Recent Advances in Hydrogel Technology in Delivering Mesenchymal Stem Cell for Osteoarthritis Therapy
Abstract
1. Introduction
2. Osteoarthritis (OA)
2.1. Pidemiological Characteristics and Physiopathological Mechanisms of OA
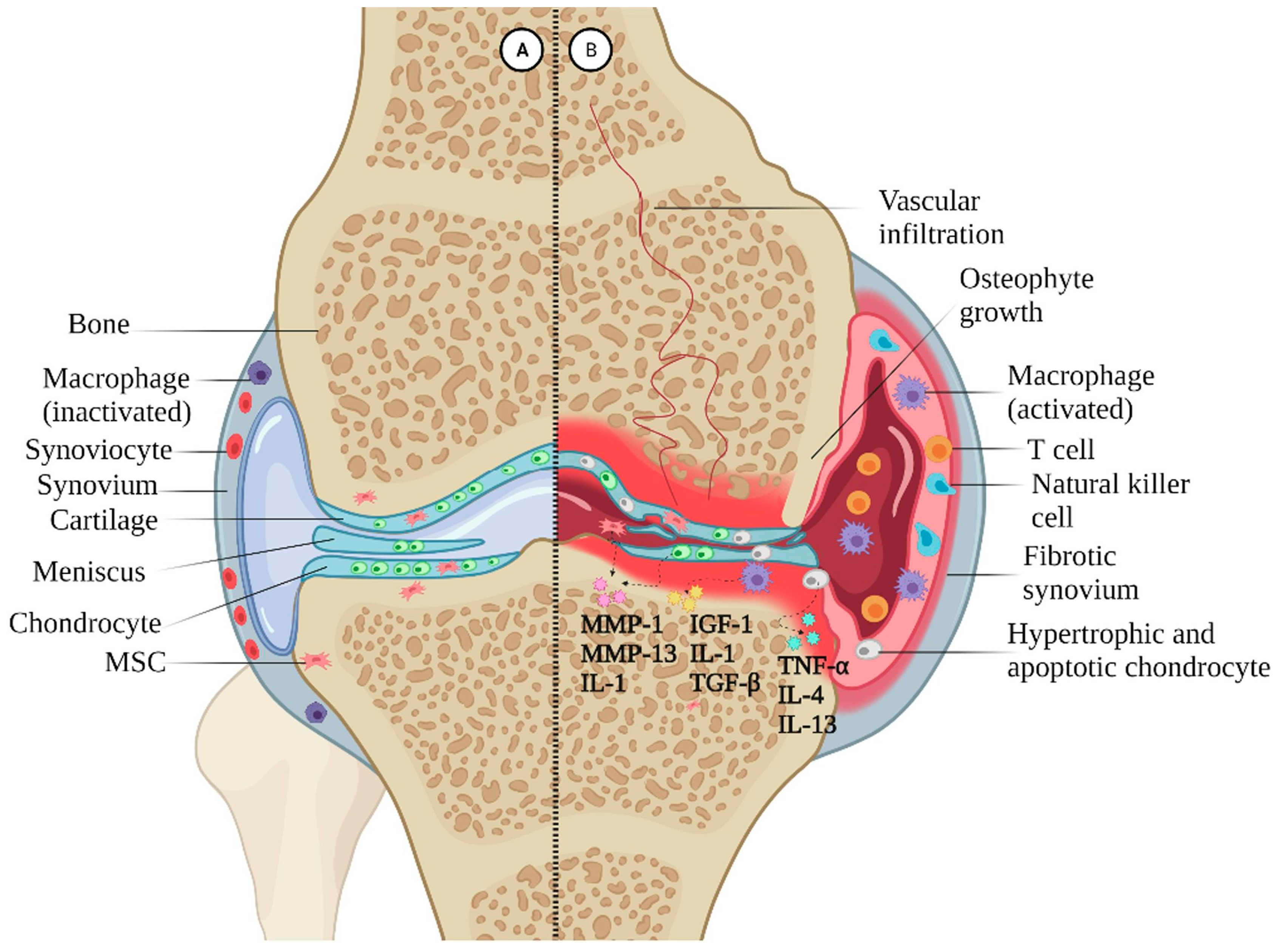
2.2. Current Dilemma in OA Treatment
| Treaments | Therapeutic Effects | Ref. | |
|---|---|---|---|
| Pharmacotherapy | Diacerein | Induces chondrogenesis; has analgesic, anti-inflammatory and antipyretic effects; and improves joint function in patients with osteoarthritis | [29,30,31,32] |
| Chondroitin/glucosamine | Pain reliever, promotes cartilage regeneration | ||
| Acetaminophen | Pain reliever | ||
| Opioids | Pain reliever | ||
| NSAIDs | Suppresses the degradation of cartilage ECM, increases ECM anabolism, and reduces chondrocytes apoptosis | ||
| Physical modalities | Exercise, Tai Chi | Reduces weight load and maintains body balance | [33,34,35,36,37,38,39,40] |
| Crutches | Reduces joint loads | ||
| Acupuncture, balneotherapy/spa, hydrotherapy, therapeutic ultrasound | Reduces local inflammatory stimuli by decreasing the expression of inflammatory factors, enhances the muscle strength around the knee to balance the stress | ||
| NMES, TENS | Relieves pain, improves blood circulation, reduces edema, promotes bone and wound healing, etc. | ||
| Surgical treatments | Total joint arthroplasty, hemiarthroplasty, arthroscopy | Reconstruction of joints to restore normal motor function | [41,42] |
3. Roles of MSCs in OA Therapy
3.1. Physiological Characteristics of MSCs
| MSCs | Origin | Differentiation Potential | Applications |
|---|---|---|---|
| BM-MSCs | Bone marrow | Osteocytes, chondrocytes, and adipocytes | Nonunion fractures, spinal cord injuries, and amyotrophic lateral sclerosis (ALS) [56,57,58,59,60,61] |
| Placenta-MSCs | Newborn placental tissue | Osteocytes, chondrocytes, adipocytes, and smooth muscle cells | Multiple sclerosis, knee osteoarthritis, preterm infant lung disease, and ovarian function restoration [62,63,64,65,66,67] |
| UC-MSCs | Intervascular, perivascular, and subamniotic area of Wharton’s jelly | Osteocytes, chondrocytes, and adipocytes | Treatment of neurological disorders, cardiovascular diseases, and autoimmune diseases [68,69,70,71] |
| ADSCs | Adipose tissue | Osteocytes, adipocytes, chondrocytes, and smooth muscle cells | Skin regeneration, soft tissue repair, and treatment of diabetes [72,73,74,75] |
| Sy-MSCs | Synovial fluid in the joint cavity | Osteocytes, chondrocytes, adipocytes, muscle cells, and neurons | Osteoarthritis treatment, cartilage injuries, systemic autoimmune diseases, and tissue engineering [76,77,78,79,80,81] |
| DPSCs | Dental pulp tissue of permanent teeth, deciduous teeth, and wisdom teeth in adults | Osteocytes, chondrocytes, adipocytes, muscle cells, and neurons | Dental treatment, neural repair, cardiovascular diseases, and bone tissue engineering [82,83,84,85,86] |
| AMSCs | Amniotic membrane tissue from the placenta | Osteocytes, chondrocytes, adipocytes, and smooth muscle cells | Skeletal tissue repair, autoimmune diseases, neurodegenerative diseases, liver diseases, and corneal repair [87,88,89,90,91] |
3.2. The Therapeutic Potential of MSCs for OA
3.2.1. AD-MSCs
3.2.2. BM-MSCs
3.3. Difficulties of Applying MSCs in OA Treatment
4. Bio-Application of Hydrogel Technologies
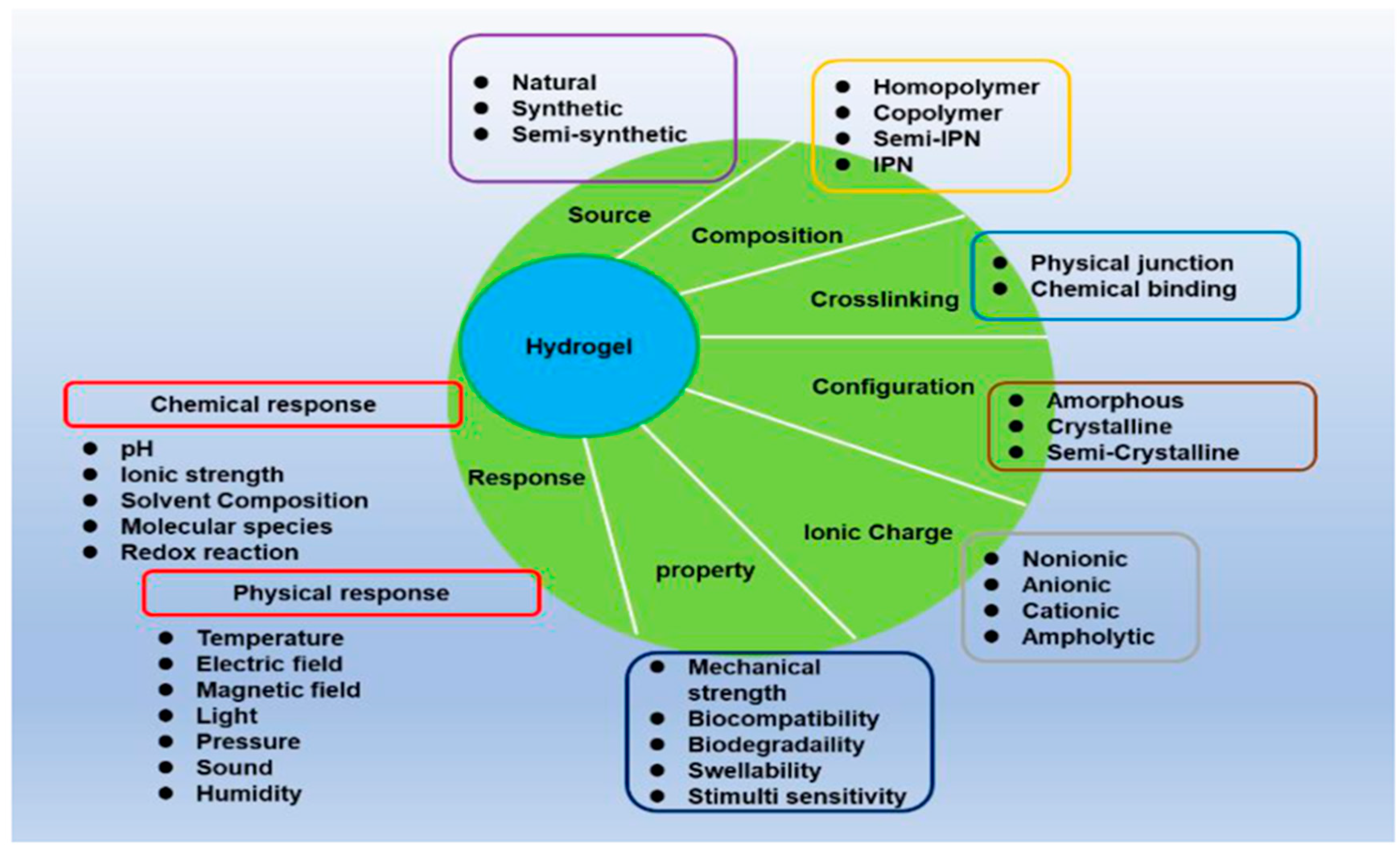
4.1. Characteristics of Hydrogel Technologies and Their Biomedical Application
4.2. Biofabrication of Hydrogel
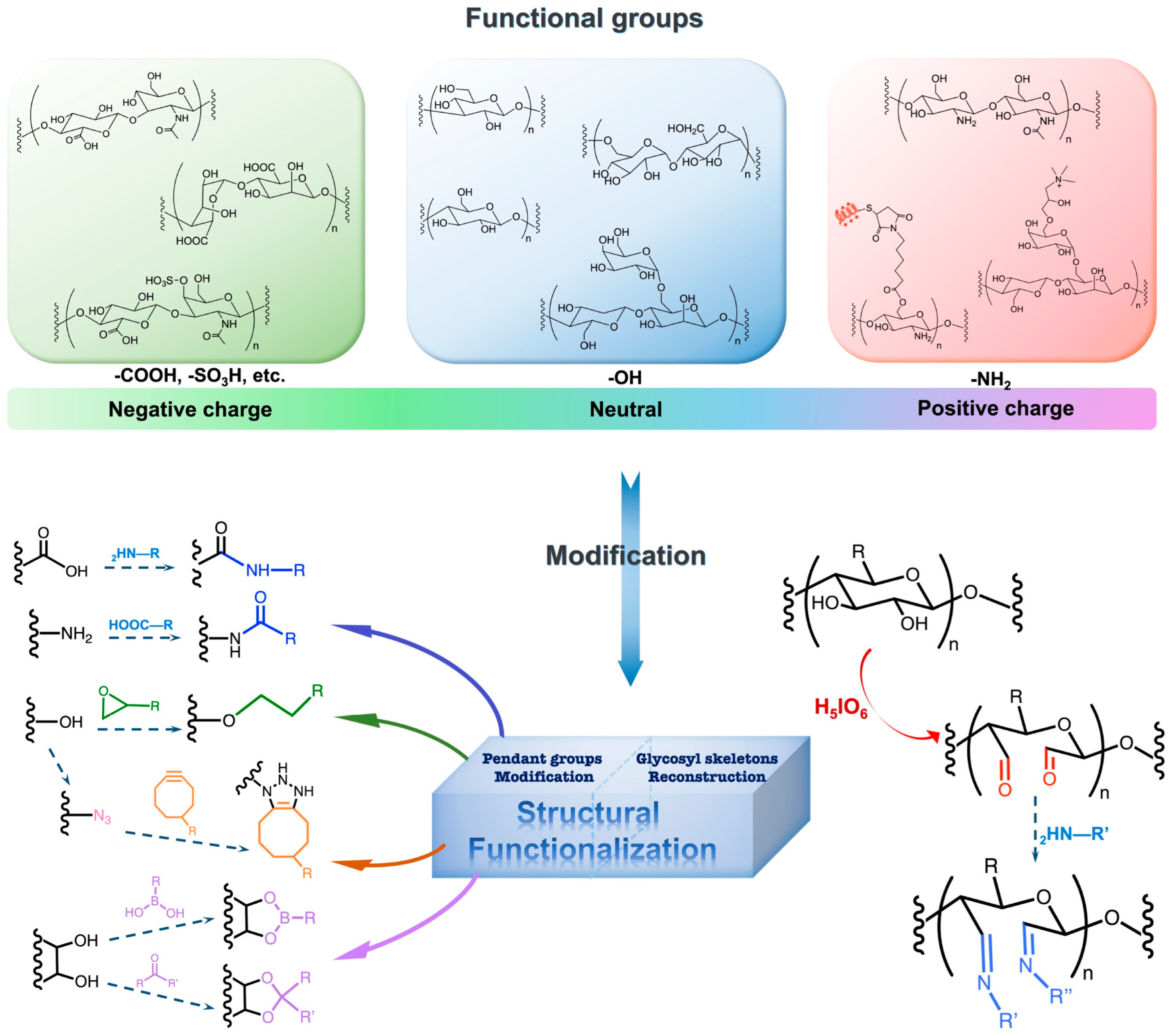
4.3. Functionalization of Hydrogel
4.4. Strategies Based on Combination of MSCs with Hydrogels for OA Treatment
4.5. Recent Advances in Application of Combining MSCs with Hydrogel in OA Treatment
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Wei, G.; Lu, K.; Umar, M.; Zhu, Z.; Lu, W.W.; Speakman, J.R.; Chen, Y.; Tong, L.; Chen, D. Risk of Metabolic Abnormalities in Osteoarthritis: A New Perspective to Understand Its Pathological Mechanisms. Bone Res. 2023, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Han, X.; Li, C.; Chen, Z.; Huang, H.; Chen, J.; Wu, C.; Fan, T.; Li, T.; Huang, W.; et al. 2D Materials for Bone Therapy. Adv. Drug Deliv. Rev. 2021, 178, 113970. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yang, F.; Chen, R.; Yang, C.; Xiao, H.; Geng, B.; Xia, Y. TRPV Channels in Osteoarthritis: A Comprehensive Review. Biomolecules 2024, 14, 292. [Google Scholar] [CrossRef] [PubMed]
- Margiana, R.; Markov, A.; Zekiy, A.O.; Hamza, M.U.; Al-Dabbagh, K.A.; Al-Zubaidi, S.H.; Hameed, N.M.; Ahmad, I.; Sivaraman, R.; Kzar, H.H.; et al. Clinical Application of Mesenchymal Stem Cell in Regenerative Medicine: A Narrative Review. Stem Cell Res. Ther. 2022, 13, 366. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Han, X.; Qu, G.; Su, L.; Zhao, B.; Miao, J. A pH Probe Inhibits Senescence in Mesenchymal Stem Cells. Stem Cell Res. Ther. 2018, 9, 343. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Fernandez, P.; Simula, L.; Jenni, S.; Jordan, O.; Allémann, E. Hyaluronan-Based Hydrogel Delivering Glucose to Mesenchymal Stem Cells Intended to Treat Osteoarthritis. Int. J. Pharm. 2024, 657, 124139. [Google Scholar] [CrossRef]
- Correa, S.; Grosskopf, A.K.; Lopez Hernandez, H.; Chan, D.; Yu, A.C.; Stapleton, L.M.; Appel, E.A. Translational Applications of Hydrogels. Chem. Rev. 2021, 121, 11385–11457. [Google Scholar] [CrossRef]
- Chen, J.; Pan, C.; Gao, Y.; Chen, Q.; An, X.; Liu, Z. Reactive Oxygen Species Scavenging Injectable Hydrogel Potentiates the Therapeutic Potential of Mesenchymal Stem Cells in Skin Flap Regeneration. ACS Appl. Mater. Interfaces 2024, 16, 17120–17128. [Google Scholar] [CrossRef]
- Kesharwani, P.; Alexander, A.; Shukla, R.; Jain, S.; Bisht, A.; Kumari, K.; Verma, K.; Sharma, S. Tissue Regeneration Properties of Hydrogels Derived from Biological Macromolecules: A Review. Int. J. Biol. Macromol. 2024, 271, 132280. [Google Scholar] [CrossRef]
- Gupta, A.; Lee, J.; Ghosh, T.; Nguyen, V.Q.; Dey, A.; Yoon, B.; Um, W.; Park, J.H. Polymeric Hydrogels for Controlled Drug Delivery to Treat Arthritis. Pharmaceutics 2022, 14, 540. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, C.; Zeng, C.; Wang, Z.; Wang, H.; Lu, J.; Liu, X.; Shao, Y.; Zhao, C.; Pan, J.; et al. Synovial Macrophage M1 Polarisation Exacerbates Experimental Osteoarthritis Partially through R-Spondin-2. Ann. Rheum. Dis. 2018, 77, 1524–1534. [Google Scholar] [CrossRef] [PubMed]
- Guillemin, F.; Rat, A.C.; Mazieres, B.; Pouchot, J.; Fautrel, B.; Euller-Ziegler, L.; Fardellone, P.; Morvan, J.; Roux, C.H.; Verrouil, E.; et al. Prevalence of Symptomatic Hip and Knee Osteoarthritis: A Two-Phase Population-Based Survey. Osteoarthr. Cartil. 2011, 19, 1314–1322. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Wu, X.; Tao, C.; Gong, W.; Chen, M.; Qu, M.; Zhong, Y.; He, T.; Chen, S.; Xiao, G. Osteoarthritis: Pathogenic Signaling Pathways and Therapeutic Targets. Signal Transduct. Target. Ther. 2023, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Leifer, V.P.; Katz, J.N.; Losina, E. The Burden of OA-Health Services and Economics. Osteoarthr. Cartil. 2022, 30, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-R.; Yoo, J.J.; Kim, H.A. Therapeutics in Osteoarthritis Based on an Understanding of Its Molecular Pathogenesis. Int. J. Mol. Sci. 2018, 19, 674. [Google Scholar] [CrossRef]
- Egloff, C.; Hügle, T.; Valderrabano, V. Biomechanics and Pathomechanisms of Osteoarthritis. Swiss Med. Wkly. 2012, 142, w13583. [Google Scholar] [CrossRef] [PubMed]
- Finnilä, M.A.J.; Das Gupta, S.; Turunen, M.J.; Hellberg, I.; Turkiewicz, A.; Lutz-Bueno, V.; Jonsson, E.; Holler, M.; Ali, N.; Hughes, V.; et al. Mineral Crystal Thickness in Calcified Cartilage and Subchondral Bone in Healthy and Osteoarthritic Human Knees. J. Bone Min. Res. 2022, 37, 1700–1710. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Liu, W.; Sun, C.; Wang, Q.; Yang, W.; Zhang, Z.; Xia, Z.; Shao, Z.; Wang, B. Endogenous Repair and Regeneration of Injured Articular Cartilage: A Challenging but Promising Therapeutic Strategy. Aging Dis. 2021, 12, 886–901. [Google Scholar] [CrossRef] [PubMed]
