Silk Sericin and Its Composite Materials with Antibacterial Properties to Enhance Wound Healing: A Review
Abstract
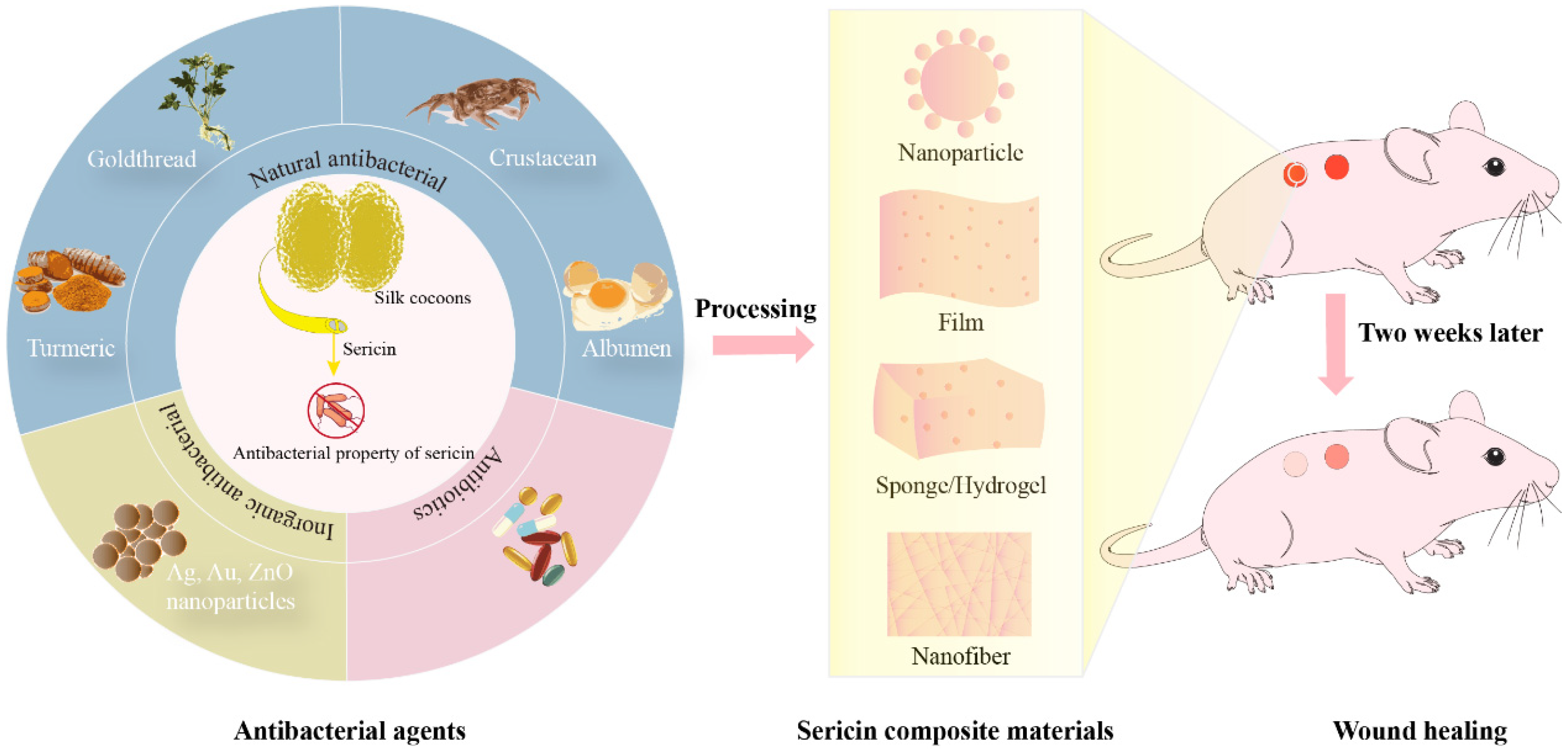
1. Introduction
2. The Antibacterial Properties of the Cocoon Silk Sericin Layer
3. Preparation and Antimicrobial Properties of SS Antimicrobial Composites
3.1. SS Nanoparticle Composite Materials and Antimicrobial Properties
3.1.1. SS Composites Loaded with Ag Nanoparticles
3.1.2. SS-ZnONPs Composite Materials
3.1.3. SS-AuNP Composite Materials
3.1.4. SS Composite Materials Loaded with Multiple Metal Nanoparticles
3.2. SS Materials for Slow Release of Drugs
3.3. SS Materials Loaded with Natural Antimicrobial Products
3.3.1. SS Composites Loaded with Plant Extracts
3.3.2. Composites of SS with Animal Extracts
4. SS-Based Composite Material Promotes Wound Healing
4.1. Wound Healing Stages
4.2. SS Composites Loaded with Metal Nanoparticles Promote Wound Healing
4.3. SS Composites Loaded with Natural Antimicrobial Products Promote Wound Healing and Mechanism
4.4. SS Composites Loaded with Antibiotics Promote Wound Healing
5. Conclusions and Future Trends
Funding
Conflicts of Interest
References
- Goldberg, S.R.; Diegelmann, R.F. What Makes Wounds Chronic. Surg. Clin. N. Am. 2020, 100, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Brook, I.; Randolph, J.G. Aerobic and anaerobic bacterial flora of burns in children. J. Trauma 1981, 21, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Brook, I. Microbiological studies of decubitus ulcers in children. J. Pediatr. Surg. 1991, 26, 207–209. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Hsia, H.C. The Impact of Microbial Communities on Wound Healing: A Review. Ann. Plast. Surg. 2018, 81, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Bessa, L.J.; Fazii, P.; Di Giulio, M.; Cellini, L. Bacterial isolates from infected wounds and their antibiotic susceptibility pattern: Some remarks about wound infection. Int. Wound J. 2015, 12, 47–52. [Google Scholar] [CrossRef]
- Bowler, P.G. Wound pathophysiology, infection and therapeutic options. Ann. Med. 2002, 34, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Rahim, K.; Saleha, S.; Zhu, X.; Huo, L.; Basit, A.; Franco, O.L. Bacterial Contribution in Chronicity of Wounds. Microb. Ecol. 2017, 73, 710–721. [Google Scholar] [CrossRef]
- Hammerschmidt, S. Adherence molecules of pathogenic pneumococci. Curr. Opin. Microbiol. 2006, 9, 12–20. [Google Scholar] [CrossRef]
- Horn, C.M.; Kielian, T. Crosstalk between Staphylococcus aureus and Innate Immunity: Focus on Immunometabolism. Front. Immunol. 2021, 11, 621750. [Google Scholar] [CrossRef]
- Gajula, B.; Munnamgi, S.; Basu, S. How bacterial biofilms affect chronic wound healing: A narrative review. IJS Glob. Health 2020, 3, e16. [Google Scholar] [CrossRef]
- Cavaillon, J.-M. Exotoxins and endotoxins: Inducers of inflammatory cytokines. Toxicon 2018, 149, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Tam, K.; Torres, V.J. Staphylococcus aureus Secreted Toxins and Extracellular Enzymes. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Nichols, R.L.; Florman, S. Clinical presentations of soft-tissue infections and surgical site infections. Clin. Infect. Dis. 2001, 33 (Suppl. S2), S84–S93. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.C.; Kufera, J.A.; Myers, R.A. Necrotizing soft tissue infections. Risk factors for mortality and strategies for management. Ann. Surg. 1996, 224, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Sussman, G. An update on wound management. Aust. Prescr. 2023, 46, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Boateng, J.; Catanzano, O. Advanced Therapeutic Dressings for Effective Wound Healing—A Review. J. Pharm. Sci. 2015, 104, 3653–3680. [Google Scholar] [CrossRef] [PubMed]
- Winter, G.D. Formation of the Scab and the Rate of Epithelization of Superficial Wounds in the Skin of the Young Domestic Pig. Nature 1962, 193, 293–294. [Google Scholar] [CrossRef]
- Farahani, M.; Shafiee, A. Wound Healing: From Passive to Smart Dressings. Adv. Healthc. Mater. 2021, 10, 2100477. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Sousa, D.; Teixeira, J.A.; Ferreira-Santos, P.; Botelho, C.M. Polymeric biomaterials for wound healing. Front. Bioeng. Biotechnol. 2023, 11, 1136077. [Google Scholar] [CrossRef]
- Liu, B.; Song, Y.W.; Jin, L.; Wang, Z.J.; Pu, D.Y.; Lin, S.Q.; Zhou, C.; You, H.J.; Ma, Y.; Li, J.M.; et al. Silk structure and degradation. Colloids Surf. B Biointerfaces 2015, 131, 122–128. [Google Scholar] [CrossRef]
- Silva, A.S.; Costa, E.C.; Reis, S.; Spencer, C.; Calhelha, R.C.; Miguel, S.P.; Ribeiro, M.P.; Barros, L.; Vaz, J.A.; Coutinho, P. Silk Sericin: A Promising Sustainable Biomaterial for Biomedical and Pharmaceutical Applications. Polymers 2022, 14, 4931. [Google Scholar] [CrossRef]
- Ghalei, S.; Handa, H. A review on antibacterial silk fibroin-based biomaterials: Current state and prospects. Mater. Today Chem. 2022, 23, 100673. [Google Scholar] [CrossRef]
- Kumar, J.P.; Mandal, B.B. Antioxidant potential of mulberry and non-mulberry silk sericin and its implications in biomedicine. Free Radic. Biol. Med. 2017, 108, 803–818. [Google Scholar] [CrossRef]
