Extracellular Vesicles Induce Nuclear Factor-κB Activation and Interleukin-8 Synthesis through miRNA-191-5p Contributing to Inflammatory Processes: Potential Implications in the Pathogenesis of Chronic Obstructive Pulmonary Disease
Abstract
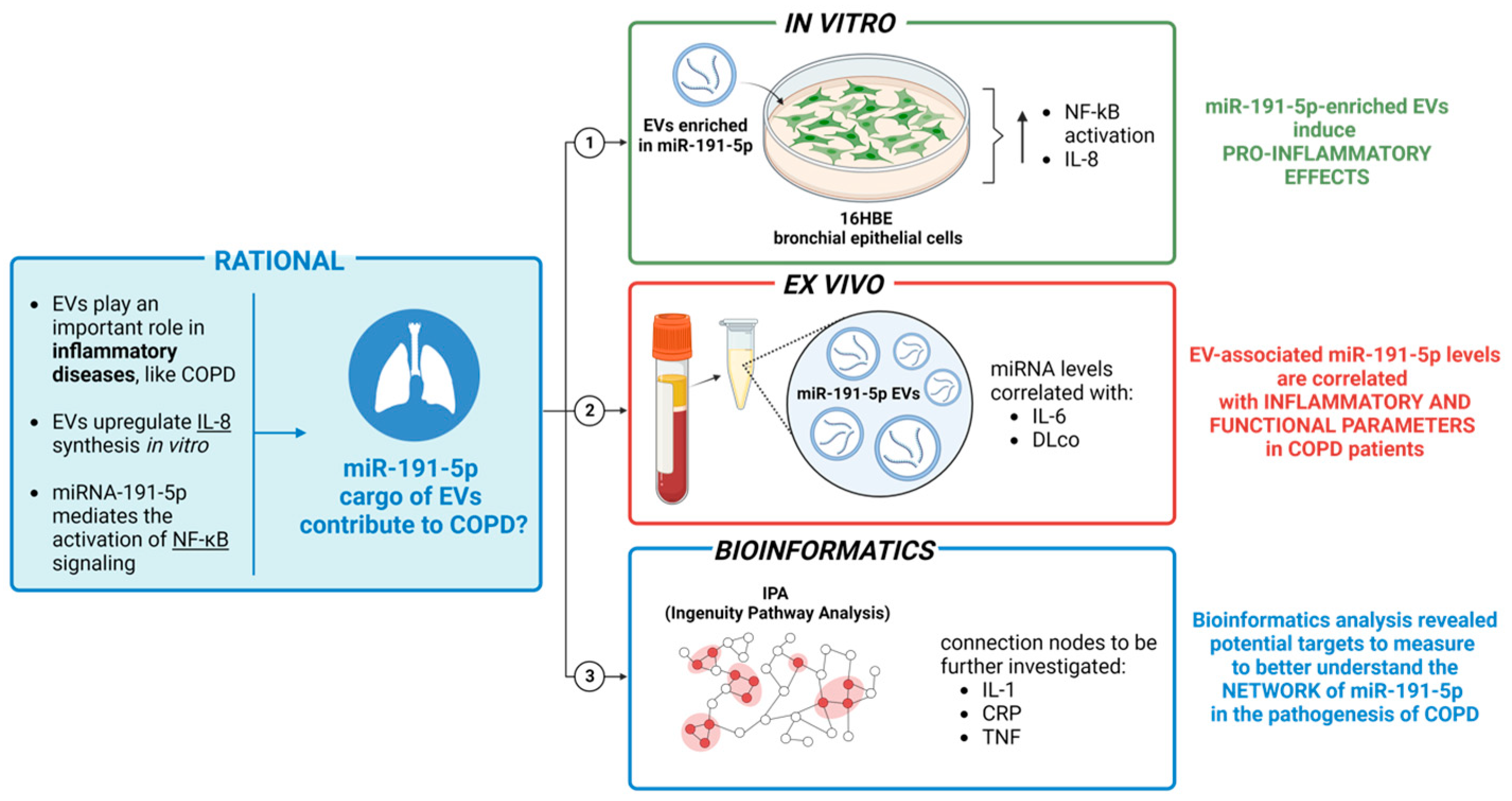
:1. Introduction
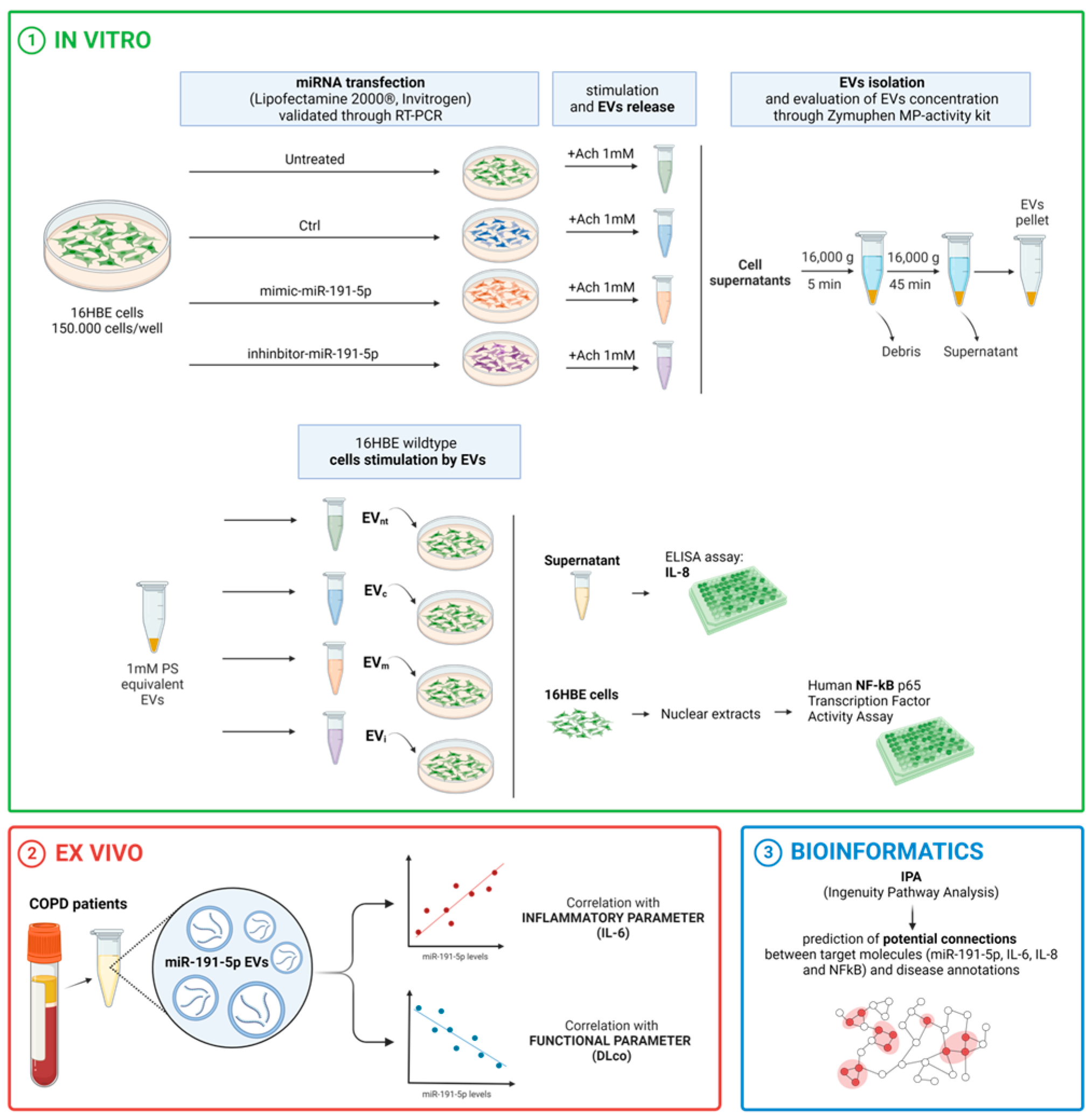
2. Materials and Methods
2.1. Study Design
2.2. Cell Culture
2.3. Cell Transfection with miR-191-5p Mimic and miR-191-5p Inhibitor
2.4. Evaluation of miR-191-5p Expression
2.5. Elisa for IL-6 and IL-8 Detection
2.6. NF-kB Activity Evaluation
2.7. Study Cohort and Functional Tests
2.8. Isolation and Characterization of Extracellular Vesicles
2.8.1. From Plasma of Patients
2.8.2. From Cell Medium
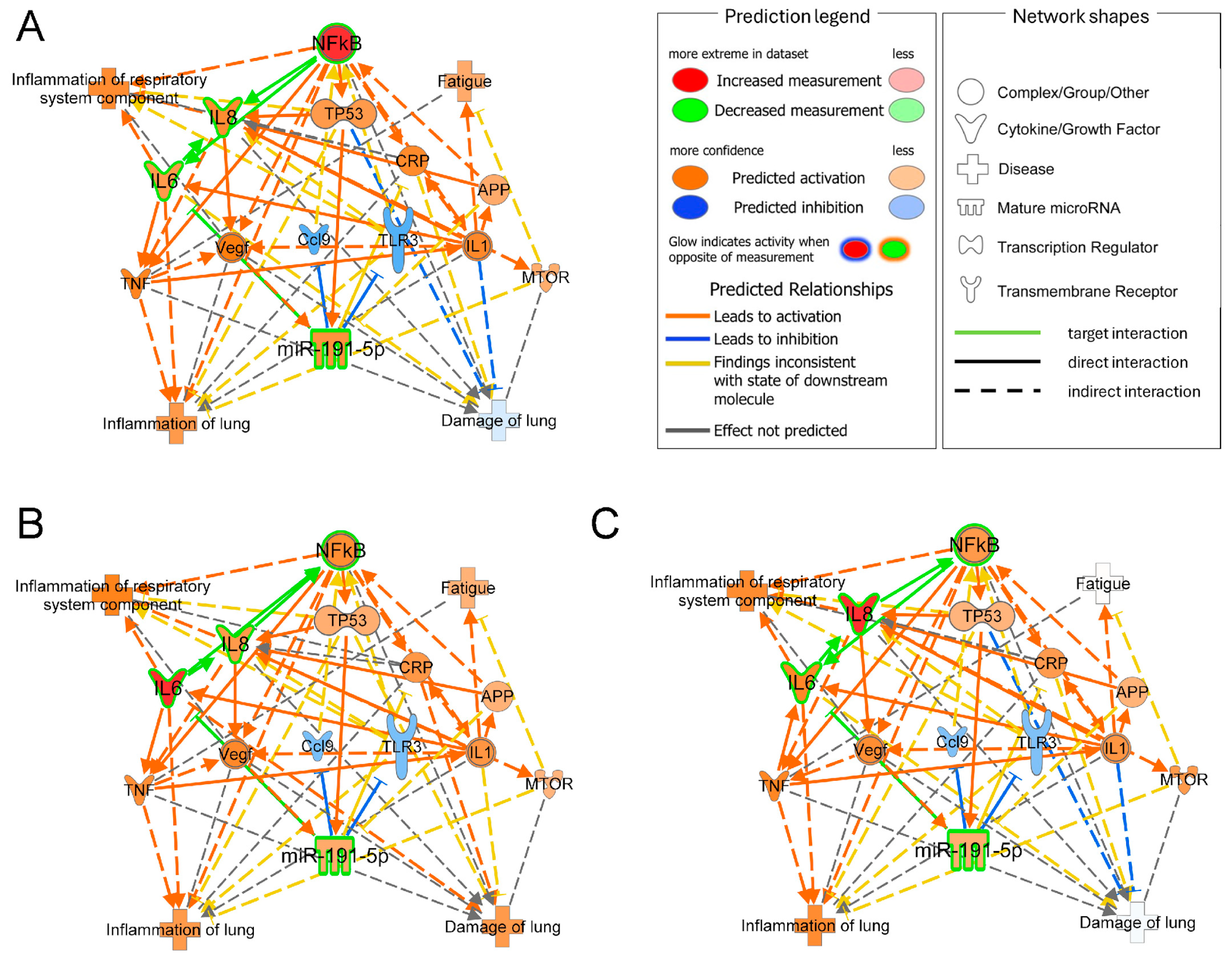
2.9. Network Analysis and Molecules Activity Prediction
2.10. Statistical Analysis
3. Results
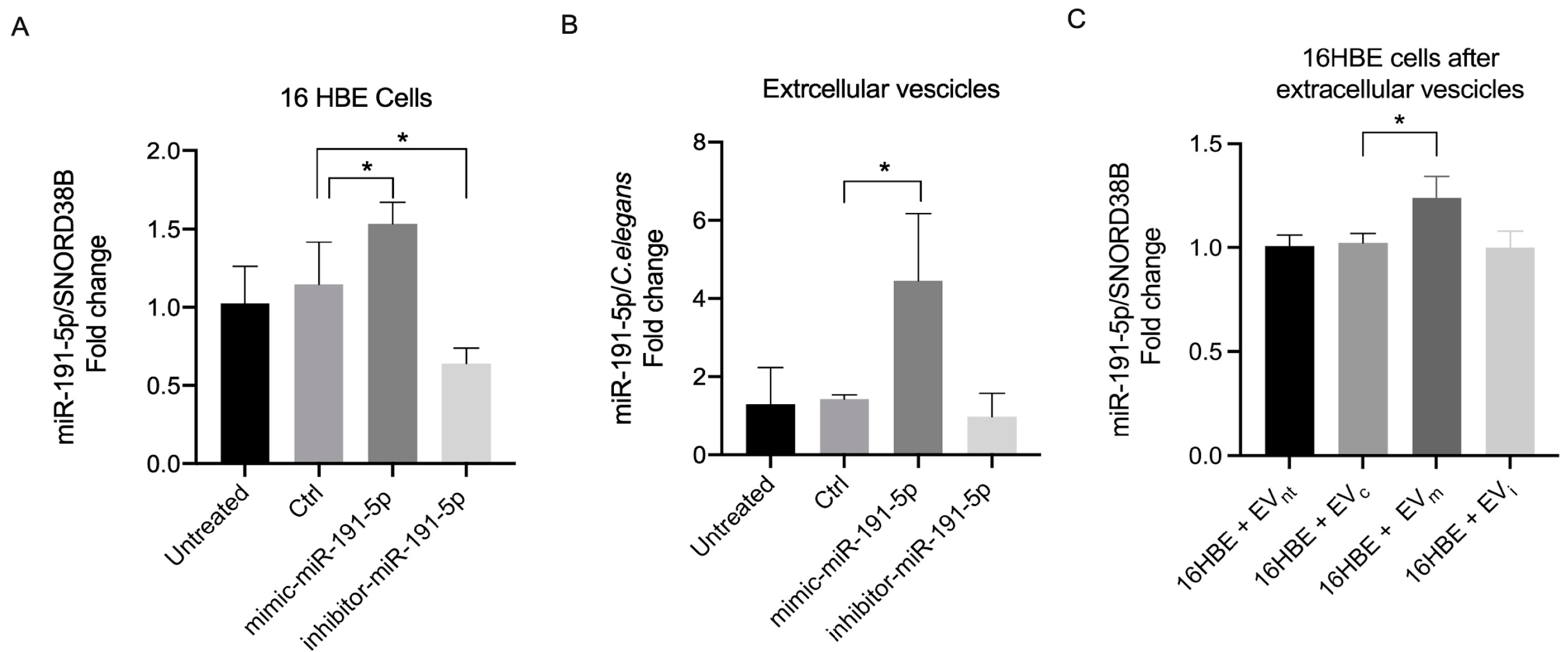
3.1. miR-191-5p-Enriched EV Increased miR-191-5p in Recipient Cells
