Evaluation of the Effect of β-Wrapin AS69 in a Mouse Model Based on Alpha-Synuclein Overexpression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Recombinant rAAV Production and Purification
2.3. Stereotactic Surgery and Tissue Processing
2.4. Cylinder Test
2.5. Immunofluorescence Staining
2.6. Quantification of TH-Positive Neurons, Analysis of TH-Positive Dendrites and Striatal TH-Positive Axon Terminals
2.7. Evaluation of αSyn Pathology
2.8. Analysis of Astrogliosis and Microgliosis
2.9. Statistical Analysis
3. Results
3.1. Overexpression of αSyn in Mouse SN Induced a Subtle Motor Deficit
3.2. rAAV-AS69 Did Not Prevent the Degeneration of TH-Positive Neurons
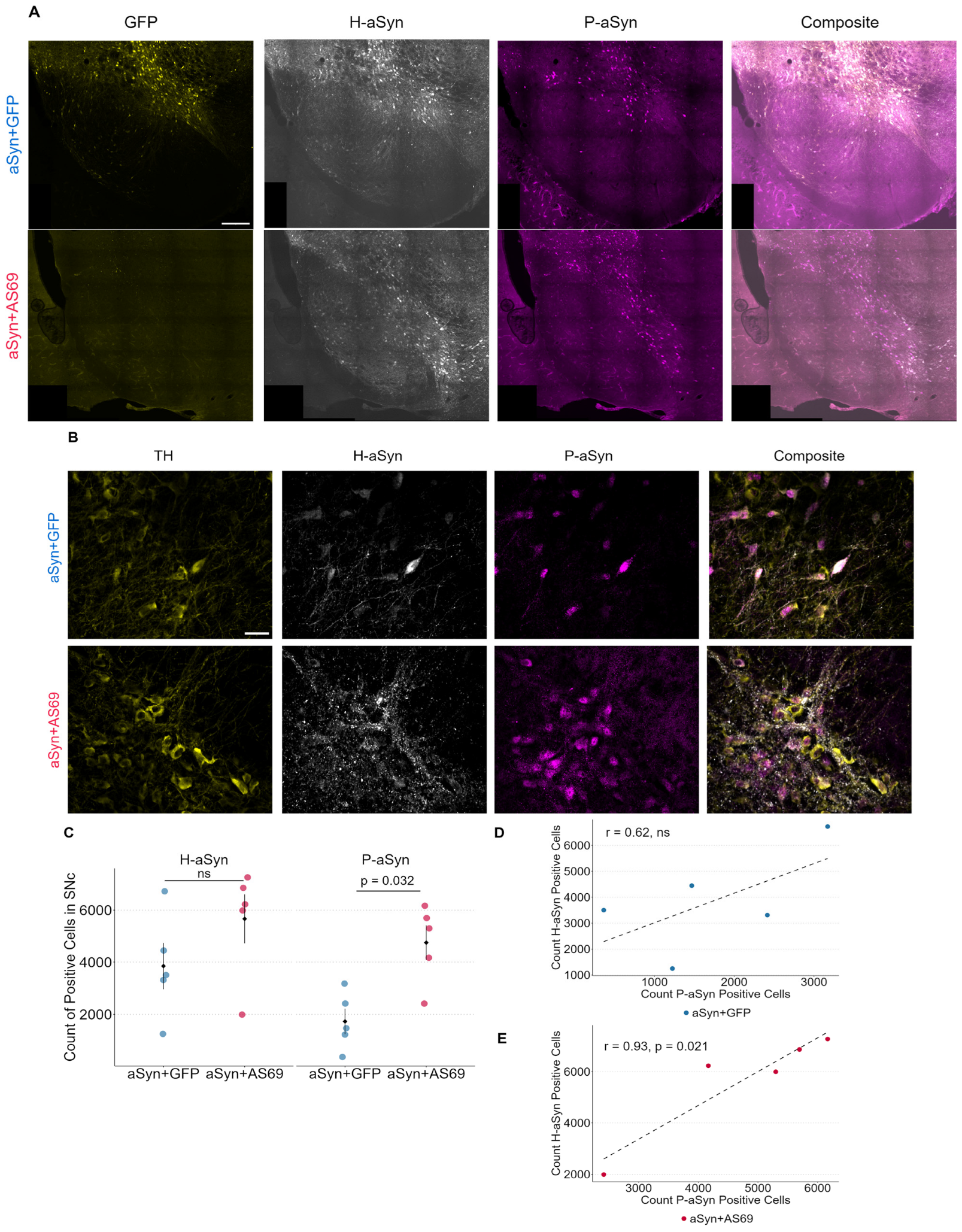
3.3. Transduction with rAAV-AS69 Is Associated with an Increase in αSyn Pathology
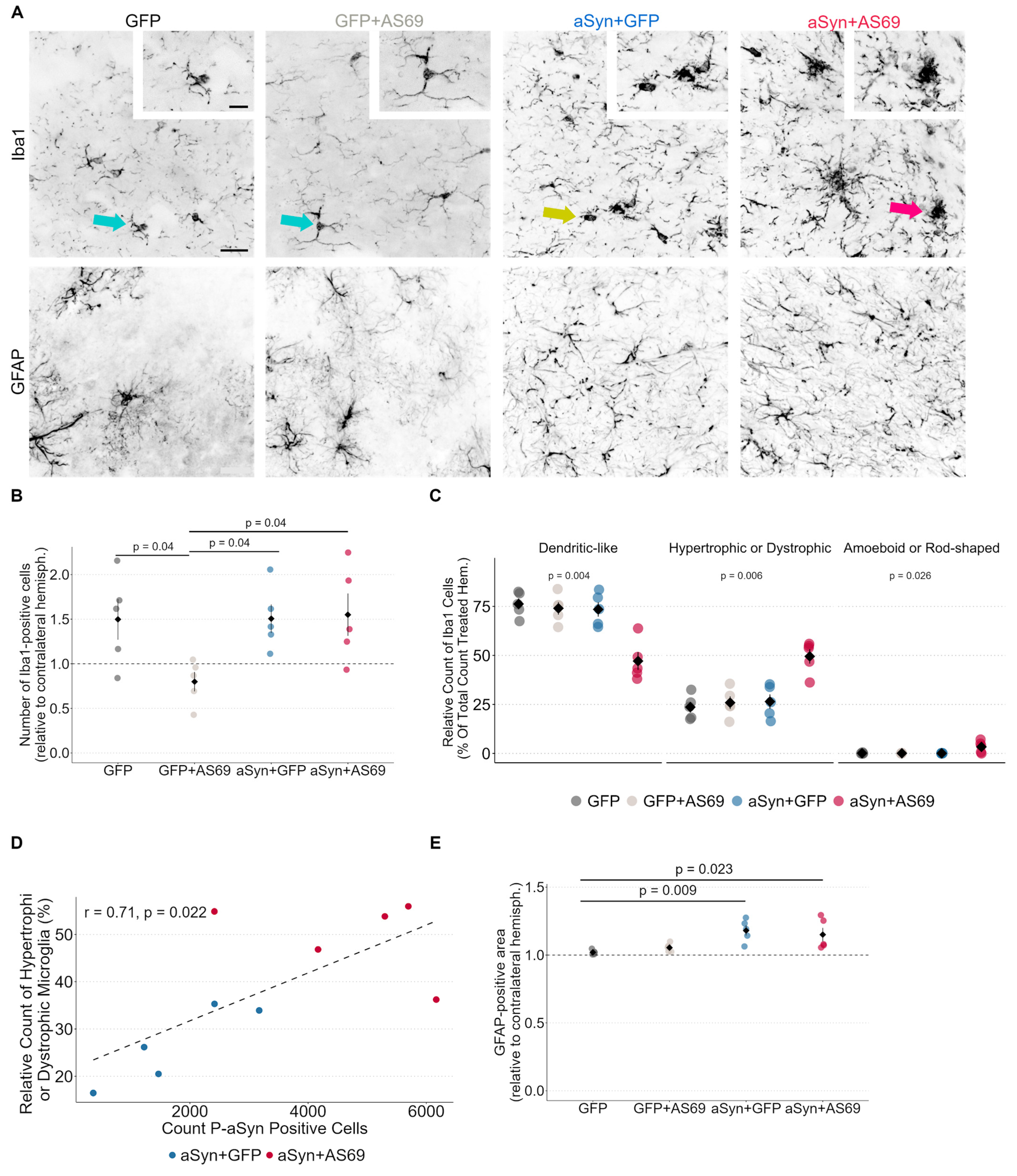
3.4. Microglia Are Activated in Mice Transduced with rAAV-αSyn + rAAV-AS69
Astrogliosis Is Not Attenuated by rAAV-AS69
4. Discussion
4.1. Degeneration of the Dopaminergic Neurons
4.2. Microglial Response to Transduction with rAAV-αSyn and rAAV-AS69
4.3. Transduction with rAAV-AS69 Did Not Reduce αSyn Pathology
4.4. Potential Increase in Synuclein Pathology Due to rAAV-AS69
4.5. Comparison of AS69 to Other αSyn Aggregation Inhibitors
4.6. AS69′s Structure and Stability
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Obeso, J.A.; Stamelou, M.; Goetz, C.G.; Poewe, W.; Lang, A.E.; Weintraub, D.; Burn, D.; Halliday, G.M.; Bezard, E.; Przedborski, S.; et al. Past, Present, and Future of Parkinson’s Disease: A Special Essay on the 200th Anniversary of the Shaking Palsy. Mov. Disord. 2017, 32, 1264–1310. [Google Scholar] [CrossRef] [PubMed]
