Lumbrokinase Extracted from Earthworms Synergizes with Bevacizumab and Chemotherapeutics in Treating Non-Small Cell Lung Cancer by Targeted Inactivation of BPTF/VEGF and NF-κB/COX-2 Signaling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Reagents and Antibodies
2.3. Cell Viability Assay
2.4. Colony Formation Assay
2.5. Western Blot
2.6. RT-PCR
2.7. ELISA
2.8. Flow Cytometry
2.9. Wound Healing Assay
2.10. Transwell Assay
2.11. Angiogenesis Assay
2.12. Immunofluorescence Assay
2.13. Pull down Assay
2.14. Chromatin Immunoprecipitation (ChIP) Assay
2.15. Dual-Luciferase Reporter Assay
2.16. Animal Experiments
2.17. Immunohistochemistry Staining
2.18. Statistical Analysis
3. Results
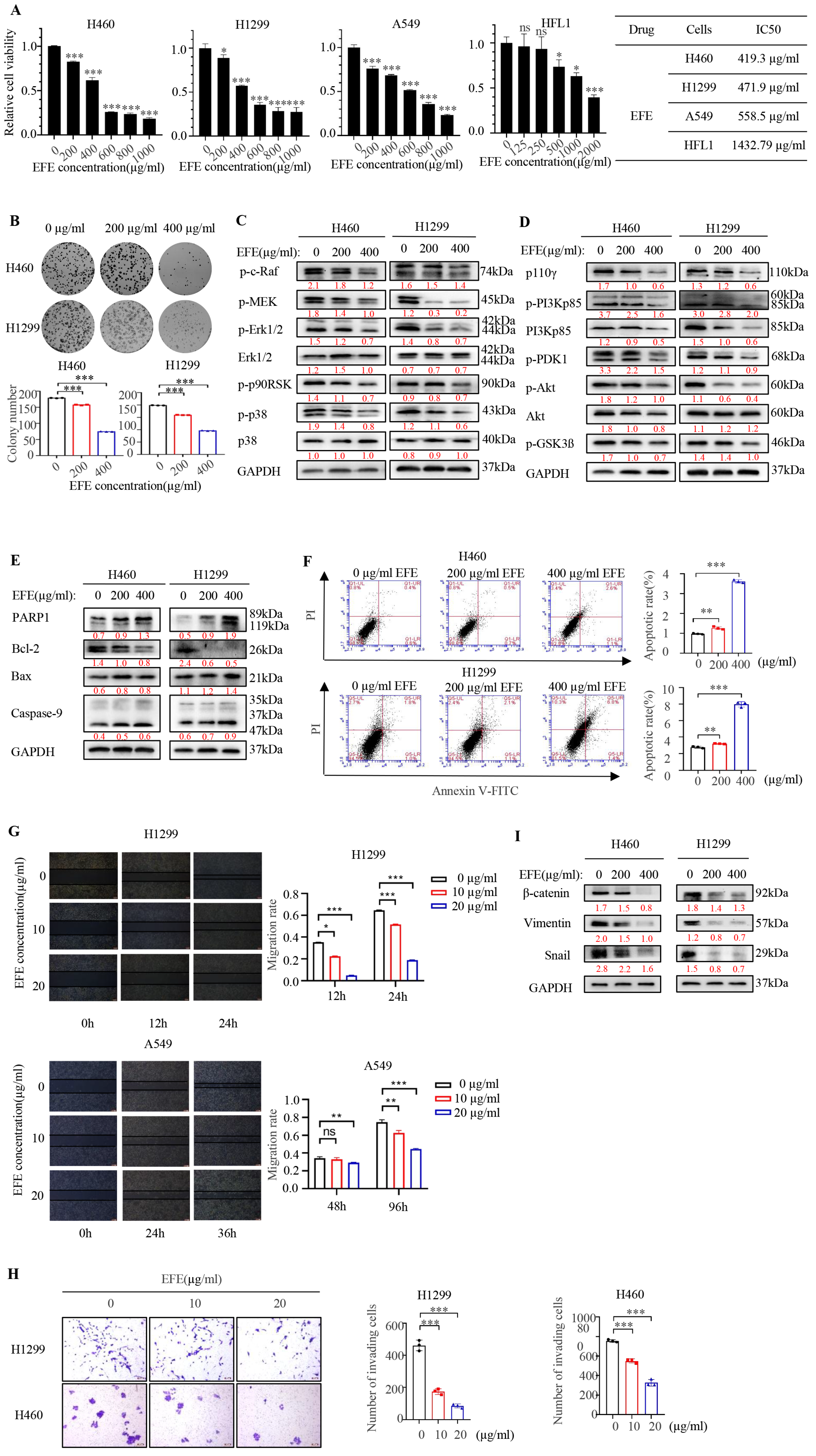
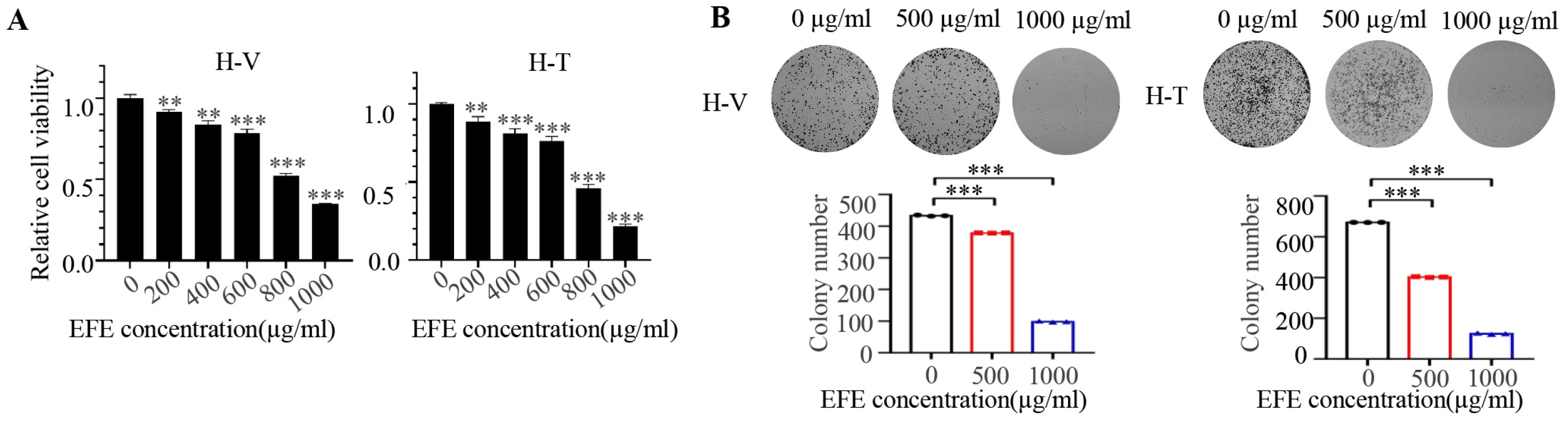
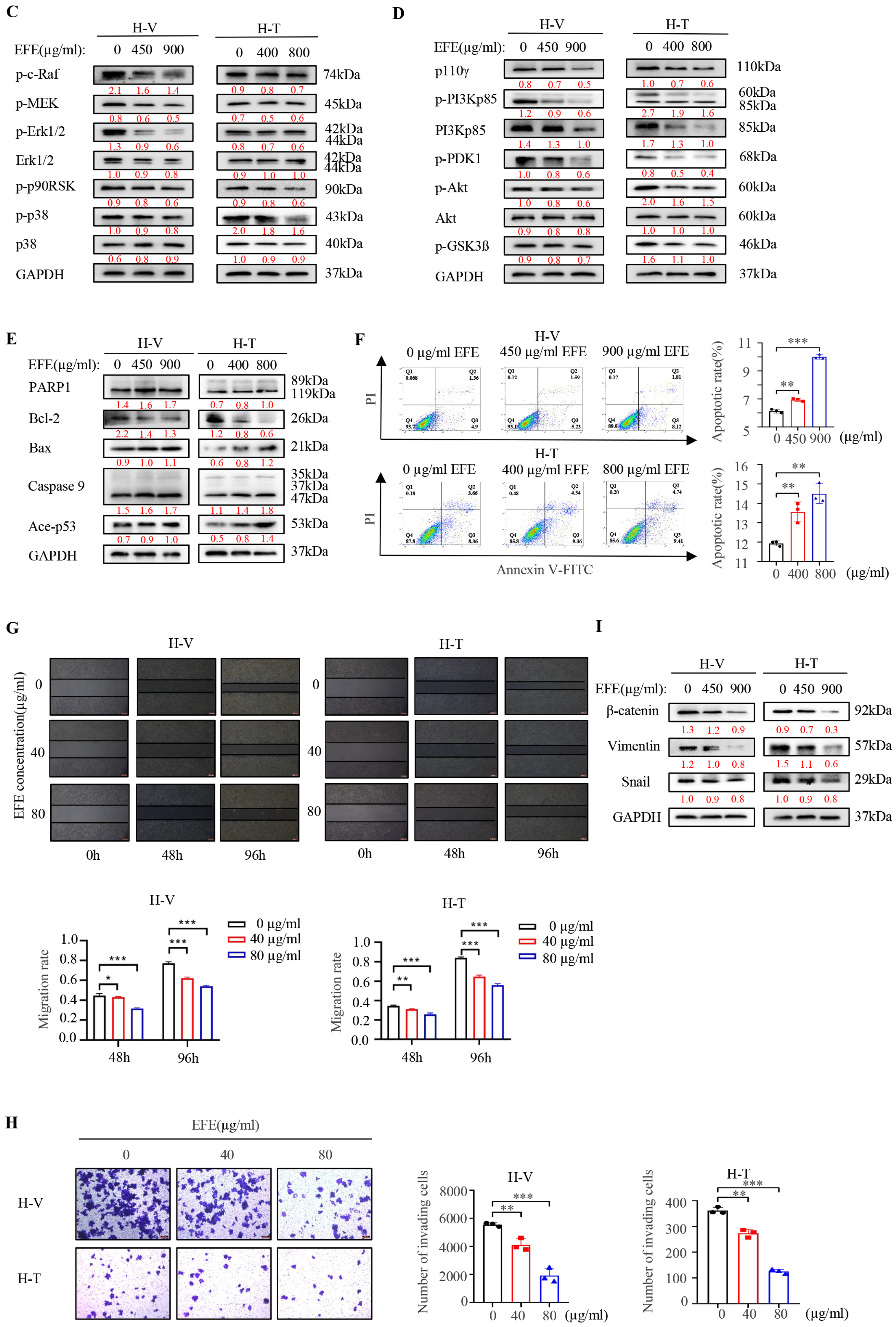
3.1. Lumbrokinase Induces Cellular Apoptosis, Proliferative, and Metastatic Inhibition in NSCLC Cells
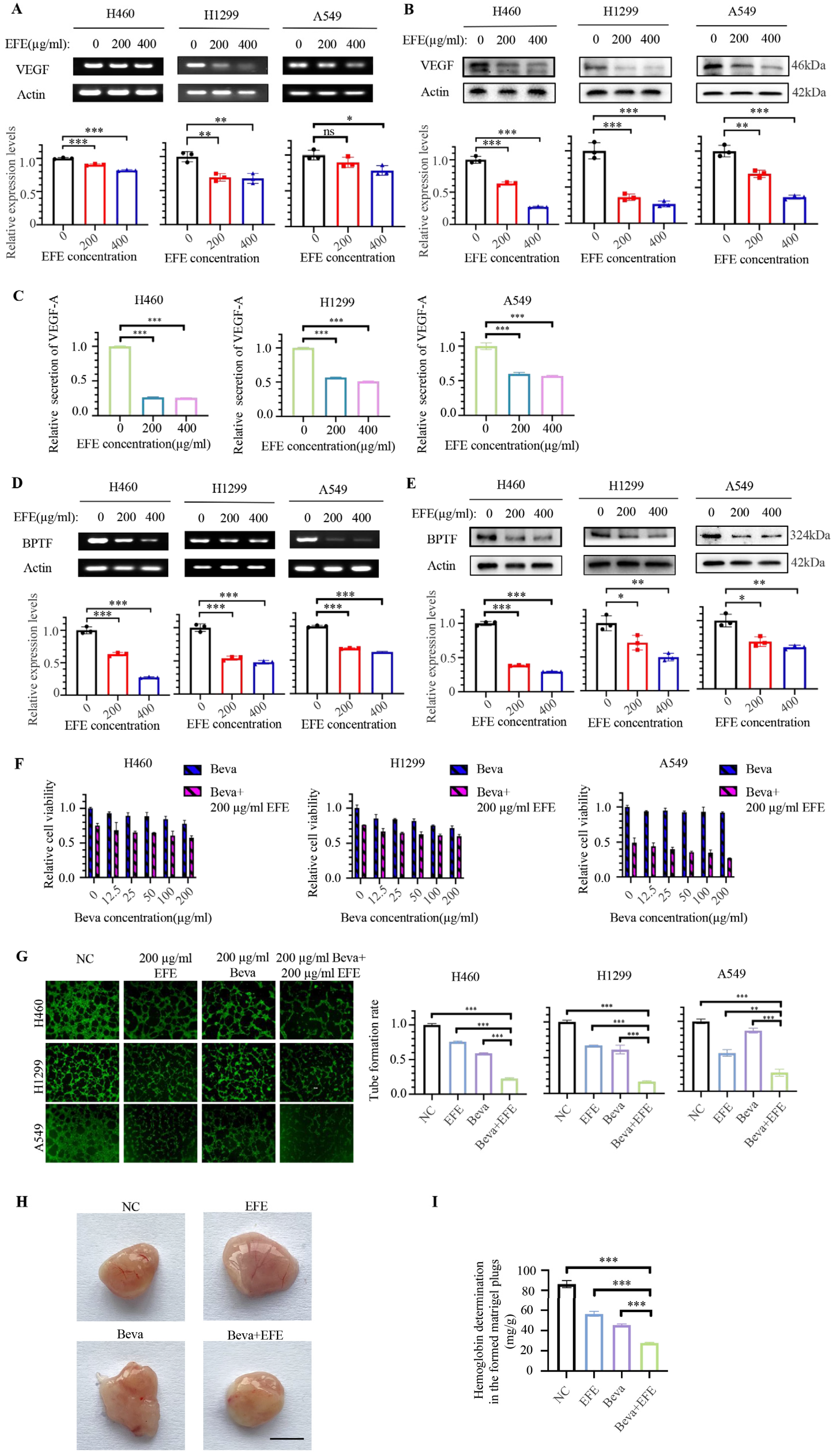
3.2. Lumbrokinase Synergizes with Bevacizumab in Anti-NSCLC by Targeting VEGF to Inhibit Angiogenesis
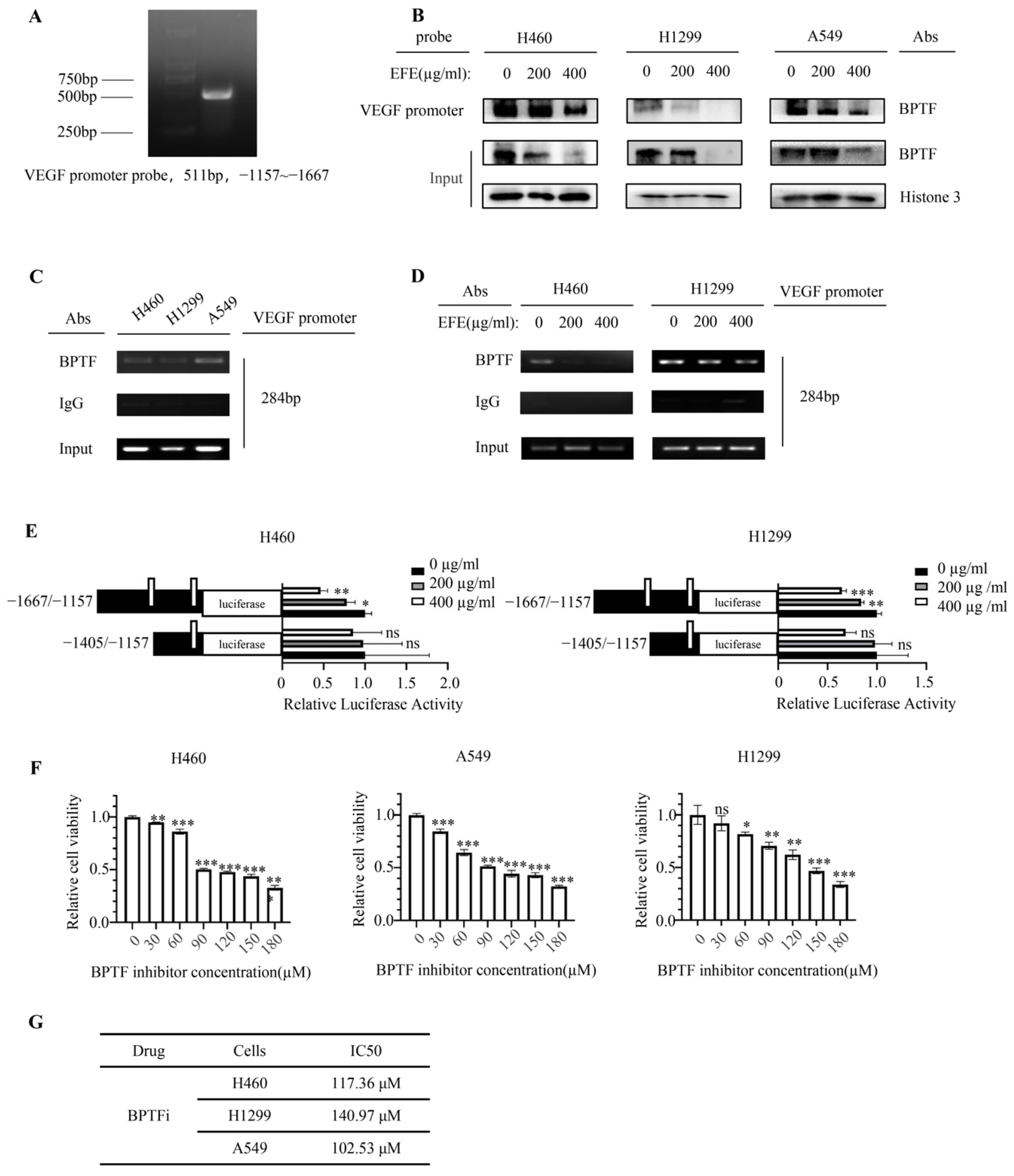
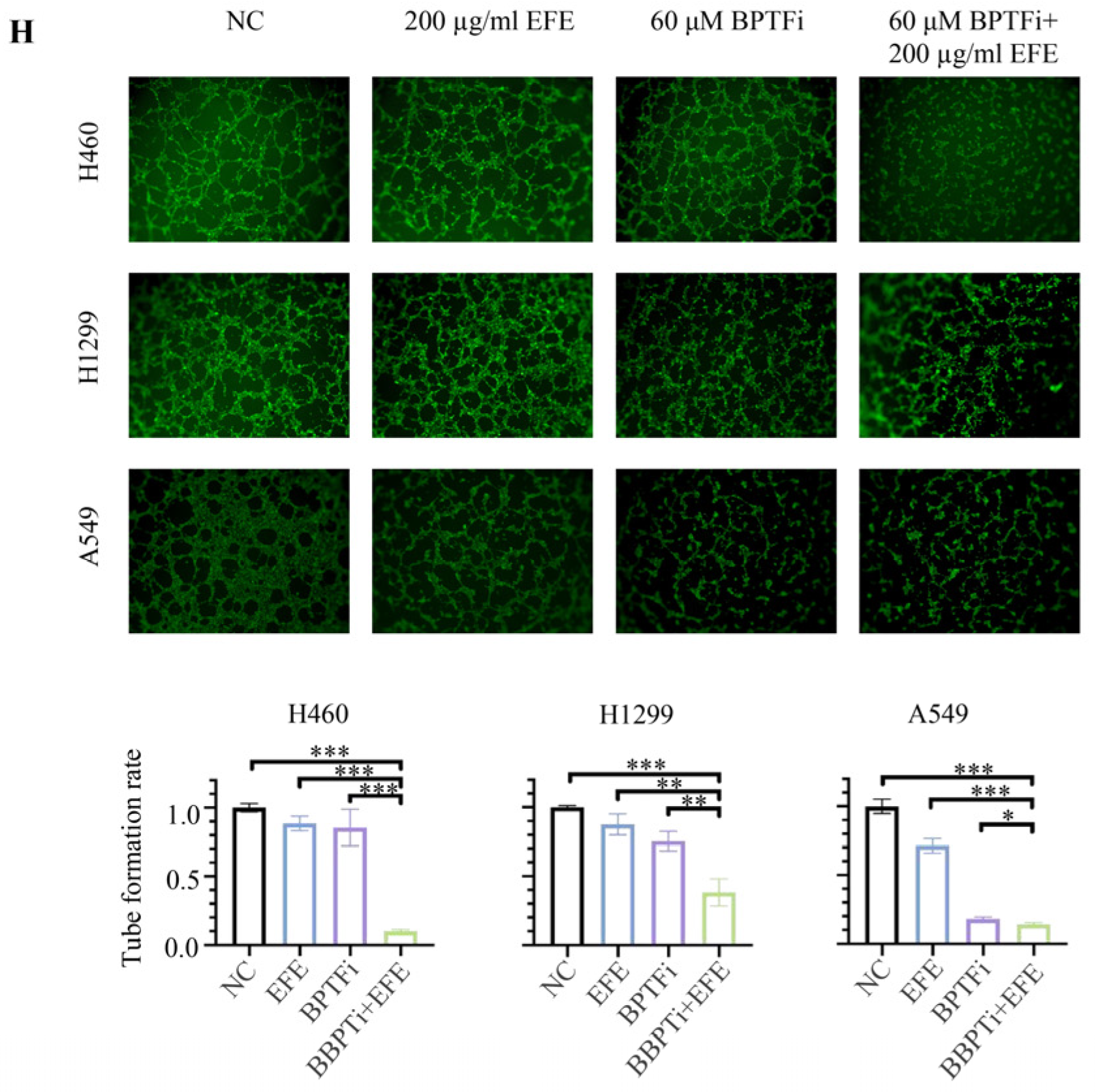
3.3. Lumbrokinase Inhibits VEGF Expression in NSCLC Cells by Alleviating the Binding of BPTF at VEGF Promoter
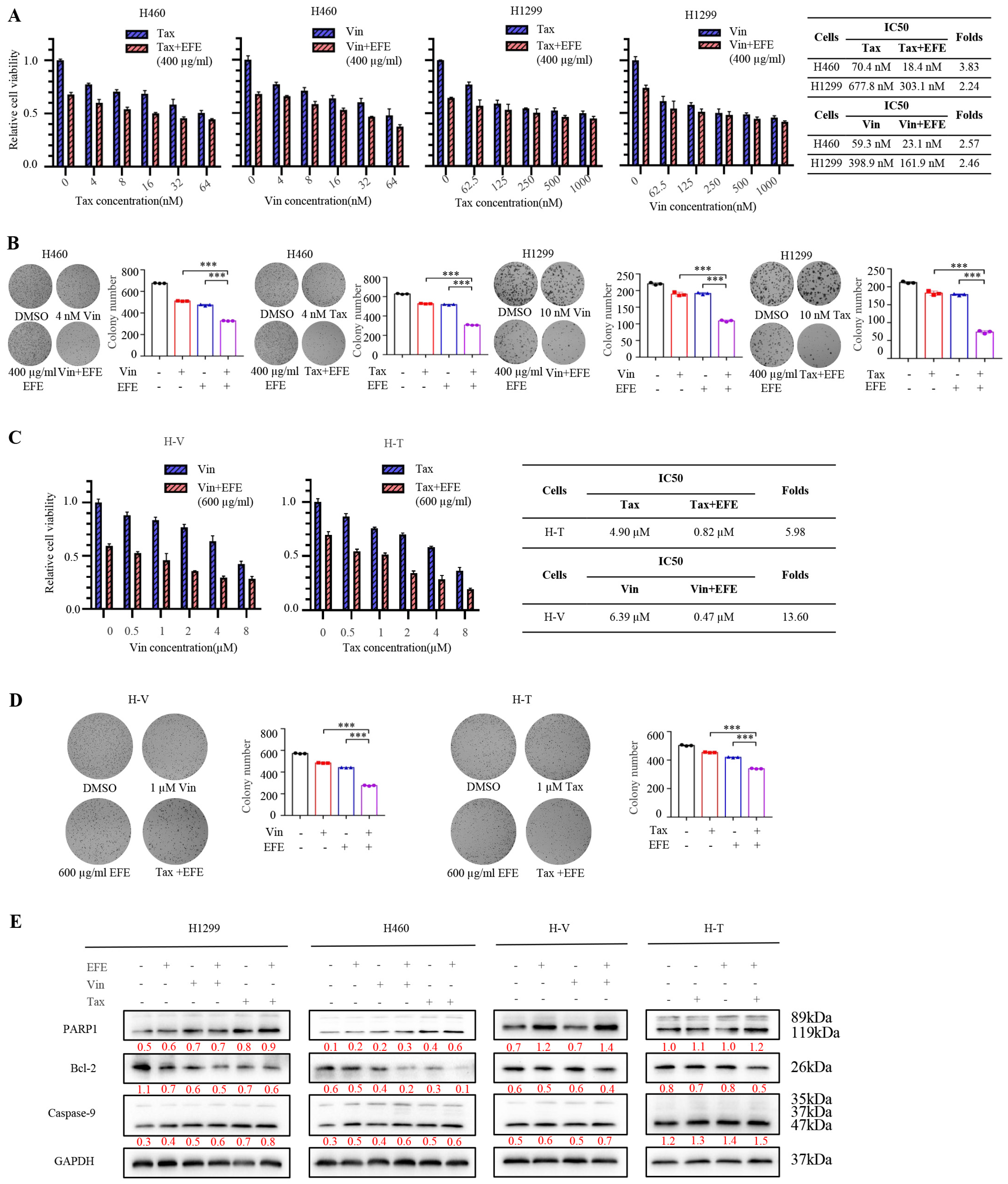
3.4. Lumbrokinase Sensitizes NSCLC Cells to Chemotherapeutics
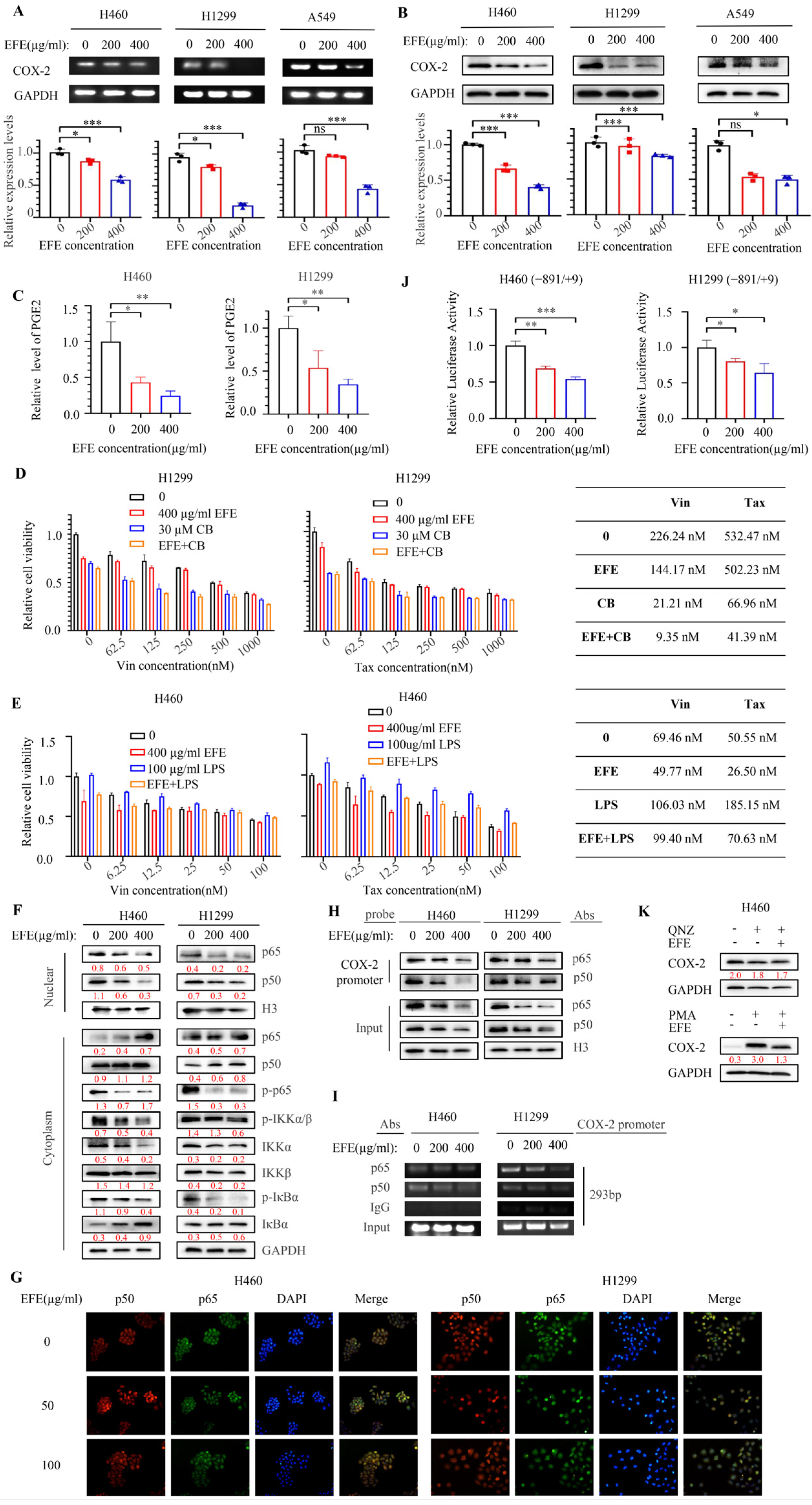
3.5. Lumbrokinase Sensitizes NSCLC Cells to Chemotherapeutics by Targeting NF-κB/COX-2 Signaling
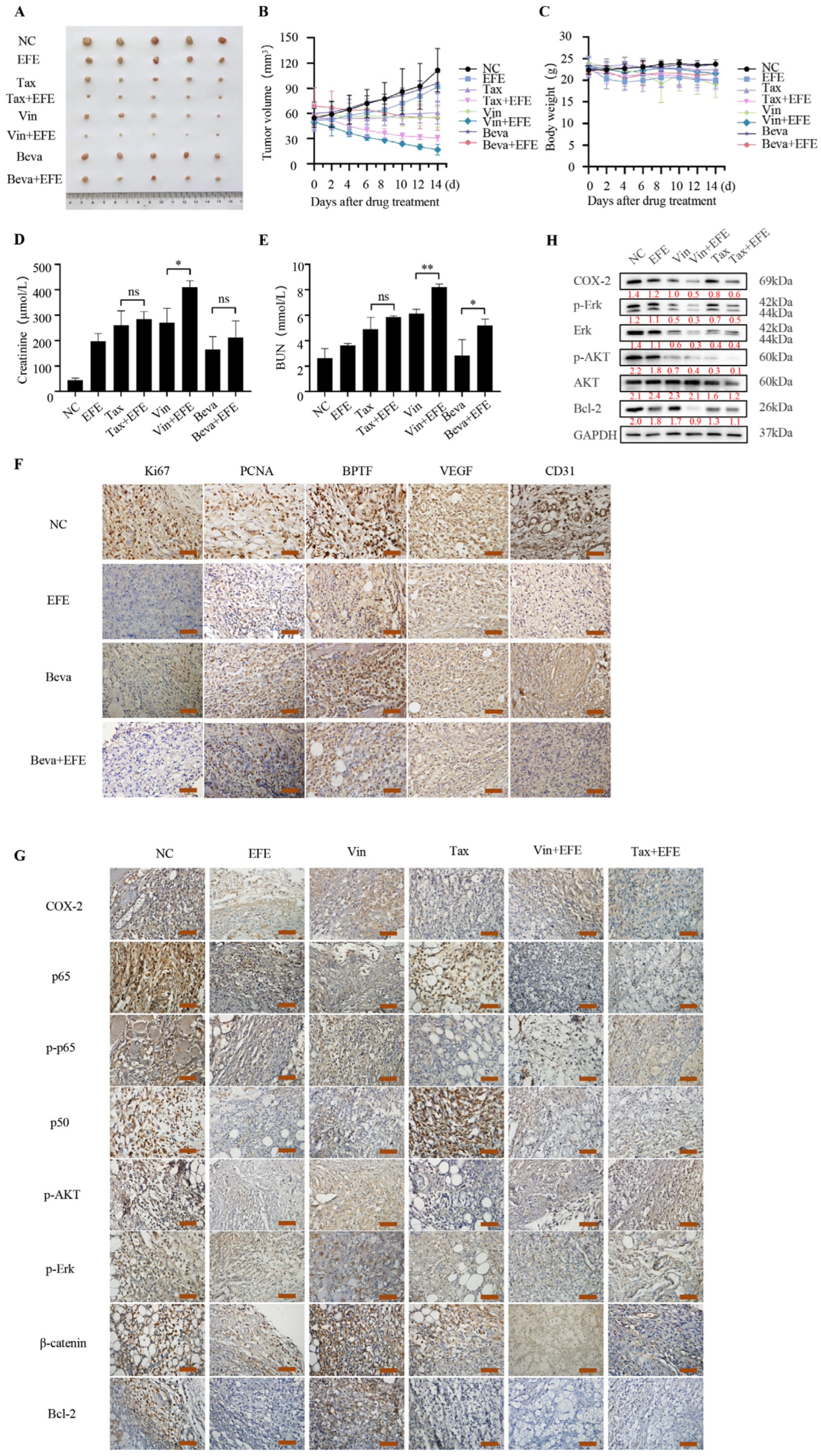
3.6. Lumbrokinase Inhibits NSCLC Survival In Vivo via Combination with Bevacizumab or Chemotherapeutics
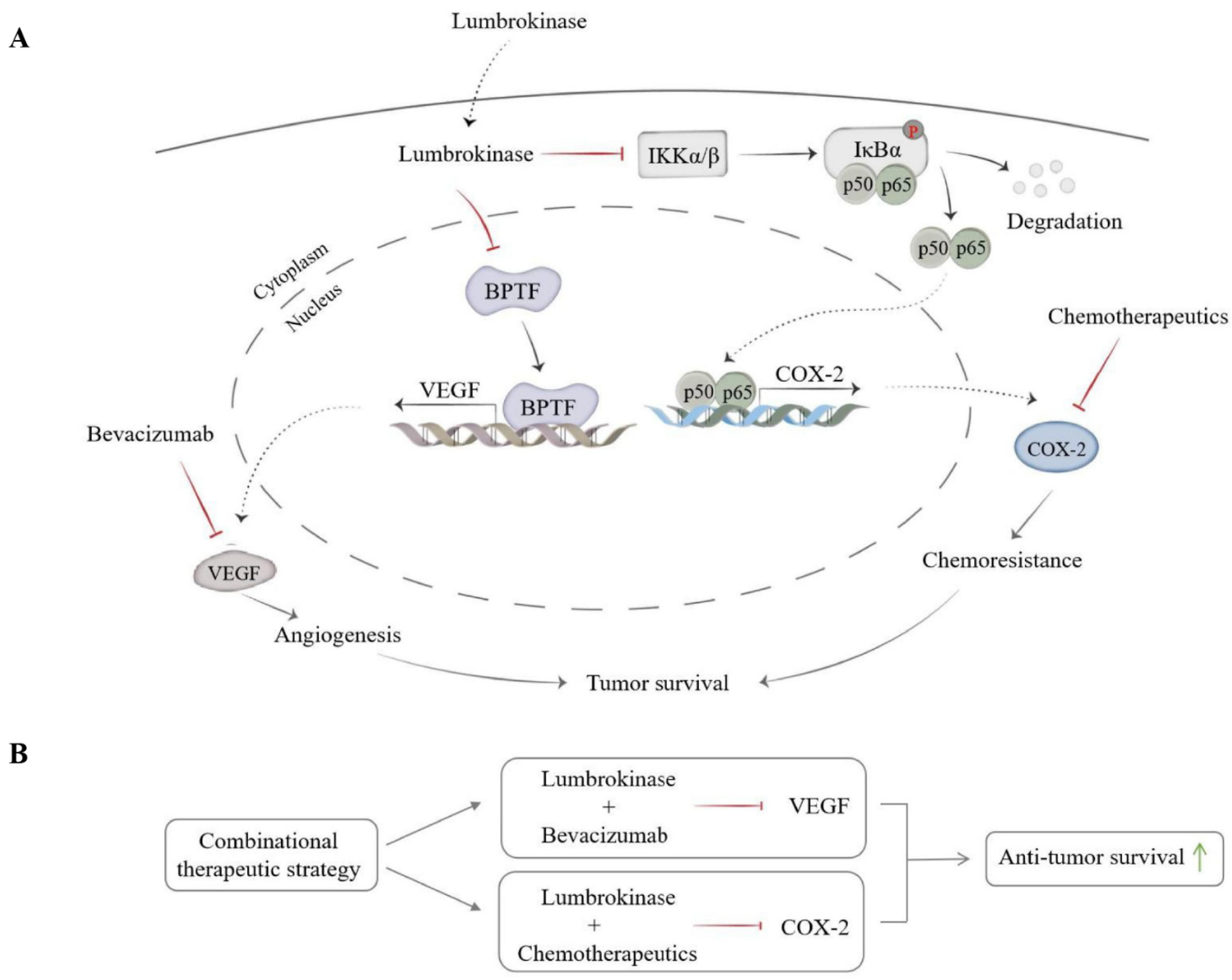
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Rahib, L.; Wehner, M.R.; Matrisian, L.M.; Nead, K.T. Estimated Projection of US Cancer Incidence and Death to 2040. JAMA Netw. Open 2021, 4, e214708. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ma, Q.; Yao, R.; Liu, J. Current status and development of anti-PD-1/PD-L1 immunotherapy for lung cancer. Int. Immunopharmacol. 