Identification of Potential New Genes Related to the SREBP Pathway in Xanthophyllomyces dendrorhous
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains, Plasmids, Primers, and Enzymes
2.2. X. dendrorhous Transformation
2.3. Random Mutagenesis
2.4. Purification and Extraction of Nucleic Acids
2.5. DNA Amplification, cDNA Synthesis (RT), and Real-Time PCR (qPCR)
2.6. Bioinformatic Analyses, Genome Mapping, SNP Calling, and Annotation
3. Results
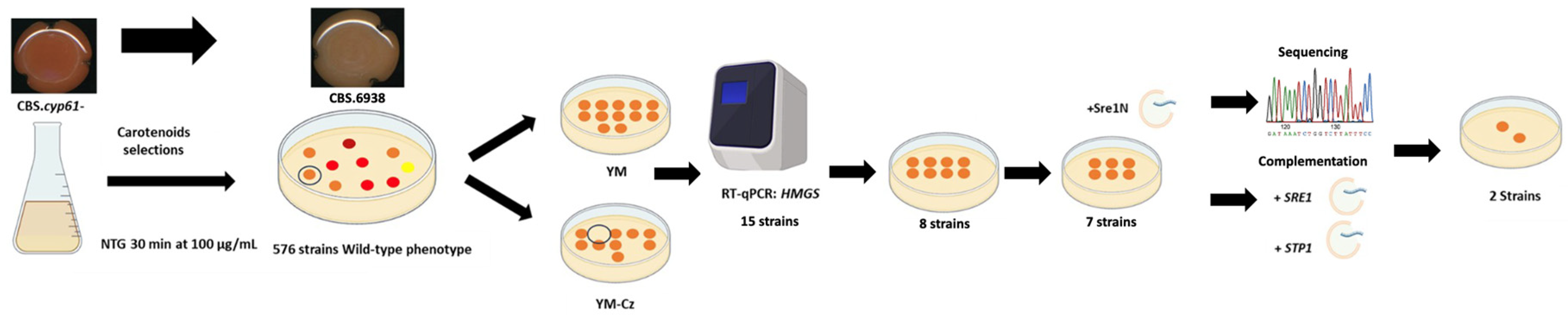
3.1. Evaluation of Mutagenesis Conditions and Effectiveness of Screening for SREBP Pathway Mutants
3.2. CBS.cyp61-.SRE1.FLAG Strain Mutation and SREBP Pathway Mutant Selection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Espenshade, P.J. SREBPs: Sterol-regulated transcription factors. J. Cell Sci. 2006, 119, 973–976. [Google Scholar] [CrossRef] [PubMed]
- Shimano, H.; Sato, R. SREBP-regulated lipid metabolism: Convergent physiology—Divergent pathophysiology. Nat. Rev. Endocrinol. 2017, 13, 710–730. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, X.; Ding, Y.; Liu, X.; Diggle, K.; Kisseleva, T.; Brenner, D.A. SREBP Regulation of Lipid Metabolism in Liver Disease, and Therapeutic Strategies. Biomedicines 2023, 11, 3280. [Google Scholar] [CrossRef] [PubMed]
- Naguib, Y.M. Antioxidant activities of astaxanthin and related carotenoids. J. Agric. Food Chem. 2000, 48, 1150–1154. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, I.; Schewe, H.; Gassel, S.; Jin, C.; Buckingham, J.; Hümbelin, M.; Sandmann, G.; Schrader, J. Biotechnological production of astaxanthin with Phaffia rhodozyma/Xanthophyllomyces dendrorhous. Appl. Microbiol. Biotechnol. 2011, 89, 555–571. [Google Scholar] [CrossRef]
- Rodriguez-Saiz, M.; de la Fuente, J.L.; Barredo, J.L. Xanthophyllomyces dendrorhous for the industrial production of astaxanthin. Appl. Microbiol. Biotechnol. 2010, 88, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Gassel, S.; Schewe, H.; Schmidt, I.; Schrader, J.; Sandmann, G. Multiple improvement of astaxanthin biosynthesis in Xanthophyllomyces dendrorhous by a combination of conventional mutagenesis and metabolic pathway engineering. Biotechnol. Lett. 2013, 35, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Torres-Haro, A.; Verdín, J.; Kirchmayr, M.R.; Arellano-Plaza, M. Metabolic engineering for high yield synthesis of astaxanthin in Xanthophyllomyces dendrorhous. Microb. Cell Fact. 2021, 20, 175. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, M.S.; Campusano, S.; González, A.M.; Gómez, M.; Barahona, S.; Sepúlveda, D.; Espenshade, P.J.; Fernández-Lobato, M.; Baeza, M.; Cifuentes, V.; et al. Sterol Regulatory Element-Binding Protein (Sre1) promotes the synthesis of carotenoids and sterols in Xanthophyllomyces dendrorhous. Front. Microbiol. 2019, 10, 586. [Google Scholar] [CrossRef]
- Gómez, M.; Campusano, S.; Gutiérrez, M.S.; Sepúlveda, D.; Barahona, S.; Baeza, M.; Cifuentes, V.; Alcaíno, J. Sterol regulatory element-binding protein Sre1 regulates carotenogenesis in the red yeast Xanthophyllomyces dendrorhous. J. Lipid Res. 2020, 61, 1658–1674. [Google Scholar] [CrossRef]
- Gómez, M.; Gutiérrez, M.S.; González, A.M.; Gárate-Castro, C.; Sepúlveda, D.; Barahona, S.; Baeza, M.; Cifuentes, V.; Alcaíno, J. Metallopeptidase Stp1 activates the transcription factor Sre1 in the carotenogenic yeast Xanthophyllomyces dendrorhous. J. Lipid Res. 2020, 61, 229–243. [Google Scholar] [CrossRef]
- Loto, I.; Gutiérrez, M.S.; Barahona, S.; Sepúlveda, D.; Martínez-Moya, P.; Baeza, M.; Cifuentes, V.; Alcaíno, J. Enhancement of carotenoid production by disrupting the C22-sterol desaturase gene (CYP61) in Xanthophyllomyces dendrorhous. BMC Microbiol. 2012, 12, 235. [Google Scholar] [CrossRef]
- Hughes, A.L.; Todd, B.L.; Espenshade, P.J. SREBP pathway responds to sterols and functions as an oxygen sensor in fission yeast. Cell 2005, 120, 831–842. [Google Scholar] [CrossRef]
- Maguire, S.L.; Wang, C.; Holland, L.M.; Brunel, F.; Neuvéglise, C.; Nicaud, J.M.; Zavrel, M.; White, T.C.; Wolfe, K.H.; Butler, G. Zinc finger transcription factors displaced SREBP proteins as the major Sterol regulators during Saccharomycotina evolution. PLoS Genet. 2014, 10, e1004076. [Google Scholar] [CrossRef] [PubMed]
- Willger, S.D.; Puttikamonkul, S.; Kim, K.H.; Burritt, J.B.; Grahl, N.; Metzler, L.J.; Barbuch, R.; Bard, M.; Lawrence, C.B.; Cramer, R.A.J. A sterol-regulatory element binding protein is required for cell polarity, hypoxia adaptation, azole drug resistance, and virulence in Aspergillus fumigatus. PLoS Pathog. 2008, 4, e1000200. [Google Scholar] [CrossRef]
- Dhingra, S.; Cramer, R.A. Regulation of sterol biosynthesis in the human fungal pathogen Aspergillus fumigatus: Opportunities for therapeutic development. Front. Microbiol. 2017, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Ingavale, S.S.; Bien, C.; Espenshade, P.; Kwon-Chung, K.J. Conservation of the sterol regulatory element-binding protein pathway and its pathobiological importance in Cryptococcus neoformans. Eukaryot. Cell 2009, 8, 1770–1779. [Google Scholar] [CrossRef] [PubMed]
