Human Hepatobiliary Organoids: Recent Advances in Drug Toxicity Verification and Drug Screening
Abstract
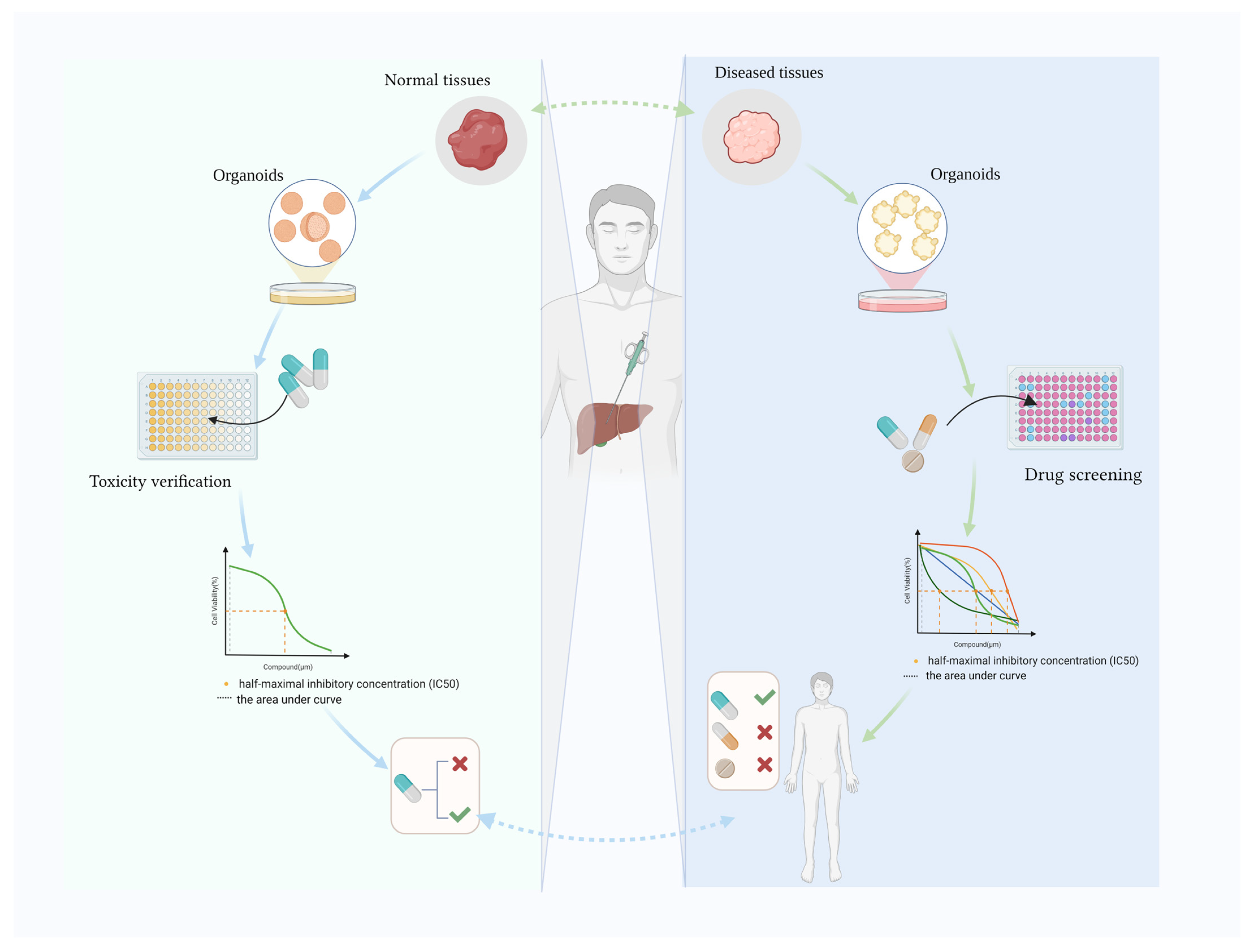
:1. Introduction
2. Hepatic Organoids Used to Verify Toxicity
2.1. Validating Drug Toxicity in Western Medicine
2.2. Herbal Medicine Toxicity Validation
2.3. Medical Device Toxicity Validation
3. Hepatobiliary Organoids for Drug Screening
3.1. Primary Liver Cancer Drug Screening
3.2. Hepatoblastoma Drug Screening
3.3. Fibrolamellar Carcinoma Drug Screening
3.4. Metastatic Hepatic Carcinoma Drug Screening
3.5. Extrahepatic Biliary Tract Cancer Drug Screening
3.6. Hepatic Organoids for Viral Hepatitis Drug Screening
3.7. Liver Fibrosis Drug Screening
3.8. Obesity-Related Non-Alcoholic Fatty Liver Disease Drug Screening
3.9. Hepatic Fibrosis in Primary Sclerosing Cholangitis
3.10. Hyperuricemia Drug Screening
3.11. Ischemic Cholangiopathy Drug Screening
4. Hopes and Future Challenges
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Fatehullah, A.; Tan, S.H.; Barker, N. Organoids as an in vitro model of human development and disease. Nat. Cell Biol. 2016, 18, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Vries, R.G.; Snippert, H.J.; van de Wetering, M.; Barker, N.; Stange, D.E.; van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Bartfeld, S.; Clevers, H. Stem cell-derived organoids and their application for medical research and patient treatment. J. Mol. Med. Berl. Ger. 2017, 95, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Clevers, H. Growing self-organizing mini-guts from a single intestinal stem cell: Mechanism and applications. Science 2013, 340, 1190–1194. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, H. Trends in the development of human stem cell-based non-animal drug testing models. Korean J. Physiol. Pharmacol. 2020, 24, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Shinozawa, T. Human Organoids for Predictive Toxicology Research and Drug Development. Front Genet. 2021, 12, 767621. [Google Scholar] [CrossRef] [PubMed]
- Stanger, B.Z. Cellular homeostasis and repair in the mammalian liver. Annu. Rev. Physiol. 2015, 77, 179–200. [Google Scholar] [CrossRef] [PubMed]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Shiota, J.; Samuelson, L.C.; Razumilava, N. Hepatobiliary Organoids and Their Applications for Studies of Liver Health and Disease: Are We There Yet? Hepatology 2021, 74, 2251–2263. [Google Scholar] [CrossRef] [PubMed]
- Tysoe, O.C.; Justin, A.W.; Brevini, T.; Chen, S.E.; Mahbubani, K.T.; Frank, A.K.; Zedira, H.; Melum, E.; Saeb-Parsy, K.; Markaki, A.E.; et al. Isolation and propagation of primary human cholangiocyte organoids for the generation of bioengineered biliary tissue. Nat. Protoc. 2019, 14, 1884–1925. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Verstegen, M.M.A.; Roest, H.P.; Ardisasmita, A.I.; Cao, W.; Roos, F.J.M.; de Ruiter, P.E.; Niemeijer, M.; Pan, Q.; IJzermans, J.N.M.; et al. Recapitulating Cholangiopathy-Associated Necroptotic Cell Death In Vitro Using Human Cholangiocyte Organoids. Cell. Mol. Gastroenterol. Hepatol. 2022, 13, 541–564. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Hui, L. Progress in human liver organoids. J. Mol. Cell Biol. 2020, 12, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Brooks, A.; Liang, X.; Zhang, Y.; Zhao, C.-X.; Roberts, M.S.; Wang, H.; Zhang, L.; Crawford, D.H.G. Liver organoid as a 3D in vitro model for drug validation and toxicity assessment. Pharmacol. Res. 2021, 169, 105608. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Stange, D.E.; Ferrante, M.; Vries, R.G.J.; Van Es, J.H.; Van den Brink, S.; Van Houdt, W.J.; Pronk, A.; Van Gorp, J.; Siersema, P.D.; et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 2011, 141, 1762–1772. [Google Scholar] [CrossRef] [PubMed]
- Jung, P.; Sato, T.; Merlos-Suárez, A.; Barriga, F.M.; Iglesias, M.; Rossell, D.; Auer, H.; Gallardo, M.; Blasco, M.A.; Sancho, E.; et al. Isolation and in vitro expansion of human colonic stem cells. Nat. Med. 2011, 17, 1225–1227. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Nadauld, L.; Ootani, A.; Corney, D.C.; Pai, R.K.; Gevaert, O.; Cantrell, M.A.; Rack, P.G.; Neal, J.T.; Chan, C.W.-M.; et al. Oncogenic transformation of diverse gastrointestinal tissues in primary organoid culture. Nat. Med. 2014, 20, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Bhang, D.H.; Beede, A.; Huang, T.L.; Stripp, B.R.; Bloch, K.D.; Wagers, A.J.; Tseng, Y.-H.; Ryeom, S.; Kim, C.F. Lung stem cell differentiation in mice directed by endothelial cells via a BMP4-NFATc1-thrombospondin-1 axis. Cell 2014, 156, 440–455. [Google Scholar] [CrossRef] [PubMed]
