Biochemical and Functional Profiling of Thioredoxin-Dependent Cytosolic GPX-like Proteins in Euglena gracilis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. RNA Extraction from Euglena
2.3. Cloning of Cytosolic EgGPXLs
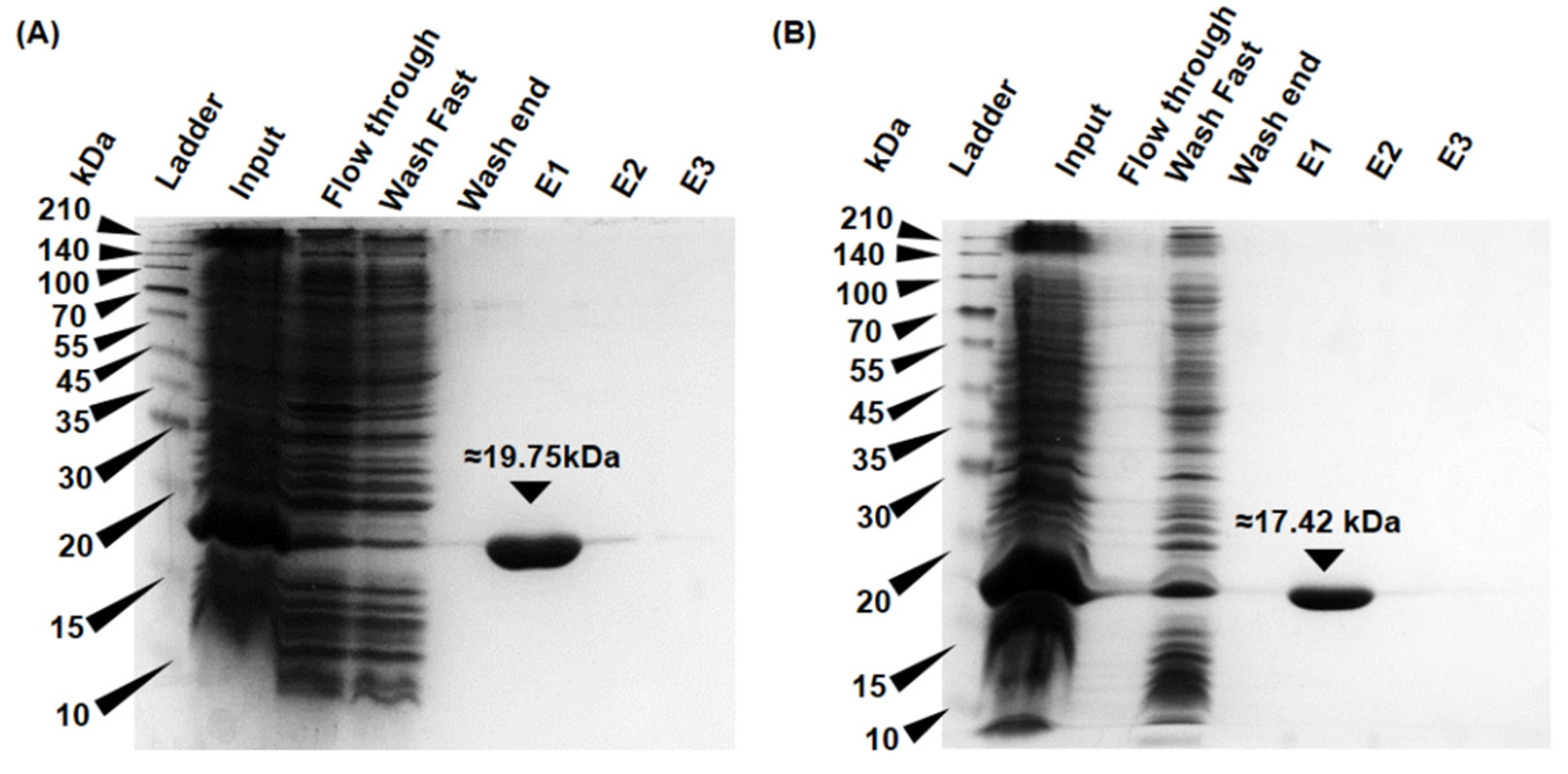
2.4. Expression and Purification of Recombinant Proteins
2.5. Detection of Recombinant EgGPXLs (rEgGPXLs) Activity
2.6. Preparation of Crude Extract of Euglena
2.7. Generation of Cytosolic EgGPXL Mutants through RNA Interference
2.8. Spot Assay
2.9. Intracellular H2O2 Detection
2.10. Glutathione and Chlorophyll Determination
2.11. Statistical Analysis
3. Results and Discussion
3.1. Molecular Identification and Structural Analysis of EgGPXLs
3.2. Characterization of Primary Structure of EgGPXLs
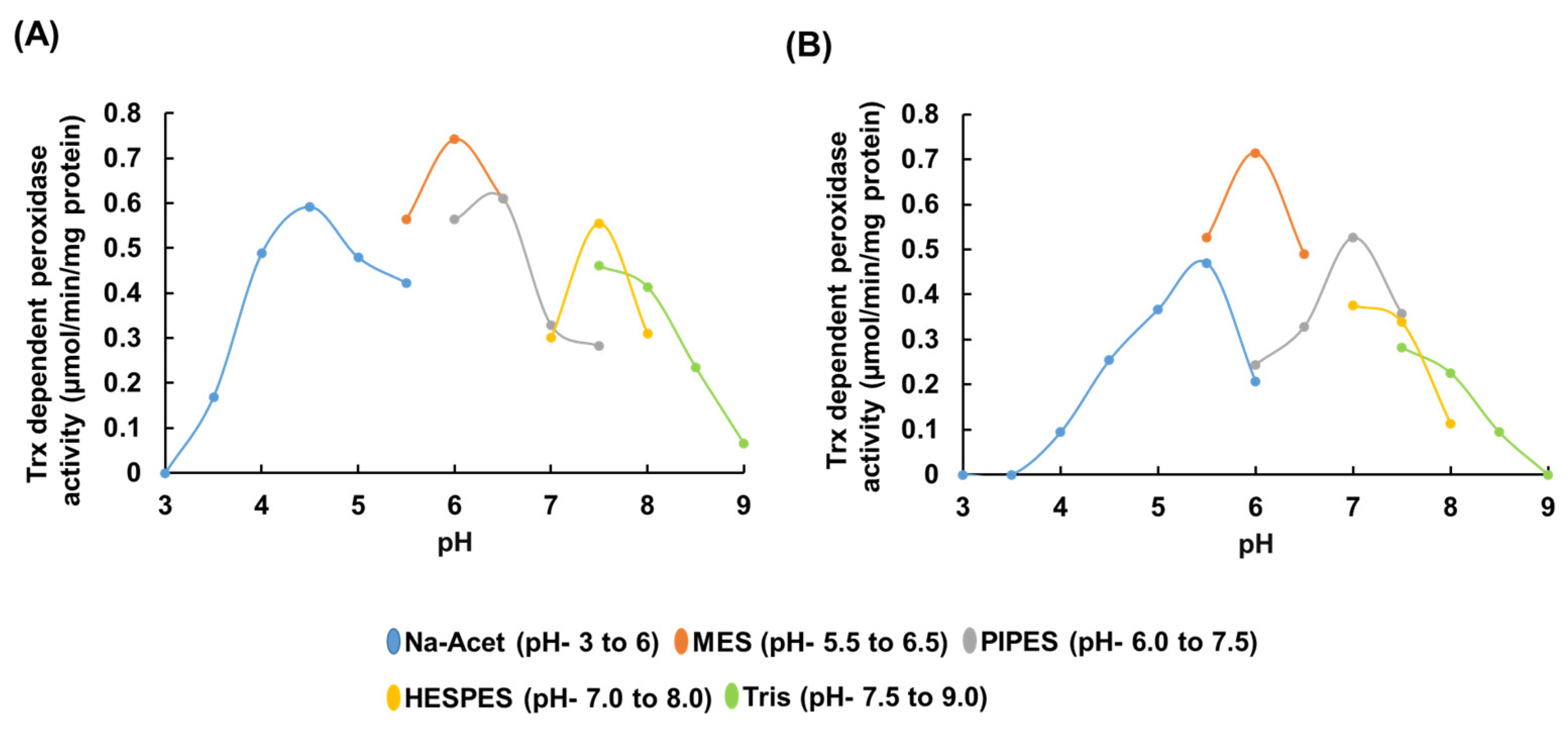
3.3. Substrates and Electron Donor Profiling of rEgGPXLs
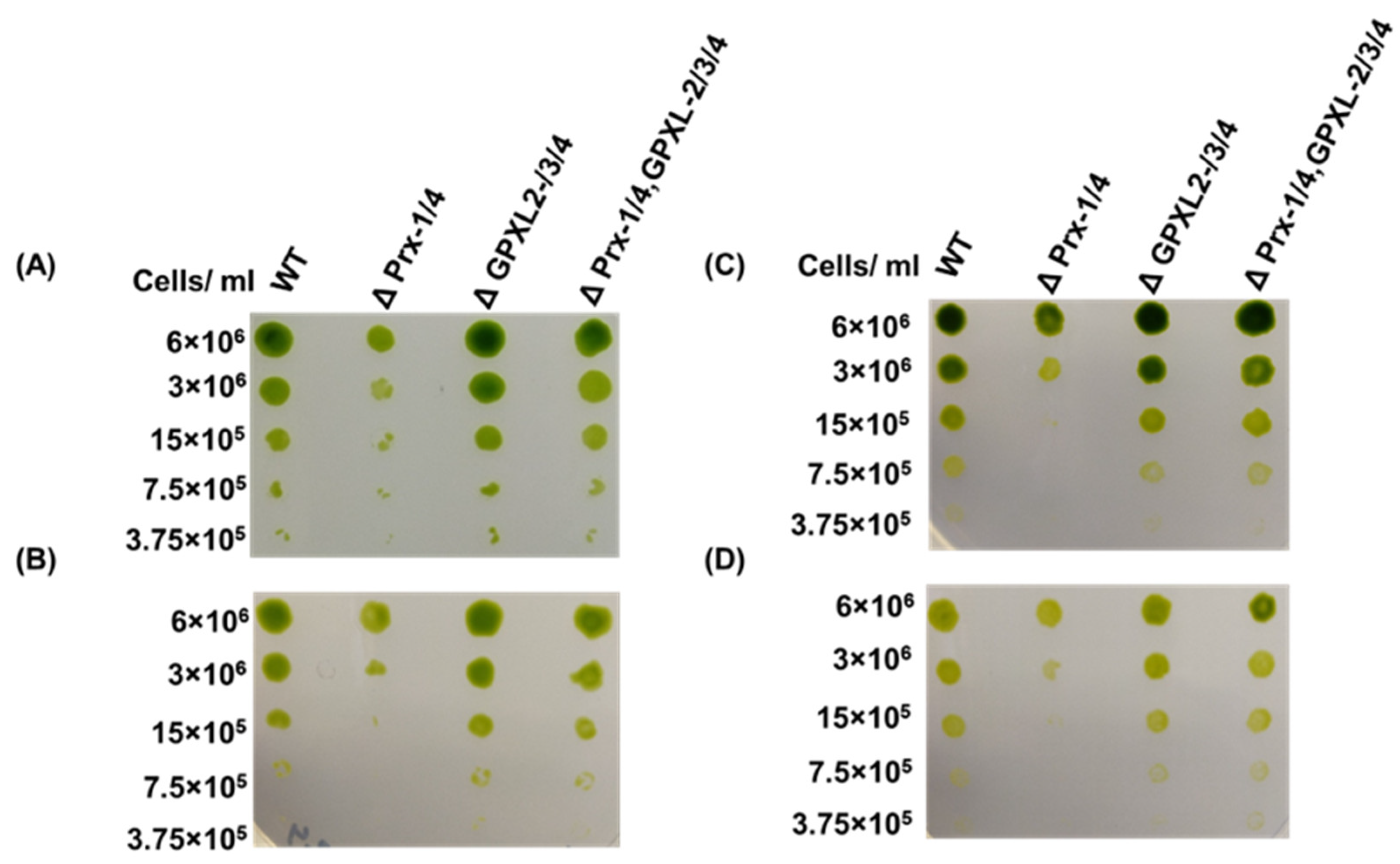
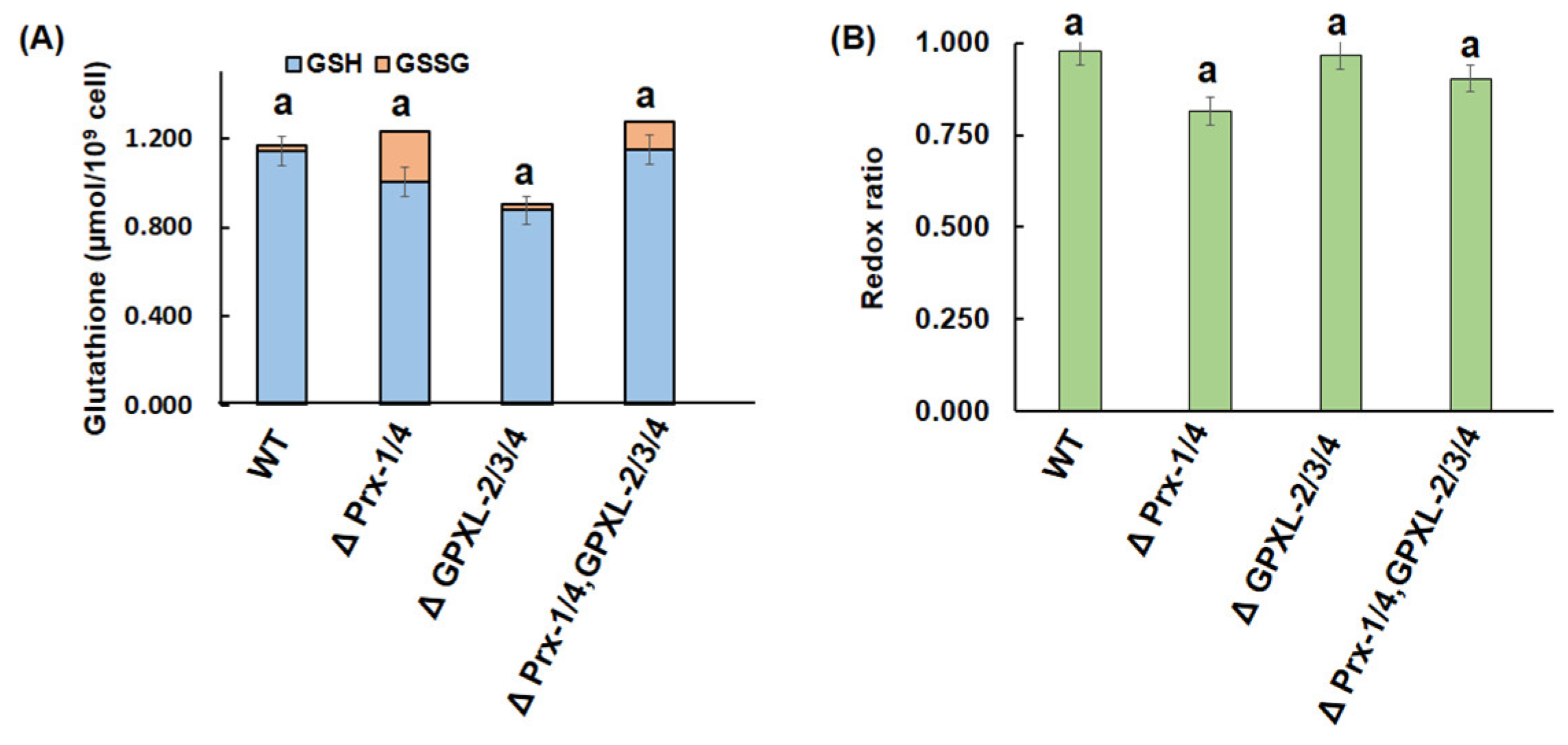
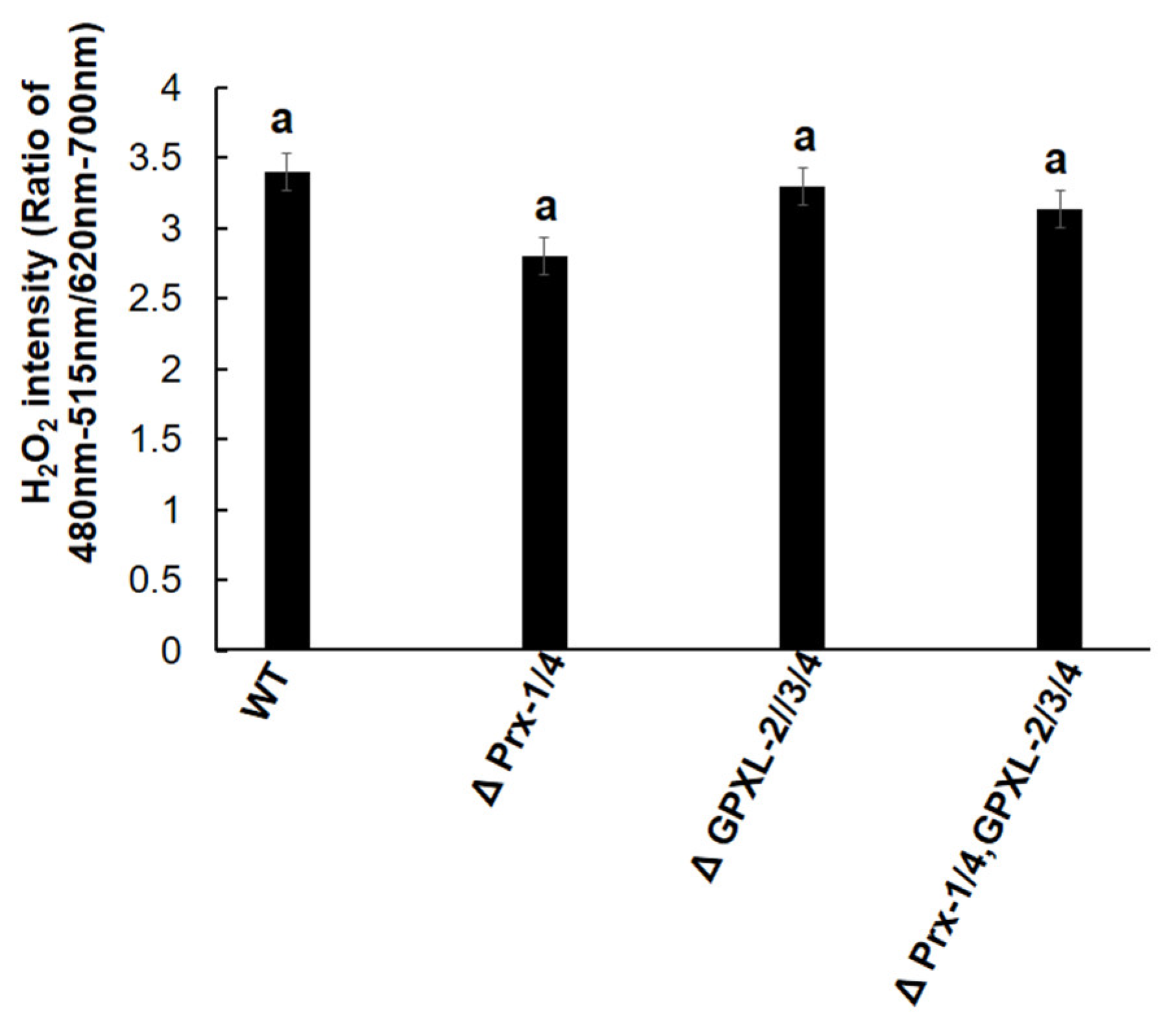
3.4. Impacts of GPXL Suppression on E. gracilis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive Oxygen Species Signalling in Plant Stress Responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Waszczak, C.; Akter, S.; Jacques, S.; Huang, J.; Messens, J.; Van Breusegem, F. Oxidative Post-Translational Modifications of Cysteine Residues in Plant Signal Transduction. J. Exp. Bot. 2015, 66, 2923–2934. [Google Scholar] [CrossRef]
- Medrano-Macías, J.; Flores-Gallegos, A.C.; Nava-Reyna, E.; Morales, I.; Tortella, G.; Solís-Gaona, S.; Benavides-Mendoza, A. Reactive Oxygen, Nitrogen, and Sulfur Species (RONSS) as a Metabolic Cluster for Signaling and Biostimulation of Plants: An Overview. Plants 2022, 23, 3203. [Google Scholar] [CrossRef]
- Manta, B.; Comini, M.; Medeiros, A.; Hugo, M.; Trujillo, M.; Radi, R. Trypanothione: A Unique Bis-Glutathionyl Derivative in Trypanosomatids. Biochim. Biophys. Acta 2013, 1830, 3199–3216. [Google Scholar] [CrossRef]
- Navrot, N.; Collin, V.; Gualberto, J.; Gelhaye, E.; Hirasawa, M.; Rey, P.; Knaff, D.B.; Issakidis, E.; Jacquot, J.P.; Rouhier, N. Plant Glutathione Peroxidases are Functional Peroxiredoxins Distributed in Several Subcellular Compartments and Regulated During Biotic and Abiotic Stresses. Plant Physiol. 2006, 142, 1364–1379. [Google Scholar] [CrossRef]
- Tripathy, B.C.; Oelmüller, R. Reactive oxygen species generation and signaling in plants. Plant Signal Behav. 2012, 7, 1621–1633. [Google Scholar] [CrossRef]
- Tian, R.; Geng, Y.; Yang, Y.; Seim, I.; Yang, G. Oxidative Stress Drives Divergent Evolution of The Glutathione Peroxidase (GPX) Gene Family in Mammals. Integr. Zool. 2021, 16, 696–711. [Google Scholar] [CrossRef]
