Development of SSR Markers and Evaluation of Genetic Diversity of Endangered Plant Saussurea involucrata
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Sampling and Preservation
2.2. Identification and Development of Genomic SSRs
2.3. PCR Amplification and Electrophoresis Detection
2.4. Data Analysis
3. Results
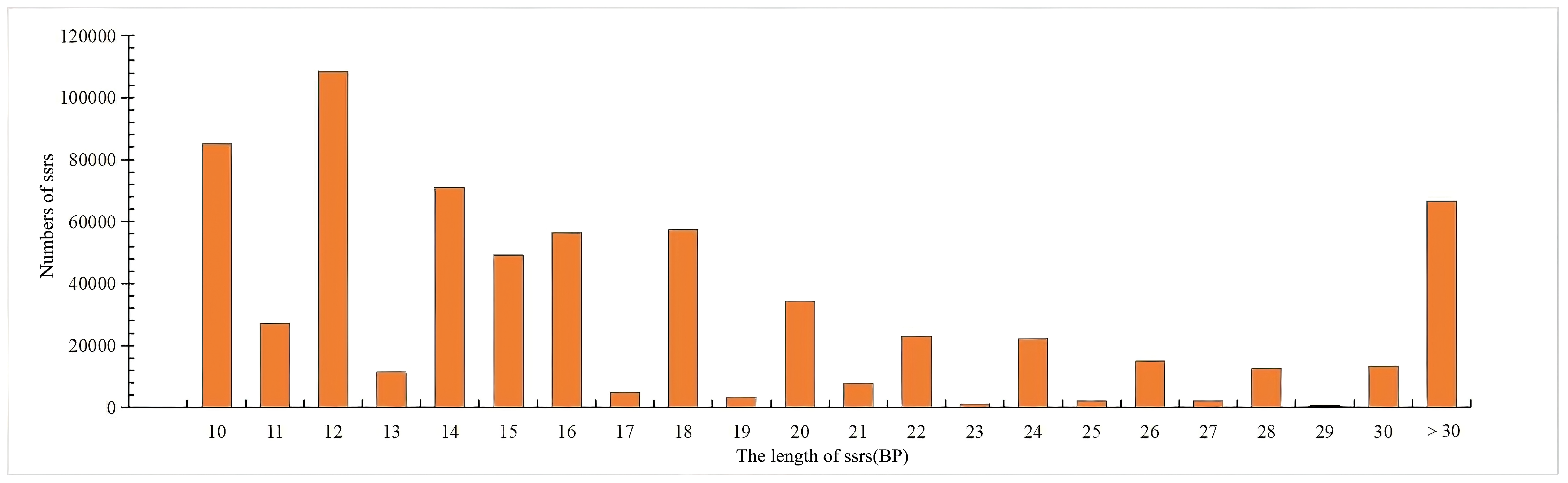
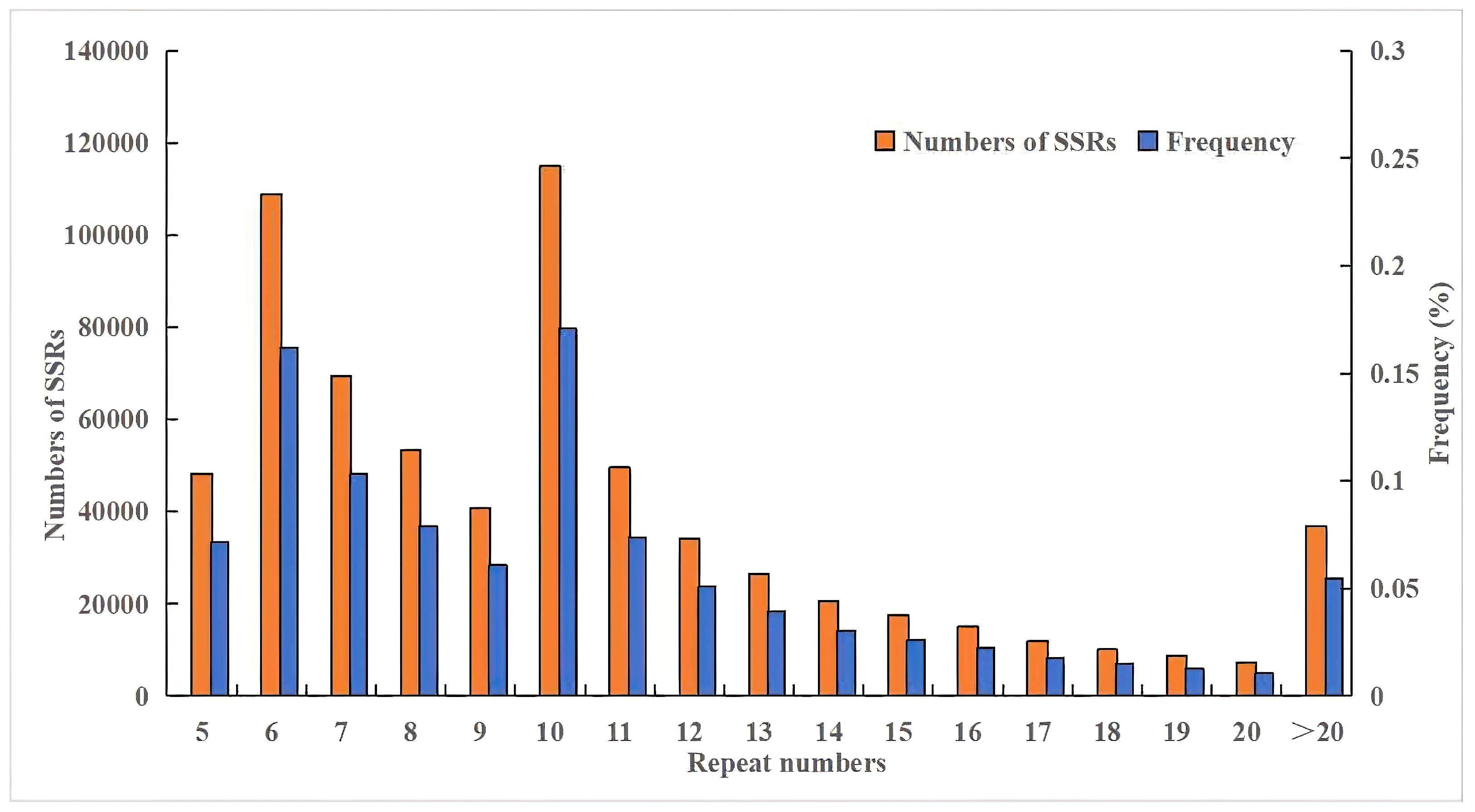
3.1. Analysis of the Distribution of SSRs in the Genome of S. involucrata
3.2. Linkage Disequilibrium Tests
3.3. Genetic Diversity of the S. involucrata
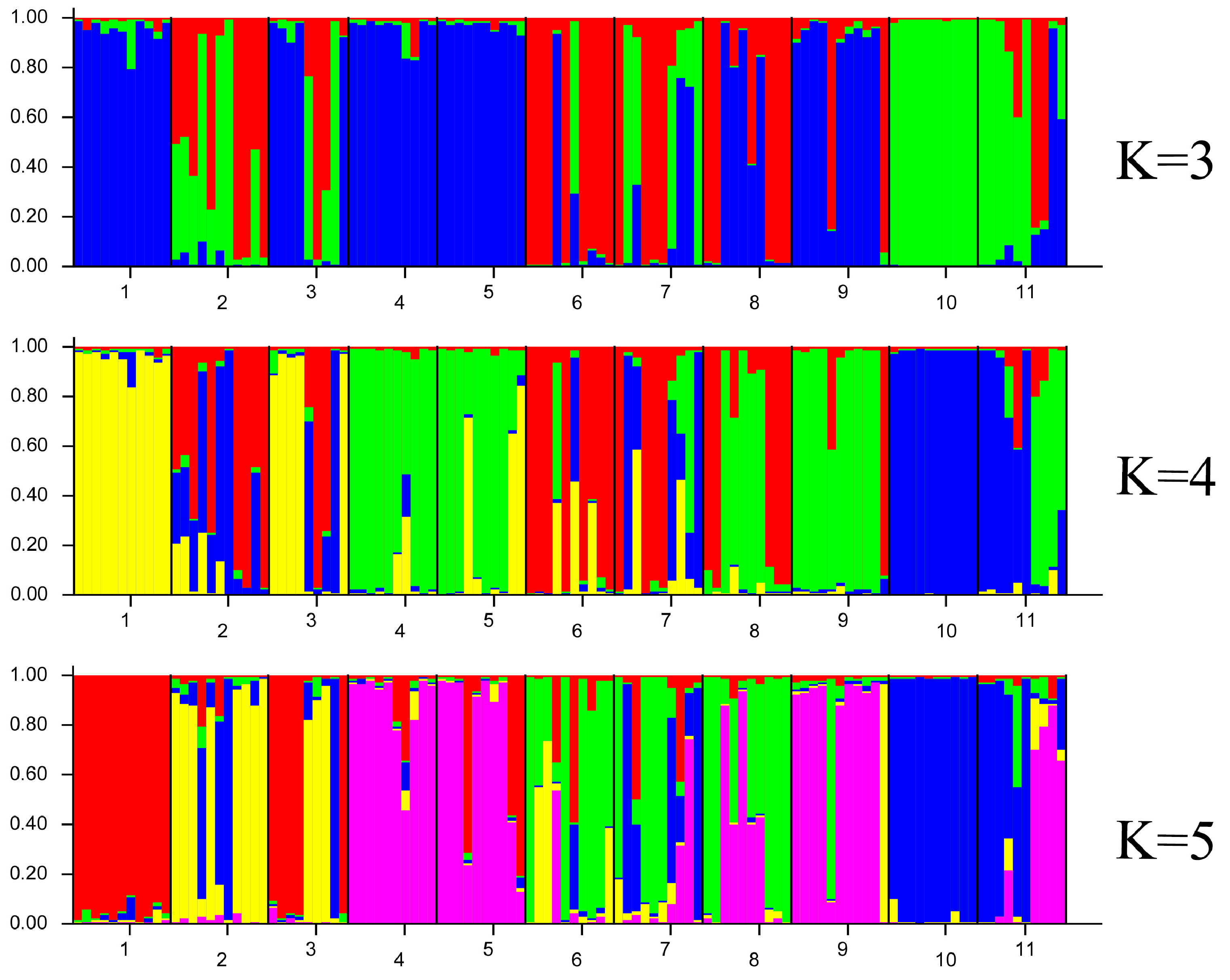
3.4. Genetic Relationship and Population Structure Analysis
4. Discussion
4.1. Genetic Diversity of S. involucrata
4.2. Genetic Differentiation of S. involucrata
4.3. Conservation of S. involucrata
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rawat, U.; Agarwal, N. Biodiversity: Concept, threats and conservation. Environ. Conserv. J. 2015, 16, 19–28. [Google Scholar] [CrossRef]
- Pauls, S.U.; Nowak, C.; Bálint, M.; Pfenninger, M. The impact of global climate change on genetic diversity within populations and species. Mol. Ecol. 2013, 22, 925–946. [Google Scholar] [CrossRef]
- Jump, A.S.; Marchant, R.; Peñuelas, J. Environmental change and the option value of genetic diversity. Trends Plant Sci. 2009, 14, 51–58. [Google Scholar] [CrossRef]
- Ellegren, H.; Galtier, N. Determinants of genetic diversity. Nat. Rev. Genet. 2016, 17, 422–433. [Google Scholar] [CrossRef] [PubMed]
- De Kort, H.; Prunier, J.G.; Ducatez, S.; Honnay, O.; Baguette, M.; Stevens, V.M.; Blanchet, S. Life history, climate and biogeography interactively affect worldwide genetic diversity of plant and animal populations. Nat. Commun. 2021, 12, 516. [Google Scholar] [CrossRef] [PubMed]
- Chik, W.-I.; Zhu, L.; Fan, L.-L.; Yi, T.; Zhu, G.-Y.; Gou, X.-J.; Tang, Y.-N.; Xu, J.; Yeung, W.-P.; Zhao, Z.-Z.; et al. Saussurea involucrata: A review of the botany, phytochemistry and ethnopharmacology of a rare traditional herbal medicine. J. Ethnopharmacol. 2015, 172, 44–60. [Google Scholar] [CrossRef]
- He, L.; Fan, S. From Saussurea bracteata to Saussurea tianschanica. For. Hum. 2018, 4, 66–73. [Google Scholar]
- Dai, P. Study on Reproductive Ecology of Saussurea involucrata. Master’s Thesis, Xinjiang Agricultural University, Urumqi, China, 2008. [Google Scholar]
- Xie, X.; Zhu, Y. Survival wisdom of Saussurea involucrata. Xinjiang For. 2023, 3, 31–32. [Google Scholar] [CrossRef]
- Yi, T.; Chen, H.-B.; Zhao, Z.-Z.; Jiang, Z.-H.; Cai, S.-Q.; Wang, T.-M. Identification and Determination of the Major Constituents in the Traditional Uighur Medicinal Plant Saussurea involucrata by LC-DAD-MS. Chromatographia 2009, 69, 537–542. [Google Scholar] [CrossRef][Green Version]