- Barker, N.; Huch, M.; Kujala, P.; van de Wetering, M.; Snippert, H.J.; van Es, J.H.; Sato, T.; Stange, D.E.; Begthel, H.; van den Born, M.; et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 2010, 6, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Stange, D.E.; Koo, B.-K.; Huch, M.; Sibbel, G.; Basak, O.; Lyubimova, A.; Kujala, P.; Bartfeld, S.; Koster, J.; Geahlen, J.H.; et al. Differentiated Troy+ chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell 2013, 155, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Chen, W.; Yu, Y.; Gao, F.; Zhou, J.; Wu, J.; Pan, Q.; Yang, J.; Zhou, L.; Yu, J.; et al. Human Placental Mesenchymal Stem Cells Relieve Primary Sclerosing Cholangitis via Upregulation of TGR5 in Mdr2-/- Mice and Human Intrahepatic Cholangiocyte Organoid Models. Research 2023, 6, 0207. [Google Scholar] [CrossRef] [PubMed]
- Huch, M.; Dorrell, C.; Boj, S.F.; van Es, J.H.; Li, V.S.W.; van de Wetering, M.; Sato, T.; Hamer, K.; Sasaki, N.; Finegold, M.J.; et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 2013, 494, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Huch, M.; Gehart, H.; van Boxtel, R.; Hamer, K.; Blokzijl, F.; Verstegen, M.M.A.; Ellis, E.; van Wenum, M.; Fuchs, S.A.; de Ligt, J.; et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell 2015, 160, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Sampaziotis, F.; Justin, A.W.; Tysoe, O.C.; Sawiak, S.; Godfrey, E.M.; Upponi, S.S.; Gieseck, R.L.; de Brito, M.C.; Berntsen, N.L.; Gómez-Vázquez, M.J.; et al. Reconstruction of the mouse extrahepatic biliary tree using primary human extrahepatic cholangiocyte organoids. Nat. Med. 2017, 23, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Soroka, C.J.; Assis, D.N.; Alrabadi, L.S.; Roberts, S.; Cusack, L.; Jaffe, A.B.; Boyer, J.L. Bile-Derived Organoids From Patients With Primary Sclerosing Cholangitis Recapitulate Their Inflammatory Immune Profile. Hepatology 2019, 70, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Hu, X.; Luo, J.; Huang, J.; Sun, Y.; Li, H.; Qiao, Y.; Wu, H.; Li, J.; Zhou, L.; et al. Liver organoid culture methods. Cell Biosci. 2023, 13, 197. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.R.; Lynch, S.; Goldring, C.; Sharma, P. Current Perspective: 3D Spheroid Models Utilizing Human-Based Cells for Investigating Metabolism-Dependent Drug-Induced Liver Injury. Front. Med. Technol. 2020, 2, 611913. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Shen, J.X.; Lauschke, V.M. Comprehensive Evaluation of Organotypic and Microphysiological Liver Models for Prediction of Drug-Induced Liver Injury. Front. Pharmacol. 2019, 10, 1093. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Jiang, D.; Yang, Y.; Li, S.; Ding, Q. Modeling drug-induced liver injury and screening for anti-hepatofibrotic compounds using human PSC-derived organoids. Cell Regen. 2023, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Mun, S.J.; Ryu, J.-S.; Lee, M.-O.; Son, Y.S.; Oh, S.J.; Cho, H.-S.; Son, M.-Y.; Kim, D.-S.; Kim, S.J.; Yoo, H.J.; et al. Generation of expandable human pluripotent stem cell-derived hepatocyte-like liver organoids. J. Hepatol. 2019, 71, 970–985. [Google Scholar] [CrossRef] [PubMed]
- Shinozawa, T.; Kimura, M.; Cai, Y.; Saiki, N.; Yoneyama, Y.; Ouchi, R.; Koike, H.; Maezawa, M.; Zhang, R.-R.; Dunn, A.; et al. High-Fidelity Drug-Induced Liver Injury Screen Using Human Pluripotent Stem Cell-Derived Organoids. Gastroenterology 2021, 160, 831–846.e10. [Google Scholar] [CrossRef] [PubMed]
- De Crignis, E.; Hossain, T.; Romal, S.; Carofiglio, F.; Moulos, P.; Khalid, M.M.; Rao, S.; Bazrafshan, A.; Verstegen, M.M.; Pourfarzad, F.; et al. Application of human liver organoids as a patient-derived primary model for HBV infection and related hepatocellular carcinoma. eLife 2021, 10, e60747. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, T.; Li, R.; Wang, J.; Li, P.; Niu, M.; Zhang, L.; Li, C.; Wang, T.; Xiao, X.; et al. Hepatic Organoid-Based High-Content Imaging Boosts Evaluation of Stereoisomerism-Dependent Hepatotoxicity of Stilbenes in Herbal Medicines. Front. Pharmacol. 2022, 13, 862830. [Google Scholar] [CrossRef] [PubMed]