- Espinosa-Diez, C.; Miguel, V.; Mennerich, D.; Kietzmann, T.; Sánchez-Pérez, P.; Cadenas, S.; Lamas, S. Antioxidant Responses and Cellular Adjustments to Oxidative Stress. Redox Biol. 2015, 6, 183–197. [Google Scholar] [CrossRef]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.I.; Neukermans, J.; Marquez-Garcia, B.E.; Queval, G.; Foyer, C.H. Glutathione in Plants: An Integrated Overview. Plant Cell Environ. 2012, 35, 454–484. [Google Scholar] [CrossRef]
- Bela, K.; Riyazuddin, R.; Csiszár, J. Plant Glutathione Peroxidases: Non-Heme Peroxidases with Large Functional Flexibility as a Core Component of ROS-Processing Mechanisms and Signalling. Antioxidants 2022, 21, 1624. [Google Scholar] [CrossRef]
- Dayer, R.; Fischer, B.B.; Eggen, R.I.; Lemaire, S.D. The peroxiredoxin and glutathione peroxidase families in Chlamydomonas reinhardtii. Genetics 2008, 179, 41–57. [Google Scholar] [CrossRef]
- Mariotti, M.; Salinas, G.; Gabaldón, T.; Gladyshev, V.N. Utilization of Selenocysteine in Early-Branching Fungal Phyla. Nat. Microbiol. 2019, 4, 759–765. [Google Scholar] [CrossRef]
- Bela, K.; Horváth, E.; Gallé, Á.; Szabados, L.; Tari, I.; Csiszár, J. Plant glutathione peroxidases: Emerging role of the antioxidant enzymes in plant development and stress responses. J. Plant Physiol. 2015, 176, 192–201. [Google Scholar] [CrossRef]
- Koh, S.C.; Didierjean, C.; Navrot, N.; Panjikar, S.; Mulliert, G.; Rouhier, N.; Jacquot, J.P.; Aubry, A.; Shawkataly, O.; Corbier, C. Crystal structures of a poplar thioredoxin peroxidase that exhibits the structure of glutathione peroxidases: Insights into redox-driven conformational changes. J. Mol. Biol. 2007, 13, 512–529. [Google Scholar] [CrossRef]
- Imai, H.; Nakagawa, Y. Biological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in mammalian cells. Free Radic. Biol. Med. 2003, 15, 145–169. [Google Scholar] [CrossRef]
- Miao, Y.; Lv, D.; Wang, P.; Wang, X.C.; Chen, J.; Miao, C.; Song, C.P. An Arabidopsis Glutathione Peroxidase Functions as Both a Redox Transducer and a Scavenger in Abscisic Acid and Drought Stress Responses. Plant Cell 2006, 18, 2749–2766. [Google Scholar] [CrossRef]
- Gaber, A.; Ogata, T.; Maruta, T.; Yoshimura, K.; Tamoi, M.; Shigeoka, S. The Involvement of Arabidopsis Glutathione Peroxidase 8 in the Suppression of Oxidative Damage in The Nucleus and Cytosol. Plant Cell Physiol. 2012, 53, 1596–1606. [Google Scholar] [CrossRef]
