Third-Generation Tetracyclines: Current Knowledge and Therapeutic Potential
Abstract
:1. Introduction
2. Literature Search Methodology
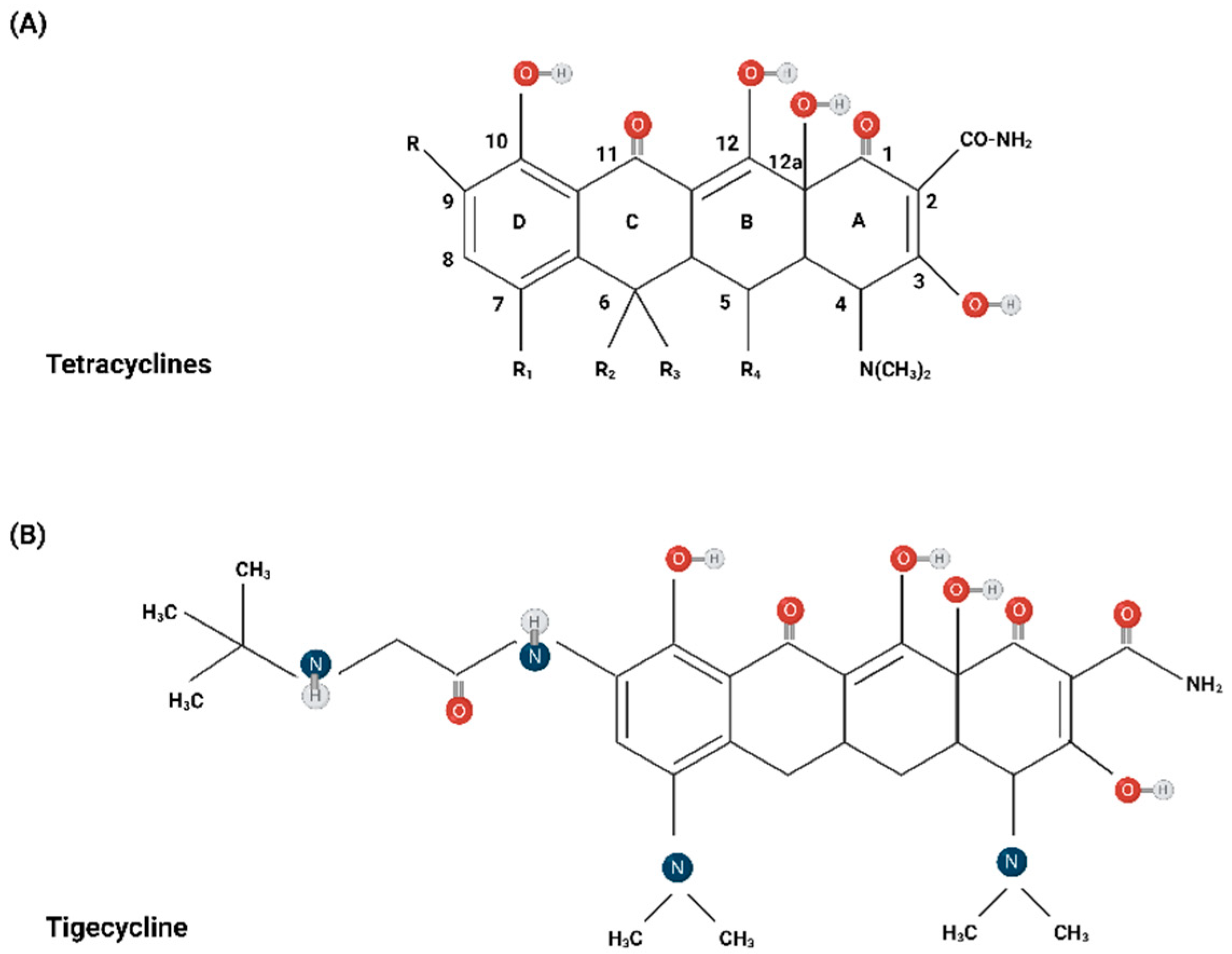
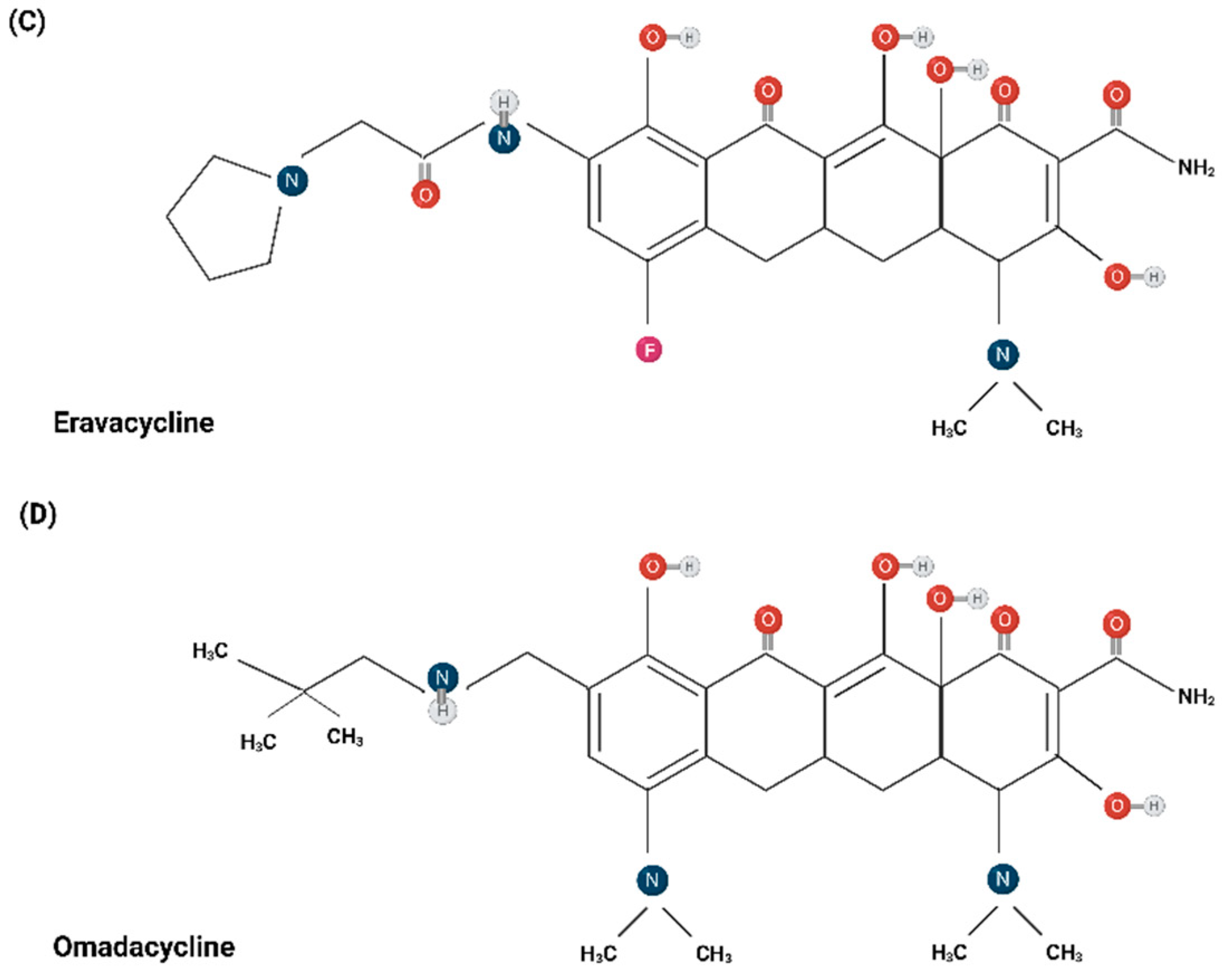
3. Chemical Structure
4. Mechanism of Action
5. Clinical Pharmacology
5.1. Tigecycline
5.2. Eravacycline
5.3. Omadacycline
6. Spectrum of Activity
6.1. Tigecycline
6.2. Eravacycline
6.3. Omadacycline
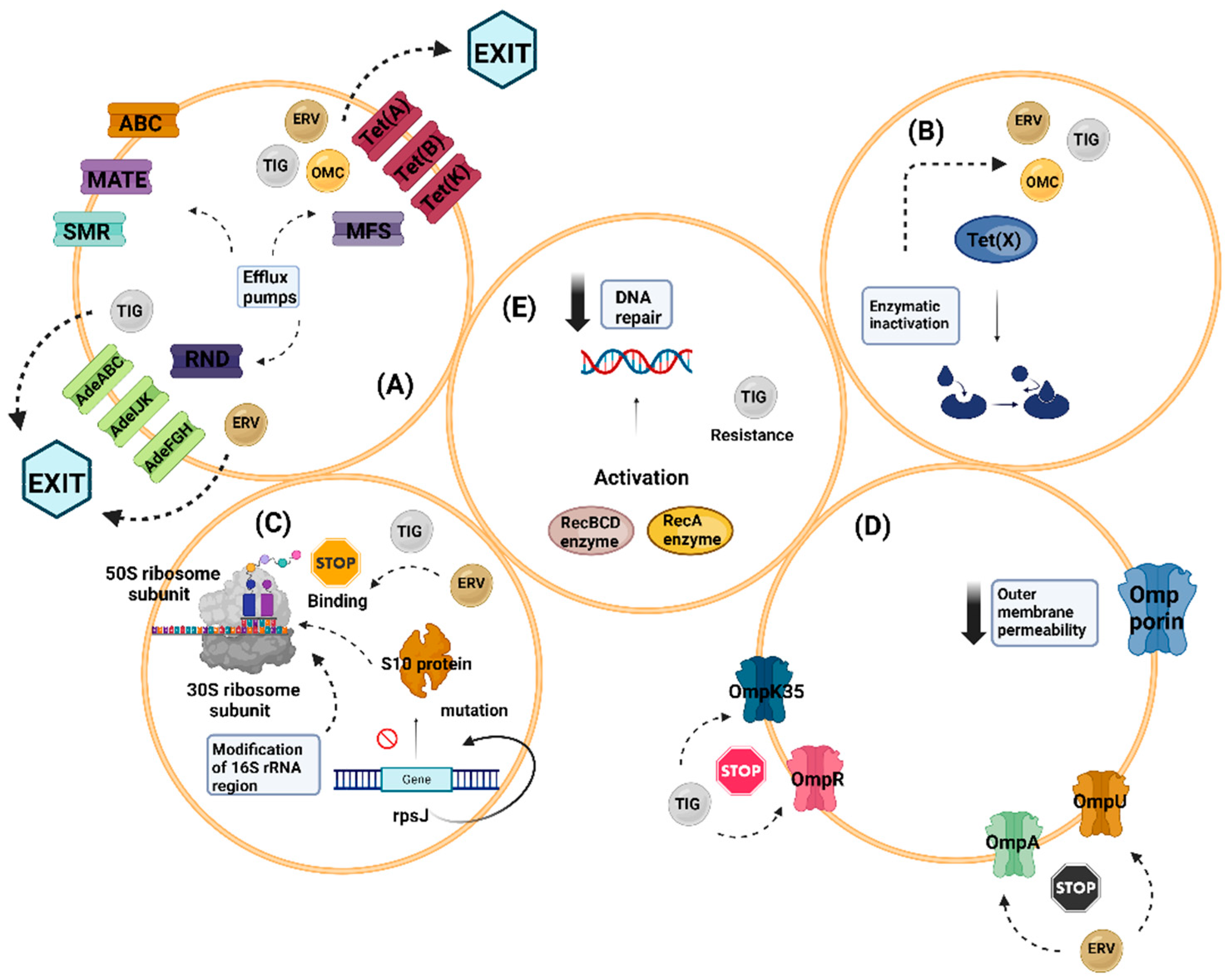
7. Resistance Mechanisms
7.1. Efflux Pumps
7.2. Enzymatic Inactivation
7.3. Modification in the Target of Action of Tetracyclines
7.4. Decreased Outer Membrane Permeability
7.5. Defective DNA Repair Mechanisms
8. Established Indications of Prescription
8.1. Tigecycline
8.2. Eravacycline
8.3. Omadacycline
9. Potential Indications of Administration
9.1. Mycobacterial Infections
9.2. Clostridioides Difficile Infection
9.3. Infection from Helicobacter pylori
9.4. Urinary Tract Infections
10. Synergistic Benefits in Combination Therapy
11. Non-Antibiotic Properties: Focusing on Immunomodulation and Malignancy
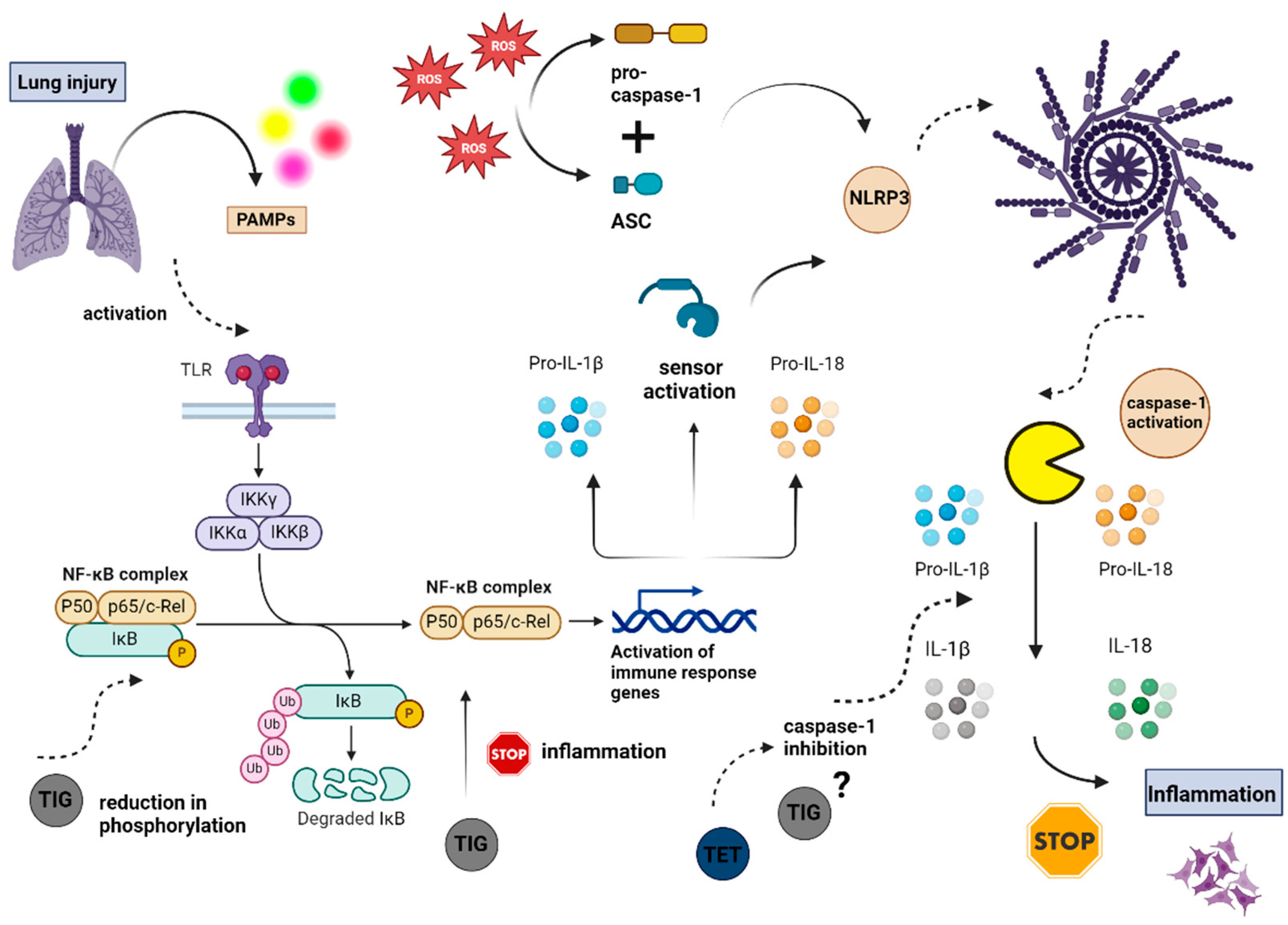
11.1. Immunomodulation
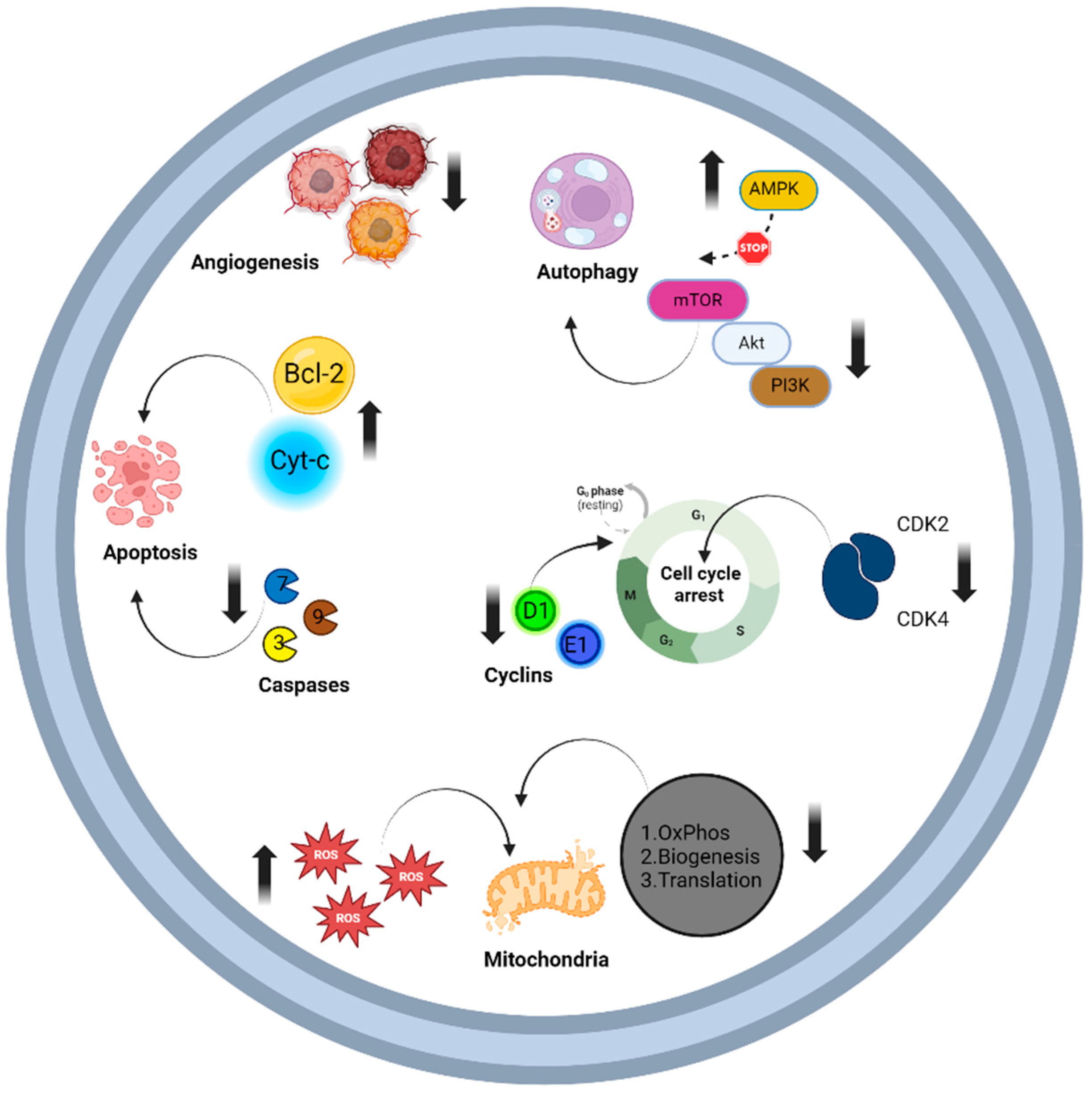
11.2. Malignancy
12. Highlighting the Future Perspectives of Third-Generation Tetracyclines
13. Side Effects of Third-Generation Tetracyclines
14. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Nelson, M.L.; Levy, S.B. The history of the tetracyclines. Ann. N. Y. Acad. Sci. 2011, 1241, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Linsell, W.D.; Fletcher, A.P. Laboratory and clinical experience with terramycin hydrochloride. Br. Med. J. 1950, 2, 1190–1195. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.C. Tetracycline therapy: Update. Clin. Infect. Dis. 2003, 36, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Miell, J.; Dhanjal, P.; Jamookeeah, C. Evidence for the use of demeclocycline in the treatment of hyponatraemia secondary to SIADH: A systematic review. Int. J. Clin. Pract. 2015, 69, 1396–1417. [Google Scholar] [CrossRef]
- Navarro-Triviño, F.J.; Pérez-López, I.; Ruiz-Villaverde, R. Doxycycline, an Antibiotic or an Anti-Inflammatory Agent? The Most Common Uses in Dermatology. Actas Dermosifiliogr. (Engl. Ed.) 2020, 111, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Mesa, N.; Zarzuelo, A.; Gálvez, J. Minocycline: Far beyond an antibiotic. Br. J. Pharmacol. 2013, 169, 337–352. [Google Scholar] [CrossRef]
- Singh, S.; Khanna, D.; Kalra, S. Minocycline and Doxycycline: More than Antibiotics. Curr. Mol. Pharmacol. 2021, 14, 1046–1065. [Google Scholar] [CrossRef]
- Rassouli, A.; Shihmani, B.; Mehrzad, J.; Shokrpoor, S. The immunomodulatory effect of minocycline on gene expression of inflammation related cytokines in lipopolysaccharide-treated human peripheral blood mononuclear cells. Anim. Biotechnol. 2023, 34, 2159–2165. [Google Scholar] [CrossRef]
- Jung, E.; Gademann, K. Clinically Approved Antibiotics from 2010 to 2022. Chimia 2023, 77, 230–234. [Google Scholar] [CrossRef]
- Reynolds, R.V.; Yeung, H.; Cheng, C.E.; Cook-Bolden, F.; Desai, S.R.; Druby, K.M.; Freeman, E.E.; Keri, J.E.; Stein Gold, L.F.; Tan, J.K.L.; et al. Guidelines of care for the management of acne vulgaris. J. Am. Acad. Dermatol. 2024, 90, 1006.e1–1006.e30. [Google Scholar] [CrossRef]
- Fuoco, D. Classification Framework and Chemical Biology of Tetracycline Structure-Based Drugs. Antibiotics 2012, 1, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chopra, I.; Roberts, M. Tetracycline antibiotics: Mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef] [PubMed]
- Barrenechea, V.; Vargas-Reyes, M.; Quiliano, M.; Milón, P. A Complementary Mechanism of Bacterial mRNA Translation Inhibition by Tetracyclines. Front. Microbiol. 2021, 12, 682682. [Google Scholar] [CrossRef] [PubMed]
- LaPlante, K.L.; Dhand, A.; Wright, K.; Lauterio, M. Re-establishing the utility of tetracycline-class antibiotics for current challenges with antibiotic resistance. Ann. Med. 2022, 54, 1686–1700. [Google Scholar] [CrossRef] [PubMed]
- Greer, N.D. Tigecycline (Tygacil): The first in the glycylcycline class of antibiotics. Proc. Bayl. Univ. Med. Cent. 2006, 19, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Zhanel, G.G.; Cheung, D.; Adam, H.; Zelenitsky, S.; Golden, A.; Schweizer, F.; Gorityala, B.; Lagacé-Wiens, P.R.; Walkty, A.; Gin, A.S.; et al. Review of Eravacycline, a Novel Fluorocycline Antibacterial Agent. Drugs 2016, 76, 567–588. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Esquivel, J.; Zelenitsky, S.; Lawrence, C.K.; Adam, H.J.; Golden, A.; Hink, R.; Berry, L.; Schweizer, F.; Zhanel, M.A.; et al. Omadacycline: A Novel Oral and Intravenous Aminomethylcycline Antibiotic Agent. Drugs 2020, 80, 285–313. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Homenuik, K.; Nichol, K.; Noreddin, A.; Vercaigne, L.; Embil, J.; Gin, A.; Karlowsky, J.A.; Hoban, D.J. The glycylcyclines: A comparative review with the tetracyclines. Drugs 2004, 64, 63–88. [Google Scholar] [CrossRef]
- Alegun, O.; Pandeya, A.; Cui, J.; Ojo, I.; Wei, Y. Donnan Potential across the Outer Membrane of Gram-Negative Bacteria and Its Effect on the Permeability of Antibiotics. Antibiotics 2021, 10, 701. [Google Scholar] [CrossRef]
- Prajapati, J.D.; Kleinekathöfer, U.; Winterhalter, M. How to Enter a Bacterium: Bacterial Porins and the Permeation of Antibiotics. Chem. Rev. 2021, 121, 5158–5192. [Google Scholar] [CrossRef]
- Olson, M.W.; Ruzin, A.; Feyfant, E.; Rush, T.S., 3rd; O’Connell, J.; Bradford, P.A. Functional, biophysical, and structural bases for antibacterial activity of tigecycline. Antimicrob. Agents Chemother. 2006, 50, 2156–2166. [Google Scholar] [CrossRef] [PubMed]
- Stein, G.E.; Babinchak, T. Tigecycline: An update. Diagn. Microbiol. Infect. Dis. 2013, 75, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Grossman, T.H.; Starosta, A.L.; Fyfe, C.; O’Brien, W.; Rothstein, D.M.; Mikolajka, A.; Wilson, D.N.; Sutcliffe, J.A. Target- and resistance-based mechanistic studies with TP-434, a novel fluorocycline antibiotic. Antimicrob. Agents Chemother. 2012, 56, 2559–2564. [Google Scholar] [CrossRef] [PubMed]