2019, 79, 106088. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.L.; Pernemalm, M.; Crosbie, P.A.; Whetton, A.D. The role of the tumor-microenvironment in lung cancer-metastasis and its relationship to potential therapeutic targets. Cancer Treat. Rev. 2014, 40, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.; Kim, S.Y.; Cheng, H. Update 2020: Management of Non-Small Cell Lung Cancer. Lung 2020, 198, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Sankar, K.; Gadgeel, S.M.; Qin, A. Molecular therapeutic targets in non-small cell lung cancer. Expert Rev. Anticancer. Ther. 2020, 20, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.M.; Thomas, S.M.; Sartoski, S.; Scott, J.G.; Sobilo, K.; Bewley, S.; Salvador, L.K.; Salazar-Abshire, M. Strategies to Mitigate Chemotherapy and Radiation Toxicities That Affect Eating. Nutrients 2021, 13, 4397. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Chen, Y.; Jiang, X.; Peng, M.; Liu, Y.; Mo, Y.; Ren, D.; Hua, Y.; Yu, B.; Zhou, Y.; et al. Mechanisms of vasculogenic mimicry in hypoxic tumor microenvironments. Mol. Cancer 2021, 20, 7. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, H.; Baluk, P.; Morikawa, S.; McLean, J.W.; Thurston, G.; Roberge, S.; Jain, R.K.; McDonald, D.M. Openings between Defective Endothelial Cells Explain Tumor Vessel Leakiness. Am. J. Pathol. 2000, 156, 1363–1380. [Google Scholar] [CrossRef]
- Chen, L.; Xie, X.; Wang, T.; Xu, L.; Zhai, Z.; Wu, H.; Deng, L.; Lu, Q.; Chen, Z.; Yang, X.; et al. ARL13B promotes angiogenesis and glioma growth by activating VEGFA-VEGFR2 signaling. Neuro-Oncology 2023, 25, 871–885. [Google Scholar] [CrossRef]
- Liu, Z.-L.; Chen, H.-H.; Zheng, L.-L.; Sun, L.-P.; Shi, L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct. Target. Ther. 2023, 8, 198. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Yuan, M.; Bu, H.; Jin, C. Antiangiogenic Strategies in Epithelial Ovarian Cancer: Mechanism, Resistance, and Combination Therapy. J. Oncol. 2022, 2022, 4880355. [Google Scholar] [CrossRef] [PubMed]
