Guanidinium Chloride-Induced Haemolysis Assay to Measure New Permeation Pathway Functionality in Rodent Malaria Plasmodium berghei
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement and Mice
2.2. Culturing of Plasmodium berghei Parasites
2.3. Synchronising P. berghei Parasites
2.4. Osmotic Lysis Assay
3. Results
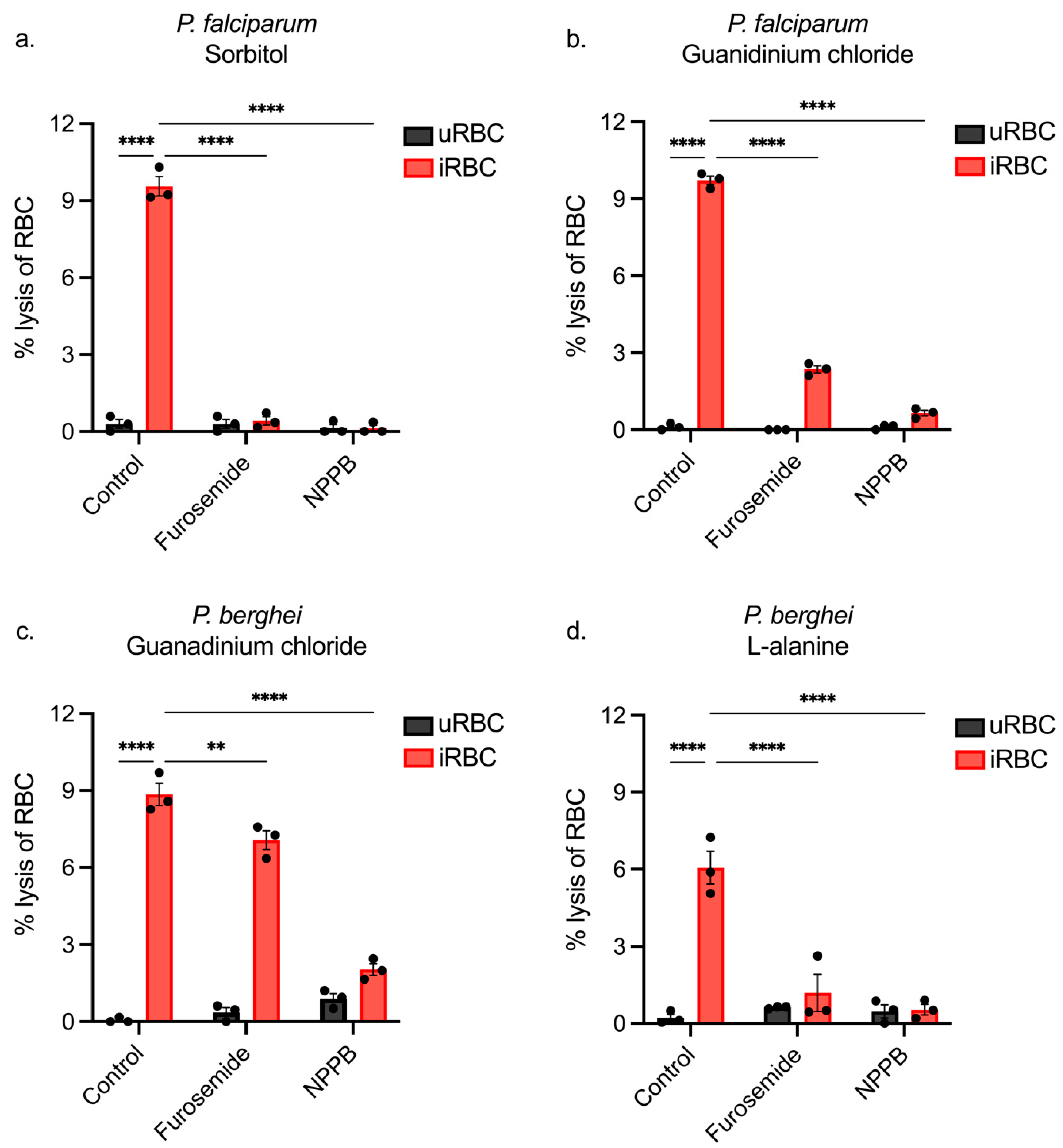
3.1. Sorbitol Lyses Both Infected and Uninfected Rodent RBCs
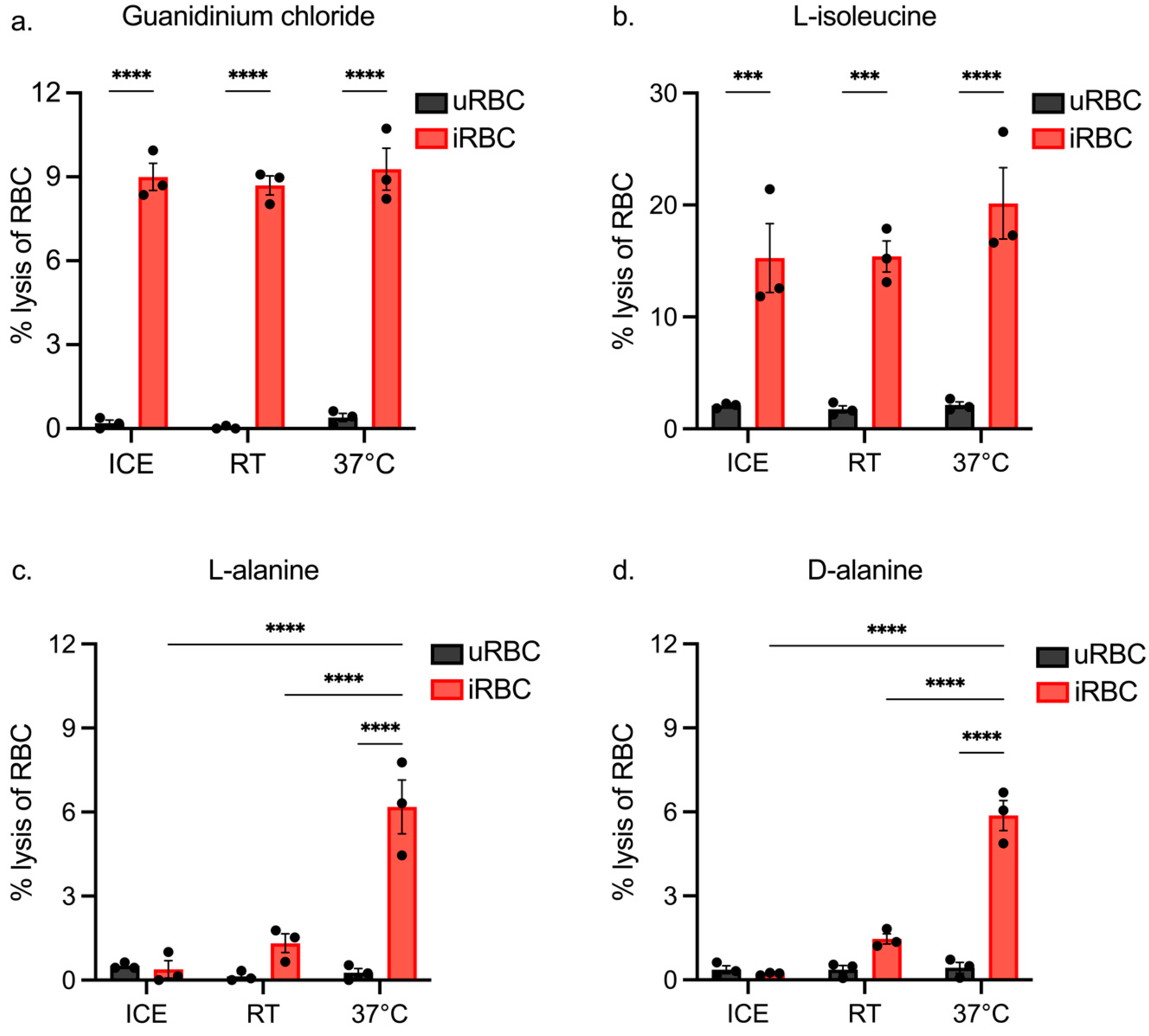
3.2. Assessing iRBC Lysis of Isotonic Solutions at Different Temperatures
3.3. Assessing the Impact of Incubation Length on iRBC and uRBC Lysis by Isotonic Solutions
3.4. Using NPP Inhibitors to Determine the Pathway of iRBC Lysis
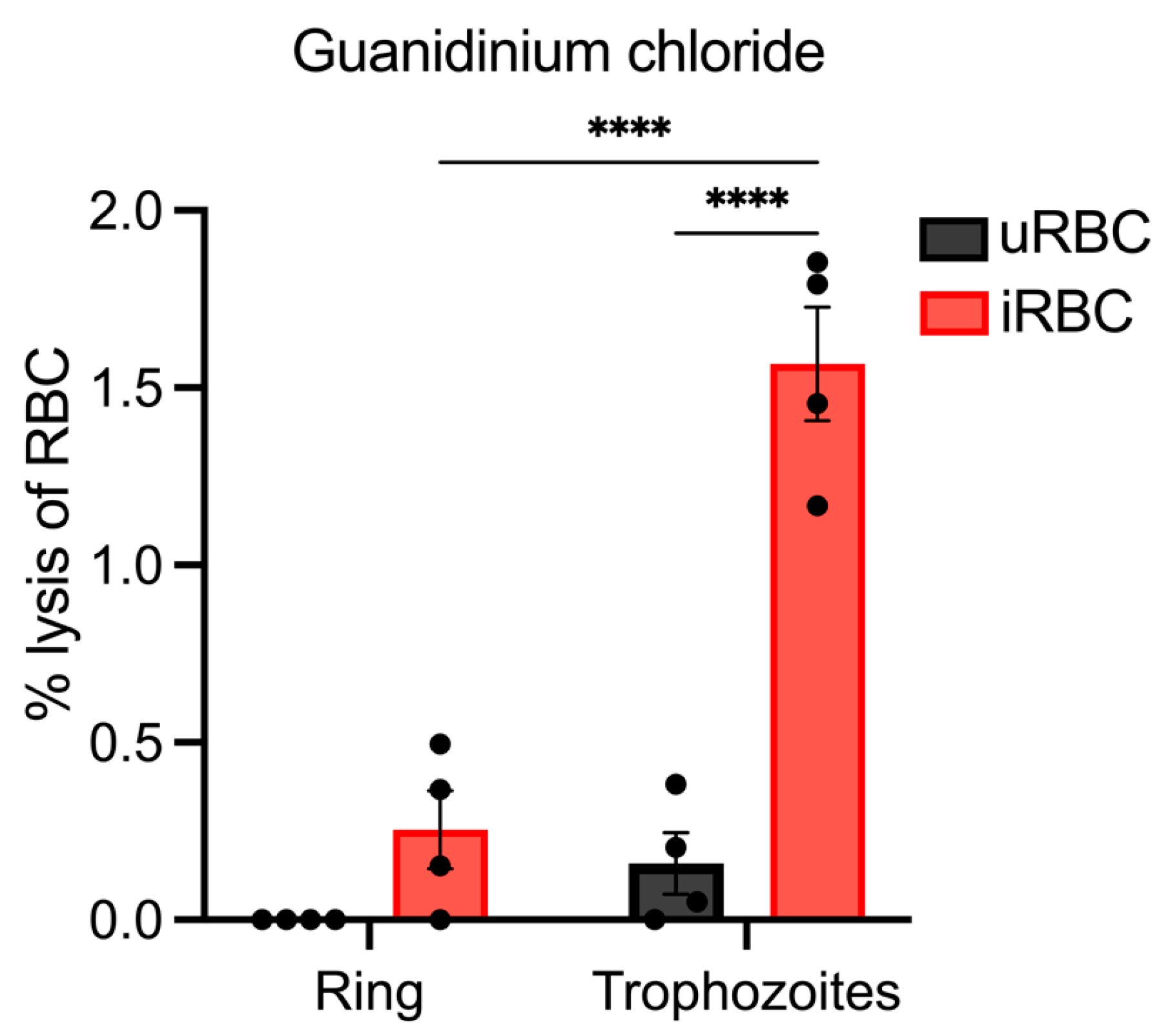
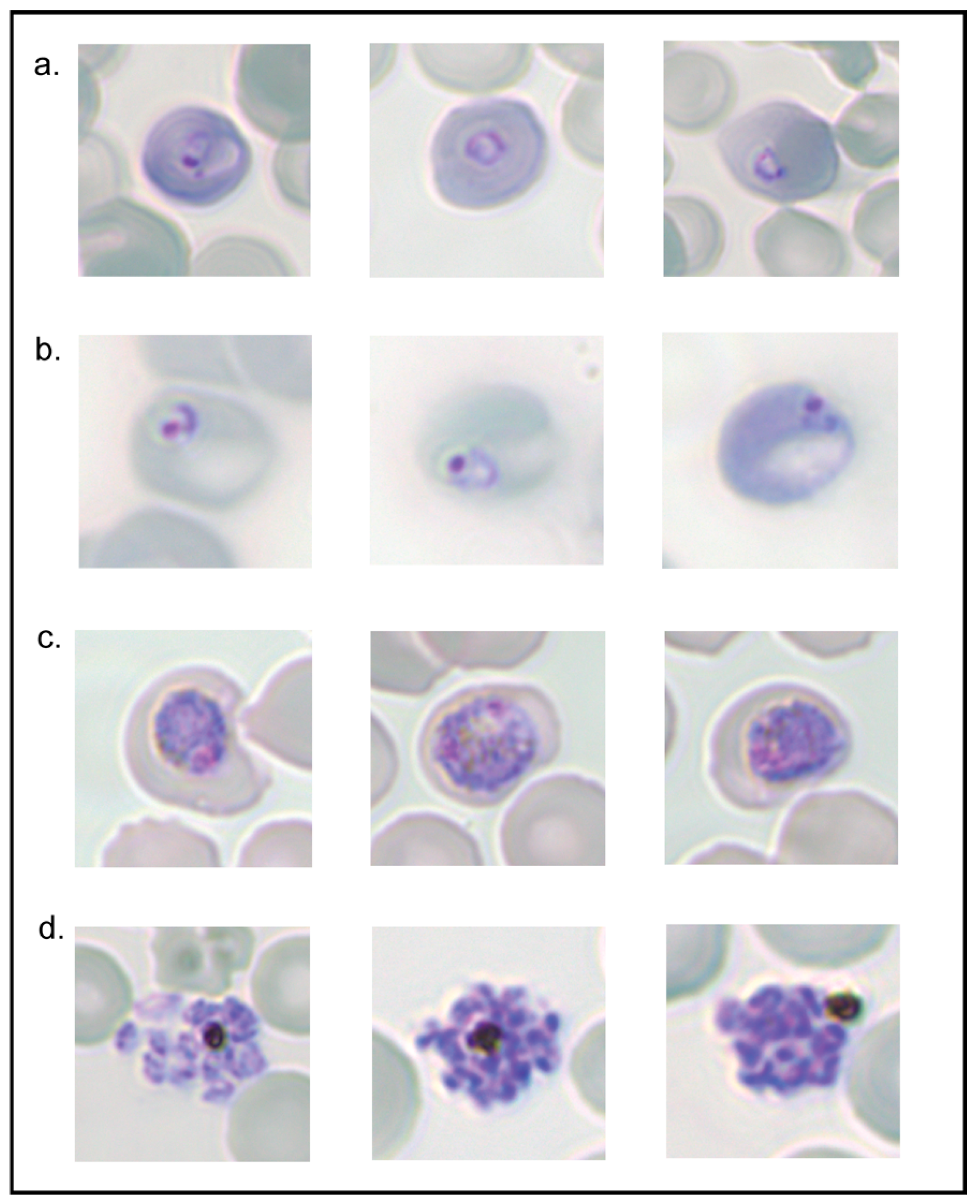
3.5. Lysis of Synchronous Populations of P. berghei iRBCs
3.6. P. berghei Survival and Growth following Guanidinium Lysis Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Straimer, J.; Gnädig, N.F.; Witkowski, B.; Amaratunga, C.; Duru, V.; Ramadani, A.P.; Dacheux, M.; Khim, N.; Zhang, L.; Lam, S.; et al. K13-propeller mutations confer artemisinin resistance in Plasmodium falciparum clinical isolates. Science 2015, 347, 428–431. [Google Scholar] [CrossRef] [PubMed]
- Uwimana, A.; Legrand, E.; Stokes, B.H.; Ndikumana, J.-L.M.; Warsame, M.; Umulisa, N.; Ngamije, D.; Munyaneza, T.; Mazarati, J.-B.; Munguti, K.; et al. Emergence and clonal expansion of in vitro artemisinin-resistant Plasmodium falciparum kelch13 R561H mutant parasites in Rwanda. Nat. Med. 2020, 26, 1602–1608. [Google Scholar] [CrossRef] [PubMed]