- Draper, M.P.; Weir, S.; Macone, A.; Donatelli, J.; Trieber, C.A.; Tanaka, S.K.; Levy, S.B. Mechanism of action of the novel aminomethylcycline antibiotic omadacycline. Antimicrob. Agents Chemother. 2014, 58, 1279–1283. [Google Scholar] [CrossRef] [PubMed]
- Meagher, A.K.; Ambrose, P.G.; Grasela, T.H.; Ellis-Grosse, E.J. The pharmacokinetic and pharmacodynamic profile of tigecycline. Clin. Infect. Dis. 2005, 41 (Suppl. S5), S333–S340. [Google Scholar] [CrossRef] [PubMed]
- Barbour, A.; Schmidt, S.; Ma, B.; Schiefelbein, L.; Rand, K.H.; Burkhardt, O.; Derendorf, H. Clinical pharmacokinetics and pharmacodynamics of tigecycline. Clin. Pharmacokinet. 2009, 48, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.C.; Huang, F.; Zhang, J.M.; Zhuang, Y.G. Population Pharmacokinetics of Tigecycline: A Systematic Review. Drug Des. Devel. Ther. 2022, 16, 1885–1896. [Google Scholar] [CrossRef] [PubMed]
- Li, M.X.; Li, N.; Zhu, L.Q.; Liu, W. Optimization of tigecycline dosage regimen for different infections in the patients with hepatic or renal impairment. J. Chemother. 2020, 32, 420–428. [Google Scholar] [CrossRef]
- Rafailidis, P.; Panagopoulos, P.; Koutserimpas, C.; Samonis, G. Current Therapeutic Approaches for Multidrug-Resistant and Extensively Drug-Resistant Acinetobacter baumannii Infections. Antibiotics 2024, 13, 261. [Google Scholar] [CrossRef]
- McCarthy, M.W. Clinical Pharmacokinetics and Pharmacodynamics of Eravacycline. Clin. Pharmacokinet. 2019, 58, 1149–1153. [Google Scholar] [CrossRef]
- Zou, X.; Jin, S.; Chen, L.; Li, J.; Zhang, X.; Zhou, H.; Li, X.; Huang, H. Antibacterial Activity of Eravacycline Against Carbapenem-Resistant Gram-Negative Isolates in China: An in vitro Study. Infect. Drug Resist. 2023, 16, 2271–2279. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.J. Eravacycline: A Review in Complicated Intra-Abdominal Infections. Drugs 2019, 79, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Rodvold, K.A.; Pai, M.P. Pharmacokinetics and Pharmacodynamics of Oral and Intravenous Omadacycline. Clin. Infect. Dis. 2019, 69 (Suppl. S1), S16–S22. [Google Scholar] [CrossRef] [PubMed]
- Rodvold, K.A.; Burgos, R.M.; Tan, X.; Pai, M.P. Omadacycline: A Review of the Clinical Pharmacokinetics and Pharmacodynamics. Clin. Pharmacokinet. 2020, 59, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Trang, M.; Lakota, E.A.; Safir, M.C.; Bhavnani, S.M.; Friedrich, L.; Steenbergen, J.N.; McGovern, P.C.; Tzanis, E.; Rubino, C.M. Evaluation of the Impact of Comorbidities on Omadacycline Pharmacokinetics. Antimicrob. Agents Chemother. 2023, 67, e0239721. [Google Scholar] [CrossRef] [PubMed]
- Cilloniz, C.; Torres, A. The pharmacokinetic evaluation of omadacycline (Oral Only Dosing Regimen) for the treatment of Community-Acquired Bacterial Pneumonia (CABP). Expert Opin. Drug Metab. Toxicol. 2023, 19, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubi, S.; Zekiy, A.O.; Krutova, M.; Gholami, M.; Kouhsari, E.; Sholeh, M.; Ghafouri, Z.; Maleki, F. Tigecycline antibacterial activity, clinical effectiveness, and mechanisms and epidemiology of resistance: Narrative review. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 1003–1022. [Google Scholar] [CrossRef] [PubMed]
- Betriu, C.; Culebras, E.; Gómez, M.; Rodríguez-Avial, I.; Picazo, J.J. In vitro activity of tigecycline against Bacteroides species. J. Antimicrob. Chemother. 2005, 56, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Sorlózano, A.; Gutiérrez, J.; Salmerón, A.; Luna, J.D.; Martínez-Checa, F.; Román, J.; Piédrola, G. Activity of tigecycline against clinical isolates of Staphylococcus aureus and extended-spectrum beta-lactamase-producing Escherichia coli in Granada, Spain. Int. J. Antimicrob. Agents 2006, 28, 532–536. [Google Scholar] [CrossRef]
- Kechagias, K.S.; Chorepsima, S.; Triarides, N.A.; Falagas, M.E. Tigecycline for the treatment of patients with Clostridium difficile infection: An update of the clinical evidence. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1053–1058. [Google Scholar] [CrossRef]
- Zhang, T.; Du, J.; Dong, L.; Wang, F.; Zhao, L.; Jia, J.; Wang, C.; Cheng, M.; Yu, X.; Huang, H. In Vitro Antimicrobial Activities of Tigecycline, Eravacycline, Omadacycline, and Sarecycline against Rapidly Growing Mycobacteria. Microbiol. Spectr. 2023, 11, e0323822. [Google Scholar] [CrossRef]
- Morrissey, I.; Olesky, M.; Hawser, S.; Lob, S.H.; Karlowsky, J.A.; Corey, G.R.; Bassetti, M.; Fyfe, C. In Vitro Activity of Eravacycline against Gram-Negative Bacilli Isolated in Clinical Laboratories Worldwide from 2013 to 2017. Antimicrob. Agents Chemother. 2020, 64, e01699-19. [Google Scholar] [CrossRef]
- Zeng, W.; Zhang, X.; Liu, Y.; Zhang, Y.; Xu, M.; Wang, S.; Sun, Y.; Zhou, T.; Chen, L. In vitro antimicrobial activity and resistance mechanisms of the new generation tetracycline agents, eravacycline, omadacycline, and tigecycline against clinical Staphylococcus aureus isolates. Front. Microbiol. 2022, 13, 1043736. [Google Scholar] [CrossRef] [PubMed]
- Brauncajs, M.; Bielec, F.; Macieja, A.; Pastuszak-Lewandoska, D. In Vitro Activity of Eravacycline against Carbapenemase-Producing Gram-Negative Bacilli Clinical Isolates in Central Poland. Biomedicines 2023, 11, 1784. [Google Scholar] [CrossRef] [PubMed]
- Alosaimy, S.; Morrisette, T.; Lagnf, A.M.; Rojas, L.M.; King, M.A.; Pullinger, B.M.; Hobbs, A.L.V.; Perkins, N.B., 3rd; Veve, M.P.; Bouchard, J.; et al. Clinical Outcomes of Eravacycline in Patients Treated Predominately for Carbapenem-Resistant Acinetobacter baumannii. Microbiol. Spectr. 2022, 10, e0047922. [Google Scholar] [CrossRef]
- Alexander, C.; Hill, D. A Retrospective Case-Control Study of Eravacycline for the Treatment of Carbapenem-Resistant Acinetobacter Infections in Patients With Burn Injuries. J. Burn Care Res. 2024, 45, 487–492. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, G.; Zhao, Q.; Wang, L.; Yang, J.; Cui, J. In vitro Antimicrobial Activity and Dose Optimization of Eravacycline and Other Tetracycline Derivatives Against Levofloxacin-Non-Susceptible and/or Trimethoprim-Sulfamethoxazole-Resistant Stenotrophomonas maltophilia. Infect. Drug Resist. 2023, 16, 6005–6015. [Google Scholar] [CrossRef]
