Novel Plasma Biomarkers Associated with Future Peripheral Atherosclerotic Disease and Abdominal Aortic Aneurysm—Insights from Contemporary Prospective Studies from the Malmö Diet and Cancer Study
Abstract
:1. Introduction
1.1. Biomarkers
1.2. Old and New Plasma Biomarkers for Cardiovascular Disease
1.3. The Malmö Diet and Cancer Study Cohort—Cardiovascular Arm
1.4. Objectives
2. Methods
2.1. Study Design
2.2. Plasma Biomarkers
2.3. Endpoint Ascertainment
2.4. Validation of Cardiovascular Disease
2.5. Statistics
2.6. Ethics
3. Results
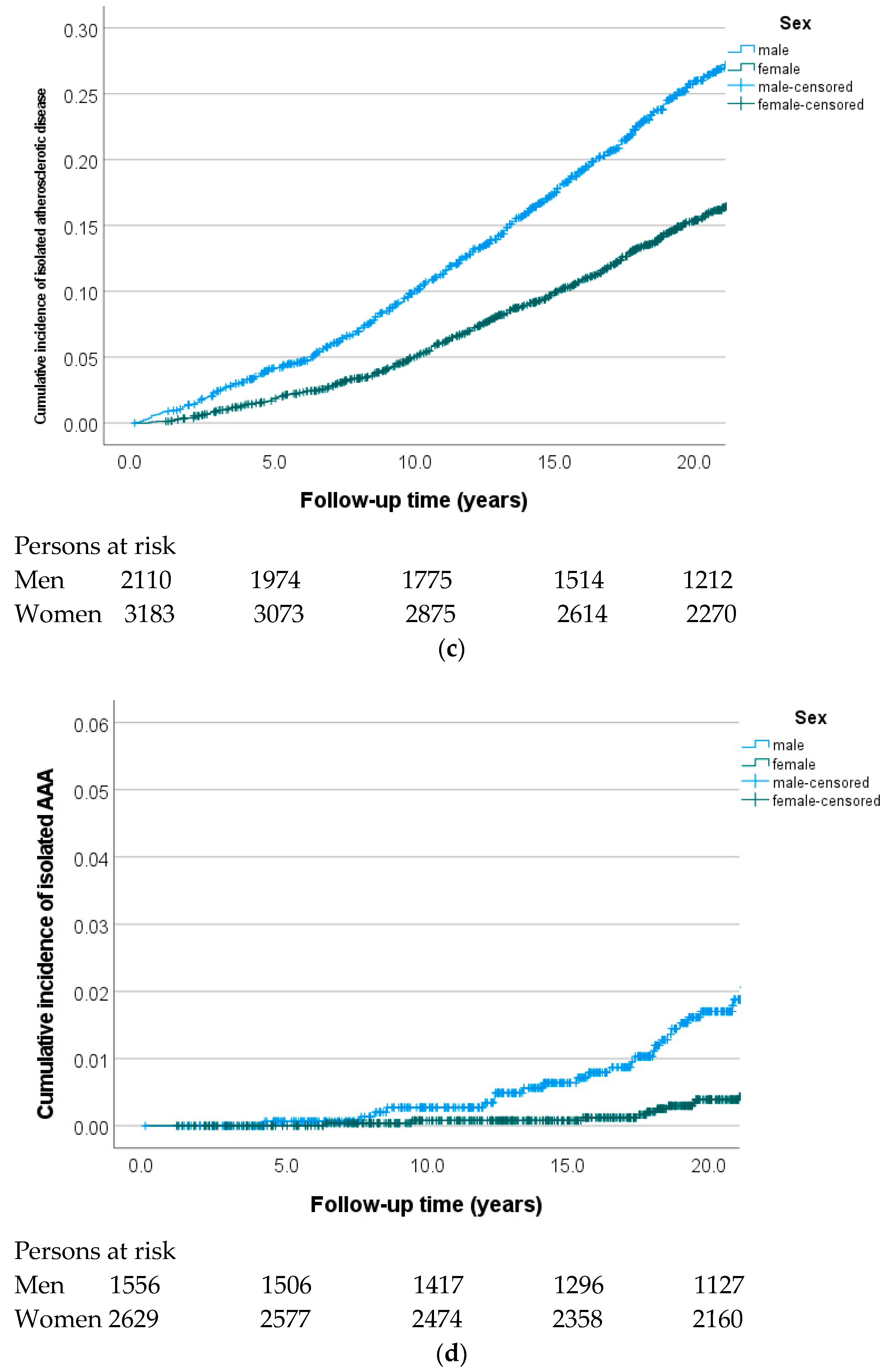
3.1. Cumulative Incidence of Incident Cardiovascular Diseases
3.2. Plasma Biomarkers Associated with Incident Cardiovascular Disease
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Engelberger, R.P.; Limacher, A.; Kucher, N.; Baumann, F.; Silbernagel, G.; Benghozi, R.; Do, D.-D.; Willenberg, T.; Baumgartner, I. Biological variation of established and novel biomarkers for atherosclerosis: Results from a prospective, parallel-group cohort study. Clin. Chim. Acta 2015, 447, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhong, X.; Cheng, G.; Zhao, C.; Zhang, L.; Hong, Y.; Wan, Q.; He, R.; Wang, Z. Hs-CRP and all-cause, cardiovascular, and cancer mortality risk: A meta-analysis. Atherosclerosis 2017, 259, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.P.; Morris, D.R.; Smith, S.; Moxon, J.V.; Golledge, J. Systematic Review and Meta-Analysis of the Association Between C-Reactive Protein and Major Cardiovascular Events in Patients with Peripheral Artery Disease. Eur. J. Vasc. Endovasc. Surg. 2017, 54, 220–223. [Google Scholar] [CrossRef]
- Badger, S.A.; Soong, C.V.; O’ Donnell, M.E.; Mercer, C.; Young, I.S.; Hughes, A.E. C-reactive protein (CRP) elevation in patients with abdominal aortic aneurysm is independent of the most important CRP genetic polymorphism. J. Vasc. Surg. 2009, 49, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Charniot, J.C.; Khani-Bittar, R.; Albertini, J.P.; Giral, P.; Cherfils, C.; Cosson, C.; Bonnefont-Rousselot, D. Interpretation of lipoprotein-associated phospho-lipase A2 levels is influenced by cardiac disease, comorbidities, extension of atherosclerosis and treatments. Int. J. Cardiol. 2013, 168, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-B.; Yang, F.; Jing, L.; Ma, J.; Jia, Y.-D.; Dong, S.-Y.; Zheng, W.-F.; Zhao, L.-S. Correlation between plasma lipoprotein-associated phospholipase A(2) and peripheral arterial disease. Exp. Ther. Med. 2013, 5, 1451–1455. [Google Scholar] [CrossRef]
- Liu, H.; Yao, Y.; Wang, Y.; Ji, L.; Zhu, K.; Hu, H.; Zhou, Y. Association between high sensitivity C-reactive protein, lipoprotein-associated phospholipase A2 and carotid atherosclerosis: A crosssectional study. J. Cell Mol. Med. 2018, 22, 5145–5150. [Google Scholar] [CrossRef]
