Advances in Molecular Research on Hip Joint Impingement—A Vascular Perspective
Abstract
:1. Introduction
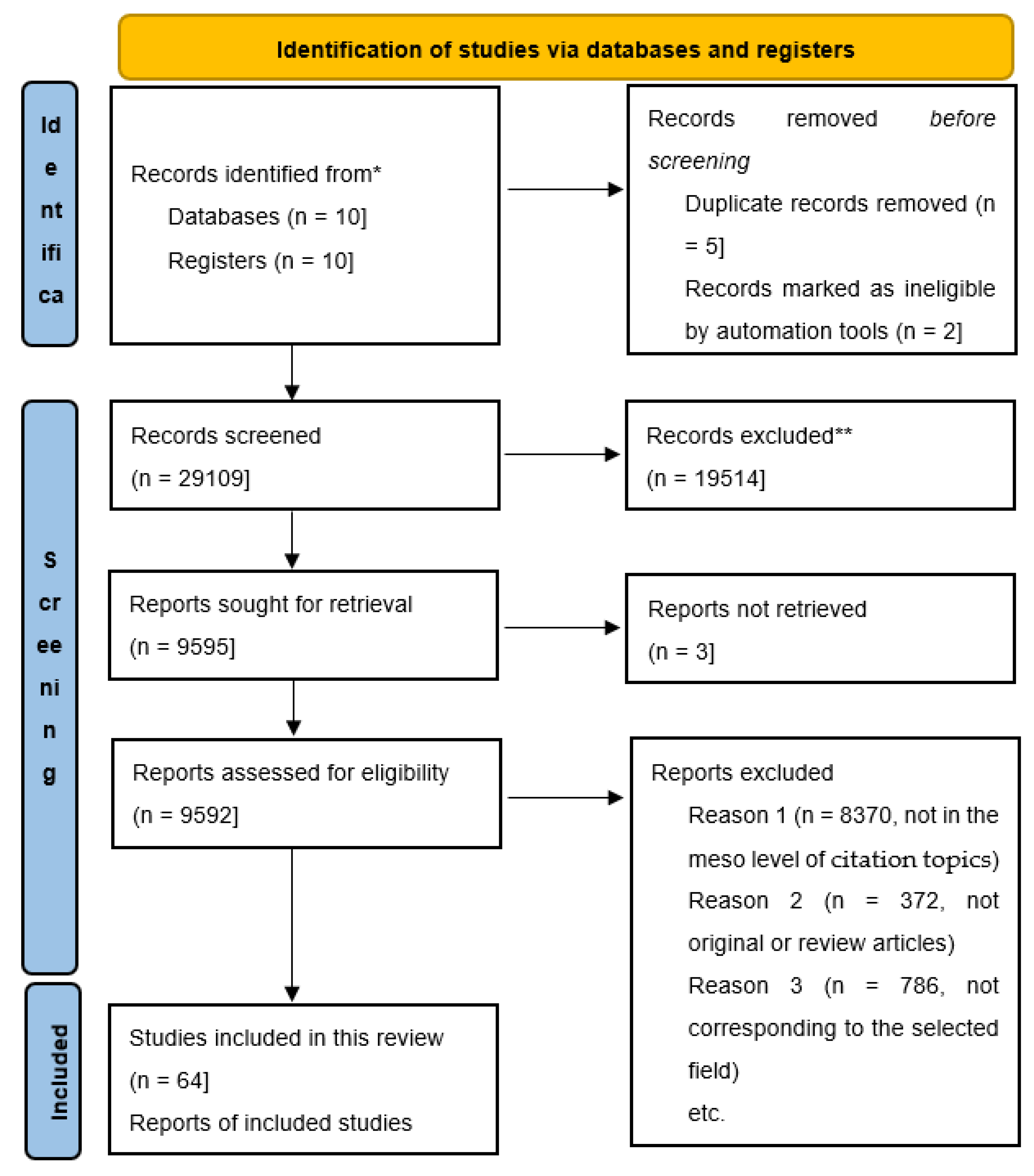
2. Materials and Methods
- I.
- Five years of molecular research on hip joint labrum, capsule, and synovial cellular senescence, with a total of 31 articles;
- II.
- Related research on similar molecular markers, totaling 51 articles;
- III.
- Perspectives, comprising 21 articles.
2.1. Five Years of Molecular Research on Hip Joint Labrum, Capsule, and Synovial Cellular Senescence
2.1.1. Hip Joint Labral Research
2.1.2. Hip Joint Capsule Research
2.1.3. Hip Joint Synovium Research
2.2. Related Research on Similar Molecular Markers
2.3. Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Liu, Y.; Zhang, Z.; Li, T.; Xu, H.; Zhang, H. Senescence in osteoarthritis: From mechanism to potential treatment. Arthritis Res. Ther. 2022, 24, 174. [Google Scholar] [CrossRef]
- Watanabe, J.; Sakai, K.; Urata, Y.; Toyama, N.; Nakamichi, E.; Hibi, H. Extracellular Vesicles of Stem Cells to Prevent BRONJ. J. Dent. Res. 2020, 99, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.N.; Xiong, J.; MacLeod, R.S.; Iyer, S.; Fujiwara, Y.; Cawley, K.M.; Han, L.; He, Y.; Thostenson, J.D.; Ferreira, E.; et al. Osteocyte RANKL is required for cortical bone loss with age and is induced by senescence. JCI Insight 2020, 5, e138815. [Google Scholar] [CrossRef]
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef]
- Narita, M.; Lowe, S.W. Senescence comes of age. Nat. Med. 2005, 11, 920–922. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Ke, Z.; Gorbunova, V.; Seluanov, A. Replicatively senescent cells are arrested in G1 and G2 phases. Aging 2012, 4, 431–435. [Google Scholar] [CrossRef]
- Dimri, G.P.; Hara, E.; Campisi, J. Regulation of two E2F-related genes in presenescent and senescent human fibroblasts. J. Biol. Chem. 1994, 269, 16180–16186. [Google Scholar] [CrossRef] [PubMed]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O.; et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef]
- Aguayo-Mazzucato, C.; Andle, J.; Lee, T.B., Jr.; Midha, A.; Talemal, L.; Chipashvili, V.; Hollister-Lock, J.; van Deursen, J.; Weir, G.; Bonner-Weir, S. Acceleration of β Cell Aging Determines Diabetes and Senolysis Improves Disease Outcomes. Cell Metab. 2019, 30, 129–142.e124. [Google Scholar] [CrossRef]
- Desdín-Micó, G.; Soto-Heredero, G.; Aranda, J.F.; Oller, J.; Carrasco, E.; Gabandé-Rodríguez, E.; Blanco, E.M.; Alfranca, A.; Cussó, L.; Desco, M.; et al. T cells with dysfunctional mitochondria induce multimorbidity and premature senescence. Science 2020, 368, 1371–1376. [Google Scholar] [CrossRef]
- d’Adda di Fagagna, F. Living on a break: Cellular senescence as a DNA-damage response. Nat. Rev. Cancer 2008, 8, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Di Micco, R.; Fumagalli, M.; Cicalese, A.; Piccinin, S.; Gasparini, P.; Luise, C.; Schurra, C.; Garre, M.; Nuciforo, P.G.; Bensimon, A.; et al. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature 2006, 444, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; d’Adda di Fagagna, F. Cellular senescence in ageing: From mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 2021, 22, 75–95. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J. Senescent cells, tumor suppression, and organismal aging: Good citizens, bad neighbors. Cell 2005, 120, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Dorronsoro, A.; Santiago, F.E.; Grassi, D.; Zhang, T.; Lai, R.C.; McGowan, S.J.; Angelini, L.; Lavasani, M.; Corbo, L.; Lu, A.; et al. Mesenchymal stem cell-derived extracellular vesicles reduce senescence and extend health span in mouse models of aging. Aging Cell 2021, 20, e13337. [Google Scholar] [CrossRef] [PubMed]
- Kuilman, T.; Peeper, D.S. Senescence-messaging secretome: SMS-ing cellular stress. Nat. Rev. Cancer 2009, 9, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Burton, D.G.; Sheerin, A.N.; Ostler, E.L.; Smith, K.; Giles, P.J.; Lowe, J.; Rhys-Williams, W.; Kipling, D.G.; Faragher, R.G. Cyclin D1 overexpression permits the reproducible detection of senescent human vascular smooth muscle cells. Ann. N. Y Acad. Sci. 2007, 1119, 20–31. [Google Scholar] [CrossRef]
- Mohaddes, M.; Nauclé, R.E.; Kärrholm, J.; Malchau, H.; Odin, D.; Rolfson, O. Implant survival and patient-reported outcome following total hip arthroplasty in patients 30 years or younger: A matched cohort study of 1,008 patients in the Swedish Hip Arthroplasty Register. Acta Orthop. 2019, 90, 249–252. [Google Scholar] [CrossRef]
- Koobatian, M.T.; Liang, M.S.; Swartz, D.D.; Andreadis, S.T. Differential effects of culture senescence and mechanical stimulation on the proliferation and leiomyogenic differentiation of MSC from different sources: Implications for engineering vascular grafts. Tissue Eng. Part A 2015, 21, 1364–1375. [Google Scholar] [CrossRef]
- Kubo, Y.; Drescher, W.; Fragoulis, A.; Tohidnezhad, M.; Jahr, H.; Gatz, M.; Driessen, A.; Eschweiler, J.; Tingart, M.; Wruck, C.J.; et al. Adverse Effects of Oxidative Stress on Bone and Vasculature in Corticosteroid-Associated Osteonecrosis: Potential Role of Nuclear Factor Erythroid 2-Related Factor 2 in Cytoprotection. Antioxid. Redox Signal 2021, 35, 357–376. [Google Scholar] [CrossRef]