- Gaber, A.; Yoshimura, K.; Yamamoto, T.; Yabuta, Y.; Takeda, T.; Miyasaka, H.; Nakano, Y.; Shigeoka, S. Glutathione peroxidase-like protein of Synechocystis PCC 6803 confers tolerance to oxidative and environmental stresses in transgenic Arabidopsis. Physiol. Plant 2006, 128, 251–262. [Google Scholar] [CrossRef]
- Paiva, A.L.; Passaia, G.; Jardim-Messeder, D.; Nogueira, F.C.; Domont, G.B.; Margis-Pinheiro, M. The Mitochondrial Isoform Glutathione Peroxidase 3 (Osgpx3) is Involved in ABA Responses in Rice Plants. J. Proteom. 2021, 232, 104029. [Google Scholar] [CrossRef]
- He, Z.; Wu, M.; Tian, H.; Wang, L.; Hu, Y.; Han, F.; Zhou, J.; Wang, Y.; Zhou, L. Euglena’s Atypical Respiratory Chain Adapts to the Discoidal Cristae and Flexible Metabolism. Nat. Commun. 2024, 15, 1628. [Google Scholar] [CrossRef]
- Montrichard, F.; Le Guen, F.; Laval-Martin, D.L.; Davioud-Charvet, E. Evidence for The Co-Existence of Glutathione Reductase and Trypanothione Reductase in the Non-Trypanosomatid Euglenozoa: Euglena gracilis Z. FEBS Lett. 1999, 442, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Tamaki, S.; Maruta, T.; Shigeoka, S. Biochemistry and Physiology of Reactive Oxygen Species in Euglena. Adv. Exp. Med. Biol. 2017, 979, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Tajima, N.; Nishikawa, H.; Gao, Y.; Rapolu, M.; Shibata, H.; Sawa, Y.; Shigeoka, S. Euglena Gracilis Ascorbate Peroxidase forms an Intramolecular Dimeric Structure: Its Unique Molecular Characterization. Biochem. J. 2010, 426, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, S.; Maruta, T.; Sawa, Y.; Shigeoka, S.; Ishikawa, T. Identification and Functional Analysis of Peroxiredoxin Isoforms in Euglena gracilis. Biosci. Biotech. Biochem. 2014, 78, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, S.; Maruta, T.; Sawa, Y.; Shigeoka, S.; Ishikawa, T. Biochemical and Physiological Analyses of NADPH-Dependent Thioredoxin Reductase Isozymes in Euglena gracilis. Plant Sci. 2015, 236, 29–36. [Google Scholar] [CrossRef]
- Yoshida, Y.; Tomiyama, T.; Maruta, T.; Tomita, M.; Ishikawa, T.; Arakawa, K. De Novo Assembly and Comparative Transcriptome Analysis of Euglena gracilis in Response to Anaerobic Conditions. BMC Genom. 2016, 17, 182. [Google Scholar] [CrossRef] [PubMed]
- Raihan, M.T.; Tanaka, Y.; Ishikawa, T. Characterization of Chloroplastic Thioredoxin Dependent Glutathione Peroxidase like Protein in Euglena gracilis: Biochemical and Functional Perspectives. Biosci Biotechnol Biochem, 2024; in press. [Google Scholar] [CrossRef]
- Kruger, N.J. The Bradford Method for Protein Quantitation. In The Protein Protocols Handbook; Springer: Berlin/Heidelberg, Germany, 2009; pp. 17–24. [Google Scholar] [CrossRef]