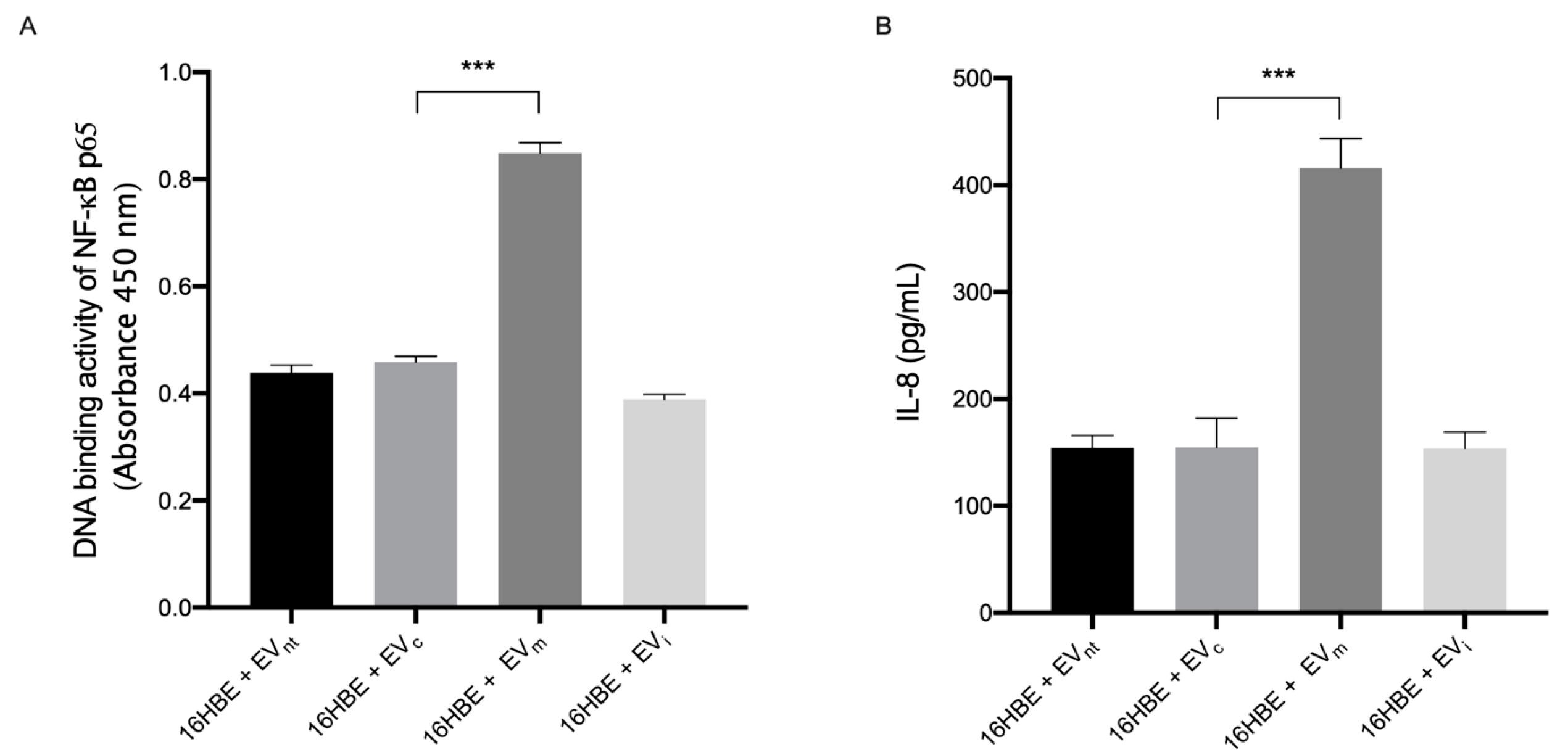
3.2. miR-191-5p-Enriched EVs Activate the Inflammatory Pathway
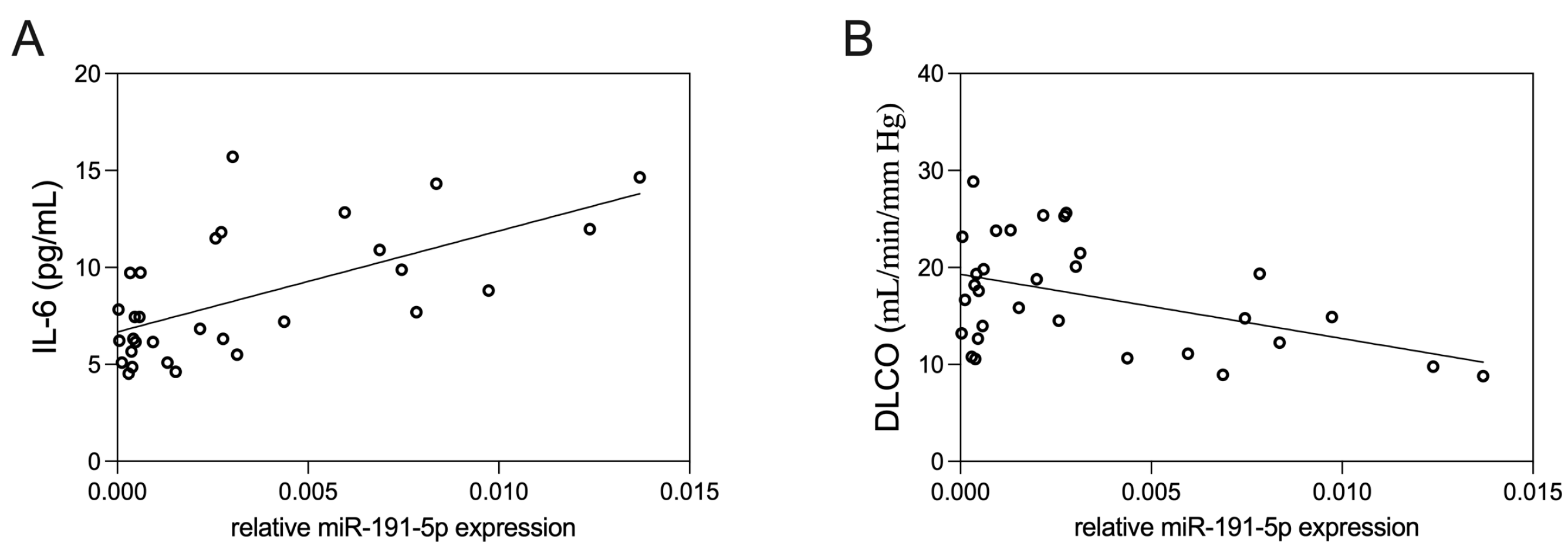
3.3. Correlation of EV-Derived miR-191-5p with Clinical Parameters
3.4. Bioinformatic Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agustí, A.; Celli, B.R.; Criner, G.J.; Halpin, D.; Anzueto, A.; Barnes, P.; Bourbeau, J.; Han, M.K.; Martinez, F.J.; Montes de Oca, M.; et al. Global Initiative for Chronic Obstructive Lung Disease 2023 Report: GOLD Executive Summary. Eur. Respir. J. 2023, 61, 2300239. [Google Scholar] [CrossRef] [PubMed]
- Quaderi, S.A.; Hurst, J.R. The unmet global burden of COPD. Glob. Health Epidemiol. Genom. 2018, 3, e4. [Google Scholar] [CrossRef]
- Barnes, P.J.; Burney, P.G.; Silverman, E.K.; Celli, B.R.; Vestbo, J.; Wedzicha, J.A.; Wouters, E.F. Chronic obstructive pulmonary disease. Nat. Rev. Dis. Primers 2015, 1, 15076. [Google Scholar] [CrossRef]