- Zhai, K.; Wang, C. Research progress of Saussurea involucrata. Hubei Agric. Sci. 2009, 48, 2869–2873. [Google Scholar]
- Zhuang, L.; Li, W. Utilization, development and protection of Saussurea involucrata resources in Xinjiang. Resour. Environ. Arid. Areas 2006, 2, 195–202. [Google Scholar] [CrossRef]
- Kuo, C.-L.; Agrawal, D.-C.; Chang, H.-C.; Chiu, Y.-T.; Huang, C.-P.; Chen, Y.-L.; Huang, S.-H.; Tsay, H.-S. In vitro culture and production of syringin and rutin in Saussurea involucrata (Kar. et Kir.)—An endangered medicinal plant. Bot. Stud. 2015, 56, 12. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Liu, J.; Liu, S.; Guan, S.; Jiao, P. Characterization of the complete chloroplast genome of Saussurea involuerata (Compositae), an endangered species endemic to China. Mitochondrial DNA Part B 2020, 5, 511–512. [Google Scholar] [CrossRef]
- Yuan, X.F.; Dai, Z.H.; Wang, X.D.; Zhao, B. Assessment of genetic stability in tissue-cultured products and seedlings of Saussurea involucrata by RAPD and ISSR markers. Biotechnol. Lett. 2009, 31, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Yang, W.; Wang, X.; Hou, Y. High genetic diversity in an endangered medicinal plant, Saussurea involucrata (Saussurea, Asteraceae), in western Tianshan Mountains, China. Conserv. Genet. 2017, 18, 1435–1447. [Google Scholar] [CrossRef]
- Hu, L.; Lu, T.; Wang, X.; Wang, J.; Shi, W. Conservation Priorities and Demographic History of Saussurea involucrata in the Tianshan Mountains and Altai Mountains. Life 2023, 13, 2209. [Google Scholar] [CrossRef]
- Zhang, B.; Yao, Y.; Cheng, W.; Zhou, C.; Lu, Z.; Chen, X.; Alshir, K.; ErDowlet, I.; Zhang, L.; Shi, Q. Human-Induced Changes to Biodiversity and Alpine Pastureland in the Bayanbulak Region of the East Tienshan Mountains. Mt. Res. Dev. 2002, 22, 383–389. [Google Scholar] [CrossRef]
- Tautz, D.; Renz, M. Simple sequences are ubiquitous repetitive components of eukaryotic genomes. Nucleic Acids Res. 1984, 12, 4127–4138. [Google Scholar] [CrossRef]
- Litt, M.; Luty, J.A. A hypervariable microsatellite revealed by in vitro amplification of a dinucleotide repeat within the cardiac muscle actin gene. Am. J. Hum. Genet. 1989, 44, 397–401. [Google Scholar]
- Tóth, G.; Gáspári, Z.; Jurka, J. Microsatellites in different eukaryotic genomes: Survey and analysis. Genome Res. 2000, 10, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Amiteye, S. Basic concepts and methodologies of DNA marker systems in plant molecular breeding. Heliyon 2021, 7, e08093. [Google Scholar] [CrossRef]