- Krizhanovsky, V.; Yon, M.; Dickins, R.A.; Hearn, S.; Simon, J.; Miething, C.; Yee, H.; Zender, L.; Lowe, S.W. Senescence of activated stellate cells limits liver fibrosis. Cell 2008, 134, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Ren, Y.; Park, M.S.; Kim, H.K.W. Damage associated molecular patterns in necrotic femoral head inhibit osteogenesis and promote fibrogenesis of mesenchymal stem cells. Bone 2022, 154, 116215. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.; Lagnado, A.B.; Farr, J.N.; Monroe, D.G.; Park, S.; Hachfeld, C.; Tchkonia, T.; Kirkland, J.L.; Khosla, S.; Passos, J.F.; et al. Targeted Reduction of Senescent Cell Burden Alleviates Focal Radiotherapy-Related Bone Loss. J. Bone Miner. Res. 2020, 35, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Murali, B.; Ren, Q.; Luo, X.; Faget, D.V.; Cole, T.; Ricci, B.; Thotala, D.; Monahan, J.; van Deursen, J.M.; et al. Therapy-Induced Senescence Drives Bone Loss. Cancer Res. 2020, 80, 1171–1182. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, J.; Torrice, C.; Ramsey, M.R.; Kovalev, G.I.; Al-Regaiey, K.; Su, L.; Sharpless, N.E. Ink4a/Arf expression is a biomarker of aging. J. Clin. Investig. 2004, 114, 1299–1307. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Nakashima, H.; Sakai, K.; Takegami, Y.; Osawa, Y.; Watanabe, J.; Ito, S.; Hibi, H.; Imagama, S. Cellular senescence is associated with osteonecrosis of the femoral head while mesenchymal stem cell conditioned medium inhibits bone collapse. Sci. Rep. 2024, 14, 3329. [Google Scholar] [CrossRef]
- Smith, J.R.; Pereira-Smith, O.M. Replicative senescence: Implications for in vivo aging and tumor suppression. Science 1996, 273, 63–67. [Google Scholar] [CrossRef]
- Loeser, R.F. Aging and osteoarthritis: The role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthr. Cartil. 2009, 17, 971–979. [Google Scholar] [CrossRef]
- Hu, L.; Li, H.; Zi, M.; Li, W.; Liu, J.; Yang, Y.; Zhou, D.; Kong, Q.P.; Zhang, Y.; He, Y. Why Senescent Cells Are Resistant to Apoptosis: An Insight for Senolytic Development. Front. Cell Dev. Biol. 2022, 10, 822816. [Google Scholar] [CrossRef]
- Schellnegger, M.; Hofmann, E.; Carnieletto, M.; Kamolz, L.P. Unlocking longevity: The role of telomeres and its targeting interventions. Front. Aging 2024, 5, 1339317. [Google Scholar] [CrossRef]
- Jeyapalan, J.C.; Ferreira, M.; Sedivy, J.M.; Herbig, U. Accumulation of senescent cells in mitotic tissue of aging primates. Mech. Ageing Dev. 2007, 128, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Nesovic Ostojic, J.; Kovacevic, S.; Ivanov, M.; Brkic, P.; Zivotic, M.; Mihailovic-Stanojevic, N.; Karanovic, D.; Vajic, U.J.; Jeremic, R.; Jovovic, D.; et al. Hyperbaric Oxygen Reduces Oxidative Stress Impairment and DNA Damage and Simultaneously Increases HIF-1α in Ischemia-Reperfusion Acute Kidney Injury. Int. J. Mol. Sci. 2024, 25, 3870. [Google Scholar] [CrossRef] [PubMed]
- Tomioka, Y.; Kitazawa, K.; Numa, K.; Hughes, J.B.; Yokoi, N.; Sotozono, C. The existence of senescent cells in conjunctival epithelium from elderly individuals. JPN J. Ophthalmol. 2024, 68, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Childs, B.G.; Baker, D.J.; Wijshake, T.; Conover, C.A.; Campisi, J.; van Deursen, J.M. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 2016, 354, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Minamino, T.; Miyauchi, H.; Yoshida, T.; Ishida, Y.; Yoshida, H.; Komuro, I. Endothelial cell senescence in human atherosclerosis: Role of telomere in endothelial dysfunction. Circulation 2002, 105, 1541–1544. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yuan, T.; Wang, Y.; Liu, C.; Li, D.; Li, Z.; Sun, S. Osteoclasts and osteoarthritis: Novel intervention targets and therapeutic potentials during aging. Aging Cell 2024, 23, e14092. [Google Scholar] [CrossRef] [PubMed]
- Price, J.S.; Waters, J.G.; Darrah, C.; Pennington, C.; Edwards, D.R.; Donell, S.T.; Clark, I.M. The role of chondrocyte senescence in osteoarthritis. Aging Cell 2002, 1, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Poudel, S.B.; Ruff, R.R.; Yildirim, G.; Miller, R.A.; Harrison, D.E.; Strong, R.; Kirsch, T.; Yakar, S. Development of primary osteoarthritis during aging in genetically diverse UM-HET3 mice. Arthritis Res. Ther. 2024, 26, 118. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Bradley, E.W.; Weivoda, M.M.; Hwang, S.M.; Pirtskhalava, T.; Decklever, T.; Curran, G.L.; Ogrodnik, M.; Jurk, D.; Johnson, K.O.; et al. Transplanted Senescent Cells Induce an Osteoarthritis-Like Condition in Mice. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 780–785. [Google Scholar] [CrossRef]
- Jasiński, T.; Turek, B.; Kaczorowski, M.; Brehm, W.; Skierbiszewska, K.; Bonecka, J.; Domino, M. Equine Models of Temporomandibular Joint Osteoarthritis: A Review of Feasibility, Biomarkers, and Molecular Signaling. Biomedicines 2024, 12, 542. [Google Scholar] [CrossRef]