- Melincovici, C.S.; Boca, A.B.; Uman, S.; Mrginean, M.; Mihu, C.M.; Istrate, M.; Moldovan, I.M.; Roman, A.L.; Mihu, C.M. Vascular endothelial growth factor (VEGF)—Key factor in normal and pathological angiogenesis. Rom. J. Morphol. Embryol. 2018, 59, 455–467. [Google Scholar] [PubMed]
- Dvorak, H.F. Vascular Permeability Factor/Vascular Endothelial Growth Factor: A Critical Cytokine in Tumor Angiogenesis and a Potential Target for Diagnosis and Therapy. J. Clin. Oncol. 2002, 20, 4368–4380. [Google Scholar] [CrossRef]
- Villaruz, L.C.; Socinski, M.A. The Role of Anti-angiogenesis in Non-small-cell Lung Cancer: An Update. Curr. Oncol. Rep. 2015, 17, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Willett, C.G.; Boucher, Y.; Di Tomaso, E.; Duda, D.G.; Munn, L.L.; Tong, R.T.; Chung, D.C.; Sahani, D.V.; Kalva, S.P.; Kozin, S.V. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat. Med. 2004, 10, 145. [Google Scholar] [CrossRef] [PubMed]
- Mody, K.; Baldeo, C.; Bekaii-Saab, T. Antiangiogenic Therapy in Colorectal Cancer. Cancer J. 2018, 24, 165–170. [Google Scholar] [CrossRef]
- Le, X.; Nilsson, M.; Goldman, J.; Reck, M.; Nakagawa, K.; Kato, T.; Ares, L.P.; Frimodt-Moller, B.; Wolff, K.; Visseren-Grul, C.; et al. Dual EGFR-VEGF Pathway Inhibition: A Promising Strategy for Patients with EGFR-Mutant NSCLC. J. Thorac. Oncol. 2021, 16, 205–215. [Google Scholar] [CrossRef]
- Gabriella, L.; Nunzia, C.; Melania, O.; Martina, C.; Carla, M.; Vincenzo, B.; Roberto, A.; Mario, S.; Ferdinando, N.; Carmelina, D.A. Anti-angiogenic Therapy in Cancer: Downsides and New Pivots for Precision Medicine. Front. Pharmacol. 2016, 7, 519. [Google Scholar]
- Singh, T.; Hassanabad, M.F.; Hassanabad, A.F. Non-small cell lung cancer: Emerging molecular targeted and immunotherapeutic agents. Biochim. Biophys. Acta (BBA)—Rev. Cancer 2021, 1876, 188636. [Google Scholar] [CrossRef]
- Kamba, T.; Mcdonald, D.M. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br. J. Cancer 2007, 2007, 1788–1795. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Hanna, N. Advances in systemic therapy for non-small cell lung cancer. BMJ 2021, 2021, n2363. [Google Scholar] [CrossRef] [PubMed]