- Garg, P.K.; Arnold, A.M.; Hinckley Stukovsky, K.D.; Koro, C.; Jenny, N.S.; Mukamal, K.J.; Cushman, M. Lipoprotein-Associated Phospholipase A2 and Incident Peripheral Arterial Disease in Older Adults: The Cardiovascular Health Study. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 750–756. [Google Scholar] [CrossRef]
- Garg, P.K.; Norby, F.L.; Polfus, L.M.; Boerwinkle, E.; Gibbs, R.A.; Grove, M.L.; Folsom, A.R.; Garimella, P.S.; Matsushita, K.; Hoogeveen, R.C.; et al. Lipoprotein-associated phospholipase A(2) and risk of incident peripheral arterial disease: Findings from The Atherosclerosis Risk in Communities study (ARIC). Atherosclerosis 2018, 268, 12–18. [Google Scholar] [CrossRef]
- Garg, P.K.; Jorgensen, N.W.; McClelland, R.L.; Jenny, N.S.; Criqui, M.H.; A Allison, M.; Greenland, P.; Rosenson, R.S.; Siscovick, D.S.; Cushman, M. Lipoprotein-associated phospholipase A(2) and risk of incident peripheral arterial disease in a multi-ethnic cohort: The Multi-Ethnic Study of Atherosclerosis. Vasc. Med. 2017, 22, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Motiwala, S.R.; Sarma, A.; Januzzi, J.L.; O’Donoghue, M.L. Biomarkers in ACS and Heart Failure: Should Men and Women Be In-terpreted Differently? Clin. Chem. 2014, 60, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Öner, Ö.; Deveci, F.; Telo, S.; Kuluöztürk, M.; Balin, M. MR-proADM and MR-proANP levels in patients with acute pulmonary embolism. J. Med. Biochem. 2019, 38, 328. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.; Ricci, F.; Di Martino, G.; Rogmark, C.; Sutton, R.; Hamrefors, V.; Melander, O.; Fedorowski, A. Cardiovascular biomarkers predict fragility fractures in older adults. Heart 2019, 105, 449–454. [Google Scholar] [CrossRef]
- Kollerits, B.; Sturm, G.; Lamina, C.; Hammerer-Lercher, A.; Rantner, B.; Stadler, M.; Kronenberg, F. Comparison and evaluation of cardiac bi-omarkers in patients with intermittent claudication: Results from the CAVASIC study. Clin. Chem. 2013, 59, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Falkensammer, J.; Frech, A.; Duschek, N.; Gasteiger, S.; Stojakovic, T.; Scharnagl, H.; Greiner, A. Prognostic relevance of ischemia-modified albumin and NT-proBNP in patients with peripheral arterial occlusive disease. Clin. Chim. Acta 2015, 438, 255–260. [Google Scholar] [CrossRef]
- Sasaki, N.; Yamamoto, H.; Ozono, R.; Maeda, R.; Kihara, Y. Association of common carotid artery measurements with N-terminal pro B-type natriuretic peptide in elderly participants. Intern. Med. 2020, 59, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; Kwak, L.; Yang, C.; Pang, Y.; Ballew, S.H.; Sang, Y.; Hoogeveen, R.C.; Jaar, B.G.; Selvin, E.; Ballantyne, C.M.; et al. High-sensitivity cardiac troponin and natriuretic peptide with risk of lower-extremity peripheral artery disease: The Atherosclerosis Risk in Communities (ARIC) Study. Eur. Heart J. 2018, 39, 2412–2419. [Google Scholar] [CrossRef]
