Abstract
Mastitis is a significant inflammatory condition of the mammary gland in dairy cows. It is caused by bacterial infections and leads to substantial economic losses worldwide. The disease can be either clinical or sub-clinical and presents challenges such as reduced milk yield, increased treatment costs, and the need to cull affected cows. The pathogenic mechanisms of mastitis involve the activation of Toll-like receptors (TLRs), specifically TLR2 and TLR4. These receptors play crucial roles in recognizing pathogen-associated molecular patterns (PAMPs) and initiating immune responses through the NF-κB signaling pathway. Recent in vitro studies have emphasized the importance of the TLR2/TLR4/NF-κB signaling pathway in the development of mastitis, suggesting its potential as a therapeutic target. This review summarizes recent research on the role of the TLR2/TLR4/NF-κB signaling pathway in mastitis. It focuses on how the activation of TLRs leads to the production of proinflammatory cytokines, which, in turn, exacerbate the inflammatory response by activating the NF-κB signaling pathway in mammary gland tissues. Additionally, the review discusses various bioactive compounds and probiotics that have been identified as potential therapeutic agents for preventing and treating mastitis by targeting TLR2/TLR4/NF-κB signaling pathway. Overall, this review highlights the significance of targeting the TLR2/TLR4/NF-κB signaling pathway to develop effective therapeutic strategies against mastitis, which can enhance dairy cow health and reduce economic losses in the dairy industry.
1. Introduction
Dairy cow mastitis is a condition characterized by inflammation and a weakened immune response in the mammary gland tissue [1,2]. This occurs when harmful microorganisms invade the gland, causing damage and impairing milk production. In severe cases, affected cows may need to be culled, leading to substantial economic losses for the dairy industry. Multiple studies have shown that mastitis poses significant challenges, including decreased milk yield, the need for treatment, reproductive issues, and the necessity of culling [3,4,5,6,7]. Globally, mastitis results in an estimated annual economic cost of between US$19.7 billion and US$32 billion. In the United States, the annual economic loss due to mastitis is around US$2 billion, while in Canada, the dairy industry faces a Can$400 million loss (equivalent to US$318 million). China experiences annual fiscal losses ranging from 15 to 45 billion Chinese Yuan (CNY) due to mastitis [8,9,10].
The multifaceted nature of mastitis as a disease is widely recognized in the scientific community [11]. Mastitis is typically classified into clinical and sub-clinical forms. Clinical mastitis is characterized by pronounced pathological and physiological changes in mammary gland tissues, while sub-clinical mastitis, particularly when caused by Staphylococcus aureus, often presents more subtly, with no obvious symptoms except for elevated milk somatic cell counts and a decrease in milk yield [12,13,14,15,16,17]. The primary bacterial pathogens associated with mastitis include Escherichia coli, Streptococcus uberis (S. uberis), S. dysgalactiae, and S. aureus [18,19,20]. Toll-like receptors (TLRs) are considered the important pathogen sensors of Pattern recognition receptors (PRRs) [21,22]. Toll like Receptors are the major sensors stimulated during bacterial infection that begins the inflammatory process first by sensing pathogen-associated–molecular-patterns (PAMPs). The PAMPs then start to attract the immune cells towards the site of infection [23,24].
Among TLRs, TLR2 and TLR4 are particularly important in bacterial induced mastitis [25,26,27,28]. Notably, the TLR4 is more specifically utilized by LPS produced bacteria [29,30,31], while TLR2 are predominantly utilized by S. aureus and other gram-positive bacteria to induced inflammatory changes [32,33,34,35]. Activation of PRRs (i.e., TLR2 and TLR4) stimulates Nuclear Factor kappa-light-chain-enhancer of activated B cells (NF-κB) and Mitogen-activated protein kinase (MAPK) signaling pathways, thereby triggering the production of proinflammatory cytokines [36]. NF-κB is a family of key transcription factors that translocate to the nucleus in response to the activation of this signaling pathway. It is one of the major signaling pathways, transmitting pro-inflammatory response during infection and inflammation, as well as tumor growth and metastasis [37,38]. Under steady state, NF-κB is retained in the cytosol by a family of inhibitory proteins, such as IκB. However, upon stimulation, IKK is phosphorylated and degraded; therefore, the free NF-κB is translocated to the nucleus to induce the expression of its target genes. NF-κB is activated via two signaling pathways: the canonical and non-canonical (or alternative) signaling pathways. The canonical signaling pathway is activated by a broad range of stimuli, including TLRs, which induces phosphorylation of IκB and triggers its degradation by the proteasome [39]. On the other hand, the non-canonical signaling pathway responds to selective stimuli and does not depend on the degradation of IκB, but rather relies on the processing of the NF-κB precursor protein p100 [40]. During the processing of p100, its C-terminal IκB-like structure undergoes degradation, thereby triggering the nuclear translocation of mature p100 [41,42]. TLRs trigger the NF-κB pathways, further activating the proinflammatory cytokine cascade. The production of proinflammatory cytokines (TNF-α, IL-1β, and IL-6) is particularly increased in the infected mammary gland [43,44,45,46].
Bovine mastitis is commonly treated with antibiotics, but the excessive and indiscriminate use of antibiotics may contribute to antimicrobial resistance [47,48]. In order to combat this resistance, researchers are exploring alternative options to prevent bovine mastitis [48,49,50,51,52,53]. Recent studies have identified several bioactive compounds and probiotics that show promise in preventing and treating mastitis [35,41,54,55,56,57,58]. These compounds have been found to regulate the expression of key signaling molecules involved in the inflammatory response of mammary gland cells. More specifically, they appear to inhibit the activation of the NF-κB and TLRs signaling pathways [41,55,58], which play a crucial role in initiating and propagating inflammation in the mammary gland. These inflammatory processes are recognized as the main pathological mechanisms in mastitis development. Based on available data, this review aims to summarize the research progress on the critical role of TLR2/TLR4/NF-κB signaling pathway activation in the prevention and treatment of mastitis. Additionally, it delves into the details of how probiotics and bioactive compounds can mitigate mastitis by targeting the NF-κB signaling pathway.
2. Methodology
This review article was completed based on published findings related to activated TLR2/TLR4/NF-κB signaling pathway role in mastitis prevention research in last four years (April 2020–April 2024). The major searching esteemed academic databases data basis used for this review article were “Google Scholar, Web of Science, X-MOL, and PubMed”. The selection of literature was guided by a set of predetermined keywords: “Proinflammatory cytokines”, “Mammalian Mammary gland Cells”, “TLR2/TLR4/NF-κB Signaling pathways”, “Bioactive compounds”, ‘’Probiotics” and “Bacterial Induced mastitis”. To ensure the credibility and relevance of the sourced information, only articles published in English and indexed in Science Citation Index (SCI) Journals were considered for this review. In addition, book chapters and articles published in non-English languages were excluded from this review to maintain a focused and high-quality dataset for analysis.
3. Role of TLR2/TLR4/NF-κB Signaling Pathways in Mastitis Pathogenesis
3.1. TLRs Role in Mastitis Pathogenesis
TLRs, are essential sensors that detect pathogens through pattern recognition receptors (PRRs) [21,22]. Among these sensors, Toll-like receptors play a prominent role in initiating the inflammatory process during bacterial infections by recognizing PAMPs. This recognition leads to the mobilization of immune cells to the site of infection [23,24]. TLRs are composed of multiple leucine-rich repeat (LRR) sequences, which can be divided into three domains: the ectodomain, responsible for pathogen recognition; the transmembrane domain, facilitating localization; and the TIR domain, which recruits different downstream signaling adaptors. There are total of ten TLRs that have evolved and are characterized based on their ability to detect different pathogen loads in livestock. Based on their cellular localization, TLRs can be classified into two groups. The first group (TLRs 1, 2, 4, 5, and 6) is found on the cell membrane, while the second group (TLRs 3, 7, 8, and 9) is located in intracellular endosomes. Upon exposure to pathogens, all TLRs activate the myeloid differentiation primary response 88 (MyD88) signaling pathway, except for TLRs 3 and 4, which recruit the TRIF signaling pathway [33].
TLRs play a crucial role in immune system by recognizing pathogens and activating immune responses. NF-κB is a transcription factor that regulates the immune response to infection. Activation of NF-κB leads to the transcription of pro-inflammatory genes, including cytokines, chemokines, and adhesion molecules. Recently a study reported the polymorphisms in TLR4 gene on promoter and 5′ untranslated region (5′UTR) were significantly associated with mastitis in cattle mammary gland tissue [30]. Consistently another study documented that SNPs at 5′UTR (c.1-g27) and coding region (c.87 C>A, c.575 A>G, and c.576 T>G were responsible for mastitis susceptibility in Egyptian buffalo breed [29].
3.2. NF-κB Signaling Pathways Role in Mastitis Pathogenesis
Upon recognition of specific stimulant, TLR2 and TLR4 undergo conformational changes and initiate downstream signaling cascades. Both TLR2 and TLR4 activate a common adaptor protein called MyD88, which recruits and activates IRAKs (interleu-kin-1 receptor-associated kinases), leading to the activation of TNF receptor-associated factor 6(TRAF6). This results in the activation of the IKK complex (IκB kinase), which phosphorylates IκB, the inhibitor of NF-κB. The phosphorylation of IκB by the IKK complex, target it for proteasomal degradation and thereby allowing NF-κB translocation to the nucleus. TLR4 also stimulate the non-canonical NF-κB signaling pathway in latent stage during persistent infection and inflammation [40]. In the nucleus, NF-κB induces the expression of pro-inflammatory genes, leading to the production of cytokines, chemokines, and other inflammatory mediators. The activation of TLR2 and TLR4 can have synergistic effects, amplifying the inflammatory response and ensuring a robust and rapid response to a wide range of pathogens. However, excessive activation and crosstalk can lead to an overproduction of inflammatory mediators, contributing to the pathology of mastitis.
Negative feedback mechanisms exist to prevent excessive inflammation. For instance, certain cytokines produced in response to NF-κB activation can inhibit further TLR signaling. Crosstalk also involves interactions with other signaling pathways, such as those mediated by cytokines like IL-6 and TNF-α, which can further modulate the immune response. Upon infection, TLR2 and TLR4 are activated, leading to NF-κB activation and a strong inflammatory response (Figure 1). This results in the production of cytokines, chemokines, and other inflammatory mediators that help fight off the infection but also cause tissue damage and symptoms of mastitis. If the infection is not resolved, persistent activation of these pathways can lead to chronic inflammation, tissue remodeling, and fibrosis, contributing to long-term damage to the mammary gland.