- Uprety, D.; Mandrekar, S.J.; Wigle, D.; Roden, A.C.; Adjei, A.A. Neoadjuvant Immunotherapy for NSCLC: Current Concepts and Future Approaches. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2020, 15, 1281–1297. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Humeau, J.; Buqué, A.; Zitvogel, L.; Kroemer, G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2020, 17, 725–741. [Google Scholar] [CrossRef] [PubMed]
- Sumi, H.; Nakajima, N.; Mihara, H. A very stable and potent fibrinolytic enzyme found in earthworm Lumbricus rubellus autolysate. Comp. Biochem. Physiol. Part B Comp. Biochem. 1993, 106, 763–766. [Google Scholar] [CrossRef]
- Chen, H.; Takahashi, S.; Imamura, M.; Okutani, E.; Zhang, Z.-G.; Chayama, K.; Chen, B.-A. Earthworm fibrinolytic enzyme: Anti-tumor activity on human hepatoma cells in vitro and in vivo. Chin. Med. J. 2007, 120, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, C.; Li, M.; Gui, L.; Zhang, J.; Chang, W. Purification, characterization and crystallization of a group of earthworm fibrinolytic enzymes from Eisenia fetida. Biotechnol. Lett. 2003, 25, 1105–1109. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.G.; Hua, L.I.; William, X.U.; Luo, J.; Rui-An, X.U. An Overview of the Fibrinolytic Enzyme from Earthworm. Chin. J. Nat. Med. 2010, 8, 301–308. [Google Scholar] [CrossRef]
- Stephani, L.; Rahayu, P.; Retnoningrum, D.; Suhartono, M.T.; Rachmawati, H.; Tjandrawinata, R.R. Purification and proteomic analysis of potent fibrinolytic enzymes extracted from Lumbricus rubellus. Proteome Sci. 2023, 2023, 8. [Google Scholar] [CrossRef]
- Kacimi, S.E.O.; Moeinafshar, A.; Haghighi, S.S.; Saghazadeh, A.; Rezaei, N. Venous thromboembolism in cancer and cancer immunotherapy. Crit. Rev. Oncol./Hematol. 2022, 178, 103782. [Google Scholar] [CrossRef]
- Mukai, M.; Oka, T. Mechanism and management of cancer-associated thrombosis. J. Cardiol. 2018, 72, 89–93. [Google Scholar] [CrossRef]