- Kremers, B.; Wübbeke, L.; Mees, B.; Ten Cate, H.; Spronk, H.; Ten Cate-Hoek, A. Plasma Biomarkers to Predict Cardiovascular Outcome in Patients with Peripheral Artery Disease: A Systematic Review and Meta-Analysis. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2018–2032. [Google Scholar] [CrossRef]
- Chan DC, S.; Cao, T.H.; Ng, L.L. Proenkephalin in Heart Failure. Heart Fail. Clin. 2018, 14, 1–11. [Google Scholar] [CrossRef]
- Mueller, C.; Möckel, M.; Giannitsis, E.; Huber, K.; Mair, J.; Plebani, M.; Thygesen, K.; ESC Study Group on Biomarkers in Cardiology of the Acute Cardiovascular Care Association. Use of copeptin for rapid rule-out of acute myocardial infarction. Eur. Heart J. Acute Cardiovasc. Care 2018, 7, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Fenske, W.; Störk, S.; Blechschmidt, A.; Maier, S.G.K.; Morgenthaler, N.G.; Allolio, B. Copeptin in the differential diagnosis of hypo-natremia. J. Clin. Endocrinol. Metab. 2009, 94, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Herget-Rosenthal, S.; Cystatin, C. Encyclopedia of Intensive Care Medicine; Vincent, J.L., Hall, J.B., Eds.; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Yang, C.; Kwak, L.; Ballew, S.H.; Garimella, P.S.; Jaar, B.G.; Folsom, A.R.; Heiss, G.; Selvin, E.; Lutsey, P.L.; Coresh, J.; et al. Kidney function, bone-mineral metabolism markers, and future risk of peripheral artery disease. Atherosclerosis 2017, 267, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Urbonaviciene, G.; Shi, G.P.; Urbonavicius, S.; Henneberg, E.W.; Lindholt, J.S. Higher cystatin C level predicts long-term mortality in patients with peripheral arterial disease. Atherosclerosis 2011, 216, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Umemura, T.; Kawamura, T.; Mashita, S.; Kameyama, T.; Sobue, G. Higher levels of cystatin c are associated with extracranial carotid artery steno-occlusive disease in patients with noncardioembolic ischemic stroke. Cerebrovasc. Dis. Extra 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Lindholt, J.S.; Erlandsen, E.J.; Henneberg, E.W. Cystatin C deficiency is associated with the progression of small abdominal aortic aneurysms. Br. J. Surg. 2001, 88, 1472–1475. [Google Scholar] [CrossRef] [PubMed]
- Hedblad, B.; Nilsson, P.; Janzon, L.; Berglund, G. Relation between insulin resistance and carotid intima-media thickness and stenosis in non-diabetic subjects. Results from a cross-sectional study in Malmo, Sweden. Diabet. Med. 2000, 17, 299–307. [Google Scholar] [CrossRef]
- Wirfält, E.; Mattisson, I.; Johansson, U.; Gullberg, B.; Wallström, P.; Berglund, G. A methodological report from the Malmö Diet and Cancer study: Development and evaluation of altered routines in dietary data processing. Nutr. J. 2002, 1, 3. [Google Scholar] [CrossRef]