- Long, C.; Li, Z.; Liang, S.; Yao, S.; Zhu, S.; Lu, L.; Cao, R.; Chen, Y.; Huang, Y.; Ma, Y.; et al. Resveratrol reliefs DEHP-induced defects during human decidualization. Ecotoxicol Environ Saf. 2023, 258, 114931. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.J.; Meyer, S.R.; O’Meara, M.J.; Huang, S.; Capeling, M.M.; Ferrer-Torres, D.; Childs, C.J.; Spence, J.R.; Fontana, R.J.; Sexton, J.Z. A human liver organoid screening platform for DILI risk prediction. J Hepatol. 2023, 78, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Gaitantzi, H.; Hakenberg, P.; Theobald, J.; Heinlein, H.; Cai, C.; Loff, S.; Wölfl, S.; Ebert, M.P.; Breitkopf-Heinlein, K.; Subotic, U. Di (2-Ethylhexyl) Phthalate and Its Role in Developing Cholestasis: An In Vitro Study on Different Liver Cell Types. J. Pediatr. Gastroenterol. Nutr. 2018, 66, e28–e35. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Hossain, T.; Mahmoudi, T. 3D human liver organoids: An in vitro platform to investigate HBV infection, replication and liver tumorigenesis. Cancer Lett. 2021, 506, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, Y.; Wang, Y.; Liu, J.; Lavrijsen, M.; Li, Y.; Zhang, R.; Verstegen, M.M.A.; Wang, Y.; Li, T.-C.; et al. Recapitulating hepatitis E virus-host interactions and facilitating antiviral drug discovery in human liver-derived organoids. Sci. Adv. 2022, 8, eabj5908. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R. Clinical presentation of hepatitis E. Virus Res. 2011, 161, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Fitzmaurice, C.; Naghavi, M. Exclusion of Kaposi Sarcoma From Analysis of Cancer Burden-Reply. JAMA Oncol. 2017, 3, 1429–1430. [Google Scholar] [CrossRef] [PubMed]
- Broutier, L.; Mastrogiovanni, G.; Verstegen, M.M.; Francies, H.E.; Gavarró, L.M.; Bradshaw, C.R.; Allen, G.E.; Arnes-Benito, R.; Sidorova, O.; Gaspersz, M.P.; et al. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat. Med. 2017, 23, 1424–1435. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Tong, M.; Tong, C.S.W.; Chan, B.K.C.; Chu, H.Y.; Wong, T.L.; Fong, J.H.C.; Cheung, M.S.H.; Mak, K.H.-M.; Pardeshi, L.; et al. A Combinatorial CRISPR-Cas9 Screen Identifies Ifenprodil as an Adjunct to Sorafenib for Liver Cancer Treatment. Cancer Res. 2021, 81, 6219–6232. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Qin, W.; Jiang, Y.; Yang, Z.; Yuan, B.; Dai, R.; Shen, H.; Chen, Y.; Fu, J.; Wang, H. ACADL plays a tumor-suppressor role by targeting Hippo/YAP signaling in hepatocellular carcinoma. NPJ Precis. Oncol. 2020, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Karkampouna, S.; van der Helm, D.; Gray, P.C.; Chen, L.; Klima, I.; Grosjean, J.; Burgmans, M.C.; Farina-Sarasqueta, A.; Snaar-Jagalska, E.B.; Stroka, D.M.; et al. CRIPTO promotes an aggressive tumour phenotype and resistance to treatment in hepatocellular carcinoma. J. Pathol. 2018, 245, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, Y.; Xun, X.; Zhang, C.; Xiang, X.; Cheng, Q.; Hu, S.; Li, Z.; Zhu, J. Hedgehog signaling promotes sorafenib resistance in hepatocellular carcinoma patient-derived organoids. J. Exp. Clin. Cancer Res. 2020, 39, 22. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Halpert, G.; Lerner, M.G.; Hu, H.; Dimitrion, P.; Weiss, M.J.; He, J.; Philosophe, B.; Burkhart, R.; Burns, W.R.; et al. Protein synthesis inhibitor omacetaxine is effective against hepatocellular carcinoma. JCI Insight 2021, 6, 138197. [Google Scholar] [CrossRef] [PubMed]
- Lampis, A.; Carotenuto, P.; Vlachogiannis, G.; Cascione, L.; Hedayat, S.; Burke, R.; Clarke, P.; Bosma, E.; Simbolo, M.; Scarpa, A.; et al. MIR21 Drives Resistance to Heat Shock Protein 90 Inhibition in Cholangiocarcinoma. Gastroenterology 2018, 154, 1066–1079.e5. [Google Scholar] [CrossRef] [PubMed]
- Maier, C.F.; Zhu, L.; Nanduri, L.K.; Kühn, D.; Kochall, S.; Thepkaysone, M.-L.; William, D.; Grützmann, K.; Klink, B.; Betge, J.; et al. Patient-Derived Organoids of Cholangiocarcinoma. Int. J. Mol. Sci. 2021, 22, 8675. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jin, Y.; Guo, Y.; Tan, Z.; Zhang, X.; Ye, D.; Yu, Y.; Peng, S.; Zheng, L.; Li, J. Conversion Therapy of Intrahepatic Cholangiocarcinoma Is Associated with Improved Prognosis and Verified by a Case of Patient-Derived Organoid. Cancers 2021, 13, 1179. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Guo, Y.; Jin, Y.; Zhang, X.; Geng, H.; Xie, G.; Ye, D.; Yu, Y.; Liu, D.; Zhou, D.; et al. Establishment and drug screening of patient-derived extrahepatic biliary tract carcinoma organoids. Cancer Cell Int. 2021, 21, 519. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Muramatsu, T.; Kanai, Y.; Ojima, H.; Sukeda, A.; Hiraoka, N.; Arai, E.; Sugiyama, Y.; Matsuzaki, J.; Uchida, R.; et al. Establishment of Patient-Derived Organoids and Drug Screening for Biliary Tract Carcinoma. Cell Rep. 2019, 27, 1265–1276.e4. [Google Scholar] [CrossRef] [PubMed]