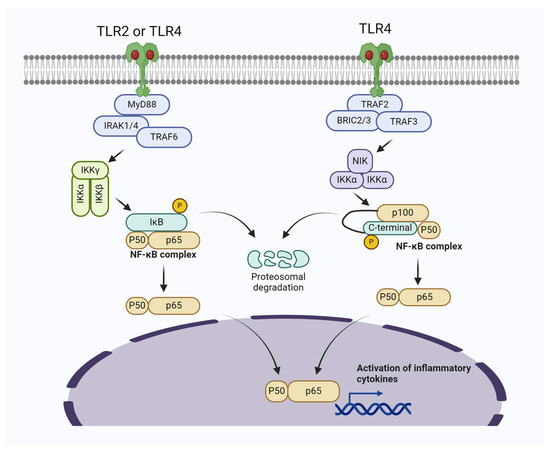
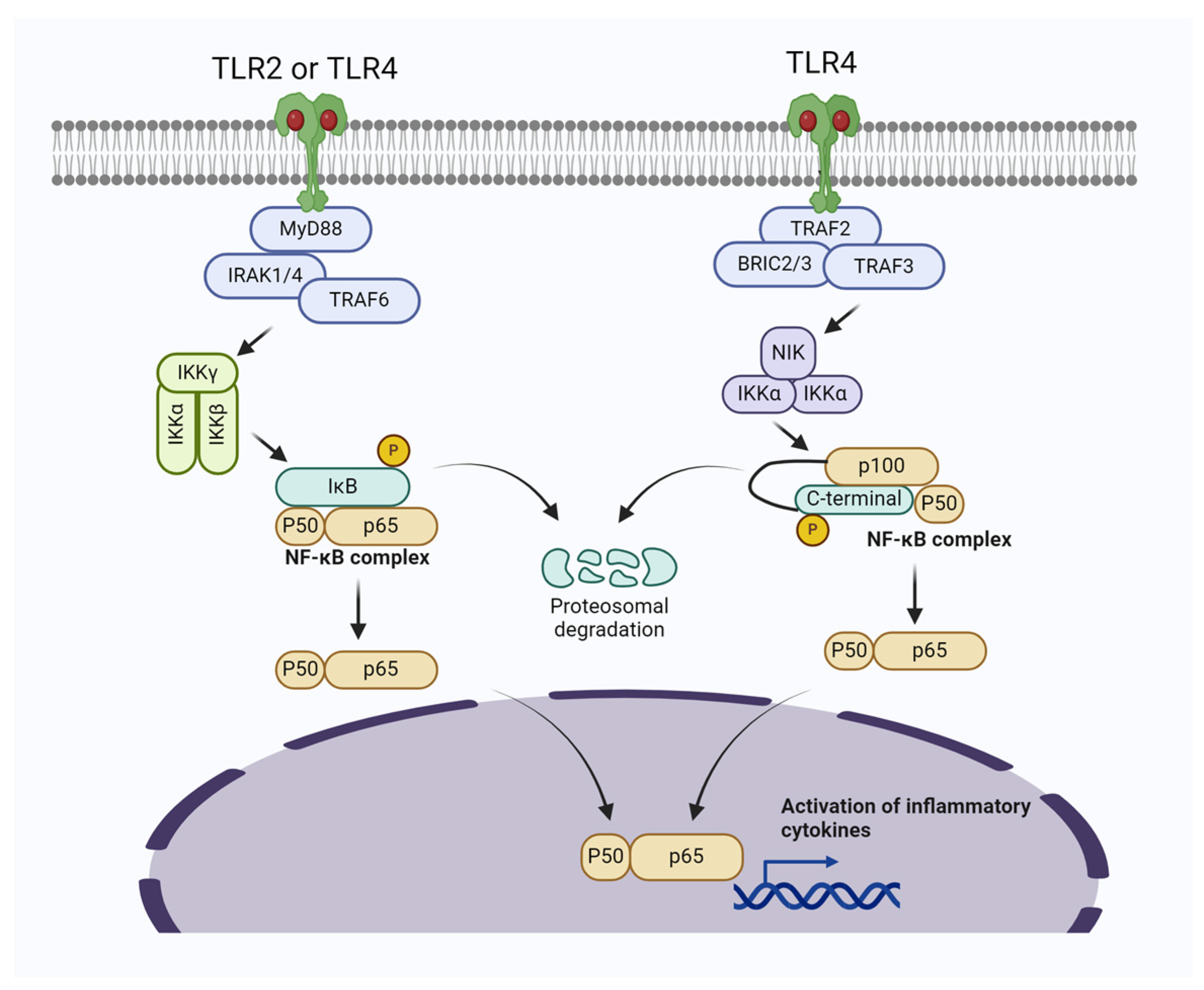
Figure 1.
Bacterial induced mastitis via activating TLR2/TLR4/NF-κB signaling pathways in mammary gland cells. The activation of Myeloid differentiation primary response 88 (MyD88)-dependent signal transduction initiates the recruitment of IRAK1/4 and TRAF6, subsequently leading to the activation of the TAK1-IKK complex. This complex then triggers the activation of NF-κB. In the noncanonical NF-κB pathway, specific subsets of the TNFR superfamily interact with their ligands, resulting in the recruitment of TRAF2/3. Following the ubiquitination and degradation of TRAF3, NF-κB-inducing kinase (NIK) accumulates in the cytoplasm and associates with IKKα, promoting the phosphorylation of p100.
Molecular mechanisms have revealed that the NF-κB signaling pathway plays a key role in the pathogenesis of mastitis in mammary gland cells [2,57]. In line with this, a study reported elevated phosphorylation of MAPKs and AKT/NF-κB P65 signaling pathways, resulting in upregulation of IL-6 and IL-1β and subsequent inflammatory changes in mouse mammary gland cells [41]. Similarly, Akhter et al. [58] found that S. aureus upregulates the expressions of TLR2, TLR4, p-65 IκBα, TNF-α, IL-1β, and IL-6, leading to the activation of NF-κB/MAPKs pathways in bovine mammary epithelial cells (BMECs), which ultimately results in mastitis. Other studies have also shown that pathogenic bacteria induce inflammatory changes and mastitis through the activation of NF-κB in the mammary gland [59,60]. It is worth noting that during pathogen-induced mastitis, there is an increase in reactive oxygen species (ROS) levels, oxidative stress, apoptosis of cells, and significant inflammatory changes. Furthermore, these changes are associated with the upregulation of the NF-κB signaling pathway and the prevention of the activation of the Nrf2/HO-1 pathway [59].
By using transcriptomic analysis, Liu J et al. reported several key signalings including NF-κB signaling pathway in LPS infected BMECs [2]. Similarly, a methylomic screening of milk somatic cell counts from infected cattle’s udder revealed extensive methylation of genes involved in the activation of the NF-κB signaling pathway [61]. Consistently other studies reported that exosomal miR-155 and miR-223 inhibitors downregulate the expression of TLR2, TLR4, TNF-α, and IL-1β, along with the suppression of phosphorylation NF-κB signaling pathway [26,62,63]. The summary of study highlighting the role of TLR2/TLR4/NF-κB signaling pathways in mastitis pathogenesis has been provided in Table 1.

Table 1.
Bacterial activated TLRs/NF-κB signaling pathways role in mastitis pathogenesis.
4. Role of Bioactive Compounds and Probiotics Targeting TLR2/TLR4/NF-κB Signaling Pathways in Mastitis Mitigation
Despite efforts to prevent and treat mastitis, it remains the most common health problem in dairy herds. Therefore, there has been an increasing interest in exploring alternative therapeutic procedures as a substitute for traditional treatment [69,70]. In recent years, there has been a significant rise in research dedicated to finding non-antibiotic alternatives for managing bovine mastitis [47]. The excessive and incorrect use of antibiotics in treating mastitis has caused bacteria strains to become resistant to antibiotics. This resistance can make antibiotics less effective or completely ineffective, which is a major challenge in treating mastitis. Moreover, using antibiotics to treat mastitis in dairy cows can result in antibiotic residues in milk, which raises concerns about food safety. These residues not only pose health risks to consumers but also lead to milk disposal, causing economic losses. Therefore, it is important to explore alternative treatments. Probiotics and bioactive compounds offer promising alternatives that do not contribute to antibiotic resistance. In addition, these beneficial substances do not leave harmful residues, resulting in the production of safer milk and reducing the economic impact. It has been demonstrated that probiotics and bioactive compounds have the ability to modulate the TLR2/TLR4/NF-κB pathway, potentially reducing the inflammatory response and aiding in the prevention of mastitis.
4.1. Bioactive Compounds Targeting TLR2/TLR4/NF-κB Signaling Pathways in Mastitis Mitigation
Extensive research has been conducted on the potential of bioactive compounds to mitigate mastitis by reducing inflammation and fighting against microbes (Table 2, Figure 2) [71,72]. Consistently, Sodium butyrate has been shown to have positive effects in reducing oxidative stress induced by LPS. This includes decreasing ROS and MDA levels, increasing the activity of SOD, GSH-Px, and CAT, as well as reducing inflammation by lowering IL-6, IL-1β, and TNF-α levels. Additionally, Sodium butyrate has demonstrated its anti-apoptotic properties by reducing caspase and Bax levels, while increasing Bcl-2 levels [73]. Sodium butyrate also inhibits NF-κB and caspase/Bax signaling pathways and increases the expression of Nrf2, Keap1, NQO-1, and HO-1 in bovine mammary epithelial cells [73]. Lipoteichoic acid (LTA), a bacterial endotoxin found in the cytoderm of S. aureus, contributes to inflammatory changes associated with to mastitis. Arab et al. [74] have demonstrated that metformin significantly suppresses the expression of NF-κBp65, cyclooxygenase 2 (COX2), IL-1β, and IL-6 in BMECs when exposed to LTA. Furthermore, they found that metformin enhanced the levels of AMPK, Nrf2, HO-1, and Gpx1, thereby promoting the antioxidant response in BMECs [74]. Overall, their study concludes that metformin prevents inflammatory changes and mastitis by activating the AMPK/Nrf2/NF-κB signaling pathway in BMECs [74]. Similarly, another study reported that Brazilin (isolated from the traditional herbal medicine Caesalpinia sappan L.) suppressed TLR2, TNF-α, IL-1β, and IL-6 levels by inhibiting the TLR2/NF-κB/MAPK signaling pathways in mammary gland tissues, thus preventing S. aureus-induced mastitis [75]. A recent study found that daidzein significantly alleviated LPS-induced mastitis in mouse mammary gland epithelial cells by inhibiting IL-6 and IL-1β levels and suppressing the activation of MAPK/NF-κB signaling pathways [76]. Furthermore, it has been documented that diosmetin relieved S. aureus-induced mastitis in mice by inhibiting the expression of MPO, TNF-α, IL-1β, and NF-κB [76]. In addition, diosmetin enhanced the antioxidant response by upregulating the levels of glutathione (GSH), glutathione peroxidase 4 (GPX4), sirtuin 1 (SIRT1), nuclear factor erythroid2-related factor 2 (Nrf2), and heme oxygenase 1 (HO-1), which were decreased by S. aureus in in mouse mammary epithelial cells (MMECs) [76]. It has also been demonstrated that Abrus cantoniensis total flavonoids (ATFs) can prevent lipopolysaccharide-induced mastitis in mouse mammary gland cells. This is achieved by enhancing gut microbiota and inhibiting the expression of the CD14/TLR4/NF-κB/MAPK pathway [77]. In addition, they noticed significant suppression of TNF-α, IL-1β, and IL-6 in response to ATFs [77]. Similarly, another study found that pedunculoside, a bioactive component of Aquifoliaceae, prevents LPS-induced mastitis and maintains the integrity of the blood-milk barrier. This is achieved through downregulation of the AKT/NF-κB/MAPK signaling pathways [78]. Furthermore, the study explored the reversal of inflammatory changes in MMECs through suppression of INF-α, IL-6, IL-1β, MPO, and iNOS levels [78]. Li et al. [79] revealed that jingfang (JF) granules also prevent LPS-induced mastitis. This is accomplished by reducing MPO activity and inhibiting the expression of IL-1β, IL-6, and TNF-α. Additionally, JF treatment downregulates TLR4, P-NF-κB, NLRP3, ASC, Caspase-1, IL-1β, and MAPK signaling pathways. To maintain the integrity of the blood-milk barrier, JF treatment also promotes the expression of ZO-1, claudin-3, and occludin [79]. Evodiamine [80] and oxymatrine [81], among other bioactive compounds, have also been reported to prevent mastitis in MMECs induced by LPS. They achieve this through the suppression of AKT/NF-κB p65/MAPK signaling pathways and pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6). Similarly, rosmarinic acid and nuciferine alleviate LPS-induced mastitis by inhibiting the TLR4/MyD88/NF-κB signaling pathway and production of TNF-α, IL-1β, and IL-6 in MMECs [82,83]. Studies have reported that leonurine [84] and magnolol [85] suppress pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6), iNOS, COX-2, and TLR4, while enhancing IL-10 levels. This is followed by the downregulation of NF-κB-p38 and TLR4/NF-κB/MAPK signaling to mitigate mastitis in MMECs. Gambogic acid (GA) was also reported to alleviate LPS-induced mastitis by reducing IL-6, TNF-α, and IL-1β levels. This is achieved through inhibiting the phosphorylation of the NF-κB/MAPK pathway in MMECs [86]. Furthermore, other bioactive compounds (sodium propionate, curcumin, hederacoside-C, palmatine, sodium butyrate, allicin, oligomeric proanthocyanidins, and dehydroandrographolide) have been reported to inhibit the NF-κB/MAPK signaling pathways and pro-inflammatory cytokines to prevent mastitis [56,73,87,88,89,90,91,92,93]. Based on the existing findings, it has been concluded that bioactive compounds targeting the TLR2/TLR4/NF-κB signaling pathways show promise in mitigating mastitis. This is achieved by reducing inflammation and oxidative stress, as well as combating microbes.