- Bien, C.M.; Chang, Y.C.; Nes, W.D.; Kwon-Chung, K.J.; Espenshade, P.J. Cryptococcus neoformans Site-2 protease is required for virulence and survival in the presence of azole drugs. Mol. Microbiol. 2009, 74, 672–690. [Google Scholar] [CrossRef]
- Dhingra, S.; Kowalski, C.H.; Thammahong, A.; Beattie, S.R.; Bultman, K.M.; Cramer, R.A. RbdB, a Rhomboid Protease Critical for SREBP Activation and Virulence in Aspergillus fumigatus. mSphere 2016, 1, 1–10. [Google Scholar] [CrossRef]
- Hwang, J.; Ribbens, D.; Raychaudhuri, S.; Cairns, L.; Gu, H.; Frost, A.; Urban, S.; Espenshade, P.J. A Golgi rhomboid protease Rbd2 recruits Cdc48 to cleave yeast SREBP. EMBO J. 2016, 35, 2332–2349. [Google Scholar] [CrossRef]
- Bat-Ochir, C.; Kwak, J.Y.; Koh, S.K.; Jeon, M.H.; Chung, D.; Lee, Y.W.; Chae, S.K. The signal peptide peptidase SppA is involved in sterol regulatory element-binding protein cleavage and hypoxia adaptation in Aspergillus nidulans. Mol. Microbiol. 2016, 100, 635–655. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, S.; Mihaylova, M.M.; Zheng, B.; Hou, X.; Jiang, B.; Park, O.; Luo, Z.; Lefai, E.; Shyy, J.Y.; et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011, 13, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Shyy, J.Y. Sterol regulatory element-binding protein 1 is negatively modulated by PKA phosphorylation. Am. J. Physiol. Cell Physiol. 2006, 290, C1477–C1486. [Google Scholar] [CrossRef] [PubMed]
- Peterson, T.R.; Sengupta, S.S.; Harris, T.E.; Carmack, A.E.; Kang, S.A.; Balderas, E.; Guertin, D.A.; Madden, K.L.; Carpenter, A.E.; Finck, B.N.; et al. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell 2011, 146, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Düvel, K.; Yecies, J.L.; Menon, S.; Raman, P.; Lipovsky, A.I.; Souza, A.L.; Triantafellow, E.; Ma, Q.; Gorski, R.; Cleaver, S.; et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol. Cell 2010, 39, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Beurel, E.; Grieco, S.F.; Jope, R.S. Glycogen synthase kinase-3 (GSK3): Regulation, actions, and diseases. Pharmacol. Ther. 2015, 148, 114–131. [Google Scholar] [CrossRef] [PubMed]
- Sundqvist, A.; Bengoechea-Alonso, M.T.; Ye, X.; Lukiyanchuk, V.; Jin, J.; Harper, J.W.; Ericsson, J. Control of lipid metabolism by phosphorylation-dependent degradation of the SREBP family of transcription factors by SCF(Fbw7). Cell Metab. 2005, 1, 379–391. [Google Scholar] [CrossRef]
- Giandomenico, V.; Simonsson, M.; Grönroos, E.; Ericsson, J. Coactivator-dependent acetylation stabilizes members of the SREBP family of transcription factors. Mol. Cell Biol. 2003, 23, 2587–2599. [Google Scholar] [CrossRef]
- Walker, A.K.; Yang, F.; Jiang, K.; Ji, J.Y.; Watts, J.L.; Purushotham, A.; Boss, O.; Hirsch, M.L.; Ribich, S.; Smith, J.J.; et al. Conserved role of SIRT1 orthologs in fasting-dependent inhibition of the lipid/cholesterol regulator SREBP. Genes. Dev. 2010, 24, 1403–1417. [Google Scholar] [CrossRef]
- Horton, J.D.; Cuthbert, J.A.; Spady, D.K. Regulation of Hepatic 7α-Hydroxylase Expression and Response to Dietary Cholesterol in the Rat and Hamster. J. Biol. Chem. 1995, 270, 5381–5387. [Google Scholar] [CrossRef]
- Misawa, K.; Horiba, T.; Arimura, N.; Hirano, Y.; Inoue, J.; Emoto, N.; Shimano, H.; Shimizu, M.; Sato, R. Sterol regulatory element-binding protein-2 interacts with hepatocyte nuclear factor-4 to enhance sterol isomerase gene expression in hepatocytes. J. Biol. Chem. 2003, 278, 36176–36182. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2001; Volume 1–3, p. 2100. [Google Scholar]
- Adrio, J.L.; Veiga, M. Transformation of the astaxanthin-producing yeast Phaffia Rhodozyma. Biotechnol. Tech. 1995, 9, 509–512. [Google Scholar] [CrossRef]
- Cifuentes, V.; Hermosilla, G.; Martínez, C.; León, R.; Pincheira, G.; Jiménez, A. Genetics and electrophoretic karyotyping of wild-type and astaxanthin mutant strains of Phaffia rhodozyma. Antonie Leeuwenhoek 1997, 72, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Retamales, P.; León, R.; Martínez, C.; Hermosilla, G.; Pincheira, G.; Cifuentes, V. Complementation analysis with new genetic markers in Phaffia rhodozyma. Antonie Leeuwenhoek 1998, 73, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Hermosilla, G.; Martínez, C.; Retamales, P.; León, R.; Cifuentes, V. Genetic determination of ploidy level in Xanthophyllomyces dendrorhous. Antonie Leeuwenhoek 2003, 84, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Boyle, J.S.; Lew, A.M. An inexpensive alternative to glassmilk for DNA purification. Trends Genet. 1995, 11, 8. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-∆∆CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Binns, D.; Chang, H.Y.; Fraser, M.; Li, W.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-scale protein function classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 25 May 2024).