- Pfander, D.; Körtje, D.; Zimmermann, R.; Weseloh, G.; Kirsch, T.; Gesslein, M.; Cramer, T.; Swoboda, B. Vascular endothelial growth factor in articular cartilage of healthy and osteoarthritic human knee joints. Ann. Rheum. Dis. 2001, 60, 1070–1073. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Y.; Wei, Y. Osteoarthritis animal models for biomaterial-assisted osteochondral regeneration. Biomater. Transl. 2022, 3, 264–279. [Google Scholar] [CrossRef]
- Malakootian, M.; Gholipour, A.; Oveisee, M. CD19, ALDH18A1, and CACNA1G as Significant Hub Genes in End-Stage Osteoarthritis. Iran. J. Public. Health 2023, 52, 2651–2662. [Google Scholar] [CrossRef] [PubMed]
- Neuhold, L.A.; Killar, L.; Zhao, W.; Sung, M.L.; Warner, L.; Kulik, J.; Turner, J.; Wu, W.; Billinghurst, C.; Meijers, T.; et al. Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice. J. Clin. Investig. 2001, 107, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Tvaroška, I. Glycosylation Modulates the Structure and Functions of Collagen: A Review. Molecules 2024, 29, 1417. [Google Scholar] [CrossRef] [PubMed]
- Ewald, C.Y.; Landis, J.N.; Porter Abate, J.; Murphy, C.T.; Blackwell, T.K. Dauer-independent insulin/IGF-1-signalling implicates collagen remodelling in longevity. Nature 2015, 519, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S.D.; Endicott, S.K.; Province, M.A.; Pierce, J.A.; Campbell, E.J. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weapons-related radiocarbon. J. Clin. Investig. 1991, 87, 1828–1834. [Google Scholar] [CrossRef] [PubMed]
- Shek, N.; Choy, A.M.; Lang, C.C.; Miller, B.E.; Tal-Singer, R.; Bolton, C.E.; Thomson, N.C.; Chalmers, J.D.; Bown, M.J.; Newby, D.E.; et al. Accelerated elastin degradation by age-disease interaction: A common feature in age-related diseases. NPJ Aging 2024, 10, 15. [Google Scholar] [CrossRef]
- Duca, L.; Blaise, S.; Romier, B.; Laffargue, M.; Gayral, S.; El Btaouri, H.; Kawecki, C.; Guillot, A.; Martiny, L.; Debelle, L.; et al. Matrix ageing and vascular impacts: Focus on elastin fragmentation. Cardiovasc. Res. 2016, 110, 298–308. [Google Scholar] [CrossRef]
- Faleeva, M.; Ahmad, S.; Theofilatos, K.; Lynham, S.; Watson, G.; Whitehead, M.; Marhuenda, E.; Iskratsch, T.; Cox, S.; Shanahan, C.M. Sox9 Accelerates Vascular Aging by Regulating Extracellular Matrix Composition and Stiffness. Circ. Res. 2024, 134, 307–324. [Google Scholar] [CrossRef]
- Pascual-Garrido, C.; Kikuchi, K.; Clohisy, J.C.; O’Keefe, R.J.; Kamenaga, T. Revealing a Natural Model of Pre-Osteoarthritis of the Hip Through Study of Femoroacetabular Impingement. Hss J. 2023, 19, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Chinzei, N.; Hashimoto, S.; Hayashi, S.; Nakano, N.; Haneda, M.; Kuroda, Y.; Matsumoto, T.; Kuroda, R. Patients’ Characteristics Can Predict Clinical Outcomes Following Hip Arthroscopy by Reflecting the Patterns of Labral Tears: A Retrospective Observational Study. Indian J. Orthop. 2022, 56, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Dean, M.C.; Cherian, N.J.; LaPorte, Z.L.; Eberlin, C.T.; Wang, C.; Torabian, K.A.; Dowley, K.S.; Kucharik, M.P.; Abraham, P.F.; Nazal, M.R.; et al. Association Between Chondrolabral Junction Breakdown and Conversion to Total Hip Arthroplasty After Hip Arthroscopy for Symptomatic Labral Tears: Minimum 8-Year Follow-up. Am. J. Sports Med. 2024, 52, 1153–1164. [Google Scholar] [CrossRef]
- Diwan, S.; Shivamallappa, S.; Timane, R.; Pai, P.; Gupta, A. Anatomic evaluation to compare the dye spread with ultrasound-guided pericapsular nerve group (PENG) injection with or without an additional suprainguinal fascia iliaca (SIFI) injection in soft embalmed cadavers. J. Anesth. 2024. Online ahead of print. [Google Scholar] [CrossRef]
- Sato, Y.; Tetsunaga, T.; Yamada, K.; Kawamura, Y.; Yoshida, A.; Ozaki, T. Expression of Acetabular Labral Vascular Endothelial Growth Factor and Nerve Growth Factor Is Directly Associated with Hip Osteoarthritis Pain: Investigation by Immunohistochemical Staining. Int. J. Mol. Sci. 2023, 24, 2926. [Google Scholar] [CrossRef]
- Cherian, N.J.; Eberlin, C.T.; Kucharik, M.P.; Abraham, P.F.; Nazal, M.R.; Dean, M.C.; Martin, S.D. Labral Reconstruction via Capsular Augmentation Maintains Perfusion to the Acetabular Labrum and Locally Transferred Autograft: An in Vivo Laser Doppler Flowmetry Analysis. JB JS Open Access 2023, 8, e23.00026. [Google Scholar] [CrossRef] [PubMed]
- Emblom, B.A.; Walters, B.L.; Mast, L.E.; Beason, D.P.; Ruder, J.A.; Ryan, M.K.; Gould, S.A.; Schwartz, M.L. Fixation strength in arthroscopic labral repair of the hip: A head-to-head comparison of the biomechanical performance of a biocompatible vs. all-suture anchor in the setting of acetabuloplasty. PLoS ONE 2023, 18, e0293738. [Google Scholar] [CrossRef]
- Radha, S.; Hutt, J.; Lall, A.; Domb, B.; Lynch, T.S.; Griffin, D.; Field, R.E.; Chuck-Cakic, J. Best practice guidelines for clinical and radiological assessment of patients with femoroacetabular impingement. Results from the ISHA International Delphi Consensus Project-Phase 2. J. Hip Preserv. Surg. 2024, 11, 44–50. [Google Scholar] [CrossRef]
- Rosenthal, R.M.; Featherall, J.; Parkes, C.W.; Khalil, A.Z.; Genuario, J.W.; Maak, T.G.; Aoki, S.K. Acetabular Labral Reconstruction: Review of Techniques and Outcomes. Curr. Rev. Musculoskelet. Med. 2023, 16, 470–479. [Google Scholar] [CrossRef]