Table 2.
Summary of bioactive compounds targeting TLR2/TLR4/NF-κB signaling pathways in mastitis mitigation.
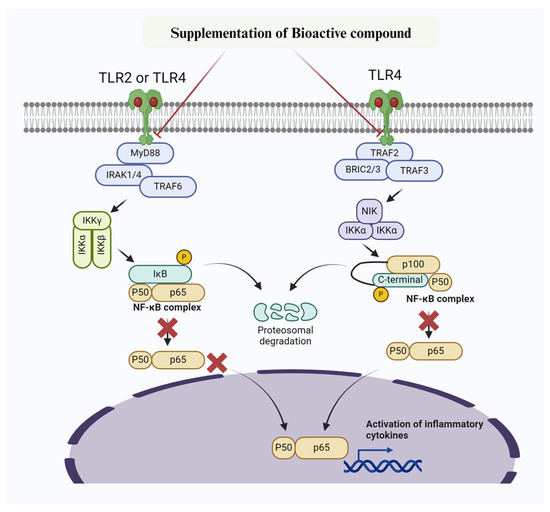
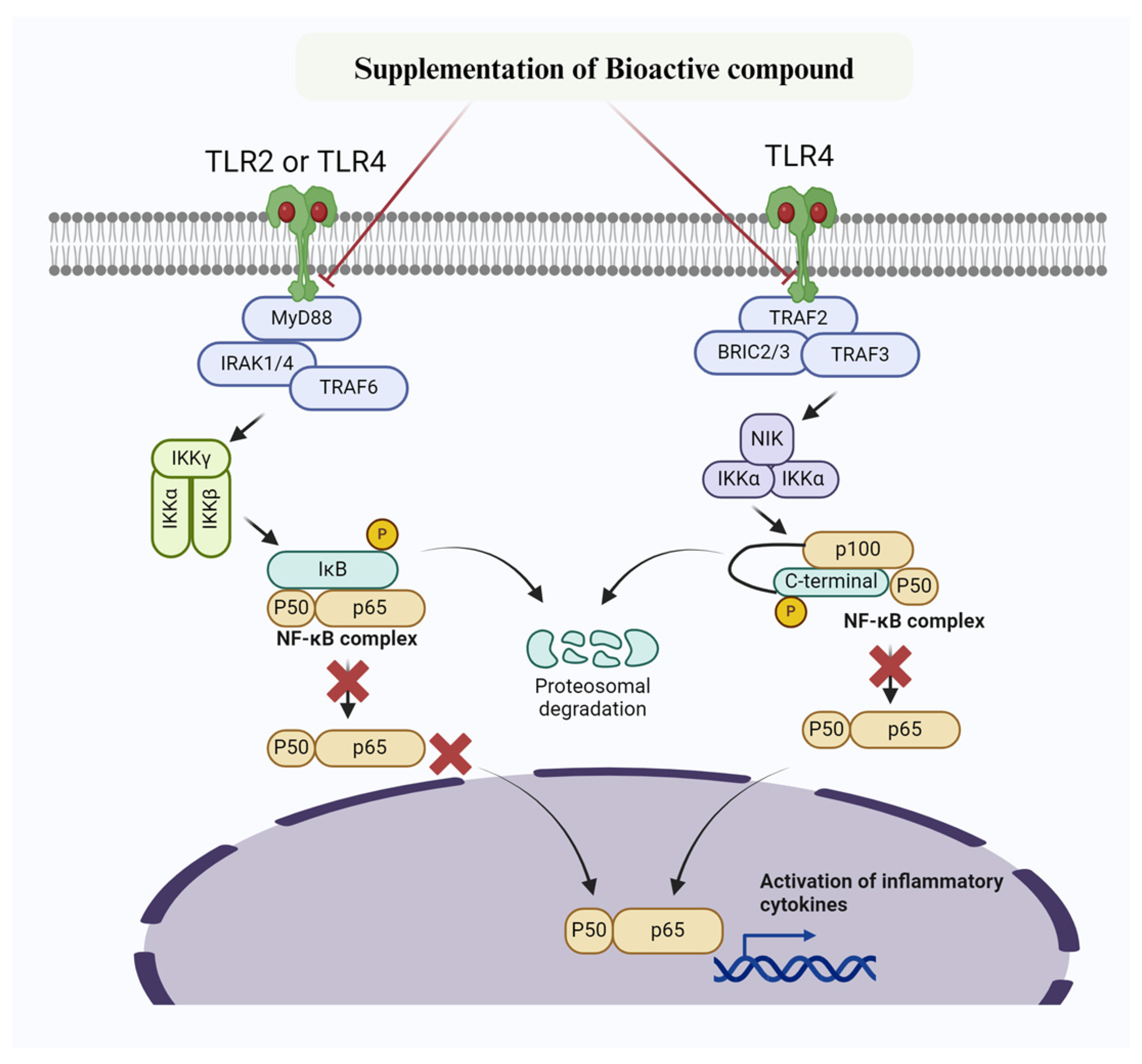
Figure 2.
Mechanism of bioactive compounds targeting NF-κB signaling pathway to prevent inflammatory changes and mastitis. Bioactive compounds inhibit IKK, thereby preventing phosphorylation of IκBα. Furthermore, IκBα super-repressor specifically prevent NF-κB subunits p65 and p50 and other members from entering the nucleus.
4.2. Role of Probiotics Supplementation in Targeting TLR2/TLR4/NF-κB Signaling to Mitigate Mastitis
Probiotics are live microorganisms that, when consumed in sufficient amounts, provide health benefits to the host [108]. They exert their positive effects through various mechanisms, such as modifying the composition of the local microbiota or directly acting on pathogenic microorganisms. Probiotics can adhere to and interact with epithelial cells, improving their barrier function and promoting cell renewal. Additionally, they can enhance the host’s immune system response [109,110]. Touza-Otero and colleagues have previously published a comprehensive review on the role of probiotics in relieving mastitis [47]. However, this study specifically examines the molecular mechanism of probiotics in preventing mastitis, with a particular emphasis on targeting the NF-κB signaling pathway.
The anti-inflammatory effect of probiotics in several diseases, including mastitis, has been well-documented [111,112,113,114,115]. Consequently, the association of gut microbiota with mastitis has been well studied [116,117]. Consistently, several probiotics, such as Lactobacillus plantarum (L. plantarum 17-5) [118,119], Lactobacillus casei [120], Lactobacillus rhamnosus GR-1 [114,121], and Bacillus subtilis [114,122], have been found to prevent inflammatory changes in MMECs and subsequently prevent mastitis caused by E. coli. Furthermore, Bacillus subtilis was found to prevent inflammatory changes in MMECs by inhibiting TNF-α, IL-1β, IL-6, and TLR4 and blocking the TLR4/NF-κB/MAPK signaling pathway [122]. Consequently, Li K et al. [118,119] reported that Lactobacillus plantarum 17-5 and Lactobacillus casei attenuate E. coli-induced inflammatory changes by suppressing the NF-κB/MAPK signaling pathway, leading to decreased production of IL-1β, IL-6, and TNF-α in MMECs [119,121]. Furthermore, in their studies on Lactobacillus plantarum KLDS 1.0344A, Chen et al. [123] experimentally demonstrated its effectiveness in alleviating LPS-induced mastitis through in vitro (BMECs) and in vivo (mastitis mouse model) experiments. They discovered that pre-treatment with Lactobacillus plantarum KLDS 1.0344A significantly reduced the phosphorylated expression levels of p65 and IκBα in the NF-κB/MAPK signaling pathway, thereby controlling the expression of downstream inflammatory factors. Additionally, Lactobacillus plantarum KLDS 1.0344A specifically suppressed the expression of pro-inflammatory cytokines such as IL-6, IL-1β, and TNF-α [123]. Most mastitis-causing pathogens are known to inhibit lactic acid bacteria. However, it has been reported that Lactobacillus plantarum KLDS 1.0344 enhances lactic acid bacteria and reduces MPO activity, thereby mitigating mastitis [123]. Another study by Qiu et al. [124] found that the probiotic Enterococcus mundtii H81 can prevent S. aureus-induced mastitis. It achieves this by inhibiting the activation of the NF-κB signaling pathway and improving the integrity of the blood-milk barrier. Furthermore, they discovered that E. mundtii H81 suppressed the levels of TNF-α and IL-1β, MPO activity, and downregulated the phosphorylation of p65 NF-κB and IκB. It also elevated the expression of tight junction proteins claudin 3 and ZO-1, thus protecting the mammary gland from S. aureus infection [124]. The role of probiotics in mitigating mastitis by targeting NF-κB signaling pathway has been summarized in Figure 3.