- Schuurman, W.; Klein, T.J.; Dhert, W.J.A.; van Weeren, P.R.; Hutmacher, D.W.; Malda, J. Cartilage Regeneration Using Zonal Chondrocyte Subpopulations: A Promising Approach or an Overcomplicated Strategy? J. Tissue Eng. Regen. Med. 2015, 9, 669–678. [Google Scholar] [CrossRef]
- Loeser, R.F.; Collins, J.A.; Diekman, B.O. Ageing and the Pathogenesis of Osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 412–420. [Google Scholar] [CrossRef]
- Yuan, X.L.; Meng, H.Y.; Wang, Y.C.; Peng, J.; Guo, Q.Y.; Wang, A.Y.; Lu, S.B. Bone-Cartilage Interface Crosstalk in Osteoarthritis: Potential Pathways and Future Therapeutic Strategies. Osteoarthr. Cartil. 2014, 22, 1077–1089. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Fernandez, P.; Rodríguez-Nogales, C.; Jordan, O.; Allémann, E. Combination of Mesenchymal Stem Cells and Bioactive Molecules in Hydrogels for Osteoarthritis Treatment. Eur. J. Pharm. Biopharm. 2022, 172, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shah, K.M.; Luo, J. Strategies for Articular Cartilage Repair and Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 770655. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Yuan, S.; Zeng, Y.; Wang, C.; Yu, N.; Ding, C. New Trends in Pharmacological Treatments for Osteoarthritis. Front. Pharmacol. 2021, 12, 645842. [Google Scholar] [CrossRef] [PubMed]
- Szwed-Georgiou, A.; Płociński, P.; Kupikowska-Stobba, B.; Urbaniak, M.M.; Rusek-Wala, P.; Szustakiewicz, K.; Piszko, P.; Krupa, A.; Biernat, M.; Gazińska, M.; et al. Bioactive Materials for Bone Regeneration: Biomolecules and Delivery Systems. ACS Biomater. Sci. Eng. 2023, 9, 5222–5254. [Google Scholar] [CrossRef] [PubMed]
- Richard, M.J.; Driban, J.B.; McAlindon, T.E. Pharmaceutical Treatment of Osteoarthritis. Osteoarthr. Cartil. 2023, 31, 458–466. [Google Scholar] [CrossRef] [PubMed]
- DeRogatis, M.; Anis, H.K.; Sodhi, N.; Ehiorobo, J.O.; Chughtai, M.; Bhave, A.; Mont, M.A. Non-Operative Treatment Options for Knee Osteoarthritis. Ann. Transl. Med. 2019, 7, S245. [Google Scholar] [CrossRef] [PubMed]
- de l’Escalopier, N.; Anract, P.; Biau, D. Surgical Treatments for Osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Rannou, F.; Pelletier, J.-P.; Martel-Pelletier, J. Efficacy and Safety of Topical NSAIDs in the Management of Osteoarthritis: Evidence from Real-Life Setting Trials and Surveys. Semin. Arthritis Rheum. 2016, 45, S18–S21. [Google Scholar] [CrossRef]
- Hochberg, M.C.; Altman, R.D.; April, K.T.; Benkhalti, M.; Guyatt, G.; McGowan, J.; Towheed, T.; Welch, V.; Wells, G.; Tugwell, P. American College of Rheumatology 2012 Recommendations for the Use of Nonpharmacologic and Pharmacologic Therapies in Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res. 2012, 64, 465–474. [Google Scholar] [CrossRef]
- Savvidou, O.; Milonaki, M.; Goumenos, S.; Flevas, D.; Papagelopoulos, P.; Moutsatsou, P. Glucocorticoid Signaling and Osteoarthritis. Mol. Cell Endocrinol. 2019, 480, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Nees, T.A.; Rosshirt, N.; Zhang, J.A.; Reiner, T.; Sorbi, R.; Tripel, E.; Walker, T.; Schiltenwolf, M.; Hagmann, S.; Moradi, B. Synovial Cytokines Significantly Correlate with Osteoarthritis-Related Knee Pain and Disability: Inflammatory Mediators of Potential Clinical Relevance. J. Clin. Med. 2019, 8, 1343. [Google Scholar] [CrossRef] [PubMed]
- Messier, S.P.; Resnik, A.E.; Beavers, D.P.; Mihalko, S.L.; Miller, G.D.; Nicklas, B.J.; deVita, P.; Hunter, D.J.; Lyles, M.F.; Eckstein, F.; et al. Intentional Weight Loss in Overweight and Obese Patients with Knee Osteoarthritis: Is More Better? Arthritis Care Res. 2018, 70, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Vincent, K.R.; Vasilopoulos, T.; Montero, C.; Vincent, H.K. Eccentric and Concentric Resistance Exercise Comparison for Knee Osteoarthritis. Med. Sci. Sports Exerc. 2019, 51, 1977–1986. [Google Scholar] [CrossRef] [PubMed]
- Franco, M.R.; Morelhão, P.K.; de Carvalho, A.; Pinto, R.Z. Aquatic Exercise for the Treatment of Hip and Knee Osteoarthritis. Phys. Ther. 2017, 97, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.; Lefèvre-Colau, M.-M.; Poiraudeau, S.; Rannou, F. Rehabilitation (Exercise and Strength Training) and Osteoarthritis: A Critical Narrative Review. Ann. Phys. Rehabil. Med. 2016, 59, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.M.; Cisneros, L.; Dias, R.; Fritsch, C.; Gomes, W.; Pereira, L.; Santos, M.L.; Ferreira, P.H. Hydrotherapy Improves Pain and Function in Older Women with Knee Osteoarthritis: A Randomized Controlled Trial. Braz. J. Phys. Ther. 2017, 21, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Dantas, L.O.; Osani, M.C.; Bannuru, R.R. Therapeutic Ultrasound for Knee Osteoarthritis: A Systematic Review and Meta-Analysis with Grade Quality Assessment. Braz. J. Phys. Ther. 2021, 25, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Chen, J.; Ma, J.; Shen, B.; Pei, F.; Kraus, V.B. Effectiveness of Low-Level Laser Therapy in Patients with Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Osteoarthr. Cartil. 2015, 23, 1437–1444. [Google Scholar] [CrossRef]
- Flynn, D.M. Chronic Musculoskeletal Pain: Nonpharmacologic, Noninvasive Treatments. Am. Fam. Phys. 2020, 102, 465–477. [Google Scholar]
- Lo, C.W.T.; Tsang, W.W.N.; Yan, C.H.; Lord, S.R.; Hill, K.D.; Wong, A.Y.L. Risk Factors for Falls in Patients with Total Hip Arthroplasty and Total Knee Arthroplasty: A Systematic Review and Meta-Analysis. Osteoarthr. Cartil. 2019, 27, 979–993. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.N.; Brownlee, S.A.; Jones, M.H. The Role of Arthroscopy in the Management of Knee Osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2014, 28, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Xie, N.; Li, W.; Yuan, B.; Shi, Y.; Wang, Y. Immunobiology of Mesenchymal Stem Cells. Cell Death Differ. 2014, 21, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, M.; Jing, X.; Guo, W.; Hao, C.; Zhang, Y.; Gao, S.; Chen, M.; Zhang, Z.; Zhang, X.; et al. Bone Marrow- and Adipose Tissue-Derived Mesenchymal Stem Cells: Characterization, Differentiation, and Applications in Cartilage Tissue Engineering. Crit. Rev. Eukaryot. Gene Expr. 2018, 28, 285–310. [Google Scholar] [CrossRef] [PubMed]
- Aprile, D.; Patrone, D.; Peluso, G.; Galderisi, U. Multipotent/Pluripotent Stem Cell Populations in Stromal Tissues and Peripheral Blood: Exploring Diversity, Potential, and Therapeutic Applications. Stem Cell Res. Ther. 2024, 15, 139. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Sun, S.; Fu, W.; Yang, Z.; Yao, H.; Zhang, Z. The Role and Prospects of Mesenchymal Stem Cells in Skin Repair and Regeneration. Biomedicines 2024, 12, 743. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yang, J.; Fang, J.; Zhou, Y.; Candi, E.; Wang, J.; Hua, D.; Shao, C.; Shi, Y. The Secretion Profile of Mesenchymal Stem Cells and Potential Applications in Treating Human Diseases. Signal Transduct. Target. Ther. 2022, 7, 92. [Google Scholar] [CrossRef] [PubMed]
- Widera, D. Recent Advances in Translational Adipose-Derived Stem Cell Biology. Biomolecules 2021, 11, 1660. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.; Liu, D.D.; Thakor, A.S. Mesenchymal Stromal Cell Homing: Mechanisms and Strategies for Improvement. iScience 2019, 15, 421–438. [Google Scholar] [CrossRef]
- Gnecchi, M.; Melo, L.G. Bone Marrow-Derived Mesenchymal Stem Cells: Isolation, Expansion, Characterization, Viral Transduction, and Production of Conditioned Medium. Methods Mol. Biol. 2009, 482, 281–294. [Google Scholar] [CrossRef]
- Abu-El-Rub, E.; Khaswaneh, R.R.; Almahasneh, F.A.; Almazari, R.; Alzu’bi, A. Adipose Tissue and Bone Marrow-Derived Mesenchymal Stem Cells Are Not Really the Same: Investigating the Differences in Their Immunomodulatory, Migratory, and Adhesive Profile. Biochem. Genet. 2024; Online ahead of print. [Google Scholar] [CrossRef]
- Aldrich, E.D.; Cui, X.; Murphy, C.A.; Lim, K.S.; Hooper, G.J.; McIlwraith, C.W.; Woodfield, T.B.F. Allogeneic Mesenchymal Stromal Cells for Cartilage Regeneration: A Review of in Vitro Evaluation, Clinical Experience, and Translational Opportunities. Stem Cells Transl. Med. 2021, 10, 1500–1515. [Google Scholar] [CrossRef] [PubMed]
- Adipose-Derived Stem Cells in Immune-Related Skin Disease: A Review of Current Research and Underlying Mechanisms. Available online: https://pubmed.ncbi.nlm.nih.gov/38331803/ (accessed on 27 May 2024).