- Uwimana, A.; Umulisa, N.; Venkatesan, M.; Svigel, S.S.; Zhou, Z.; Munyaneza, T.; Habimana, R.M.; Rucogoza, A.; Moriarty, L.F.; Sandford, R.; et al. Association of Plasmodium falciparum kelch13 R561H genotypes with delayed parasite clearance in Rwanda: An open-label, single-arm, multicentre, therapeutic efficacy study. Lancet Infect. Dis. 2021, 21, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.A.; Krogstad, D.J.; McCleskey, E.W. A nutrient-permeable channel on the intraerythrocytic malaria parasite. Nature 1993, 362, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Kirk, K.; Horner, H.; Elford, B.; Ellory, J.; Newbold, C. Transport of diverse substrates into malaria-infected erythrocytes via a pathway showing functional characteristics of a chloride channel. J. Biol. Chem. 1994, 269, 3339–3347. [Google Scholar] [CrossRef] [PubMed]
- Pillai, A.D.; Pain, M.; Solomon, T.; Bokhari, A.A.B.; Desai, S.A. A Cell-Based High-Throughput Screen Validates the Plasmodial Surface Anion Channel As an Antimalarial Target. Mol. Pharmacol. 2010, 77, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Overman, R.R. Reversible cellular permeability alterations in disease; in vivo studies on sodium, potassium and chloride concentrations in erythrocytes of the malarious monkey. Am. J. Physiol. 1948, 152, 113–121. [Google Scholar] [CrossRef]
- Ginsburg, H.; Krugliak, M.; Eidelman, O.; Cabantchik, Z.I. New permeability pathways induced in membranes of Plasmodium falciparum infected erythrocytes. Mol. Biochem. Parasitol. 1983, 8, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.A.; Bezrukov, S.M.; Zimmerberg, J. A voltage-dependent channel involved in nutrient uptake by red blood cells infected with the malaria parasite. Nature 2000, 406, 1001–1005. [Google Scholar] [CrossRef]