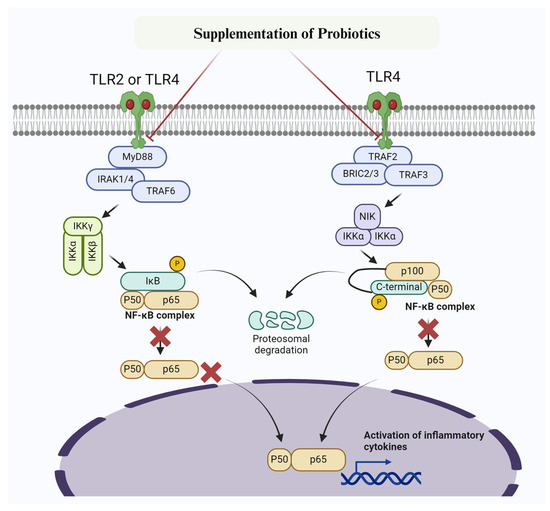
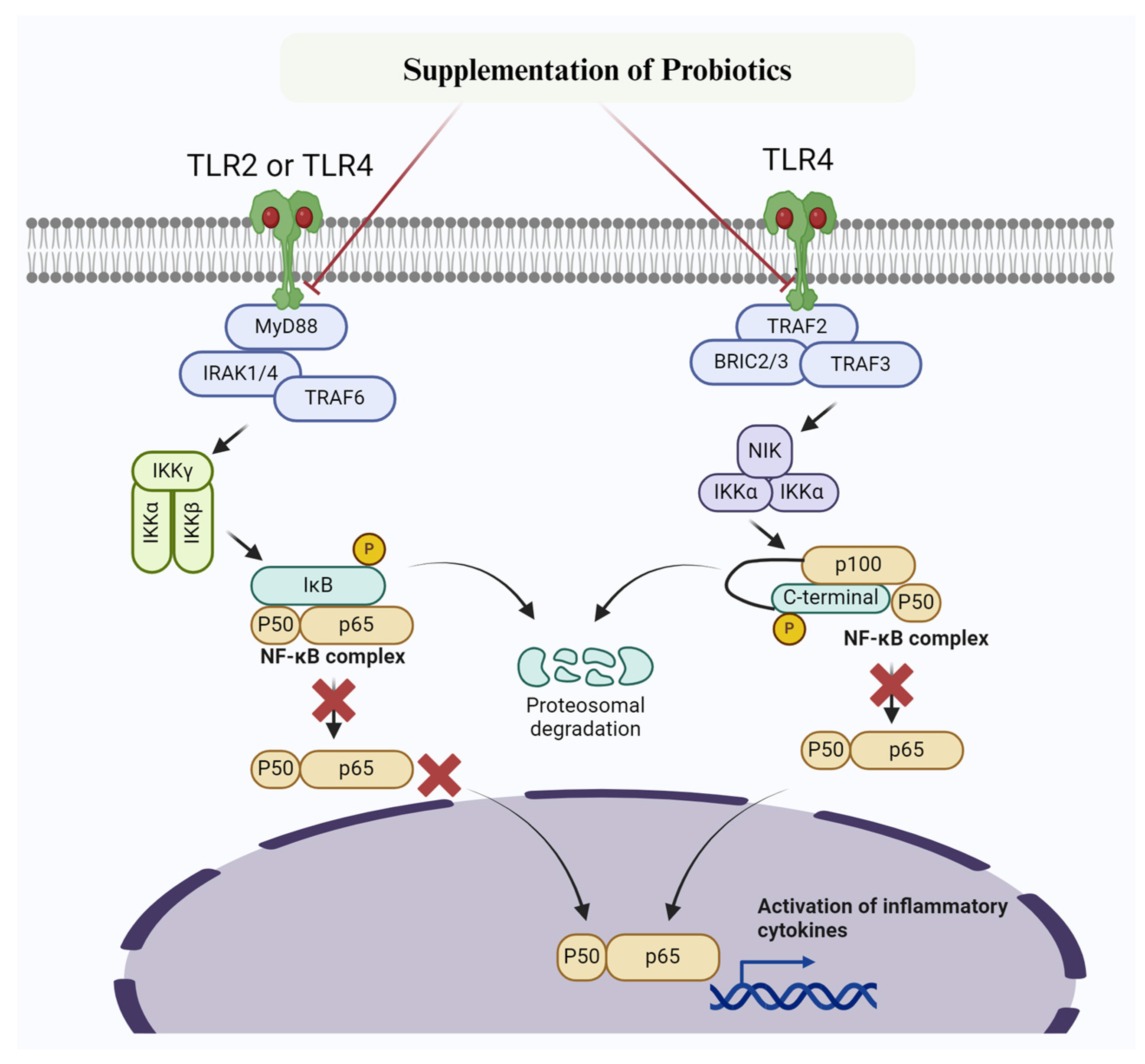
Figure 3.
Mechanism of probiotic targetingTLR2/TLR4 /NF-κB signaling pathway to prevent inflammatory changes and mastitis. Probiotics inhibit IKK, thereby preventing phosphorylation of IκBα. Furthermore, IκBα super-repressor specifically prevent NF-κB subunits p65 and p50 and other members from entering the nucleus.
5. Summary and Future Perspective
Currently, the primary approach to treating mastitis is through the use of antibiotics. Although antibiotics are effective in fighting bacterial pathogens, however, their extensive use has raised concerns regarding antibiotic resistance. The development of antibiotic resistance not only weakens the effectiveness of antibiotics over time but also poses a major risk to public health. Additionally, the presence of antibiotic residues in milk raises concerns about its safety, potentially posing health risks to consumers and creating regulatory complications for dairy producers.
Given these challenges, there is growing interest in alternative therapies, particularly bioactive compounds and probiotics. Bioactive compounds include various plant derivatives, peptides, and other natural molecules, which have shown potential in reducing inflammation and oxidative stress. These compounds can modulate immune responses, thereby helping manage the symptoms of mastitis without inducing antibiotic resistance. For instance, certain flavonoids and essential oils have demonstrated anti-inflammatory and antioxidant properties, capable of alleviating the symptoms of mastitis. Similarly, probiotics, as beneficial microorganisms, offer another promising alternative. These microorganisms can inhibit key inflammatory pathways, enhance the host’s immune response, and compete with pathogenic bacteria in the mammary gland. By maintaining a healthy microbial balance, probiotics can help prevent infections or reduce their severity. For example, certain strains of lactic acid bacteria and bifidobacteria have been shown to enhance mammary gland health and reduce the incidence of mastitis in dairy cows.
Although these alternative methods are promising, further research is needed to fully understand the exact mechanisms by which bioactive compounds and probiotics exert their beneficial effects. Detailed studies are necessary to determine the optimal doses, delivery methods, and combinations of these agents to maximize their efficacy. Additionally, large-scale clinical trials and field studies are required to confirm their safety and effectiveness in different dairy production systems. Future research should focus on elucidating the molecular and cellular pathways affected by these alternative treatments.
Bioactive compounds and probiotics may exert their effects by modulating the TLR2/TLR4/NF-κB signaling pathways. They may influence TLR2/TLR4/NF-κB signaling pathway by interacting with mammary immune cells, potentially enhancing anti-inflammatory responses and promoting tissue repair. By optimizing the use of bioactive compounds and probiotics, we can develop effective strategies for the prevention and treatment of mastitis, thereby reducing dependence on antibiotics. This shift not only addresses the issue of antibiotic resistance but also ensures the safety and quality of milk, benefiting both producers and consumers. Understanding and targeting the TLR2/TLR4/NF-κB signaling pathways will be a key part of this research, providing new insights into the mechanisms of mastitis and how alternative therapies can combat this common dairy disease. Additionally, studying other cellular signaling pathways involved in mastitis and their activation mechanisms in response to probiotics and bioactive compounds will contribute to a more comprehensive understanding of their roles and potential therapeutic targets.
6. Conclusions
The in vitro investigations discussed in this review strongly support the idea that the TLR2/TLR4/NF-κB signaling pathway plays a crucial role in the development of mastitis in dairy cows. When this pathway is activated, it triggers the production of pro-inflammatory cytokines, which worsen the inflammatory response in the mammary gland tissues. By targeting the TLR2/TLR4/NF-κB signaling pathway, we can potentially find a promising therapeutic approach to reduce mastitis. The review also highlights the potential of various bioactive compounds and probiotics to prevent and treat mastitis by influencing this signaling pathway. Utilizing probiotics and bioactive compounds to mitigate mastitis could be the optimal alternative to decrease antibiotic usage and combat the growing problem of antibiotic resistance, which poses a serious threat to public health. These findings emphasize the need for further research on the TLR2/TLR4/NF-κB signaling pathway to improve dairy cow mammary gland health and minimize economic losses in the dairy industry. In addition, future studies must consider the specific sites of action of bioactive compounds in regulating the TLR2/TLR4/NF-κB signaling pathway. Understanding these mechanisms is crucial for the further development of potential therapeutic molecules.
Author Contributions
Conceptualization, methodology, supervision, writing—original draft, project administration: M.Z.K. and C.W.; formal analysis and interpretation, software, data curation, validation, writing—review and editing: M.Z.K., M.Z., X.L., Q.M., T.W., W.C., L.L. and C.W.; resources and funding: C.W. and M.Z.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key R&D Program of China (grant numbers 2022YFD1600103; 2023YFD1302004), The Shandong Province Modern Agricultural Technology System Donkey Industrial Innovation Team (grant no. SDAIT-27), Livestock and Poultry Breeding Industry Project of the Ministry of Agriculture and Rural Affairs (grant number 19211162), the National Natural Science Foundation of China (grant no. 31671287), The Open Project of Liaocheng University Animal Husbandry Discipline (grant no. 319312101-14), The Open Project of Shandong Collaborative Innovation Center for Donkey Industry Technology (grant no. 3193308), Research on Donkey Pregnancy Improvement (grant no. K20LC0901), Key R&D Program Project of Shandong Province (2021TZXD012) and Liaocheng University scientific research fund (grant no. 318052025).