- Kim, J.A.; Park, S.; Kim, K.; Rhee, S.G.; Kang, S.W. Activity Assay of Mammalian 2-Cys Peroxiredoxins Using Yeast Thioredoxin Reductase System. Anal. Biochem. 2005, 338, 216–223. [Google Scholar] [CrossRef]
- Ishikawa, T.; Nishikawa, H.; Gao, Y.; Sawa, Y.; Shibata, H.; Yabuta, Y.; Maruta, T.; Shigeoka, S. The Pathway via D-Galacturonate/L-Galactonate is Significant for Ascorbate Biosynthesis in Euglena gracilis: Identification and Functional Characterization of Aldonolactonase. J. Biol. Chem. 2008, 283, 31133–31141. [Google Scholar] [CrossRef]
- Noctor, G.; Mhamdi, A.; Foyer, C.H. Oxidative Stress and Antioxidative Systems: Recipes for Successful Data Collection and Interpretation. Plant Cell Envir. 2016, 39, 1140–1160. [Google Scholar] [CrossRef]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of Accurate Extinction Coefficients and Simultaneous Equations for Assaying Chlorophylls A and B Extracted with Four Different Solvents: Verification of the Concentration of Chlorophyll Standards by Atomic Absorption Spectroscopy. Biochim. Et Biophys. Acta 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Tessier, L.H.; Keller, M.; Chan, R.L.; Fournier, R.; Weil, J.H.; Imbault, P. Short Leader Sequences May Be Transferred From Small Rnas to Pre-Mature Mrnas by Trans-Splicing in Euglena. EMBO J. 1991, 10, 2621–2625. [Google Scholar] [CrossRef]
- Bitar, M.; Boroni, M.; Macedo, A.M.; Machado, C.R.; Franco, G.R. The spliced leader trans-splicing mechanism in different organisms: Molecular details and possible biological roles. Front. Genet. 2013, 11, 199. [Google Scholar] [CrossRef]
- Chen, M.; Li, K.; Li, H.; Song, C.P.; Miao, Y. The Glutathione Peroxidase Gene Family in Gossypium hirsutum: Genome-Wide Identification, Classification, Gene Expression and Functional Analysis. Sci. Rep. 2017, 7, 44743. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; An, Y.Q.; Zhang, H.; Meng, D.; Jin, Y.; Huo, H.; Yu, L.; Zhang, J. Identification of Glutathione Peroxidase Gene Family in Ricinus communis and Functional Characterization of Rcgpx4 in Cold Tolerance. Front. Plant Sci. 2021, 12, 707127. [Google Scholar] [CrossRef]
- Evans, R.M.; Krahn, N.; Murphy, B.J.; Lee, H.; Armstrong, F.A.; Söll, D. Selective cysteine-to-selenocysteine changes in a [NiFe]-hydrogenase confirm a special position for catalysis and oxygen tolerance. Proc. Natl. Acad. Sci. USA 2021, 118, e2100921118. [Google Scholar] [CrossRef]