- Tan, L.; Liu, X.; Dou, H.; Hou, Y. Characteristics and Regulation of Mesenchymal Stem Cell Plasticity by the Microenvironment—Specific Factors Involved in the Regulation of MSC Plasticity. Genes Dis. 2022, 9, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Rosa, I.; Romano, E.; Fioretto, B.S.; Matucci-Cerinic, M.; Manetti, M. Adipose-Derived Stem Cells: Pathophysiologic Implications vs Therapeutic Potential in Systemic Sclerosis. World J. Stem Cells 2021, 13, 30–48. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, V.V.; Patel, N.H.; Smith, E.E.; Barnes, C.L.; Gustafson, M.P.; Rao, R.R.; Samsonraj, R.M. Clinical Utility of Mesenchymal Stem/Stromal Cells in Regenerative Medicine and Cellular Therapy. J. Biol. Eng. 2023, 17, 44. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Xiao, H.; Shi, Q. Mesenchymal Stem Cells Derived from the Fibrotic Tissue of Atrophic Nonunion or the Bone Marrow of Iliac Crest: A Donor-Matched Comparison. Regen. Ther. 2023, 24, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Granero-Moltó, F.; Weis, J.A.; Miga, M.I.; Landis, B.; Myers, T.J.; O’Rear, L.; Longobardi, L.; Jansen, E.D.; Mortlock, D.P.; Spagnoli, A. Regenerative Effects of Transplanted Mesenchymal Stem Cells in Fracture Healing. Stem Cells 2009, 27, 1887–1898. [Google Scholar] [CrossRef] [PubMed]
- Cofano, F.; Boido, M.; Monticelli, M.; Zenga, F.; Ducati, A.; Vercelli, A.; Garbossa, D. Mesenchymal Stem Cells for Spinal Cord Injury: Current Options, Limitations, and Future of Cell Therapy. Int. J. Mol. Sci. 2019, 20, 2698. [Google Scholar] [CrossRef]
- Yousefifard, M.; Nasirinezhad, F.; Shardi Manaheji, H.; Janzadeh, A.; Hosseini, M.; Keshavarz, M. Human Bone Marrow-Derived and Umbilical Cord-Derived Mesenchymal Stem Cells for Alleviating Neuropathic Pain in a Spinal Cord Injury Model. Stem Cell Res. Ther. 2016, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.R.; Kim, H.Y.; Park, J.-Y.; Lee, T.Y.; Baik, C.S.; Chai, Y.G.; Jung, K.H.; Park, K.S.; Roh, W.; Kim, K.S.; et al. Selection of Optimal Passage of Bone Marrow-Derived Mesenchymal Stem Cells for Stem Cell Therapy in Patients with Amyotrophic Lateral Sclerosis. Neurosci. Lett. 2010, 472, 94–98. [Google Scholar] [CrossRef]
- Moonshi, S.S.; Adelnia, H.; Wu, Y.; Ta, H.T. Placenta-Derived Mesenchymal Stem Cells for Treatment of Diseases: A Clinically Relevant Source. Adv. Ther. 2022, 5, 2200054. [Google Scholar] [CrossRef]
- Rhim, J.; Ha, C.-W.; Park, Y.-B.; Kim, J.-A.; Han, W.-J.; Choi, S.; Lee, K.; Park, H.; Park, H.-J. Cartilage Repair by Various Concentrations of Placenta-Derived Mesenchymal Stem Cells and Hyaluronic Acid Hydrogels in a Rabbit Model. Osteoarthr. Cartil. 2017, 25, S160. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhong, Y.; Zou, L.; Liu, X. Significance of Placental Mesenchymal Stem Cell in Placenta Development and Implications for Preeclampsia. Front. Pharmacol. 2022, 13, 896531. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, R.; Zou, Q.; Chen, Y.; Zhou, M.; Li, X.; Ran, R.; Chen, Q. Comparison of the Biological Characteristics of Mesenchymal Stem Cells Derived from the Human Placenta and Umbilical Cord. Sci. Rep. 2018, 8, 5014. [Google Scholar] [CrossRef] [PubMed]
- James, J.L.; Umapathy, A.; Srinivasan, S.; Barker, C.N.; Brooks, A.; Hearn, J.; Chhana, A.; Williams, E.; Sheppard, H.; McGlashan, S.R. The Chondrogenic Potential of First-Trimester and Term Placental Mesenchymal Stem/Stromal Cells. Cartilage 2021, 13, 544S–558S. [Google Scholar] [CrossRef]
- Vellasamy, S.; Sandrasaigaran, P.; Vidyadaran, S.; George, E.; Ramasamy, R. Isolation and Characterisation of Mesenchymal Stem Cells Derived from Human Placenta Tissue. World J. Stem Cells 2012, 4, 53–61. [Google Scholar] [CrossRef]
- Ahani-Nahayati, M.; Niazi, V.; Moradi, A.; Pourjabbar, B.; Roozafzoon, R.; Keshel, S.H.; Baradaran-Rafii, A. Umbilical Cord Mesenchymal Stem/Stromal Cells Potential to Treat Organ Disorders; An Emerging Strategy. Curr. Stem Cell Res. Ther. 2022, 17, 126–146. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, H.; Zheng, Z.; Liu, Q.; Xu, L. Umbilical Cord-Derived Mesenchymal Stem Cell Therapy for Neurological Disorders via Inhibition of Mitogen-Activated Protein Kinase Pathway-Mediated Apoptosis. Mol. Med. Rep. 2015, 11, 1807–1812. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, Y.; Shen, H.; Ding, Y.; Yu, Y.; Shao, L.; Shen, Z. The Application of Umbilical Cord-Derived MSCs in Cardiovascular Diseases. J. Cell Mol. Med. 2021, 25, 8103–8114. [Google Scholar] [CrossRef] [PubMed]
- Abbaspanah, B.; Reyhani, S.; Mousavi, S.H. Applications of Umbilical Cord Derived Mesenchymal Stem Cells in Autoimmune and Immunological Disorders: From Literature to Clinical Practice. Curr. Stem Cell Res. Ther. 2021, 16, 454–464. [Google Scholar] [CrossRef]
- Gadelkarim, M.; Abushouk, A.I.; Ghanem, E.; Hamaad, A.M.; Saad, A.M.; Abdel-Daim, M.M. Adipose-Derived Stem Cells: Effectiveness and Advances in Delivery in Diabetic Wound Healing. Biomed. Pharmacother. 2018, 107, 625–633. [Google Scholar] [CrossRef]
- Sheng, L.; Yang, M.; Liang, Y.; Li, Q. Adipose Tissue-Derived Stem Cells (ADSCs) Transplantation Promotes Regeneration of Expanded Skin Using a Tissue Expansion Model. Wound Repair. Regen. 2013, 21, 746–754. [Google Scholar] [CrossRef]
- Cherubino, M.; Marra, K.G. Adipose-Derived Stem Cells for Soft Tissue Reconstruction. Regen. Med. 2009, 4, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Song, Y.; Zhang, B.; Cao, G.; Zhou, H.; Li, H.; Sun, H.; Deng, M.; Qiu, Y.; Yi, W.; et al. Progress and Application of Adipose-Derived Stem Cells in the Treatment of Diabetes and Its Complications. Stem Cell Res. Ther. 2024, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraman, M.; Muthu, S.; Jeyaraman, N.; Ranjan, R.; Jha, S.K.; Mishra, P. Synovium Derived Mesenchymal Stromal Cells (Sy-MSCs): A Promising Therapeutic Paradigm in the Management of Knee Osteoarthritis. Indian J. Orthop. 2022, 56, 1–15. [Google Scholar] [CrossRef]
- Yields and Chondrogenic Potential of Primary Synovial Mesenchymal Stem Cells Are Comparable between Rheumatoid Arthritis and Osteoarthritis Patients. Available online: https://pubmed.ncbi.nlm.nih.gov/28511664/ (accessed on 28 May 2024).
- Li, N.; Gao, J.; Mi, L.; Zhang, G.; Zhang, L.; Zhang, N.; Huo, R.; Hu, J.; Xu, K. Synovial Membrane Mesenchymal Stem Cells: Past Life, Current Situation, and Application in Bone and Joint Diseases. Stem Cell Res. Ther. 2020, 11, 381. [Google Scholar] [CrossRef]
- Jorgenson, K.D.; Hart, D.A.; Krawetz, R.; Sen, A. Production of Adult Human Synovial Fluid-Derived Mesenchymal Stem Cells in Stirred-Suspension Culture. Stem Cells Int. 2018, 2018, 8431053. [Google Scholar] [CrossRef]
- Insensitive Effects of Inflammatory Cytokines on the Reference Genes of Synovial Fluid Resident-Mesenchymal Stem Cells Derived from Rheumatoid Arthritis Patients. Available online: https://pubmed.ncbi.nlm.nih.gov/37894839/ (accessed on 28 May 2024).
