Reversible Histone Modifications Contribute to the Frozen and Thawed Recovery States of Wood Frog Brains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Experiments
2.2. Total Histone Extraction—Modified from [24]
2.3. Western Immunoblotting
2.4. Quantification and Statistics
3. Results
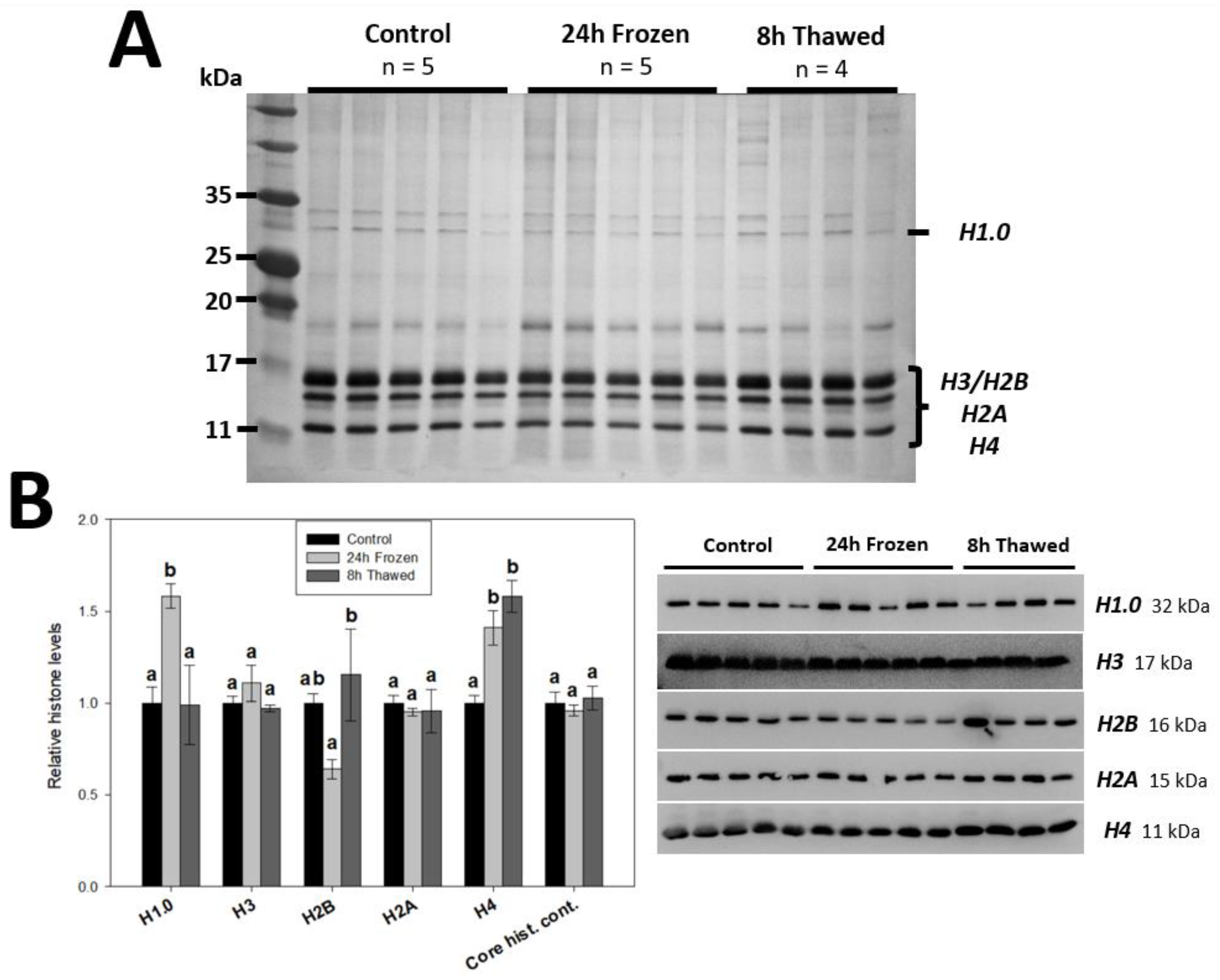
3.1. Histone H1.0, H2B and H4 Are Responsive to the Wood Frog Brain Freeze-Thaw Cycle
3.2. H2BK5ac and H3K14ac Are Reduced across Chromatin of Frozen Wood Frog Brains
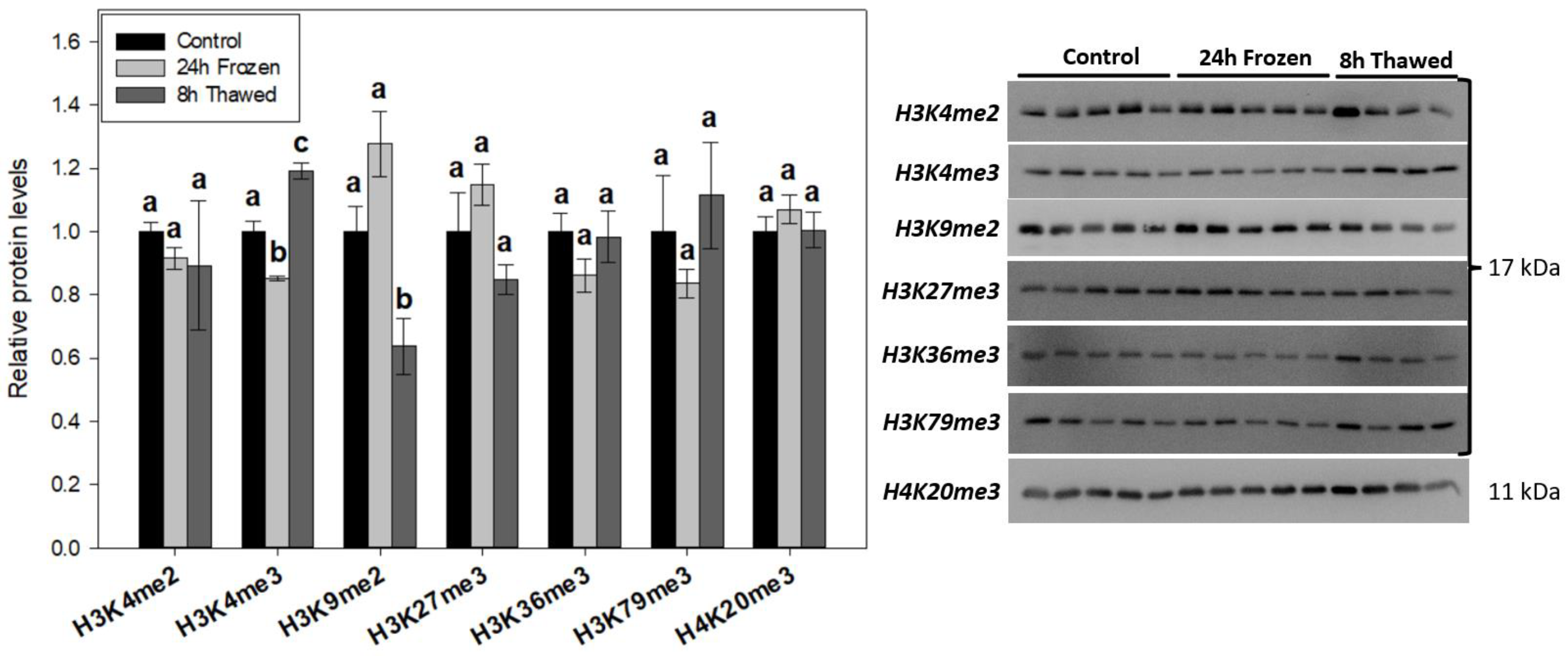
3.3. Permissive H3K4me3 and Repressive H3K9me2 Display Opposing Trends during Freeze-Thaw
3.4. Freeze-Thaw Led to No Significant Changes in Histone Methyl-Arginine Modifications
4. Discussion
4.1. Freeze-Thaw Stimulates Dynamic Changes of Some Histone Proteins in Wood Frog Brain Chromatin
4.2. Histone H3 PTMs Provide Evidence in Favour of Silenced Gene Transcription under Frozen Conditions
4.3. Rebounded Transcriptional State during Wood Frog Brain Thawing?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mancarella, D.; Plass, C. Epigenetic Signatures in Cancer: Proper Controls, Current Challenges and the Potential for Clinical Translation. Genome Med. 2021, 13, 23. [Google Scholar] [CrossRef] [PubMed]
- Allis, C.D.; Jenuwein, T. The Molecular Hallmarks of Epigenetic Control. Nat. Rev. Genet. 2016, 17, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, G.; Heard, E. Advances in Epigenetics Link Genetics to the Environment and Disease. Nature 2019, 571, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Ingelson-Filpula, W.A.; Bloskie, T.; Storey, K.B. Epigenetics and the Extreme Stress Response. In Epigenetics, Development, Ecology and Evolution; Springer: Cham, Switzerland, 2022; pp. 177–213. [Google Scholar]
- Storey, K.B.; Storey, J.M. Molecular Physiology of Freeze Tolerance in Vertebrates. Physiol. Rev. 2017, 97, 623–665. [Google Scholar] [CrossRef] [PubMed]
- Bloskie, T.; Storey, K.B. Epigenetics of the Frozen Brain: Roles for Lysine Methylation in Hypometabolism. FEBS Lett. 2022, 596, 2007–2020. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.W.; Tessier, S.N.; Storey, K.B. Stress-Induced Antioxidant Defense and Protein Chaperone Response in the Freeze-Tolerant Wood Frog Rana sylvatica. Cell Stress Chaperones 2018, 23, 1205–1217. [Google Scholar] [CrossRef]
