The Evaluation of Drugs as Potential Modulators of the Trafficking and Maturation of ACE2, the SARS-CoV-2 Receptor
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Cell Culture
2.2. Compounds and Drugs Screened for Inhibition of ACE2 Maturation
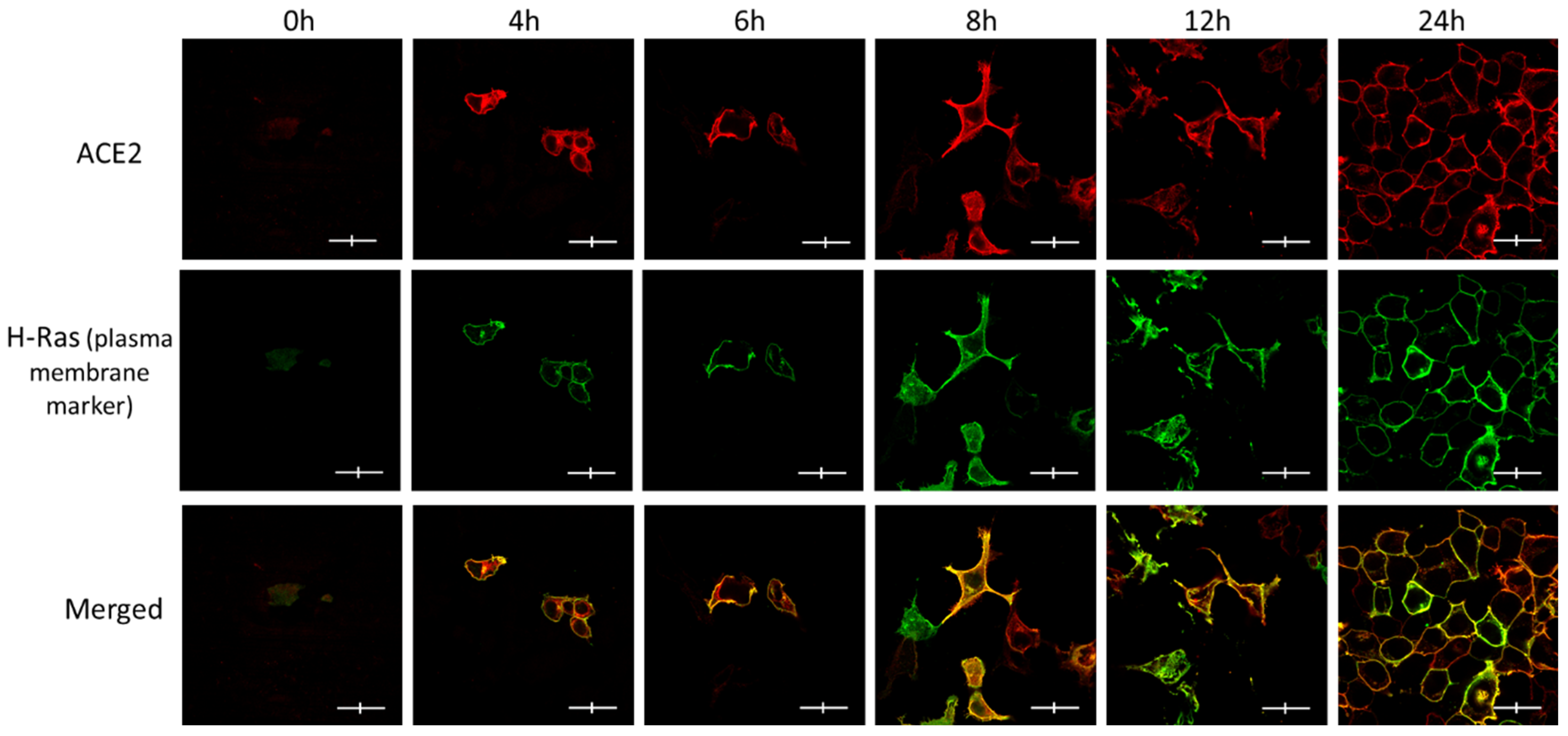
2.3. Immunofluorescence and Confocal Microscopy
2.4. The Development and Optimization of ACE2 Maturation Assay
2.5. ACE2 Maturation Rate Calculation
2.6. Statistics
2.7. The Development of ACE2 Maturation Assay
3. Results
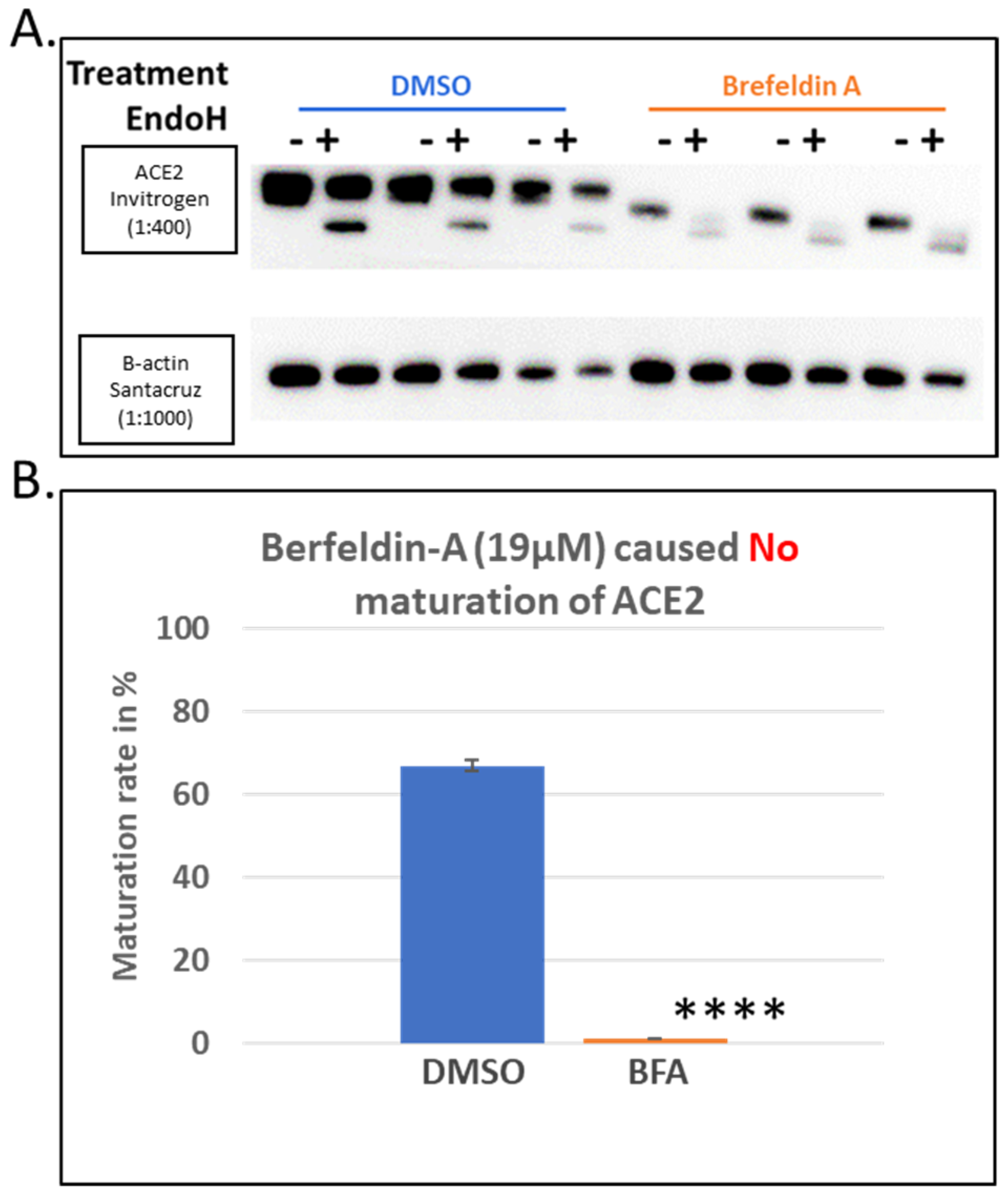
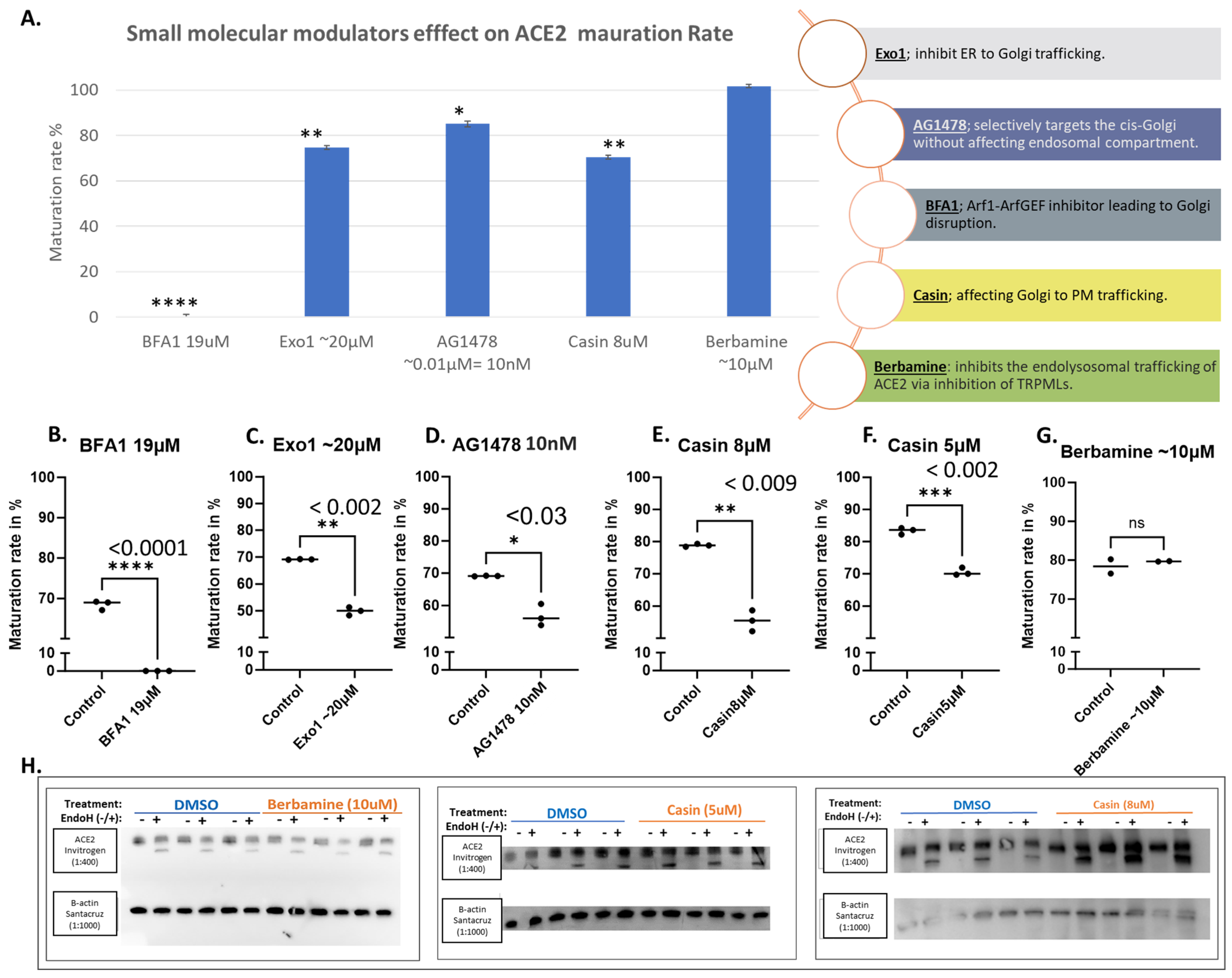
3.1. Brefeldin A Disrupted ACE2 Maturation While the Other Trafficking Modulator Showed Modest Effects
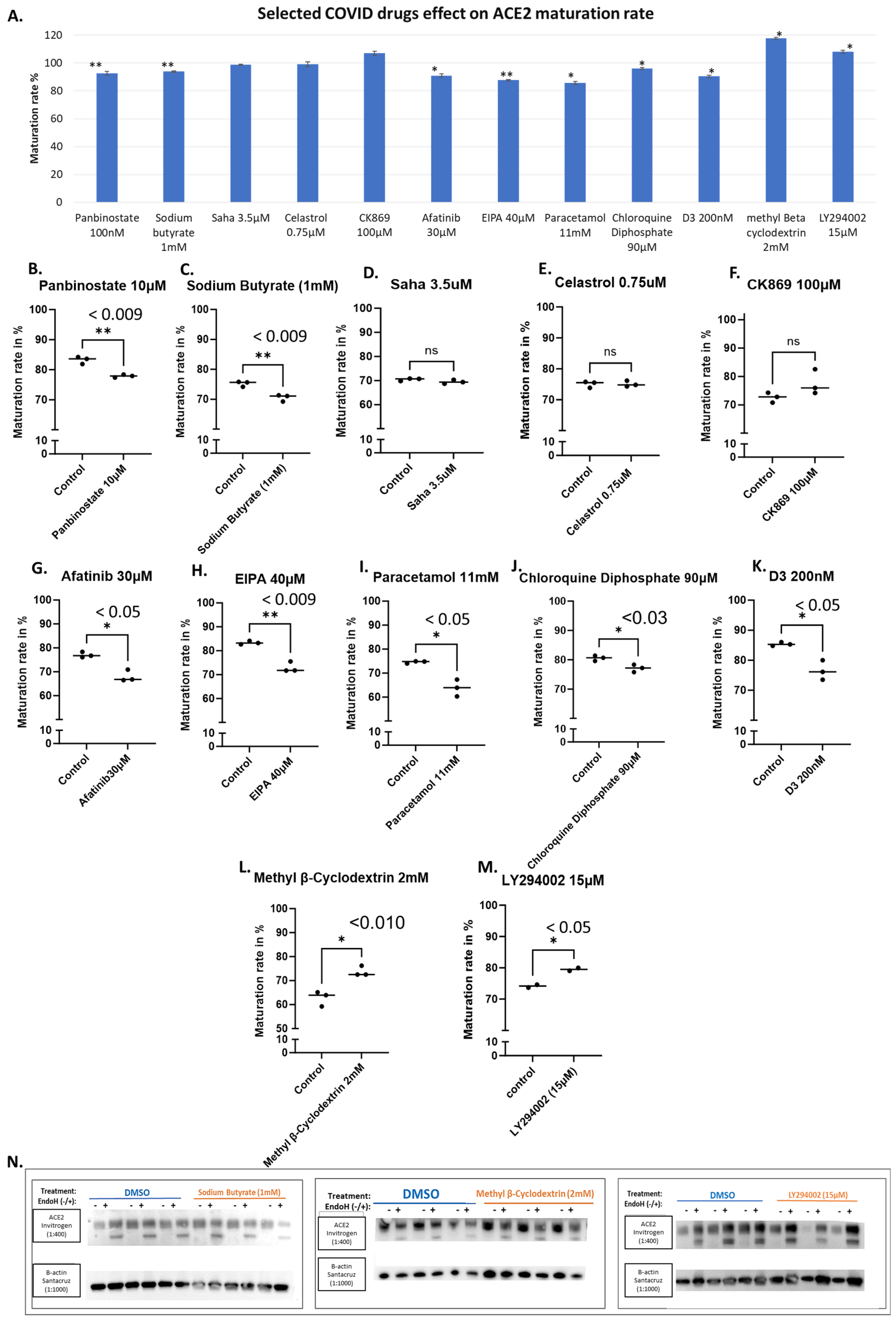
3.2. Evaluating the Effects of Some Reported Drugs Affecting SARS-CoV-2 Virus or COVID-19 Severity Demonstrated Modest Impacts on ACE2 Maturation
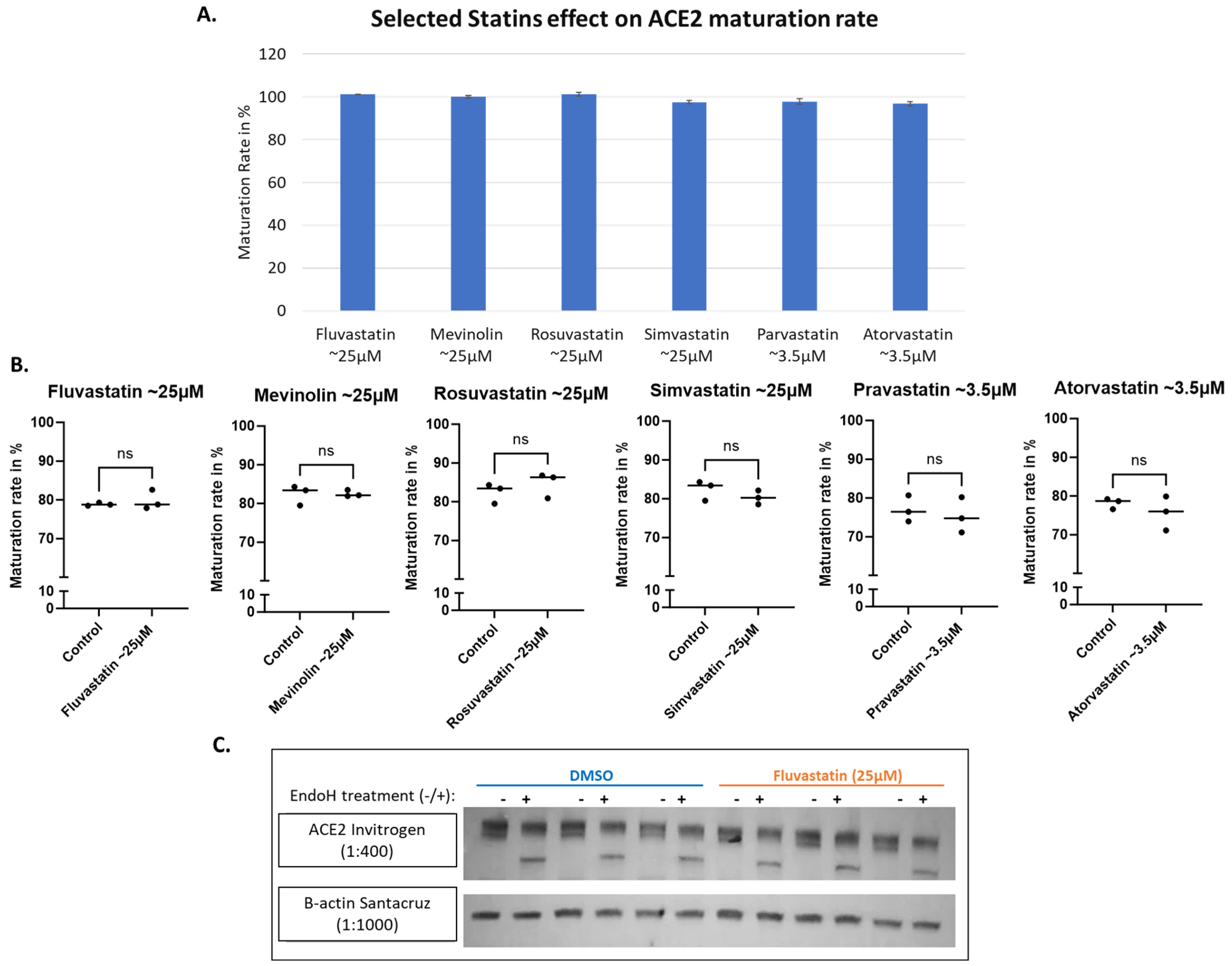
3.3. ACE2 Maturation Is Not Influenced by Statins
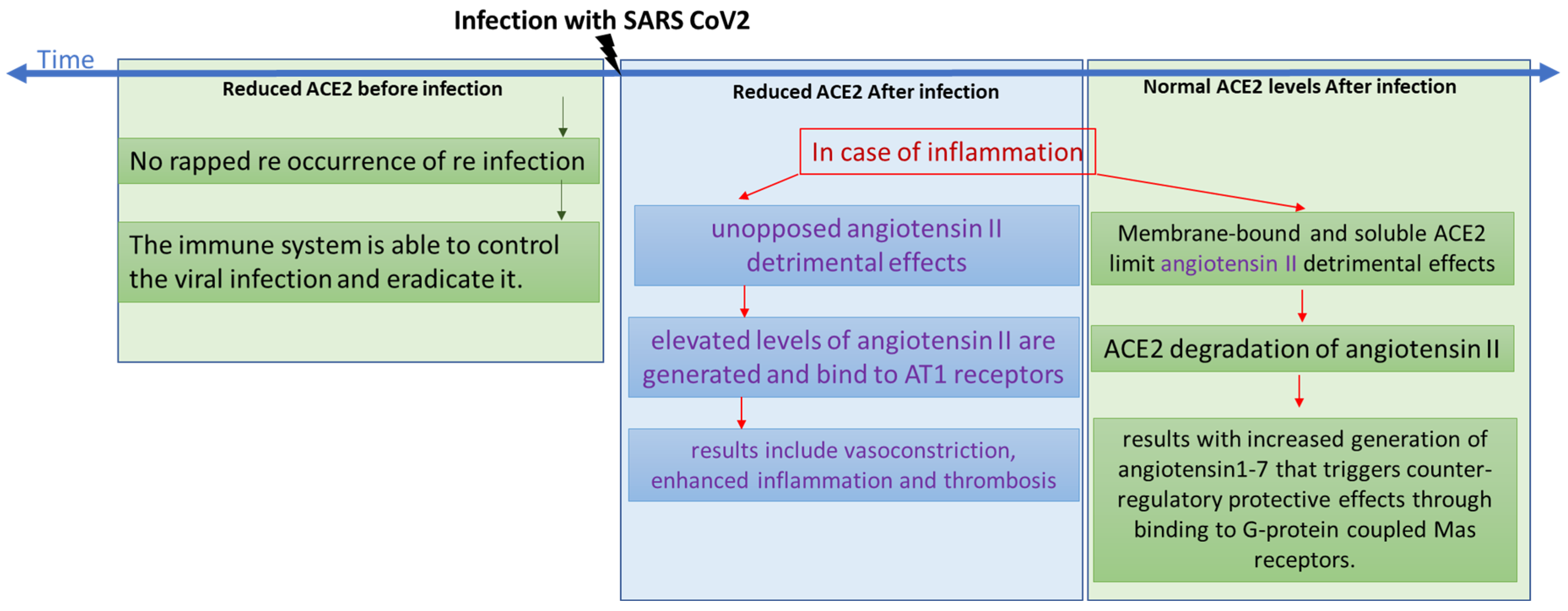
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walls, A.C.; Park, Y.-J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292.e6. [Google Scholar] [CrossRef] [PubMed]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.-L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM Structure of the 2019-nCoV Spike in the Prefusion Conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef] [PubMed]
- Samadizadeh, S.; Masoudi, M.; Rastegar, M.; Salimi, V.; Shahbaz, M.B.; Tahamtan, A. COVID-19: Why does disease severity vary among individuals? Respir. Med. 2021, 180, 106356. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Fernández, L.; Sawalha, A.H. Genetic variability in the expression of the SARS-CoV-2 host cell entry factors across populations. Genes Immun. 2020, 21, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Praissman, J.L.; Grant, O.C.; Cai, Y.; Xiao, T.; Rosenbalm, K.E.; Aoki, K.; Kellman, B.P.; Bridger, R.; Barouch, D.H.; et al. Virus-Receptor Interactions of Glycosylated SARS-CoV-2 Spike and Human ACE2 Receptor. Cell Host Microbe 2020, 28, 586. [Google Scholar] [CrossRef]
- Badawi, S.; Ali, B.R. ACE2 Nascence, trafficking, and SARS-CoV-2 pathogenesis: The saga continues. Hum. Genom. 2021, 15, 8. [Google Scholar] [CrossRef] [PubMed]
- Samavati, L.; Uhal, B.D. ACE2, Much More Than Just a Receptor for SARS-COV-2. Front. Cell. Infect. Microbiol. 2020, 10, 317. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.J.; Tipnis, S.R.; Guy, J.L.; Rice, G.I.; Hooper, N.M. ACEH/ACE2 is a novel mammalian metallocarboxypeptidase and a homologue of angiotensin-converting enzyme insensitive to ACE inhibitors. Can. J. Physiol. Pharmacol. 2011, 80, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.U.; Tabish, M. Alternative splicing of ACE2 possibly generates variants that may limit the entry of SARS-CoV-2: A potential therapeutic approach using SSOs. Clin. Sci. 2020, 134, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2012, 579, 270–273. [Google Scholar] [CrossRef]