- Varki, A. Trousseau’s syndrome: Multiple definitions and multiple mechanisms. Blood 2007, 110, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Yan, Y.; Tong, F.; Xu, L.; Zhu, J.; Xu, G.; Shen, R. Lumbrokinase/paclitaxel nanoparticle complex: Potential therapeutic applications in bladder cancer. Int. J. Nanomed. 2018, 13, 3625–3640. [Google Scholar] [CrossRef] [PubMed]
- Nagano, T.; Tachihara, M.; Nishimura, Y. Molecular Mechanisms and Targeted Therapies Including Immunotherapy for Non-Small Cell Lung Cancer. Curr. Cancer Drug Targets 2019, 19, 595–630. [Google Scholar] [CrossRef] [PubMed]
- Langenfeld, E.M.; Langenfeld, J. Bone morphogenetic protein-2 stimulates angiogenesis in developing tumors. Mol. Cancer Res. 2004, 2, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Lu, J.J.; Guo, W.; Yu, W.; Wu, T. BPTF promotes tumor growth and predicts poor prognosis in lung adenocarcinomas. Oncotarget 2015, 6, 33878–33892. [Google Scholar] [CrossRef]
- Wang, Y.H.; Chen, K.M.; Chiu, P.S.; Lai, S.C.; Huang, S.S. Lumbrokinase attenuates myocardial ischemia-reperfusion injury by inhibiting TLR4 signaling. J. Mol. Cell. Cardiol. 2016, 99, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Ooki, A.; Begum, A.; Marchionni, L.; Vandenbussche, C.J.; Mao, S.; Max, K.; Hoque, M.O. Arsenic promotes the COX2/PGE2–SOX2 axis to increase the malignant stemness properties of urothelial cells. Int. J. Cancer 2018, 143, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Lin, F.; Wang, Z.; Yang, L.; Meng, J.; Ou, Z.; Shao, Z.; Di, G.; Yang, G. CXCR2 promotes breast cancer metastasis and chemoresistance via suppression of AKT1 and activation of COX2. Cancer Lett. 2018, 412, 69–80. [Google Scholar] [CrossRef]
- Lawrence, T. The Nuclear Factor NF- B Pathway in Inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, 1651–1660. [Google Scholar] [CrossRef]
- Ulivi, V.; Giannoni, P.; Gentili, C.; Cancedda, R.; Descalzi, F. p38/NF-kB-dependent expression of COX-2 during differentiation and inflammatory response of chondrocytes. J. Cell. Biochem. 2010, 104, 1393–1406. [Google Scholar] [CrossRef] [PubMed]