- Rodger, A.T.; ALNasser, M.; Carter, W.G. Are Therapies That Target α-Synuclein Effective at Halting Parkinson’s Disease Progression? A Systematic Review. Int. J. Mol. Sci. 2023, 24, 11022. [Google Scholar] [CrossRef] [PubMed]
- Mirecka, E.A.; Shaykhalishahi, H.; Gauhar, A.; Akgül, Ş.; Lecher, J.; Willbold, D.; Stoldt, M.; Hoyer, W. Sequestration of a β-Hairpin for Control of α-Synuclein Aggregation. Angew. Chem. Int. Ed. 2014, 53, 4227–4230. [Google Scholar] [CrossRef] [PubMed]
- Agerschou, E.D.; Flagmeier, P.; Saridaki, T.; Galvagnion, C.; Komnig, D.; Heid, L.; Prasad, V.; Shaykhalishahi, H.; Willbold, D.; Dobson, C.M.; et al. An Engineered Monomer Binding-Protein for α-Synuclein Efficiently Inhibits the Proliferation of Amyloid Fibrils. eLife 2019, 8, e46112. [Google Scholar] [CrossRef] [PubMed]
- Szegő, É.M.; Boß, F.; Komnig, D.; Gärtner, C.; Höfs, L.; Shaykhalishahi, H.; Wördehoff, M.M.; Saridaki, T.; Schulz, J.B.; Hoyer, W.; et al. A β-Wrapin Targeting the N-Terminus of α-Synuclein Monomers Reduces Fibril-Induced Aggregation in Neurons. Front. Neurosci. 2021, 15, 696440. [Google Scholar] [CrossRef] [PubMed]
- Luk, K.C.; Kehm, V.; Carroll, J.; Zhang, B.; O’Brien, P.; Trojanowski, J.Q.; Lee, V.M.-Y. Pathological α-Synuclein Transmission Initiates Parkinson-like Neurodegeneration in Nontransgenic Mice. Science 2012, 338, 949–953. [Google Scholar] [CrossRef]
- Brundin, P.; Melki, R. Prying into the Prion Hypothesis for Parkinson’s Disease. J. Neurosci. Off. J. Soc. Neurosci. 2017, 37, 9808–9818. [Google Scholar] [CrossRef] [PubMed]
- Stopschinski, B.E.; Diamond, M.I. The Prion Model for Progression and Diversity of Neurodegenerative Diseases. Lancet Neurol. 2017, 16, 323–332. [Google Scholar] [CrossRef]