- Kuba, K.; Imai, Y.; Rao, S.; Gao, H.; Guo, F.; Guan, B.; Huan, Y.; Yang, P.; Zhang, Y.; Deng, W.; et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat. Med. 2005, 11, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Deng, W.; Huang, B.; Gao, H.; Liu, J.; Ren, L.; Wei, Q.; Yu, P.; Xu, Y.; Qi, F.; et al. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature 2020, 583, 830–833. [Google Scholar] [CrossRef] [PubMed]
- Poduri, R.; Joshi, G.; Jagadeesh, G. Drugs targeting various stages of the SARS-CoV-2 life cycle: Exploring promising drugs for the treatment of COVID-19. Cell. Signal. 2020, 74, 109721. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Jabeen, N.; Amanullah, A.; Baig, A.A.; Aziz, B.; Shabbir, S.; Raza, F.; Uddin, N. Molecular docking between human TMPRSS2 and SARS-CoV-2 spike protein: Conformation and intermolecular interactions. AIMS Microbiol. 2020, 6, 350. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Duarte, M.; Estevinho, M.M.; Duarte-Araújo, M.; Magro, F.; Morato, M. Unraveling the Role of ACE2, the Binding Receptor for SARS-CoV-2, in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2020, 26, 1787–1795. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, S.; Niu, S. ACE2 and COVID-19 and the resulting ARDS. Postgrad. Med. J. 2020, 96, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Scalise, M.; Indiveri, C. Repurposing Nimesulide, a Potent Inhibitor of the B0AT1 Subunit of the SARS-CoV-2 Receptor, as a Therapeutic Adjuvant of COVID-19. SLAS Discov. 2020, 25, 1171–1173. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, Y.; Xu, H.; Wei, Z.; Yang, Y.; Jin, F.; Zhang, M.; Wang, C.; Song, W.; Huo, J.; et al. ACE2 attenuates epithelial-mesenchymal transition in MLE-12 cells induced by silica. Drug Des. Devel. Ther. 2020, 14, 1547–1559. [Google Scholar] [CrossRef] [PubMed]
- Lambert, D.W.; Yarski, M.; Warner, F.J.; Thornhill, P.; Parkin, E.T.; Smith, A.I.; Hooper, N.M.; Turner, A.J. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2). J. Biol. Chem. 2005, 280, 30113–30119. [Google Scholar] [CrossRef]
- Hong, P.J.; Look, D.C.; Tan, P.; Shi, L.; Hickey, M.; Gakhar, L.; Chappell, M.C.; Wohlford-Lenane, C.; McCray, P.B. Ectodomain shedding of angiotensin converting enzyme 2 in human airway epithelia. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2009, 297, L84. [Google Scholar] [CrossRef]