- Bohaček, I.; Plečko, M.; Duvančić, T.; Smoljanović, T.; Vukasović Barišić, A.; Delimar, D. Current knowledge on the genetic background of developmental dysplasia of the hip and the histomorphological status of the cartilage. Croat. Med. J. 2020, 61, 260–270. [Google Scholar] [CrossRef]
- Gómez-Hoyos, J.; Márquez, W.H.; Gallo, J.A.; Khoury, A.; Bernal-Sierra, S.; Martin, H.D. Iliopsoas Muscle/Tendon Proportions at Three Levels of Described Arthroscopic Tenotomy: An Anatomic Study in Fresh Cadaveric Specimens. Arthrosc. Sports Med. Rehabil. 2021, 3, e297–e303. [Google Scholar] [CrossRef] [PubMed]
- Seldes, R.M.; Tan, V.; Hunt, J.; Katz, M.; Winiarsky, R.; Fitzgerald, R.H., Jr. Anatomy, histologic features, and vascularity of the adult acetabular labrum. Clin. Orthop. Relat. Res. 2001, 382, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Lage, L.A.; Patel, J.V.; Villar, R.N. The acetabular labral tear: An arthroscopic classification. Arthroscopy 1996, 12, 269–272. [Google Scholar] [CrossRef]
- Mayer, S.W.; Fauser, T.R.; Marx, R.G.; Ranawat, A.S.; Kelly, B.T.; Lyman, S.; Nawabi, D.H. Reliability of the classification of cartilage and labral injuries during hip arthroscopy. J. Hip Preserv. Surg. 2020, 7, 448–457. [Google Scholar] [CrossRef]
- Nogami, R.; Kaku, N.; Shimada, T.; Tabata, T.; Tagomori, H.; Tsumura, H. Three-dimensional architecture of the acetabular labrum in the human hip joint. Med. Mol. Morphol. 2020, 53, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, S.; Han, X.; Zhang, Y.; Gao, L.; Wang, X.; Wang, G.; Chen, Z. A rapid VEGF-gene-sequence photoluminescence detector for osteoarthritis. Front. Bioeng. Biotechnol. 2024, 12, 1338901. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Singh, G.; Wang, J.; O-Sullivan, I.; Votta-Velis, G.; Bruce, B.; Anbazhagan, A.N.; van Wijnen, A.J.; Im, H.J. Targeting Vascular Endothelial Growth Factor Receptors as a Therapeutic Strategy for Osteoarthritis and Associated Pain. Int. J. Biol. Sci. 2023, 19, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Shirogane, Y.; Homma, Y.; Yanagisawa, N.; Higano, M.; Hirasawa, Y.; Nakamura, S.; Baba, T.; Kaneko, K.; Taneda, H.; Ishijima, M. Relationship between labral length and symptoms in patients with acetabular dysplasia before rotational acetabular osteotomy. J. Hip Preserv. Surg. 2022, 9, 240–251. [Google Scholar] [CrossRef]
- Ertürk, C.; Koçarslan, S.; Büyükdoğan, H.; Altay, M.A. Investigation of sensory nerve endings in pulvinar, ligamentum teres, and hip joint capsule: A prospective immunohistochemical study of 36 cases with developmental hip dysplasia. Acta Orthop. Traumatol. Turc. 2021, 55, 33–37. [Google Scholar] [CrossRef]
- Mishra, G.; Townsend, K.L. The metabolic and functional roles of sensory nerves in adipose tissues. Nat. Metab. 2023, 5, 1461–1474. [Google Scholar] [CrossRef]
- Chen, B.; Sun, Y.; Xu, G.; Jiang, J.; Zhang, W.; Wu, C.; Xue, P.; Cui, Z. Role of crosstalk between synovial cells and chondrocytes in osteoarthritis (Review). Exp. Ther. Med. 2024, 27, 201. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Peng, Z.; Zhou, Y.; Su, Y.; Bu, P.; Meng, X.; Li, B.; Xu, Y. Comprehensive analysis of pathological changes in hip joint capsule of patients with developmental dysplasia of the hip. Bone Joint Res. 2021, 10, 558–570. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Tsuda, M. Nociceptive signaling mediated by P2X3, P2X4 and P2X7 receptors. Biochem. Pharmacol. 2021, 187, 114309. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, C.; Boffa, A.; Salerno, M.; Merli, G.; Grigolo, B.; Filardo, G. Adipose Tissue-Derived Products May Present Inflammatory Properties That Affect Chondrocytes and Synoviocytes from Patients with Knee Osteoarthritis. Int. J. Mol. Sci. 2023, 24, 12401. [Google Scholar] [CrossRef] [PubMed]
- Antoniadis, A.; Wegrzyn, J.; Omoumi, P.; Loisay, L.; Hügle, T.; Geurts, J. Elevated secretion of pro-collagen I-alpha and vascular endothelial growth factor as biomarkers of acetabular labrum degeneration and calcification in hip osteoarthritis: An explant study. J. Orthop. Transl. 2024, 44, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Bay-Jensen, A.C.; Mobasheri, A.; Thudium, C.S.; Kraus, V.B.; Karsdal, M.A. Blood and urine biomarkers in osteoarthritis—An update on cartilage associated type II collagen and aggrecan markers. Curr. Opin. Rheumatol. 2022, 34, 54–60. [Google Scholar] [CrossRef]
- Hasan, S.; van Schie, P.; Kaptein, B.L.; Schoones, J.W.; Marang-van de Mheen, P.J.; Nelissen, R. Biomarkers to discriminate between aseptic loosened and stable total hip or knee arthroplasties: A systematic review. EFORT Open Rev. 2024, 9, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Koyama, T.; Uchida, K.; Fukushima, K.; Ohashi, Y.; Uchiyama, K.; Inoue, G.; Takahira, N.; Takaso, M. Elevated levels of TNF-α, IL-1β and IL-6 in the synovial tissue of patients with labral tear: A comparative study with hip osteoarthritis. BMC Musculoskelet. Disord. 2021, 22, 33. [Google Scholar] [CrossRef] [PubMed]