- Fatemi, S.; Gottsäter, A.; Zarrouk, M.; Engström, G.; Melander, O.; Persson, M.; Acosta, S. Lp-PLA2 activity and mass and CRP are associated with incident symptomatic peripheral arterial disease. Sci. Rep. 2019, 9, 5609. [Google Scholar] [CrossRef]
- Fatemi, S.; Acosta, S.; Gottsäter, A.; Melander, O.; Engström, G.; Dakhel, A.; Zarrouk, M. Copeptin, B-type natriuretic peptide and Cystatin C are associated with incident symptomatic PAD. Biomarkers 2019, 24, 615–621. [Google Scholar] [CrossRef]
- Fatemi, S.; Acosta, S.; Zarrouk, M.; Engström, G.; Melander, O.; Gottsäter, A. Pro B-type natriuretic peptide and midregional proadrenomedullin are associated with incident carotid stenosis during long term follow-up. J. Stroke Cerebrovasc. Dis. 2021, 30, 105403. [Google Scholar] [CrossRef]
- Fatemi, S.; Acosta, S.; Zarrouk, M.; Engström, G.; Melander, O.; Gottsäter, A. Circulating biomarkers predict symptomatic but not asymptomatic carotid artery stenosis. Cerebrovasc. Dis. 2022, 51, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Acosta, S.; Fatemi, S.; Melander, O.; Engström, G.; Gottsäter, A. Prospective comparison of plasma biomarker and traditional risk factor profiles for incident isolated atherosclerotic disease and incident isolated abdominal aortic aneurysm. Front. Cardiovasc. Med. 2022, 8, 818656. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, S. The Value of Novel Biomarkers for Prediction of Arterial Disease during Long Term Follow-Up. Ph.D. Thesis, Department of Clinical Sciences, Malmö, Lund University, Faculty of Medicine, Lund, Sweden, 2024. [Google Scholar]
- Melander, O.; Newton-Cheh, C.; Almgren, P.; Hedblad, B.; Berglund, G.; Engström, G.; Persson, M.; Smith, J.G.; Magnusson, M.; Christensson, A.; et al. Novel and conventional biomarkers for prediction of incident cardiovascular events in the community. JAMA 2009, 302, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Persson, M.; Nilsson, J.; Nelson, J.J.; Hedblad, B.; Berglund, G. The epidemiology of Lp-PLA2: Distribution and correlation with cardiovascular risk factors in a population-based cohort. Atherosclerosis 2007, 190, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Melander, O.; Maisel, A.S.; Almgren, P.; Manjer, J.; Belting, M.; Hedblad, B.; Engström, G.; Kilger, U.; Nilsson, P.; Bergmann, A.; et al. Plasma proneurotensin and incidence of diabetes, cardiovascular disease, breast cancer, and mortality. JAMA 2012, 308, 1469–1475. [Google Scholar] [CrossRef] [PubMed]
- Morgenthaler, N.G.; Struck, J.; Alonso, C.; Bergmann, A. Measurement of midregional proadrenomedullin in plasma with an im-munoluminometric assay. Clin. Chem. 2005, 51, 1823–1829. [Google Scholar] [CrossRef] [PubMed]