- Chang, C.C.; Slesak, I.; Jordá, L.; Sotnikov, A.; Melzer, M.; Miszalski, Z.; Mullineaux, P.M.; Parker, J.E.; Karpinska, B.; Karpinski, S. Arabidopsis Chloroplastic Glutathione Peroxidases Play a Role in Cross Talk Between Photooxidative Stress and Immune Responses. Plant Physiol. 2009, 150, 670–683. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J.; Gonzalez-Gordo, S.; Rodríguez-Ruiz, M.; Muñoz-Vargas, M.A.; Palma, J.M. Thiol-Based Oxidative Posttranslational Modifications (OxiPTMs) of Plant Proteins. Plant Cell Physiol. 2022, 63, 889–900. [Google Scholar] [CrossRef] [PubMed]
- Begara-Morales, J.C.; Sánchez-Calvo, B.; Chaki, M.; Valderrama, R.; MataPérez, C.; Ló pez-Jaramillo, J.; Padilla, M.N.; Carreras, A.; Corpas, F.J.; Barroso, J.B. Dual Regulation of Cytosolic Ascorbate Peroxidase (APX) by Tyrosine Nitration And S-Nitrosylation. J. Exp. Bot. 2014, 65, 527–538. [Google Scholar] [CrossRef]
- Puyaubert, J.; Fares, A.; Rézé, N.; Peltier, J.B.; Baudouin, E. Identification of Endogenously S-Nitrosylated Proteins in Arabidopsis Plantlets: Effect of Cold Stress on Cysteine Nitrosylation Level. Plant Sci. 2014, 215–216, 150–156. [Google Scholar] [CrossRef]
- Yang, H.; Mu, J.; Chen, L.; Feng, J.; Hu, J.; Li, L.; Zhou, J.M.; Zuo, J. S-Nitrosylation Positively Regulates Ascorbate Peroxidase Activity during Plant Stress Responses. Plant Physiol. 2015, 167, 1604–1615. [Google Scholar] [CrossRef]
- Begara-Morales, J.C.; Sánchez-Calvo, B.; Chaki, M.; Mata-Pérez, C.; Valderrama, R.; Padilla, M.N.; López-Jaramillo, J.; Luque, F.; Corpas, F.J.; Barroso, J.B. Differential Molecular Response of Monodehydroascorbate Reductase and Glutathione Reductase by Nitration and S-nitrosylation. J. Exp. Bot. 2015, 66, 5983–5996. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lin, P.; Luo, Y.; Bai, H.; Liao, X.; Li, X.; Tian, Y.; Jiang, B.; Pan, Y.; Zhang, F.; et al. Lysine decrotonylation of glutathione peroxidase at lysine 220 site increases glutathione peroxidase activity to resist cold stress in chrysanthemum. Ecotoxicol. Environ. Saf. 2022, 232, 113295. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Yang, F.; Li, Q. Post-Translational Modification of GPX4 is a Promising Target for Treating Ferroptosis-Related Diseases. Front. Mol. Biosci. 2022, 9, 901565. [Google Scholar] [CrossRef] [PubMed]