- Singleton, A.B.; Farrer, M.; Johnson, J.; Singleton, A.; Hague, S.; Kachergus, J.; Hulihan, M.; Peuralinna, T.; Dutra, A.; Nussbaum, R.; et al. α-Synuclein Locus Triplication Causes Parkinson’s Disease. Science 2003, 302, 841. [Google Scholar] [CrossRef]
- Maraganore, D.M. Collaborative Analysis of α-Synuclein Gene Promoter Variability and Parkinson Disease. JAMA 2006, 296, 661. [Google Scholar] [CrossRef]
- Nalls, W. Imputation of Sequence Variants for Identification of Genetic Risks for Parkinson’s Disease: A Meta-Analysis of Genome-Wide Association Studies. Lancet 2011, 377, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Van der Perren, A.; Toelen, J.; Carlon, M.; Van den Haute, C.; Coun, F.; Heeman, B.; Reumers, V.; Vandenberghe, L.H.; Wilson, J.M.; Debyser, Z.; et al. Efficient and Stable Transduction of Dopaminergic Neurons in Rat Substantia Nigra by rAAV 2/1, 2/2, 2/5, 2/6.2, 2/7, 2/8 and 2/9. Gene Ther. 2011, 18, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Stirling, D.R.; Swain-Bowden, M.J.; Lucas, A.M.; Carpenter, A.E.; Cimini, B.A.; Goodman, A. CellProfiler 4: Improvements in Speed, Utility and Usability. BMC Bioinform. 2021, 22, 433. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open Source Software for Digital Pathology Image Analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [PubMed]
- Lier, J.; Streit, W.J.; Bechmann, I. Beyond Activation: Characterizing Microglial Functional Phenotypes. Cells 2021, 10, 2236. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Kassambara, A. Ggpubr: “ggplot2” Based Publication Ready Plots. 2023. Available online: https://cran.r-project.org/web/packages/ggpubr/index.html (accessed on 24 April 2024).