- Sinclair, B.J.; Stinziano, J.R.; Williams, C.M.; MacMillan, H.A.; Marshall, K.E.; Storey, K.B. Real-Time Measurement of Metabolic Rate during Freezing and Thawing of the Wood Frog, Rana sylvatica: Implications for Overwinter Energy Use. J. Exp. Biol. 2013, 216, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Rolfe, D.F.S.; Brown, G.C. Cellular Energy Utilization and Molecular Origin of Standard Metabolic Rate in Mammals. Physiol. Rev. 1997, 77, 731–758. [Google Scholar] [CrossRef] [PubMed]
- Hochachka, P.W.; Buck, L.T.; Doll, C.J.; Land, S.C. Unifying Theory of Hypoxia Tolerance: Molecular/Metabolic Defense and Rescue Mechanisms for Surviving Oxygen Lack. Proc. Natl. Acad. Sci. USA 1996, 93, 9493. [Google Scholar] [CrossRef]
- Fraser, K.P.P.; Houlihan, D.F.; Lutz, P.L.; Leone-Kabler, S.; Manuel, L.; Brechin, J.G. Complete Suppression of Protein Synthesis during Anoxia with No Post-Anoxia Protein Synthesis Debt in the Red-Eared Slider Turtle Trachemys scripta elegans. J. Exp. Biol. 2001, 204, 4353–4360. [Google Scholar] [CrossRef]
- Frerichs, K.U.; Smith, C.B.; Brenner, M.; Degracia, D.J.; Krause, G.S.; Marrone, L.; Dever, T.E.; Hallenbeck, J.M. Suppression of Protein Synthesis in Brain during Hibernation Involves Inhibition of Protein Initiation and Elongation. Proc. Natl. Acad. Sci. USA 1998, 95, 14511–14516. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Storey, K.B. Freezing-Induced Genes in Wood Frog (Rana sylvatica): Fibrinogen Upregulation by Freezing and Dehydration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1997, 272, R1480–R1492. [Google Scholar] [CrossRef] [PubMed]
- McNally, J.D.; Wu, S.B.; Sturgeon, C.M.; Storey, K.B. Identification and Characterization of a Novel Freezing Inducible Gene, Li16, in the Wood Frog Rana sylvatica. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2002, 16, 902–904. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, K.J.; Biggar, K.K.; Storey, K.B. Transcript Expression of the Freeze Responsive Gene Fr10 in Rana sylvatica during Freezing, Anoxia, Dehydration, and Development. Mol. Cell. Biochem. 2015, 399, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, L.J.; Storey, K.B. Histone Methylation in the Freeze-Tolerant Wood Frog (Rana sylvatica). J. Comp. Physiol. B 2018, 188, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Naranjo Vera, M. Histone Arginine Methylation in the Freeze-Tolerant Wood Frog, Rana sylvatica. Master Thesis, Carleton University, Ottawa, ON, Canada, 2022. [Google Scholar]