- Yang, H.; Li, X.; Liu, D.; Chen, X.; Li, F.; Qi, X.; Luo, Z.; Wang, C. Genetic diversity and population structure of the endangered medicinal plant Phellodendron amurense in China revealed by SSR markers. Biochem. Syst. Ecol. 2016, 66, 286–292. [Google Scholar] [CrossRef]
- Jiao, Y.; Li, X.-W.; Chai, M.-L.; Jia, H.-J.; Chen, Z.; Wang, G.-Y.; Chai, C.-Y.; van de Weg, E.; Gao, Z.-S. Development of simple sequence repeat (SSR) markers from a genome survey of Chinese bayberry (Myrica rubra). BMC Genom. 2012, 13, 201. [Google Scholar] [CrossRef] [PubMed]
- Sethy, N.K.; Shokeen, B.; Edwards, K.J.; Bhatia, S. Development of microsatellite markers and analysis of intraspecific genetic variability in chickpea (Cicer arietinum L.). Theor. Appl. Genet. 2006, 112, 1416–1428. [Google Scholar] [CrossRef]
- Du, Q.; Wang, B.; Wei, Z.; Zhang, D.; Li, B. Genetic Diversity and Population Structure of Chinese White Poplar (Populus tomentosa) Revealed by SSR Markers. J. Hered. 2012, 103, 853–862. [Google Scholar] [CrossRef]
- Ramakrishnan, M.; Ceasar, S.A.; Duraipandiyan, V.; Al-Dhabi, N.A.; Ignacimuthu, S. Assessment of genetic diversity, population structure and relationships in Indian and non-Indian genotypes of finger millet (Eleusine coracana (L.) Gaertn) using genomic SSR markers. SpringerPlus 2016, 5, 120. [Google Scholar] [CrossRef]
- Gil, J.; Um, Y.; Kim, S.; Kim, O.T.; Koo, S.C.; Reddy, C.S.; Kim, S.-C.; Hong, C.P.; Park, S.-G.; Kim, H.B.; et al. Development of Genome-Wide SSR Markers from Angelica gigas Nakai Using Next Generation Sequencing. Genes 2017, 8, 238. [Google Scholar] [CrossRef]
- Liu, F.; Hong, Z.; Xu, D.; Jia, H.; Zhang, N.; Liu, X.; Yang, Z.; Lu, M. Genetic Diversity of the Endangered Dalbergia odorifera Revealed by SSR Markers. Forests 2019, 10, 225. [Google Scholar] [CrossRef]
- Wang, S.-Q. Genetic diversity and population structure of the endangered species Paeonia decomposita endemic to China and implications for its conservation. BMC Plant Biol. 2020, 20, 510. [Google Scholar] [CrossRef]
- Serrote, C.M.L.; Reiniger, L.R.S.; Silva, K.B.; Rabaiolli, S.M.d.S.; Stefanel, C.M. Determining the Polymorphism Information Content of a Molecular Marker. Gene 2020, 726, 144175. [Google Scholar] [CrossRef]
- Falk, D.A. Integrated Strategies for Conserving Plant Genetic Diversity. Ann. Mo. Bot. Gard. 1990, 77, 38–47. [Google Scholar] [CrossRef]
- Escudero, A.; Iriondo, J.M.; Torres, M. Spatial analysis of genetic diversity as a tool for plant conservation. Biol. Conserv. 2003, 113, 351–365. [Google Scholar] [CrossRef]