- Hawser, S.; Kothari, N.; Monti, F.; Morrissey, I.; Siegert, S.; Hodges, T. In vitro activity of eravacycline and comparators against Gram-negative and Gram-positive bacterial isolates collected from patients globally between 2017 and 2020. J. Glob. Antimicrob. Resist. 2023, 33, 304–320. [Google Scholar] [CrossRef] [PubMed]
- Karlowsky, J.A.; Steenbergen, J.; Zhanel, G.G. Microbiology and Preclinical Review of Omadacycline. Clin. Infect. Dis. 2019, 69 (Suppl. S1), S6–S15. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Huband, M.D.; Shortridge, D.; Flamm, R.K. Surveillance of Omadacycline Activity Tested against Clinical Isolates from the United States and Europe: Report from the SENTRY Antimicrobial Surveillance Program, 2016 to 2018. Antimicrob. Agents Chemother. 2020, 64, e02488-19. [Google Scholar] [CrossRef]
- Abbey, T.; Vialichka, A.; Jurkovic, M.; Biagi, M.; Wenzler, E. Activity of Omadacycline Alone and in Combination against Carbapenem-Nonsusceptible Acinetobacter baumannii with Varying Minocycline Susceptibility. Microbiol. Spectr. 2022, 10, e0054222. [Google Scholar] [CrossRef] [PubMed]
- Deolankar, M.S.; Carr, R.A.; Fliorent, R.; Roh, S.; Fraimow, H.; Carabetta, V.J. Evaluating the Efficacy of Eravacycline and Omadacycline against Extensively Drug-Resistant Acinetobacter baumannii Patient Isolates. Antibiotics 2022, 11, 1298. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, J.N.; Putra, V.; Maring, B.L.; Ozer, E.A.; Belfiore, G.M.; Rhodes, N.J. Effect of omadacycline alone and in combination with meropenem against carbapenem-resistant Acinetobacter baumannii isolates. J. Glob. Antimicrob. Resist. 2022, 29, 147–149. [Google Scholar] [CrossRef]
- Grossman, T.H. Tetracycline Antibiotics and Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025387. [Google Scholar] [CrossRef]
- Vanbaelen, T.; Manoharan-Basil, S.S.; Kenyon, C. 45 years of tetracycline post exposure prophylaxis for STIs and the risk of tetracycline resistance: A systematic review and meta-analysis. BMC Infect. Dis. 2024, 24, 376. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.S.; Unemo, M. Antimicrobial treatment and resistance in sexually transmitted bacterial infections. Nat. Rev. Microbiol. 2024, 22, 435–450. [Google Scholar] [CrossRef]
- Sun, C.; Yu, Y.; Hua, X. Resistance mechanisms of tigecycline in Acinetobacter baumannii. Front. Cell. Infect. Microbiol. 2023, 13, 1141490. [Google Scholar] [CrossRef]
- Roch, M.; Sierra, R.; Andrey, D.O. Antibiotic heteroresistance in ESKAPE pathogens, from bench to bedside. Clin. Microbiol. Infect. 2023, 29, 320–325. [Google Scholar] [CrossRef]
- Khlaif, M.M.; Hussein, N.H. Sequencing analysis of tigecycline resistance among non-susceptible in three species of G-ve bacteria isolated from clinical specimens in Baghdad. Mol. Biol. Rep. 2022, 49, 11811–11820. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, B.; Xu, G.; Chen, J.; Shang, Y.; Lin, Z.; Yu, Z.; Zheng, J.; Bai, B. In Vitro Activity of the Novel Tetracyclines, Tigecycline, Eravacycline, and Omadacycline, Against Moraxella catarrhalis. Ann. Lab. Med. 2021, 41, 293–301. [Google Scholar] [CrossRef]
- Okada, U.; Murakami, S. Structural and functional characteristics of the tripartite ABC transporter. Microbiology 2022, 168, 001257. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.; Zeng, Y.; Wang, Y.; Liu, C.; Lu, J.; Cai, C.; Liu, X.; Chen, Y.; Wu, Y.; Fang, Y.; et al. Distribution and spread of the mobilized RND efflux pump gene cluster tmexCD-toprJ in clinical Gram-negative bacteria: A molecular epidemiological study. Lancet Microbe 2022, 3, e846–e856. [Google Scholar] [CrossRef] [PubMed]
- Burata, O.E.; Yeh, T.J.; Macdonald, C.B.; Stockbridge, R.B. Still rocking in the structural era: A molecular overview of the small multidrug resistance (SMR) transporter family. J. Biol. Chem. 2022, 298, 102482. [Google Scholar] [CrossRef]
- Shi, Y.; Hua, X.; Xu, Q.; Yang, Y.; Zhang, L.; He, J.; Mu, X.; Hu, L.; Leptihn, S.; Yu, Y. Mechanism of eravacycline resistance in Acinetobacter baumannii mediated by a deletion mutation in the sensor kinase adeS, leading to elevated expression of the efflux pump AdeABC. Infect. Genet. Evol. 2020, 80, 104185. [Google Scholar] [CrossRef] [PubMed]
- Anyanwu, M.U.; Nwobi, O.C.; Okpala, C.O.R.; Ezeonu, I.M. Mobile Tigecycline Resistance: An Emerging Health Catastrophe Requiring Urgent One Health Global Intervention. Front. Microbiol. 2022, 13, 808744. [Google Scholar] [CrossRef]
- Sun, J.; Chen, C.; Cui, C.Y.; Zhang, Y.; Liu, X.; Cui, Z.H.; Ma, X.Y.; Feng, Y.; Fang, L.X.; Lian, X.L.; et al. Plasmid-encoded tet(X) genes that confer high-level tigecycline resistance in Escherichia coli. Nat. Microbiol. 2019, 4, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Jiang, Y.; Peng, K.; Wang, Y.; Wang, M.; Liu, Y.; Wang, Z. Phenotypic and genomic analysis reveals Riemerella anatipestifer as the potential reservoir of tet(X) variants. J. Antimicrob. Chemother. 2022, 77, 374–380. [Google Scholar] [CrossRef]
- Hsieh, Y.C.; Wu, J.W.; Chen, Y.Y.; Quyen, T.L.T.; Liao, W.C.; Li, S.W.; Chen, Y.C.; Pan, Y.J. An Outbreak of tet(X6)-Carrying Tigecycline-Resistant Acinetobacter baumannii Isolates with a New Capsular Type at a Hospital in Taiwan. Antibiotics 2021, 10, 1239. [Google Scholar] [CrossRef]
- Wang, Q.; Lei, C.; Cheng, H.; Yang, X.; Huang, Z.; Chen, X.; Ju, Z.; Zhang, H.; Wang, H. Widespread Dissemination of Plasmid-Mediated Tigecycline Resistance Gene tet(X4) in Enterobacterales of Porcine Origin. Microbiol. Spectr. 2022, 10, e0161522. [Google Scholar] [CrossRef]
- Chirabhundhu, N.; Luk-In, S.; Phuadraksa, T.; Wichit, S.; Chatsuwan, T.; Wannigama, D.L.; Yainoy, S. Occurrence and mechanisms of tigecycline resistance in carbapenem- and colistin-resistant Klebsiella pneumoniae in Thailand. Sci. Rep. 2024, 14, 5215. [Google Scholar] [CrossRef]
- Alosaimy, S.; Abdul-Mutakabbir, J.C.; Kebriaei, R.; Jorgensen, S.C.J.; Rybak, M.J. Evaluation of Eravacycline: A Novel Fluorocycline. Pharmacotherapy 2020, 40, 221–238. [Google Scholar] [CrossRef]