- Shukla, V.; Kaushal, J.B.; Sankhwar, P.; Manohar, M.; Dwivedi, A. Inhibition of TPPP3 attenuates β-catenin/NF-κB/COX-2 signaling in endometrial stromal cells and impairs decidualization. J. Endocrinol. 2019, 240, 417–429. [Google Scholar] [CrossRef]
- Ghosh, S.; Javia, A.; Shetty, S.; Bardoliwala, D.; Maiti, K.; Banerjee, S.; Khopade, A.; Misra, A.; Sawant, K.; Bhowmick, S. Triple negative breast cancer and non-small cell lung cancer: Clinical challenges and nano-formulation approaches. J. Control. Release 2021, 337, 27–58. [Google Scholar] [CrossRef]
- Nguyen, Q.T.T.; Rhee, H.; Kim, M.; Lee, M.Y.; Lee, E.-J.; Chiarini, A. Lumbrokinase, a Fibrinolytic Enzyme, Prevents Intra-Abdominal Adhesion by Inhibiting the Migrative and Adhesive Activities of Fibroblast via Attenuation of the AP-1/ICAM-1 Signaling Pathway. BioMed. Res. Int. 2023, 2023, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Akazawa, S.-I.; Ikarashi, Y.; Yokoyama, K.; Shida, Y.; Ogasawara, W. Characterization of earthworm α-amylases for dietary supplement development and biomass utilization. Environ. Sci. Pollut. Res. 2019, 27, 33458–33463. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P. VEGF as a Key Mediator of Angiogenesis in Cancer. Oncology 2005, 69, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Bradley, M.; Philip, L.; Thomas, C.; Ira, J. Barriers to the Access of Bevacizumab in Patients with Solid Tumors and the Potential Impact of Biosimilars: A Physician Survey. Pharmaceuticals 2017, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.; Hurwitz, H.I.; Sandler, A.B.; Miles, D.; Coleman, R.L.; Deurloo, R.; Chinot, O.L. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat. Rev. 2020, 86, 102017. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Zhou, X.; Liang, C.; Li, X.; Ge, M.; Chen, Y.; Yin, J.; Zhu, J.; Zhong, C. Apatinib triggers autophagic and apoptotic cell death via VEGFR2/STAT3/PD-L1 and ROS/Nrf2/p62 signaling in lung cancer. J. Exp. Clin. Cancer Res. 2021, 40, 266. [Google Scholar] [CrossRef]