- Bannister, A.J.; Kouzarides, T. Regulation of Chromatin by Histone Modifications. Cell Res. 2011, 21, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Barski, A.; Cuddapah, S.; Cui, K.; Roh, T.Y.; Schones, D.E.; Wang, Z.; Wei, G.; Chepelev, I.; Zhao, K. High-Resolution Profiling of Histone Methylations in the Human Genome. Cell 2007, 129, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Bedford, M.T.; Clarke, S.G. Protein Arginine Methylation in Mammals: Who, What, and Why. Mol. Cell 2009, 33, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Hoang, S.; Mayo, M.W.; Bekiranov, S. Application of Machine Learning Methods to Histone Methylation ChIP-Seq Data Reveals H4R3me2 Globally Represses Gene Expression. BMC Bioinform. 2010, 11, 396. [Google Scholar] [CrossRef]
- Shvedunova, M.; Akhtar, A. Modulation of Cellular Processes by Histone and Non-Histone Protein Acetylation. Nat. Rev. Mol. Cell Biol. 2022, 23, 329–349. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.; Yi, S.J.; Kim, K. Gene Regulation by Histone-Modifying Enzymes under Hypoxic Conditions: A Focus on Histone Methylation and Acetylation. Exp. Mol. Med. 2022, 54, 878–889. [Google Scholar] [CrossRef] [PubMed]
- Shechter, D.; Dormann, H.L.; Allis, C.D.; Hake, S.B. Extraction, Purification and Analysis of Histones. Nat. Protoc. 2007, 2, 1445–1457. [Google Scholar] [CrossRef] [PubMed]
- Layne, J.R.; Lee, R.E. Freeze Tolerance and the Dynamics of Ice Formation in Wood Frogs (Rana sylvatica) from Southern Ohio. Can. J. Zool. 1987, 65, 2062–2065. [Google Scholar] [CrossRef]
- Brown, D.T.; Gunjan, A.; Alexander, B.T.; Sittman, D.B. Differential Effect of H1 Variant Overproduction on Gene Expression Is Due to Differences in the Central Globular Domain. Nucleic Acids Res. 1997, 25, 5003–5009. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Brown, D.T.; Lu, Z.H.; Leno, G.H.; Wellman, S.E.; Sittman, D.B. Histone H1 Variants Differentially Inhibit DNA Replication through an Affinity for Chromatin Mediated by Their Carboxyl-Terminal Domains. Gene 2002, 292, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Chitsazian, F.; Sadeghi, M.; Elahi, E. Confident Gene Activity Prediction Based on Single Histone Modification H2BK5ac in Human Cell Lines. BMC Bioinform. 