- Yu, Y.-L.; Wang, H.-C.; Yu, Z.-X.; Schinnerl, J.; Tang, R.; Geng, Y.-P.; Chen, G. Genetic diversity and structure of the endemic and endangered species Aristolochia delavayi growing along the Jinsha River. Plant Divers. 2021, 43, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Gaudeul, M.; Taberlet, P.; Till-Bottraud, I. Genetic diversity in an endangered alpine plant, Eryngium alpinum L. (Apiaceae), inferred from amplified fragment length polymorphism markers. Mol. Ecol. 2000, 9, 1625–1637. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Y.; Liao, L.; Li, W.; Li, Z.-Z. Genetic diversity and population structure of the endangered alpine quillwort Isoetes hypsophila Hand.-Mazz. revealed by AFLP markers. Plant Syst. Evol. 2010, 290, 127–139. [Google Scholar] [CrossRef]
- Milarska, S.E.; Androsiuk, P.; Bednarek, P.T.; Larson, K.; Giełwanowska, I. Genetic variation of Cerastium alpinum L. from Babia Góra, a critically endangered species in Poland. J. Appl. Genet. 2023, 64, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liang, Q.; Hao, G.; Chen, C.; Liu, J. Population genetic analyses of the endangered alpine Sinadoxa corydalifolia (Adoxaceae) provide insights into future conservation. Biodivers. Conserv. 2018, 27, 2275–2291. [Google Scholar] [CrossRef]
- Yun, S.A.; Kim, S.-C. Genetic diversity and structure of Saussurea polylepis (Asteraceae) on continental islands of Korea: Implications for conservation strategies and management. PLoS ONE 2021, 16, e0249752. [Google Scholar] [CrossRef]
- Zawko, G.; Krauss, S.L.; Dixon, K.W.; Sivasithamparam, K. Conservation genetics of the rare and endangered Leucopogon obtectus(Ericaceae). Mol. Ecol. 2001, 10, 2389–2396. [Google Scholar] [CrossRef]
- Booy, G.; Hendriks, R.J.J.; Smulders, M.J.M.; Van Groenendael, J.M.; Vosman, B. Genetic Diversity and the Survival of Populations. Plant Biol. 2000, 2, 379–395. [Google Scholar] [CrossRef]
- Young, A.G.; Brown, A.H.D.; Zich, F.A. Genetic Structure of Fragmented Populations of the Endangered Daisy Rutidosis leptorrhynchoides. Conserv. Biol. 1999, 13, 256–265. [Google Scholar] [CrossRef]
- Friedman, J.; Barrett, S.C.H. High Outcrossing in the Annual Colonizing Species Ambrosia artemisiifolia (Asteraceae). Ann. Bot. 2008, 101, 1303–1309. [Google Scholar] [CrossRef]
- Ferrer, M.M.; Eguiarte, L.E.; Montaña, C. Genetic structure and outcrossing rates in Flourensia cernua (Asteraceae) growing at different densities in the South-western Chihuahuan Desert. Ann. Bot. 2004, 94, 419–426. [Google Scholar] [CrossRef]