- González-Domínguez, Á.; Visiedo, F.; Domínguez-Riscart, J.; Durán-Ruiz, M.C.; Saez-Benito, A.; Lechuga-Sancho, A.M.; Mateos, R.M. Catalase Post-Translational Modifications as Key Targets in the Control of Erythrocyte Redox Homeostasis in Children with Obesity and Insulin Resistance. Free Radic. Biol. Med. 2022, 191, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Overbaugh, J.M.; Fall, R. Characterization of A Selenium-Independent Glutathione Peroxidase from Euglena gracilis. Plant Physiol. 1985, 77, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Herbette, S.; Lenne, C.; Leblanc, N.; Julien, J.L.; Drevet, J.R.; Roeckel-Drevet, P. Two GPX-Like Proteins from Lycopersicon esculentum and Helianthus annuus are Antioxidant Enzymes with Phospholipid Hydroperoxide Glutathione Peroxidase and Thioredoxin Peroxidase Activities. Eur. J. Biochem. 2002, 269, 2414–2420. [Google Scholar] [CrossRef] [PubMed]
- Jung, B.G.; Lee, K.O.; Lee, S.S.; Chi, Y.H.; Jang, H.H.; Kang, S.S.; Lee, K.; Lim, D.; Yoon, S.C.; Yun, D.J.; et al. A Chinese Cabbage cDNA with High Sequence Identity to Phospholipid Hydroperoxide Glutathione Peroxidases Encodes a Novel Isoform of Thioredoxin-dependent Peroxidase. J. Biol. Chem. 2002, 277, 12572–12578. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Yabuta, Y.; Takeda, T.; Nakano, Y.; Shigeoka, S. Hydroperoxide Reduction by Thioredoxin-Specific Glutathione Peroxidase Isoenzymes of Arabidopsis thaliana. FEBS J. 2006, 273, 5589–5597. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.B.; Dayer, R.; Schwarzenbach, Y.; Lemaire, S.D.; Behra, R.; Liedtke, A.; Eggen, R.I. Function and Regulation of the Glutathione Peroxidase Homologous Gene GPXH/GPX5 in Chlamydomonas reinhardtii. Plant Mol. Biol. 2009, 71, 569–583. [Google Scholar] [CrossRef]
- Wang, X.; Xu, X. Molecular Cloning and Functional Analyses of Glutathione Peroxidase Homologous Genes from Chlorella sp. NJ-18. Gene 2012, 501, 17–23. [Google Scholar] [CrossRef]
- Fan, Z.; Yan, Q.; Song, J.; Wei, J. Reactive Human Plasma Glutathione Peroxidase Mutant with Diselenide Bond Succeeds in Tetramer Formation. Antioxidants 2022, 11, 1083. [Google Scholar] [CrossRef] [PubMed]
- Al-Madboly, L.A.; Ali, S.M.; Fakharany, E.M.E.; Ragab, A.E.; Khedr, E.G.; Elokely, K.M. Stress-Based Production, and Characterization of Glutathione Peroxidase and Glutathione S-Transferase Enzymes from Lactobacillus plantarum. Front. Bioeng. Biotechnol. 2020, 27, 78. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Han, H.; Cui, B.; Hou, Y.; Wang, Y.; Wang, Q. A glutathione peroxidase from Antarctic psychrotrophic bacterium Pseudoalteromonas sp. ANT506: Cloning and Heterologous Expression of the Gene and Characterization of Recombinant Enzyme. Bioengineered 2017, 8, 742–749. [Google Scholar] [CrossRef]
- Takeda, T.; Yokota, A.; Shigeoka, S. Resistance of Photosynthesis to Hydrogen Peroxide in Algae. Plant Cell Physiol. 1995, 36, 1089–1095. [Google Scholar] [CrossRef]