- Lynch, T.S.; O’Connor, M.; Minkara, A.A.; Westermann, R.W.; Rosneck, J.T. Biomarkers for Femoroacetabular Impingement and Hip Osteoarthritis: A Systematic Review and Meta-analysis. Am. J. Sports Med. 2019, 47, 2242–2250. [Google Scholar] [CrossRef]
- Mobasheri, A.; Lambert, C.; Henrotin, Y. Coll2-1 and Coll2-1NO2 as exemplars of collagen extracellular matrix turnover— biomarkers to facilitate the treatment of osteoarthritis? Expert. Rev. Mol. Diagn. 2019, 19, 803–812. [Google Scholar] [CrossRef]
- Oppl, B.; Datz, C.; Huber-Schönauer, U.; Husar-Memmer, E.; Brozek, W.; Zenz, P.; Gollob, E.; Wurnig, C.; Engel, A.; Klaushofer, K.; et al. Vascular cell adhesion molecule 1 in patients with severe osteoarthritis of the hip: A prospective cross-sectional study. Wien. Klin. Wochenschr. 2019, 131, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, Y.; Uchida, K.; Fukushima, K.; Satoh, M.; Koyama, T.; Tsuchiya, M.; Saito, H.; Takahira, N.; Inoue, G.; Takaso, M. NGF Expression and Elevation in Hip Osteoarthritis Patients with Pain and Central Sensitization. Biomed. Res. Int. 2021, 2021, 9212585. [Google Scholar] [CrossRef] [PubMed]
- Schon, J.; Chahla, J.; Paudel, S.; Manandhar, L.; Feltham, T.; Huard, J.; Philippon, M.; Zhang, Z. Expression profile of matrix metalloproteinases in the labrum of femoroacetabular impingement. Bone Joint Res. 2020, 9, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Stattin, E.L.; Lindblom, K.; Struglics, A.; Önnerfjord, P.; Goldblatt, J.; Dixit, A.; Sarkar, A.; Randell, T.; Suri, M.; Raggio, C.; et al. Novel missense ACAN gene variants linked to familial osteochondritis dissecans cluster in the C-terminal globular domain of aggrecan. Sci. Rep. 2022, 12, 5215. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Chen, Y.; Ji, J.; Chang, J.; Yu, S.; Yu, B. The relationship between serum vitamin D and fracture risk in the elderly: A meta-analysis. J. Orthop. Surg. Res. 2020, 15, 81. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.C.G.; Jeffers, J.R.T.; Beaulé, P.E. Hip Joint Capsular Anatomy, Mechanics, and Surgical Management. J. Bone Joint Surg. Am. 2019, 101, 2141–2151. [Google Scholar] [CrossRef]
- Zhao, Z.; Fan, C.; Wang, S.; Wang, H.; Deng, H.; Zeng, S.; Tang, S.; Li, L.; Xiong, Z.; Qiu, X. Single-nucleus RNA and multiomics in situ pairwise sequencing reveals cellular heterogeneity of the abnormal ligamentum teres in patients with developmental dysplasia of the hip. Heliyon 2024, 10, e27803. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Zhang, Y.; Guo, L.; Li, P.; Wang, D.; Huang, L.; Zhao, X.; Wu, G.; Li, L.; Wei, X. Effect of Moderate Exercise on the Superficial Zone of Articular Cartilage in Age-Related Osteoarthritis. Diagnostics 2023, 13, 3193. [Google Scholar] [CrossRef]
- Shaw, C.; Warwick, H.; Nguyen, K.H.; Link, T.M.; Majumdar, S.; Souza, R.B.; Vail, T.P.; Zhang, A.L. Correlation of hip capsule morphology with patient symptoms from femoroacetabular impingement. J. Orthop. Res. 2021, 39, 590–596. [Google Scholar] [CrossRef]
- Luitjens, J.; Gassert, F.G.; Patwardhan, V.; Bhattacharjee, R.; Joseph, G.B.; Zhang, A.L.; Souza, R.B.; Majumdar, S.; Link, T.M. Is hip capsule morphology associated with hip pain in patients without another structural correlate? Eur. Radiol. 2024. Online ahead of print. [Google Scholar] [CrossRef]
- Turner, E.H.G.; Markhardt, B.K.; Cotter, E.J.; Hetzel, S.J.; Kanarek, A.; Lang, M.H.; Mintz, D.N.; Spiker, A.M. Patients With Generalized Joint Hypermobility Have Thinner Superior Hip Capsules and Greater Hip Internal Rotation on Physical Examination. Arthrosc. Sports Med. Rehabil. 2022, 4, e1417–e1427. [Google Scholar] [CrossRef]
- Jiang, N.; Liu, H.X.; Liang, H.Y.; Feng, X.H.; Liu, B.Y.; Zhou, Y.Y. Osteogenic differentiation characteristics of hip joint capsule fibroblasts obtained from patients with ankylosing spondylitis. Ann. Transl. Med. 2021, 9, 331. [Google Scholar] [CrossRef]
- Zhang, S.; Song, J.; Wu, Q.; Fang, J.; Ning, B. Collagen I in the Hip Capsule Plays a Role in Postoperative Clinical Function in Patients With Developmental Dysplasia of the Hip. Front. Pediatr. 2022, 10, 918660. [Google Scholar] [CrossRef]
- Tomlinson, J.; Ondruschka, B.; Prietzel, T.; Zwirner, J.; Hammer, N. A systematic review and meta-analysis of the hip capsule innervation and its clinical implications. Sci. Rep. 2021, 11, 5299. [Google Scholar] [CrossRef] [PubMed]
- Duquesne, K.; Pattyn, C.; Vanderstraeten, B.; Audenaert, E.A. Handle With Care: The Anterior Hip Capsule Plays a Key Role in Daily Hip Performance. Orthop. J. Sports Med. 2022, 10, 23259671221078254. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, A.S.; Brennick, C.; Occhialini, A.P.; Leet, J.G.; Clark, T.S.; Rahimi, O.B.; Hulk, K.; Bickelhaupt, B.; Eckmann, M.S. Innervation of the Posterior Hip Capsule: A Cadaveric Study. Pain. Med. 2021, 22, 1072–1079. [Google Scholar] [CrossRef]
- Gao, G.; Fang, H.; Zhou, K.; Mo, Z.; Liu, J.; Meng, L.; Wang, J.; Xu, Y. Ultrasound had high accuracy in measuring hip joint capsule thickness. BMC Musculoskelet. Disord. 2024, 25, 101. [Google Scholar] [CrossRef]