- Saltsman, J.A.; Hammond, W.J.; Narayan, N.J.C.; Requena, D.; Gehart, H.; Lalazar, G.; LaQuaglia, M.P.; Clevers, H.; Simon, S. A Human Organoid Model of Aggressive Hepatoblastoma for Disease Modeling and Drug Testing. Cancers 2020, 12, 2668. [Google Scholar] [CrossRef] [PubMed]
- Skardal, A.; Devarasetty, M.; Rodman, C.; Atala, A.; Soker, S. Liver-Tumor Hybrid Organoids for Modeling Tumor Growth and Drug Response In Vitro. Ann. Biomed. Eng. 2015, 43, 2361–2373. [Google Scholar] [CrossRef] [PubMed]
- Bruun, J.; Kryeziu, K.; Eide, P.W.; Moosavi, S.H.; Eilertsen, I.A.; Langerud, J.; Røsok, B.; Totland, M.Z.; Brunsell, T.H.; Pellinen, T.; et al. Patient-Derived Organoids from Multiple Colorectal Cancer Liver Metastases Reveal Moderate Intra-patient Pharmacotranscriptomic Heterogeneity. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 4107–4119. [Google Scholar] [CrossRef] [PubMed]
- Kryeziu, K.; Moosavi, S.H.; Bergsland, C.H.; Guren, M.G.; Eide, P.W.; Totland, M.Z.; Lassen, K.; Abildgaard, A.; Nesbakken, A.; Sveen, A.; et al. Increased sensitivity to SMAC mimetic LCL161 identified by longitudinal ex vivo pharmacogenomics of recurrent, KRAS mutated rectal cancer liver metastases. J. Transl. Med. 2021, 19, 384. [Google Scholar] [CrossRef] [PubMed]
- Boos, S.L.; Loevenich, L.P.; Vosberg, S.; Engleitner, T.; Öllinger, R.; Kumbrink, J.; Rokavec, M.; Michl, M.; Greif, P.A.; Jung, A.; et al. Disease Modeling on Tumor Organoids Implicates AURKA as a Therapeutic Target in Liver Metastatic Colorectal Cancer. Cell. Mol. Gastroenterol. Hepatol. 2022, 13, 517–540. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Enejder, A.; Wang, M.; Fang, Z.; Cui, L.; Chen, S.-Y.; Wang, J.; Tan, Y.; Wu, M.; Chen, X.; et al. A human multi-lineage hepatic organoid model for liver fibrosis. Nat. Commun. 2021, 12, 6138. [Google Scholar] [CrossRef] [PubMed]
- Gwag, T.; Ma, E.; Zhou, C.; Wang, S. Anti-CD47 antibody treatment attenuates liver inflammation and fibrosis in experimental non-alcoholic steatohepatitis models. Liver Int. Off. J. Int. Assoc. Study Liver 2022, 42, 829–841. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, M.; Yu, B.; Shi, S.; Liu, J.; Zhang, R.; Ayada, I.; Verstegen, M.M.A.; van der Laan, L.J.W.; Peppelenbosch, M.P.; et al. Recapitulating lipid accumulation and related metabolic dysregulation in human liver-derived organoids. J. Mol. Med. Berl. Ger. 2022, 100, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Sha, W.; Xu, Z.; Hu, Y.; Amakye, W.K.; Yao, M.; Ren, J. Culture and establishment of self-renewing human liver 3D organoids with high uric acid for screening antihyperuricemic functional compounds. Food Chem. 2022, 374, 131634. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Hu, Y.; Jiang, H.; Xu, Z.; Sha, W.; Liu, J.; Ren, J.; Yao, M. Establishment of a 3D hyperuricemia model based on cultured human liver organoids. Free Radic. Biol. Med. 2022, 178, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Knutsdottir, H.; Hui, K.; Weiss, M.J.; He, J.; Philosophe, B.; Cameron, A.M.; Wolfgang, C.L.; Pawlik, T.M.; Ghiaur, G.; et al. Human primary liver cancer organoids reveal intratumor and interpatient drug response heterogeneity. JCI Insight 2019, 4, 121490. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.L.; Wong, N.; Lam, W.K.J.; Kuang, M. Personalized treatment for hepatocellular carcinoma: Current status and future perspectives. J. Gastroenterol. Hepatol. 2022, 37, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Xian, L.; Zhao, P.; Chen, X.; Wei, Z.; Ji, H.; Zhao, J.; Liu, W.; Li, Z.; Liu, D.; Han, X.; et al. Heterogeneity, inherent and acquired drug resistance in patient-derived organoid models of primary liver cancer. Cell. Oncol. 2022, 45, 1019–1036. [Google Scholar] [CrossRef]
- Lim, J.J.; Hooi, L.; Dan, Y.Y.; Bonney, G.K.; Zhou, L.; Chow, P.K.-H.; Chee, C.E.; Toh, T.B.; Chow, E.K.-H. Rational drug combination design in patient-derived avatars reveals effective inhibition of hepatocellular carcinoma with proteasome and CDK inhibitors. J. Exp. Clin. Cancer Res. 2022, 41, 249. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Zhou, Y.; Hu, H.; Hu, Y.; Georgiades, C.; Mao, H.-Q.; Selaru, F.M. Quaternary Nanoparticles Enable Sustained Release of Bortezomib for Hepatocellular Carcinoma. Hepatology 2022, 76, 1660–1672. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Quan, Y.; Zeng, J.; Lyu, X.; Wang, H.; Lei, J.H.; Feng, Y.; Xu, J.; Chen, Q.; Sun, H.; et al. Cullin3 deficiency shapes tumor microenvironment and promotes cholangiocarcinoma in liver-specific Smad4/Pten mutant mice. Int. J. Biol. Sci. 2021, 17, 4176–4191. [Google Scholar] [CrossRef] [PubMed]