- Schäfer, S.; Aavani, F.; Köpf, M.; Drinic, A.; Stürmer, E.K.; Fuest, S.; Grust, A.L.C.; Gosau, M.; Smeets, R. Silk proteins in reconstructive surgery: Do they possess an inherent antibacterial activity? A systematic review. Wound Repair Regen. 2023, 31, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.P.; Mandal, B.B. Silk sericin induced pro-oxidative stress leads to apoptosis in human cancer cells. Food Chem. Toxicol. 2019, 123, 275–287. [Google Scholar] [CrossRef]
- Kumar, J.P.; Alam, S.; Jain, A.K.; Ansari, K.M.; Mandal, B.B. Protective Activity of Silk Sericin against UV Radiation-Induced Skin Damage by Downregulating Oxidative Stress. ACS Appl. Bio Mater. 2018, 1, 2120–2132. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jiang, M.; Zhang, Y.; Xie, X.; Li, W.; Ming, P.; Jiang, X.; Yang, B.; He, Y.; Chen, J.; et al. Multi-functional carboxymethyl chitosan/sericin protein/halloysite composite sponge with efficient antibacterial and hemostatic properties for accelerating wound healing. Int. J. Biol. Macromol. 2023, 234, 123357. [Google Scholar] [CrossRef] [PubMed]
- Tao, G.; Wang, Y.; Cai, R.; Chang, H.; Song, K.; Zuo, H.; Zhao, P.; Xia, Q.; He, H. Design and performance of sericin/poly(vinyl alcohol) hydrogel as a drug delivery carrier for potential wound dressing application. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 101, 341–351. [Google Scholar] [CrossRef]
- Tuentam, K.; Aramwit, P.; Reamtong, O.; Supasai, S.; Chaisri, U.; Fongsodsri, K.; Yamdech, R.; Tirawanchai, N.; Sukphopetch, P.; Ampawong, S. Sericin-Based Poly(Vinyl) Alcohol Relieves Plaque and Epidermal Lesions in Psoriasis; a Chance for Dressing Development in a Specific Area. Int. J. Mol. Sci. 2022, 24, 145. [Google Scholar] [CrossRef]
- Tao, G.; Cai, R.; Wang, Y.; Zuo, H.; He, H. Fabrication of antibacterial sericin based hydrogel as an injectable and mouldable wound dressing. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111597. [Google Scholar] [CrossRef]
- Ai, L.; Wang, Y.; Tao, G.; Zhao, P.; Umar, A.; Wang, P.; He, H. Polydopamine-Based Surface Modification of ZnO Nanoparticles on Sericin/Polyvinyl Alcohol Composite Film for Antibacterial Application. Molecules 2019, 24, 503. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.; He, H.; Wang, P.; Cai, R.; Tao, G.; Yang, M.; Liu, L.; Zuo, H.; Zhao, P.; Wang, Y. Rational Design and Fabrication of ZnONPs Functionalized Sericin/PVA Antimicrobial Sponge. Int. J. Mol. Sci. 2019, 20, 4796. [Google Scholar] [CrossRef]
- Muhammad Tahir, H.; Saleem, F.; Ali, S.; Ain, Q.u.; Fazal, A.; Summer, M.; Mushtaq, R.; Tariq Zahid, M.; Liaqat, I.; Murtaza, G. Synthesis of sericin-conjugated silver nanoparticles and their potential antimicrobial activity. J. Basic Microbiol. 2020, 60, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Bakadia, B.M.; Boni, B.O.O.; Ahmed, A.A.Q.; Zheng, R.; Shi, Z.; Ullah, M.W.; Lamboni, L.; Yang, G. In Situ Synthesized Porous Bacterial Cellulose/Poly(vinyl alcohol)-Based Silk Sericin and Azithromycin Release System for Treating Chronic Wound Biofilm. Macromol. Biosci. 2022, 22, 2200201. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Liang, J.; Fang, H.; Meng, X.; Chen, J.; Zhong, Z.; Liu, Q.; Hu, H.; Zhang, X. Fabrication and Evaluation of Silk Sericin-Derived Hydrogel for the Release of the Model Drug Berberine. Gels 2021, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Seo, S.; Yang, I.-J.; Nguyen, L.T.H.; Shin, H.-S.; Patra, J.K. Synthesis of Biogenic Gold Nanoparticles by Using Sericin Protein from Bombyx mori Silk Cocoon and Investigation of Its Wound Healing, Antioxidant, and Antibacterial Potentials. Int. J. Nanomed. 2023, 18, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Tao, G.; Cai, R.; Wang, Y.; Liu, L.; Zuo, H.; Zhao, P.; Umare, A.; Mao, C.; Xia, Q.; He, H. Bioinspired design of AgNPs embedded silk sericin-based sponges for efficiently combating bacteria and promoting wound healing. Mater. Des. 2019, 180, 107940. [Google Scholar] [CrossRef]
- Wang, H.Y.; Wang, Y.J.; Zhou, L.X.; Zhu, L.; Zhang, Y.Q. Isolation and bioactivities of a non-sericin component from cocoon shell silk sericin of the silkworm Bombyx mori. Food Funct. 2012, 3, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Xia, Q.; Zhao, P. Antimicrobial components in the cocoon silk of silkworm, Bombyx mori. Int. J. Biol. Macromol. 2023, 224, 68–78. [Google Scholar] [CrossRef]
- Takasu, Y.; Hata, T.; Uchino, K.; Zhang, Q. Identification of Ser2 proteins as major sericin components in the non-cocoon silk of Bombyx mori. Insect Biochem. Mol. Biol. 2010, 40, 339–344. [Google Scholar] [CrossRef]
- Takasu, Y.; Yamada, H.; Tsubouchi, K. Isolation of three main sericin components from the cocoon of the silkworm, Bombyx mori. Biosci. Biotechnol. Biochem. 2002, 66, 2715–2718. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Guo, K.; Zhang, X.; Zhang, T.; Zhang, Y.; Ma, S.; Chang, H.; Tang, M.; An, L.; Xia, Q.; et al. Identification of Bombyx mori sericin 4 protein as a new biological adhesive. Int. J. Biol. Macromol. 2019, 132, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Zhang, X.; Zhao, D.; Qin, L.; Jiang, W.; Hu, W.; Liu, X.; Xia, Q.; Dong, Z.; Zhao, P. Identification and characterization of sericin5 reveals non-cocoon silk sericin components with high β-sheet content and adhesive strength. Acta Biomater. 2022, 150, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Shitole, M.; Dugam, S.; Tade, R.; Nangare, S. Pharmaceutical applications of silk sericin. Ann. Pharm. Françaises 2020, 78, 469–486. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.; El-Samad, L.M.; Gomaa, R.A.; Augustyniak, M.; Hassan, M.A. A comprehensive review of recent advances in silk sericin: Extraction approaches, structure, biochemical characterization, and biomedical applications. Int. J. Biol. Macromol. 2023, 250, 126067. [Google Scholar] [CrossRef] [PubMed]