- Deshotels, M.R.; Xia, H.; Sriramula, S.; Lazartigues, E.; Filipeanu, C.M. Angiotensin II mediates angiotensin converting enzyme type 2 internalization and degradation through an Angiotensin II type I receptor-dependent mechanism. Hypertension 2014, 64, 1368–1375. [Google Scholar] [CrossRef] [PubMed]
- Banu, N.; Panikar, S.S.; Leal, L.R.; Leal, A.R. Protective role of ACE2 and its downregulation in SARS-CoV-2 infection leading to Macrophage Activation Syndrome: Therapeutic implications. Life Sci. 2020, 256, 117905. [Google Scholar] [CrossRef] [PubMed]
- Badawi, S.; Mohamed, F.E.; Alkhofash, N.R.; John, A.; Ali, A.; Ali, B.R. Characterization of ACE2 naturally occurring missense variants: Impact on subcellular localization and trafficking. Hum. Genom. 2022, 16, 35. [Google Scholar] [CrossRef] [PubMed]
- Freeze, H.H.; Kranz, C. Endoglycosidase and Glycoamidase Release of N-Linked Glycans. Curr. Protoc. Mol. Biol. 2010, 89, 17.13A.1–17.13A.25. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.L.; Ertelt, J.M. Endoglycosidase assay using enzymatically synthesized fluorophore-labeled glycans as substrates to uncover enzyme substrate specificities. Commun. Biol. 2022, 5, 501. [Google Scholar] [CrossRef]
- Hume, A.N.; Buttgereit, J.; Al-Awadhi, A.M.; Al-Suwaidi, S.S.; John, A.; Bader, M.; Seabra, M.C.; Al-gazali, L.; Ali, B.R. Defective cellular trafficking of missense NPR-B mutants is the major mechanism underlying acromesomelic dysplasia-type Maroteaux. Hum. Mol. Genet. 2009, 18, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.R.; Ben-Rebeh, I.; John, A.; Akawi, N.A.; Milhem, R.M.; Al-Shehhi, N.A.; Al-Ameri, M.M.; Al-Shamisi, S.A.; Al-Gazali, L. Endoplasmic Reticulum Quality Control Is Involved in the Mechanism of Endoglin-Mediated Hereditary Haemorrhagic Telangiectasia. PLoS ONE 2011, 6, e26206. [Google Scholar] [CrossRef] [PubMed]
- Gariballa, N.; Badawi, S.; Ali, B.R. Endoglin mutants retained in the endoplasmic reticulum exacerbate loss of function in hereditary hemorrhagic telangiectasia type 1 (HHT1) by exerting dominant negative effects on the wild type allele. Traffic 2024, 25, e12928. [Google Scholar] [CrossRef]
- Feng, Y.; Yu, S.; Lasell, T.K.R.; Jadhav, A.P.; Macia, E.; Chardin, P.; Melancon, P.; Roth, M.; Mitchison, T.; Kirchhausen, T. Exo1: A new chemical inhibitor of the exocytic pathway. Proc. Natl. Acad. Sci. USA 2003, 100, 6469–6474. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Yu, J.; Zhang, L.; Carpenter, A.; Zhu, H.; Li, L.; Ma, D.; Yuan, J. A novel small molecule regulator of guanine nucleotide exchange activity of the ADP-ribosylation factor and golgi membrane trafficking. J. Biol. Chem. 2008, 283, 31087–31096. [Google Scholar] [CrossRef]
