Cancer Using In Vitro Cell Models. Molecules 2021, 26, 4069. [Google Scholar] [CrossRef]
- Uğuz, A.C.; Rocha-Pimienta, J.; Martillanes, S.; Garrido, M.; Espino, J.; Delgado-Adámez, J. Chlorophyll Pigments of Olive Leaves and Green Tea Extracts Differentially Affect Their Antioxidant and Anticancer Properties. Molecules 2023, 28, 2779. [Google Scholar] [CrossRef]
- Shah, Z.A.; Mujawah, A.A.H.; Ullah, I.; Rauf, A.; Rashid, U.; Khalil, A.A.; Shah, S.M.M.; Pervaiz, A.; Shaheen, F.; Al-Awthan, Y.S.; et al. Antioxidant and Cytotoxic Activity of a New Ferruginan A from Olea ferruginea: In Vitro and In Silico Studies. Oxid. Med. Cell. Longev. 2022, 2022, e8519250. [Google Scholar] [CrossRef]
- Samet, I.; Han, J.; Jlaiel, L.; Sayadi, S.; Isoda, H. Olive (Olea europaea) Leaf Extract Induces Apoptosis and Monocyte/Macrophage Differentiation in Human Chronic Myelogenous Leukemia K562 Cells: Insight into the Underlying Mechanism. Oxid. Med. Cell. Longev. 2014, 2014, e927619. [Google Scholar] [CrossRef]
- Morsy, F.S.; Abdel-Aziz, M.E. Efficiency of olive (Olea europaea L.) leaf extract as antioxidant and anticancer agents. J. Agroaliment. Process. Technol. 2014, 20, 46–53. [Google Scholar]
- Essafi Rhouma, H.; Trabelsi, N.; Chimento, A.; Benincasa, C.; Tamaalli, A.; Perri, E.; Zarrouk, M.; Pezzi, V. Olea Europaea L. Flowers as a New Promising Anticancer Natural Product: Phenolic Composition, Antiproliferative Activity and Apoptosis Induction. Nat. Prod. Res. 2021, 35, 1836–1839. [Google Scholar] [CrossRef]
- Alzandi, A.A.; Taher, E.A.; Al-Sagheer, N.A.; Al-Khulaidi, A.W.; Azizi, M.; Naguib, D.M. Phytochemical components, antioxidant and anticancer activity of 18 major medicinal plants in Albaha region, Saudi Arabia. Biocatal. Agric. Biotechnol. 2021, 34, 102020. [Google Scholar] [CrossRef]
- Goldsmith, C.D.; Vuong, Q.V.; Sadeqzadeh, E.; Stathopoulos, C.E.; Roach, P.D.; Scarlett, C.J. Phytochemical Properties and Anti-Proliferative Activity of Olea europaea L. Leaf Extracts against Pancreatic Cancer Cells. Molecules 2015, 20, 12992–13004. [Google Scholar] [CrossRef]
- Najibullah, S.N.M.; Ahamad, J.; Sultana, S.; Uthirapathy, S. Potential anticancer activity of chemically characterized extract of Olea europaea (Olive) leaves. Emir. J. Food Agric. 2023, 35, 890–896. [Google Scholar] [CrossRef]
- Mutlu, M.; Tunca, B.; Aksoy, S.A.; Tekin, C.; Cecener, G.; Egeli, U. Olea europaea leaf extract decreases tumour size by affecting the LncRNA expression status in glioblastoma 3D cell cultures. Eur. J. Integr. Med. 2021, 45, 101345. [Google Scholar] [CrossRef]
- Fontana, G.; Bruno, M.; Notarbartolo, M.; Labbozzetta, M.; Poma, P.; Spinella, A.; Rosselli, S. Cytotoxicity of oleanolic and ursolic acid derivatives toward hepatocellular carcinoma and evaluation of NF-κB involvement. Bioorganic Chem. 2019, 90, 103054. [Google Scholar] [CrossRef]
- Clewell, A.E.; Béres, E.; Vértesi, A.; Glávits, R.; Hirka, G.; Endres, J.R.; Murbach, T.S.; Szakonyiné, I.P. A Comprehensive Toxicological Safety Assessment of an Extract of Olea europaea L. Leaves (BonoliveTM). Int. J. Toxicol. 2016, 35, 208–221. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).