- Siritientong, T.; Bonani, W.; Motta, A.; Migliaresi, C.; Aramwit, P. The effects of Bombyx mori silk strain and extraction time on the molecular and biological characteristics of sericin. Biosci. Biotechnol. Biochem. 2016, 80, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Bascou, R.; Hardouin, J.; Ben Mlouka, M.A.; Guénin, E.; Nesterenko, A. Detailed investigation on new chemical-free methods for silk sericin extraction. Mater. Today Commun. 2022, 33, 104491. [Google Scholar] [CrossRef]
- Liu, J.; Shi, L.; Deng, Y.; Zou, M.; Cai, B.; Song, Y.; Wang, Z.; Wang, L. Silk sericin-based materials for biomedical applications. Biomaterials 2022, 287, 121638. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Liu, Y.; Zhang, Q.; Liang, C.; Qin, H.; Liu, P.; Wang, K.; Zhang, X.; Chen, L.; Wei, Y. Shape Changes and Interaction Mechanism of Escherichia coli Cells Treated with Sericin and Use of a Sericin-Based Hydrogel for Wound Healing. Appl. Environ. Microbiol. 2016, 82, 4663–4672. [Google Scholar] [CrossRef]
- Aramwit, P.; Kanokpanont, S.; Nakpheng, T.; Srichana, T. The effect of sericin from various extraction methods on cell viability and collagen production. Int. J. Mol. Sci. 2010, 11, 2200–2211. [Google Scholar] [CrossRef]
- Bungthong, C.; Wrigley, C.; Sonteera, T.; Siriamornpun, S. Amino Acid Profile and Biological Properties of Silk Cocoon as Affected by Water and Enzyme Extraction. Molecules 2021, 26, 3455. [Google Scholar] [CrossRef] [PubMed]
- Doakhan, S.; Montazer, M.; Rashidi, A.; Moniri, R.; Moghadam, M.B. Influence of sericin/TiO2 nanocomposite on cotton fabric: Part 1. Enhanced antibacterial effect. Carbohydr. Polym. 2013, 94, 737–748. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, X.; Lu, M.; Chen, H.; Chen, S.; Han, J.; Zhang, Y.; Zhao, P.; Dong, Z. Antibacterial Mechanism of Silkworm Seroins. Polymers 2020, 12, 2985. [Google Scholar] [CrossRef]
- Guo, X.; Dong, Z.; Zhang, Y.; Li, Y.; Liu, H.; Xia, Q.; Zhao, P. Proteins in the Cocoon of Silkworm Inhibit the Growth of Beauveria bassiana. PLoS ONE 2016, 11, e0151764. [Google Scholar] [CrossRef]
- Zhang, X.; Ni, Y.; Guo, K.; Dong, Z.; Chen, Y.; Zhu, H.; Xia, Q.; Zhao, P. The mutation of SPI51, a protease inhibitor of silkworm, resulted in the change of antifungal activity during domestication. Int. J. Biol. Macromol. 2021, 178, 63–70. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, P.; Liu, H.; Guo, X.; He, H.; Zhu, R.; Xiang, Z.; Xia, Q. TIL-type protease inhibitors may be used as targeted resistance factors to enhance silkworm defenses against invasive fungi. Insect Biochem. Mol. Biol. 2015, 57, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, P.; Liu, S.; Dong, Z.; Chen, J.; Xiang, Z.; Xia, Q. A novel protease inhibitor in Bombyx mori is involved in defense against Beauveria bassiana. Insect Biochem. Mol. Biol. 2012, 42, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.P.; Vaishna, R.L.; Kakkar, A.; Arunkumar, K.P.; Nagaraju, J. Characterization of antiviral and antibacterial activity of Bombyx mori seroin proteins. Cell Microbiol. 2014, 16, 1354–1365. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, P.; Wang, Q.; Zhang, Y.; Xu, H.; Zhao, P. Overexpression of Gloverin2 in the Bombyx mori silk gland enhances cocoon/silk antimicrobial activity. Dev. Comp. Immunol. 2019, 98, 6–12. [Google Scholar] [CrossRef]
- Chuysinuan, P.; Pengsuk, C.; Lirdprapamongkol, K.; Thanyacharoen, T.; Techasakul, S.; Svasti, J.; Nooeaid, P. Turmeric Herb Extract-Incorporated Biopolymer Dressings with Beneficial Antibacterial, Antioxidant and Anti-Inflammatory Properties for Wound Healing. Polymers 2023, 15, 1090. [Google Scholar] [CrossRef]
- Karahaliloglu, Z.; Kilicay, E.; Denkbas, E.B. Antibacterial chitosan/silk sericin 3D porous scaffolds as a wound dressing material. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1172–1185. [Google Scholar] [CrossRef]
- Singh, S.; Supaweera, N.; Nwabor, O.F.; Chaichompoo, W.; Suksamrarn, A.; Chittasupho, C.; Chunglok, W. Poly (vinyl alcohol)-gelatin-sericin copolymerized film fortified with vesicle-entrapped demethoxycurcumin/bisdemethoxycurcumin for improved stability, antibacterial, anti-inflammatory, and skin tissue regeneration. Int. J. Biol. Macromol. 2024, 258, 129071. [Google Scholar] [CrossRef]
- Karthick, S.A.; Manjari, K.; Devi, M.G. Biocompatible and bioactive PVA/Sericin/Chitosan nanofibrous wound dressing matrix. Appl. Surf. Sci. Adv. 2023, 13, 100362. [Google Scholar] [CrossRef]
- Chu, W.; Wang, P.; Ma, Z.; Peng, L.; Guo, C.; Fu, Y.; Ding, L. Lupeol-loaded chitosan-Ag(+) nanoparticle/sericin hydrogel accelerates wound healing and effectively inhibits bacterial infection. Int. J. Biol. Macromol. 2023, 243, 125310. [Google Scholar] [CrossRef]