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
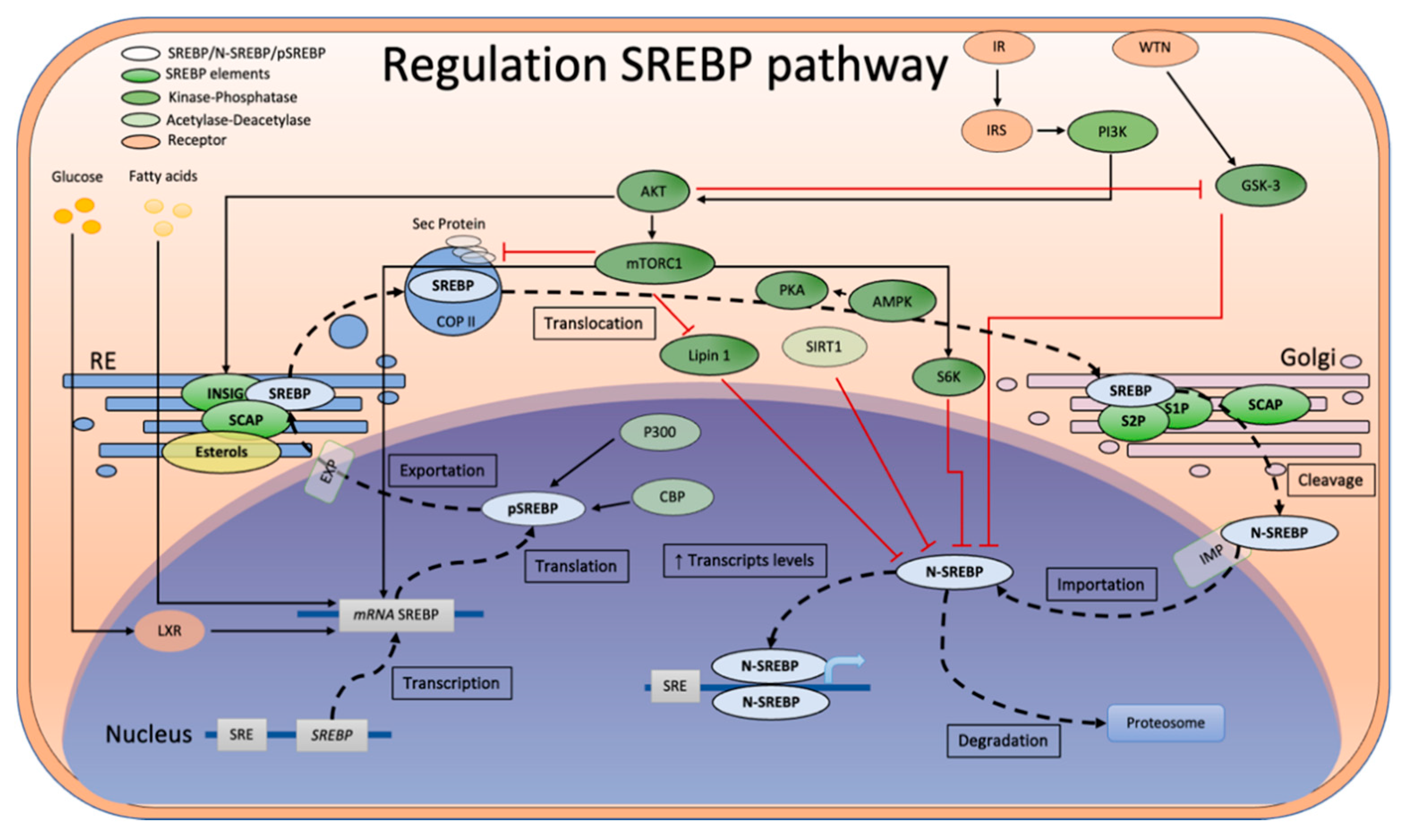
- Gómez, M.; Baeza, M.; Cifuentes, V.; Alcaíno, J. The SREBP (Sterol Regulatory Element-Binding Protein) pathway: A regulatory bridge between carotenogenesis and sterol biosynthesis in the carotenogenic yeast Xanthophyllomyces dendrorhous. Biol. Res. 2021, 54, 34. [Google Scholar] [CrossRef]
- Stewart, E.V.; Nwosu, C.C.; Tong, Z.; Roguev, A.; Cummins, T.D.; Kim, D.U.; Hayles, J.; Park, H.O.; Hoe, K.L.; Powell, D.W.; et al. Yeast SREBP cleavage activation requires the Golgi Dsc E3 ligase complex. Mol. Cell 2011, 42, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Van der Auwera, G.; Carneiro, M.; Hartl, C.; Poplin, R.; del Angel, G.; Levy-Moonshine, A.; Jordan, T.; Shakir, K.; Roazen, D.; Thibault, J.; et al. From FastQ data to high confidence variant calls: The Genome Analysis Toolkit best practices pipeline. Curr. Protoc. Bioinform. 2013, 43, 1. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 2010, 26, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Cingolani, P.; Platts, A.; Wang, L.L.; Coon, M.; Nguyen, T.; Wang, L.; Land, S.J.; Lu, X.; Ruden, D.M. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 2012, 6, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Ng, P.C.; Henikoff, S. SIFT: Predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003, 31, 3812–3814. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Whitwham, A.; Keane, T.; McCarthy, S.A.; Davies, R.M.; et al. Twelve years of SAMtools and BCFtools. Gigascience 2021, 10, giab008. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Smyth, G.K.; Shi, W. The R package Rsubread is easier, faster, cheaper and better for alignment and quantification of RNA sequencing reads. Nucleic Acids Res. 2019, 47, e47. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Chang, Y.C.; Bien, C.M.; Lee, H.; Espenshade, P.J.; Kwon-Chung, K.J. Sre1p, a regulator of oxygen sensing and sterol homeostasis, is required for virulence in Cryptococcus neoformans. Mol. Microbiol. 2007, 64, 614–629. [Google Scholar] [CrossRef]
- Blatzer, M.; Barker, B.M.; Willger, S.D.; Beckmann, N.; Blosser, S.J.; Cornish, E.J.; Mazurie, A.; Grahl, N.; Haas, H.; Cramer, R.A. SREBP coordinates iron and ergosterol homeostasis to mediate triazole drug and hypoxia responses in the human fungal pathogen Aspergillus fumigatus. PLoS Genet. 2011, 7, e1002374. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, J.; Mizoguchi, H.; Takeno, S.; Ikeda, M. Characterization of mutations induced by N-methyl-N’-nitro-N-nitrosoguanidine in an industrial Corynebacterium glutamicum strain. Mutat. Res. 2008, 649, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Sandesh Kamath, B.; Vidhyavathi, R.; Sarada, R.; Ravishankar, G.A. Enhancement of carotenoids by mutation and stress induced carotenogenic genes in Haematococcus pluvialis mutants. Bioresour. Technol. 2008, 99, 8667–8673. [Google Scholar] [CrossRef]
- Lee, S.J.; Sekimoto, T.; Yamashita, E.; Nagoshi, E.; Nakagawa, A.; Imamoto, N.; Yoshimura, M.; Sakai, H.; Chong, K.T.; Tsukihara, T.; et al. The structure of importin-beta bound to SREBP-2: Nuclear import of a transcription factor. Science 2003, 302, 1571–1575. [Google Scholar] [CrossRef]
- Zhu, C.; Dong, X.; Wang, X.; Zheng, Y.; Qiu, J.; Peng, Y.; Xu, J.; Chai, Z.; Liu, C. Multiple Roles of SIRT2 in Regulating Physiological and Pathological Signal Transduction. Genet Res. 2022, 2022, 9282484. [Google Scholar] [CrossRef]
- Zhang, Y.; Iratni, R.; Erdjument-Bromage, H.; Tempst, P.; Reinberg, D. Histone deacetylases and SAP18, a novel polypeptide, are components of a human Sin3 complex. Cell 1997, 89, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Wang, Z.; Xia, Y.; Shao, F.; Xia, W.; Wei, Y.; Li, X.; Qian, X.; Lee, J.H.; Du, L.; et al. The gluconeogenic enzyme PCK1 phosphorylates INSIG1/2 for lipogenesis. Nature 2020, 580, 530–535. [Google Scholar] [CrossRef]
- Rangwala, A.M.; Mingione, V.R.; Georghiou, G.; Seeliger, M.A. Kinases on Double Duty: A Review of UniProtKB Annotated Bifunctionality within the Kinome. Biomolecules 2022, 12, 685. [Google Scholar] [CrossRef]
- Muszewska, A.; Stepniewska-Dziubinska, M.M.; Steczkiewicz, K.; Pawlowska, J.; Dziedzic, A.; Ginalski, K. Fungal lifestyle reflected in serine protease repertoire. Sci. Rep. 2017, 7, 9147. [Google Scholar] [CrossRef]