- McDermott, M.M.; Greenland, P.; Green, D.; Guralnik, J.M.; Criqui, M.H.; Liu, K.; Chan, C.; Pearce, W.H.; Taylor, L.; Ridker, P.M.; et al. D-Dimer, inflammatory markers, and lower extremity functioning in patients with and without peripheral arterial disease. Circulation 2003, 107, 3191–3198. [Google Scholar] [CrossRef]
- Pande, R.L.; Brown, J.; Buck, S.; Redline, W.; Doyle, J.; Plutzky, J.; Creager, M.A. Association of monocyte tumor necrosis factor α expression and serum inflammatory biomarkers with walking impairment in peripheral artery disease. J. Vasc. Surg. 2015, 61, 155–161. [Google Scholar] [CrossRef]
- Vainas, T.; Stassen, F.R.; de Graaf, R.; Twiss, E.L.; Herngreen, S.B.; Welten, R.J.T.; Akker, L.H.v.D.; van Dieijen-Visser, M.P.; Bruggeman, C.A.; Kitslaar, P.J. C-Reactive protein in peripheral arterial disease: Relation to severity of the disease and to future cardiovascular events. J. Vasc. Surg. 2005, 42, 243–251. [Google Scholar] [CrossRef]
- Clemens, R.K.; Annema, W.; Baumann, F.; Roth-Zetzsche, S.; Seifert, B.; von Eckardstein, A.; Amann-Vesti, B.R. Cardiac biomarkers but not measures of vascular atherosclerosis predict mortality in patients with peripheral artery disease. Clin. Chim. Acta 2019, 495, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.L. Medical (nonsurgical) intervention alone is now best treatment for prevention of stroke associated with asymp-tomatic severe carotid stenosis. Results of a systematic review and analysis. Stroke 2009, 40, e573–e583. [Google Scholar] [CrossRef]
- Naylor, A.R.; Gaines, P.A.; Rothwell, P.M. Who benefits most from intervention for asymptomatic carotid stenosis: Patients or professional? Eur. J. Vasc. Endovasc. Surg. 2009, 37, 625–632. [Google Scholar] [CrossRef]
- Marquardt, L.; Geraghty, O.C.; Mehta, Z.; Rothwell, P.M. Low risk of ipsilateral stroke in patients with asymptomatic carotid stenosis on best medical treatment. A prospective, poulation-based study. Stroke 2010, 41, E11–E17. [Google Scholar] [CrossRef] [PubMed]
- Stenudd, I.; Sjödin, E.; Nyman, E.; Wester, P.; Johansson, E.; Grönlund, C. Ultrasound risk marker variability in symptomatic carotid plaque: Impact on risk reclassification and association with temporal variation pattern. Int. J. Cardiovasc. Imaging 2020, 36, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Naylor, A.; Ricco, J.-B.; de Borst, G.; Debus, S.; de Haro, J.; Halliday, A.; Hamilton, G.; Kakisis, J.; Kakkos, S.; Lepidi, S.; et al. Editor’s Choice—Management of Atherosclerotic Carotid and Vertebral Artery Disease: 2017 Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2018, 55, 3–81. [Google Scholar] [CrossRef] [PubMed]