- Darbari, A.; Sabin, K.M.; Shapiro, C.N.; Schwarz, K.B. Epidemiology of primary hepatic malignancies in U.S. children. Hepatology 2003, 38, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Narayan, N.J.C.; Requena, D.; Lalazar, G.; Ramos-Espiritu, L.; Ng, D.; Levin, S.; Shebl, B.; Wang, R.; Hammond, W.J.; Saltsman, J.A.; et al. Human liver organoids for disease modeling of fibrolamellar carcinoma. Stem Cell Rep. 2022, 17, 1874–1888. [Google Scholar] [CrossRef] [PubMed]
- Young, M.; Ordonez, L.; Clarke, A.R. What are the best routes to effectively model human colorectal cancer? Mol. Oncol. 2013, 7, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.H.; Rogatto, S.R.; Lindebjerg, J.; Havelund, B.; Abildgaard, C.; do Canto, L.M.; Vagn-Hansen, C.; Dam, C.; Rafaelsen, S.; Hansen, T.F. Precision medicine applied to metastatic colorectal cancer using tumor-derived organoids and in-vitro sensitivity testing: A phase 2, single-center, open-label, and non-comparative study. J. Exp. Clin. Cancer Res. CR 2023, 42, 115. [Google Scholar] [CrossRef] [PubMed]
- Martini, G.; Belli, V.; Napolitano, S.; Ciaramella, V.; Ciardiello, D.; Belli, A.; Izzo, F.; Avallone, A.; Selvaggi, F.; Menegon Tasselli, F.; et al. Establishment of patient-derived tumor organoids to functionally inform treatment decisions in metastatic colorectal cancer. ESMO Open 2023, 8, 101198. [Google Scholar] [CrossRef]
- Mo, S.; Tang, P.; Luo, W.; Zhang, L.; Li, Y.; Hu, X.; Ma, X.; Chen, Y.; Bao, Y.; He, X.; et al. Patient-Derived Organoids from Colorectal Cancer with Paired Liver Metastasis Reveal Tumor Heterogeneity and Predict Response to Chemotherapy. Adv. Sci. 2022, 9, 2204097. [Google Scholar] [CrossRef] [PubMed]
- Okano, K.; Yoshizawa, T.; Miura, T.; Ishido, K.; Kudo, D.; Kimura, N.; Wakiya, T.-I.; Wu, Y.; Morohashi, S.; Hakamada, K.; et al. Impact of the histological phenotype of extrahepatic bile duct carcinoma. Mol. Clin. Oncol. 2018, 8, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Feng, F.; Yang, Y.; Liu, K.; Duan, J.; Liu, H.; Yang, J.; Wu, M.; Liu, C.; Chang, Y. High-throughput screening identified miR-7-2-3p and miR-29c-3p as metastasis suppressors in gallbladder carcinoma. J. Gastroenterol. 2020, 55, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Akita, M.; Ajiki, T.; Ueno, K.; Tsugawa, D.; Tanaka, M.; Kido, M.; Toyama, H.; Fukumoto, T. Benefits and limitations of middle bile duct segmental resection for extrahepatic cholangiocarcinoma. Hepatobiliary Pancreat. Dis. Int. 2020, 19, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Tantau, A.I.; Mandrutiu, A.; Pop, A.; Zaharie, R.D.; Crisan, D.; Preda, C.M.; Tantau, M.; Mercea, V. Extrahepatic cholangiocarcinoma: Current status of endoscopic approach and additional therapies. World J. Hepatol. 2021, 13, 166–186. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.-B.; Weng, J.-Y.; Hu, Y.-Y.; Deng, G.-L.; Wan, X.-J. Feasibility and efficacy evaluation of metallic biliary stents eluting gemcitabine and cisplatin for extrahepatic cholangiocarcinoma. World J. Gastroenterol. 2020, 26, 4589–4606. [Google Scholar] [CrossRef] [PubMed]
- van Tienderen, G.S.; Willemse, J.; van Loo, B.; van Hengel, E.V.A.; de Jonge, J.; van der Laan, L.J.W.; Leijten, J.; Verstegen, M.M.A. Scalable Production of Size-Controlled Cholangiocyte and Cholangiocarcinoma Organoids within Liver Extracellular Matrix-Containing Microcapsules. Cells 2022, 11, 3657. [Google Scholar] [CrossRef] [PubMed]
- Schinazi, R.F.; Ehteshami, M.; Bassit, L.; Asselah, T. Towards HBV curative therapies. Liver Int. Off. J. Int. Assoc. Study Liver 2018, 38 (Suppl. 1), 102–114. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.P.; Huynh, T.; Rao, S.; Mackiewicz, L.; Mason, H.; Romal, S.; Stutz, M.D.; Ahn, S.H.; Earnest, L.; Sozzi, V.; et al. Clinical stage drugs targeting inhibitor of apoptosis proteins purge episomal Hepatitis B viral genome in preclinical models. Cell Death Dis. 2021, 12, 641. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Chen, R.; Li, F.; Xu, B.; Han, L.; Liu, C.; Tong, Y.; Jiu, Y.; Zhong, J.; Zhou, G.C. Discovery of a fused bicyclic derivative of 4-hydroxypyrrolidine and imidazolidinone as a new anti-HCV agent. Virology 2023, 586, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Meyers, N.L.; Ashuach, T.; Lyons, D.E.; Khalid, M.M.; Simoneau, C.R.; Erickson, A.L.; Bouhaddou, M.; Nguyen, T.T.; Kumar, G.R.; Taha, T.Y.; et al. Hepatitis C virus infects and perturbs liver stem cells. mBio. 2023, 14, e0131823. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, P.; He, Q.; Zhang, R.; Li, Y.; Kamar, N.; Peppelenbosch, M.P.; de Man, R.A.; Wang, L.; Pan, Q. Niclosamide inhibits hepatitis E virus through suppression of NF-kappaB signalling. Antiviral Res. 2022, 197, 105228. [Google Scholar] [CrossRef] [PubMed]