- Hua, X.; He, J.; Wang, J.; Zhang, L.; Zhang, L.; Xu, Q.; Shi, K.; Leptihn, S.; Shi, Y.; Fu, X.; et al. Novel tigecycline resistance mechanisms in Acinetobacter baumannii mediated by mutations in adeS, rpoB and rrf. Emerg. Microbes Infect. 2021, 10, 1404–1417. [Google Scholar] [CrossRef] [PubMed]
- Heidrich, C.G.; Mitova, S.; Schedlbauer, A.; Connell, S.R.; Fucini, P.; Steenbergen, J.N.; Berens, C. The Novel Aminomethylcycline Omadacycline Has High Specificity for the Primary Tetracycline-Binding Site on the Bacterial Ribosome. Antibiotics 2016, 5, 32. [Google Scholar] [CrossRef]
- Shi, J.; Cheng, J.; Liu, S.; Zhu, Y.; Zhu, M. Acinetobacter baumannii: An evolving and cunning opponent. Front. Microbiol. 2024, 15, 1332108. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, H.; Ko, K.S. Reduced virulence in tigecycline-resistant Klebsiella pneumoniae caused by overexpression of ompR and down-regulation of ompK35. J. Biomed. Sci. 2023, 30, 22. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wei, X.; Jin, Y.; Bai, F.; Cheng, Z.; Chen, S.; Pan, X.; Wu, W. Development of Resistance to Eravacycline by Klebsiella pneumoniae and Collateral Sensitivity-Guided Design of Combination Therapies. Microbiol. Spectr. 2022, 10, e0139022. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Skiebe, E.; Wilharm, G. Contributions of RecA and RecBCD DNA repair pathways to the oxidative stress response and sensitivity of Acinetobacter baumannii to antibiotics. Int. J. Antimicrob. Agents 2018, 52, 629–636. [Google Scholar] [CrossRef]
- Babinchak, T.; Ellis-Grosse, E.; Dartois, N.; Rose, G.M.; Loh, E.; Tigecycline 301 Study Group; Tigecycline 306 Study Group. The efficacy and safety of tigecycline for the treatment of complicated intra-abdominal infections: Analysis of pooled clinical trial data. Clin. Infect. Dis. 2005, 41 (Suppl. S5), S354–S367. [Google Scholar] [CrossRef]
- Ellis-Grosse, E.J.; Babinchak, T.; Dartois, N.; Rose, G.; Loh, E.; Tigecycline 300 cSSSI Study Group; Tigecycline 305 cSSSI Study Group. The efficacy and safety of tigecycline in the treatment of skin and skin-structure infections: Results of 2 double-blind phase 3 comparison studies with vancomycin-aztreonam. Clin. Infect. Dis. 2005, 41 (Suppl. S5), S341–S353. [Google Scholar] [CrossRef]
- Tanaseanu, C.; Bergallo, C.; Teglia, O.; Jasovich, A.; Oliva, M.E.; Dukart, G.; Dartois, N.; Cooper, C.A.; Gandjini, H.; Mallick, R.; et al. Integrated results of 2 phase 3 studies comparing tigecycline and levofloxacin in community-acquired pneumonia. Diagn. Microbiol. Infect. Dis. 2008, 61, 329–338. [Google Scholar] [CrossRef]
- Solomkin, J.; Evans, D.; Slepavicius, A.; Lee, P.; Marsh, A.; Tsai, L.; Sutcliffe, J.A.; Horn, P. Assessing the Efficacy and Safety of Eravacycline vs Ertapenem in Complicated Intra-abdominal Infections in the Investigating Gram-Negative Infections Treated With Eravacycline (IGNITE 1) Trial: A Randomized Clinical Trial. JAMA Surg. 2017, 152, 224–232. [Google Scholar] [CrossRef]
- Solomkin, J.S.; Gardovskis, J.; Lawrence, K.; Montravers, P.; Sway, A.; Evans, D.; Tsai, L. IGNITE4: Results of a Phase 3, Randomized, Multicenter, Prospective Trial of Eravacycline vs Meropenem in the Treatment of Complicated Intraabdominal Infections. Clin. Infect. Dis. 2019, 69, 921–929. [Google Scholar] [CrossRef]
- Meng, R.; Guan, X.; Sun, L.; Fei, Z.; Li, Y.; Luo, M.; Ma, A.; Li, H. The efficacy and safety of eravacycline compared with current clinically common antibiotics in the treatment of adults with complicated intra-abdominal infections: A Bayesian network meta-analysis. Front. Med. 2022, 9, 935343. [Google Scholar] [CrossRef] [PubMed]
- Lan, S.H.; Chang, S.P.; Lai, C.C.; Lu, L.C.; Chao, C.M. The Efficacy and Safety of Eravacycline in the Treatment of Complicated Intra-Abdominal Infections: A Systemic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2019, 8, 866. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, G.D.; Warren, N.; Yashayev, R.; Chitra, S.; Amodio-Groton, M.; Wright, K. Omadacycline in the treatment of community-acquired bacterial pneumonia in patients with comorbidities: A post-hoc analysis of the phase 3 OPTIC trial. Front. Med. 2023, 10, 1225710. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Bassetti, M.; Concia, E.; De Simone, G.; De Rosa, F.G.; Grossi, P.; Novelli, A.; Menichetti, F.; Petrosillo, N.; Tinelli, M.; et al. Italian Society of Infectious and Tropical Diseases. Diagnosis and management of skin and soft-tissue infections (SSTI). A literature review and consensus statement: An update. J. Chemother. 2017, 29, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Alsaad, N.; Wilffert, B.; van Altena, R.; de Lange, W.C.; van der Werf, T.S.; Kosterink, J.G.; Alffenaar, J.W. Potential antimicrobial agents for the treatment of multidrug-resistant tuberculosis. Eur. Respir. J. 2014, 43, 884–897. [Google Scholar] [CrossRef]
- Daley, C.L.; Iaccarino, J.M.; Lange, C.; Cambau, E.; Wallace, R.J., Jr.; Andrejak, C.; Böttger, E.C.; Brozek, J.; Griffith, D.E.; Guglielmetti, L.; et al. Treatment of nontuberculous mycobacterial pulmonary disease: An official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur. Respir. J. 2020, 56, 2000535. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Levin, A.; Kasperbauer, S.H.; Huitt, G.A.; Daley, C.L. Efficacy and safety of tigecycline for Mycobacterium abscessus disease. Respir. Med. 2019, 158, 89–91. [Google Scholar] [CrossRef]
- Kaushik, A.; Ammerman, N.C.; Martins, O.; Parrish, N.M.; Nuermberger, E.L. In Vitro Activity of New Tetracycline Analogs Omadacycline and Eravacycline against Drug-Resistant Clinical Isolates of Mycobacterium abscessus. Antimicrob. Agents Chemother. 2019, 63, e00470-19. [Google Scholar] [CrossRef]
- Brown-Elliott, B.A.; Wallace, R.J., Jr. In Vitro Susceptibility Testing of Eravacycline against Nontuberculous Mycobacteria. Antimicrob. Agents Chemother. 2022, 66, e0068922. [Google Scholar] [CrossRef]
- Rizzo, A.R.; Moniri, N.H. Omadacycline for management of Mycobacterium abscessus infections: A review of its effectiveness, place in therapy, and considerations for use. BMC Infect. Dis. 2022, 22, 874. [Google Scholar] [CrossRef]