- Xiao, Y.; Jiang, X.; Lu, C.; Liu, J.; Diao, S.; Jiang, J. Genetic Diversity and Population Structure Analysis in the Chinese Endemic Species Michelia crassipes Based on SSR Markers. Forests 2023, 14, 508. [Google Scholar] [CrossRef]
- Duminil, J.; Fineschi, S.; Hampe, A.; Jordano, P.; Salvini, D.; Vendramin, G.G.; Petit, R.J. Can population genetic structure be predicted from life-history traits? Am. Nat. 2007, 169, 662–672. [Google Scholar] [CrossRef]
- Hmeljevski, K.V.; Wolowski, M.; Forzza, R.C.; Freitas, L. High outcrossing rates and short-distance pollination in a species restricted to granitic inselbergs. Aust. J. Bot. 2017, 65, 315–326. [Google Scholar] [CrossRef]
- Szczecińska, M.; Sramko, G.; Wołosz, K.; Sawicki, J. Genetic Diversity and Population Structure of the Rare and Endangered Plant Species Pulsatilla patens (L.) Mill in East Central Europe. PLoS ONE 2016, 11, e0151730. [Google Scholar] [CrossRef] [PubMed]
- Storfer, A. Gene flow and endangered species translocations: A topic revisited. Biol. Conserv. 1999, 87, 173–180. [Google Scholar] [CrossRef]
- Govindaraju, D.R. Relationship between Dispersal Ability and Levels of Gene Flow in Plants. Oikos 1988, 52, 31–35. [Google Scholar] [CrossRef]
- Gerber, S.; Chadœuf, J.; Gugerli, F.; Lascoux, M.; Buiteveld, J.; Cottrell, J.; Dounavi, A.; Fineschi, S.; Forrest, L.L.; Fogelqvist, J.; et al. High rates of gene flow by pollen and seed in oak populations across Europe. PLoS ONE 2014, 9, e85130. [Google Scholar] [CrossRef]
- Tang, J.; Fan, X.; Milne, R.I.; Yang, H.; Tao, W.; Zhang, X.; Guo, M.; Li, J.; Mao, K. Across two phylogeographic breaks: Quaternary evolutionary history of a mountain aspen (Populus rotundifolia) in the Hengduan Mountains. Plant Divers. 2024, 46, 321–332. [Google Scholar] [CrossRef]
- Su, R. Study on tourism climate resources and comfort in Hejing County. Rural. Technol. 2017, 19, 87–88. [Google Scholar] [CrossRef]
- Stronen, A.V.; Norman, A.J.; Wal, E.V.; Paquet, P.C. The relevance of genetic structure in ecotype designation and conservation management. Evol. Appl. 2022, 15, 185–202. [Google Scholar] [CrossRef]
- Dobrowski, S.Z. A climatic basis for microrefugia: The influence of terrain on climate. Glob. Change Biol. 2011, 17, 1022–1035. [Google Scholar] [CrossRef]
- Lande, R.; Barrowclough, G.F. Effective population size, genetic variation, and their use in population management. Viable Popul. Conserv. 1987, 87, 87–124. [Google Scholar]
- Schemske, D.W.; Husband, B.C.; Ruckelshaus, M.H.; Goodwillie, C.; Parker, I.M.; Bishop, J.G. Evaluating Approaches to the Conservation of Rare and Endangered Plants. Ecology 1994, 75, 584–606. [Google Scholar] [CrossRef]