Data Availability Statement
All the data are included in the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Khan, M.Z.; Huang, B.; Kou, X.; Chen, Y.; Liang, H.; Ullah, Q.; Khan, I.M.; Khan, A.; Chai, W.; Wang, C. Enhancing Bovine Immune, Antioxidant and Anti-Inflammatory Responses with Vitamins, Rumen-Protected Amino Acids, and Trace Minerals to Prevent Periparturient Mastitis. Front. Immunol. 2024, 14, 1290044. [Google Scholar] [CrossRef]
- Liu, J.; Gao, Y.; Zhang, X.; Hao, Z.; Zhang, H.; Gui, R.; Liu, F.; Tong, C.; Wang, X. Transcriptome Sequencing Analysis of Bovine Mammary Epithelial Cells Induced by Lipopolysaccharide. Anim. Biotechnol. 2024, 35, 2290527. [Google Scholar] [CrossRef]
- Corrêa, D.C.; Nunes, G.T.; Barcelos, R.A.; Dos Santos, J.R.; Vogel, F.S.; Cargnelutti, J.F. Economic Losses Caused by Mastitis and the Influence of Climate Variation on the Occurrence of the Disease in a Dairy Cattle Farm in Southern Brazil. Trop. Anim. Health Prod. 2024, 56, 78. [Google Scholar] [CrossRef]
- Kour, S.; Sharma, N.; N, B.; Kumar, P.; Soodan, J.S.; Santos, M.V.; Son, Y.O. Advances in Diagnostic Approaches and Therapeutic Management in Bovine Mastitis. Vet. Sci. 2023, 10, 449. [Google Scholar] [CrossRef]
- Richardet, M.; Solari, H.G.; Cabrera, V.E.; Vissio, C.; Agüero, D.; Bartolomé, J.A.; Bó, G.A.; Bogni, C.I.; Larriestra, A.J. The Economic Evaluation of Mastitis Control Strategies in Holstein-Friesian Dairy Herds. Animals 2023, 13, 1701. [Google Scholar] [CrossRef]
- Samaraweera, A.M.; van der Werf, J.H.; Boerner, V.; Hermesch, S. Economic Values for Production, Fertility and Mastitis Traits for Temperate Dairy Cattle Breeds in Tropical Sri Lanka. J. Anim. Breed. Genet. 2022, 139, 330–341. [Google Scholar] [CrossRef]
- Hogeveen, H.; Steeneveld, W.; Wolf, C.A. Production Diseases Reduce the Efficiency of Dairy Production: A Review of the Results, Methods, and Approaches Regarding the Economics of Mastitis. Annu. Rev. Resour. Econ. 2019, 11, 289–312. [Google Scholar] [CrossRef]
- Puerto, M.A.; Shepley, E.; Cue, R.I.; Warner, D.; Dubuc, J.; Vasseur, E. The Hidden Cost of Disease: I. Impact of the First Incidence of Mastitis on Production and Economic Indicators of Primiparous Dairy Cows. J. Dairy Sci. 2021, 104, 7932–7943. [Google Scholar] [CrossRef]
- Wang, L.; Yang, F.; Wei, X.J.; Luo, Y.J.; Guo, W.Z.; Zhou, X.Z.; Guo, Z.T. Prevalence and Risk Factors of Subclinical Mastitis in Lactating Cows in Northwest China. Isr. J. Vet. Med. 2019, 74, 17–22. [Google Scholar]
- Rollin, E.; Dhuyvetter, K.C.; Overton, M.W. The Cost of Clinical Mastitis in the First 30 Days of Lactation: An Economic Modeling Tool. Prev. Vet. Med. 2015, 122, 257–264. [Google Scholar] [CrossRef]
- De Vliegher, S.; Ohnstad, I.; Piepers, S. Management and Prevention of Mastitis: A Multifactorial Approach with a Focus on Milking, Bedding and Data-Management. J. Integr. Agric. 2018, 17, 1214–1233. [Google Scholar] [CrossRef]
- Cobirka, M.; Tancin, V.; Slama, P. Epidemiology and Classification of Mastitis. Animals 2020, 10, 2212. [Google Scholar] [CrossRef]
- Khan, M.Z.; Dari, G.; Khan, A.; Yu, Y. Genetic Polymorphisms of TRAPPC9 and CD4 Genes and Their Association with Milk Production and Mastitis Resistance Phenotypic Traits in Chinese Holstein. Front. Vet. Sci. 2022, 9, 1008497. [Google Scholar] [CrossRef]
- Khan, M.Z.; Khan, A.; Xiao, J.; Ma, Y.; Ma, J.; Gao, J.; Cao, Z. Role of the JAK-STAT Pathway in Bovine Mastitis and Milk Production. Animals 2020, 10, 2107. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.Z.; Khan, A.; Xiao, J.; Ma, J.; Ma, Y.; Chen, T.; Shao, D.; Cao, Z. Overview of Research Development on the Role of NF-κB Signaling in Mastitis. Animals 2020, 10, 1625. [Google Scholar] [CrossRef]
- Wang, D.; Wei, Y.; Shi, L.; Khan, M.Z.; Fan, L.; Wang, Y.; Yu, Y. Genome-Wide DNA Methylation Pattern in a Mouse Model Reveals Two Novel Genes Associated with Staphylococcus aureus Mastitis. Asian Australas. J. Anim. Sci. 2020, 33, 203. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.Z.; Wang, D.; Liu, L.; Usman, T.; Wen, H.; Zhang, R.; Liu, S.; Shi, L.; Mi, S.; Xiao, W.; et al. Significant Genetic Effects of JAK2 and DGAT1 Mutations on Milk Fat Content and Mastitis Resistance in Holsteins. J. Dairy Res. 2019, 86, 388–393. [Google Scholar] [CrossRef]
- Srithanasuwan, A.; Tata, L.; Tananupak, W.; Jaraja, W.; Suriyasathaporn, W.; Chuammitri, P. Exploring the Distinct Immunological Reactions of Bovine Neutrophils towards Major and Minor Pathogens Responsible for Mastitis. Int. J. Vet. Sci. Med. 2023, 11, 106–120. [Google Scholar] [CrossRef]
- Schneider, P.; Salamon, H.; Weizmann, N.; Nissim-Eliraz, E.; Lysnyansky, I.; Shpigel, N.Y. Immune Profiling of Experimental Murine Mastitis Reveals Conserved Response to Mammary Pathogenic Escherichia coli, Mycoplasma bovis, and Streptococcus uberis. Front. Microbiol. 2023, 14, 1126896. [Google Scholar] [CrossRef]
- Tommasoni, C.; Fiore, E.; Lisuzzo, A.; Gianesella, M. Mastitis in Dairy Cattle: On-Farm Diagnostics and Future Perspectives. Animals 2023, 13, 2538. [Google Scholar] [CrossRef]
- Maurić Maljković, M.; Vlahek, I.; Piplica, A.; Ekert Kabalin, A.; Sušić, V.; Stevanović, V. Prospects of Toll-Like Receptors in Dairy Cattle Breeding. Anim. Genet. 2023, 54, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Atli, M.O.; Hitit, M.; Özbek, M.; Köse, M.; Bozkaya, F. Cell-Specific Expression Pattern of Toll-Like Receptors and Their Roles in Animal Reproduction. Toll Like Recept. Health Dis. 2022, 276, 65–93. [Google Scholar]
- Rainard, P.; Gilbert, F.B.; Germon, P. Immune Defenses of the Mammary Gland Epithelium of Dairy Ruminants. Front. Immunol. 2022, 13, 1031785. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, D.; Worku, T.; Dad, R.; Rehman, Z.U.; Gong, X.; Zhang, S. Mechanism of Pattern Recognition Receptors (PRRs) and Host Pathogen Interplay in Bovine Mastitis. Microb. Pathog. 2018, 120, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.Z.; Wang, J.; Ma, Y.; Chen, T.; Ma, M.; Ullah, Q.; Khan, I.M.; Khan, A.; Cao, Z.; Liu, S. Genetic Polymorphisms in Immune-and Inflammation-Associated Genes and Their Association with Bovine Mastitis Resistance/Susceptibility. Front. Immunol. 2023, 14, 1082144. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Gu, B.; Han, X.; Feng, Y. Mammary Epithelial Cell-Derived Exosomal miR-155-Inhibitor Played a Key Role in the Treatment of Mastitis via Down-Regulation of TLRs/NF-κB Signaling Pathway to Inhibit Inflammatory Response. Cell. Mol. Biol. 2023, 69, 160–166. [Google Scholar] [PubMed]
- Islam, M.A.; Takagi, M.; Fukuyama, K.; Komatsu, R.; Albarracin, L.; Nochi, T.; Suda, Y.; Ikeda-Ohtsubo, W.; Rutten, V.; Eden, V.W.; et al. Transcriptome Analysis of the Inflammatory Responses of Bovine Mammary Epithelial Cells: Exploring Immunomodulatory Target Genes for Bovine Mastitis. Pathogens 2020, 9, 200. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, K.; Islam, M.A.; Takagi, M.; Ikeda-Ohtsubo, W.; Kurata, S.; Aso, H.; Vignolo, G.; Villena, J.; Kitazawa, H. Evaluation of the Immunomodulatory Ability of Lactic Acid Bacteria Isolated from Feedlot Cattle against Mastitis Using a Bovine Mammary Epithelial Cells In Vitro Assay. Pathogens 2020, 9, 410. [Google Scholar] [CrossRef] [PubMed]
- Abou Mossallam, A.; El Nahas, S.M.; Osman, N.M.; Shahwan, E.H.; Sabry, N.M. Sequence Analysis of TLR4 Gene in River Buffalo (Egyptian Breed) and SNPs Association with Mastitis. Bull. Natl. Res. Cent. 2023, 47, 110. [Google Scholar] [CrossRef]
- Bhat, R.R.; Bhat, N.N.; Shabir, A.; Mir, M.U.; Ahmad, S.B.; Hussain, I.; Hussain, S.A.; Ali, A.; Shamim, K.; Rehman, M.U. SNP Analysis of TLR 4 Promoter and Its Transcriptional Factor Binding Profile in Relevance to Bovine Subclinical Mastitis. Biochem. Genet. 2023, 1–9. [Google Scholar] [CrossRef]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 Trafficking and Its Influence on LPS-Induced Pro-Inflammatory Signaling. Cell. Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef]
- Simpson, M.E.; Petri, W.A. TLR2 as a Therapeutic Target in Bacterial Infection. Trends Mol. Med. 2020, 26, 715–717. [Google Scholar] [CrossRef]
- Li, B.; Wan, Z.; Wang, Z.; Zuo, J.; Xu, Y.; Han, X.; Phouthapane, V.; Miao, J. TLR2 Signaling Pathway Combats Streptococcus uberis Infection by Inducing Mitochondrial Reactive Oxygen Species Production. Cells 2020, 9, 494. [Google Scholar] [CrossRef]