- Shah, A.; Ali Buabeid, M.; Arafa, E.-S.A.; Hussain, I.; Li, L.; Murtaza, G. The wound healing and antibacterial potential of triple-component nanocomposite (chitosan-silver-sericin) films loaded with moxifloxacin. Int. J. Pharm. 2019, 564, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, H.; Qin, S.; Yang, C.; Lv, Q.; Wang, Z.; Wang, L. Antibacterial Sericin Cryogels Promote Hemostasis by Facilitating the Activation of Coagulation Pathway and Platelets. Adv. Healthc. Mater. 2022, 11, 2102717. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, D.; Ji, N.; Lee, S.; Wang, G.; Zheng, Y.; Zhang, X.; Yang, L.; Qin, Z.; Yang, Y. Bioinspired Design of Sericin/Chitosan/Ag@MOF/GO Hydrogels for Efficiently Combating Resistant Bacteria, Rapid Hemostasis, and Wound Healing. Polymers 2021, 13, 2812. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Cai, R.; Wang, Y.; Tao, G.; Ai, L.; Wang, P.; Yang, M.; Zuo, H.; Zhao, P.; He, H. Polydopamine-Assisted Silver Nanoparticle Self-Assembly on Sericin/Agar Film for Potential Wound Dressing Application. Int. J. Mol. Sci. 2018, 19, 2875. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cai, R.; Tao, G.; Wang, P.; Zuo, H.; Zhao, P.; Umar, A.; He, H. A Novel AgNPs/Sericin/Agar Film with Enhanced Mechanical Property and Antibacterial Capability. Molecules 2018, 23, 1821. [Google Scholar] [CrossRef]
- Cai, R.; Tao, G.; He, H.; Song, K.; Zuo, H.; Jiang, W.; Wang, Y. One-Step Synthesis of Silver Nanoparticles on Polydopamine-Coated Sericin/Polyvinyl Alcohol Composite Films for Potential Antimicrobial Applications. Molecules 2017, 22, 721. [Google Scholar] [CrossRef]
- Liu, L.; Cai, R.; Wang, Y.; Tao, G.; Ai, L.; Wang, P.; Yang, M.; Zuo, H.; Zhao, P.; Shen, H.; et al. Preparation and Characterization of AgNPs In Situ Synthesis on Polyelectrolyte Membrane Coated Sericin/Agar Film for Antimicrobial Applications. Materials 2018, 11, 1205. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Cai, R.; Wang, Y.; Tao, G.; Guo, P.; Zuo, H.; Chen, L.; Liu, X.; Zhao, P.; Xia, Q. Preparation and characterization of silk sericin/PVA blend film with silver nanoparticles for potential antimicrobial application. Int. J. Biol. Macromol. 2017, 104, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Boonpavanitchakul, K.; Pimpha, N.; Kangwansupamonkon, W.; Magaraphan, R. Processing and antibacterial application of biodegradable sponge nano-composite materials of silver nanoparticles and silk sericin. Eur. Polym. J. 2020, 130, 109649. [Google Scholar] [CrossRef]
- He, H.; Tao, G.; Wang, Y.; Cai, R.; Guo, P.; Chen, L.; Zuo, H.; Zhao, P.; Xia, Q. In situ green synthesis and characterization of sericin-silver nanoparticle composite with effective antibacterial activity and good biocompatibility. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 80, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Bian, L.; Hu, X. Synergic Fabrication of Gold Nanoparticles Embedded Dextran/ Silk Sericin Nanomaterials for the Treatment and Care of Wound Healing. J. Clust. Sci. 2022, 33, 2147–2156. [Google Scholar] [CrossRef]
- Li, W.; Huang, Z.; Cai, R.; Yang, W.; He, H.; Wang, Y. Rational Design of Ag/ZnO Hybrid Nanoparticles on Sericin/Agarose Composite Film for Enhanced Antimicrobial Applications. Int. J. Mol. Sci. 2020, 22, 105. [Google Scholar] [CrossRef]
- Sapru, S.; Ghosh, A.K.; Kundu, S.C. Non-immunogenic, porous and antibacterial chitosan and Antheraea mylitta silk sericin hydrogels as potential dermal substitute. Carbohydr. Polym. 2017, 167, 196–209. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Li, X.; Sun, B.; Zhang, Y.; Zhang, D.; Tang, Z.; Chen, X.; Wang, C. Electrospun chitosan/sericin composite nanofibers with antibacterial property as potential wound dressings. Int. J. Biol. Macromol. 2014, 68, 92–97. [Google Scholar] [CrossRef]
- Yang, M.; Wang, Y.; Tao, G.; Cai, R.; Wang, P.; Liu, L.; Ai, L.; Zuo, H.; Zhao, P.; Umar, A.; et al. Fabrication of Sericin/Agrose Gel Loaded Lysozyme and Its Potential in Wound Dressing Application. Nanomaterials 2018, 8, 235. [Google Scholar] [CrossRef]
- Bakadia, B.M.; Zhong, A.; Li, X.; Boni, B.O.O.; Ahmed, A.A.Q.; Souho, T.; Zheng, R.; Shi, Z.; Shi, D.; Lamboni, L.; et al. Biodegradable and injectable poly(vinyl alcohol) microspheres in silk sericin-based hydrogel for the controlled release of antimicrobials: Application to deep full-thickness burn wound healing. Adv. Compos. Hybrid Mater. 2022, 5, 2847–2872. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Ismail, A.F.; Aziz, M.; Akbari, M.; Hadisi, Z.; Omidi, M.; Chen, X. Development of the PVA/CS nanofibers containing silk protein sericin as a wound dressing: In vitro and in vivo assessment. Int. J. Biol. Macromol. 2020, 149, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Chao, S.; Li, Y.; Zhao, R.; Zhang, L.; Li, Y.; Wang, C.; Li, X. Synthesis and characterization of tigecycline-loaded sericin/poly(vinyl alcohol) composite fibers via electrospinning as antibacterial wound dressings. J. Drug Deliv. Sci. Technol. 2018, 44, 440–447. [Google Scholar] [CrossRef]
- Diab, S.E.; Tayea, N.A.; Elwakil, B.H.; Gad, A.A.E.M.; Ghareeb, D.A.; Olama, Z.A. Novel Amoxicillin-Loaded Sericin Biopolymeric Nanoparticles: Synthesis, Optimization, Antibacterial and Wound Healing Activities. Int. J. Mol. Sci. 2022, 23, 11654. [Google Scholar] [CrossRef] [PubMed]
- Yah, C.S.; Simate, G.S. Nanoparticles as potential new generation broad spectrum antimicrobial agents. DARU J. Pharm. Sci. 2015, 23, 43. [Google Scholar] [CrossRef] [PubMed]