- Azad, A.K.; Ahmed, J.; Hakim, A.; Hasan, M.M.; Alum, M.A.; Hasan, M.; Raihan, T.; Ishikawa, T.; Sawa, Y. Genome-Wide Characterization Deciphers Distinct Properties of Aquaporins in Six Phytophthora species. Curr. Bioinform. 2021, 16, 880–898. [Google Scholar] [CrossRef]
- Capel, C.; Albaladejo, I.; Egea, I.; Massaretto, I.L.; Yuste-Lisbona, F.J.; Pineda, B.; García-Sogo, B.; Angosto, T.; Flores, F.B.; Moreno, V.; et al. The Res (Restored Cell Structure by Salinity) Tomato Mutant Reveals The Role of the DEAD-box RNA Helicase SlDEAD39 in Plant Development and Salt Response. Plant Cell Environ. 2020, 43, 1722–1739. [Google Scholar] [CrossRef] [PubMed]
- Passaia, G.; Queval, G.; Bai, J.; Margis-Pinheiro, M.; Foyer, C.H. The Effects of Redox Controls Mediated by Glutathione Peroxidases on Root Architecture in Arabidopsis thaliana. J. Exp. Bot. 2014, 65, 1403–1413. [Google Scholar] [CrossRef] [PubMed]
- Bonifacio, A.; Martins, M.O.; Ribeiro, C.W.; Fontenele, A.V.; Carvalho, F.E.; Margis-Pinheiro, M.Á.; Silveira, J.A. Role of Peroxidases in the Compensation of Cytosolic Ascorbate Peroxidase Knockdown in Rice Plants under Abiotic Stress. Plant Cell Environ. 2011, 34, 1705–1722. [Google Scholar] [CrossRef] [PubMed]
- Jardim-Messeder, D.; Caverzan, A.; Balbinott, N.; Menguer, P.K.; Paiva, A.L.S.; Lemos, M.; Cunha, J.R.; Gaeta, M.L.; Costa, M.; Zamocky, M.; et al. Stromal Ascorbate Peroxidase (OsAPX7) Modulates Drought Stress Tolerance in Rice (Oryza sativa). Antioxidants 2023, 12, 387. [Google Scholar] [CrossRef] [PubMed]
- Maruta, T.; Sawa, Y.; Shigeoka, S.; Ishikawa, T. Diversity and Evolution of Ascorbate Peroxidase Functions in Chloroplasts: More Than Just a Classical Antioxidant Enzyme? Plant Cell Physiol. 2016, 57, 1377–1386. [Google Scholar] [CrossRef]
- Ribeiro, C.W.; Korbes, A.P.; Garighan, J.A.; Jardim-Messeder, D.; Carvalho, F.E.; Sousa, R.H.; Caverzan, A.; Teixeira, F.K.; Silveira, J.A.; Margis-Pinheiro, M. Rice Peroxisomal Ascorbate Peroxidase Knockdown Affects ROS Signaling and Triggers Early Leaf Senescence. Plant Sci. 2017, 263, 55–65. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Q.; Fang, X.; Yuan, M.; Hu, W.; Liang, X.; Liu, J.; Yang, Y.; Fang, C. Destroying Glutathione Peroxidase Improves the Oxidative Stress Resistance and Pathogenicity of Listeria monocytogenes. Front. Microbiol. 2023, 14, 1122623. [Google Scholar] [CrossRef]


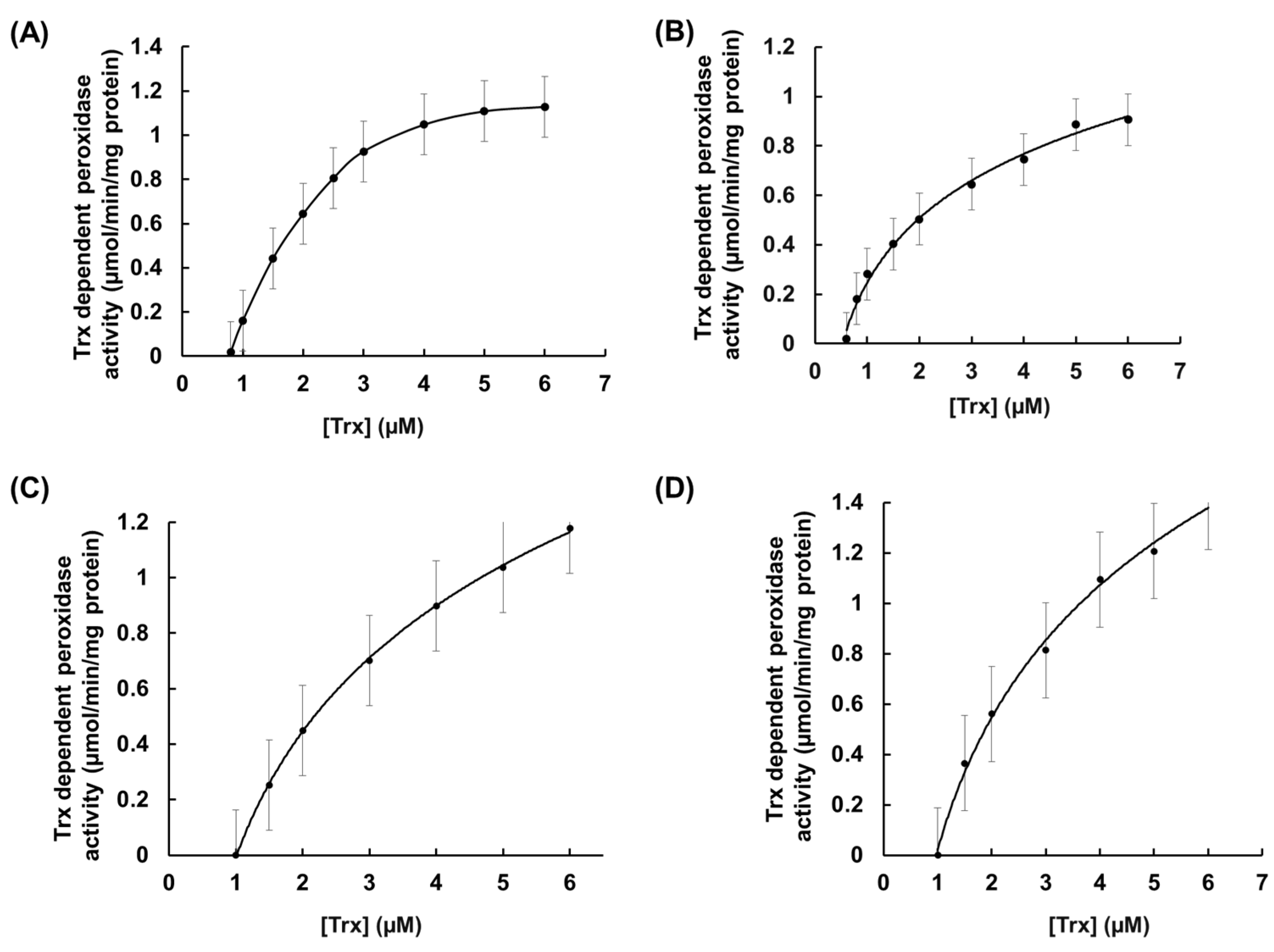




| Organisms | Substrate | Vmax (µmol/min/mg Protein) | Km (µM) | kcat(s−1) | kcat/Km (M−1 s−1) | Reference | |