- Li, J.; Cai, S. Advances in chemical and pharmacological studies of Saussurea involucrata. Chin. J. Pharm. 1998, 8, 3–6. [Google Scholar]
- Chen, F.; Yang, Y. Advances in species, habitat distribution and chemical constituents of Saussurea involucrata in China. Bot. Bull. 1999, 5, 561–566. [Google Scholar] [CrossRef]
- Aiguly, S.; Adiri, S. The research status of Hami ‘Snow Mountain Flower King’—S. involucrata. Chin. J. Ethn. Med. 2016, 22, 26–28. [Google Scholar]
- Wei, S.; Wu, Y. Research progress on endangered medicinal plant Saussurea involucrate resources. J. Minzu Univ. China (Nat. Sci. Ed.) 2014, 23, 10–15. [Google Scholar]
- Feng, J. Protection and Development of Saussurea involucrata. For. Pract. Technol. 2004, 10, 33. [Google Scholar] [CrossRef]


| Items | Numbers |
|---|---|
| Total size of the genome (Mb) | 168.12 |
| Total number of identified SSRs | 673,244 |
| Total length of SSRs (bp) | 12,818,069 |
| Frequency (SSRs/Mb) | 4004.61 |
| Density (bp/Mb) | 76,244.85 |
| Total content of genome SSRs (%) | 7.62 |
| Repeat Type | Predominant Type | Number | Proportion (%) | Frequency (SSRs/Mb) | Total Length (bp) | Average Length (bp) |
|---|---|---|---|---|---|---|
| Mono | A/T | 184,196 | 27.36 | 1095.64 | 2,264,331 | 12.29 |
| Di | AT/AT | 405,972 | 60.30 | 2414.82 | 8,661,590 | 21.34 |
| Tri | ATC/ATG | 71,601 | 10.64 | 425.90 | 1,338,753 | 18.70 |
| Tetra | ACAT/ATGT | 6062 | 0.90 | 36.06 | 360,580 | 59.48 |
| Penta | AACCC/GGGTT | 2242 | 0.33 | 13.34 | 61,925 | 27.62 |
| Hexa | AAGGAG/CCTTCT | 3171 | 0.47 | 18.86 | 130,890 | 41.28 |
| Total | 673,244 | 100 | 4004.61 | 12,818,069 | 19.04 |
| S4 | S10 | S11 | S15 | S16 | S20 | S23 | S24 | S25 | S26 | S29 | S30 | S31 | S32 | S35 | S36 | S37 | S38 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S4 | ||||||||||||||||||
| S10 | 0.474 | |||||||||||||||||
| S11 | 0.977 | 0.981 | ||||||||||||||||
| S15 | 0.414 | 0.457 | 0.966 | |||||||||||||||
| S16 | 0.370 | 0.819 | 0.970 | 0.139 | ||||||||||||||
| S20 | 0.024 * | 0.487 | 0.994 | 0.911 | 0.210 | |||||||||||||
| S23 | 0.906 | 0.373 | 1.000 | 0.848 | 0.950 | 0.558 | ||||||||||||
| S24 | 0.637 | 0.388 | 1.000 | 0.727 | 0.875 | 0.495 | 0.841 | |||||||||||
| S25 | 0.060 | 0.529 | 0.788 | 0.618 | 0.775 | 0.110 | 0.131 | 0.083 | ||||||||||
| S26 | 0.315 | 0.506 | 0.703 | 0.922 | 0.289 | 0.382 | 0.917 | 0.979 | 0.057 | |||||||||
| S29 | 0.995 | 0.667 | 0.992 | 0.925 | 0.913 | 0.999 | 0.864 | 0.999 | 0.908 | 0.869 | ||||||||
| S30 | 0.297 | 0.837 | 0.837 | 0.232 | 0.937 | 1.000 | 0.999 | 0.883 | 0.166 | 0.130 | 0.971 | |||||||
| S31 | 0.277 | 0.393 | 0.979 | 0.520 | 1.000 | 0.744 | 0.694 | 0.565 | 0.026 * | 0.390 | 0.996 | 0.198 | ||||||