- Pelish, H.E.; Peterson, J.R.; Salvarezza, S.B.; Rodriguez-Boulan, E.; Chen, J.-L.; Stamnes, M.; Macia, E.; Feng, Y.; Shair, M.D.; Kirchhausen, T. Secramine inhibits Cdc42-dependent functions in cells and Cdc42 activation in vitro. Nat. Chem. Biol. 2006, 2, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yuen, T.T.T.; Ye, Z.; Liu, S.; Zhang, G.; Chu, H.; Yue, J. Berbamine inhibits SARS-CoV-2 infection by compromising TRPMLs-mediated endolysosomal trafficking of ACE2. Signal Transduct. Target. Ther. 2021, 6, 168. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zeng, Z.; Cao, Y.; Liu, Y.; Ping, F.; Liang, M.; Xue, Y.; Xi, C.; Zhou, M.; Jiang, W. Angiotensin-converting enzyme 2 prevents lipopolysaccharide-induced rat acute lung injury via suppressing the ERK1/2 and NF-κB signaling pathways. Sci. Rep. 2016, 6, 27911. [Google Scholar] [CrossRef] [PubMed]
- Pinto, B.G.G.; Oliveira, A.E.R.; Singh, Y.; Jimenez, L.; Gonçalves, A.N.A.; Ogava, R.L.T.; Creighton, R.; Peron, J.P.S.; Nakaya, H.I. ACE2 expression is increased in the lungs of patients with comorbidities associated with severe COVID-19. J. Infect. Dis. 2020, 222, 556–563. [Google Scholar] [CrossRef]
- Takahashi, Y.; Hayakawa, A.; Sano, R.; Fukuda, H.; Harada, M.; Kubo, R.; Okawa, T.; Kominato, Y. Histone deacetylase inhibitors suppress ACE2 and ABO simultaneously, suggesting a preventive potential against COVID-19. Sci. Rep. 2021, 11, 3379. [Google Scholar] [CrossRef] [PubMed]
- Fuzo, C.A.; Martins, R.B.; C Fraga-Silva, T.F.; Amstalden, M.K.; Canassa De Leo, T.; Souza, J.P.; Lima, T.M.; Faccioli, L.H.; Noma Okamoto, D.; Aparecida Juliano, M.; et al. Celastrol: A lead compound that inhibits SARS-CoV-2 replication, the activity of viral and human cysteine proteases, and virus-induced IL-6 secretion. Drug Dev. Res. 2022, 83, 1623–1640. [Google Scholar] [CrossRef] [PubMed]
- Hetrick, B.; Han, M.S.; Helgeson, L.A.; Nolen, B.J. Small molecules CK-666 and CK-869 inhibit actin-related protein 2/3 complex by blocking an activating conformational change. Chem. Biol. 2013, 20, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Hickerson, B.T.; Ilyushina, N.A.; Mohan, N.; Peng, H.; Takeda, K.; Donnelly, R.P.; Wu, W.J. Identification of a pharmacological approach to reduce ACE2 expression and development of an in vitro COVID-19 viral entry model. J. Virus Erad. 2022, 8, 100307. [Google Scholar] [CrossRef] [PubMed]
- Schwab, C.L.; Bellone, S.; English, D.P.; Roque, D.M.; Lopez, S.; Cocco, E.; Nicoletti, R.; Bortolomai, I.; Bonazzoli, E.; Ratner, E.; et al. Afatinib demonstrates remarkable activity against HER2-amplified uterine serous endometrial cancer in vitro and in vivo. Br. J. Cancer 2014, 111, 1750–1756. [Google Scholar] [CrossRef]