- Ogle, D.H.; Doll, J.C.; Wheeler, A.P.; Dinno, A. FSA: Simple Fisheries Stock Assessment Methods. 2023. Available online: https://cran.r-project.org/web/packages/FSA/citation.html (accessed on 24 April 2024).
- Wilke, C.; Fox, S.J.; Bates, T.; Manalo, K.; Lang, B.; Barrett, M.; Stoiber, M.; Philipp, A.; Denney, B.; Hesselberth, J.; et al. Wilkelab/Cowplot: 1.1.1 2021. Available online: https://wilkelab.org/cowplot/authors.html#citation (accessed on 24 April 2024).
- Wei, T.; Simko, V. R Package “Corrplot”: Visualization of a Correlation Matrix. 2021. Available online: https://cran.r-project.org/web/packages/corrplot/citation.html (accessed on 24 April 2024).
- Kolde, R. Others Pheatmap: Pretty Heatmaps. R Package Version 2012, 1, 726. Available online: https://cran.r-project.org/web/packages/pheatmap/index.html (accessed on 24 April 2024).
- Oliveras-Salvá, M.; Van der Perren, A.; Casadei, N.; Stroobants, S.; Nuber, S.; D’Hooge, R.; Van den Haute, C.; Baekelandt, V. rAAV2/7 Vector-Mediated Overexpression of Alpha-Synuclein in Mouse Substantia Nigra Induces Protein Aggregation and Progressive Dose-Dependent Neurodegeneration. Mol. Neurodegener. 2013, 8, 44. [Google Scholar] [CrossRef]
- Mahul-Mellier, A.-L.; Burtscher, J.; Maharjan, N.; Weerens, L.; Croisier, M.; Kuttler, F.; Leleu, M.; Knott, G.W.; Lashuel, H.A. The Process of Lewy Body Formation, Rather than Simply α-Synuclein Fibrillization, Is One of the Major Drivers of Neurodegeneration. Proc. Natl. Acad. Sci. USA 2020, 117, 4971–4982. [Google Scholar] [CrossRef]
- Weston, L.J.; Stackhouse, T.L.; Spinelli, K.J.; Boutros, S.W.; Rose, E.P.; Osterberg, V.R.; Luk, K.C.; Raber, J.; Weissman, T.A.; Unni, V.K. Genetic Deletion of Polo-like Kinase 2 Reduces Alpha-Synuclein Serine-129 Phosphorylation in Presynaptic Terminals but Not Lewy Bodies. J. Biol. Chem. 2021, 296, 100273. [Google Scholar] [CrossRef]
- Fujiwara, H.; Hasegawa, M.; Dohmae, N.; Kawashima, A.; Masliah, E.; Goldberg, M.S.; Shen, J.; Takio, K.; Iwatsubo, T. α-Synuclein Is Phosphorylated in Synucleinopathy Lesions. Nat. Cell Biol. 2002, 4, 160–164. [Google Scholar] [CrossRef]
- Savage, J.C.; Carrier, M.; Tremblay, M.-È. Morphology of Microglia across Contexts of Health and Disease. In Microglia; Garaschuk, O., Verkhratsky, A., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2019; Volume 2034, pp. 13–26. ISBN 978-1-4939-9657-5. [Google Scholar]
- Szegö, E.M.; den Haute, C.V.; Höfs, L.; Baekelandt, V.; der Perren, A.V.; Falkenburger, B.H. Rab7 Reduces α-Synuclein Toxicity in Rats and Primary Neurons. Exp. Neurol. 2022, 347, 113900. [Google Scholar] [CrossRef]
- Szego, E.M.; Malz, L.; Bernhardt, N.; Rösen-Wolff, A.; Falkenburger, B.H.; Luksch, H. Constitutively Active STING Causes Neuroinflammation and Degeneration of Dopaminergic Neurons in Mice. eLife 2022, 11, e81943. [Google Scholar] [CrossRef]