- Quinteros, M.A.; Cano Aristizábal, V.; Dalmasso, P.R.; Paraje, M.G.; Páez, P.L. Oxidative stress generation of silver nanoparticles in three bacterial genera and its relationship with the antimicrobial activity. Toxicol. Vitr. 2016, 36, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Tee, J.K.; Ong, C.N.; Bay, B.H.; Ho, H.K.; Leong, D.T. Oxidative stress by inorganic nanoparticles. WIREs Nanomed. Nanobiotechnol. 2016, 8, 414–438. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, B.; Kar, S.; Dey, S.K.; Bhandary, S.; Roy, D.; Mukhopadhyay, T.K.; Das, S.; Nandy, P. In situ synthesis and antibacterial activity of copper nanoparticle loaded natural montmorillonite clay based on contact inhibition and ion release. Colloids Surf. B Biointerfaces 2013, 108, 358–365. [Google Scholar] [CrossRef]
- Kittler, S.; Greulich, C.; Diendorf, J.; Köller, M.; Epple, M. Toxicity of Silver Nanoparticles Increases during Storage Because of Slow Dissolution under Release of Silver Ions. Chem. Mater. 2010, 22, 4548–4554. [Google Scholar] [CrossRef]
- Khan, Z.; Al-Thabaiti, S.A.; Obaid, A.Y.; Al-Youbi, A.O. Preparation and characterization of silver nanoparticles by chemical reduction method. Colloids Surf. B Biointerfaces 2011, 82, 513–517. [Google Scholar] [CrossRef]
- Das, G.; Patra, J.K. Evaluation of Antibacterial Mechanism of Action, Tyrosinase Inhibition, and Photocatalytic Degradation Potential of Sericin-Based Gold Nanoparticles. Int. J. Mol. Sci. 2023, 24, 9477. [Google Scholar] [CrossRef]
- Al Masud, M.A.; Shaikh, H.; Alam, M.S.; Karim, M.M.; Momin, M.A.; Islam, M.A.; Khan, G.M.A. Green synthesis of silk sericin-embedded silver nanoparticles and their antibacterial application against multidrug-resistant pathogens. J. Genet. Eng. Biotechnol. 2021, 19, 74. [Google Scholar] [CrossRef] [PubMed]
- Sharmin, S.; Rahaman, M.M.; Sarkar, C.; Atolani, O.; Islam, M.T.; Adeyemi, O.S. Nanoparticles as antimicrobial and antiviral agents: A literature-based perspective study. Heliyon 2021, 7, e06456. [Google Scholar] [CrossRef] [PubMed]
- Baptista, P.V.; McCusker, M.P.; Carvalho, A.; Ferreira, D.A.; Mohan, N.M.; Martins, M.; Fernandes, A.R. Nano-Strategies to Fight Multidrug Resistant Bacteria—“A Battle of the Titans”. Front. Microbiol. 2018, 9, 1441. [Google Scholar] [CrossRef] [PubMed]
- Arango, M.C.; Montoya, Y.; Peresin, M.S.; Bustamante, J.; Álvarez-López, C. Silk sericin as a biomaterial for tissue engineering: A review. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 1115–1129. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Szymczak, M.; Maciejewska, M.; Laskowski, Ł.; Laskowska, M.; Ostaszewski, R.; Skiba, G.; Franiak-Pietryga, I. All That Glitters Is Not Silver—A New Look at Microbiological and Medical Applications of Silver Nanoparticles. Int. J. Mol. Sci. 2021, 22, 854. [Google Scholar] [CrossRef] [PubMed]
- Htwe, Y.; Chow, W.; Suda, Y.; Mariatti, M. Effect of silver nitrate concentration on the production of silver nanoparticles by green method. Mater. Today Proc. 2019, 17, 568–573. [Google Scholar] [CrossRef]
- Abram, S.-L.; Fromm, K.M. Handling (Nano)Silver as Antimicrobial Agent: Therapeutic Window, Dissolution Dynamics, Detection Methods and Molecular Interactions. Chem. Eur. J. 2020, 26, 10948–10971. [Google Scholar] [CrossRef] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liao, C.; Tjong, S.C. Recent Advances in Zinc Oxide Nanostructures with Antimicrobial Activities. Int. J. Mol. Sci. 2020, 21, 8836. [Google Scholar] [CrossRef]
- Stoimenov, P.K.; Klinger, R.L.; Marchin, G.L.; Klabunde, K.J. Metal oxide nanoparticles as bactericidal agents. Langmuir 2002, 18, 6679–6686. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, A.Z.; Xie, S.; Li, X.; Cen, T.; Li, D.; Chen, J. Nano-metal oxides induce antimicrobial resistance via radical-mediated mutagenesis. Environ. Int. 2018, 121, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Asharani, P.V.; Lianwu, Y.; Gong, Z.; Valiyaveettil, S. Comparison of the toxicity of silver, gold and platinum nanoparticles in developing zebrafish embryos. Nanotoxicology 2011, 5, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef] [PubMed]
- Sathiyaraj, S.; Suriyakala, G.; Dhanesh Gandhi, A.; Babujanarthanam, R.; Almaary, K.S.; Chen, T.W.; Kaviyarasu, K. Biosynthesis, characterization, and antibacterial activity of gold nanoparticles. J. Infect. Public Health 2021, 14, 1842–1847. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Seo, S.; Yang, I.-J.; Nguyen, L.T.H.; Shin, H.-S.; Patra, J.K. Sericin mediated gold/silver bimetallic nanoparticles and exploration of its multi-therapeutic efficiency and photocatalytic degradation potential. Environ. Res. 2023, 229, 115935. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.S.; Patil, R.H.; Kale, S.B.; Tamboli, M.S.; Ambekar, J.D.; Gade, W.N.; Kolekar, S.S.; Kale, B.B. Nanostructured microspheres of silver @ zinc oxide: An excellent impeder of bacterial growth and biofilm. J. Nanoparticle Res. 2014, 16, 2717. [Google Scholar] [CrossRef]
- Rajaboopathi, S.; Thambidurai, S. Synthesis of bio-surfactant based Ag/ZnO nanoparticles for better thermal, photocatalytic and antibacterial activity. Mater. Chem. Phys. 2019, 223, 512–522. [Google Scholar] [CrossRef]
- Riaz Ahmed, K.B.; Nagy, A.M.; Brown, R.P.; Zhang, Q.; Malghan, S.G.; Goering, P.L. Silver nanoparticles: Significance of physicochemical properties and assay interference on the interpretation of in vitro cytotoxicity studies. Toxicol. In Vitro 2017, 38, 179–192. [Google Scholar] [CrossRef]