- Meng, J.; Adkin, C.F.; Arechavala-Gomeza, V.; Boldrin, L.; Muntoni, F.; Morgan, J.E. The Contribution of Human Synovial Stem Cells to Skeletal Muscle Regeneration. Neuromuscul. Disord. 2010, 20, 6–15. [Google Scholar] [CrossRef]
- Botelho, J.; Cavacas, M.A.; Machado, V.; Mendes, J.J. Dental Stem Cells: Recent Progresses in Tissue Engineering and Regenerative Medicine. Ann. Med. 2017, 49, 644–651. [Google Scholar] [CrossRef]
- Chalisserry, E.P.; Nam, S.Y.; Park, S.H.; Anil, S. Therapeutic Potential of Dental Stem Cells. J. Tissue Eng. 2017, 8, 2041731417702531. [Google Scholar] [CrossRef] [PubMed]
- Potdar, P.D.; Jethmalani, Y.D. Human Dental Pulp Stem Cells: Applications in Future Regenerative Medicine. World J. Stem Cells 2015, 7, 839–851. [Google Scholar] [CrossRef]
- Al-Maswary, A.A.; O’Reilly, M.; Holmes, A.P.; Walmsley, A.D.; Cooper, P.R.; Scheven, B.A. Exploring the Neurogenic Differentiation of Human Dental Pulp Stem Cells. PLoS ONE 2022, 17, e0277134. [Google Scholar] [CrossRef]
- Song, B.; Jiang, W.; Alraies, A.; Liu, Q.; Gudla, V.; Oni, J.; Wei, X.; Sloan, A.; Ni, L.; Agarwal, M. Bladder Smooth Muscle Cells Differentiation from Dental Pulp Stem Cells: Future Potential for Bladder Tissue Engineering. Stem Cells Int. 2016, 2016, 6979368. [Google Scholar] [CrossRef]
- Corradetti, B.; Meucci, A.; Bizzaro, D.; Cremonesi, F.; Lange Consiglio, A. Mesenchymal Stem Cells from Amnion and Amniotic Fluid in the Bovine. Reproduction 2013, 145, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Lee, K.-B.; Kim, M.K. The Potential of Mesenchymal Stem Cells Derived from Amniotic Membrane and Amniotic Fluid for Neuronal Regenerative Therapy. BMB Rep. 2014, 47, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.-W.; Ying, Y.-M.; Zhou, J.-X.; Zhang, W.-J.; Liu, Z.; Jia, B.-B.; Gu, H.-C.; Zhao, C.-Y.; Guan, X.-H.; Deng, K.-Y.; et al. Human Amniotic Mesenchymal Stem Cells-Derived IGFBP-3, DKK-3, and DKK-1 Attenuate Liver Fibrosis through Inhibiting Hepatic Stellate Cell Activation by Blocking Wnt/β-Catenin Signaling Pathway in Mice. Stem Cell Res. Ther. 2022, 13, 224. [Google Scholar] [CrossRef]
- Navas, A.; Magaña-Guerrero, F.S.; Domínguez-López, A.; Chávez-García, C.; Partido, G.; Graue-Hernández, E.O.; Sánchez-García, F.J.; Garfias, Y. Anti-Inflammatory and Anti-Fibrotic Effects of Human Amniotic Membrane Mesenchymal Stem Cells and Their Potential in Corneal Repair. Stem Cells Transl. Med. 2018, 7, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Amniotic Membrane Mesenchymal Cells-Derived Factors Skew T Cell Polarization toward Treg and Downregulate Th1 and Th17 Cells Subsets. Available online: https://pubmed.ncbi.nlm.nih.gov/25348066/ (accessed on 28 May 2024).
- Nombela-Arrieta, C.; Ritz, J.; Silberstein, L.E. The Elusive Nature and Function of Mesenchymal Stem Cells. Nat. Rev. Mol. Cell Biol. 2011, 12, 126–131. [Google Scholar] [CrossRef]
- Maged, G.; Abdelsamed, M.A.; Wang, H.; Lotfy, A. The Potency of Mesenchymal Stem/Stromal Cells: Does Donor Sex Matter? Stem Cell Res. Ther. 2024, 15, 112. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.-C.; Shyu, W.-C.; Lin, S.-Z. Mesenchymal Stem Cells. Cell Transpl. 2011, 20, 5–14. [Google Scholar] [CrossRef]
- Samsonraj, R.M.; Raghunath, M.; Nurcombe, V.; Hui, J.H.; van Wijnen, A.J.; Cool, S.M. Concise Review: Multifaceted Characterization of Human Mesenchymal Stem Cells for Use in Regenerative Medicine. Stem Cells Transl. Med. 2017, 6, 2173–2185. [Google Scholar] [CrossRef]
- Koç, O.N.; Gerson, S.L.; Cooper, B.W.; Dyhouse, S.M.; Haynesworth, S.E.; Caplan, A.I.; Lazarus, H.M. Rapid Hematopoietic Recovery after Coinfusion of Autologous-Blood Stem Cells and Culture-Expanded Marrow Mesenchymal Stem Cells in Advanced Breast Cancer Patients Receiving High-Dose Chemotherapy. J. Clin. Oncol. 2000, 18, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Rojas, M.; Xu, J.; Woods, C.R.; Mora, A.L.; Spears, W.; Roman, J.; Brigham, K.L. Bone Marrow-Derived Mesenchymal Stem Cells in Repair of the Injured Lung. Am. J. Respir. Cell Mol. Biol. 2005, 33, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Kim, J.-H.; Yim, C.; Lee, M.; Kang, H.J.; Choi, D. Therapeutic Effects of a Mesenchymal Stem Cell-based Insulin-like Growth Factor-1/Enhanced Green Fluorescent Protein Dual Gene Sorting System in a Myocardial Infarction Rat Model. Mol. Med. Rep. 2018, 18, 5563–5571. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Wu, Z.; Li, L. Mesenchymal Stromal Cells Promote Liver Regeneration through Regulation of Immune Cells. Int. J. Biol. Sci. 2020, 16, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Peired, A.J.; Sisti, A.; Romagnani, P. Mesenchymal Stem Cell-Based Therapy for Kidney Disease: A Review of Clinical Evidence. Stem Cells Int. 2016, 2016, 4798639. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.M.; Kalamegam, G.; Pushparaj, P.N.; Matta, C.; Memic, A.; Khademhosseini, A.; Mobasheri, R.; Poletti, F.L.; Hoyland, J.A.; Mobasheri, A. Mesenchymal Stem Cells in Regenerative Medicine: Focus on Articular Cartilage and Intervertebral Disc Regeneration. Methods 2016, 99, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Song, X.; Li, T.; Xiao, J.; Chen, Y.; Gong, X.; Zeng, W.; Yang, L.; Chen, C. Pellet Coculture of Osteoarthritic Chondrocytes and Infrapatellar Fat Pad-Derived Mesenchymal Stem Cells with Chitosan/Hyaluronic Acid Nanoparticles Promotes Chondrogenic Differentiation. Stem Cell Res. Ther. 2017, 8, 264. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Criado, I.; Meseguer-Ripolles, J.; Mellado-López, M.; Alastrue-Agudo, A.; Griffeth, R.J.; Forteza-Vila, J.; Cugat, R.; García, M.; Moreno-Manzano, V. Human Suprapatellar Fat Pad-Derived Mesenchymal Stem Cells Induce Chondrogenesis and Cartilage Repair in a Model of Severe Osteoarthritis. Stem Cells Int. 2017, 2017, 4758930. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, Y.; Wang, Q.; Fang, C.; Sun, Y.; Yuan, T.; Wang, Y.; Bao, R.; Zhao, N. Effect of Bone Marrow-Derived Stem Cells on Chondrocytes from Patients with Osteoarthritis. Mol. Med. Rep. 2016, 13, 1795–1800. [Google Scholar] [CrossRef]
- Li, K.; Yan, G.; Huang, H.; Zheng, M.; Ma, K.; Cui, X.; Lu, D.; Zheng, L.; Zhu, B.; Cheng, J.; et al. Anti-Inflammatory and Immunomodulatory Effects of the Extracellular Vesicles Derived from Human Umbilical Cord Mesenchymal Stem Cells on Osteoarthritis via M2 Macrophages. J. Nanobiotechnol. 2022, 20, 38. [Google Scholar] [CrossRef]
- Vahedi, P.; Roshangar, L.; Jarolmasjed, S.; Shafaei, H.; Samadi, N.; Soleimanirad, J. Effect of Low-Intensity Pulsed Ultrasound on Regenerative Potential of Transplanted ASCs €“PCL Construct in Articular Cartilage Defects in Sheep. Indian J. Anim. Sci. 2016, 86, 111–1114. [Google Scholar] [CrossRef]
- Lopa, S.; Mondadori, C.; Mainardi, V.L.; Talò, G.; Costantini, M.; Candrian, C.; Święszkowski, W.; Moretti, M. Translational Application of Microfluidics and Bioprinting for Stem Cell-Based Cartilage Repair. Stem Cells Int. 2018, 2018, 6594841. [Google Scholar] [CrossRef] [PubMed]
- Fink, T.; Rasmussen, J.G.; Emmersen, J.; Pilgaard, L.; Fahlman, Å.; Brunberg, S.; Josefsson, J.; Arnemo, J.M.; Zachar, V.; Swenson, J.E.; et al. Adipose-Derived Stem Cells from the Brown Bear (Ursus Arctos) Spontaneously Undergo Chondrogenic and Osteogenic Differentiation in Vitro. Stem Cell Res. 2011, 7, 89–95. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Derfoul, A.; Perkins, G.L.; Hall, D.J.; Tuan, R.S. Glucocorticoids Promote Chondrogenic Differentiation of Adult Human Mesenchymal Stem Cells by Enhancing Expression of Cartilage Extracellular Matrix Genes. Stem Cells 2006, 24, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Xu, M.; Zhu, H.; Dong, C.; Ji, J.; Liu, Y.; Deng, A.; Gu, Z. Therapeutic Effects of Bone Marrow Mesenchymal Stem Cells-Derived Exosomes on Osteoarthritis. J. Cell Mol. Med. 2021, 25, 9281–9294. [Google Scholar] [CrossRef]
- Molnar, V.; Pavelić, E.; Vrdoljak, K.; Čemerin, M.; Klarić, E.; Matišić, V.; Bjelica, R.; Brlek, P.; Kovačić, I.; Tremolada, C.; et al. Mesenchymal Stem Cell Mechanisms of Action and Clinical Effects in Osteoarthritis: A Narrative Review. Genes 2022, 13, 949. [Google Scholar] [CrossRef]
- Wei, Z.-J.; Wang, Q.-Q.; Cui, Z.-G.; Inadera, H.; Jiang, X.; Wu, C.-A. Which Is the Most Effective One in Knee Osteoarthritis Treatment from Mesenchymal Stem Cells Obtained from Different Sources?—A Systematic Review with Conventional and Network Meta-Analyses of Randomized Controlled Trials. Ann. Transl. Med. 2021, 9, 452. [Google Scholar] [CrossRef] [PubMed]
- Jo, C.H.; Lee, Y.G.; Shin, W.H.; Kim, H.; Chai, J.W.; Jeong, E.C.; Kim, J.E.; Shim, H.; Shin, J.S.; Shin, I.S.; et al. Intra-Articular Injection of Mesenchymal Stem Cells for the Treatment of Osteoarthritis of the Knee: A Proof-of-Concept Clinical Trial. Stem Cells 2014, 32, 1254–1266. [Google Scholar] [CrossRef] [PubMed]