- Xu, J.; Jia, Z.; Chen, A.; Wang, C. Curcumin Ameliorates Staphylococcus aureus-Induced Mastitis Injury through Attenuating TLR2-Mediated NF-κB Activation. Microb. Pathog. 2020, 142, 104054. [Google Scholar] [CrossRef]
- Ge, B.J.; Zhao, P.; Li, H.T.; Sang, R.; Wang, M.; Zhou, H.Y.; Zhang, X.M. Taraxacum mongolicum Protects against Staphylococcus aureus-Infected Mastitis by Exerting Anti-Inflammatory Role via TLR2-NF-κB/MAPKs Pathways in Mice. J. Ethnopharmacol. 2021, 268, 113595. [Google Scholar] [CrossRef]
- Guo, W.; Liu, B.; Hu, G.; Kan, X.; Li, Y.; Gong, Q.; Xu, D.; Ma, H.; Cao, Y.; Huang, B.; et al. Vanillin Protects the Blood-Milk Barrier and Inhibits the Inflammatory Response in LPS-Induced Mastitis in Mice. Toxicol. Appl. Pharmacol. 2019, 365, 9–18. [Google Scholar] [CrossRef]
- Bochniarz, M.; Hahaj-Siembida, A.; Krajewska-Wędzina, M.; Osińska, M.; Tracz, A.; Trościańczyk, A.; Brodzki, P.; Krakowski, L.; Kosior-Korzecka, U.; Nowakiewicz, A. Cytokine Inflammatory Response in Dairy Cows with Mastitis Caused by Streptococcus agalactiae. J. Vet. Res. 2024, 68, 115. [Google Scholar] [CrossRef]
- Lyu, C.C.; Ji, X.Y.; Che, H.Y.; Meng, Y.; Wu, H.Y.; Zhang, J.B.; Zhang, Y.H.; Yuan, B. CGA Alleviates LPS-Induced Inflammation and Milk Fat Reduction in BMECs through the NF-κB Signaling Pathway. Heliyon 2024, 10, e25004. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, C.; Zhang, Z.; Zhang, H.; Hu, H. NF-κB Signaling in Inflammation and Cancer. MedComm 2021, 2, 618–653. [Google Scholar] [CrossRef]
- Cai, X.; Zhou, Z.; Kan, X.; Xu, P.; Guo, W.; Fu, S.; Liu, J.; Jia, Y. Daidzein Relieves Lipopolysaccharide-Induced Mastitis through Inhibiting MAPKs and AKT/NF-κB P65 Signaling Pathways. Rev. Bras. Farmacogn. 2024, 29, 1–2. [Google Scholar] [CrossRef]
- Wu, J.; Li, L.; Sun, Y.; Huang, S.; Tang, J.; Yu, P.; Wang, G. Altered Molecular Expression of the TLR4/NF-κB Signaling Pathway in Mammary Tissue of Chinese Holstein Cattle with Mastitis. PLoS ONE 2015, 10, e0118458. [Google Scholar] [CrossRef]
- Gao, S.; Tang, L.; Ma, J.; Wang, K.; Yao, H.; Tong, J.; Zhang, H. Evaluation of the Mechanism of Gong Ying San Activity on Dairy Cows Mastitis by Network Pharmacology and Metabolomics Analysis. PLoS ONE 2024, 19, e0299234. [Google Scholar] [CrossRef]
- Shen, J.; Yang, F.; Wang, G.; Mou, X.; Li, J.; Ding, X.; Wang, X.; Li, H. Paeoniflorin Alleviates Inflammation in Bovine Mammary Epithelial Cells Induced by Staphylococcus haemolyticus through TLR2/NF-κB Signaling Pathways. Res. Vet. Sci. 2023, 156, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Wang, X.; Lin, T.; Dong, W.; Gao, Y.; Ji, P.; Zhang, Y.; Zhao, X.; Zhang, Q. Toll-Like Receptor 2 is Associated with the Immune Response, Apoptosis, and Angiogenesis in the Mammary Glands of Dairy Cows with Clinical Mastitis. Int. J. Mol. Sci. 2022, 23, 10717. [Google Scholar] [CrossRef]
- Khan, M.Z.; Zhang, Z.; Liu, L.; Wang, D.; Mi, S.; Liu, X.; Liu, G.; Guo, G.; Li, X.; Wang, Y.; et al. Folic Acid Supplementation Regulates Key Immunity-Associated Genes and Pathways during the Periparturient Period in Dairy Cows. Asian Australas. J. Anim. Sci. 2020, 33, 1507. [Google Scholar] [CrossRef]
- Touza-Otero, L.; Landin, M.; Diaz-Rodriguez, P. Fighting Antibiotic Resistance in the Local Management of Bovine Mastitis. Biomed. Pharmacother. 2024, 170, 115967. [Google Scholar] [CrossRef]
- Cantekin, Z.; Özmen, G.Ö.; Demir, M.; Er, Z.Y.; Gürtürk, K.; Solmaz, H.; Öztürk, D.; Ergün, Y. Presence of Antibiotic Resistance Genes in Staphylococci Isolated from Bovine Subclinical Mastitis. Large Anim. Rev. 2024, 30, 99–104. [Google Scholar]
- Langhorne, C.; Wood, B.J.; Wood, C.; Henning, J.; McGowan, M.; Schull, D.; Ranjbar, S.; Gibson, J.S. Understanding Barriers to Reducing Antimicrobials on Australian Dairy Farms: A Qualitative Analysis. Aust. Vet. J. 2024. [CrossRef]
- Gomes, F.; Henriques, M. Control of Bovine Mastitis: Old and Recent Therapeutic Approaches. Curr. Microbiol. 2016, 72, 377–382. [Google Scholar] [CrossRef]
- Khan, M.Z.; Liu, S.; Ma, Y.; Ma, M.; Ullah, Q.; Khan, I.M.; Wang, J.; Xiao, J.; Chen, T.; Khan, A.; et al. Overview of the Effect of Rumen-Protected Limiting Amino Acids (Methionine and Lysine) and Choline on the Immunity, Antioxidative, and Inflammatory Status of Periparturient Ruminants. Front. Immunol. 2023, 13, 1042895. [Google Scholar] [CrossRef]
- Khan, M.Z.; Ma, Y.; Xiao, J.; Chen, T.; Ma, J.; Liu, S.; Wang, Y.; Khan, A.; Gibson Alugongo, M.; Cao, Z. Role of Selenium and Vitamins E and B9 in the Alleviation of Bovine Mastitis During the Periparturient Period. Antioxidants 2022, 11, 657. [Google Scholar] [CrossRef]
- Xiao, J.; Khan, M.Z.; Ma, Y.; Alugongo, G.M.; Ma, J.; Chen, T.; Khan, A.; Cao, Z. The Antioxidant Properties of Selenium and Vitamin E; Their Role in Periparturient Dairy Cattle Health Regulation. Antioxidants 2021, 10, 1555. [Google Scholar] [CrossRef]
- Khan, M.Z.; Khan, A.; Xiao, J.; Dou, J.; Liu, L.; Yu, Y. Overview of Folic Acid Supplementation Alone or in Combination with Vitamin B12 in Dairy Cattle During Periparturient Period. Metabolites 2020, 10, 263. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wang, J.; Sun, L.; Ma, W.; Li, M.; Yu, S.; Zhou, Q.; Jiang, J. Wogonin Attenuates Inflammation and Oxidative Stress in Lipopolysaccharide-Induced Mastitis by Inhibiting Akt/NF-κB Pathway and Activating the Nrf2/HO-1 Signaling. Cell Stress Chaperones 2023, 28, 989–999. [Google Scholar] [CrossRef]
- Che, H.Y.; Zhou, C.H.; Lyu, C.C.; Meng, Y.; He, Y.T.; Wang, H.Q.; Wu, H.Y.; Zhang, J.B.; Yuan, B. Allicin Alleviated LPS-Induced Mastitis via the TLR4/NF-κB Signaling Pathway in Bovine Mammary Epithelial Cells. Int. J. Mol. Sci. 2023, 24, 3805. [Google Scholar] [CrossRef]
- Wu, F.; Du, T.; Jiang, X.; Liu, S.; Cheng, Y.; Zhang, Z.; Miao, W.; Wang, T. Lactococcus garvieae Exerts a Critical Role in Inducing Inflammation in Dairy Mastitis by Triggering NLRP3 Inflammasome-Mediated Pyroptosis in MAC-T Cells. World J. Microbiol. Biotechnol. 2024, 40, 132. [Google Scholar] [CrossRef]
- Akhtar, M.; Guo, S.; Guo, Y.F.; Zahoor, A.; Shaukat, A.; Chen, Y.; Umar, T.; Deng, G.; Guo, M. Upregulated-Gene Expression of Pro-Inflammatory Cytokines (TNF-α, IL-1β and IL-6) via TLRs Following NF-κB and MAPKs in Bovine Mastitis. Acta Trop. 2020, 207, 105458. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Ding, Y.; Wu, J.; Miao, Z.; Wang, J.; Wang, F. Staphylococcus aureus Induces Mammary Gland Fibrosis through Activating the TLR/NF-κB and TLR/AP-1 Signaling Pathways in Mice. Microb. Pathog. 2020, 148, 104427. [Google Scholar] [CrossRef]
- Zahoor, A.; Yang, Y.; Yang, C.; Khan, S.B.; Reix, C.; Anwar, F.; Guo, M.Y.; Deng, G. MerTK Negatively Regulates Staphylococcus aureus-Induced Inflammatory Response via Toll-Like Receptor Signaling in the Mammary Gland. Mol. Immunol. 2020, 122, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Giannuzzi, D.; Capra, E.; Bisutti, V.; Vanzin, A.; Marsan, P.A.; Cecchinato, A.; Pegolo, S. Methylome-Wide Analysis of Milk Somatic Cells upon Subclinical Mastitis in Dairy Cattle. J. Dairy Sci. 2024, 107, 1805–1820. [Google Scholar] [CrossRef]
- Li, Y.X.; Jiao, P.; Wang, X.P.; Wang, J.P.; Feng, F.; Bao, B.W.; Dong, Y.W.; Luoreng, Z.M.; Wei, D.W. RNA-seq Reveals the Role of miR-223 in Alleviating Inflammation of Bovine Mammary Epithelial Cells. Res. Vet. Sci. 2023, 159, 257–266. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, T.; Hu, X.; Xie, Y.; Wu, R.; Lian, S.; Wang, J. Exosomal miR-193b-5p as a Regulator of LPS-Induced Inflammation in Dairy Cow Mammary Epithelial Cells. Vitr. Cell. Dev. Biol. Anim. 2021, 57, 695–703. [Google Scholar] [CrossRef]
- Imaizumi, N.; Gondaira, S.; Kamioka, M.; Sugiura, T.; Eguchi, A.; Nishi, K.; Fujiki, J.; Iwano, H.; Higuchi, H. Innate Immune Response of Bovine Mammary Epithelial Cells in Mycoplasma bovis Mastitis Using an In Vitro Model of Bovine Mammary Gland Infection. J. Vet. Med. Sci. 2024, 86, 712–720. [Google Scholar] [CrossRef]
- Schneider, P.; Brill, R.E.; Schouten, I.; Nissim-Eliraz, E.; Lysnyansky, I.; Shpigel, N.Y. Lipoproteins are Potent Activators of Nuclear Factor Kappa B in Mammary Epithelial Cells and Virulence Factors in Mycoplasma bovis Mastitis. Microorganisms 2022, 10, 2209. [Google Scholar] [CrossRef]