- Deng, X.; Zhang, W.; O-Sullivan, I.; Williams, J.B.; Dong, Q.; Park, E.A.; Raghow, R.; Unterman, T.G.; Elam, M.B. FoxO1 inhibits sterol regulatory element-binding protein-1c (SREBP-1c) gene expression via transcription factors Sp1 and SREBP-1c. J. Biol. Chem. 2012, 287, 20132–20143. [Google Scholar] [CrossRef]
- Takeuchi, K.; Reue, K. Biochemistry, physiology, and genetics of GPAT, AGPAT, and lipin enzymes in triglyceride synthesis. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1195–E1209. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.K.; Tunison, K.; Dalal, J.S.; Nagamma, S.S.; Hamra, F.K.; Sankella, S.; Shao, X.; Auchus, R.J.; Garg, A. Metabolic, reproductive, and neurologic abnormalities in Agpat1-null mice. Endocrinology 2017, 158, 3954–3973. [Google Scholar] [CrossRef] [PubMed]
- Robinet, P.; Fradagrada, A.; Monier, M.N.; Marchetti, M.; Cogny, A.; Moatti, N.; Paul, J.L.; Vedie, B.; Lamaze, C. Dynamin is involved in endolysosomal cholesterol delivery to the endoplasmic reticulum: Role in cholesterol homeostasis. Traffic 2006, 7, 811–823. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ma, M.Y.; Sun, M.; Jiang, L.Y.; Zhao, X.T.; Fang, X.X.; Man Lam, S.; Shui, G.H.; Luo, J.; Shi, X.J.; et al. Endogenous sterol intermediates of the mevalonate pathway regulate HMGCR degradation and SREBP-2 processing. J. Lipid Res. 2019, 60, 1765–1775. [Google Scholar] [CrossRef] [PubMed]
- Alcaíno, J.; Barahona, S.; Carmona, M.; Lozano, C.; Marcoleta, A.; Niklitschek, M.; Sepúlveda, D.; Baeza, M.; Cifuentes, V. Cloning of the cytochrome P450 reductase (crtR) gene and its involvement in the astaxanthin biosynthesis of Xanthophyllomyces dendrorhous. BMC Microbiol. 2008, 8, 169. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, M.S.; Rojas, M.C.; Sepúlveda, D.; Baeza, M.; Cifuentes, V.; Alcaíno, J. Molecular characterization and functional analysis of cytochrome b5 reductase (CBR) encoding genes from the carotenogenic yeast Xanthophyllomyces dendrorhous. PLoS ONE 2015, 10, e0140424. [Google Scholar] [CrossRef] [PubMed]
- González, A.M.; Venegas, M.; Barahona, S.; Gómez, M.; Gutiérrez, M.S.; Sepúlveda, D.; Baeza, M.; Cifuentes, V.; Alcaíno, J. Damage response protein 1 (Dap1) functions in the synthesis of carotenoids and sterols in Xanthophyllomyces dendrorhous. J. Lipid Res. 2022, 63, 100175. [Google Scholar] [CrossRef] [PubMed]
- Hirano, Y.; Yoshida, M.; Shimizu, M.; Sato, R. Direct demonstration of rapid degradation of nuclear sterol regulatory element-binding proteins by the ubiquitin-proteasome pathway. J. Biol. Chem. 2001, 276, 36431–36437. [Google Scholar] [CrossRef]
- Venegas, M.; Barahona, S.; González, A.M.; Sepúlveda, D.; Zúñiga, G.E.; Baeza, M.; Cifuentes, V.; Alcaíno, J. Phenotypic analysis of mutants of ergosterol biosynthesis genes (ERG3 and ERG4) in the red yeast Xanthophyllomyces dendrorhous. Front. Microbiol. 2020, 11, 1312. [Google Scholar] [CrossRef]

| Strain/Plasmid | Description | Reference |
|---|---|---|
| Strains | ||
| DH5α | Strain of E. coli used for molecular cloning and plasmid propagation. | [32] |
| CBS 6938 | X. dendrorhous wild-type strain. Strain with wild-type pigmentation and resistant to clotrimazole. | ATCC 96594 |
| CBS.cyp61- | Mutant derived from CBS 6938. The CYP61 locus was interrupted by the zeocin resistance cassette. This strain overproduces carotenoids and is resistant to clotrimazole. | [12] |
| CBS.cyp61-.FLAG.SRE1 | Mutant derived from CBS 6938. The native SRE1 gene was replaced by a gene variant that expresses the Sre1 protein fused to the 3xFLAG epitope at its N-terminus, followed by the zeocin resistance cassette used for transformant selection. This strain overproduces carotenoids and is resistant to clotrimazole. | [9] |
| G4, K8, C3, Ñ1, A14, A2, Ñ34, K21, T1, X4, Ñ18, T16, K20, M6 and D1 | Initial 15 selected strains obtained by NTG mutagenesis from CBS.cyp61- with a wild-type pigmentation and sensible to clotrimazole. Strain K20 was included for whole-genome sequencing and SNP analysis (Sample_K20). | This work |
| 001-019 | Nineteen strains obtained by NTG mutagenesis from CBS.cyp61-.FLAG.SRE1, with a wild-type pigmentation and sensible to clotrimazole. These strains were used for whole-genome sequencing and SNP analysis (Samples 001-019). | This work |
| Plasmids | ||
| pBluescript SK- (pBS) | Cloning vector | Agilent Technologies Inc. |