2017, 18, 67. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Huang, C.M.; Nakatsuji, T.; Thiboutot, D.; Kang, S.A.; Monestier, M.; Gallo, R.L. Histone H4 Is a Major Component of the Antimicrobial Action of Human Sebocytes. J. Investig. Dermatol. 2009, 129, 2489–2496. [Google Scholar] [CrossRef] [PubMed]
- Dorrington, T.; Villamil, L.; Gómez-chiarri, M. Upregulation in Response to Infection and Antibacterial Activity of Oyster Histone H4. Fish Shellfish. Immunol. 2011, 30, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Bouma, H.R.; Carey, H.V.; Kroese, F.G.M. Hibernation: The Immune System at Rest? J. Leukoc. Biol. 2010, 88, 619–624. [Google Scholar] [CrossRef]
- Wang, R.; Zheng, X.; Zhang, L.; Zhou, B.; Hu, H.; Li, Z.; Zhang, L.; Lin, Y.; Wang, X. Histone H4 Expression Is Cooperatively Maintained by IKKβ and Akt1 Which Attenuates Cisplatin-Induced Apoptosis through the DNA-PK/RIP1/IAPs Signaling Cascade. Sci. Rep. 2017, 7, 41715. [Google Scholar] [CrossRef]
- Zhang, J.; Storey, K.B. Akt Signaling and Freezing Survival in the Wood Frog, Rana sylvatica. Biochim. Biophys. Acta 2013, 1830, 4828–4837. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Brooks, C.; Storey, K.B. Regulation of NF-ΚB, FHC and SOD2 in Response to Oxidative Stress in the Freeze Tolerant Wood Frog, Rana sylvatica. Cryobiology 2020, 97, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Bloskie, T.; Storey, K.B. Histone H3 and H4 Modifications Point to Transcriptional Suppression as a Component of Winter Freeze Tolerance in the Gall Fly Eurosta solidaginis. Int. J. Mol. Sci. 2023, 24, 10153. [Google Scholar] [CrossRef] [PubMed]
- Prado, F.; Jimeno-González, S.; Reyes, J.C. Histone Availability as a Strategy to Control Gene Expression. RNA Biol. 2017, 14, 281. [Google Scholar] [CrossRef] [PubMed]
- Jimeno-González, S.; Payán-Bravo, L.; Muñoz-Cabello, A.M.; Guijo, M.; Gutierrez, G.; Prado, F.; Reyes, J.C. Defective Histone Supply Causes Changes in RNA Polymerase II Elongation Rate and Cotranscriptional Pre-MRNA Splicing. Proc. Natl. Acad. Sci. USA 2015, 112, 14840–14845. [Google Scholar] [CrossRef] [PubMed]