- Santosa, A.; Franzén, S.; Nåtman, J.; Wettermark, B.; Parmryd, I.; Nyberg, F. Protective effects of statins on COVID-19 risk, severity and fatal outcome: A nationwide Swedish cohort study. Sci. Rep. 2022, 12, 12047. [Google Scholar] [CrossRef]
- Vitiello, A.; Ferrara, F. Plausible Positive Effects of Statins in COVID-19 Patient. Cardiovasc. Toxicol. 2021, 21, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Kollias, A.; Kyriakoulis, K.G.; Kyriakoulis, I.G.; Nitsotolis, T.; Poulakou, G.; Stergiou, G.S.; Syrigos, K. Statin use and mortality in COVID-19 patients: Updated systematic review and meta-analysis. Atherosclerosis 2021, 330, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Minz, M.M.; Bansal, M.; Kasliwal, R.R. Statins and SARS-CoV-2 disease: Current concepts and possible benefits. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 2063–2067. [Google Scholar] [CrossRef] [PubMed]
- Zapatero-Belinchón, F.J.; Moeller, R.; Lasswitz, L.; van Ham, M.; Becker, M.; Brogden, G.; Rosendal, E.; Bi, W.; Carriquí-Madroñal, B.; Islam, K.; et al. Fluvastatin mitigates SARS-CoV-2 infection in human lung cells. iScience 2021, 24, 103469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.R.; Zhang, Y.N.; Zhang, H.Q.; Zhang, Q.Y.; Li, N.; Li, Q.; Deng, C.L.; Zhang, B.; Li, X.D.; Ye, H.Q. Berbamine hydrochloride potently inhibits SARS-CoV-2 infection by blocking S protein-mediated membrane fusion. PLoS Negl. Trop. Dis. 2022, 16, e0010363. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Pawara, R.; Surana, S.; Patel, H. The Repurposed ACE2 Inhibitors: SARS-CoV-2 Entry Blockers of COVID-19. Top. Curr. Chem. 2021, 379, 40. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.T.; Chuang, Y.W.; Huang, C.P.; Chang, M.H. Loss of angiotensin converting enzyme II (ACE2) accelerates the developmentof liver injury induced by thioacetamide. Exp. Anim. 2018, 67, 41. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Forrester, S.J.; O’Brien, S.; Baggett, A.; Rizzo, V.; Eguchi, S. AT1 receptor signaling pathways in the cardiovascular system. Pharmacol. Res. 2017, 125, 4–13. [Google Scholar] [CrossRef]
- Angeli, F.; Zappa, M.; Reboldi, G.; Trapasso, M.; Cavallini, C.; Spanevello, A.; Verdecchia, P. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection: One year later. Eur. J. Intern. Med. 2021, 93, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Chen, Y.; Lin, R.; Han, K. Clinical features of COVID-19 in elderly patients: A comparison with young and middle-aged patients. J. Infect. 2020, 80, 14–18. [Google Scholar] [CrossRef]