| pBS-gSTP1up-down | Plasmid used to replace the X. dendrorhous STP1 gene with the wild-type sequence of the gene by homologous recombination in strains obtained by random mutagenesis. | [11] |
| pXd-gSRE1-zeo | Plasmid used to replace the X. dendrorhous SRE1 gene with the wild-type sequence of the gene by homologous recombination in strains obtained by random mutagenesis. | [11] |
| pXd-gSRE1N-zeo | Plasmid used to replace the X. dendrorhous SRE1 gene with the gene variant that expresses Sre1N (Sre1 N-terminal domain) by homologous recombination in strains obtained by random mutagenesis. | [9] |
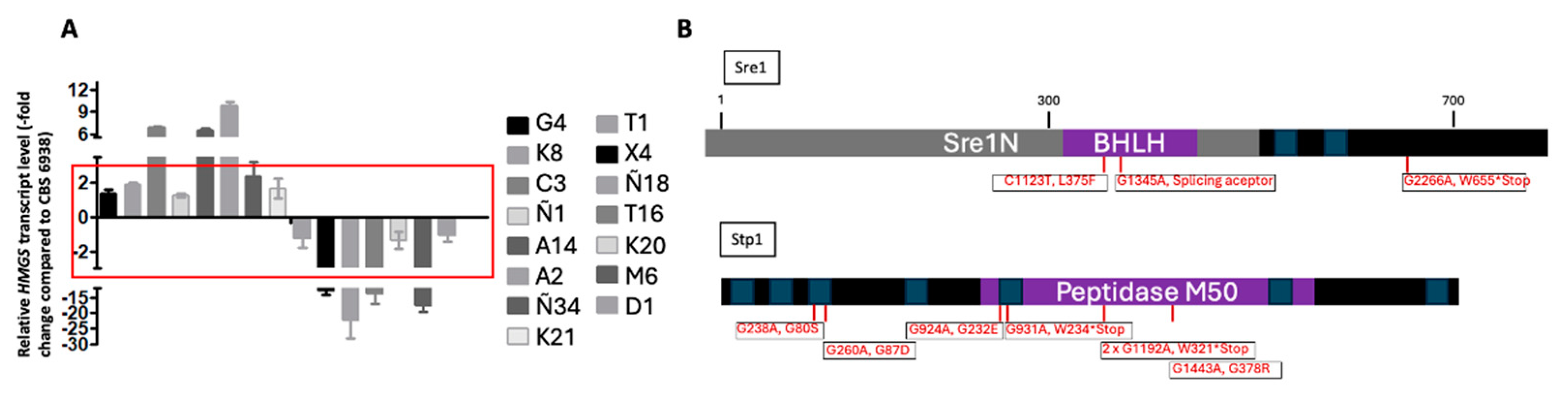
| Mutant | HMGS Transcript Levels Compared to wt Strain | Sre1N Complementation | Mutation in Genes (Sequencing) | SRE1 Complementation | STP1 Complementation |
|---|---|---|---|---|---|
| G4 | similar | Yes | SRE1 | Yes | - |
| K8 | similar | No | - | - | - |
| C3 | upregulated | - | - | - | - |
| Ñ1 | similar | Yes | STP1 | No | Yes |
| A14 | upregulated | - | - | - | - |
| A2 | upregulated | - | - | - | - |
| Ñ34 | similar | Yes | None | No | No |
| K21 | similar | Yes | STP1 | No | Yes |
| T1 | similar | Yes | STP1 | No | Yes |
| X4 | downregulated | - | - | - | - |
| Ñ18 | downregulated | - | - | - | - |
| T16 | downregulated | - | - | - | - |
| K20 | Similar | Yes | None | No | No |
| M6 | downregulated | - | - | - | - |
| D1 | similar | Yes | STP1 | No | Yes |
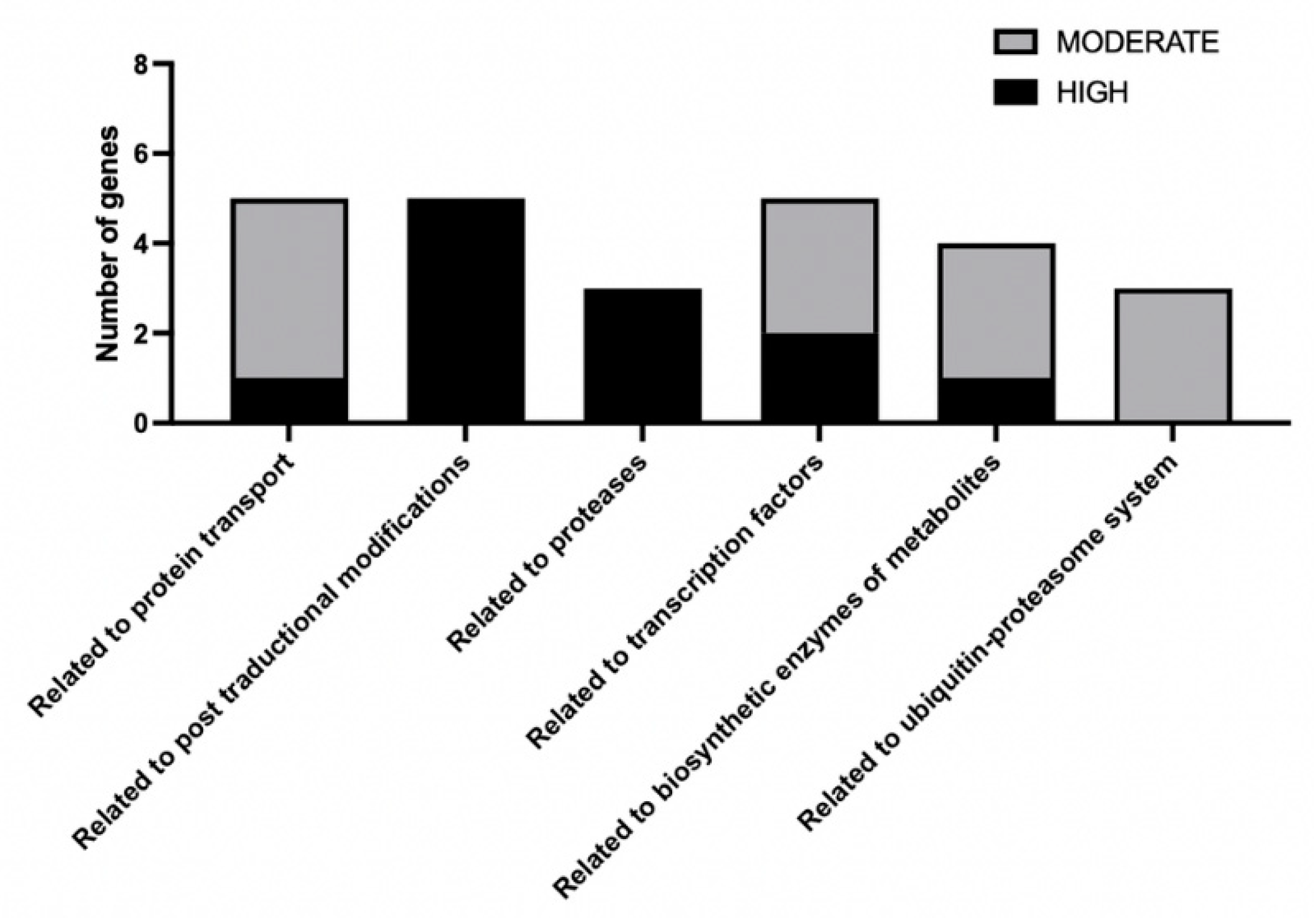
| Gene Code (#) | Protein Effect | Blast Hit (Protein Ref_seq) | InterProScan Hit | Log2 Change | p-Value Adjust |
|---|---|---|---|---|---|
| g904 | Arg288 * | delta-sterol C-methyltransferase | Erg6/SMT methyltransferase (IPR050447) | 4.48 × 100 | 4.28 × 10−36 |
| g5458 | Trp371 * | pyridoxal kinase | Pyridoxamine kinase/Phosphomethylpyrimidine kinase (IPR013749) | 1.47 × 100 | 2.75× 10−9 |
| g5041 | Splicing | ND | ND | 1.29 × 100 | 1.11 × 10−7 |
| g751 | Splicing | ND | ND | 1.05 × 100 | 1.05 × 10−5 |
| g2375 | Splicing | ND | ND | 1.02 × 100 | 6.45 × 10−3 |
| g6106 | Glu46 * | ND | ND | 8.70 × 10−1 | 4.92 × 10−1 |
| g5290 | Arg480 * | ND | Chromatin SPT2 (IPR013256) | 7.75 × 10−1 | 5.10 × 10−3 |
| g1695 | Met1? | predicted protein | Signal peptidase complex subunit 2 (IPR009582) | 7.09 × 10−1 | 2.79 × 10−3 |
| g6091 | Splicing | urea transporter | Sodium/solute symporter (IPR001734) | 6.84 × 10−1 | 1.10 × 10−1 |
| g3457 | Splicing | Ribokinase-like protein | Carbohydrate kinase PfkB (IPR011611) | 6.64 × 10−1 | 1.28 × 10−2 |
| g2927 | Leu248 * | ND | ND | 6.10 × 10−1 | 1.72 × 10−1 |
| g5215 | Splicing | glutathione peroxidase | Glutathione peroxidase (IPR000889) | 5.52 × 10−1 | 9.42 × 10−3 |
| g4522 | Met1? | hypothetical protein TREMEDRAFT_69977 | Protein Zds1, C-terminal (IPR013941) | 3.78 × 10−1 | 1.65 × 10−1 |
| g3946 | Splicing | 2-acylglycerol O-acyltransferase 2 | Diacylglycerol acyl transferase (IPR007130) | 3.38 × 10−1 | 2.36 × 10−1 |
| g4324 (2) | Splicing (both) | predicted protein | ND | 2.67 × 10−1 | 1.88 × 10−1 |
| g3664 | Trp16 * | hypothetical protein TREMEDRAFT_73274 | ND | 2.58 × 10−1 | 4.42 × 10−1 |
| g108 | Splicing | ARM repeat-containing protein | Exportin-1/Importin-beta-like (IPR013598) | 1.66 × 10−1 | 4.75 × 10−1 |
| g671 | Splicing | electron-transferring-flavoprotein dehydrogenase | ND | 1.58 × 10−1 | 6.36 × 10−1 |
| g716 | Splicing | Mov34-domain-containing protein | Cop9 signalosome subunit 5C-terminal domain (IPR040961) | 1.32 × 10−1 | 6.27 × 10−1 |
| g3597 | Splicing | predicted protein | ND | 7.96 × 10−2 | 8.89 × 10−1 |
| g2198 | Splicing | DNA repair protein | ERCC4 domain (IPR006166) | 6.56 × 10−2 | 8.35 × 10−1 |
| g5577 (2) | Gln669 * Trp724 * | transcription factor | Ankyrin repeat-containing domain superfamily (IPR036770) | 4.79 × 10−3 | 9.90 × 10−1 |
| g3833 | Splicing | ND | ND | −5.05 × 10−2 | 8.92 × 10−1 |
| g5637 | Splicing | membrane organization and biogenesis-related protein | TB2/DP1/HVA22-related protein (IPR004345) | −7.98 × 10−2 | 7.78 × 10−1 |
| g4193 | Splicing | hypothetical protein I206_05628 | Transcription factor domain, fungi (IPR007219) | −9.16 × 10−2 | 8.37 × 10−1 |
| g3231 | Trp239 * | hypothetical protein TRAVEDRAFT_171989 | Peptidase M50 (IPR008915) | −9.57 × 10−2 | 7.89 × 10−1 |
| g2846 | Splicing | NAD-dependent protein deacetylase sirtuin-2-like | Sirtuin, class II (IPR026587) | −2.05 × 10−1 | 3.30 × 10−1 |
| g4683 | Met1? | predicted protein | Sin3 associated polypeptide p18 (IPR010516) | −2.68 × 10−1 | 4.21 × 10−1 |