- Szmyd, R.; Goralczyk, A.G.; Skalniak, L.; Cierniak, A.; Lipert, B.; Filon, F.L.; Crosera, M.; Borowczyk, J.; Laczna, E.; Drukala, J.; et al. Effect of silver nanoparticles on human primary keratinocytes. Biol. Chem. 2013, 394, 113–123. [Google Scholar] [CrossRef]
- Nishida, A.; Yamada, M.; Kanazawa, T.; Takashima, Y.; Ouchi, K.; Okada, H. Sustained-release of protein from biodegradable sericin film, gel and sponge. Int. J. Pharm. 2011, 407, 44–52. [Google Scholar] [CrossRef]
- Nishida, A.; Yamada, M.; Kanazawa, T.; Takashima, Y.; Ouchi, K.; Okada, H. Use of silk protein, sericin, as a sustained-release material in the form of a gel, sponge and film. Chem. Pharm. Bull. 2010, 58, 1480–1486. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Tao, G.; He, H.; Guo, P.; Yang, M.; Ding, C.; Zuo, H.; Wang, L.; Zhao, P.; Wang, Y. In Situ Synthesis of Silver Nanoparticles on the Polyelectrolyte-Coated Sericin/PVA Film for Enhanced Antibacterial Application. Materials 2017, 10, 967. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, Y.; Zhang, J.; Huang, L.; Liu, J.; Li, Y.; Zhang, G.; Kundu, S.C.; Wang, L. Exploring natural silk protein sericin for regenerative medicine: An injectable, photoluminescent, cell-adhesive 3D hydrogel. Sci. Rep. 2014, 4, 7064. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, J.; Huang, L.; Wang, Z.; Wang, L. Design and performance of a sericin-alginate interpenetrating network hydrogel for cell and drug delivery. Sci. Rep. 2015, 5, 12374. [Google Scholar] [CrossRef] [PubMed]
- Bakadia, B.M.; He, F.; Souho, T.; Lamboni, L.; Ullah, M.W.; Boni, B.O.; Ahmed, A.A.Q.; Mukole, B.M.; Yang, G. Prevention and treatment of COVID-19: Focus on interferons, chloroquine/hydroxychloroquine, azithromycin, and vaccine. Biomed. Pharmacother. Biomed. Pharmacother. 2021, 133, 111008. [Google Scholar] [CrossRef] [PubMed]
- Kanoujia, J.; Faizan, M.; Parashar, P.; Singh, N.; Saraf, S.A. Curcumin loaded sericin nanoparticles: Assessment for biomedical application. Food Hydrocoll. Health 2021, 1, 100029. [Google Scholar] [CrossRef]
- Bayer, I.S. A Review of Sustained Drug Release Studies from Nanofiber Hydrogels. Biomedicines 2021, 9, 1612. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, L.; Mao, C.; Jin, L.; Wu, S.; Zheng, Y.; Cui, Z.; Li, Z.; Zhang, Y.; Zhu, S.; et al. Natural Extracts for Antibacterial Applications. Small 2023, 20, 2306553. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Umar, S.; Ashafaq, M.; Akhtar, M.; Iqbal, Z.; Samim, M.; Ahmad, F.J. A comparative study of PNIPAM nanoparticles of curcumin, demethoxycurcumin, and bisdemethoxycurcumin and their effects on oxidative stress markers in experimental stroke. Protoplasma 2013, 250, 1327–1338. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, J.; Li, X.; Chen, T.; Park, S.; Bae, M.; Jung, D.; Lin, L.; Paek, S.H.; Piao, Y. Anticancer pH-Responsive Supramolecular Vesicles Fabricated Using Water-Soluble Pillar[5]arene and Curcumin Derivative. Mater. Des. 2022, 222, 111084. [Google Scholar] [CrossRef]
- Anthony, A.A.; Mumuni, A.M.; Philip, F.B. Lipid Nanoparticulate Drug Delivery Systems: A Revolution in Dosage Form Design and Development. In Recent Advances in Novel Drug Carrier Systems; Ali Demir, S., Ed.; IntechOpen: Rijeka, Croatia, 2012; Chapter 5. [Google Scholar] [CrossRef]
- Zhang, P.; He, L.; Zhang, J.; Mei, X.; Zhang, Y.; Tian, H.; Chen, Z. Preparation of novel berberine nano-colloids for improving wound healing of diabetic rats by acting Sirt1/NF-κB pathway. Colloids Surf. B Biointerfaces 2020, 187, 110647. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Wang, S.; Xie, J.; Fu, T.; Li, S. In situ gelling hydrogel loaded with berberine liposome for the treatment of biofilm-infected wounds. Front. Bioeng. Biotechnol. 2023, 11, 1189010. [Google Scholar] [CrossRef]
- Cometa, S.; Bonifacio, M.A.; Licini, C.; Bellissimo, A.; Pinto, L.; Baruzzi, F.; Mattioli-Belmonte, M.; De Giglio, E. Innovative Eco-Friendly Hydrogel Film for Berberine Delivery in Skin Applications. Molecules 2021, 26, 4901. [Google Scholar] [CrossRef]
- Vedovatto, S.; Facchini, J.C.; Batista, R.K.; Paim, T.C.; Lionzo, M.I.Z.; Wink, M.R. Development of chitosan, gelatin and liposome film and analysis of its biocompatibility in vitro. Int. J. Biol. Macromol. 2020, 160, 750–757. [Google Scholar] [CrossRef]
- Elnaggar, Y.S.R.; El-Refaie, W.M.; El-Massik, M.A.; Abdallah, O.Y. Lecithin-based nanostructured gels for skin delivery: An update on state of art and recent applications. J. Control. Release 2014, 180, 10–24. [Google Scholar] [CrossRef]
- Qi, L.; Xu, Z.; Jiang, X.; Hu, C.; Zou, X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr. Res. 2004, 339, 2693–2700. [Google Scholar] [CrossRef]
- Chollakup, R.; Uttayarat, P.; Chworos, A.; Smitthipong, W. Noncovalent Sericin-Chitosan Scaffold: Physical Properties and Low Cytotoxicity Effect. Int. J. Mol. Sci. 2020, 21, 775. [Google Scholar] [CrossRef]
- Xiao, L.; Ni, W.; Zhao, X.; Guo, Y.; Li, X.; Wang, F.; Luo, G.; Zhan, R.; Xu, X. A moisture balanced antibacterial dressing loaded with lysozyme possesses antibacterial activity and promotes wound healing. Soft Matter 2021, 17, 3162–3173. [Google Scholar] [CrossRef]