- El Ghali, A.; Morrisette, T.; Alosaimy, S.; Lucas, K.; Tupayachi-Ortiz, M.G.; Vemula, R.; Wadle, C.; Philley, J.V.; Mejia-Chew, C.; Hamad, Y.; et al. Long-term evaluation of clinical success and safety of omadacycline in nontuberculous mycobacteria infections: A retrospective, multicenter cohort of real-world health outcomes. Antimicrob. Agents Chemother. 2023, 67, e0082423. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Gumbo, T.; Boorgula, G.D.; Thomas, T.A.; Philley, J.V.; Srivastava, S. Omadacycline pharmacokinetics/pharmacodynamics and efficacy against multidrug-resistant Mycobacterium tuberculosis in the hollow fiber system model. Antimicrob. Agents Chemother. 2024, 68, e0108023. [Google Scholar] [CrossRef] [PubMed]
- Jump, R.L.; Li, Y.; Pultz, M.J.; Kypriotakis, G.; Donskey, C.J. Tigecycline exhibits inhibitory activity against Clostridium difficile in the colon of mice and does not promote growth or toxin production. Antimicrob. Agents Chemother. 2011, 55, 546–569. [Google Scholar] [CrossRef] [PubMed]
- Phillips, E.C.; Warren, C.A.; Ma, J.Z.; Madden, G.R. Impact of Tigecycline on C. difficile Outcomes: Case Series and Propensity-Matched Retrospective Study. Antimicrob. Agents Chemother. 2022, 66, e0000122. [Google Scholar] [CrossRef] [PubMed]
- Buckley, A.M.; Altringham, J.; Clark, E.; Bently, K.; Spittal, W.; Ewin, D.; Wilkinson, V.; Davis, G.; Moura, I.B.; Wilcox, M.H. Eravacycline, a novel tetracycline derivative, does not induce Clostridioides difficile infection in an in vitro human gut model. J. Antimicrob. Chemother. 2021, 76, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Bassères, E.; Begum, K.; Lancaster, C.; Gonzales-Luna, A.J.; Carlson, T.J.; Miranda, J.; Rashid, T.; Alam, M.J.; Eyre, D.W.; Wilcox, M.H.; et al. In vitro activity of eravacycline against common ribotypes of Clostridioides difficile. J. Antimicrob. Chemother. 2020, 75, 2879–2884. [Google Scholar] [CrossRef] [PubMed]
- Skinner, A.M.; Petrella, L.A.; Cheknis, A.; Johnson, S. Antimicrobial susceptibility of Clostridioides difficile to omadacycline and comparator antimicrobials. J. Antimicrob. Chemother. 2023, 78, 1779–1784. [Google Scholar] [CrossRef] [PubMed]
- Budi, N.D.; Godfrey, J.J.; Safdar, N.; Shukla, S.K.; Rose, W.E. Efficacy of Omadacycline or Vancomycin Combined With Germinants for Preventing Clostridioides difficile Relapse in a Murine Model. J. Infect. Dis. 2023, 227, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.; Hu, C.; Begum, K.; Wang, W.; Le, T.M.; Agyapong, S.; Hanson, B.M.; Ayele, H.; Lancaster, C.; Jahangir Alam, M.; et al. Fecal Pharmacokinetics and Gut Microbiome Effects of Oral Omadacycline Versus Vancomycin in Healthy Volunteers. J. Infect. Dis. 2024, 229, 273–281. [Google Scholar] [CrossRef]
- Yang, J.C.; Lee, P.I.; Hsueh, P.R. In vitro activity of nemonoxacin, tigecycline, and other antimicrobial agents against Helicobacter pylori isolates in Taiwan, 1998–2007. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 1369–1375. [Google Scholar] [CrossRef]
- Monno, R.; Fumarola, L.; Capolongo, C.; Calia, C.; Pazzani, C.; Ierardi, E.; Miragliotta, G. Susceptibility of Helicobacter pylori to Antibiotics Including Tigecycline. J. Med. Microbiol. Diagn. 2015, S5, 005. [Google Scholar] [CrossRef]
- Yang, Y.; Bian, L.; Hang, X.; Yan, C.; Huang, Y.; Ye, F.; Zhang, G.; Jin, G.; Bi, H. In vitro activity of new tetracycline analogues omadacycline and eravacycline against clinical isolates of Helicobacter pylori collected in China. Diagn. Microbiol. Infect. Dis. 2020, 98, 115129. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Abraham, T.; Saad, N. Role of Tigecycline for the Treatment of Urinary Tract Infections. J. Pharm. Technol. 2014, 30, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.X.; Le, K.J.; Shi, H.Y.; Zhang, Z.L.; Cui, M.; Zhong, H.; Yu, Y.T.; Gu, Z.C. Efficacy and safety of tigecycline for complicated urinary tract infection: A systematic review. Transl. Androl. Urol. 2021, 10, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Charles, R.; Adhikari, S.D.; Mittal, A.; Chaudhuri, S.; Gupta, M.; Khot, W.; Schito, M.; Gupta, N. Role of tigecycline in the treatment of urinary tract infections: A systematic review of published case reports. InfezMed 2022, 30, 516–524. [Google Scholar] [CrossRef]
- Stone, T.J.; Kilic, A.; Williamson, J.C.; Palavecino, E.L. In Vitro Activity of Omadacycline and Comparator Antibiotics against Extended-Spectrum Beta-Lactamase-Producing Escherichia coli and Klebsiella pneumoniae Urinary Isolates. Antibiotics 2023, 12, 953. [Google Scholar] [CrossRef]
- Kim, S.R.; Jang, M.; Kim, S.Y.; Kim, D.H.; Jhun, B.W. Outcomes of Short-Term Tigecycline-Containing Regimens for Mycobacterium abscessus Pulmonary Disease. Antimicrob. Agents Chemother. 2022, 66, e0077422. [Google Scholar] [CrossRef] [PubMed]
- Karau, M.J.; Schmidt-Malan, S.M.; Cunningham, S.A.; Mandrekar, J.N.; Pritt, B.S.; Keepers, T.R.; Serio, A.W.; Chitra, S.; Patel, R. Activity of Omadacycline in Rat Methicillin-Resistant Staphylococcus aureus Osteomyelitis. Antimicrob. Agents Chemother. 2022, 66, e0170321. [Google Scholar] [CrossRef] [PubMed]
- Morrisette, T.; Stamper, K.C.; Lev, K.L.; Kebriaei, R.; Holger, D.J.; Abdul-Mutakabbir, J.C.; Kunz Coyne, A.J.; Rybak, M.J. Evaluation of Omadacycline Alone and in Combination with Rifampin against Staphylococcus aureus and Staphylococcus epidermidis in an In Vitro Pharmacokinetic/Pharmacodynamic Biofilm Model. Antimicrob. Agents Chemother. 2023, 67, e0131722. [Google Scholar] [CrossRef]
- Ni, W.; Yang, D.; Guan, J.; Xi, W.; Zhou, D.; Zhao, L.; Cui, J.; Xu, Y.; Gao, Z.; Liu, Y. In vitro and in vivo synergistic effects of tigecycline combined with aminoglycosides on carbapenem-resistant Klebsiella pneumoniae. J. Antimicrob. Chemother. 2021, 76, 2097–2105. [Google Scholar] [CrossRef]
- Nulsopapon, P.; Nasomsong, W.; Pongchaidecha, M.; Changpradub, D.; Juntanawiwat, P.; Santimaleeworagun, W. The Synergistic Activity and Optimizing Doses of Tigecycline in Combination with Aminoglycosides against Clinical Carbapenem-Resistant Klebsiella pneumoniae Isolates. Antibiotics 2021, 10, 736. [Google Scholar] [CrossRef] [PubMed]