- Thi Lai, T.; Kim, Y.E.; Nguyen, L.T.N.; Thi Nguyen, T.; Kwak, I.H.; Richter, F.; Kim, Y.J.; Ma, H. Microglial Inhibition Alleviates Alpha-Synuclein Propagation and Neurodegeneration in Parkinson’s Disease Mouse Model. NPJ Park. Dis. 2024, 10, 32. [Google Scholar] [CrossRef]
- Scheres, S.H.W.; Ryskeldi-Falcon, B.; Goedert, M. Molecular Pathology of Neurodegenerative Diseases by Cryo-EM of Amyloids. Nature 2023, 621, 701–710. [Google Scholar] [CrossRef]
- Konnova, E.A.; Swanberg, M. Animal Models of Parkinson’s Disease. In Parkinson’s Disease: Pathogenesis and Clinical Aspects; Stoker, T.B., Greenland, J.C., Eds.; John Van Geest Centre for Brain Repair, Department of Clinical Neurosciences, University of Cambridge: Cambridge, UK; Codon Publications: Singapore, 2018; pp. 83–106. ISBN 978-0-9944381-6-4. [Google Scholar]
- Peelaerts, W.; Bousset, L.; Van Der Perren, A.; Moskalyuk, A.; Pulizzi, R.; Giugliano, M.; Van Den Haute, C.; Melki, R.; Baekelandt, V. α-Synuclein Strains Cause Distinct Synucleinopathies after Local and Systemic Administration. Nature 2015, 522, 340–344. [Google Scholar] [CrossRef]
- Miller, D.W.; Hague, S.M.; Clarimon, J.; Baptista, M.; Gwinn-Hardy, K.; Cookson, M.R.; Singleton, A.B. α-Synuclein in Blood and Brain from Familial Parkinson Disease with SNCA Locus Triplication. Neurology 2004, 62, 1835–1838. [Google Scholar] [CrossRef]
- Van der Perren, A.; Van den Haute, C.; Baekelandt, V. Viral Vector-Based Models of Parkinson’s Disease. In Behavioral Neurobiology of Huntington’s Disease and Parkinson’s Disease; Nguyen, H.H.P., Cenci, M.A., Eds.; Current Topics in Behavioral Neurosciences; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2014; Volume 22, pp. 271–301. ISBN 978-3-662-46343-7. [Google Scholar]
- Karpinar, D.P.; Balija, M.B.G.; Kügler, S.; Opazo, F.; Rezaei-Ghaleh, N.; Wender, N.; Kim, H.-Y.; Taschenberger, G.; Falkenburger, B.H.; Heise, H.; et al. Pre-Fibrillar Alpha-Synuclein Variants with Impaired Beta-Structure Increase Neurotoxicity in Parkinson’s Disease Models. EMBO J. 2009, 28, 3256–3268. [Google Scholar] [CrossRef]
- Prots, I.; Grosch, J.; Brazdis, R.-M.; Simmnacher, K.; Veber, V.; Havlicek, S.; Hannappel, C.; Krach, F.; Krumbiegel, M.; Schütz, O.; et al. α-Synuclein Oligomers Induce Early Axonal Dysfunction in Human iPSC-Based Models of Synucleinopathies. Proc. Natl. Acad. Sci. USA 2018, 115, 7813–7818. [Google Scholar] [CrossRef] [PubMed]
- Rockenstein, E.; Nuber, S.; Overk, C.R.; Ubhi, K.; Mante, M.; Patrick, C.; Adame, A.; Trejo-Morales, M.; Gerez, J.; Picotti, P.; et al. Accumulation of Oligomer-Prone α-Synuclein Exacerbates Synaptic and Neuronal Degeneration in Vivo. Brain J. Neurol. 2014, 137, 1496–1513. [Google Scholar] [CrossRef] [PubMed]
- Masuda, M.; Suzuki, N.; Taniguchi, S.; Oikawa, T.; Nonaka, T.; Iwatsubo, T.; Hisanaga, S.; Goedert, M.; Hasegawa, M. Small Molecule Inhibitors of α-Synuclein Filament Assembly. Biochemistry 2006, 45, 6085–6094. [Google Scholar] [CrossRef]