- Spasovski, D.; Spasovski, V.; Baščarević, Z.; Stojiljković, M.; Vreća, M.; Anđelković, M.; Pavlović, S. Intra-Articular Injection of Autologous Adipose-Derived Mesenchymal Stem Cells in the Treatment of Knee Osteoarthritis. J. Gene Med. 2018, 20, e3002. [Google Scholar] [CrossRef]
- Zhu, C.; Wu, W.; Qu, X. Mesenchymal Stem Cells in Osteoarthritis Therapy: A Review. Am. J. Transl. Res. 2021, 13, 448–461. [Google Scholar]
- Cheng, J.-H.; Hsu, C.-C.; Hsu, S.-L.; Chou, W.-Y.; Wu, Y.-N.; Kuo, C.-E.A.; Hsu, T.-C.; Shiu, L.-Y.; Jhan, S.-W. Adipose-Derived Mesenchymal Stem Cells-Conditioned Medium Modulates the Expression of Inflammation Induced Bone Morphogenetic Protein-2, -5 and -6 as Well as Compared with Shockwave Therapy on Rat Knee Osteoarthritis. Biomedicines 2021, 9, 1399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Meng, F.; Zhang, Q.; Zou, Z.; Xiao, K.; Zhu, T.; Li, H.; Zhang, W.; Ma, J.; Ma, J. Allogeneic Adipose-Derived Mesenchymal Stem Cells Promote the Expression of Chondrocyte Redifferentiation Markers and Retard the Progression of Knee Osteoarthritis in Rabbits. Am. J. Transl. Res. 2021, 13, 632–645. [Google Scholar] [PubMed]
- Song, Y.; Du, H.; Dai, C.; Zhang, L.; Li, S.; Hunter, D.J.; Lu, L.; Bao, C. Human Adipose-Derived Mesenchymal Stem Cells for Osteoarthritis: A Pilot Study with Long-Term Follow-up and Repeated Injections. Regen. Med. 2018, 13, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Kouroupis, D.; Willman, M.A.; Best, T.M.; Kaplan, L.D.; Correa, D. Infrapatellar Fat Pad-Derived Mesenchymal Stem Cell-Based Spheroids Enhance Their Therapeutic Efficacy to Reverse Synovitis and Fat Pad Fibrosis. Stem Cell Res. Ther. 2021, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Platas, J.; Guillén, M.I.; del Caz, M.D.P.; Gomar, F.; Mirabet, V.; Alcaraz, M.J. Conditioned Media from Adipose-Tissue-Derived Mesenchymal Stem Cells Downregulate Degradative Mediators Induced by Interleukin-1β in Osteoarthritic Chondrocytes. Mediat. Inflamm. 2013, 2013, 357014. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, R.; Ishida, K.; Matsumoto, T.; Akisue, T.; Fujioka, H.; Mizuno, K.; Ohgushi, H.; Wakitani, S.; Kurosaka, M. Treatment of a Full-Thickness Articular Cartilage Defect in the Femoral Condyle of an Athlete with Autologous Bone-Marrow Stromal Cells. Osteoarthr. Cartil. 2007, 15, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Orozco, L.; Munar, A.; Soler, R.; Alberca, M.; Soler, F.; Huguet, M.; Sentís, J.; Sánchez, A.; García-Sancho, J. Treatment of Knee Osteoarthritis with Autologous Mesenchymal Stem Cells: Two-Year Follow-up Results. Transplantation 2014, 97, e66–e68. [Google Scholar] [CrossRef] [PubMed]
- Chahal, J.; Gómez-Aristizábal, A.; Shestopaloff, K.; Bhatt, S.; Chaboureau, A.; Fazio, A.; Chisholm, J.; Weston, A.; Chiovitti, J.; Keating, A.; et al. Bone Marrow Mesenchymal Stromal Cell Treatment in Patients with Osteoarthritis Results in Overall Improvement in Pain and Symptoms and Reduces Synovial Inflammation. Stem Cells Transl. Med. 2019, 8, 746–757. [Google Scholar] [CrossRef] [PubMed]
- Carlo, D.; Stefano, B.; Nicholas, E.; Roberto, V.; Elena, M.S.; Bruno, M. Mesenchymal Stem Cells Injection in Hip Osteoarthritis: Preliminary Results. Acta Biomed. 2019, 90, 75–80. [Google Scholar] [CrossRef]
- Denoeud, C.; Luo, G.; Paquet, J.; Boisselier, J.; Wosinski, P.; Moya, A.; Diallo, A.; Larochette, N.; Marinesco, S.; Meiller, A.; et al. Enzyme-Controlled, Nutritive Hydrogel for Mesenchymal Stromal Cell Survival and Paracrine Functions. Commun. Biol. 2023, 6, 1266. [Google Scholar] [CrossRef]
- Amer, M.H.; Rose, F.R.A.J.; Shakesheff, K.M.; Modo, M.; White, L.J. Translational Considerations in Injectable Cell-Based Therapeutics for Neurological Applications: Concepts, Progress and Challenges. NPJ Regen. Med. 2017, 2, 23. [Google Scholar] [CrossRef] [PubMed]
- Levy, O.; Kuai, R.; Siren, E.M.J.; Bhere, D.; Milton, Y.; Nissar, N.; De Biasio, M.; Heinelt, M.; Reeve, B.; Abdi, R.; et al. Shattering Barriers toward Clinically Meaningful MSC Therapies. Sci. Adv. 2020, 6, eaba6884. [Google Scholar] [CrossRef] [PubMed]
- Music, E.; Futrega, K.; Doran, M.R. Sheep as a Model for Evaluating Mesenchymal Stem/Stromal Cell (MSC)-Based Chondral Defect Repair. Osteoarthr. Cartil. 2018, 26, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Sabapathy, V.; Kumar, S. hiPSC-Derived iMSCs: NextGen MSCs as an Advanced Therapeutically Active Cell Resource for Regenerative Medicine. J. Cell Mol. Med. 2016, 20, 1571–1588. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Salman, S.; Khan, S.A.; Amin, A.; Rahman, Z.U.; Al-Ghamdi, Y.O.; Akhtar, K.; Bakhsh, E.M.; Khan, S.B. Versatility of Hydrogels: From Synthetic Strategies, Classification, and Properties to Biomedical Applications. Gels 2022, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.-C.; Chang, C.-C.; Chan, H.-P.; Chung, T.-W.; Shu, C.-W.; Chuang, K.-P.; Duh, T.-H.; Yang, M.-H.; Tyan, Y.-C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, B.; Jayakrishnan, A. Self-Cross-Linking Biopolymers as Injectable in Situ Forming Biodegradable Scaffolds. Biomaterials 2005, 26, 3941–3951. [Google Scholar] [CrossRef] [PubMed]
- Mahinroosta, M.; Jomeh Farsangi, Z.; Allahverdi, A.; Shakoori, Z. Hydrogels as Intelligent Materials: A Brief Review of Synthesis, Properties and Applications. Mater. Today Chem. 2018, 8, 42–55. [Google Scholar] [CrossRef]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of Natural Hydrogels for Regenerative Medicine Applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef]
- Vijayavenkataraman, S.; Vialli, N.; Fuh, J.Y.H.; Lu, W.F. Conductive Collagen/Polypyrrole-b-Polycaprolactone Hydrogel for Bioprinting of Neural Tissue Constructs. Int. J. Bioprint 2019, 5, 229. [Google Scholar] [CrossRef] [PubMed]
- Bonelli, N.; Poggi, G.; Chelazzi, D.; Giorgi, R.; Baglioni, P. Poly(Vinyl Alcohol)/Poly(Vinyl Pyrrolidone) Hydrogels for the Cleaning of Art. J. Colloid. Interface Sci. 2019, 536, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Saffer, E.M.; Tew, G.N.; Bhatia, S.R. Poly(Lactic Acid)-Poly(Ethylene Oxide) Block Copolymers: New Directions in Self-Assembly and Biomedical Applications. Curr. Med. Chem. 2011, 18, 5676–5686. [Google Scholar] [CrossRef] [PubMed]
- Atta, S.; Khaliq, S.; Islam, A.; Javeria, I.; Jamil, T.; Athar, M.M.; Shafiq, M.I.; Ghaffar, A. Injectable Biopolymer Based Hydrogels for Drug Delivery Applications. Int. J. Biol. Macromol. 2015, 80, 240–245. [Google Scholar] [CrossRef]
- Rodríguez Rodríguez, R.; Garcia, Z.; Jimenez-Palomar, I.; Avalos, J.; Espinosa-Andrews, H. Development of Gelatin/Chitosan/PVA Hydrogels: Thermal Stability, Water State, Viscoelasticity, and Cytotoxicity Assays. J. Appl. Polym. Sci. 2019, 136, 47149. [Google Scholar] [CrossRef]
- Nagase, K.; Yamato, M.; Kanazawa, H.; Okano, T. Poly(N-Isopropylacrylamide)-Based Thermoresponsive Surfaces Provide New Types of Biomedical Applications. Biomaterials 2018, 153, 27–48. [Google Scholar] [CrossRef] [PubMed]
- McMasters, J.; Poh, S.; Lin, J.B.; Panitch, A. Delivery of Anti-Inflammatory Peptides from Hollow PEGylated Poly(NIPAM) Nanoparticles Reduces Inflammation in an Ex Vivo Osteoarthritis Model. J. Control Release 2017, 258, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.-Y.; Lin, Y.-C.; Lu, H.-T.; Ho, Y.-C.; Weng, S.-C.; Tsai, M.-L.; Mi, F.-L. A Novel Injectable in Situ Forming Gel Based on Carboxymethyl Hexanoyl Chitosan/Hyaluronic Acid Polymer Blending for Sustained Release of Berberine. Carbohydr. Polym. 2019, 206, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Arunkumar, P.; Indulekha, S.; Vijayalakshmi, S.; Srivastava, R. Poly (Caprolactone) Microparticles and Chitosan Thermogels Based Injectable Formulation of Etoricoxib for the Potential Treatment of Osteoarthritis. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 534–544. [Google Scholar] [CrossRef]
- Agnihotri, S.A.; Aminabhavi, T.M. Novel Interpenetrating Network Chitosan-Poly(Ethylene Oxide-g-Acrylamide) Hydrogel Microspheres for the Controlled Release of Capecitabine. Int. J. Pharm. 2006, 324, 103–115. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Peng, J.; Xiao, H.; Xu, X.; Qian, Z. Polysaccharide Hydrogels: Functionalization, Construction and Served as Scaffold for Tissue Engineering. Carbohydr. Polym. 2022, 278, 118952. [Google Scholar] [CrossRef] [PubMed]
- Caló, E.; Khutoryanskiy, V.V. Biomedical Applications of Hydrogels: A Review of Patents and Commercial Products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Gan, X.; Wang, X.; Huang, Y.; Li, G.; Kang, H. Applications of Hydrogels in Osteoarthritis Treatment. Biomedicines 2024, 12, 923. [Google Scholar] [CrossRef] [PubMed]
- Waresindo, W.X.; Luthfianti, H.R.; Priyanto, A.; Hapidin, D.A.; Edikresnha, D.; Aimon, A.H.; Suciati, T.; Khairurrijal, K. Freeze–Thaw Hydrogel Fabrication Method: Basic Principles, Synthesis Parameters, Properties, and Biomedical Applications. Mater. Res. Express 2023, 10, 024003. [Google Scholar] [CrossRef]