- Romero-Gómez, M.; Zelber-Sagi, S.; Trenell, M. Treatment of NAFLD with diet, physical activity and exercise. J. Hepatol. 2017, 67, 829–846. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, D.; Brouwers, J.F.; Hamer, K.; Geurts, M.H.; Luciana, L.; Massalini, S.; López-Iglesias, C.; Peters, P.J.; Rodríguez-Colman, M.J.; Chuva de Sousa Lopes, S.; et al. Engineered human hepatocyte organoids enable CRISPR-based target discovery and drug screening for steatosis. Nat. Biotechnol. 2023, 41, 1567–1581. [Google Scholar] [CrossRef]
- Du, Y.; de Jong, I.E.M.; Gupta, K.; Waisbourd-Zinman, O.; Har-Zahav, A.; Soroka, C.J.; Boyer, J.L.; Llewellyn, J.; Liu, C.; Naji, A.; et al. Human vascularized bile duct-on-a chip: A multi-cellular micro-physiological system for studying cholestatic liver disease. Biofabrication 2023, 16, 015004. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lin, F.; Feng, X.; Yao, Q.; Yu, Y.; Gao, F.; Zhou, J.; Pan, Q.; Wu, J.; Yang, J.; et al. MSC-derived exosomes attenuate hepatic fibrosis in primary sclerosing cholangitis through inhibition of Th17 differentiation. Asian J. Pharm. Sci. 2024, 19, 100889. [Google Scholar] [CrossRef] [PubMed]
- George, C.; Leslie, S.W.; Minter, D.A. Hyperuricemia. In StatPearls [Internet]; StatPearls Publishing: St. Petersburg, FL, USA, 2024; Bookshelf ID: NBK459218. [Google Scholar] [PubMed]
- Shi, S.; Roest, H.P.; van den Bosch, T.P.P.; Bijvelds, M.J.C.; Boehnert, M.U.; de Jonge, J.; Dekker, S.O.; de Vries, A.A.F.; de Jonge, H.R.; Verstegen, M.M.A.; et al. Modeling bile duct ischemia and reoxygenation injury in human cholangiocyte organoids for screening of novel cholangio-protective agents. eBioMedicine 2023, 88, 104431. [Google Scholar] [CrossRef] [PubMed]
- Theobald, J.; Ghanem, A.; Wallisch, P.; Banaeiyan, A.A.; Andrade-Navarro, M.A.; Taškova, K.; Haltmeier, M.; Kurtz, A.; Becker, H.; Reuter, S.; et al. Liver-Kidney-on-Chip To Study Toxicity of Drug Metabolites. ACS Biomater. Sci. Eng. 2018, 4, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Schimek, K.; Frentzel, S.; Luettich, K.; Bovard, D.; Rütschle, I.; Boden, L.; Rambo, F.; Erfurth, H.; Dehne, E.-M.; Winter, A.; et al. Human multi-organ chip co-culture of bronchial lung culture and liver spheroids for substance exposure studies. Sci. Rep. 2020, 10, 7865. [Google Scholar] [CrossRef] [PubMed]
- Shroff, T.; Aina, K.; Maass, C.; Cipriano, M.; Lambrecht, J.; Tacke, F.; Mosig, A.; Loskill, P. Studying metabolism with multi-organ chips: New tools for disease modelling, pharmacokinetics and pharmacodynamics. Open Biol. 2022, 12, 210333. [Google Scholar] [CrossRef] [PubMed]
- Unal, A.; Jeffs, S.; West, J. 3D Co-Culture with Vascular Cells Supports Long-Term Hepatocyte Phenotype and Function In Vitro. Regen. Eng. Transl. Med. 2018, 4, 21–34. [Google Scholar] [CrossRef]
- Obeid, D.A.; Mir, T.A.; Alzhrani, A.; Altuhami, A.; Shamma, T.; Ahmed, S.; Kazmi, S.; Fujitsuka, I.; Ikhlaq, M.; Shabab, M.; et al. Using Liver Organoids as Models to Study the Pathobiology of Rare Liver Diseases. Biomedicines 2024, 12, 446. [Google Scholar] [CrossRef] [PubMed]
- Wensink, G.E.; Elias, S.G.; Mullenders, J.; Koopman, M.; Boj, S.F.; Kranenburg, O.W.; Roodhart, J.M.L. Patient-derived organoids as a predictive biomarker for treatment response in cancer patients. NPJ Precis. Oncol. 2021, 5, 30. [Google Scholar] [CrossRef] [PubMed]
- Schneeberger, K.; Sánchez-Romero, N.; Ye, S.; van Steenbeek, F.G.; Oosterhoff, L.A.; Pla Palacin, I.; Chen, C.; van Wolferen, M.E.; van Tienderen, G.; Lieshout, R.; et al. Large-Scale Production of LGR5-Positive Bipotential Human Liver Stem Cells. Hepatology 2020, 72, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Im, I.; Jeon, J.S.; Kang, E.-H.; Lee, H.-A.; Jo, S.; Kim, J.-W.; Woo, D.-H.; Choi, Y.J.; Kim, H.J.; et al. Development of human pluripotent stem cell-derived hepatic organoids as an alternative model for drug safety assessment. Biomaterials 2022, 286, 121575. [Google Scholar] [CrossRef] [PubMed]
- Berkers, G.; van Mourik, P.; Vonk, A.M.; Kruisselbrink, E.; Dekkers, J.F.; de Winter-de Groot, K.M.; Arets, H.G.M.; Marck-van der Wilt, R.E.P.; Dijkema, J.S.; Vanderschuren, M.M.; et al. Rectal Organoids Enable Personalized Treatment of Cystic Fibrosis. Cell Rep. 2019, 26, 1701–1708.e3. [Google Scholar] [CrossRef]