- Terry, P.D.; Dhand, R. The 2023 GOLD Report: Updated Guidelines for Inhaled Pharmacological Therapy in Patients with Stable COPD. Pulm. Ther. 2023, 9, 345–357. [Google Scholar] [CrossRef]
- Papaioannou, A.I.; Hillas, G.; Loukides, S.; Vassilakopoulos, T. Mortality prevention as the centre of COPD management. ERJ Open Res. 2024, 10, 00850–02023. [Google Scholar] [CrossRef]
- Cazzola, M.; Rogliani, P.; Barnes, P.J.; Blasi, F.; Celli, B.; Hanania, N.A.; Martinez, F.J.; Miller, B.E.; Miravitlles, M.; Page, C.P.; et al. An Update on Outcomes for COPD Pharmacological Trials: A COPD Investigators Report—Reassessment of the 2008 American Thoracic Society/European Respiratory Society Statement on Outcomes for COPD Pharmacological Trials. Am. J. Respir. Crit. Care Med. 2023, 208, 374–394. [Google Scholar] [CrossRef] [PubMed]
- György, B.; Szabó, T.G.; Pásztói, M.; Pál, Z.; Misják, P.; Aradi, B.; László, V.; Pállinger, E.; Pap, E.; Kittel, A.; et al. Membrane vesicles, current state-of-the-art: Emerging role of extracellular vesicles. Cell. Mol. Life Sci. 2011, 68, 2667–2688. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Pease, J.E.; Sabroe, I. The role of interleukin-8 and its receptors in inflammatory lung disease: Implications for therapy. Am. J. Respir. Med. 2002, 1, 19–25. [Google Scholar] [CrossRef]
- Cerri, C.; Chimenti, D.; Conti, I.; Neri, T.; Paggiaro, P.; Celi, A. Monocyte/macrophage-derived microparticles up-regulate inflammatory mediator synthesis by human airway epithelial cells. J. Immunol. 2006, 177, 1975–1980. [Google Scholar] [CrossRef]
- Neri, T.; Armani, C.; Pegoli, A.; Cordazzo, C.; Carmazzi, Y.; Brunelleschi, S.; Bardelli, C.; Breschi, M.C.; Paggiaro, P.; Celi, A. Role of NF-kappaB and PPAR-gamma in lung inflammation induced by monocyte-derived microparticles. Eur. Respir. J. 2011, 37, 1494–1502. [Google Scholar] [CrossRef] [PubMed]
- Neri, T.; Scalise, V.; Passalacqua, I.; Giusti, I.; Lombardi, S.; Balia, C.; D’alessandro, D.; Berrettini, S.; Pedrinelli, R.; Paggiaro, P.; et al. CD18-mediated adhesion is required for the induction of a proinflammatory phenotype in lung epithelial cells by mononuclear cell-derived extracellular vesicles. Exp. Cell Res. 2018, 365, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Gou, Z.; Yang, H.; Wang, R.; Wang, S.; Chen, Q.; Liu, Z.; Zhang, Y. A new frontier in precision medicine: Exploring the role of extracellular vesicles in chronic obstructive pulmonary disease. Biomed. Pharmacother. 2024, 174, 116443. [Google Scholar] [CrossRef] [PubMed]
- Nieri, D.; Morani, C.; De Francesco, M.; Gaeta, R.; Niceforo, M.; De Santis, M.; Giusti, I.; Dolo, V.; Daniele, M.; Papi, A.; et al. Enhanced prothrombotic and proinflammatory activity of circulating extracellular vesicles in acute exacerbations of chronic obstructive pulmonary disease. Respir. Med. 2024, 223, 107563. [Google Scholar] [CrossRef]
- Stefani, G.; Slack, F.J. Small non-coding RNAs in animal development. Nat. Rev. Mol. Cell Biol. 2008, 9, 219–230. [Google Scholar] [CrossRef]