- Saam, T.; Cai, J.; Ma, L.; Cai, Y.-Q.; Ferguson, M.S.; Polissar, N.L.; Hatsukami, T.S.; Yuan, C. Comparison of symptomatic and asymptomatic atherosclerotic carotid plaque features with in vivo MR imaging. Radiology 2006, 240, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Shaalan, W.E.; Cheng, H.; Gewertz, B.; McKinsey, J.F.; Schwartz, L.B.; Katz, D.; Cao, D.; Desai, T.; Glagov, S.; Bassiouny, H.S. Degree of carotid plaque calcification in relation to symptomatic outcome and plaque inflammation. J. Vasc. Surg. 2004, 40, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Acosta, S.; Taimour, S.; Gottsäter, A.; Persson, M.; Engström, G.; Melander, O.; Zarrouk, M.; Nilsson, P.M.; Smith, J.G. Lp-PLA2 activity and mass for prediction of incident abdominal aortic aneurysms: A prospective longitudinal cohort study. Atherosclerosis 2017, 262, 14–18. [Google Scholar] [CrossRef]
- Acosta, S.; Gottsäter, A.; Engström, G.; Melander, O.; Zarrouk, M.; Nilsson, P.M.; Smith, J.G. Circulating Midregional Proadrenomedullin and Risk of Incident Abdominal Aortic Aneurysm: A Prospective Longitudinal Cohort Study. Angiology 2018, 69, 333–338. [Google Scholar] [CrossRef]
- Taimour, S.; Zarrouk, M.; Holst, J.; Melander, O.; Engström, G.; Smith, J.G.; Gottsäter, A. No relation between biomarkers at age 47–49 and aortic diameter after 14–19 years of follow-up–a population-based study. Vasa 2017, 46, 291–295. [Google Scholar] [CrossRef] [PubMed]
- A Memon, A.; Zarrouk, M.; Ågren-Witteschus, S.; Sundquist, J.; Gottsäter, A.; Sundquist, K. Identification of novel diagnostic and prognostic biomarkers for abdominal aortic aneurysm. Eur. J. Prev. Cardiol. 2020, 27, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Nana, P.; Dakis, K.; Brodis, A.; Spanos, K.; Kouvelos, G. Circulating biomarkers for the prediction of abdominal aortic aneurysm growth. J. Clin. Med. 2021, 10, 1718. [Google Scholar] [CrossRef] [PubMed]
- Acosta, S.; Gottsäter, A.; Engström, G.; Melander, O.; Zarrouk, M.; Nilsson, P.M.; Smith, J.G. B-type natriuretic peptide for prediction of incident clinically significant abdominal aortic aneurysm: A population-based prospective study. Vasc. Med. 2018, 23, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Miceli, G.; Basso, M.G.; Rizzo, G.; Pintus, C.; Tuttolomondo, A. The role of the coagulation system in peripheral arterial disease: In-teractions with the arterial wall and its vascular microenvironment and implications for rational therapies. Int. J. Mol. Sci. 2022, 23, 14914. [Google Scholar] [CrossRef] [PubMed]
- Alhadad, A.; Wictorsson, C.; Alhadad, H.; Lindblad, B.; Gottsäter, A. Medical risk factor in peripheral arterial disease. Need for further improvement. Int. Angiol. 2013, 32, 332–338. [Google Scholar] [PubMed]