| g3594 | Trp641 * | vacuolar membrane protein, putative | PQ-loop repeat (IPR006603) | −2.79 × 10−1 | 1.35 × 10−1 |
| g2091 | Splicing | acid phosphatase, putative | Histidine phosphatase superfamily, clade-2 (IPR000560) | −3.96 × 10−1 | 2.53 × 10−1 |
| g6351 | Trp489 * | ND | Pentatrico peptide repeat (IPR002885) | −4.42 × 10−1 | 8.19 × 10−2 |
| g1730 | Trp413 * | hypothetical protein EHS24_009014 | Zinc finger C2H2-type (IPR013087) | −4.94 × 10−1 | 2.25 × 10−2 |
| g2575 | Trp241 * | kinesin-domain-containing protein | Kinesin motor domain (IPR001752) | −5.01 × 10−1 | 1.17 × 10−2 |
| g1263 | Splicing | predicted protein | ND | −6.28 × 10−1 | 7.80 × 10−3 |
| g1367 | Trp254 * | glucocorticoid receptor-like protein | ND | −6.97 × 10−1 | 2.01 × 10−2 |
| g3954 | Cys548 * | hypothetical protein L202_04737 | ND | −7.10 × 10−1 | 7.20 × 10−5 |
| g5918 | Splicing | predicted protein | Integral membrane bound transporter domain (IPR049453) | −7.59 × 10−1 | 7.59 × 10−4 |
| g6306 | Arg929 * | RNB-domain-containing protein | Nucleic acid-binding, OB-fold (IPR012340) | −8.12 × 10−1 | 4.54 × 10−4 |
| g4290 | Splicing | serine-type endopeptidase, putative | Peptidase S8/S53 domain superfamily (IPR036852) | −9.27 × 10−1 | 6.99 × 10−9 |
| g4519 | Splicing | amino acid transporter, putative | Amino acid permease/SLC12A domain (IPR004841) | −9.82 × 10−1 | 1.55 × 10−4 |
| g1606 | Lys1419 * | multi-sensor hybrid histidine kinase | Histidine kinase/HSP90-like ATPase (IPR003594) | −1.01 × 100 | 6.96 × 10−5 |
| g5825 | Trp373 * | glucan endo-1,3-alpha-glucosidase agn1 | Glycoside hydrolase family 71 (IPR005197) | −1.25 × 100 | 2.04 × 10−7 |
| g912 | Splicing | putative alcohol dehydrogenase | Alcohol dehydrogenase-like, N-terminal (IPR013154) | −1.28 × 100 | 1.21 × 10−9 |
| g5575 | Splicing | signal sequence binding protein | Sortilin, C-terminal (IPR031777) | −1.55 × 100 | 1.04 × 10−14 |
| g890 | Splicing | putative alcohol dehydrogenase | Alcohol dehydrogenase-like, C-terminal (IPR013149) | −1.82 × 100 | 1.42 × 10−15 |
| g693 | Splicing | predicted protein | Band7/SPFH domain superfamily (IPR036013) | −2.20 × 100 | 2.31 × 10−15 |
| g1908 | Trp59 * | glutamine synthetase | Glutamine synthetase, N-terminal domain superfamily (IPR036651) | −2.63 × 100 | 9.99 × 10−25 |
| g5769 | Glu626 * | DDE-type integrase/transposase/recombinase | Zinc finger, CCHC-type (IPR001878) | −3.16 × 100 | 1.21 × 10−22 |
| g4483 | Gln126 * | dihydrodipicolinate synthase | ND | −3.59 × 100 | 1.41 × 10−24 |
| Gene Code (#) | Protein Effect | Blast Hit | InterProScan Hit | Log2 Change | p-Value Adjust |
|---|---|---|---|---|---|
| g904 | Pro265Leu | delta-sterol C-methyltransferase | Erg6/SMT methyltransferase (IPR050447) | 4.48 × 100 | 4.28 × 10−36 |
| g5928 | Val363Met | cytochrome P450 oxidoreductase | NADPH-cytochrome P450 reductase (IPR023208) | 2.56 × 100 | 1.19 × 10−13 |
| g6107 (5) | Gly263Arg Ala209Val Ala203Asp Gly171Glu Gly3Glu | ATP synthase F0 subunit 6 (mitochondrion) | ATP synthase, F0 complex, subunit A (IPR000568) | 2.56 × 100 | 1.49 × 10−1 |
| g1377 | Val32Met | hydroxymethylglutaryl-CoA reductase (NADPH) | Hydroxymethylglutaryl-CoA reductase, class I/II (IPR002202) | 1.49 × 100 | 4.40 × 10−9 |
| g5142 | Val805Ile | ribosome biogenesis protein tsr1 | Ribosome biogenesis protein BMS1/TSR1, C-terminal (IPR007034) | 1.40 × 100 | 2.07 × 10−6 |
| g1155 | Ala310Val | P-loop containing nucleoside triphosphate hydrolase protein | P-loop containing nucleoside triphosphate hydrolase (IPR027417) | 1.32 × 100 | 6.28× 10−9 |
| g3604 | Pro158Leu | ribulose-5-phosphate 3-epimerase | Ribulose-phosphate 3-epimerase-like (IPR000056) | 1.31 × 100 | 8.59× 10−7 |
| g5830 | Thr68Ile | FK506-binding protein 2 | N/D | 1.23 × 100 | 1.02 × 10−4 |
| g4594 | Gly246Asp | pyruvate kinase | Pyruvate kinase, C-terminal (IPR015795) | 1.08 × 100 | 9.34 × 10−3 |
| g2552 | Val68Ile | Phosphoribosyl aminoimidazole carboxamide formyl transferase/IMP cyclohydrolase | Bifunctional purine biosynthesis protein PurH-like (IPR002695) | 1.08 × 100 | 2.17 × 10−4 |
| g368 | Ser6Ile | 1-alkyl-2-acetyl glycerol phospho choline esterase | WD40 repeat (IPR001680) | 1.01 × 100 | 5.35 × 10−6 |
| g3129 | Tyr686Cys | ATP-dependent RNA helicase DDX35 | Helicase associated domain (HA2), winged-helix domain (IPR048333) | 9.80 × 10−1 | 8.76 × 10−5 |