| S32 | 0.875 | 0.503 | 1.000 | 0.812 | 0.384 | 0.940 | 0.825 | 0.249 | 0.111 | 0.950 | 0.649 | 0.351 | 0.016 * | |||||
| S35 | 0.013 * | 0.940 | 1.000 | 0.431 | 0.423 | 0.202 | 0.351 | 0.169 | 0.001 * | 0.332 | 0.679 | 0.054 | 0.018 * | 0.016 * | ||||
| S36 | 0.963 | 0.186 | 0.987 | 0.567 | 0.995 | 0.954 | 0.548 | 1.000 | 0.385 | 0.998 | 1.000 | 0.557 | 0.836 | 0.976 | 0.404 | |||
| S37 | 0.782 | 0.722 | 1.000 | 0.405 | 0.648 | 0.934 | 0.535 | 0.782 | 0.250 | 0.926 | 0.941 | 0.711 | 0.003 * | 0.079 | 0.004 * | 0.642 | ||
| S38 | 0.978 | 0.161 | 0.990 | 0.727 | 0.991 | 0.844 | 0.706 | 0.433 | 0.997 | 0.774 | 0.893 | 0.826 | 0.597 | 0.923 | 0.175 | 1.000 | 0.956 |
| Locus | Na | Ne | I | Nm | Ho | He | uHe | Fst | PIC | Nei’s |
|---|---|---|---|---|---|---|---|---|---|---|
| S10 | 7 | 5.700 | 1.818 | 1.664 | 0.702 | 0.811 | 0.863 | 0.131 | 0.971 | 0.811 |
| S11 | 3 | 1.901 | 0.754 | 1.120 | 0.008 | 0.438 | 0.461 | 0.182 | 0.978 | 0.438 |
| S15 | 6 | 3.812 | 1.437 | 3.552 | 1.000 | 0.697 | 0.737 | 0.066 | 0.984 | 0.697 |
| S16 | 2 | 1.452 | 0.376 | 0.522 | 0.000 | 0.234 | 0.246 | 0.324 | 0.980 | 0.234 |
| S20 | 4 | 2.736 | 1.011 | 1.255 | 0.125 | 0.549 | 0.580 | 0.166 | 0.983 | 0.549 |
| S23 | 2 | 1.639 | 0.529 | 1.191 | 0.000 | 0.345 | 0.366 | 0.173 | 0.971 | 0.345 |
| S24 | 3 | 2.028 | 0.804 | 0.750 | 0.151 | 0.455 | 0.482 | 0.250 | 0.968 | 0.455 |
| S25 | 3 | 2.168 | 0.796 | 0.998 | 0.498 | 0.456 | 0.482 | 0.200 | 0.982 | 0.456 |
| S26 | 2 | 1.645 | 0.510 | 0.583 | 0.170 | 0.325 | 0.359 | 0.300 | 0.883 | 0.325 |
| S29 | 3 | 2.507 | 1.026 | 1.233 | 0.709 | 0.592 | 0.631 | 0.169 | 0.955 | 0.592 |
| S30 | 3 | 2.643 | 1.032 | 2.570 | 0.991 | 0.607 | 0.639 | 0.089 | 0.990 | 0.607 |
| S32 | 3 | 2.289 | 0.848 | 1.923 | 1.000 | 0.553 | 0.585 | 0.115 | 0.983 | 0.553 |
| S36 | 2 | 1.618 | 0.552 | 2.444 | 0.000 | 0.342 | 0.360 | 0.093 | 0.982 | 0.342 |
| S37 | 2 | 1.478 | 0.430 | 0.264 | 0.193 | 0.277 | 0.293 | 0.486 | 0.977 | 0.277 |
| S38 | 2 | 1.964 | 0.631 | 0.774 | 0.350 | 0.368 | 0.389 | 0.244 | 0.978 | 0.368 |
| Pop | N | Na | Ne | I | Ho | He | uHe | F | Percentage of Deviation from HWE Site (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Mean | 9.867 | 3.267 | 2.490 | 0.854 | 0.374 | 0.480 | 0.506 | 0.333 | 66.667 |
| SE | 0.307 | 0.672 | 0.473 | 0.140 | 0.116 | 0.058 | 0.061 | 0.192 | ||
| 2 | Mean | 8.400 | 3.200 | 2.458 | 0.848 | 0.426 | 0.475 | 0.506 | 0.193 | 53.333 |
| SE | 0.335 | 0.518 | 0.424 | 0.137 | 0.110 | 0.060 | 0.064 | 0.193 | ||
| 3 | Mean | 7.667 | 3.800 | 2.651 | 0.950 | 0.403 | 0.501 | 0.538 | 0.326 | 53.333 |