- Kulezic, A.; Bergwall, S.; Fatemi, S.; Sonestedt, E.; Zarrouk, M.; Gottsäter, A.; Acosta, S. Healthy diet and fibre intake are associated with decreased risk of incident symptomatic peripheral arterial disease—A prospective cohort study. Vasc. Med. 2019, 24, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Sara, J.D.S.; Toya, T.; Ahmad, A.; Clark, M.M.; Gilliam, W.P.; Lerman, L.O.; Lerman, A. Mental Stress and Its Effects on Vascular Health. Mayo Clin. Proc. 2022, 97, 951–990. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A. A review of family history of cardiovascular disease: Risk factor and research tool. Int. J. Clin. Pract. 2012, 66, 536–543. [Google Scholar] [CrossRef]
- Brunkwall, L.; Jönsson, D.; Ericson, U.; Hellstrand, S.; Kennbäck, C.; Östling, G.; Nilsson, P.M. The Malmö Offspring Study (MOS): Design, methods and first results. Eur. J. Epidemiol. 2021, 36, 103–116. [Google Scholar] [CrossRef]

| Name | Cell Origin | Type of Marker | Disease Marker |
|---|---|---|---|
| CRP [2,3,4,5] | Liver | Inflammatory | Cardiovascular Obesity Diabetes |
| Lp-PLA2 [6,7,8,9,10,11] (mass) | Monocytes | Inflammatory | Atherogenesis |
| Lp-PLA2 (activity) | Monocytes | Inflammatory | Atherogenesis |
| Proneurotensin [12] | Gut | Fat metabolism | Satiety Obesity regulation |
| MR-proADM [13,14,15] | Vascular smooth muscle and endothelial cells, cardiomyocytes | Vasodilatation | Endothelial dysfunction |
| MR-proANP [13,14,15] | Atria | Cardiac | Heart failure, volume overload |
| NT pro-BNP [16,17,18,19] | Atria | Cardiac | Heart failure, volume overload |
| Copeptin [20,21,22] | Hypothalamus | Inflammatory | Heart failure, acute myocardial infarction, ischemic stroke |
| Cystatin C [23,24,25,26,27] | Body fluids | Renal | Kidney failure |
| Name | Assay | Manufacturer | CV (%) |
|---|---|---|---|
| CRP | High sensitivity Tina-quant® latex | Roche Diagnostics, Rotkreuz, Switzerland | 4.59 |
| Lp-PLA2 (mass) [37] | Sandwich enzyme immuno- | diaDexus Inc., San Francisco, California, United States | 4.62 |
| Lp-PLA2 (activity) [37] | Enzyme-linked immunosorbent | Non-commercial | 5.78 |
| Proneurotensin [38] | Chemiluminescence | Non-commercial | 4.1–6.2 |
| MR-proADM [39] | Immunoluminometric sandwich | Brahms AG, Hennigsdorf, Germany | ≤10 |
| MR-proANP | Immunoluminometric sandwich | Brahms AG, Hennigsdorf, Germany | ≤10 |
| NT pro-BNP [36] | Automated Dimension Vista Intelligent Lab System | Siemens diagnostics, Erlangen, Germany | 2.7 |
| Copeptin [22] | Chemiluminescence | Brahms AG, Hennigsdorf, Germany | <20 |
| Cystatin C | Particle-enhanced immune-nephelometric | Siemens diagnostics, Erlangen, Germany | 4.3 |
| Plasma Inflammatory Biomarkers | PAD n = 244. HR * (95% CI) |
|---|---|
| C-reactive protein (n = 5300) | 1.55 (1.36–1.76) |
| Proneurotensin (n = 4627) | 0.94 (0.80–1.09) |
| Lipoprotein-associated phospholipase A2 (mass) (n = 5390) | 1.20 (1.05–1.37) |
| Lipoprotein-associated phospholipase A2 (activity) (n = 5395) | 1.33 (1.17–1.52) |
| Plasma hemodynamic biomarkers | |
| Cystatin C (n = 5150) | 1.19 (1.10–1.29) |