- Lv, X.; Nuertai, Y.; Wang, Q.; Zhang, D.; Hu, X.; Liu, J.; Zeng, Z.; Huang, R.; Huang, Z.; Zhao, Q.; et al. Multilevel Pedicle Subtraction Osteotomy for Correction of Thoracolumbar Kyphosis in Ankylosing Spondylitis: Clinical Effect and Biomechanical Evaluation. Neurospine 2024, 21, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Haubruck, P.; Pinto, M.M.; Moradi, B.; Little, C.B.; Gentek, R. Monocytes, Macrophages, and Their Potential Niches in Synovial Joints—Therapeutic Targets in Post-Traumatic Osteoarthritis? Front. Immunol. 2021, 12, 763702. [Google Scholar] [CrossRef]
- Kung, M.S.; Markantonis, J.; Nelson, S.D.; Campbell, P. The Synovial Lining and Synovial Fluid Properties after Joint Arthroplasty. Lubricants 2015, 3, 394–412. [Google Scholar] [CrossRef]
- Soontararak, S.; Ardaum, P.; Senarat, N.; Yangtara, S.; Lekcharoensuk, C.; Putchong, I.; Kashemsant, N.; Vijarnsorn, M.; Chow, L.; Dow, S.; et al. In Vitro Anti-Inflammatory and Regenerative Effects of Autologous Conditioned Serum from Dogs with Osteoarthritis. Animals 2022, 12, 2717. [Google Scholar] [CrossRef]
- Subaşı, İ.; Veizi, E.; Çepni, Ş.; Alkan, H.; Oğuz, T.; Fırat, A. Clinical Examination Findings Can Accurately Diagnose Developmental Dysplasia of The Hip-A Large, Single-Center Cohort. Children 2023, 10, 304. [Google Scholar] [CrossRef] [PubMed]
- Kamml, J.; Acevedo, C.; Kammer, D. Mineral and cross-linking in collagen fibrils: The mechanical behavior of bone tissue at the nano-scale. arXiv 2024, arXiv:2403.11753. [Google Scholar]
- Gahlawat, S.; Nanda, V.; Shreiber, D.I. Purification of recombinant bacterial collagens containing structural perturbations. PLoS ONE 2023, 18, e0285864. [Google Scholar] [CrossRef]
- Perino, G.; Sunitsch, S.; Huber, M.; Ramirez, D.; Gallo, J.; Vaculova, J.; Natu, S.; Kretzer, J.P.; Müller, S.; Thomas, P.; et al. Diagnostic guidelines for the histological particle algorithm in the periprosthetic neo-synovial tissue. BMC Clin. Pathol. 2018, 18, 7. [Google Scholar] [CrossRef]
- Coryell, P.R.; Diekman, B.O.; Loeser, R.F. Mechanisms and therapeutic implications of cellular senescence in osteoarthritis. Nat. Rev. Rheumatol. 2021, 17, 47–57. [Google Scholar] [CrossRef]
- Batushansky, A.; Zhu, S.; Komaravolu, R.K.; South, S.; Mehta-D’souza, P.; Griffin, T.M. Fundamentals of OA. An initiative of Osteoarthritis and Cartilage. Obesity and metabolic factors in OA. Osteoarthr. Cartil. 2022, 30, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Carlson, A.K.; Rawle, R.A.; Wallace, C.W.; Brooks, E.G.; Adams, E.; Greenwood, M.C.; Olmer, M.; Lotz, M.K.; Bothner, B.; June, R.K. Characterization of synovial fluid metabolomic phenotypes of cartilage morphological changes associated with osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1174–1184. [Google Scholar] [CrossRef]
- Kim, S.; Hwang, J.; Kim, J.; Lee, S.H.; Cheong, Y.E.; Lee, S.; Kim, K.H.; Cha, H.S. Metabolic discrimination of synovial fluid between rheumatoid arthritis and osteoarthritis using gas chromatography/time-of-flight mass spectrometry. Metabolomics 2022, 18, 48. [Google Scholar] [CrossRef]
- Laus, F.; Gialletti, R.; Bazzano, M.; Laghi, L.; Dini, F.; Marchegiani, A. Synovial Fluid Metabolome Can Differentiate between Healthy Joints and Joints Affected by Osteoarthritis in Horses. Metabolites 2023, 13, 913. [Google Scholar] [CrossRef]
- Murillo-Saich, J.D.; Coras, R.; Meyer, R.; Llorente, C.; Lane, N.E.; Guma, M. Synovial tissue metabolomic profiling reveal biomarkers of synovial inflammation in patients with osteoarthritis. Osteoarthr. Cartil. Open 2022, 4, 100295. [Google Scholar] [CrossRef]
- Stabile, M.; Girelli, C.R.; Lacitignola, L.; Samarelli, R.; Crovace, A.; Fanizzi, F.P.; Staffieri, F. (1)H-NMR metabolomic profile of healthy and osteoarthritic canine synovial fluid before and after UC-II supplementation. Sci. Rep. 2022, 12, 19716. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Koga, H.; Nakagawa, Y.; Nakamura, T.; Katagiri, H.; Takada, R.; Katakura, M.; Tsuji, K.; Sekiya, I.; Miyatake, K. Characteristics of the synovial microenvironment and synovial mesenchymal stem cells with hip osteoarthritis of different bone morphologies. Arthritis Res. Ther. 2024, 26, 17. [Google Scholar] [CrossRef] [PubMed]
- Wallace, C.W.; Hislop, B.; Hahn, A.K.; Erdogan, A.E.; Brahmachary, P.P.; June, R.K. Correlations between metabolites in the synovial fluid and serum: A mouse injury study. J. Orthop. Res. 2022, 40, 2792–2802. [Google Scholar] [CrossRef]
- Zhan, X.; Wu, H.; Wu, H. Joint Synovial Fluid Metabolomics Method to Decipher the Metabolic Mechanisms of Adjuvant Arthritis and Geniposide Intervention. J. Proteome Res. 2020, 19, 3769–3778. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ouyang, Z.; Huang, Y.; Lin, S.; Li, S.; Xu, J.; Liu, T.; Wu, J.; Guo, P.; Chen, Z.; et al. NOD2 attenuates osteoarthritis via reprogramming the activation of synovial macrophages. Arthritis Res. Ther. 2023, 25, 249. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, F.; Xu, L.; Wang, J.; Zhai, J.; Ren, L.; Zhu, G. C-C Motif Chemokine Ligand 5 (CCL5) Promotes Irradiation-Evoked Osteoclastogenesis. Int. J. Mol. Sci. 2023, 24, 16168. [Google Scholar] [CrossRef]
- Wang, S.; Yang, J.; Xiang, R.; Li, C.; Li, J.; Shen, X.; Liu, W.; Xu, X. Research and publication trends on knee osteoarthritis and cellular senescence: A bibliometric analysis. Front. Physiol. 2023, 14, 1269338. [Google Scholar] [CrossRef]