- Celona, B.; Weiner, A.; Di Felice, F.; Mancuso, F.M.; Cesarini, E.; Rossi, R.L.; Gregory, L.; Baban, D.; Rossetti, G.; Grianti, P.; et al. Substantial Histone Reduction Modulates Genomewide Nucleosomal Occupancy and Global Transcriptional Output. PLoS Biol. 2011, 9, 1001086. [Google Scholar] [CrossRef]
- Rao, R.C.; Dou, Y. Hijacked in Cancer: The MLL/KMT2 Family of Methyltransferases. Nat. Rev. Cancer 2015, 15, 334. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fan, Z.; Shliaha, P.V.; Miele, M.; Hendrickson, R.C.; Jiang, X.; Helin, K. H3K4me3 Regulates RNA Polymerase II Promoter-Proximal Pause-Release. Nature 2023, 615, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Wijenayake, S.; Hawkins, L.J.; Storey, K.B. Dynamic Regulation of Six Histone H3 Lysine (K) Methyltransferases in Response to Prolonged Anoxia Exposure in a Freshwater Turtle. Gene 2018, 649, 50–57. [Google Scholar] [CrossRef]
- Karmodiya, K.; Krebs, A.R.; Oulad-Abdelghani, M.; Kimura, H.; Tora, L. H3K9 and H3K14 Acetylation Co-Occur at Many Gene Regulatory Elements, While H3K14ac Marks a Subset of Inactive Inducible Promoters in Mouse Embryonic Stem Cells. BMC Genom. 2012, 13, 424. [Google Scholar] [CrossRef]
- Tsukamoto, D.; Ito, M.; Takamatsu, N. HNF-4 Participates in the Hibernation-Associated Transcriptional Regulation of the Chipmunk Hibernation-Related Protein Gene. Sci. Rep. 2017, 7, 44279. [Google Scholar] [CrossRef] [PubMed]
- Wijenayake, S.; Storey, K.B. Dynamic Regulation of Histone H3 Lysine (K) Acetylation and Deacetylation during Prolonged Oxygen Deprivation in a Champion Anaerobe. Mol. Cell. Biochem. 2020, 474, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Krivoruchko, A.; Storey, K.B. Epigenetics in Anoxia Tolerance: A Role for Histone Deacetylases. Mol. Cell. Biochem. 2010, 342, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yan, Y.; Davidson, T.L.; Shinkai, Y.; Costa, M. Hypoxic Stress Induces Dimethylated Histone H3 Lysine 9 through Histone Methyltransferase G9a in Mammalian Cells. Cancer Res. 2006, 66, 9009–9016. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Storey, K.B. Coordinated Expression of Jumonji and AHCY under OCT Transcription Factor Control to Regulate Gene Methylation in Wood Frogs during Anoxia. Gene 2021, 788, 145671. [Google Scholar] [CrossRef] [PubMed]
- Rathert, P.; Dhayalan, A.; Murakami, M.; Zhang, X.; Tamas, R.; Jurkowska, R.; Komatsu, Y.; Shinkai, Y.; Cheng, X.; Jeltsch, A. Protein Lysine Methyltransferase G9a Acts on Non-Histone Targets. Nat. Chem. Biol. 2008, 4, 344. [Google Scholar] [CrossRef]