- Gupta, A.; Marzook, H.; Ahmad, F. Comorbidities and clinical complications associated with SARS-CoV-2 infection: An overview. Clin. Exp. Med. 2023, 23, 313–331. [Google Scholar] [CrossRef] [PubMed]
- Santos Leal, N.; Yu, Y.; Chen, Y.; Fedele, G.; Martins, L.M.; Poulas, K. Paracetamol Is Associated with a Lower Risk of COVID-19 Infection and Decreased ACE2 Protein Expression: A Retrospective Analysis. COVID 2021, 1, 218–229. [Google Scholar] [CrossRef]
- Suter, F.; Consolaro, E.; Pedroni, S.; Moroni, C.; Past, E.; Paganini, M.V.; Pravettoni, G.; Cantarelli, U.; Rubis, N.; Perico, N.; et al. A simple, home-therapy algorithm to prevent hospitalisation for COVID-19 patients: A retrospective observational matched-cohort study. EClinicalMedicine 2021, 37, 100941. [Google Scholar] [CrossRef] [PubMed]
- Rejman, J.; Bragonzi, A.; Conese, M. Role of Clathrin-and Caveolae-Mediated Endocytosis in Gene Transfer Mediated by Lipo-and Polyplexes. Mol. Ther. 2005, 12, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yuan, Z.; Pavel, M.A.; Jablonski, S.M.; Jablonski, J.; Hobson, R.; Valente, S.; Reddy, C.B.; Hansen, S.B. The role of high cholesterol in SARS-CoV-2 infectivity. J. Biol. Chem. 2023, 299, 104763. [Google Scholar] [CrossRef] [PubMed]
- Gautret, P.; Lagier, J.C.; Parola, P.; Hoang, V.T.; Meddeb, L.; Mailhe, M.; Doudier, B.; Courjon, J.; Giordanengo, V.; Vieira, V.E.; et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents 2020, 56, 105949. [Google Scholar] [CrossRef] [PubMed]
- Vincent, M.J.; Bergeron, E.; Benjannet, S.; Erickson, B.R.; Rollin, P.E.; Ksiazek, T.G.; Seidah, N.G.; Nichol, S.T. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005, 2, 69. [Google Scholar] [CrossRef]
- Li, X.C.; Zhang, J.; Zhuo, J.L. The vasoprotective axes of the renin-angiotensin system: Physiological relevance and therapeutic implications in cardiovascular, hypertensive and kidney diseases. Pharmacol. Res. 2017, 125, 21–38. [Google Scholar] [CrossRef]
- Ferrario, C.M.; Jessup, J.; Chappell, M.C.; Averill, D.B.; Brosnihan, K.B.; Tallant, E.A.; Diz, D.I.; Gallagher, P.E. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation 2005, 111, 2605–2610. [Google Scholar] [CrossRef]
- Zisman, L.S. ACE and ACE2: A tale of two enzymes. Eur. Heart J. 2005, 26, 322–324. [Google Scholar] [CrossRef]
- Xie, K.Q.; Zhang, L.M.; Cao, Y.; Zhu, J.; Feng, L.Y. Adenosine A 1 receptor-mediated transactivation of the EGF receptor produces a neuroprotective effect on cortical neurons in vitro. Acta Pharmacol. Sin. 2009, 30, 889–898. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yan, Y.; Jiang, K.; Liu, P.; Zhang, X.; Dong, X.; Gao, J.; Liu, Q.; Barr, M.P.; Zhang, Q.; Hou, X.; et al. Bafilomycin A1 induces caspase-independent cell death in hepatocellular carcinoma cells via targeting of autophagy and MAPK pathways. Sci. Rep. 2016, 6, 37052. [Google Scholar] [CrossRef]