- Zhu, Y.; Tang, S.; Yuan, Q.; Fu, J.; He, J.; Liu, Z.; Zhao, X.; Li, Y.; Zhao, Y.; Zhang, Y.; et al. Integrated characterization of hepatobiliary tumor organoids provides a potential landscape of pharmacogenomic interactions. Cell Rep. Med. 2024, 5, 101375. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.P.; Baumgarten, S.F.; Verma, R.; Lunov, O.; Dejneka, A.; Sullivan, G.J. Liver Organoids: Recent Developments, Limitations and Potential. Front. Med. 2021, 8, 574047. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zhang, W.; Wu, X.; Zhang, Y.; Xu, K.; Su, J. Organoid assessment technologies. Clin. Transl. Med. 2023, 13, e1499. [Google Scholar] [CrossRef] [PubMed]
- Brancati, G.; Treutlein, B.; Camp, J.G. Resolving Neurodevelopmental and Vision Disorders Using Organoid Single-Cell Multi-omics. Neuron 2020, 107, 1000–1013. [Google Scholar] [CrossRef] [PubMed]
| Organoid Sources | Characteristics | Test Substances | Toxic Endpoints | Ref. |
|---|---|---|---|---|
| Pluripotent stem cells | Exhibit self-renewal (expandable and further able to differentiate) while maintaining their mature hepatic characteristics over long-term culture | Troglitazone, APAP, trovafloxacin and levofloxacin | Cell viability | [29] |
| Induced pluripotent stem cell | Contain polarized immature hepatocytes with bile canaliculi-like architecture, establishing the unidirectional bile acid-transport pathway | Hepatotoxicity library (Enzo SCREEN-WELL®) | Cell viability | [30] |
| Healthy and HBV-infected liver tissue | Support the full replication cycle of HBV, may serve as an ideal novel primary platform for drug screening as well as elucidation of the molecular events underlying HBV replication | Tenofovir and fialuridine | Relative cellular viability | [31] |
| HepaRG cells | Suitable to test the repeated or accumulative intoxication of drugs at low concentrations due to its long-term culturing nature | PM (trans-SG and its cis-isomer, cis-SG) | Cell viability 50, ROS, intracellular glutathione | [32] |
| UHCs, upcyte LSECs and UHSCs | Have typical functional characteristics of liver parenchyma including activity of cytochromes P450 | Di (2-ethylhexyl) phthalate (DEHP) | mRNA expression levels, Cyp450 activities | [33] |
| Diseases | Org Type | Characteristics | Org Sources | Test Substances | Test Endpoints | Ref. |
|---|---|---|---|---|---|---|
| Hepatitis B | Liver org | Support the full replication cycle of HBV, may serve as an ideal novel primary platform for drug screening as well as elucidation of the molecular events underlying HBV replication | Healthy and HBV-infected liver tissue | Tenofovir and fialuridine | Relative cellular viability | [31] |
| —— | Liver specimens from healthy donors | The monovalent IAP antagonist LCL-161 and recombinant TNF or the combination of both | HBV DNA levels, the levels of cleaved caspase 3 | [36] | ||
| Hepatitis E | Harboring subgenomic genotype 1 or3 HEV replicon | Tissue samples of donor liver biopsies | Niclosamide, ribavirin | Luciferase activity, HEV RLU | [37] | |
| ICOs | Support the full life cycle of HEV infection and able to anti-HEV drug discovery | Tissue samples of donor liver biopsies and fetal liver tissues | Ribavirin, mycophenolic acid, homoharringtonine and a library of 94 broad-spectrum antiviral agents | Cell viability and HEV replication–related luciferase activity | [38] | |
| PLC | Tumor org | 1. Recapitulate the histological architecture and expression profiles of the corresponding parent tumour; 2. Retain the specific differences between patients as well as between tumour subtypes | Liver tumour tissue from untreated PLC patients | 29 anti-cancer compounds | Relative cellular viability, AUC and IC50 | [39] |
| 1. Display marker profiles similar to the original primary human tumors; 2. Reflect cell-intrinsic sensitivity to drugs | Human HCC or CCA tissue | FDA-approved cancer drug library (https://wiki.nci.nih.gov/display/NCIDTPdata/Compound+Sets, accessed on 28 August 2022) | Relative cellular viability 50 | [40] | ||
| HCC | Model the pathophysiology of a growing tumor in vivo, able to evaluate inhibitions of growth and self-renewal brought by the drug combination | Patient liver specimens | NMDAR antagonist ifenprodil and sorafenib | Relative cellular viability | [41] | |
| Display consistent expression patterns of ACADL and YAP with original tissues | Primary HCC specimens, nontumorous liver tissues | YAP inhibitor verteporfin | Relative viability of control (dose-response curves) | [42] | ||
| —— | PDX tumors | Sorafenib, N20 (GRP78 blocking peptide) | ATP content | [43] | ||
| Maintain the histological features of the corresponding tumors and responded to drug treatment | HCC specimens | Sorafenib, Hedgehog signaling inhibitor (GANT61) | Cell viability, IC 50 | [44] | ||