- Zavascki, A.P.; Klee, B.O.; Bulitta, J.B. Aminoglycosides against carbapenem-resistant Enterobacteriaceae in the critically ill: The pitfalls of aminoglycoside susceptibility. Expert Rev. Anti. Infect. Ther. 2017, 15, 519–526. [Google Scholar] [CrossRef]
- Yang, F.; Chen, P.; Wang, H.; Xing, X.; Wang, S.; Ishaq, H.M.; Liao, W. Comparative Minimal Inhibitory and Mutant Prevention Concentration of Eight Antimicrobial Agents Against Klebsiella pneumoniae. Microb. Drug Resist. 2022, 28, 229–235. [Google Scholar] [CrossRef]
- Rahul, R.; Maheswary, D.; Damodaran, N.; Leela, K.V. Eravacycline -Synergistic activity with other antimicrobials in carbapenem resistant isolates of Escherichia coli and Acinetobacter baumannii. Diagn. Microbiol. Infect. Dis. 2023, 107, 116006. [Google Scholar] [CrossRef]
- Li, J.; Fu, Y.; Zhang, J.; Wang, Y.; Zhao, Y.; Fan, X.; Yu, L.; Wang, Y.; Zhang, X.; Li, C. Efficacy of tigecycline monotherapy versus combination therapy with other antimicrobials against carbapenem-resistant Acinetobacter baumannii sequence type 2 in Heilongjiang Province. Ann. Palliat. Med. 2019, 8, 651–659. [Google Scholar] [CrossRef]
- Ozger, H.S.; Cuhadar, T.; Yildiz, S.S.; Demirbas Gulmez, Z.; Dizbay, M.; Guzel Tunccan, O.; Kalkanci, A.; Simsek, H.; Unaldi, O. In vitro activity of eravacycline in combination with colistin against carbapenem-resistant A. baumannii isolates. J. Antibiot. 2019, 72, 600–604. [Google Scholar] [CrossRef]
- Li, Y.; Cui, L.; Xue, F.; Wang, Q.; Zheng, B. Synergism of eravacycline combined with other antimicrobial agents against carbapenem-resistant Enterobacteriaceae and Acinetobacter baumannii. J. Glob. Antimicrob. Resist. 2022, 30, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Bich Hanh, B.T.; Quang, N.T.; Park, Y.; Heo, B.E.; Jeon, S.; Park, J.W.; Jang, J. Omadacycline Potentiates Clarithromycin Activity Against Mycobacterium abscessus. Front. Pharmacol. 2021, 12, 790767. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, K.; Aono, A.; Asami, T.; Morimoto, K.; Kamada, K.; Morishige, Y.; Igarashi, Y.; Chikamatsu, K.; Murase, Y.; Yamada, H.; et al. In Vitro Synergistic Effects of Omadacycline with Other Antimicrobial Agents against Mycobacterium abscessus. Antimicrob. Agents Chemother. 2023, 67, e0157922. [Google Scholar] [CrossRef]
- Rimal, B.; Nicklas, D.A.; Panthi, C.M.; Lippincott, C.K.; Belz, D.C.; Ignatius, E.H.; Deck, D.H.; Serio, A.W.; Lamichhane, G. Efficacy of Omadacycline-Containing Regimen in a Mouse Model of Pulmonary Mycobacteroides abscessus Disease. mSphere 2023, 8, e0066522. [Google Scholar] [CrossRef]
- Aziz, D.B.; Teo, J.W.P.; Dartois, V.; Dick, T. Teicoplanin—Tigecycline Combination Shows Synergy Against Mycobacterium abscessus. Front. Microbiol. 2018, 9, 932. [Google Scholar] [CrossRef] [PubMed]
- Chew, K.L.; Octavia, S.; Yeoh, S.F.; Teo, J.W.P. In Vitro Synergy Testing of Eravacycline in Combination with Clarithromycin and Rifabutin against Mycobacterium abscessus Complex. Microbiol. Spectr. 2021, 9, e0004521. [Google Scholar] [CrossRef] [PubMed]
- Ku, T.S.; Palanisamy, S.K.; Lee, S.A. Susceptibility of Candida albicans biofilms to azithromycin, tigecycline and vancomycin and the interaction between tigecycline and antifungals. Int. J. Antimicrob. Agents 2010, 36, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Hooper, R.W.; Ashcraft, D.S.; Pankey, G.A. In vitro synergy with fluconazole plus doxycycline or tigecycline against clinical Candida glabrata isolates. Med. Mycol. 2019, 57, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, H.; Duan, X.; Cui, M.; Xing, W.; Zheng, S. Synergistic effect of eravacycline combined with fluconazole against resistant Candida albicans in vitro and in vivo. Expert Rev. Anti. Infect. Ther. 2023, 21, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Shigemi, H.; Tanaka, Y.; Yamauchi, T.; Ueda, T.; Iwasaki, H. Tetracyclines downregulate the production of LPS-induced cytokines and chemokines in THP-1 cells via ERK, p38, and nuclear factor-κB signaling pathways. Biochem. Biophys. Rep. 2015, 4, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Peukert, K.; Fox, M.; Schulz, S.; Feuerborn, C.; Frede, S.; Putensen, C.; Wrigge, H.; Kümmerer, B.M.; David, S.; Seeliger, B.; et al. Inhibition of Caspase-1 with Tetracycline Ameliorates Acute Lung Injury. Am. J. Respir. Crit. Care Med. 2021, 204, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Dikshit, N.; Kale, S.D.; Khameneh, H.J.; Balamuralidhar, V.; Tang, C.Y.; Kumar, P.; Lim, T.P.; Tan, T.T.; Kwa, A.L.; Mortellaro, A.; et al. NLRP3 inflammasome pathway has a critical role in the host immunity against clinically relevant Acinetobacter baumannii pulmonary infection. Mucosal. Immunol. 2018, 11, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Yagnik, R.M.; Benzeroual, K.E. Tigecycline prevents LPS-induced release of pro-inflammatory and apoptotic mediators in neuronal cells. Toxicol. In Vitro 2013, 27, 686–693. [Google Scholar] [CrossRef]
- Cockeran, R.; Mutepe, N.D.; Theron, A.J.; Tintinger, G.R.; Steel, H.C.; Stivaktas, P.I.; Richards, G.A.; Feldman, C.; Anderson, R. Calcium-dependent potentiation of the pro-inflammatory functions of human neutrophils by tigecycline in vitro. J. Antimicrob. Chemother. 2012, 67, 130–137. [Google Scholar] [CrossRef]