- Srithanasuwan, A.; Schukken, Y.H.; Pangprasit, N.; Chuammitri, P.; Suriyasathaporn, W. Different Cellular and Molecular Responses of Bovine Milk Phagocytes to Persistent and Transient Strains of Streptococcus uberis Causing Mastitis. PLoS ONE 2024, 19, e0295547. [Google Scholar] [CrossRef]
- Zhang, K.; Jia, Y.; Qian, Y.; Jiang, X.; Zhang, S.; Liu, B.; Cao, J.; Song, Y.; Mao, W. Staphylococcus aureus Increases Prostaglandin E2 Secretion in Cow Neutrophils by Activating TLR2, TLR4, and NLRP3 Inflammasome Signaling Pathways. Front. Microbiol. 2023, 14, 1163261. [Google Scholar] [CrossRef]
- Xu, P.; Xu, X.; Fotina, H.; Fotina, T. Anti-Inflammatory Effects of Chlorogenic Acid from Taraxacum officinale on LTA-Stimulated Bovine Mammary Epithelial Cells via the TLR2/NF-κB Pathway. PLoS ONE 2023, 18, e0282343. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, C.; Liang, B.; Kastelic, J.P.; Han, B.; Tong, X.; Gao, J. Alternatives to Antibiotics for Treatment of Mastitis in Dairy Cows. Front. Vet. Sci. 2023, 10, 1160350. [Google Scholar] [CrossRef] [PubMed]
- Tomanić, D.; Samardžija, M.; Kovačević, Z. Alternatives to Antimicrobial Treatment in Bovine Mastitis Therapy: A Review. Antibiotics 2023, 12, 683. [Google Scholar] [CrossRef] [PubMed]
- Erhabor, R.C.; Erhabor, J.O.; Nkadimeng, S.M.; Petzer, I.M.; Dzoyem, J.P.; McGaw, L.J. In Vitro Biological Activities of Combretum molle R. Br. ex G. Don (Combretaceae) against Mastitis-Causing Organisms. S. Afr. J. Bot. 2024, 165, 228–236. [Google Scholar] [CrossRef]
- Nelson, V.K.; Nuli, M.V.; Ausali, S.; Gupta, S.; Sanga, V.; Mishra, R.; Kumar Jaini, P.; Kallam, S.D.; Sudhan, H.H.; Mayasa, V.; et al. Dietary Anti-Inflammatory and Anti-Bacterial Medicinal Plants and Its Compounds in Bovine Mastitis Associated Impact on Human Life: A Comprehensive Review. Microb. Pathog. 2024, 192, 106687. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Li, C.; Kuang, M.; Shah, A.U.; Shafiq, M.; Ahmad, M.A.; Abdalmegeed, D.; Li, L.; Wang, G. Nrf2 Activation and NF-κB & Caspase/Bax Signaling Inhibition by Sodium Butyrate Alleviates LPS-Induced Cell Injury in Bovine Mammary Epithelial Cells. Mol. Immunol. 2022, 148, 54–67. [Google Scholar]
- Arbab, A.A.; Lu, X.; Abdalla, I.M.; Idris, A.A.; Chen, Z.; Li, M.; Mao, Y.; Xu, T.; Yang, Z. Metformin Inhibits Lipoteichoic Acid–Induced Oxidative Stress and Inflammation through AMPK/NRF2/NF-κB Signaling Pathway in Bovine Mammary Epithelial Cells. Front. Vet. Sci. 2021, 8, 661380. [Google Scholar] [CrossRef]
- Gao, X.J.; Wang, T.C.; Zhang, Z.C.; Cao, Y.G.; Zhang, N.S.; Guo, M.Y. Brazilin Plays an Anti-Inflammatory Role with Regulating Toll-Like Receptor 2 and TLR2 Downstream Pathways in Staphylococcus aureus-Induced Mastitis in Mice. Int. Immunopharmacol. 2015, 27, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Jin, L.; Yang, B. Diosmetin Alleviates, S. aureus-Induced Mastitis by Inhibiting SIRT1/GPX4 Mediated Ferroptosis. Life Sci. 2023, 331, 122060. [Google Scholar] [CrossRef]
- Sun, W.J.; Wu, E.Y.; Zhang, G.Y.; Xu, B.C.; Chen, X.G.; Hao, K.Y.; Wang, Y.; He, L.Z.; Lv, Q.Z. Total Flavonoids of Abrus cantoniensis Inhibit CD14/TLR4/NF-κB/MAPK Pathway Expression and Improve Gut Microbiota Disorders to Reduce Lipopolysaccharide-Induced Mastitis in Mice. Front. Microbiol. 2022, 13, 985529. [Google Scholar] [CrossRef]
- Kan, X.; Hu, G.; Huang, B.; Guo, W.; Huang, Y.; Chen, Y.; Xu, P.; Cai, X.; Fu, S.; Liu, J. Pedunculoside Protects against LPS-Induced Mastitis in Mice by Inhibiting Inflammation and Maintaining the Integrity of Blood-Milk Barrier. Aging 2021, 13, 19460. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Yang, T.; Pan, L.; Xu, Y.; Wang, L.; Jiang, M.; Zhou, J.; Sun, C.; Yao, J.; et al. Jingfang Granules Alleviate LPS-Induced Mastitis by Inhibiting Inflammation, Protecting the Blood-Milk Barrier Structure and Regulating Cell Apoptosis. Pharmacol. Res. Mod. Chin. Med. 2022, 2, 100072. [Google Scholar] [CrossRef]
- Yang, Y.; Ran, X.; Wang, H.; Chen, Y.; Hou, S.; Yang, Z.; Fu, S.; Liu, J.; Hu, G.; Guo, W. Evodiamine Relieve LPS-Induced Mastitis by Inhibiting AKT/NF-κB p65 and MAPK Signaling Pathways. Inflammation 2021, 45, 129–142. [Google Scholar] [CrossRef]
- Yang, Z.; Yin, R.; Cong, Y.; Yang, Z.; Zhou, E.; Wei, Z.; Liu, Z.; Cao, Y.; Zhang, N. Oxymatrine Lightened the Inflammatory Response of LPS-Induced Mastitis in Mice through Affecting NF-κB and MAPKs Signaling Pathways. Inflammation 2014, 37, 2047–2055. [Google Scholar] [CrossRef]
- Jiang, K.; Ma, X.; Guo, S.; Zhang, T.; Zhao, G.; Wu, H.; Wang, X.; Deng, G. Anti-Inflammatory Effects of Rosmarinic Acid in Lipopolysaccharide-Induced Mastitis in Mice. Inflammation 2018, 41, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zheng, X.; Zhang, M.; Yin, H.; Jiang, K.; Wu, H.; Dai, A.; Yang, S. Nuciferine Alleviates LPS-Induced Mastitis in Mice via Suppressing the TLR4-NF-κB Signaling Pathway. Inflamm. Res. 2018, 67, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wang, T.; Zhang, Z.; Jiang, H.; Wang, W.; Cao, Y.; Zhang, N. Leonurine Exerts Anti-Inflammatory Effect by Regulating Inflammatory Signaling Pathways and Cytokines in LPS-Induced Mouse Mastitis. Inflammation 2015, 38, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Dejie, L.; Xiaojing, S.; Tiancheng, W.; Yongguo, C.; Zhengtao, Y.; Naisheng, Z. Magnolol Inhibits the Inflammatory Response in Mouse Mammary Epithelial Cells and a Mouse Mastitis Model. Inflammation 2015, 38, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Liu, C.; Li, T.; Lin, C.; Hao, Z.; Zhang, H.; Zhao, G.; Chen, Y.; Guo, A.; Hu, C. Gambogic Acid Alleviates Inflammation and Apoptosis and Protects the Blood-Milk Barrier in Mastitis Induced by LPS. Int. Immunopharmacol. 2020, 86, 106697. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Yang, M.; Wang, Y.; Yang, C.; Shafiq, M.; Wang, G.; Li, L. Sodium Propionate Protects the Blood-Milk Barrier Integrity, Relieve Lipopolysaccharide-Induced Inflammatory Injury and Cells Apoptosis. Life Sci. 2021, 270, 119138. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Fang, H.; Shen, J.; Jin, Y.; Zhao, Y.; Wang, R.; Fu, Y.; Tian, Y.; Yu, H.; Zhang, J. Curcumin Alleviates LPS-Induced Oxidative Stress, Inflammation and Apoptosis in Bovine Mammary Epithelial Cells via the NFE2L2 Signaling Pathway. Toxins 2021, 13, 208. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Zhang, Y.; Wang, J.; Guo, W.; Hu, G.; Xie, S.; Yang, Z.; Liu, J.; Fu, S. Palmatine Attenuates LPS-Induced Inflammatory Response in Mouse Mammary Epithelial Cells through Inhibiting ERK1/2, P38 and Akt/NF-κB Signalling Pathways. J. Anim. Physiol. Anim. Nutr. 2021, 105, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Shaukat, A.; Zahoor, A.; Chen, Y.; Wang, Y.; Yang, M.; Umar, T.; Guo, M.; Deng, G. Hederacoside-C Inhibition of Staphylococcus aureus-Induced Mastitis via TLR2 & TLR4 and Their Downstream Signaling NF-κB and MAPKs Pathways In Vivo and In Vitro. Inflammation 2020, 43, 579–594. [Google Scholar]
- Dai, H.; Wei, G.; Wang, Y.; Ma, N.; Chang, G.; Shen, X. Sodium Butyrate Promotes Lipopolysaccharide-Induced Innate Immune Responses by Enhancing Mitogen-Activated Protein Kinase Activation and Histone Acetylation in Bovine Mammary Epithelial Cells. J. Dairy Sci. 2020, 103, 11636–11652. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Liu, J.; Zhang, Y.; Ma, H.; Li, Y.; Gong, Q.; Cao, Y.; Hu, G.; Xie, S.; Fu, S. Dehydroandrographolide Inhibits Mastitis by Activating Autophagy without Affecting Intestinal Flora. Aging 2020, 12, 14050. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, R.; Yu, S.; Lu, G.; Yu, Y.; Jiang, C. Anti-Inflammatory Activity of Oligomeric Proanthocyanidins via Inhibition of NF-κB and MAPK in LPS-Stimulated MAC-T Cells. J. Microbiol. Biotechnol. 2020, 30, 1458. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Zhang, C.; Huai, Y.; Liu, D.; Zhang, M.; Wang, H.; Zhao, X.; Bo, R.; Li, J.; Liu, M. The Inhibition Effect of Caffeic Acid on NOX/ROS-Dependent Macrophages M1-Like Polarization Contributes to Relieve the LPS-Induced Mice Mastitis. Cytokine 2024, 174, 156471. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xu, L.; Lv, L.; Wang, L.; Wang, X.; Liang, C.; Wang, C.; Qiu, Y.; Pei, X. Network Pharmacology and In Vivo and In Vitro Experiments to Determine the Mechanism Behind the Effects of Jiawei Yanghe Decoction via TLR4/Myd88/NF-κB against Mastitis. Heliyon 2023, 9, e21219. [Google Scholar] [CrossRef]