| Drug screening in HCC PDOs can be utilized for drug discovery or drug repurposing | HCC specimens | 129 drugs (Omacetaxin etc.) | Cell viability, IC 50 | [45] | ||
| CCA | Retain the same morphology of the primary tumor, the same positivity for cytokeratin 7 and 19 | CCA liver biopsies | 484 small-molecule compounds | Cell viability | [46] | |
| The patient-derived organoids retain transcriptomic features and the mutational landscape of the parental tumor | CCA surgical resection specimens | Anti-cancer agents (gemcitabine, sorafenib, cisplatin and doxorubicin) | Relative cellular viability | [47] | ||
| IHCC | Expression profiles of cancer organoid resembled original tissue, CK7 and EpCAM were highly expressed | IHCC tissue samples | Gemcitabine, 5-FU, cisplatin, paclitaxel, infigratinib or ivosidenib, FGFR inhibitor(infigratinib) | Dose-response curves and IC50 | [48] | |
| eBTC | Different PDOs exhibited diverse growth rates during in vitro culture. Most retained the original structures of adenocarcinoma | Samples of GBC and eCCA | Gemcitabine, 5-FU, cisplatin, paclitaxel, infigratinib and ivosidenib | Cell viability, IC 50 | [49] | |
| Harbored mutations of driver genes such as TP53 and KRAS | Cancer and non-cancer tissue specimens | A group of 339 medicines already in clinical use | Cell viability | [50] | ||
| Hepatoblastoma | Show morphologic and genomic similarity to human tissue from which they are derived | Hepatoblastoma tumor tissue and surrounding tissue | 12 candidate compounds | Cell viability | [51] | |
| Metastatic HCC | liver org | N-cadherin staining was observed in the tumor foci regions of the live-tumor organoids, co-localizing withHCT-116 cells | HepG2 hepatoma cells | 5-FU | Anti-caspase 3 antibodies | [52] |
| Formed clusters based primarily on the sensitivity to EGFR and/or MDM2 inhibition, as well as to SN-38 and nucleosides | Liver resection from the studied tumors | 40 anticancer agents | Cell viability | [53] | ||
| Retain the undifferentiated phenotype and expression patterns of the tumor | Samples of processed liver metastases | A custom library of 33 clinically relevant small molecule inhibitors and three 5-FU-based drug combinations with Leucovorin (FLV), Oxaliplatin (FLOX) and SN-38 (FLIRI) | Growth rate adjusted drug sensitivity scores | [54] | ||
| KRAS/NRAS and BRAF wild-type | Partial hepatectomy normal and cancerous tissue specimens | FOLFIRI, anti-EGFR antibody cetuximab (Cmab), afatinib (pan-HER inhibitor), selumetinib (MEK inhibitor), type II inhibitor alisertib (MLN8237) | Cell viability, IC50, AUG, cell viability at Emax | [55] | ||
| Liver fibrosis | Reproduce ARPKD liver pathology, which includes biliary abnormalities and extensive fibrosis | iPSCs | The PDGFR tyrosine kinase (Crenolanib, Sunitinib and Imatinib) | Fibrosis scores | [56] | |
| NAFLD | NASH org | Human hepatocytes, macrophages and stellate cells | Anti-CD47 antibody | Immunofluorescence staining and qPCR | [57] | |
| ICOs | Lipid accumulation in human liver organoids was much more robust compared to that in mouse liver organoids | Human intrahepatic bile duct tissue | Obeticholic acid (INT747) and metformin | Lipid droplet size | [58] | |
| HUA | Liver org | As a stable model of HUA for long-term studies and screening for antihyperuricemic compounds | Human liver paracarcinomatous tissues | Allopurinol, carnosine and anserine | Concentration of uric acid | [59] |
| Maintain intact purine metabolic pathways as well as mimicking the uric acid production of the human liver | Human liver paracarcinomatous tissues | Allopurinol and puerarin | Content of uric acid | [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, H.; Xu, H.; Yu, J.; Cao, H.; Li, L. Human Hepatobiliary Organoids: Recent Advances in Drug Toxicity Verification and Drug Screening. Biomolecules 2024, 14, 794. https://doi.org/10.3390/biom14070794
Fang H, Xu H, Yu J, Cao H, Li L. Human Hepatobiliary Organoids: Recent Advances in Drug Toxicity Verification and Drug Screening. Biomolecules. 2024; 14(7):794. https://doi.org/10.3390/biom14070794
Chicago/Turabian StyleFang, Haoyu, Haoying Xu, Jiong Yu, Hongcui Cao, and Lanjuan Li. 2024. "Human Hepatobiliary Organoids: Recent Advances in Drug Toxicity Verification and Drug Screening" Biomolecules 14, no. 7: 794. https://doi.org/10.3390/biom14070794
APA StyleFang, H., Xu, H., Yu, J., Cao, H., & Li, L. (2024). Human Hepatobiliary Organoids: Recent Advances in Drug Toxicity Verification and Drug Screening. Biomolecules, 14(7), 794. https://doi.org/10.3390/biom14070794