- Elhayek, S.Y.; Fararjeh, M.A.; Assaf, A.M.; Abu-Rish, E.Y.; Bustanji, Y. Immunomodulatory Effects of Tigecycline in Balb/C Mice. Acta Pharm. 2018, 68, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Bryant, A.E.; Stevens, D.L. Investigating the immunomodulatory activities of omadacycline. J. Antimicrob. Chemother. 2022, 78, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Skrtić, M.; Sriskanthadevan, S.; Jhas, B.; Gebbia, M.; Wang, X.; Wang, Z.; Hurren, R.; Jitkova, Y.; Gronda, M.; Maclean, N.; et al. Inhibition of mitochondrial translation as a therapeutic strategy for human acute myeloid leukemia. Cancer Cell 2011, 20, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Abbas, M.N.; Kausar, S.; Yang, J.; Li, L.; Tan, L.; Cui, H. Biological Functions and Molecular Mechanisms of Antibiotic Tigecycline in the Treatment of Cancers. Int. J. Mol. Sci. 2019, 20, 3577. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Yan, Y.; Li, Z.; Qian, L.; Gong, Z. The Antibiotic Drug Tigecycline: A Focus on its Promising Anticancer Properties. Front. Pharmacol. 2016, 7, 473. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Malagón, A.J.; Hidalgo-García, L.; Rodríguez-Sojo, M.J.; Molina-Tijeras, J.A.; García, F.; Diez-Echave, P.; Vezza, T.; Becerra, P.; Marchal, J.A.; Redondo-Cerezo, E.; et al. Tigecycline reduces tumorigenesis in colorectal cancer via inhibition of cell proliferation and modulation of immune response. Biomed. Pharmacother. 2023, 163, 114760. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Xu, N.; He, B.; Pan, C.; Lan, Y.; Zhou, H.; Liu, X. Inhibition of autophagy enhances the selective anti-cancer activity of tigecycline to overcome drug resistance in the treatment of chronic myeloid leukemia. J. Exp. Clin. Cancer Res. 2017, 36, 43. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Song, M.; Zhou, M.; Hu, Y. Antibiotic tigecycline enhances cisplatin activity against human hepatocellular carcinoma through inducing mitochondrial dysfunction and oxidative damage. Biochem. Biophys. Res. Commun. 2017, 483, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Guo, Y. Inhibition of mitochondrial translation as a therapeutic strategy for human ovarian cancer to overcome chemoresistance. Biochem. Biophys. Res. Commun. 2019, 509, 373–378. [Google Scholar] [CrossRef]
- Yang, R.; Yi, L.; Dong, Z.; Ouyang, Q.; Zhou, J.; Pang, Y.; Wu, Y.; Xu, L.; Cui, H. Tigecycline inhibits glioma growth by regulating miRNA-199b-5p-HES1-AKT pathway. Mol. Cancer Ther. 2016, 15, 421–429. [Google Scholar] [CrossRef]
- Zhong, X.; Zhao, E.; Tang, C.; Zhang, W.; Tan, J.; Dong, Z.; Ding, H.F.; Cui, H. Antibiotic drug tigecycline reduces neuroblastoma cells proliferation by inhibiting Akt activation in vitro and in vivo. Tumour. Biol. 2016, 37, 7615–7623. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Liu, W.; Huang, Q.; Wang, J.; Wang, Y.; Li, H.; Fu, X. Tigecycline as a dual inhibitor of retinoblastoma and angiogenesis via inducing mitochondrial dysfunctions and oxidative damage. Sci. Rep. 2018, 8, 11747. [Google Scholar] [CrossRef] [PubMed]
- Jabarin, A.; Shtar, G.; Feinshtein, V.; Mazuz, E.; Shapira, B.; Ben-Shabat, S.; Rokach, L. Eravacycline, an antibacterial drug, repurposed for pancreatic cancer therapy: Insights from a molecular-based deep learning model. Brief. Bioinform. 2024, 25, bbae108. [Google Scholar] [CrossRef] [PubMed]
- Rolston, K.; Gerges, B.; Nesher, L.; Shelburne, S.A.; Prince, R.; Raad, I. In vitro activity of eravacycline and comparator agents against bacterial pathogens isolated from patients with cancer. JAC Antimicrob. Resist. 2023, 5, dlad020. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.E.; Prajapati, S.; Pixley, J.N.; Grada, A.; Feldman, S.R. Oral Tetracycline-Class Drugs in Dermatology: Impact of Food Intake on Absorption and Efficacy. Antibiotics 2023, 12, 1152. [Google Scholar] [CrossRef] [PubMed]
- Opal, S.; File, T.M.; van der Poll, T.; Tzanis, E.; Chitra, S.; McGovern, P.C. An Integrated Safety Summary of Omadacycline, a Novel Aminomethylcycline Antibiotic. Clin. Infect. Dis. 2019, 69 (Suppl. S1), S40–S47. [Google Scholar] [CrossRef]
- Wang, P.F.; Zou, H.; Zhu, J.H.; Shi, F.E. Acute pancreatitis caused by tigecycline: A case report and literature review. Medicine 2021, 100, e28245. [Google Scholar] [CrossRef]
- Eljaaly, K.; Alghamdi, H.; Almehmadi, H.; Aljawi, F.; Hassan, A.; Thabit, A.K. Long-term gastrointestinal adverse effects of doxycycline. J. Infect. Dev. Ctries. 2023, 17, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.R.; Rogers, R.S., 3rd; Sheridan, P.J. Tetracycline and other tetracycline-derivative staining of the teeth and oral cavity. Int. J. Dermatol. 2004, 43, 709–715. [Google Scholar] [CrossRef]
- Rusu, A.; Buta, E.L. The Development of Third-Generation Tetracycline Antibiotics and New Perspectives. Pharmaceutics 2021, 13, 2085. [Google Scholar] [CrossRef]
- Lan, S.H.; Chang, S.P.; Lai, C.C.; Lu, L.C.; Chao, C.M. The efficacy and safety of omadacycline in treatment of acute bacterial infection: A systemic review and meta-analysis of randomized controlled trials. Medicine 2019, 98, e18426. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.K.; Villano, S. In Vitro and In Vivo Assessments of Cardiovascular Effects with Omadacycline. Antimicrob. Agents Chemother. 2016, 60, 5247–5253. [Google Scholar] [CrossRef] [PubMed]
- Kadoyama, K.; Sakaeda, T.; Tamon, A.; Okuno, Y. Adverse event profile of tigecycline: Data mining of the public version of the U.S. Food and Drug Administration adverse event reporting system. Biol. Pharm. Bull. 2012, 35, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhao, F.; Jin, P. Clinical Manifestations and Risk Factors of Tigecycline-Associated Thrombocytopenia. Infect. Drug Resist. 2023, 16, 6225–6235. [Google Scholar] [CrossRef] [PubMed]
- Durães, F.; Sousa, E. Omadacycline: A Newly Approved Antibacterial from the Class of Tetracyclines. Pharmaceuticals 2019, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- Leng, B.; Xue, Y.C.; Zhang, W.; Gao, T.T.; Yan, G.Q.; Tang, H. A Retrospective Analysis of the Effect of Tigecycline on Coagulation Function. Chem. Pharm. Bull. 2019, 67, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Sharma, S.; Atal, S.; Sadasivam, B.; Jhaj, R. Tigecycline-Induced Severe Hypoglycemia in a Non-Diabetic Individual: A Case Report and Brief Review of Tigecycline-Induced Severe Hypoglycemia. Am. J. Case Rep. 2020, 21, e924556. [Google Scholar] [CrossRef] [PubMed]
- Hakeam, H.A.; Sarkhi, K.A.; Iansavichene, A. Tigecycline and Hypoglycemia, When and How? J. Pharm. Technol. 2024, 40, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.; Sun, J.; Danner, R.L.; Natanson, C. Excess deaths associated with tigecycline after approval based on noninferiority trials. Clin. Infect. Dis. 2012, 54, 1699–1709. [Google Scholar] [CrossRef]