- Staines, H.M.; Alkhalil, A.; Allen, R.J.; De Jonge, H.R.; Derbyshire, E.; Egée, S.; Ginsburg, H.; Hill, D.A.; Huber, S.M.; Kirk, K.; et al. Electrophysiological studies of malaria parasite-infected erythrocytes: Current status. Int. J. Parasitol. 2007, 37, 475–482. [Google Scholar] [CrossRef]
- Nguitragool, W.; Bokhari, A.A.; Pillai, A.D.; Rayavara, K.; Sharma, P.; Turpin, B.; Aravind, L.; Desai, S.A. Malaria Parasite clag3 Genes Determine Channel-Mediated Nutrient Uptake by Infected Red Blood Cells. Cell 2011, 145, 665–677. [Google Scholar] [CrossRef]
- Nguitragool, W.; Rayavara, K.; Desai, S.A. Proteolysis at a Specific Extracellular Residue Implicates Integral Membrane CLAG3 in Malaria Parasite Nutrient Channels. PLoS ONE 2014, 9, e93759. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Bokhari, A.A.B.; Pillai, A.D.; Crater, A.K.; Gezelle, J.; Saggu, G.; Nasamu, A.S.; Ganesan, S.M.; Niles, J.C.; Desai, S.A. Complex nutrient channel phenotypes despite Mendelian inheritance in a Plasmodium falciparum genetic cross. PLoS Pathog. 2020, 16, e1008363. [Google Scholar] [CrossRef] [PubMed]
- Comeaux, C.A.; Coleman, B.I.; Bei, A.K.; Whitehurst, N.; Duraisingh, M.T. Functional analysis of epigenetic regulation of tandem RhopH1/clag genes reveals a role in Plasmodium falciparum growth. Mol. Microbiol. 2011, 80, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Pillai, A.D.; Nguitragool, W.; Lyko, B.; Dolinta, K.; Butler, M.M.; Nguyen, S.T.; Peet, N.P.; Bowlin, T.L.; Desai, S.A. Solute restriction reveals an essential role for clag3-associated channels in malaria parasite nutrient acquisition. Mol. Pharmacol. 