- Wu, C.J.; Liu, R.X.; Huan, S.W.; Tang, W.; Zeng, Y.K.; Zhang, J.C.; Yang, J.; Li, Z.Y.; Zhou, Y.; Zha, Z.G.; et al. Senescent skeletal cells cross-talk with synovial cells plays a key role in the pathogenesis of osteoarthritis. Arthritis Res. Ther. 2022, 24, 59. [Google Scholar] [CrossRef]
- Yang, J.; Li, S.; Li, Z.; Yao, L.; Liu, M.; Tong, K.-L.; Xu, Q.; Yu, B.; Peng, R.; Gui, T.; et al. Targeting YAP1-regulated Glycolysis in Fibroblast-Like Synoviocytes Impairs Macrophage Infiltration to Ameliorate Diabetic Osteoarthritis Progression. Adv. Sci. 2024, 11, e2304617. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Q.; Tao, H.; Lu, L.; Zhou, J.; Wang, Q.; Huang, W.; Yang, X. Subchondral osteoclasts and osteoarthritis: New insights and potential therapeutic avenues. Acta Biochim. Biophys. Sin. 2024, 56, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Kim, S.Y. Endothelial senescence in vascular diseases: Current understanding and future opportunities in senotherapeutics. Exp. Mol. Med. 2023, 55, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Khavinson, V.; Linkova, N.; Dyatlova, A.; Kantemirova, R.; Kozlov, K. Senescence-Associated Secretory Phenotype of Cardiovascular System Cells and Inflammaging: Perspectives of Peptide Regulation. Cells 2022, 12, 106. [Google Scholar] [CrossRef] [PubMed]
- Ghelfi, J.; Soulairol, I.; Stephanov, O.; Bacle, M.; de Forges, H.; Sanchez-Ballester, N.; Ferretti, G.; Beregi, J.P.; Frandon, J. Feasibility of Neovessel Embolization in a Large Animal Model of Tendinopathy: Safety and Efficacy of Various Embolization Agents. J. Pers. Med. 2022, 12, 1530. [Google Scholar] [CrossRef] [PubMed]
- Russu, O.; Bloj, F.; Feier, A.M.; Vunvulea, V.; Mogoș, S.; Predescu, V.; Pop, T.S. At the Edge of Orthopaedics: Initial Experience with Transarterial Periarticular Embolization for Knee Osteoarthritis in a Romanian Population. J. Clin. Med. 2022, 11, 6573. [Google Scholar] [CrossRef]
- Talaie, R.; Torkian, P.; Clayton, A.; Wallace, S.; Cheung, H.; Chalian, M.; Golzarian, J. Emerging Targets for the Treatment of Osteoarthritis: New Investigational Methods to Identify Neo-Vessels as Possible Targets for Embolization. Diagnostics 2022, 12, 1403. [Google Scholar] [CrossRef]
| Article | Histology/Other | Biomolecule | Ultrastructural Association | IRM/CT Association/Other | H/AM | Statistics/ Scoring/ Scale | Aim |
|---|---|---|---|---|---|---|---|
| Antoniadis et al. [75] | ELISA/Explant culture/Alcian blue/Alizarin Red S | Pro/Pro-Col-Iα, IL-6, COMP/VEGF, ACAN | _ | Fluorescence imaging | H | Hierarchical clustering in ClustVis 1.0/GraphPad Prism/one-way ANOVA with Tukey’s multiple-comparison test/Mann–Whitney test and Friedman’s test with Dunn’s multiple-comparison test | Putative biomarkers of OA from explanted human labrum tissues under basal and inflammatory conditions |
| Bay-Jensen et al. [76] | _ | CTX-II/TIMP | _ | _ | H | _ | Review of biomarkers derived from type II collagen and aggrecan |
| Hasan et al. [77] | _ | TNFα/ TNF mRNA/TNFbeta/IL-1/IL-1b/ N-terminal-telopeptide (NTX) | _ | _ | H | ICROMS tool (integrated quality criteria for review of multiple study designs) | Review of blood and urine degenerative biomarkers |
| Huang et al. [66] | Nanoparticles | VEGF/ SsDNA/CRISPR/Cas12a | _ | Fluorescence spectroscopy | H | _ | Rapid detection of ssDNA molecules |
| Koyama et al. [78] | Real-time polymerase chain reaction (PCR) | RNA/TNFA/IL1B/IL6/COX2 mRNA | _ | _ | H | SPSS version 19.0 | Compare synovial tissue’s inflammatory cytokine levels |
| Lynch et al. [79] | _ | COMP | _ | _ | H | Fixed-effect inverse-variance model | Review of COMP |
| Mobasheri et al. [80] | _ | Collagen | _ | _ | H | _ | Review of glycosylation of collagens |
| Nogami et al. [65] | Hematoxylin and eosin (HE) and Alcian blue (AB) | Collagen I and II | Collagen I and II | _ | H | _ | Ultrastructure of collagen fibers |
| Oppl et al. [81] | ELISA—enzyme-linked immunosorbent assay | VCAM-1 | _ | _ | H | Clinical future prediction model development | |
| Osashi et al. [82] | PCR | RNA | _ | _ | H | G∗POWER3 | NGF-expressing cells/mRNA expression of NGF, CD14, and CD90 |
| Sato et al. [55] | Hematoxylin and eosin | Anti-VEGF/ Anti-NGF/ ISOGEN reagent | _ | _ | H | Krenn score/ Kellgren–Lawrence grade/ Harris Hip Score/ Spearman’s correlation analysis | VEGF and NGF expression levels |
| Schon et al. [83] | HE/ Picrosirius Red | MMPs/TIMPs | _ | _ | H | Mann–Whitney U test | Expression profile of matrix metalloproteinases |
| Stattin et al. [84] | Cell culture/Recombinant protein expression | ACAN gene (NM_00135.2, NM_013227.2) | _ | Chemiluminescent detection and documentation (Bio-Rad ChemiDoc MP) | H | Affinity Design 1.7 software | ACAN variants linked to hereditary skeletal disorders |
| Wang et al. [85] | _ | Serum 25-hydroxyvitamin D | _ | _ | H | Categorical analysis, heterogeneity checks, publication bias analysis, and subgroup analyses | Review 25-(H)D |
| Article | Histology/Other | Biomolecule | Ultrastructural Association | IRM/CT Association/Other | H/AM | Statistics/ Scoring/ Scale | Aim |