| Copeptin (n = 5248) | 1.46 (1.19–1.80) |
| N-terminal pro-B-type natriuretic peptide (n = 5156) | 1.28 (1.11–1.48) |
| Mid-regional proatrial natriuretic peptide (n = 5255) | 1.13 (0.98–1.31) |
| Mid-regional proadrenomedullin (n = 5254) | 1.16 (1.00–1.34) |
| Plasma Biomarkers | Symptomatic CAS n = 56. HR * (95% CI) | Asymptomatic CAS n = 54. HR * (95% CI) |
|---|---|---|
| Lipoprotein-associated phospholipase A2 (mass) | 1.28 (0.97–1.69) | 0.87 (0.64–1.18) |
| Lipoprotein-associated phospholipase A2 (activity) | 1.34 (0.99–1.81) | 0.95 (0.68–1.33) |
| Proneurotensin | 0.90 (0.63–1.28) | 0.96 (0.70–1.30) |
| Mid-regional proadrenomedullin | 1.40 (1.13–1.73) | 0.98 (0.69–1.40) |
| Mid-regional proatrial natriuretic peptide | 1.12 (0.83–1.52) | 0.79 (0.58–1.09) |
| N-terminal pro-B-type natriuretic peptide | 1.59 (1.20–2.11) | 1.08 (0.80–1.47) |
| Copeptin | 1.35 (0.88–2.06) | 1.16 (0.80–1.67) |
| Cystatin C | 1.21 (1.02–1.43) | 0.75 (0.50–1.10) |
| C-reactive protein | 1.53 (1.13–2.05) | 1.08 (0.80–1.46) |
| Plasma Biomarker | Incident AD (Free from Incident AAA) HR * (95% CI) | Incident AAA (Free from Incident AD) HR * (95% CI) |
|---|---|---|
| Lipoprotein-associated phospholipase A2 (activity) | 1.12 (1.04–1.19) | 1.53 (1.11–2.11) |
| Lipoprotein-associated phospholipase A2 (mass) | 1.05 (0.99–1.12) | 1.53 (1.14–2.04) |
| Copeptin | 1.09 (1.01–1.17) | 0.98 (0.70–1.39) |
| Mid-regional proadrenomedullin | 1.17 (1.10–1.25) | 1.47 (1.15–1.88) |
| Mid-regional proatrial natriuretic peptide | 1.03 (0.97–1.11) | 1.01 (0.71–1.43) |
| N-terminal pro-B-type natriuretic peptide | 1.16 (1.08–1.24) | 1.13 (0.80–1.60) |
| Cystatin C | 1.17 (1.11–1.23) | 1.13 (0.82–1.55) |
| Proneurotensin | 1.07 (1.02–1.13) | 1.09 (0.85–1.40) |
| C-reactive protein | 1.17 (1.10–1.25) | 1.22 (0.88–1.68) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acosta, S.; Fatemi, S.; Zarrouk, M.; Gottsäter, A. Novel Plasma Biomarkers Associated with Future Peripheral Atherosclerotic Disease and Abdominal Aortic Aneurysm—Insights from Contemporary Prospective Studies from the Malmö Diet and Cancer Study. Biomolecules 2024, 14, 844. https://doi.org/10.3390/biom14070844
Acosta S, Fatemi S, Zarrouk M, Gottsäter A. Novel Plasma Biomarkers Associated with Future Peripheral Atherosclerotic Disease and Abdominal Aortic Aneurysm—Insights from Contemporary Prospective Studies from the Malmö Diet and Cancer Study. Biomolecules. 2024; 14(7):844. https://doi.org/10.3390/biom14070844
Chicago/Turabian StyleAcosta, Stefan, Shahab Fatemi, Moncef Zarrouk, and Anders Gottsäter. 2024. "Novel Plasma Biomarkers Associated with Future Peripheral Atherosclerotic Disease and Abdominal Aortic Aneurysm—Insights from Contemporary Prospective Studies from the Malmö Diet and Cancer Study" Biomolecules 14, no. 7: 844. https://doi.org/10.3390/biom14070844