- Chen, L.-L.; Shi, W.-P.; Zhang, T.-D.; Zhou, Y.-Q.; Zhao, F.-Z.; Ge, W.-Y.; Jin, X.-Q.; Lin, W.-J.; Guo, W.-H.; Yin, D.-C. Antibacterial activity of lysozyme-loaded cream against MRSA and promotion of scalded wound healing. Int. J. Pharm. 2022, 627, 122200. [Google Scholar] [CrossRef]
- Gyawali, R.; Ibrahim, S.A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Ferraboschi, P.; Ciceri, S.; Grisenti, P. Applications of Lysozyme, an Innate Immune Defense Factor, as an Alternative Antibiotic. Antibiotics 2021, 10, 1534. [Google Scholar] [CrossRef]
- AlSheikh, H.M.A.; Sultan, I.; Kumar, V.; Rather, I.A.; Al-Sheikh, H.; Tasleem Jan, A.; Haq, Q.M.R. Plant-Based Phytochemicals as Possible Alternative to Antibiotics in Combating Bacterial Drug Resistance. Antibiotics 2020, 9, 480. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Singh, S.; Young, A.; McNaught, C.-E. The physiology of wound healing. Surgery 2017, 35, 473–477. [Google Scholar] [CrossRef]
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef]
- Wynn, M. The impact of infection on the four stages of acute wound healing: An overview. Wounds UK 2021, 17, 26–32. [Google Scholar]
- Robson, M.C. Wound Infection: A Failure of Wound Healing Caused by an Imbalance of Bacteria. Surg. Clin. N. Am. 1997, 77, 637–650. [Google Scholar] [CrossRef]
- Jones, S.G.; Edwards, R.; Thomas, D.W. Inflammation and wound healing: The role of bacteria in the immuno-regulation of wound healing. Int. J. Low. Extrem. Wounds 2004, 3, 201–208. [Google Scholar] [CrossRef]
- Gilaberte, Y.; Prieto-Torres, L.; Pastushenko, I.; Juarranz, Á. Chapter 1—Anatomy and Function of the Skin. In Nanoscience in Dermatology; Hamblin, M.R., Avci, P., Prow, T.W., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 1–14. [Google Scholar] [CrossRef]
- Munir, F.; Tahir, H.M.; Ali, S.; Ali, A.; Tehreem, A.; Zaidi, S.D.E.S.; Adnan, M.; Ijaz, F. Characterization and Evaluation of Silk Sericin-Based Hydrogel: A Promising Biomaterial for Efficient Healing of Acute Wounds. ACS Omega 2023, 8, 32090–32098. [Google Scholar] [CrossRef]
- Aramwit, P.; Towiwat, P.; Srichana, T. Anti-inflammatory potential of silk sericin. Nat. Prod. Commun. 2013, 8, 501–504. [Google Scholar] [CrossRef]
- Manoharan, C.; Thomas, D.S.; Yashwant, R.S.; Mudagal, M.P.; Janadri, S.; Roy, G.; Kunjupillai, V.; Mishra, R.K.; Gopalapillai, R. Bioengineered and functionalized silk proteins accelerate wound healing in rat and human dermal fibroblasts. Integr. Biol. 2022, 14, 151–161. [Google Scholar] [CrossRef]
- Aramwit, P.; Fongsodsri, K.; Tuentam, K.; Reamtong, O.; Thiangtrongjit, T.; Kanjanapruthipong, T.; Yadavalli, V.K.; Ampawong, S. Sericin coated thin polymeric films reduce keratinocyte proliferation via the mTOR pathway and epidermal inflammation through IL17 signaling in psoriasis rat model. Sci. Rep. 2023, 13, 12133. [Google Scholar] [CrossRef]
- Pereira Beserra, F.; Sérgio Gushiken, L.F.; Vieira, A.J.; Augusto Bérgamo, D.; Luísa Bérgamo, P.; Oliveira de Souza, M.; Alberto Hussni, C.; Kiomi Takahira, R.; Henrique Nóbrega, R.; Monteiro Martinez, E.R.; et al. From Inflammation to Cutaneous Repair: Topical Application of Lupeol Improves Skin Wound Healing in Rats by Modulating the Cytokine Levels, NF-κB, Ki-67, Growth Factor Expression, and Distribution of Collagen Fibers. Int. J. Mol. Sci. 2020, 21, 4952. [Google Scholar] [CrossRef]
- Pereira Beserra, F.; Xue, M.; Maia, G.L.A.; Leite Rozza, A.; Helena Pellizzon, C.; Jackson, C.J. Lupeol, a Pentacyclic Triterpene, Promotes Migration, Wound Closure, and Contractile Effect In Vitro: Possible Involvement of PI3K/Akt and p38/ERK/MAPK Pathways. Molecules 2018, 23, 2819. [Google Scholar] [CrossRef]
- Pankey, G.A. Tigecycline. J. Antimicrob. Chemother. 2005, 56, 470–480. [Google Scholar] [CrossRef]
- Makvandi, P.; Wang, C.-y.; Zare, E.N.; Borzacchiello, A.; Niu, L.-n.; Tay, F.R. Metal-Based Nanomaterials in Biomedical Applications: Antimicrobial Activity and Cytotoxicity Aspects. Adv. Funct. Mater. 2020, 30, 1910021. [Google Scholar] [CrossRef]
- O’Toole, P.W.; Marchesi, J.R.; Hill, C. Next-generation probiotics: The spectrum from probiotics to live biotherapeutics. Nat. Microbiol. 2017, 2, 17057. [Google Scholar] [CrossRef]
- Patnode, M.L.; Beller, Z.W.; Han, N.D.; Cheng, J.; Peters, S.L.; Terrapon, N.; Henrissat, B.; Le Gall, S.; Saulnier, L.; Hayashi, D.K.; et al. Interspecies Competition Impacts Targeted Manipulation of Human Gut Bacteria by Fiber-Derived Glycans. Cell 2019, 179, 59–73.e13. [Google Scholar] [CrossRef]
- Raffatellu, M. Learning from bacterial competition in the host to develop antimicrobials. Nat. Med. 2018, 24, 1097–1103. [Google Scholar] [CrossRef]
- Chowdhury, S.; Castro, S.; Coker, C.; Hinchliffe, T.E.; Arpaia, N.; Danino, T. Programmable bacteria induce durable tumor regression and systemic antitumor immunity. Nat. Med. 2019, 25, 1057–1063. [Google Scholar] [CrossRef]
- Leventhal, D.S.; Sokolovska, A.; Li, N.; Plescia, C.; Kolodziej, S.A.; Gallant, C.W.; Christmas, R.; Gao, J.R.; James, M.J.; Abin-Fuentes, A.; et al. Immunotherapy with engineered bacteria by targeting the STING pathway for anti-tumor immunity. Nat. Commun. 2020, 11, 2739. [Google Scholar] [CrossRef]
- Ming, Z.; Han, L.; Bao, M.; Zhu, H.; Qiang, S.; Xue, S.; Liu, W. Living Bacterial Hydrogels for Accelerated Infected Wound Healing. Adv. Sci. 2021, 8, e2102545. [Google Scholar] [CrossRef]