|---|---|---|---|---|---|---|---|
| Jiang et al. [92] | Cell cultures/chromatography via an ALP detection kit/chromatography using a hydroxyproline detection kit/PCR | Cbfa1α sense/Cbfa1α anti-sense/OCα sense/OCα anti-sense/BMPR-I/BMPR-II/Smad1/Smad5/phosphorylated (p) Smad1/pSmad5/Smad4/Smad6/Cbfa1 | Osteogenic disparity characteristics of fibroblasts in hip joint capsule | ||||
| Li et al. [72] | Cell cycle, viability, apoptosis, immunofluorescence, reverse transcription polymerase chain reaction/RT-PCR/Western blotting | COL1A1/COL3A1/MMP1/MMP3/MMP9/MMP13/TGF-β1/TGF-β2/SMAD3/WNT11/αSMA/CCNB1/CCNE2/CCNA2/CDK1/E2F1/CDC6/CDC7 | _ | _ | H | Unpaired two-tailed t-test with GraphPad Prism software, USA | Molecular changes in hip dysplasia |
| Zhang et al. [93] | Monoclonal rabbit anti-human collagen I/rabbit anti-human collagen III antibody/qPCR RT | X-ray imaging | Tonnis’ classification of hip dislocation/statistical software SPSS 16.0/two-tailed Student’s t-tests | Roles of collagen I and III in the hip capsule | |||
| Zhao et al. [87] | HE/MASSON | DNBelab C Series Single-Cell Library Prep Kit/Metabolic genes | _ | _ | H | clusterProfiler R package/pseudotemporal analysis | Receptor-like cells and ligament stem cells |
| Article | Histology/Other | Biomolecule | UA | IRM/CT Association/Other | H/AM | Statistics/ Scoring/ Scale | Aim |
|---|---|---|---|---|---|---|---|
| Batushansky et al. [107] | Liquid chromatography/gas chromatography–mass spectrometry | Glycine/histidine, lysophospholipid LPC/hypoxanthine/homocysteine/urate/tryptophan/fructose/citrate/malate/methionine/3-hydroxybutyrate | _ | _ | H | BIPED classification model of OA biomarkers | Review to relate metabolic syndrome to synovium pathology |
| Carlson et al. [108] | High-performance liquid chromatography–mass spectrometry (LC-MS) | Glycine/serine/alanine/threonine/lysine, arginine/proline/urea cycle/phosphatidylinositol phosphate metabolism/carnitine shuttle/vitamin metabolism (B5 and C)/porphyrin metabolism | _ | _ | H | MetaboAnalyst/Kolmogorov–Smirnov test (KS-test)/HCA/Mummichog/Volcano plot analysis | Metabolomic phenotypes from human synovial fluid |
| Coryell et al. [106] | _ | SA-β-Gal production/p16 expression/EV secretion | _ | _ | H | _ | Review of phenotypes associated with cellular senescence |
| Haubruck et al. [99] | _ | Monocyte/Macrophage | _ | _ | H | _ | Review of synovial in situ dynamics of joint macrophages and monocytes |
| Liu et al. [1] | _ | GATA/STINGFOXD1/SIRT6/DGCR8 | _ | _ | H | _ | Systematic review/chondrocyte senescence/senescent fibroblast-like synoviocytes |
| Kim et al. [109] | Gas chromatography/time-of-flight mass spectrometry | Hypoxanthine/xanthine/adenosine/citrulline/histidine/tryptophan | _ | _ | H | MetaboAnalyst | RA and OA metabolomic differentiation |
| Koyama et al. [78] | Real-time polymerase chain reaction (PCR) | RNA/TNFA/IL1B/IL6/COX2 mRNA | _ | _ | H | SPSS version 19.0 | Compare synovial tissue inflammatory cytokine levels |
| Laus et al. [110] | 1H-NMR | Tryptophan/phenylalanine/tyrosine/glycine/asparagine/glutamine/arginine/methionine/1,3-Dihydroxyacetone | _ | _ | NH | Box–Cox transformation/t-test | Metabolomic phenotypes from horse synovial fluid |
| MurilloSaich et al. [111] | HE/ELISA | CD68/Vimentin/TNF/CXCL2/ CCL2/MMP13 | _ | A 600 MHz Bruker Avance III spectrometer 1 H NMR | H | Krenn OPLS-DA/VIP | Potential metabolomic biomarkers of joint injury in synovial fluid and serum |
| Stabile et al. [112] | 1H-NMR | Isoleucine/leucine/valine/β-hydroxybutyrate/threonine/alanine/glutamine/methionine/lactate/acetate/acetoacetate/pyruvate/citrate/creatine/creatinine/β-glucose/TMAO/lactate/histidine/phenylalanine/tyrosine/formic acid | _ | _ | NH | R Statistic software/unsupervised PCA, HCA/supervised OPLS-DA | Metabolomic phenotypes from canine synovial fluid |
| Yang et al. [113] | _ | RNA/RT-qPCR/synovial mesenchymal stem cells/VEGF/MMP9 | _ | _ | H | R software/GraphPad Prism/Shapiro–Wilk test | Characteristics of the synovium in OA |
| Wallace et al. [114] | High-performance liquid chromatography | GAG monomers/steroid hormones/NADPH | _ | _ | NH | MetaboAnalyst/hierarchical cluster analysis | Potential metabolomic biomarkers of joint injury in synovial fluid and serum |
| Zhan et al. [115] | Mass spectrometry | Geniposide-related biomarkers | _ | _ | H | MetaboAnalyst/hierarchical cluster analysis | Potential metabolomic biomarkers of joint injury in synovial fluid |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huzum, R.M.; Hînganu, M.V.; Huzum, B.; Hînganu, D. Advances in Molecular Research on Hip Joint Impingement—A Vascular Perspective. Biomolecules 2024, 14, 784. https://doi.org/10.3390/biom14070784
Huzum RM, Hînganu MV, Huzum B, Hînganu D. Advances in Molecular Research on Hip Joint Impingement—A Vascular Perspective. Biomolecules. 2024; 14(7):784. https://doi.org/10.3390/biom14070784
Chicago/Turabian StyleHuzum, Riana Maria, Marius Valeriu Hînganu, Bogdan Huzum, and Delia Hînganu. 2024. "Advances in Molecular Research on Hip Joint Impingement—A Vascular Perspective" Biomolecules 14, no. 7: 784. https://doi.org/10.3390/biom14070784