- Smit, J.W.; Basile, P.; Prato, M.K.; Detalle, L.; Mathy, F.; Schmidt, A.; Lalla, M.; Germani, M.; Domange, C.; Biere, A.; et al. Phase 1/1b Studies of UCB0599, an Oral Inhibitor of α-Synuclein Misfolding, Including a Randomized Study in Parkinson’s Disease. Mov. Disord. 2022, 37, 2045–2056. [Google Scholar] [CrossRef]
- Levin, J.; Sing, N.; Melbourne, S.; Morgan, A.; Mariner, C.; Spillantini, M.G.; Wegrzynowicz, M.; Dalley, J.W.; Langer, S.; Ryazanov, S.; et al. Safety, Tolerability and Pharmacokinetics of the Oligomer Modulator Anle138b with Exposure Levels Sufficient for Therapeutic Efficacy in a Murine Parkinson Model: A Randomised, Double-Blind, Placebo-Controlled Phase 1a Trial. eBioMedicine 2022, 80, 104021. [Google Scholar] [CrossRef]
- Wagner, J.; Ryazanov, S.; Leonov, A.; Levin, J.; Shi, S.; Schmidt, F.; Prix, C.; Pan-Montojo, F.; Bertsch, U.; Mitteregger-Kretzschmar, G.; et al. Anle138b: A Novel Oligomer Modulator for Disease-Modifying Therapy of Neurodegenerative Diseases Such as Prion and Parkinson’s Disease. Acta Neuropathol. 2013, 125, 795–813. [Google Scholar] [CrossRef]
- Levin, J.; Schmidt, F.; Boehm, C.; Prix, C.; Bötzel, K.; Ryazanov, S.; Leonov, A.; Griesinger, C.; Giese, A. The Oligomer Modulator Anle138b Inhibits Disease Progression in a Parkinson Mouse Model Even with Treatment Started after Disease Onset. Acta Neuropathol. 2014, 127, 779–780. [Google Scholar] [CrossRef]
- Price, D.L.; Khan, A.; Angers, R.; Cardenas, A.; Prato, M.K.; Bani, M.; Bonhaus, D.W.; Citron, M.; Biere, A.-L. In Vivo Effects of the Alpha-Synuclein Misfolding Inhibitor Minzasolmin Supports Clinical Development in Parkinson’s Disease. NPJ Park. Dis. 2023, 9, 114. [Google Scholar] [CrossRef]
- Bechtel, T.J.; Weerapana, E. From Structure to Redox: The Diverse Functional Roles of Disulfides and Implications in Disease. Proteomics 2017, 17, 1600391. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Höfs, L.; Geißler-Lösch, D.; Wunderlich, K.M.; Szegö, E.M.; Van den Haute, C.; Baekelandt, V.; Hoyer, W.; Falkenburger, B.H. Evaluation of the Effect of β-Wrapin AS69 in a Mouse Model Based on Alpha-Synuclein Overexpression. Biomolecules 2024, 14, 756. https://doi.org/10.3390/biom14070756
Höfs L, Geißler-Lösch D, Wunderlich KM, Szegö EM, Van den Haute C, Baekelandt V, Hoyer W, Falkenburger BH. Evaluation of the Effect of β-Wrapin AS69 in a Mouse Model Based on Alpha-Synuclein Overexpression. Biomolecules. 2024; 14(7):756. https://doi.org/10.3390/biom14070756
Chicago/Turabian StyleHöfs, Lennart, David Geißler-Lösch, Kristof M. Wunderlich, Eva M. Szegö, Chris Van den Haute, Veerle Baekelandt, Wolfgang Hoyer, and Björn H. Falkenburger. 2024. "Evaluation of the Effect of β-Wrapin AS69 in a Mouse Model Based on Alpha-Synuclein Overexpression" Biomolecules 14, no. 7: 756. https://doi.org/10.3390/biom14070756
APA StyleHöfs, L., Geißler-Lösch, D., Wunderlich, K. M., Szegö, E. M., Van den Haute, C., Baekelandt, V., Hoyer, W., & Falkenburger, B. H. (2024). Evaluation of the Effect of β-Wrapin AS69 in a Mouse Model Based on Alpha-Synuclein Overexpression. Biomolecules, 14(7), 756. https://doi.org/10.3390/biom14070756