- Wang, W.; Liu, W.; Wu, J.; Liu, M.; Wang, Y.; Liu, H.; Liu, J. Preparation and Characterization of Particle-Filled Microgels by Chemical Cross-Linking Based on Zein and Carboxymethyl Starch for Delivering the Quercetin. Carbohydr. Polym. 2024, 323, 121375. [Google Scholar] [CrossRef] [PubMed]
- Mironi-Harpaz, I.; Wang, D.Y.; Venkatraman, S.; Seliktar, D. Photopolymerization of Cell-Encapsulating Hydrogels: Crosslinking Efficiency versus Cytotoxicity. Acta Biomater. 2012, 8, 1838–1848. [Google Scholar] [CrossRef] [PubMed]
- Moreira Teixeira, L.S.; Feijen, J.; van Blitterswijk, C.A.; Dijkstra, P.J.; Karperien, M. Enzyme-Catalyzed Crosslinkable Hydrogels: Emerging Strategies for Tissue Engineering. Biomaterials 2012, 33, 1281–1290. [Google Scholar] [CrossRef]
- Li, Z.; Lu, F.; Liu, Y. A Review of the Mechanism, Properties, and Applications of Hydrogels Prepared by Enzymatic Cross-Linking. J. Agric. Food Chem. 2023, 71, 10238–10249. [Google Scholar] [CrossRef]
- Nicol, E. Photopolymerized Porous Hydrogels. Biomacromolecules 2021, 22, 1325–1345. [Google Scholar] [CrossRef]
- Oliva, N.; Conde, J.; Wang, K.; Artzi, N. Designing Hydrogels for On-Demand Therapy. Acc. Chem. Res. 2017, 50, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, C.F.; Ahmed, R.; Marques, A.P.; Reis, R.L.; Demirci, U. Engineering Hydrogel-Based Biomedical Photonics: Design, Fabrication, and Applications. Adv. Mater. 2021, 33, e2006582. [Google Scholar] [CrossRef] [PubMed]
- Dhand, A.P.; Galarraga, J.H.; Burdick, J.A. Enhancing Biopolymer Hydrogel Functionality through Interpenetrating Networks. Trends Biotechnol. 2021, 39, 519–538. [Google Scholar] [CrossRef]
- Shi, W.; Huang, J.; Fang, R.; Liu, M. Imparting Functionality to the Hydrogel by Magnetic-Field-Induced Nano-Assembly and Macro-Response. ACS Appl. Mater. Interfaces 2020, 12, 5177–5194. [Google Scholar] [CrossRef]
- Wang, Y.; Katyal, P.; Montclare, J.K. Protein-Engineered Functional Materials. Adv. Healthc. Mater. 2019, 8, e1801374. [Google Scholar] [CrossRef]
- Hacker, M.C.; Nawaz, H.A. Multi-Functional Macromers for Hydrogel Design in Biomedical Engineering and Regenerative Medicine. Int. J. Mol. Sci. 2015, 16, 27677–27706. [Google Scholar] [CrossRef] [PubMed]
- Correa, S.; Meany, E.L.; Gale, E.C.; Klich, J.H.; Saouaf, O.M.; Mayer, A.T.; Xiao, Z.; Liong, C.S.; Brown, R.A.; Maikawa, C.L.; et al. Injectable Nanoparticle-Based Hydrogels Enable the Safe and Effective Deployment of Immunostimulatory CD40 Agonist Antibodies. Adv. Sci. 2022, 9, e2103677. [Google Scholar] [CrossRef]
- Manferdini, C.; Gabusi, E.; Saleh, Y.; Lenzi, E.; D’Atri, G.; Ricotti, L.; Lisignoli, G. Mesenchymal Stromal Cells Laden in Hydrogels for Osteoarthritis Cartilage Regeneration: A Systematic Review from In Vitro Studies to Clinical Applications. Cells 2022, 11, 3969. [Google Scholar] [CrossRef]
- Lolli, A.; Sivasubramaniyan, K.; Vainieri, M.L.; Oieni, J.; Kops, N.; Yayon, A.; van Osch, G.J.V.M. Hydrogel-Based Delivery of antimiR-221 Enhances Cartilage Regeneration by Endogenous Cells. J. Control Release 2019, 309, 220–230. [Google Scholar] [CrossRef]
- Li, H.; Feng, F.; Bingham, C.O., 3rd; Elisseeff, J.H. Matrix Metalloproteinases and Inhibitors in Cartilage Tissue Engineering. J. Tissue Eng. Regen. Med. 2012, 6, 144–154. [Google Scholar] [CrossRef]
- Snyder, T.N.; Madhavan, K.; Intrator, M.; Dregalla, R.C.; Park, D. A Fibrin/Hyaluronic Acid Hydrogel for the Delivery of Mesenchymal Stem Cells and Potential for Articular Cartilage Repair. J. Biol. Eng. 2014, 8, 10. [Google Scholar] [CrossRef]
- Yan, X.; Yang, B.; Chen, Y.; Song, Y.; Ye, J.; Pan, Y.; Zhou, B.; Wang, Y.; Mao, F.; Dong, Y.; et al. Anti-Friction MSCs Delivery System Improves the Therapy for Severe Osteoarthritis. Adv. Mater. 2021, 33, e2104758. [Google Scholar] [CrossRef] [PubMed]
- Whitworth, D.J.; Frith, J.E.; Frith, T.J.R.; Ovchinnikov, D.A.; Cooper-White, J.J.; Wolvetang, E.J. Derivation of Mesenchymal Stromal Cells from Canine Induced Pluripotent Stem Cells by Inhibition of the TGFβ/Activin Signaling Pathway. Stem Cells Dev. 2014, 23, 3021–3033. [Google Scholar] [CrossRef] [PubMed]
- Weißenberger, M.; Weißenberger, M.H.; Wagenbrenner, M.; Heinz, T.; Reboredo, J.; Holzapfel, B.M.; Rudert, M.; Groll, J.; Evans, C.H.; Steinert, A.F. Different Types of Cartilage Neotissue Fabricated from Collagen Hydrogels and Mesenchymal Stromal Cells via SOX9, TGFB1 or BMP2 Gene Transfer. PLoS ONE 2020, 15, e0237479. [Google Scholar] [CrossRef] [PubMed]
- Kilmer, C.E.; Walimbe, T.; Panitch, A.; Liu, J.C. Incorporation of a Collagen-Binding Chondroitin Sulfate Molecule to a Collagen Type I and II Blend Hydrogel for Cartilage Tissue Engineering. ACS Biomater. Sci. Eng. 2022, 8, 1247–1257. [Google Scholar] [CrossRef]
- Michalopoulos, E.; Knight, R.L.; Korossis, S.; Kearney, J.N.; Fisher, J.; Ingham, E. Development of Methods for Studying the Differentiation of Human Mesenchymal Stem Cells under Cyclic Compressive Strain. Tissue Eng. Part C Methods 2012, 18, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Aisenbrey, E.A.; Bryant, S.J. Mechanical Loading Inhibits Hypertrophy in Chondrogenically Differentiating hMSCs within a Biomimetic Hydrogel. J. Mater. Chem. B 2016, 4, 3562–3574. [Google Scholar] [CrossRef]
- Wang, W.; Wan, Y.; Fu, T.; Zhou, T.; Tang, X.; Wu, H.; Liu, C.; Jagodzinski, M. Effect of Cyclic Compression on Bone Marrow Mesenchymal Stromal Cells in Tissue Engineered Cartilage Scaffold. J. Biomed. Mater. Res. A 2019, 107, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Qin, Y.; Dubaa, M.; Killion, J.; Gao, Y.; Zhao, T.; Zhou, D.; Duscher, D.; Geever, L.; Gurtner, G.C.; et al. A Rapid Crosslinking Injectable Hydrogel for Stem Cell Delivery, from Multifunctional Hyperbranched Polymers via RAFT Homopolymerization of PEGDA. Polym. Chem. 2015, 6, 6182–6192. [Google Scholar] [CrossRef]
- Martin, K.E.; Hunckler, M.D.; Chee, E.; Caplin, J.D.; Barber, G.F.; Kalelkar, P.P.; Schneider, R.S.; García, A.J. Hydrolytic Hydrogels Tune Mesenchymal Stem Cell Persistence and Immunomodulation for Enhanced Diabetic Cutaneous Wound Healing. Biomaterials 2023, 301, 122256. [Google Scholar] [CrossRef]
- He, J.; Zhang, N.; Zhu, Y.; Jin, R.; Wu, F. MSC Spheroids-Loaded Collagen Hydrogels Simultaneously Promote Neuronal Differentiation and Suppress Inflammatory Reaction through PI3K-Akt Signaling Pathway. Biomaterials 2021, 265, 120448. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.A.; Marks, W.H.; Bhatia, S.K. Hydrogel Encapsulation to Improve Cell Viability during Syringe Needle Flow. J. Long-Term Eff. Med. Implants 2014, 24, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ye, L.; Cai, X.; Li, Z.; Fan, Y.; Yang, F. Icariin-Loaded Hydrogel Regulates Bone Marrow Mesenchymal Stem Cell Chondrogenic Differentiation and Promotes Cartilage Repair in Osteoarthritis. Front. Bioeng. Biotechnol. 2022, 10, 755260. [Google Scholar] [CrossRef] [PubMed]
- Kováč, J.; Priščáková, P.; Gbelcová, H.; Heydari, A.; Žiaran, S. Bioadhesive and Injectable Hydrogels and Their Correlation with Mesenchymal Stem Cells Differentiation for Cartilage Repair: A Mini-Review. Polymers 2023, 15, 4228. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Burdick, J.A. Engineered Hydrogels for Local and Sustained Delivery of RNA-Interference Therapies. Adv. Healthc. Mater. 2017, 6, 1601041. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, C.; Niyazi, S.; Zheng, L.; Li, J.; Zhang, W.; Xu, M.; Rong, R.; Yang, C.; Zhu, T. A Novel Cytoprotective Peptide Protects Mesenchymal Stem Cells against Mitochondrial Dysfunction and Apoptosis Induced by Starvation via Nrf2/Sirt3/FoxO3a Pathway. J. Transl. Med. 2017, 15, 33. [Google Scholar] [CrossRef] [PubMed]
- Goyal, U.; Ta, M. A Novel Role of Vitronectin in Promoting Survival of Mesenchymal Stem Cells under Serum Deprivation Stress. Stem Cell Res. Ther. 2020, 11, 181. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.I.; Coimbra, L.S.; Tian, C.; Alblowi, J.; Kayal, R.A.; Einhorn, T.A.; Gerstenfeld, L.C.; Pignolo, R.J.; Graves, D.T. Diabetes Reduces Mesenchymal Stem Cells in Fracture Healing through a TNFα-Mediated Mechanism. Diabetologia 2015, 58, 633–642. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wang, H.; Jin, T.; Xu, Y.; Mei, L.; Yang, J. TLR4 Activation Promotes Bone Marrow MSC Proliferation and Osteogenic Differentiation via Wnt3a and Wnt5a Signaling. PLoS ONE 2016, 11, e0149876. [Google Scholar] [CrossRef]
- Feng, M.; Liu, W.; Ding, J.; Qiu, Y.; Chen, Q. Sonic Hedgehog Induces Mesenchymal Stromal Cell Senescence-Associated Secretory Phenotype and Chondrocyte Apoptosis in Human Osteoarthritic Cartilage. Front. Cell Dev. Biol. 2021, 9, 716610. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, P.; Liu, T.; Li, Z.; Huang, Y.; Liao, J.; Hamid, M.R.; Wen, L.; Wang, T.; Mo, C.; et al. Kartogenin Hydrolysis Product 4-Aminobiphenyl Distributes to Cartilage and Mediates Cartilage Regeneration. Theranostics 2019, 9, 7108–7121. [Google Scholar] [CrossRef] [PubMed]