- Bloskie, T.; Storey, K.B. DNA Hypomethylation May Contribute to Metabolic Recovery of Frozen Wood Frog Brains. Epigenomes 2022, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Watts, A.J.; Storey, K.B. Hibernation Impacts Lysine Methylation Dynamics in the 13-Lined Ground Squirrel, Ictidomys tridecemlineatus. J. Exp. Zool. A Ecol. Integr. Physiol. 2019, 331, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Tessier, S.N.; Luu, B.E.; Smith, J.C.; Storey, K.B. The Role of Global Histone Post-Translational Modifications during Mammalian Hibernation. Cryobiology 2017, 75, 28–36. [Google Scholar] [CrossRef]
- Cai, Y.; Zhang, Y.; Loh, Y.P.; Tng, J.Q.; Lim, M.C.; Cao, Z.; Raju, A.; Lieberman Aiden, E.; Li, S.; Manikandan, L.; et al. H3K27me3-Rich Genomic Regions Can Function as Silencers to Repress Gene Expression via Chromatin Interactions. Nat. Commun. 2021, 12, 719. [Google Scholar] [CrossRef]
- Ni, L.; Lin, B.; Zhang, Y.; Hu, L.; Lin, J.; Fu, F.; Shen, M.; Li, C.; Chen, L.; Yang, J.; et al. Histone Modification Landscape and the Key Significance of H3K27me3 in Myocardial Ischaemia/Reperfusion Injury. Sci. China Life Sci. 2023, 66, 1264–1279. [Google Scholar] [CrossRef] [PubMed]
- Reddington, J.P.; Perricone, S.M.; Nestor, C.E.; Reichmann, J.; Youngson, N.A.; Suzuki, M.; Reinhardt, D.; Dunican, D.S.; Prendergast, J.G.; Mjoseng, H.; et al. Redistribution of H3K27me3 upon DNA Hypomethylation Results in De-Repression of Polycomb Target Genes. Genome Biol. 2013, 14, R25. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bloskie, T.; Taiwo, O.O.; Storey, K.B. Reversible Histone Modifications Contribute to the Frozen and Thawed Recovery States of Wood Frog Brains. Biomolecules 2024, 14, 839. https://doi.org/10.3390/biom14070839
Bloskie T, Taiwo OO, Storey KB. Reversible Histone Modifications Contribute to the Frozen and Thawed Recovery States of Wood Frog Brains. Biomolecules. 2024; 14(7):839. https://doi.org/10.3390/biom14070839
Chicago/Turabian StyleBloskie, Tighe, Olawale O. Taiwo, and Kenneth B. Storey. 2024. "Reversible Histone Modifications Contribute to the Frozen and Thawed Recovery States of Wood Frog Brains" Biomolecules 14, no. 7: 839. https://doi.org/10.3390/biom14070839