- Malik, M.U.; Hashmi, N.; Khan, M.; Aabdin, Z.U.; Sami, R.; Aljahani, A.H.; Al-Eisa, R.A.; Moawadh, M.S.; Algehainy, N.A. Nutraceutical Effect of Resveratrol on the Mammary Gland: Focusing on the NF-κB/Nrf2 Signaling Pathways. Animals 2023, 13, 1266. [Google Scholar] [CrossRef]
- Li, K.; Ran, X.; Zeng, Y.; Li, S.; Hu, G.; Wang, X.; Li, Y.; Yang, Z.; Liu, J.; Fu, S. Maslinic Acid Alleviates LPS-Induced Mice Mastitis by Inhibiting Inflammatory Response, Maintaining the Integrity of the Blood-Milk Barrier and Regulating Intestinal Flora. Int. Immunopharmacol. 2023, 122, 110551. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jin, L. Quyu Xiaozhong Recipe Exerts Anti-Inflammatory Effect in Acute Mastitis by Inhibiting TLR4/NF-κB Signal Pathway. Trop. J. Pharm. Res. 2023, 22, 981–986. [Google Scholar] [CrossRef]
- Bao, L.; Sun, H.; Zhao, Y.; Feng, L.; Wu, K.; Shang, S.; Xu, J.; Shan, R.; Duan, S.; Qiu, M. Hexadecanamide Alleviates Staphylococcus aureus-Induced Mastitis in Mice by Inhibiting Inflammatory Responses and Restoring Blood-Milk Barrier Integrity. PLoS Pathog. 2023, 19, e1011764. [Google Scholar] [CrossRef]
- Wu, K.; Shang, S.; Bao, L.; Zhao, Y.; Guan, Z.; Xu, J.; Sun, H.; Yuan, W.; Fu, Y.; Peng, L.; et al. Retinoic Acid Ameliorates Low-Grade Endotoxemia-Induced Mastitis by Limiting Inflammatory Responses in Mice. Microb. Pathog. 2023, 185, 106426. [Google Scholar] [CrossRef]
- Amin, I.; Rashid, S.M.; Shubeena, S.; Hussain, I.; Ahmad, S.B.; Mir, M.U.; Alshehri, S.; Bukhari, S.I.; Mir, T.M.; Rehman, M.U. TLR4/NF-κB-Mediated Anti-Inflammatory and Antioxidative Effect of Hexanic and Ethanolic Extracts of Curcuma longa L. in Buffalo Mammary Epithelial Cells. Separations 2022, 9, 414. [Google Scholar] [CrossRef]
- Tong, C.; Chen, T.; Chen, Z.; Wang, H.; Wang, X.; Liu, F.; Dai, H.; Wang, X.; Li, X. Forsythiaside A Plays an Anti-Inflammatory Role in LPS-Induced Mastitis in a Mouse Model by Modulating the MAPK and NF-κB Signaling Pathways. Res. Vet. Sci. 2021, 136, 390–395. [Google Scholar] [CrossRef]
- Xu, T.; Wu, X.; Lu, X.; Liang, Y.; Mao, Y.; Loor, J.J.; Yang, Z. Metformin Activated AMPK Signaling Contributes to the Alleviation of LPS-Induced Inflammatory Responses in Bovine Mammary Epithelial Cells. BMC Vet. Res. 2021, 17, 97. [Google Scholar] [CrossRef] [PubMed]
- Jiang, A.; Zhang, Y.; Zhang, X.; Wu, D.; Liu, Z.; Li, S.; Liu, X.; Han, Z.; Wang, C.; Wang, J.; et al. Morin Alleviates LPS-Induced Mastitis by Inhibiting the PI3K/AKT, MAPK, NF-κB and NLRP3 Signaling Pathway and Protecting the Integrity of Blood-Milk Barrier. Int. Immunopharmacol. 2020, 78, 105972. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Liu, X.; Yu, D.; Changyong, E.; Yang, J. Morin Protects LPS-Induced Mastitis via Inhibiting NLRP3 Inflammasome and NF-κB Signaling Pathways. Inflammation 2020, 43, 1293–1303. [Google Scholar] [CrossRef]
- Yang, C.; Liu, P.; Wang, S.; Zhao, G.; Zhang, T.; Guo, S.; Jiang, K.; Wu, H.; Deng, G. Shikonin Exerts Anti-Inflammatory Effects in LPS-Induced Mastitis by Inhibiting NF-κB Signaling Pathway. Biochem. Biophys. Res. Commun. 2018, 505, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Hong, D.; Zhang, T.; Duan, H.; Wei, P.; Guo, X.; Mu, X. Cynatratoside-C from Cynanchum atratum Displays Anti-Inflammatory Effect via Suppressing TLR4 Mediated NF-κB and MAPK Signaling Pathways in LPS-Induced Mastitis in Mice. Chem. Biol. Interact. 2018, 279, 187–195. [Google Scholar] [CrossRef]
- Martín, R.; Langella, P. Emerging Health Concepts in the Probiotics Field: Streamlining the Definitions. Front. Microbiol. 2019, 10, 457799. [Google Scholar] [CrossRef]
- Kober, A.H.; Saha, S.; Islam, M.A.; Rajoka, M.S.; Fukuyama, K.; Aso, H.; Villena, J.; Kitazawa, H. Immunomodulatory Effects of Probiotics: A Novel Preventive Approach for the Control of Bovine Mastitis. Microorganisms 2022, 10, 2255. [Google Scholar] [CrossRef] [PubMed]
- Rainard, P.; Foucras, G. A Critical Appraisal of Probiotics for Mastitis Control. Front. Vet. Sci. 2018, 5, 251. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Tang, X.; Nan, X.; Jiang, L.; Wang, H.; Liu, J.; Yang, L.; Yao, J.; Xiong, B. Nutrition, Gastrointestinal Microorganisms and Metabolites in Mastitis Occurrence and Control. Anim. Nutr. 2024, 17, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Youn, H.Y.; Moon, J.S.; Kim, H.; Seo, K.H. Comparative Anti-Microbial and Anti-Biofilm Activities of Postbiotics Derived from Kefir and Normal Raw Milk Lactic Acid Bacteria against Bovine Mastitis Pathogens. LWT 2024, 191, 115699. [Google Scholar] [CrossRef]
- Shan, Q.; Liu, N.; Wang, X.; Zhu, Y.; Yin, J.; Wang, J. Lactobacillus rhamnosus GR-1 Attenuates Foodborne Bacillus cereus-Induced NLRP3 Inflammasome Activity in Bovine Mammary Epithelial Cells by Protecting Intercellular Tight Junctions. J. Anim. Sci. Biotechnol. 2022, 13, 101. [Google Scholar] [CrossRef]
- Urakawa, M.; Zhuang, T.; Sato, H.; Takanashi, S.; Yoshimura, K.; Endo, Y.; Katsura, T.; Umino, T.; Tanaka, K.; Watanabe, H.; et al. Prevention of Mastitis in Multiparous Dairy Cows with a Previous History of Mastitis by Oral Feeding with Probiotic Bacillus subtilis. Anim. Sci. J. 2022, 93, e13764. [Google Scholar] [CrossRef]
- Yu, Q.; Xu, C.; Wang, M.; Zhu, J.; Yu, L.; Yang, Z.; Liu, S.; Gao, X. The Preventive and Therapeutic Effects of Probiotics on Mastitis: A Systematic Review and Meta-Analysis. PLoS ONE 2022, 17, e0274467. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Wang, Y.; Kang, X.; Liu, P.; Wang, G. Research Progress on the Association between Mastitis and Gastrointestinal Microbes in Dairy Cows and the Effect of Probiotics. Microb. Pathog. 2022, 173, 105809. [Google Scholar] [CrossRef]
- Steinberg, R.S.; Silva, L.C.; de Souza, M.R.; Reis, R.B.; Bicalho, A.F.; Nunes, J.P.; Dias, A.A.; Nicoli, J.R.; Neumann, E.; Nunes, Á.C. Prospecting of Potentially Probiotic Lactic Acid Bacteria from Bovine Mammary Ecosystem: Imminent Partners from Bacteriotherapy against Bovine Mastitis. Int. Microbiol. 2021, 25, 189–206. [Google Scholar] [CrossRef]
- Li, K.; Yang, M.; Jia, L.; Tian, M.; Du, J.; Wu, Y.; Yuan, L.; Li, L.; Ma, Y. The Prevention Effect of Lactobacillus plantarum 17–5 on Escherichia coli-Induced Mastitis in Mice. Probiotics Antimicrob. Proteins 2023, 15, 1644–1652. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Yang, M.; Tian, M.; Jia, L.; Du, J.; Wu, Y.; Li, L.; Yuan, L.; Ma, Y. Lactobacillus plantarum 17–5 Attenuates Escherichia coli-Induced Inflammatory Responses via Inhibiting the Activation of the NF-κB and MAPK Signalling Pathways in Bovine Mammary Epithelial Cells. BMC Vet. Res. 2022, 18, 250. [Google Scholar] [CrossRef]
- Li, K.; Yang, M.; Tian, M.; Jia, L.; Wu, Y.; Du, J.; Yuan, L.; Li, L.; Ma, Y. The Preventive Effects of Lactobacillus casei 03 on Escherichia coli-Induced Mastitis In Vitro and In Vivo. J. Inflamm. 2024, 21, 1–9. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, Y.; Chu, B.; Liu, N.; Chen, S.; Wang, J. Lactobacillus rhamnosus GR-1 Prevents Escherichia coli-Induced Apoptosis through PINK1/Parkin-Mediated Mitophagy in Bovine Mastitis. Front. Immunol. 2021, 12, 715098. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Tang, R.; Zhao, C.; Mu, R.; Wang, Y.; Cao, Y.; Zhang, N.; Fu, Y. The Prevention Effect of Bacillus subtilis on Escherichia coli–Induced Mastitis in Mice by Suppressing the NF-κB and MAPK Signaling Pathways. Probiotics Antimicrob. Proteins 2021, 15, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, S.; Guo, J.; Xie, Q.; Evivie, S.E.; Song, Y.; Li, B.; Huo, G. The Protective Effects of Lactobacillus plantarum KLDS 1.0344 on LPS-Induced Mastitis In Vitro and In Vivo. Front. Immunol. 2021, 12, 770822. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Feng, L.; Yu, Z.; Zhao, C.; Gao, S.; Bao, L.; Zhang, N.; Fu, Y.; Hu, X. Probiotic Enterococcus mundtii H81 Inhibits the NF-κB Signaling Pathway to Ameliorate Staphylococcus aureus-Induced Mastitis in Mice. Microb. Pathog. 2022, 164, 105414. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).