2012, 82, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Counihan, N.A.; Chisholm, S.A.; Bullen, H.E.; Srivastava, A.; Sanders, P.R.; Jonsdottir, T.K.; Weiss, G.E.; Ghosh, S.; Crabb, B.S.; Creek, D.J.; et al. Plasmodium falciparum parasites deploy RhopH2 into the host erythrocyte to obtain nutrients, grow and replicate. eLife 2017, 6, 23217. [Google Scholar] [CrossRef]
- Ito, D.; Schureck, M.A.; Desai, S.A. An essential dual-function complex mediates erythrocyte invasion and channel-mediated nutrient uptake in malaria parasites. eLife 2017, 6, 23485. [Google Scholar] [CrossRef] [PubMed]
- Sherling, E.S.; Knuepfer, E.; Brzostowski, J.A.; Miller, L.H.; Blackman, M.J.; van Ooij, C. The Plasmodium falciparum rhoptry protein RhopH3 plays essential roles in host cell invasion and nutrient uptake. eLife 2017, 6, 23239. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.M.; Li, X.; Lai, M.; Terwilliger, T.C.; Beck, J.R.; Wohlschlegel, J.; Goldberg, D.E.; Fitzpatrick, A.W.P.; Zhou, Z.H. Bottom-up structural proteomics: CryoEM of protein complexes enriched from the cellular milieu. Nat. Meth. 2020, 17, 79–85. [Google Scholar] [CrossRef]
- Schureck, M.A.; Darling, J.E.; Merk, A.; Shao, J.; Daggupati, G.; Srinivasan, P.; Olinares, P.D.B.; Rout, M.P.; Chait, B.T.; Wollenberg, K.; et al. Malaria parasites use a soluble RhopH complex for erythrocyte invasion and an integral form for nutrient uptake. eLife 2021, 10, 65282. [Google Scholar] [CrossRef]