|---|---|---|---|---|---|---|---|
| E. gracilis | rEgGPXL-2 | H2O2 | 3.33 ± 1.0 | 71.43 ± 2.5 | 0.44 ± 0.33 | 6.1 × 103 | Present study |
| t-BOOH | 1.0 ± 0.2 | 28.5 ± 3.1 | 0.03 ± 0.01 | 1.0 × 103 | |||
| rEgGPXL-4 | H2O2 | 10 ± 0.9 | 25 ± 0.4 | 0.33 ± 0.03 | 1.3 × 104 | ||
| t-BOOH | 5.0 ± 1.1 | 38.5 ± 1.6 | 0.17 ± 0.03 | 4.4 × 103 | |||
| rEgGPXL-1 | H2O2 | 3.03 ± 0.74 | 7.14 ± 2.3 | 0.505 ± 0.21 | 7.0 × 104 | [27] | |
| t-BOOH | 4.25 ± 0.95 | 8.0 ± 2.7 | 0.708 ± 0.15 | 8.9 × 104 | |||
| rEgPrx-1 | H2O2 | 3.15 ± 0.34 | 39.1 ± 4.1 | 1.21 ± 0.13 | 3.1 × 104 | [24] | |
| t-BOOH | 2.21 ± 0.21 | 24.5 ± 4.9 | 0.85 ± 0.08 | 3.5 × 104 | |||
| rEgPrx-2 | H2O2 | 3.34 ± 0.51 | 44.7 ± 3.1 | 1.28 ± 0.19 | 2.9 × 104 | ||
| t-BOOH | 2.92 ± 0.13 | 38.8 ± 1.8 | 1.12 ± 0.05 | 2.9 × 104 | |||
| H2O2 | 2.29 ± 0.16 | 37.8 ± 6.5 | 0.88 ± 0.06 | 2.3 × 104 | |||
| t-BOOH | 2.22 ± 0.11 | 36.1 ± 2.6 | 0.85 ± 0.04 | 2.4 × 104 | |||
| rEgPrx-3 | H2O2 | 0.59 ± 0.04 | 3.4 ± 0.5 | 0.22 ± 0.01 | 6.6 × 104 | ||
| t-BOOH | 0.77 ± 0.09 | 12.1 ± 1.4 | 0.29 ± 0.04 | 2.4 × 104 | |||
| rEgPrx-4 | H2O2 | 3.34 ± 0.51 | 44.7 ± 3.1 | 0.44 ± 0.33 | 6.1 × 103 | ||
| t-BOOH | 2.92 ± 0.13 | 38.8 ± 1.8 | 0.03 ± 0.01 | 1.0 × 103 | |||
| * GPX | H2O2 | - | 300 | - | - | [47] | |
| t-BOOH | - | 500 | - | - | |||
| C. reinhardtii | CrGPX-3 | H2O2 | 0.26 ± 0.01 | - | 7.4 ± 0.2 | 137 × 103 | [11,51] |
| t-BOOH | 0.27 ± 0.06 | - | 11.3 ± 0.7 | 16 × 103 | |||
| CrGPX-5 | H2O2 | 0.31 ± 0.06 | 54 ± 5 | - | - | ||
| t-BOOH | 0.32 ± 0.02 | 732 ± 112 | - | - | |||
| A. thaliana | AtGPX-8 | H2O2 | 0.39 ± 0.02 | 65 ± 5.4 | - | - | [17] |
| t-BOOH | 0.30 ± 0.01 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raihan, M.T.; Ishikawa, T. Biochemical and Functional Profiling of Thioredoxin-Dependent Cytosolic GPX-like Proteins in Euglena gracilis. Biomolecules 2024, 14, 765. https://doi.org/10.3390/biom14070765
Raihan MT, Ishikawa T. Biochemical and Functional Profiling of Thioredoxin-Dependent Cytosolic GPX-like Proteins in Euglena gracilis. Biomolecules. 2024; 14(7):765. https://doi.org/10.3390/biom14070765
Chicago/Turabian StyleRaihan, Md Topu, and Takahiro Ishikawa. 2024. "Biochemical and Functional Profiling of Thioredoxin-Dependent Cytosolic GPX-like Proteins in Euglena gracilis" Biomolecules 14, no. 7: 765. https://doi.org/10.3390/biom14070765
APA StyleRaihan, M. T., & Ishikawa, T. (2024). Biochemical and Functional Profiling of Thioredoxin-Dependent Cytosolic GPX-like Proteins in Euglena gracilis. Biomolecules, 14(7), 765. https://doi.org/10.3390/biom14070765