| SE | 0.333 | 0.611 | 0.475 | 0.144 | 0.114 | 0.058 | 0.063 | 0.177 | ||
| 4 | Mean | 9.467 | 3.067 | 2.340 | 0.868 | 0.431 | 0.501 | 0.529 | 0.121 | 60.000 |
| SE | 0.215 | 0.371 | 0.225 | 0.117 | 0.120 | 0.059 | 0.063 | 0.212 | ||
| 5 | Mean | 9.333 | 3.067 | 2.350 | 0.864 | 0.420 | 0.496 | 0.525 | 0.167 | 46.667 |
| SE | 0.211 | 0.358 | 0.251 | 0.117 | 0.108 | 0.061 | 0.064 | 0.181 | ||
| 6 | Mean | 8.933 | 3.400 | 2.841 | 0.915 | 0.375 | 0.506 | 0.536 | 0.289 | 73.333 |
| SE | 0.206 | 0.668 | 0.570 | 0.160 | 0.112 | 0.068 | 0.072 | 0.205 | ||
| 7 | Mean | 9.133 | 3.267 | 2.234 | 0.847 | 0.375 | 0.480 | 0.509 | 0.299 | 73.333 |
| SE | 0.376 | 0.483 | 0.279 | 0.112 | 0.107 | 0.048 | 0.051 | 0.185 | ||
| 8 | Mean | 9.733 | 3.133 | 2.428 | 0.865 | 0.346 | 0.499 | 0.526 | 0.372 | 80.000 |
| SE | 0.153 | 0.487 | 0.319 | 0.126 | 0.116 | 0.055 | 0.059 | 0.212 | ||
| 9 | Mean | 10.667 | 3.467 | 2.283 | 0.849 | 0.415 | 0.453 | 0.476 | 0.274 | 66.667 |
| SE | 0.159 | 0.424 | 0.292 | 0.130 | 0.107 | 0.066 | 0.069 | 0.167 | ||
| 10 | Mean | 9.667 | 2.467 | 1.940 | 0.599 | 0.418 | 0.345 | 0.364 | −0.111 | 53.333 |
| SE | 0.187 | 0.435 | 0.301 | 0.143 | 0.118 | 0.075 | 0.079 | 0.182 | ||
| 11 | Mean | 9.867 | 3.267 | 2.490 | 0.854 | 0.374 | 0.480 | 0.465 | 0.403 | 66.667 |
| SE | 0.307 | 0.672 | 0.473 | 0.140 | 0.116 | 0.058 | 0.062 | 0.186 |
| Source of Variation | df | SS | Est. Var. | Variation (%) |
|---|---|---|---|---|
| Among Pops | 10 | 297.870 | 0.617 | 2.560 |
| Within Pops | 101 | 2373.701 | 23.502 | 97.440 |
| Total | 111 | 2671.571 | 24.119 | 100.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, L.; Wang, J.; Wang, X.; Zhang, D.; Sun, Y.; Lu, T.; Shi, W. Development of SSR Markers and Evaluation of Genetic Diversity of Endangered Plant Saussurea involucrata. Biomolecules 2024, 14, 1010. https://doi.org/10.3390/biom14081010
Hu L, Wang J, Wang X, Zhang D, Sun Y, Lu T, Shi W. Development of SSR Markers and Evaluation of Genetic Diversity of Endangered Plant Saussurea involucrata. Biomolecules. 2024; 14(8):1010. https://doi.org/10.3390/biom14081010
Chicago/Turabian StyleHu, Lin, Jiancheng Wang, Xiyong Wang, Daoyuan Zhang, Yanxia Sun, Ting Lu, and Wei Shi. 2024. "Development of SSR Markers and Evaluation of Genetic Diversity of Endangered Plant Saussurea involucrata" Biomolecules 14, no. 8: 1010. https://doi.org/10.3390/biom14081010
APA StyleHu, L., Wang, J., Wang, X., Zhang, D., Sun, Y., Lu, T., & Shi, W. (2024). Development of SSR Markers and Evaluation of Genetic Diversity of Endangered Plant Saussurea involucrata. Biomolecules, 14(8), 1010. https://doi.org/10.3390/biom14081010