- Gupta, A.; Thiruvengadam, G.; Desai, S.A. The conserved clag multigene family of malaria parasites: Essential roles in host–pathogen interaction. Drug Resist. Updat. 2015, 18, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Iriko, H.; Kaneko, O.; Otsuki, H.; Tsuboi, T.; Su, X.-z.; Tanabe, K.; Torii, M. Diversity and evolution of the rhoph1/clag multigene family of Plasmodium falciparum. Mol. Bioch. Parasitolo. 2008, 158, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Janse, C.J.; Ramesar, J.; Waters, A.P. High-efficiency transfection and drug selection of genetically transformed blood stages of the rodent malaria parasite Plasmodium berghei. Nat. Protoc. 2006, 1, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, N.; Nozaki, M.; Tachibana, M.; Baba, M.; Matsuoka, K.; Tsuboi, T.; Torii, M.; Ishino, T. Expression and Localization Profiles of Rhoptry Proteins in Plasmodium berghei Sporozoites. Front. Cell. Infect. Microbiol. 2019, 9, 316. [Google Scholar] [CrossRef] [PubMed]
- Homewood, C.A.; Neame, K.D. Malaria and the permeability of the host erythrocyte. Nature 1974, 252, 718–719. [Google Scholar] [CrossRef] [PubMed]
- Huber, S.M.; Duranton, C.; Henke, G.; van de Sand, C.; Heussler, V.; Shumilina, E.; Sandu, C.D.; Tanneur, V.; Brand, V.; Kasinathan, R.S.; et al. Plasmodium Induces Swelling-activated ClC-2 Anion Channels in the Host Erythrocyte. J. Biol. Chem. 2004, 279, 41444–41452. [Google Scholar] [CrossRef] [PubMed]
- Staines, H.M.; Kirk, K. Increased choline transport in erythrocytes from mice infected with the malaria parasite Plasmodium vinckei vinckei. Biochem. J. 1998, 334, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Gati, W.P.; Lin, A.N.; Wang, T.I.; Young, J.D.; Paterson, A.R. Parasite-induced processes for adenosine permeation in mouse erythrocytes infected with the malarial parasite Plasmodium yoelii. Biochem. J. 1990, 272, 277–280. [Google Scholar] [CrossRef]