- Dar, A.A.; Mehdi, N.; Vladimir, B.; David, D.S.; Shahana, M.; Suresh, T.; Vera, S.; Schuyler, T.; Leong, S.P.L.; David, M. The Role of BPTF in Melanoma Progression and in Response to BRAF-Targeted Therapy. J. Natl. Cancer Inst. 2015, 23, 5. [Google Scholar] [CrossRef]
- Pan, Y.; Yuan, F.; Li, Y.; Wang, G.; Lin, Z.; Chen, L. Bromodomain PHD-finger transcription factor promotes glioma progression and indicates poor prognosis. Oncol. Rep. 2019, 41, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Zhang, M.; Huang, X.; Xu, L.; Tang, R.; Wang, H.; Han, S. Upregulation of bromodomain PHD finger transcription factor in ovarian cancer and its critical role for cancer cell proliferation and survival. Biochem. Cell Biol. 2021, 99, 304–312. [Google Scholar] [CrossRef]
- Shuai, X.; Longfei, L.; Xianzhou, L.; Jianwu, L.; Xiaojun, Z.; Min, F. The prognostic significance of bromodomain PHD-finger transcription factor in colorectal carcinoma and association with vimentin and E-cadherin. J. Cancer Res. Clin. Oncol. 2015, 141, 1465–1474. [Google Scholar]
- Frey, W.D.; Chaudhry, A.; Slepicka, P.F.; Ouellette, A.M.; Kirberger, S.E.; Pomerantz, W.C.K.; Hannon, G.J.; Dos Santos, C.O. BPTF Maintains Chromatin Accessibility and the Self-Renewal Capacity of Mammary Gland Stem Cells. Stem Cell Rep. 2017, 9, S2213671117301856. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zheng, F.; Li, Y.; Hao, J.; Tang, Z.; Tian, C.; Yang, Q.; Zhu, T.; Diao, C.; Zhang, C. BPTF promotes hepatocellular carcinoma growth by modulating hTERT signaling and cancer stem cell traits. Redox Biol. 2019, 20, 427–441. [Google Scholar] [CrossRef] [PubMed]
- Di Nardo, P.; Lisanti, C.; Garutti, M.; Buriolla, S.; Alberti, M.; Mazzeo, R.; Puglisi, F. Chemotherapy in patients with early breast cancer: Clinical overview and management of long-term side effects. Expert. Opin. Drug Saf. 2022, 21, 1341–1355. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Ozawa, H.; Imanishi, Y.; Sekimizu, M.; Watanabe, Y.; Ito, F.; Ikari, Y.; Nakahara, N.; Kameyama, K.; Ogawa, K. Cyclooxygenase-2 expression is associated with chemoresistance through cancer stemness property in hypopharyngeal carcinoma. Spandidos Publ. 2021, 22, 533. [Google Scholar] [CrossRef] [PubMed]