- McGovern, P.C.; Wible, M.; El-Tahtawy, A.; Biswas, P.; Meyer, R.D. All-cause mortality imbalance in the tigecycline phase 3 and 4 clinical trials. Int. J. Antimicrob. Agents 2013, 41, 463–467. [Google Scholar] [CrossRef]
| First Generation | Second Generation | Third Generation |
|---|---|---|
| Naturally synthetized | Semi-chemical derivatives | Fully synthesized analogs |
| 1. Tetracycline 2. Chlortetecycline 3. Oxytetracycline 4. Demeclocycline | 1. Doxycycline 2. Lymecycline 3. Meclocycline 4. Methacycline 5. Minocycline 6. Rolitetracycline | 1. Tigecycline 2. Eravacycline 3. Omadacycline 4. Sarecycline |
| Author, Year | Agent | Purpose/Characteristics | Main Findings/Remarks |
|---|---|---|---|
| Bassères, 2020 [98] | ERV | 1. Evaluation of in vitro activity of ERV and FDX, VAN, MTZ against 6 common C. difficile ribotypes (234 strains), including isolates with ↓ VAN/MTZ susceptibility 2. Additionally tested: - MBCs - Time-kill kinetics - WGSs | 1. Robust in vitro activity of ERV against C. difficile isolates 2. ERV’s efficacy was not affected by: - Ribotype - Susceptibility to VAN - ERV’s MIC was not influenced by the presence of tetM or tetW resistance genes 3. ↓ MIC50/90 values for ERV: - ERV: ≤0.0078/0.016 mg/L - FDX: 0.016/0.063 mg/L - MTZ: 0.25/1.0 mg/L - VAN: 2.0/4.0 mg/L 4. MBCs were ↓ for ERV vs. VAN for all ribotypes tested 5. Both ERV and VAN exhibited bactericidal killing at 8×, 16× and 32× the MIC, including epidemic RT027 |
| Yang, 2020 [104] | ERV, OMC | Comparison of in vitro activity of ERV and OMC vs. TET against 201 isolates of H. pylori retrieved from biopsy samples from subjects with gastritis or gastric cancer | 1. ERV and OMC are potent in vitro against H. pylori strains: - ERV vs. TET: ↑ eightfold potency - OMC vs. TET: ↑ fourfold potency 2. ERV’s and OMC’s potency are unaffected by the TET resistance: - 6 out of 201 isolates were TET-resistant with MICs of ≥2 μg/mL - All 201 isolates had ERV and OMC MICs of ≤1 μg/mL - TET-resistant strains showed ↓↓ ERV MICs (0.063 to 0.25 μg/mL) and OMC MIC (0.125 to 1 μg/mL) values |
| Phillips, 2021 [96] | TIG | 1. Retrospective cohort of 28 CDI cases treated with TIG 2. Evaluation of the effect of TIG use on 90-day mortality and recurrency 3. In all cases, TIG was injected in combination with oral VAN +/− MTZ with a mean duration of treatment at 7.6 days | 1. Patients treated with TIG showed ↑ in-hospital mortality, particularly when suffering from fulminant disease - 90-day mortality in 35.7% of the subjects - 50% mortality rates in fulminant infection 2. ↑ rate of CDI recurrency: 43.8% of surviving patients that reached 90-day follow-up had recurrent C. difficile infection |
| Kim, 2022 [109] | TIG | 1. Evaluation of subjects with M. abscessus PD treated with multidrug regimens 2. Comparison of microbiological response within 12 months (based on sputum AFB culture negativity and negative culture conversion) after treatment between 2 groups: - Group treated with conventional regimens - Group treated with conventional regimens PLUS TIG for 2 or 4 weeks during the initial phase 3. Conventional agents used: - AMK, IMP, CFX - MAC, CFZ, LZD, RFB | 1. Short-term iv TIG treatment during a 1-month initial phase may ↑ early microbiological response in M. abscessus lung disease 2. Short-term use of TIG does not ↑ the long-term culture conversion rate of M. abscessus lung disease 3. ↑↑ AFB culture negativity rate at 1 month in the TIG group vs. non-TG group (89% vs. 50%) 4. ↑ culture conversion within 12 months in the non-TIG group vs. TG-group (44% vs. 26%) |
| Budi, 2023 [100] | OMC | 1. Evaluation of murine models using C. difficile VPI 10463 2. OMC vs. VAN: * Severe model: - Survival rates - Weight loss - Disease severity - C. difficile production * Non-severe model: Addition of Gs 3. Additional assessment: - Colon histology - Bile acid analysis - Spore shedding - 16S sequencing | 1. OMC vs. VAN: * Severe model: - Survival rates: 60% vs. 13.3% - ↓ weight loss - ↓ disease severity * Non-severe model: all mice survived with G-antibiotic therapy vs. 60% antibiotics alone 2. ↓ changes in bile acids and microbiota composition in the omadacycline group 3. Germinant–antibiotic combinations showed ↑ outcomes at preventing rCDI vs. antibiotics alone, without spore release or ↑ toxin production at 15 days |
| Singh, 2024 [94] | OMC | 1. PK/PD experiments for the treatment of MDR-TB with OMC 2. Strains that were used: - Mtb H37Rv - MDR-TB strain 16D | 1. OMC shows efficacy against both drug-susceptible TB and MDR-TB 2. PK/PD target exposure: AUC0–24/MIC of 26.93 3. MIC breakpoint for the 300 mg daily oral dose >4 mg/L 4. Routine clinical assays for slow-growing bacteria face a disadvantage when testing OMC MICs due to its ↑ degradation rate of 50% in solution at the standard incubation temperature of 37 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kounatidis, D.; Dalamaga, M.; Grivakou, E.; Karampela, I.; Koufopoulos, P.; Dalopoulos, V.; Adamidis, N.; Mylona, E.; Kaziani, A.; Vallianou, N.G. Third-Generation Tetracyclines: Current Knowledge and Therapeutic Potential. Biomolecules 2024, 14, 783. https://doi.org/10.3390/biom14070783
Kounatidis D, Dalamaga M, Grivakou E, Karampela I, Koufopoulos P, Dalopoulos V, Adamidis N, Mylona E, Kaziani A, Vallianou NG. Third-Generation Tetracyclines: Current Knowledge and Therapeutic Potential. Biomolecules. 2024; 14(7):783. https://doi.org/10.3390/biom14070783
Chicago/Turabian StyleKounatidis, Dimitris, Maria Dalamaga, Eugenia Grivakou, Irene Karampela, Petros Koufopoulos, Vasileios Dalopoulos, Nikolaos Adamidis, Eleni Mylona, Aikaterini Kaziani, and Natalia G. Vallianou. 2024. "Third-Generation Tetracyclines: Current Knowledge and Therapeutic Potential" Biomolecules 14, no. 7: 783. https://doi.org/10.3390/biom14070783
APA StyleKounatidis, D., Dalamaga, M., Grivakou, E., Karampela, I., Koufopoulos, P., Dalopoulos, V., Adamidis, N., Mylona, E., Kaziani, A., & Vallianou, N. G. (2024). Third-Generation Tetracyclines: Current Knowledge and Therapeutic Potential. Biomolecules, 14(7), 783. https://doi.org/10.3390/biom14070783


_Kwok.png)