- Lambros, C.; Vanderberg, J.P. Synchronization of Plasmodium falciparum Erythrocytic Stages in Culture. J. Parasitol. 1979, 65, 418–420. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Schulz, V.P.; Mohandas, N.; Gallagher, P.G. Human and murine erythropoiesis. Curr. Opin. Hematol. 2015, 22, 206–211. [Google Scholar] [CrossRef]
- Pasini, E.M.; Kirkegaard, M.; Salerno, D.; Mortensen, P.; Mann, M.; Thomas, A.W. Deep Coverage Mouse Red Blood Cell Proteome. Mol. Cell. Proteom. 2008, 7, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Varga, A.; Matrai, A.A.; Barath, B.; Deak, A.; Horvath, L.; Nemeth, N. Interspecies Diversity of Osmotic Gradient Deformability of Red Blood Cells in Human and Seven Vertebrate Animal Species. Cells 2022, 11, 1351. [Google Scholar] [CrossRef]
- Bokhari, A.A.B.; Mita-Mendoza, N.K.; Fuller, A.; Pillai, A.D.; Desai, S.A. High Guanidinium Permeability Reveals Dehydration-Dependent Ion Selectivity in the Plasmodial Surface Anion Channel. BioMed Res. Int. 2014, 2014, 741024. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.E.; Kirk, K. Transport of the essential nutrient isoleucine in human erythrocytes infected with the malaria parasite Plasmodium falciparum. Blood 2007, 109, 2217–2224. [Google Scholar] [CrossRef] [PubMed]
- Ngernna, S.; Chim-Ong, A.; Roobsoong, W.; Sattabongkot, J.; Cui, L.; Nguitragool, W. Efficient synchronization of Plasmodium knowlesi in vitro cultures using guanidine hydrochloride. Malar. J. 2019, 18, 147. [Google Scholar] [CrossRef]
- Lisk, G.; Desai, S.A. The plasmodial surface anion channel is functionally conserved in divergent malaria parasites. Eukaryot. Cell 2005, 4, 2153–2159. [Google Scholar] [CrossRef]