- Aizman, I.; Tirumalashetty, B.J.; McGrogan, M.; Case, C.C. Comparison of the Neuropoietic Activity of Gene-Modified versus Parental Mesenchymal Stromal Cells and the Identification of Soluble and Extracellular Matrix-Related Neuropoietic Mediators. Stem Cell Res. Ther. 2014, 5, 29. [Google Scholar] [CrossRef]
- Wang, Y.; Malcolm, D.W.; Benoit, D.S.W. Controlled and Sustained Delivery of siRNA/NPs from Hydrogels Expedites Bone Fracture Healing. Biomaterials 2017, 139, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Mitsuhashi, N.; Klein, A.; Barsky, L.W.; Weinberg, K.; Barr, M.L.; Demetriou, A.; Wu, G.D. The Role of the Hyaluronan Receptor CD44 in Mesenchymal Stem Cell Migration in the Extracellular Matrix. Stem Cells 2006, 24, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.Y.; Kim, H.; Lee, H.J.; Lee, K.; Barreda, H.; Kim, H.J.; Shin, E.; Bae, E.-H.; Kaur, G.; Zhang, Y.; et al. MHC Class I Enables MSCs to Evade NK-Cell-Mediated Cytotoxicity and Exert Immunosuppressive Activity. Stem Cells 2022, 40, 870–882. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.J.; Mittal, S.K.; Chauhan, S.K. Mesenchymal Stromal Cells Suppress T-Cell-Mediated Delayed-Type Hypersensitivity via ALCAM-CD6 Interaction. Stem Cells Transl. Med. 2023, 12, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Li, Y.; Ma, Z.; Lin, H.; Zhu, X.; Xiao, Y.; Zhang, X. Collagen Hydrogel Viscoelasticity Regulates MSC Chondrogenesis in a ROCK-Dependent Manner. Sci. Adv. 2023, 9, eade9497. [Google Scholar] [CrossRef] [PubMed]
- Ledo, A.M.; Vining, K.H.; Alonso, M.J.; Garcia-Fuentes, M.; Mooney, D.J. Extracellular Matrix Mechanics Regulate Transfection and SOX9-Directed Differentiation of Mesenchymal Stem Cells. Acta Biomater. 2020, 110, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Hodge, J.G.; Robinson, J.L.; Mellott, A.J. Novel Hydrogel System Eliminates Subculturing and Improves Retention of Nonsenescent Mesenchymal Stem Cell Populations. Regen. Med. 2023, 18, 23–36. [Google Scholar] [CrossRef]
- Pangjantuk, A.; Kaokaen, P.; Kunhorm, P.; Chaicharoenaudomrung, N.; Noisa, P. 3D Culture of Alginate-Hyaluronic Acid Hydrogel Supports the Stemness of Human Mesenchymal Stem Cells. Sci. Rep. 2024, 14, 4436. [Google Scholar] [CrossRef]
- Gao, X.; Liang, X.; Liu, B.; Hong, Y.; He, H.; Shen, Y.; Chen, J.; Huang, X.; Hu, B.; Li, W.; et al. Downregulation of ALKBH5 Rejuvenates Aged Human Mesenchymal Stem Cells and Enhances Their Therapeutic Efficacy in Myocardial Infarction. FASEB J. 2023, 37, e23294. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wang, S.; Zeng, C.; Tang, S.; Gu, H.; Wang, Z.; Li, J.; Feng, P.; Zhang, Y.; Wang, P.; et al. Silencing circSERPINE2 Restrains Mesenchymal Stem Cell Senescence via the YBX3/PCNA/P21 Axis. Cell Mol. Life Sci. 2023, 80, 325. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Li, L.; Chen, Y.; Luo, A.; Ni, Z.; Liu, J.; Yuan, Y.; Shi, M.; Chen, B.; Long, D.; et al. Mesenchymal Stem Cells Ameliorated Glucolipotoxicity in HUVECs through TSG-6. Int. J. Mol. Sci. 2016, 17, 483. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-Q.; Huang, S.-H.; Huang, X.; Li, J.-H.; Ye, P.; Xu, J.; Zheng, P.-Z.; Shen, H.-Y.; Huang, J.-R. Regulation of Human Mesenchymal Stem Cell Differentiation by TREM-2. Hum. Immunol. 2016, 77, 476–482. [Google Scholar] [CrossRef]
- Yoon, D.S.; Lee, K.-M.; Choi, Y.; Ko, E.A.; Lee, N.-H.; Cho, S.; Park, K.H.; Lee, J.-H.; Kim, H.-W.; Lee, J.W. TLR4 Downregulation by the RNA-Binding Protein PUM1 Alleviates Cellular Aging and Osteoarthritis. Cell Death Differ. 2022, 29, 1364–1378. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, S.; He, C.; Lv, T.; Zeng, L.; Zhang, F.; Chen, H.; Zhao, R.C. LncRNA LYPLAL1-AS1 Rejuvenates Human Adipose-Derived Mesenchymal Stem Cell Senescence via Transcriptional MIRLET7B Inactivation. Cell Biosci. 2022, 12, 45. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, X.; Gu, R.; Liu, X.; Wang, S.; Xia, D.; Li, Z.; Lian, X.; Zhang, P.; Liu, Y.; et al. LAMA2 Regulates the Fate Commitment of Mesenchymal Stem Cells via Hedgehog Signaling. Stem Cell Res. Ther. 2020, 11, 135. [Google Scholar] [CrossRef]
- Hu, S.; Li, J.; Xu, X.; Liu, A.; He, H.; Xu, J.; Chen, Q.; Liu, S.; Liu, L.; Qiu, H.; et al. The Hepatocyte Growth Factor-Expressing Character Is Required for Mesenchymal Stem Cells to Protect the Lung Injured by Lipopolysaccharide In Vivo. Stem Cell Res. Ther. 2016, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.-C.; Chen, W.-C.; Chiang, C.-W.; Chen, L.-Y.; Chang, Y.-C.; Chen, C.-H. Differentiation Effects of Platelet-Rich Plasma Concentrations on Synovial Fluid Mesenchymal Stem Cells from Pigs Cultivated in Alginate Complex Hydrogel. Int. J. Mol. Sci. 2015, 16, 18507–18521. [Google Scholar] [CrossRef]
- Hori, J.; Deie, M.; Kobayashi, T.; Yasunaga, Y.; Kawamata, S.; Ochi, M. Articular Cartilage Repair Using an Intra-Articular Magnet and Synovium-Derived Cells. J. Orthop. Res. 2011, 29, 531–538. [Google Scholar] [CrossRef]
- Li, J.; Huang, Y.; Song, J.; Li, X.; Zhang, X.; Zhou, Z.; Chen, D.; Ma, P.X.; Peng, W.; Wang, W.; et al. Cartilage Regeneration Using Arthroscopic Flushing Fluid-Derived Mesenchymal Stem Cells Encapsulated in a One-Step Rapid Cross-Linked Hydrogel. Acta Biomater. 2018, 79, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.Y.; Song, M.; Ha, C.-W.; Kim, J.-A.; Lee, C.-H.; Park, Y.-B. Comparison of Articular Cartilage Repair with Different Hydrogel-Human Umbilical Cord Blood-Derived Mesenchymal Stem Cell Composites in a Rat Model. Stem Cell Res. Ther. 2014, 5, 39. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-B.; Ha, C.-W.; Lee, C.-H.; Yoon, Y.C.; Park, Y.-G. Cartilage Regeneration in Osteoarthritic Patients by a Composite of Allogeneic Umbilical Cord Blood-Derived Mesenchymal Stem Cells and Hyaluronate Hydrogel: Results from a Clinical Trial for Safety and Proof-of-Concept with 7 Years of Extended Follow-Up. Stem Cells Transl. Med. 2017, 6, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-K.; Bae, H.C.; Ro, D.H.; Lee, S.; Lee, M.C.; Han, H.-S. Enhancement of Cartilage Regeneration of Synovial Stem Cells/Hydrogel by Using Transglutaminase-4. Tissue Eng. Part A 2021, 27, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Geckil, H.; Xu, F.; Zhang, X.; Moon, S.; Demirci, U. Engineering Hydrogels as Extracellular Matrix Mimics. Nanomedicine 2010, 5, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Mao, X.; Cao, Y.; Liu, Z.; Shen, Z.; Li, M.; Jia, W.; Guo, Z.; Wang, Z.; Xiang, C.; et al. 3D Bioprinting Using Synovium-Derived MSC-Laden Photo-Cross-Linked ECM Bioink for Cartilage Regeneration. ACS Appl. Mater. Interfaces 2023, 15, 8895–8913. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.L.; Kim, Y.S.; Koons, G.L.; Lam, J.; Navara, A.M.; Barrios, S.; Xie, V.Y.; Watson, E.; Smith, B.T.; Pearce, H.A.; et al. Bilayered, Peptide-Biofunctionalized Hydrogels for in Vivo Osteochondral Tissue Repair. Acta Biomater. 2021, 128, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Abramoff, B.; Caldera, F.E. Osteoarthritis: Pathology, Diagnosis, and Treatment Options. Med. Clin. N. Am. 2020, 104, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as Extracellular Matrix Mimics for 3D Cell Culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef]
- Ankrum, J.; Karp, J.M. Mesenchymal Stem Cell Therapy: Two Steps Forward, One Step Back. Trends Mol. Med. 2010, 16, 203–209. [Google Scholar] [CrossRef]
- Ankrum, J.A.; Ong, J.F.; Karp, J.M. Mesenchymal Stem Cells: Immune Evasive, Not Immune Privileged. Nat. Biotechnol. 2014, 32, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Ge, W.; Jiang, J.; Baroja, M.L.; Arp, J.; Zassoko, R.; Liu, W.; Bartholomew, A.; Garcia, B.; Wang, H. Infusion of Mesenchymal Stem Cells and Rapamycin Synergize to Attenuate Alloimmune Responses and Promote Cardiac Allograft Tolerance. Am. J. Transpl. 2009, 9, 1760–1772. [Google Scholar] [CrossRef] [PubMed]
- Carballo, C.B.; Nakagawa, Y.; Sekiya, I.; Rodeo, S.A. Basic Science of Articular Cartilage. Clin. Sports Med. 2017, 36, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Vincent, T.L.; McClurg, O.; Troeberg, L. The Extracellular Matrix of Articular Cartilage Controls the Bioavailability of Pericellular Matrix-Bound Growth Factors to Drive Tissue Homeostasis and Repair. Int. J. Mol. Sci. 2022, 23, 6003. [Google Scholar] [CrossRef] [PubMed]
- Gahunia, H.K.; Pritzker, K.P.H. Structure and Function of Articular Cartilage. In Articular Cartilage of the Knee: Health, Disease and Therapy; Gahunia, H.K., Gross, A.E., Pritzker, K.P.H., Babyn, P.S., Murnaghan, L., Eds.; Springer: New York, NY, USA, 2020; pp. 3–70. ISBN 978-1-4939-7587-7. [Google Scholar]


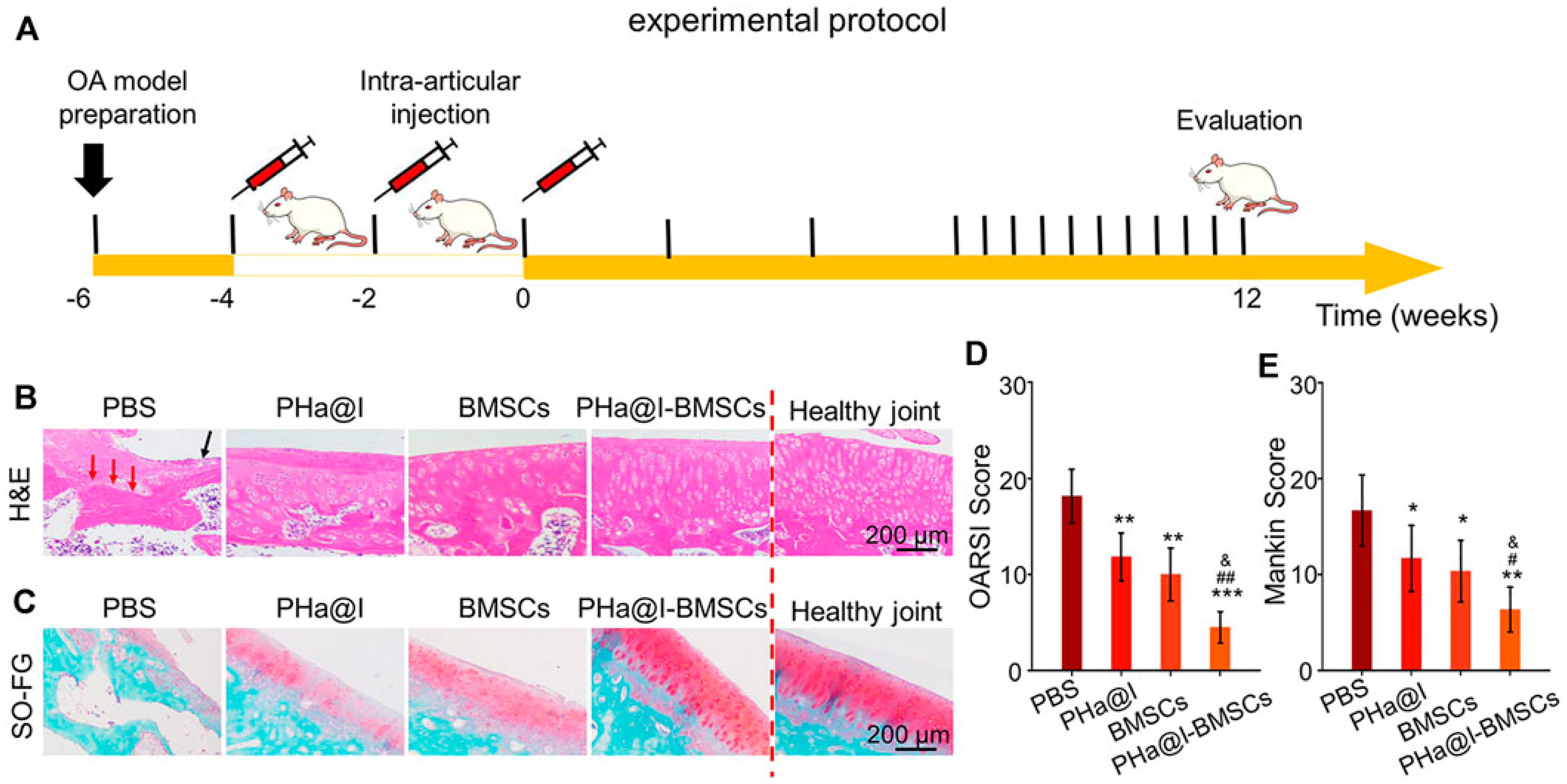
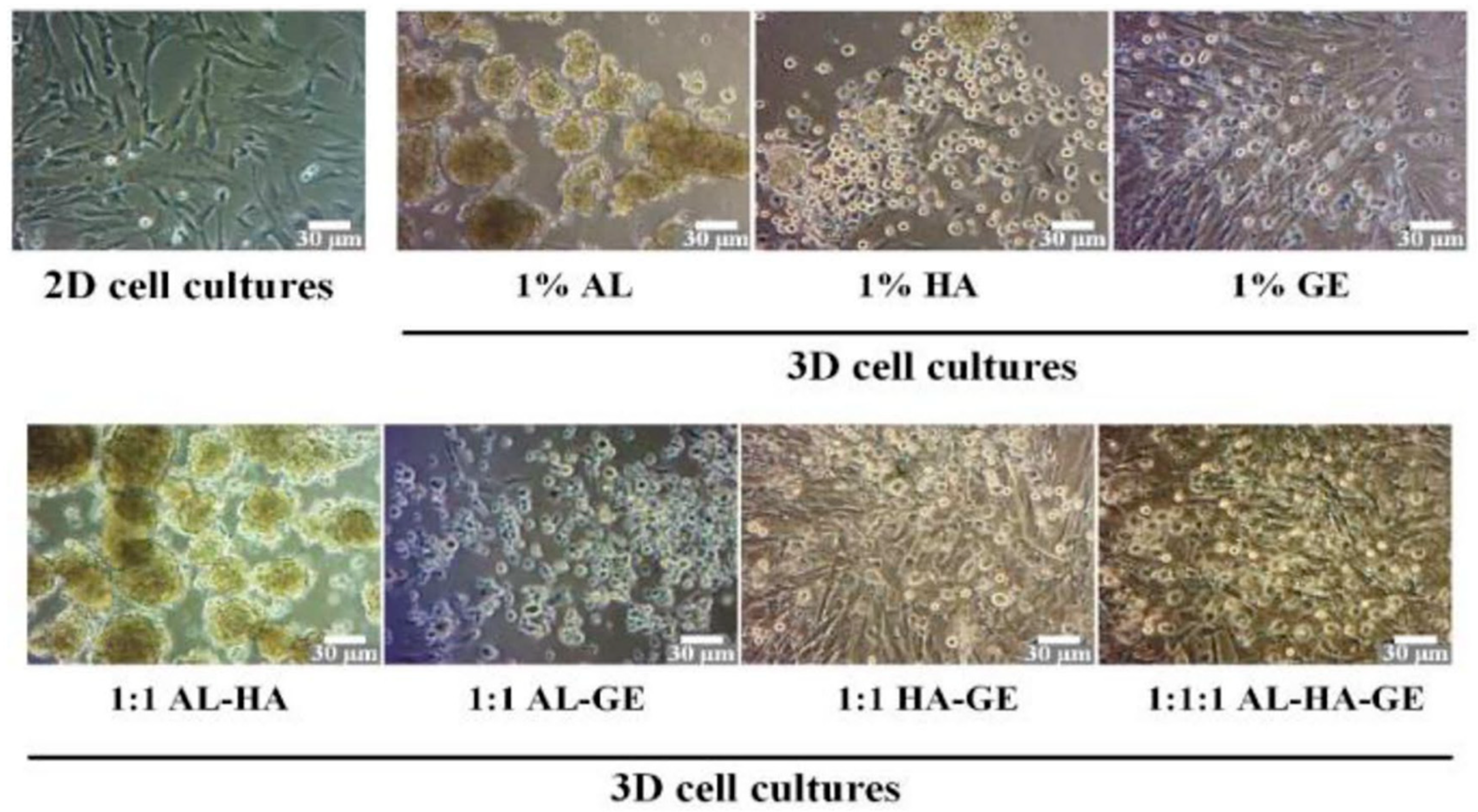
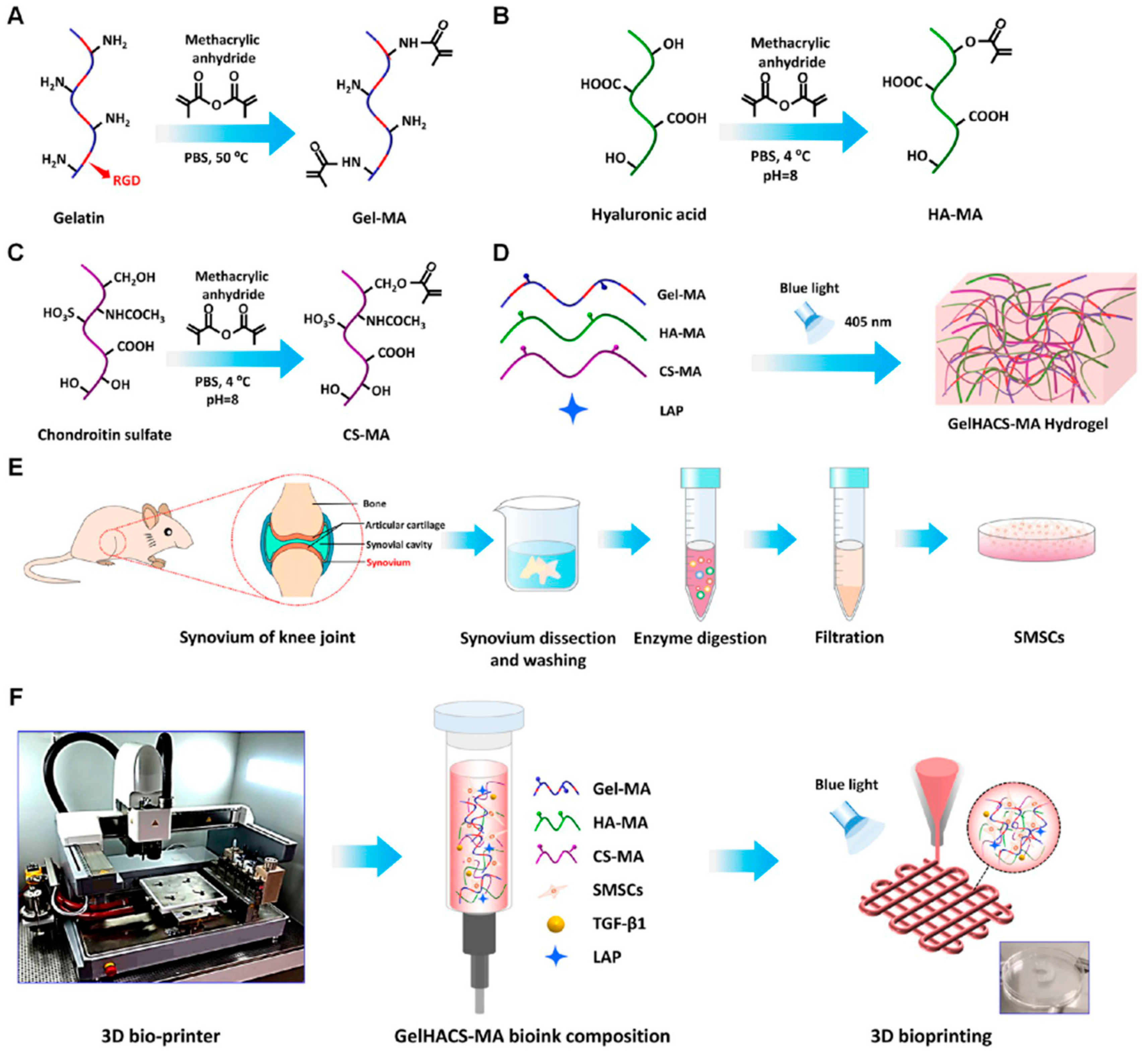
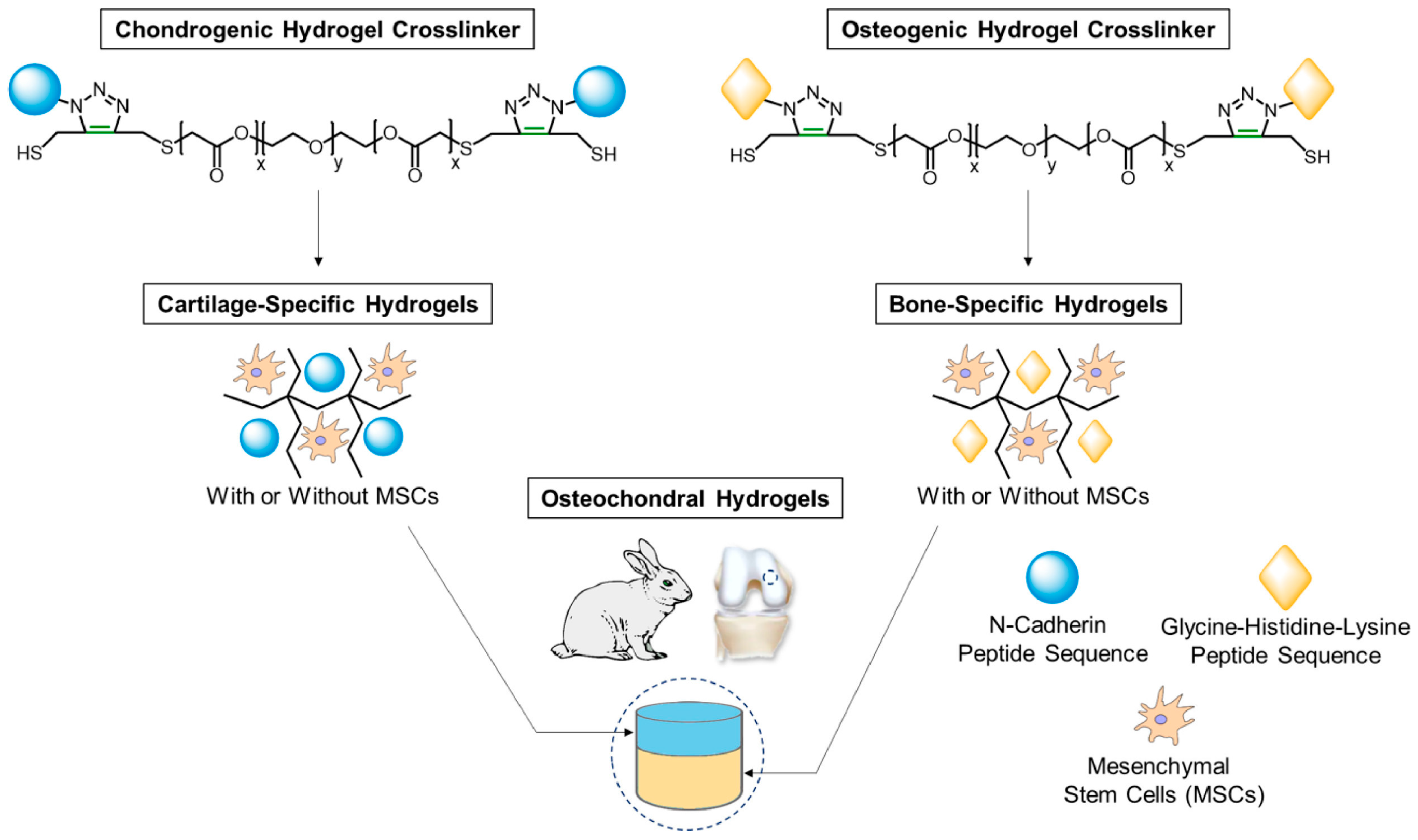
| International Society for Cellular Therapy (ISCT) Definition of MSC Properties | |
|---|---|
| Plastic adhesion or not | Yes (in standard culture conditions) |