| g2716 | Pro39Leu | U3 sno RNP-associated protein Rrp5 | Tetratricopeptide-like helical domain superfamily (IPR011990) | 9.80 × 10−1 | 1.65 × 10−4 |
| g402 | Pro596Ser | dimethylaniline monooxygenase (N-oxide forming) | FAD/NAD(P)-binding domain superfamily (IPR036188) | 6.10 × 10−1 | 1.76 × 10−2 |
| g378 | Val58Met | ribose-phosphate pyrophosphokinase | Phosphoribosyl transferase-like (IPR029057) | 5.37 × 10−1 | 1.64 × 10−2 |
| g3461 | Arg132Gln | putative CDC12-septin | P-loop containing nucleoside triphosphate hydrolase (IPR027417) | 4.73 × 10−1 | 4.02 × 10−2 |
| g6171 | Val532Met | importin-alpha export receptor | Importin-beta, N-terminal domain (IPR001494) | 4.61 × 10−1 | 5.93 × 10−2 |
| g2350 | Pro158Leu | leukotriene A-4 hydrolase/aminopeptidase | Aminopeptidase N-like, N-terminal domain (IPR045357) | 4.41 × 10−1 | 2.23 × 10−2 |
| g3216 | Val3010Ile | Midasin | Von Willebrand factor A-like domain superfamily (IPR036465) | 4.38 × 10−1 | 2.03 × 10−1 |
| g5516 | Ile204Met | gamma-tubulin complex component 3 | Gamma tubulin complex component protein, N-terminal (IPR041470) | 3.83 × 10−1 | 1.04 × 10−1 |
| g4453 | Ser987Phe | ribosome assembly protein 1 | Translation protein, beta-barrel domain superfamily (IPR009000) | 2.99 × 10−1 | 1.51 × 10−1 |
| g5781 | Pro70Ser | DEAD-domain-containing protein | Helicase, C-terminal domain-like (IPR001650) | 2.85 × 10−1 | 2.02 × 10−1 |
| g3358 | Thr358Ile | predicted protein | Csn12 family (IPR045114) | 2.24 × 10−1 | 4.31 × 10−1 |
| g6320 | Pro14Ser | proteasome subunit alpha type 4, putative | Proteasome, subunit alpha/beta (IPR001353) | 1.95 × 10−1 | 3.95 × 10−1 |
| g1143 | Thr63Ile | allantoicase, putative | Rml C-like cupin domain superfamily (IPR011051) | 1.78 × 10−1 | 5.05 × 10−1 |
| g1906 | Asn146Ser | L-fucose transporter | MFS transporter superfamily (IPR036259) | 1.78 × 10−1 | 5.57 × 10−1 |
| g1317 | Pro435Ser | hypothetical protein GLOIN_2v1436072, partial | Zinc finger C2H2-type (IPR013087) | 9.64 × 10−2 | 7.96 × 10−1 |
| g4775 | Met82Ile | pyruvate dehydrogenase (acetyl-transferring) E1 component, alpha subunit | Pyruvate dehydrogenase (acetyl-transferring) E1 component, alpha subunit, subgroup y (IPR017597) | 5.48 × 10−2 | 9.00 × 10−1 |
| g2238 | Val1270Ile | PAB-dependent poly(A)-specific ribonuclease subunit PAN2 | WD40-repeat-containing domain superfamily (IPR036322) | −3.84 × 10−2 | 8.75 × 10−1 |
| g5211 | Val213Phe | protein arginine N-methyltransferase | S-adenosyl-L-methionine-dependent methyltransferase superfamily (IPR029063) | −8.02 × 10−2 | 8.85 × 10−1 |
| g2877 | Thr322Ile | DNA polymerase zeta subunit | Ribonuclease H-like superfamily (IPR012337) | −9.34 × 10−2 | 7.57 × 10−1 |
| g5939 | Leu1427Phe | hypothetical protein PUNSTDRAFT_35157, partial | Zinc finger, RING-CH-type (IPR011016) | −1.00 × 10−1 | 7.56 × 10−1 |
| g2572 | Gly125Ser | biotin-[acetyl-CoA-carboxylase] ligase | Class II Aminoacyl-tRNA synthetase/Biotinyl protein ligase (BPL) and lipoyl protein ligase (LPL) (IPR045864) | −1.37 × 10−1 | 6.32 × 10−1 |
| g4518 | Ala331Thr | PLP-dependent transferase | Pyridoxal phosphate-dependent transferase (IPR015424) | −1.67 × 10−1 | 5.77 × 10−1 |
| g2586 | Val445Ile | ATPase, V1 complex, subunit H | Armadillo-type fold (IPR016024) | −1.70 × 10−1 | 6.08 × 10−1 |
| g5199 | Trp665Arg | CRM1 C terminal-domain-containing protein | Importin-beta, N-terminal domain (IPR001494) | −1.71 × 10−1 | 5.88 × 10−1 |
| g3195 | Val106Met | replication factor C subunit 3/5 | DNA polymerase III, clamp loader complex, gamma/delta/delta subunit, C-terminal (IPR008921) | −1.84 × 10−1 | 5.81 × 10−1 |
| g4523 | Gly63Asp | Golgi phosphoprotein 3 | Golgi phosphoprotein 3-like (IPR008628) | −2.92 × 10−1 | 3.03 × 10−1 |
| g3705 | Pro154Ser | dynamin protein dnm1, putative | Dynamin, N-terminal (IPR045063) | −3.03 × 10−1 | 2.22 × 10−1 |
| g3050 (2) | Ile10Ser Asn11Asp | 1-acylglycerol-3-phosphate O-acyltransferase, putative | 1-acyl-sn-glycerol-3-phosphate acyl transferase (IPR004552) | −3.04 × 10−1 | 4.53 × 10−1 |
| g3734 | Ser747Phe | kinesin-domain-containing protein | Kinesin motor domain (IPR001752) | −3.30 × 10−1 | 5.77 × 10−1 |
| g1433 | Ser91Phe | chaperone, putative | Tetratricopeptide repeat (IPR019734) | −3.80 × 10−1 | 1.72 × 10−1 |
| g2728 | Asp797Asn | adaptin N terminal region-domain-containing protein | Coatomer subunit gamma, C-terminal (IPR032154) | −3.93 × 10−1 | 2.40 × 10−1 |