- Ginsburg, H.; Kutner, S.; Krugliak, M.; Cabantchik, Z.I. Characterization of permeation pathways appearing in the host membrane of Plasmodium falciparum infected red blood cells. Mol. Biochem. Parasitol. 1985, 14, 313–322. [Google Scholar] [CrossRef]
- Staines, H.M.; Ellory, J.C.; Kirk, K. Perturbation of the pump-leak balance for Na(+) and K(+) in malaria-infected erythrocytes. Am. J. Physiol. Cell Physiol. 2001, 280, 1576–1587. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trickey, M.L.; Counihan, N.A.; Modak, J.K.; de Koning-Ward, T.F. Guanidinium Chloride-Induced Haemolysis Assay to Measure New Permeation Pathway Functionality in Rodent Malaria Plasmodium berghei. Biomolecules 2024, 14, 781. https://doi.org/10.3390/biom14070781
Trickey ML, Counihan NA, Modak JK, de Koning-Ward TF. Guanidinium Chloride-Induced Haemolysis Assay to Measure New Permeation Pathway Functionality in Rodent Malaria Plasmodium berghei. Biomolecules. 2024; 14(7):781. https://doi.org/10.3390/biom14070781
Chicago/Turabian StyleTrickey, Mitchell L., Natalie A. Counihan, Joyanta K. Modak, and Tania F. de Koning-Ward. 2024. "Guanidinium Chloride-Induced Haemolysis Assay to Measure New Permeation Pathway Functionality in Rodent Malaria Plasmodium berghei" Biomolecules 14, no. 7: 781. https://doi.org/10.3390/biom14070781
APA StyleTrickey, M. L., Counihan, N. A., Modak, J. K., & de Koning-Ward, T. F. (2024). Guanidinium Chloride-Induced Haemolysis Assay to Measure New Permeation Pathway Functionality in Rodent Malaria Plasmodium berghei. Biomolecules, 14(7), 781. https://doi.org/10.3390/biom14070781