| Specific antigen | CD105+ CD73+ CD90+ Stro-1+, CD29+, CD44+, CD73+, CD146+, and SSEA4+, CD14-, CD34-, CD45- or CD79a-, CD11b- or HLA-DR-, CD19- |
| In vitro diffenentiated ability | Adipocytes, osteoblasts, chondroblasts |
| Property | Kinds of MSCs | Model | Outcome of OA Treatment | Ref. |
|---|---|---|---|---|
| Anti- inflammation | IPFP-ASCs | Human | Promoting chondrogenic differentiation and preventing articular cartilage thickening and inflammation | [102] |
| Supra-hASC | Mouse | Reducing OA-associated knee inflammation and cartilage degenerative grade | [103] | |
| BM-MSCs | Human | Promoting cell proliferation of chondrocytes and inhibiting inflammatory activity in osteoarthritis | [104] | |
| hUCMSCs-EVs | Human | Promoting the polarization of M2-type macrophages, reducing the infammatory of cytokines (IL-10) response | [105] | |
| Cartilage regeneration | IPFP-ASCs | Sheep | Promoting the expression of cartilage genes | [106] |
| BM-MSCs | Human | Inducing chondrogenic differentiation | [107] | |
| Sc-ASCs | Bear | Promoting chondrogenic differentiation | [108] | |
| BM-MSCs | Human | Promoting chondrogenic differentiation by enhancing the expression of cartilage extracellular matrix genes | [109] | |
| BM-MSCs | Rat | Prevents cell apoptosis and inhibits senescence of chondrocytes by reducing the IL-1β level and improving the inflammation in joints | [110] |
| Types of Hydrogels | Characterizations | Ref. | |
|---|---|---|---|
| Natural hydrogels | ①Polysaccharide: hyaluronic acid, chondroitin sulfate, chitin, chitosan, cellulose, starch, gum, alginate, and carrageenan | 1. Low immune response 2. Low toxicity response 3. Non-toxic and non-immunogenic degradation products 4. Poor stability, rapid degradation 5. Relatively low mechanical strength | [130,133,134,135] |
| ②Protein-based materials: gelatin, collagen, fibroin, sericin | |||
| ③Polyphenols: lignin | |||
| ④Organic polyester/inorganic polyester: polyphthalamide | |||
| ⑤Polyanhydride: polyadipic acid | |||
| ⑥Biopolymer: nucleic acid, DNA | |||
| Synthetic hydrogels | ①Polycaprolactone-(PCL) ②Polyvinylpyrrolidone-(PVP) ③Polylactic acid- (PLA) ④Polyethylene glycol-(PEG) ⑤Polyvinyl alcohol- (PVA) | 1. Providing customized performance characteristics 2. Controlability, reproducibility, and excellent mechanical performance 3. Poor compatibility with host tissues 4. Low biological activity | [136,137,138,139,140,141,142] |
| Hybrid-origin hydrogels | ①Carboxymethyl chitosan-(CHC) ②Hyaluronic acid-(HA) | 1. pH-dependent drug release characteristics 2. Inhibition of cell apoptosis | [143,144,145] |
| ①Chitosan ②Polycaprolactone microspheres | 1. Dual functionality of supplementing mucus and storing drugs 2. Prolonging drug residence time in the body | ||
| ①Semi-polyacrylonitrile chitosan-poly(acrylamide-ethylene oxide) hydrogel microspheres | Used for encapsulation and delivery of anticancer drugs | ||
| Hydrogel Preparation Method | Advantage | Disadvantage | Ref. |
|---|---|---|---|
| Chemical crosslinking | ①High degree of crosslinking and stability ②Highly adjustable ③Wide range of applicability | ①Biotoxicity ②Toxic substances need to be cleared. ③Long reaction time and complex preparation | [132] |
| Physical crosslinking | ①Mildly reactive and environmentally friendly ②Prepared at room temperature ③Gel structures with reversible properties can be prepared | ①Poor gel stabilization ②Sensitive to temperature and ionic concentration conditions and structural instability ③The preparation process can be complex. | [132] |
| Enzymatic crosslinking | ①Good biocompatibility ②Mild chemical reaction, sensitive to biologically active substances ③It can be prepared under physiological conditions. | ①Enzyme stability and activity are easily affected. ②The enzyme-catalyzed reaction rate is slower and the preparation time is longer. ③The range of applicability is limited by the available enzymes and substrates. | [154] |
| Photopolymerization crosslinking | ①The preparation process is simple and easy to operate. ②A high degree of crosslinking can be achieved in a relatively short period of time. ③Better spatial and temporal control | ①Possible phototoxicity to organisms ②Limited by the depth of light penetration and the rate of reaction ③Technical and equipment support is required for photosensitive monomer selection and light source control. | [155] |
| Hydrogel Type | Cell Type and Loading | Chondrogenic Inducting Factors | Main Results | Ref. |
|---|---|---|---|---|
| Fibrin/hyaluronan hydrogel | Human BMSCs | TGF-β1 | Increasing COL2, ACAN, and GAG levels | [164] |
| 10% PEGDA | Goat BMSCs | TGF-β1 | Increasing COL2 and GAG level | [165] |
| Fibrin MeHA | Human MSCs | N.I. | Increasing SOX9 level | [166] |
| DNA supramolecular | Rabbit BMSCs | N.I | Increasing COL2, SOX9 and ACAN level, decreasing COL1 and COL10 levels | [167] |
| PEG–hyaluronic acid (HA) | Canine MSCs | TGF-β3 | Increaing proteoglycan and GAG levels | [168] |
| Collagen type 1 | Human BMSCs | No | Increasing COL2 and GAG levels and condroitin sulfate | [169] |
| Chondroitin sulfate (CS) | Rabbit BMSCs | TGF-β3 | Increasing GAG and COL2 levels | [170] |
| Collagen and alginate | Human MSCs | No | Increasing CBFA-1, Sox9, and aggrecan levels | [171] |
| Chondroitin sulfate (CS) and PEG | Human MSCs | N.I. | Increasing collagen II gene expression | [172] |
| Chitosan | Rat BMMSCs | N.I. | Promoting chondrogenesis markers expression (Sox9, aggrecan, and collagen II) | [173] |
| Gene Symbol | Kinds of MSCs | Gene Functions | Gene Manipulation | Diseases | Ref. |
|---|---|---|---|---|---|
| Sirt3 (sirtuin 3) | BMSCs | Against starvation-induced apoptosis | Knockdown | In vitro | [181] |
| ALKBH5 (AlkB homolog 5) | BMSCs | Inducer of aging in MSCs | Knockdown | Myocardial infarction | [196] |
| circSERPINE2 (serpin family E member 2) | BMSCs | Inducer of aging in MSCs | Knockdown | Osteoarthritis | [197] |
| NICD1 (notch receptor 1) | BMSCs | Enhanced neuropoietic effects | Knockdown | Ischemic stroke and Parkinson’s | [187] |
| ALCAM (activated leukocyte cell adhesion molecule) | BMSCs | Inhibiting the activation and proliferation of allogeneic CD4+ T cells | Knockdown | Allograft rejection, autoimmune diseases | [191] |
| SHH (Sonic hedgehog signaling molecule) | OA-MSC | Inducer of aging in MSCs | Knockdown | Osteoarthritis | [185] |
| FOXO1 (forkhead box O1) | BMSCs | Against TNF-α-induced apoptosis in MSCs | Knockdown | Diabetes | [183] |
| TSG-6 (tumor necrosis factor-α-stimulated protein 6) | HUC-MSCs | Against cellular damage caused by high sugar and fat | Knockdown | Diabetes | [198] |
| TREM-2 (triggering receptor expressed on myeloid cells 2) | MSCs | Critical for MSCs’ pluripotency and immunomodulatory capacity | Knockdown | In vitro | [199] |
| TLR4 (Toll-like receptor 4) | BMSCs | Promote proliferation and osteogenic differentiation of MSCs | Knockdown | Fracture healing, osteoporosis | [184] |
| VTN (vitronectin) | WJ-MSCs | Against starvation-induced apoptosis | Knockdown | Ischemic diseases and wound healing | [182] |
| RPS6KA2 (ribosomal protein S6 kinase A2) | BMSC and UC-MSC | Critical for repairing cartilage defects | Knockdown | Osteoarthritis | [186] |
| PUM1 (Pumilio RNA binding family member 1) | BMSCs | Against aging of MSC | Knockdown | Osteoarthritis | [200] |
| LYPLAL1-AS1 (LYPLAL1 antisense RNA 1) | hADSCs | Against aging of MSC | Overexpression | Senile disease | [201] |
| LAMA2 (laminin subunit alpha 2) | hASCs and hBMMSCs | Inhibiting of osteogenic differentiation but promoting adipogenic differentiation of MSCs | Knockdown | Bone defect diseases | [202] |
| HGF (hepatocyte growth factor) | BMSCs | Repair lung endothelial cell function | Knockdown | Acute lung injury | [203] |
| CD44 (CD44 molecule (IN blood group)) | BMSCs | Mediates cell adhesion to ECM, promotes cell migration | Knockdown | Tissue damage and graft fibrosis | [189] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; He, W.; Huang, H.; Han, J.; Wang, R.; Li, H.; Long, Y.; Wang, G.; Han, X. Recent Advances in Hydrogel Technology in Delivering Mesenchymal Stem Cell for Osteoarthritis Therapy. Biomolecules 2024, 14, 858. https://doi.org/10.3390/biom14070858
Wang X, He W, Huang H, Han J, Wang R, Li H, Long Y, Wang G, Han X. Recent Advances in Hydrogel Technology in Delivering Mesenchymal Stem Cell for Osteoarthritis Therapy. Biomolecules. 2024; 14(7):858. https://doi.org/10.3390/biom14070858
Chicago/Turabian StyleWang, Xiangjiang, Wentao He, Hao Huang, Jiali Han, Ruren Wang, Hongyi Li, Ying Long, Guiqing Wang, and Xianjing Han. 2024. "Recent Advances in Hydrogel Technology in Delivering Mesenchymal Stem Cell for Osteoarthritis Therapy" Biomolecules 14, no. 7: 858. https://doi.org/10.3390/biom14070858
APA StyleWang, X., He, W., Huang, H., Han, J., Wang, R., Li, H., Long, Y., Wang, G., & Han, X. (2024). Recent Advances in Hydrogel Technology in Delivering Mesenchymal Stem Cell for Osteoarthritis Therapy. Biomolecules, 14(7), 858. https://doi.org/10.3390/biom14070858