| g3299 | Val439Met | S-adenosyl-L-methionine-dependent methyltransferase | Zinc finger C2H2 superfamily (IPR036236) | −4.07 × 10−1 | 1.10 × 10−1 |
| g5836 | Gly74Glu | peptidyl-prolyl cis-trans isomerase B | Cyclophilin-like domain superfamily (IPR029000) | −4.53 × 10−1 | 5.95 × 10−2 |
| g4740 | Gly42Arg | ATPase GET3 | Anion-transporting ATPase-like domain (IPR025723) | −4.75 × 10−1 | 2.06 × 10−1 |
| g4621 | Ala48Thr | homocitrate synthase | Pyruvate carboxyltransferase (IPR000891) | −4.97 × 10−1 | 3.62 × 10−2 |
| g2740 | Ala62Val | hypothetical protein SCHCODRAFT_79669 | Aldehyde dehydrogenase domain (IPR015590) | −5.00 × 10−1 | 1.71 × 10−2 |
| g3165 | Asp98Asn | Enolase | Enolase (IPR000941) | −5.13 × 10−1 | 5.18 × 10−2 |
| g3166 | Thr388Ile | dihydroxy-acid dehydratase | Dihydroxy-acid dehydratase (IPR004404) | −5.18 × 10−1 | 9.54 × 10−2 |
| g3697 | Arg295Gln | SPOC domain-like protein | Ku, C-terminal (IPR014893) | −5.27 × 10−1 | 2.18 × 10−2 |
| g1933 | Thr278Ile | ATP-dependent RNA helicase eIF4A | Helicase, C-terminal domain-like (IPR001650) | −6.23 × 10−1 | 4.05 × 10−2 |
| g6193 | Ala163Thr | predicted protein | Armadillo-type fold (IPR016024) | −7.16 × 10−1 | 7.89 × 10−2 |
| g630 | Ala71Thr | peroxisomal assembly protein PEX3 | Peroxin-3 (IPR006966) | −8.89 × 10−1 | 8.92 × 10−5 |
| g4137 | Asp574Asn | vacuolar protein sorting protein (VPS11), putative | Vacuolar protein sorting protein 11 C-terminal (IPR024763) | −9.29 × 10−1 | 1.33 × 10−3 |
| g4083 | Val245Ile | GTP-binding protein TypA | EF-G domain III/V-like (IPR035647) | −9.93 × 10−1 | 2.31 × 10−3 |
| g767 | Glu472Lys | glycogen(starch) synthase | N/D | −1.05 × 100 | 5.17 × 10−3 |
| g3532 | Thr252Ile | Arginase | Ureohydrolase domain superfamily (IPR023696) | −1.10 × 100 | 7.19 × 10−5 |
| g2334 | Val266Met | acetyl-CoA acyltransferase | Thiolase, N-terminal (IPR020616) | −1.14 × 100 | 5.15 × 10−4 |
| g3912 | Thr184Pro | cyclin-dependent protein kinase inhibitor | SPX domain (IPR004331) | −1.31 × 100 | 7.10 × 10−5 |
| g3916 | Gly112Arg | DNA repair protein RAD51 | DNA recombination and repair protein Rad51-like, C-terminal (IPR013632) | −1.50 × 100 | 7.27 × 10−4 |
| g927 | Gly506Glu | heat shock protein 70 | ATPase, nucleotide binding domain (IPR043129) | −1.51 × 100 | 2.25 × 10−3 |
| g304 | Val201Phe | hexose transport-related protein, putative | Major facilitator, sugar transporter-like (IPR005828) | −1.52 × 100 | 1.27 × 10−5 |
| g4590 | Ser36Tyr | putative 60S ribosomal protein L19 | Large ribosomal subunit protein L19 domain (IPR000196) | −1.53 × 100 | 3.05 × 10−7 |
| g683 | Gly5Asp | solute carrier family 36 (proton-coupled amino acid transporter) | Metalloenzyme, LuxS/M16 peptidase-like (IPR011249) | −1.69 × 100 | 4.15 × 10−6 |
| g6004 (2) | Leu906Phe Val3038Met | E3 ubiquitin-protein ligase ptr1 | E3 ubiquitin ligase, domain of unknown function DUF908 (IPR010309) | −1.98 × 100 | 7.90 × 10−9 |
| g1436 | Ser64Leu | Glucan endo-1,3-alpha-glucosidase agn1 | Carbohydrate-binding WSC (IPR002889) | −2.57 × 100 | 5.10 × 10−16 |
| g5769 | Gln277Pro | DDE-type integrase/transposase/recombinase | Zinc finger, CCHC-type (IPR001878) | −3.16 × 100 | 1.21 × 10−22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venegas, M.; Durán, A.; Campusano, S.; Barahona, S.; Sepúlveda, D.; Baeza, M.; Cifuentes, V.; Alcaíno, J. Identification of Potential New Genes Related to the SREBP Pathway in Xanthophyllomyces dendrorhous. Biomolecules 2024, 14, 778. https://doi.org/10.3390/biom14070778
Venegas M, Durán A, Campusano S, Barahona S, Sepúlveda D, Baeza M, Cifuentes V, Alcaíno J. Identification of Potential New Genes Related to the SREBP Pathway in Xanthophyllomyces dendrorhous. Biomolecules. 2024; 14(7):778. https://doi.org/10.3390/biom14070778
Chicago/Turabian StyleVenegas, Maximiliano, Alejandro Durán, Sebastián Campusano, Salvador Barahona, Dionisia Sepúlveda, Marcelo Baeza, Víctor Cifuentes, and Jennifer Alcaíno. 2024. "Identification of Potential New Genes Related to the SREBP Pathway in Xanthophyllomyces dendrorhous" Biomolecules 14, no. 7: 778. https://doi.org/10.3390/biom14070778
APA StyleVenegas, M., Durán, A., Campusano, S., Barahona, S., Sepúlveda, D., Baeza, M., Cifuentes, V., & Alcaíno, J. (2024). Identification of Potential New Genes Related to the SREBP Pathway in Xanthophyllomyces dendrorhous. Biomolecules, 14(7), 778. https://doi.org/10.3390/biom14070778