- Chen, Y.; Li, M.X.; Lu, G.D.; Shen, H.M.; Zhou, J. Hydroxychloroquine/Chloroquine as Therapeutics for COVID-19: Truth under the Mystery. Int. J. Biol. Sci. 2021, 17, 1538. [Google Scholar] [CrossRef] [PubMed]
- Theobald, J.; Abu el Maaty, M.A.; Kusterer, N.; Wetterauer, B.; Wink, M.; Cheng, X.; Wölfl, S. In vitro metabolic activation of vitamin D3 by using a multi-compartment microfluidic liver-kidney organ on chip platform. Sci. Rep. 2019, 9, 4616. [Google Scholar] [CrossRef]
- Wu, W.; Wang, Y.; Deng, X.L.; Sun, H.Y.; Li, G.R. Cholesterol Down-Regulates BK Channels Stably Expressed in HEK 293 Cells. PLoS ONE 2013, 8, e79952. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.; Zheng, Y.; Tyner, J.W.; Chng, W.J.; Chien, W.W.; Gery, S.; Leong, G.; Braunstein, G.D.; Koeffler, H.P. Belinostat and panobinostat (HDACI): In vitro and in vivo studies in thyroid cancer. J Cancer Res. Clin. Oncol. 2013, 139, 1507–1514. [Google Scholar] [CrossRef] [PubMed]
- Neelima, S.; Reddy, P.D.; Sekhar, C.; Bannoth, K. Nephroprotective activity of Annona Squamosa leaves against paracetamol-induced nephrotoxicity in rats: In vitro and in vivo experiments. Future J. Pharm. Sci. 2020, 6, 1–8. [Google Scholar] [CrossRef]
- Li, L.; Sun, Y.; Liu, J.; Wu, X.; Chen, L.; Ma, L.; Wu, P. Histone deacetylase inhibitor sodium butyrate suppresses DNA double strand break repair induced by etoposide more effectively in MCF-7 cells than in HEK293 cells. BMC Biochem. 2015, 16, 2. [Google Scholar] [CrossRef]
- Banerjee, N.S.; Moore, D.W.; Broker, T.R.; Chow, L.T. Vorinostat, a pan-HDAC inhibitor, abrogates productive HPV-18 DNA amplification. Proc. Natl. Acad. Sci. USA 2018, 115, E11138–E11147. [Google Scholar] [CrossRef]


| # | Class | Drug | Note | Catalog # | Supplier | Solvent Used |
|---|---|---|---|---|---|---|
| 1 | Small molecular modulators of protein trafficking | Brefeldin A | Potent inhibitor of protein trafficking from the ER to the Golgi apparatus | B6542 | Sigma (St. Louis, MO, USA) | DMSO |
| 2 | Exo-1 | Vesicular trafficking inhibitor | ab120292 | Abcam (Cambridge, UK) | DMSO | |
| 3 | AG 1478 | EGF receptor tyrosine kinase inhibitor (the same as BFA but less cytotoxic) | ab141438 | Abcam | DMSO | |
| 4 | Casin | Rho GTPase Cdc42 inhibitor | SML12553 | Sigma | DMSO | |
| 5 | Berbamine | Natural STAT3 inhibitor | 547190 | Sigma | DMSO | |
| 6 | COVID-19 investigated drugs | Panobinostat (LBH589) | HDAC inhibitor, suppresses ACE2 expression | sml3060 | Sigma | DMSO |