| Categorization | Reagent Content | Forms | Antimicrobial Testing | Research Model | Efficiency | References | |
|---|---|---|---|---|---|---|---|
| Metal nanoparticles | Ag | 0.8 mmol/L AgNO3 | hydrogel | E. coli, S. aureus, P. aeruginosa | In vitro, rat model | antibacterial, anti-inflammatory, promoting angiogenesis | [30] |
| 0.01 M AgNO3 | hydrogel | S. aureus, MRSA, E. coli | In vitro, rat model | antibacterial, hemostasis | [66] | ||
| 0.05 wt% AgNO3 | hydrogel | E. coli, S. aureus | In vitro, rat model | antibacterial, promoting cell migration and proliferation, hemostasis | [67] | ||
| 10 mM AgNO3 | film | E. coli, S. aureus | In vitro | antibacterial, promoting cell proliferation | [68] | ||
| 50 mM AgNO3 | film | E. coli, S. aureus | In vitro | antibacterial | [69] | ||
| 30 mM AgNO3 | film | E. coli, S. aureus | In vitro | antibacterial | [70] | ||
| 20 mM AgNO3 | film | E. coli, S. aureus | In vitro | antibacterial | [71] | ||
| 100 mM AgNO3 | film | E. coli, S. aureus | In vitro | antibacterial | [72] | ||
| 0.2 mM/L AgNO3 | sponge | E. coli, S. aureus, P. aeruginosa | In vitro, rat model | antibacterial, anti-inflammatory, promoting wound healing | [37] | ||
| 5 mM AgNO3 | sponge | E. coli, S. aureus | In vitro | antibacterial | [73] | ||
| 30 mg AgNO3 | nanoparticle | E. coli, S. aureus, K. pneumoniae | In vitro | antibacterial | [33] | ||
| 4 mg/mL AgNO3 | \ | S. aureus | In vitro | antibacterial | [74] | ||
| Au | 1.0 mM HAuCl4·3H2O | nanoparticle | E. coli, E. faecium, S. enterica, S. typhimurium | In vitro | antibacterial, promoting cell proliferation, antioxidation | [36] | |
| 2 wt% HAuCl4·3H2O | nanoparticle | E. coli, S. aureus | In vitro, rat model | antibacterial, promoting cell migration and proliferation | [75] | ||
| ZnO | 61.7 mM ZnONPs | film | E. coli, S. aureus | In vitro | antibacterial | [31] | |
| 0.01 M ZnONPs | film | E. coli, S. aureus | In vitro | antibacterial | [76] | ||
| 24.7 mM ZnONPs | sponge | E. coli, S. aureus | In vitro | antibacterial | [32] | ||
| Natural extracts | plant extract | 10 mg/mL lupeol | hydrogel | E. coli, S. aureus | In vitro, rat model | antibacterial, promoting cell migration and proliferation, anti-inflammatory | [64] |
| 3 mg/mL berberine | hydrogel | E. coli, S. aureus | In vitro | antibacterial | [35] | ||
| 200 mg VDMC/VBDMC | film | A. baumannii, S. epidermidis | In vitro | antibacterial, antioxidation, promoting cell migration | [62] | ||
| 3% turmeric extract | sponge | E. coli, S. aureus | In vitro | antibacterial, antioxidation, anti-inflammatory | [60] | ||
| animal extract | 2% w/v chitosan | hydrogel | E. coli, S. aureus | In vitro | antibacterial, antioxidation, promoting cell migration and proliferation | [77] | |
| 2% w/v chitosan | scaffold | E. coli, S. aureus | In vitro | antibacterial | [61] | ||
| 0.4 mg/mL composite nanofiber solution | nanofibers | E.coli, B. subtilis | In vitro | antibacterial, promoting cell proliferation | [78] | ||
| 2% w/v chitosan | nanofibers | E. coli, S. aureus | In vitro | antibacterial | [63] | ||
| 20–75 mg/mL lysozyme | gel | E. coli, S. aureus | In vitro | antibacterial | [79] | ||
| Antibiotics | 10 mg/mL gentamicin sulfate 30 mg/mL aspirin | hydrogel | E. coli, S. aureus, P. aeruginosa | In vitro | antibacterial | [28] | |
| vancomycin gentamicin | hydrogel | MRSA, P. aeruginosa, E. coli | In vitro, rat model | antibacterial, promoting angiogenesis, collagen deposition | [80] | ||
| 10% moxifloxacin | film | A. baumannii, S. epidermidis, MRSA | In vitro, rat model | antibacterial, promoting angiogenesis, collagen deposition | [65] | ||
| 32 μg/mL azithromycin | scaffold | S. aureus, P. aeruginosa, E. coli, C. albicans | In vitro, rat model | antibacterial, promoting cell migration and proliferation, promote angiogenesis, collagen deposition | [34] | ||
| 5 wt% tetracycline | nanofibers | E. coli, S. aureus | In vitro, rat model | antibacterial, promote angiogenesis, collagen deposition, anti-inflammatory | [81] | ||
| 0.015 g tigecycline | nanofibers | E. coli, B. subtilis | In vitro, rat model | antibacterial | [82] | ||
| 10 mg/mL Amoxicillin | nanoparticle | S. aureus, K. pneumonia, E. coli, A. baumannii, P. aeruginosa | In vitro, rat model | antibacterial, promote angiogenesis, collagen deposition | [83] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.-L.; Zhuo, J.-J.; Fang, S.-M.; Xu, W.; Yu, Q.-Y. Silk Sericin and Its Composite Materials with Antibacterial Properties to Enhance Wound Healing: A Review. Biomolecules 2024, 14, 723. https://doi.org/10.3390/biom14060723
Wang S-L, Zhuo J-J, Fang S-M, Xu W, Yu Q-Y. Silk Sericin and Its Composite Materials with Antibacterial Properties to Enhance Wound Healing: A Review. Biomolecules. 2024; 14(6):723. https://doi.org/10.3390/biom14060723
Chicago/Turabian StyleWang, Sheng-Lan, Jia-Jun Zhuo, Shou-Min Fang, Wei Xu, and Quan-You Yu. 2024. "Silk Sericin and Its Composite Materials with Antibacterial Properties to Enhance Wound Healing: A Review" Biomolecules 14, no. 6: 723. https://doi.org/10.3390/biom14060723
APA StyleWang, S.-L., Zhuo, J.-J., Fang, S.-M., Xu, W., & Yu, Q.-Y. (2024). Silk Sericin and Its Composite Materials with Antibacterial Properties to Enhance Wound Healing: A Review. Biomolecules, 14(6), 723. https://doi.org/10.3390/biom14060723