- Kurtova, A.V.; Xiao, J.; Mo, Q.; Pazhanisamy, S.; Krasnow, R.; Lerner, S.P.; Chen, F.; Roh, T.T.; Lay, E.; Ho, P.L.; et al. Blocking PGE2-induced tumour repopulation abrogates bladder cancer chemoresistance. Nature 2015, 517, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jiang, M.; Wang, L.; Yu, S. Combined chemotherapy with cyclooxygenase-2 (COX-2) inhibitors in treating human cancers: Recent advancement. Biomed. Pharmacother. 2020, 129, 110389. [Google Scholar] [CrossRef]
- Yang, C.X.; Xing, L.; Chang, X.; Zhou, T.J.; Bi, Y.Y.; Yu, Z.Q.; Zhang, Z.Q.; Jiang, H.L. Synergistic Platinum(II) Prodrug Nanoparticles for Enhanced Breast Cancer Therapy. Mol. Pharm. 2020, 17, 1300–1309. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hua, C.; Guo, Z.; Dai, M.; Zhou, J.; Ge, H.; Xue, G.; Xu, F.; Ru, L.; Lv, K.; Zhang, G.; et al. Lumbrokinase Extracted from Earthworms Synergizes with Bevacizumab and Chemotherapeutics in Treating Non-Small Cell Lung Cancer by Targeted Inactivation of BPTF/VEGF and NF-κB/COX-2 Signaling. Biomolecules 2024, 14, 741. https://doi.org/10.3390/biom14070741
Hua C, Guo Z, Dai M, Zhou J, Ge H, Xue G, Xu F, Ru L, Lv K, Zhang G, et al. Lumbrokinase Extracted from Earthworms Synergizes with Bevacizumab and Chemotherapeutics in Treating Non-Small Cell Lung Cancer by Targeted Inactivation of BPTF/VEGF and NF-κB/COX-2 Signaling. Biomolecules. 2024; 14(7):741. https://doi.org/10.3390/biom14070741
Chicago/Turabian StyleHua, Chunyu, Ziyue Guo, Meng Dai, Jie Zhou, Hanxiao Ge, Guoqing Xue, Fahui Xu, Liyuan Ru, Kuan Lv, Guohui Zhang, and et al. 2024. "Lumbrokinase Extracted from Earthworms Synergizes with Bevacizumab and Chemotherapeutics in Treating Non-Small Cell Lung Cancer by Targeted Inactivation of BPTF/VEGF and NF-κB/COX-2 Signaling" Biomolecules 14, no. 7: 741. https://doi.org/10.3390/biom14070741