| 7 | Sodium butyrate | HDACIs, suppresses ABO expression in the same way as panobinostat | 303410 | Sigma-Aldrich | DMSO | |
| 8 | SAHA (Vorinostat in dimethyl sulfoxide form) | Histone deacetylase (HDAC) inhibitor | 10009929 | Cayman Chemical Company (Ann Arbor, MI, USA) | DMSO | |
| 9 | Celastrol | Disrupts Hsp90/Cdc37 complete | 34157-83-0 | Sigma | DMSO | |
| 10 | Ck869 | An inhibitor of the actin-related protein 2/3 (ARP2/3) complex | C9124 | Sigma | DMSO | |
| 11 | Afatinib | A kinase inhibitor of HER2 and EGFR | S1011 | Selleckchem (Cologne Germany) | DMSO | |
| 12 | Eipa [5-(n-ethyl-n-isopropyl)-amiloride] | An inhibitor of the Na+/H+ exchanger (NHE) | 14406 | Cayman Chemical Company | DMSO | |
| 13 | Paracetamol | Highly effective analgesic and antipyretic properties | P0300000 | edQm (Strasbourg France) | DMSO | |
| 14 | Chloroquine diphosphate | A DNA intercalator that inhibits cell growth and induces cell death | C6628 | Sigma | H2O | |
| 15 | Cholecalciferol (vitamin D3) | A total of 85% infectious virus reduction | 740292 | Sigma | DMSO | |
| 16 | LY294002 | an inhibitor of PI3K | L9908 | Sigma | DMSO | |
| 17 | Cholesterol-lowering drugs | Methyl-β cyclodextrin | Depolymerizes the actin cytoskeleton, HMG-CoA reductase inhibitor | C4555 | Sigma | DMSO |
| 18 | Fluvastatin | Statins | SML0038 | Sigma | DMSO | |
| 19 | Lovastatin (mevinolin) | Statins Hypocholesterolaemia drug HMG-CoA reductase inhibitor | M2147 | Sigma | DMSO | |
| 20 | Rosuvastatin | Statins | SML1264 | Sigma | DMSO | |
| 21 | Simvastatin | Statins | S6196 | Sigma | DMSO | |
| 22 | Pravastatin | Statins | P4498 | Sigma | H2O | |
| 23 | Atorvastatin | Statins | PZ0001 | Sigma | DMSO |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkhofash, N.F.; Ali, B.R. The Evaluation of Drugs as Potential Modulators of the Trafficking and Maturation of ACE2, the SARS-CoV-2 Receptor. Biomolecules 2024, 14, 764. https://doi.org/10.3390/biom14070764
Alkhofash NF, Ali BR. The Evaluation of Drugs as Potential Modulators of the Trafficking and Maturation of ACE2, the SARS-CoV-2 Receptor. Biomolecules. 2024; 14(7):764. https://doi.org/10.3390/biom14070764
Chicago/Turabian StyleAlkhofash, Nesreen F., and Bassam R. Ali. 2024. "The Evaluation of Drugs as Potential Modulators of the Trafficking and Maturation of ACE2, the SARS-CoV-2 Receptor" Biomolecules 14, no. 7: 764. https://doi.org/10.3390/biom14070764
APA StyleAlkhofash, N. F., & Ali, B. R. (2024). The Evaluation of Drugs as Potential Modulators of the Trafficking and Maturation of ACE2, the SARS-CoV-2 Receptor. Biomolecules, 14(7), 764. https://doi.org/10.3390/biom14070764