- O’Brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef]
- Stolzenburg, L.R.; Harris, A. The role of microRNAs in chronic respiratory disease: Recent insights. Biol. Chem. 2018, 399, 219–234. [Google Scholar] [CrossRef]
- Carpi, S.; Polini, B.; Nieri, D.; Dubbini, N.; Celi, A.; Nieri, P.; Neri, T. Expression Analysis of Muscle-Specific miRNAs in Plasma-Derived Extracellular Vesicles from Patients with Chronic Obstructive Pulmonary Disease. Diagnostics 2020, 10, 502. [Google Scholar] [CrossRef]
- Nagpal, N.; Kulshreshtha, R. miR-191: An emerging player in disease biology. Front. Genet. 2014, 5, 99. [Google Scholar] [CrossRef]
- Gu, Y.; Ampofo, E.; Menger, M.D.; Laschke, M.W. miR-191 suppresses angiogenesis by activation of NF-κB signaling. FASEB J. 2017, 31, 3321–3333. [Google Scholar] [CrossRef]
- Dangwal, S.; Stratmann, B.; Bang, C.; Lorenzen, J.M.; Kumarswamy, R.; Fiedler, J.; Falk, C.S.; Scholz, C.J.; Thum, T.; Tschoepe, D. Impairment of Wound Healing in Patients With Type 2 Diabetes Mellitus Influences Circulating MicroRNA Patterns via Inflammatory Cytokines. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1480–1488. [Google Scholar] [CrossRef] [PubMed]
- Serban, K.A.; Rezania, S.; Petrusca, D.N.; Poirier, C.; Cao, D.; Justice, M.J.; Patel, M.; Tsvetkova, I.; Kamocki, K.; Mikosz, A.; et al. Structural and functional characterization of endothelial microparticles released by cigarette smoke. Sci. Rep. 2016, 6, 31596. [Google Scholar] [CrossRef]
- Takahashi, K.; Yokota, S.; Tatsumi, N.; Fukami, T.; Yokoi, T.; Nakajima, M. Cigarette smoking substantially alters plasma microRNA profiles in healthy subjects. Toxicol. Appl. Pharmacol. 2013, 272, 154–160. [Google Scholar] [CrossRef]
- Wei, C.; Henderson, H.; Spradley, C.; Li, L.; Kim, I.K.; Kumar, S.; Hong, N.; Arroliga, A.C.; Gupta, S. Circulating miRNAs as potential marker for pulmonary hypertension. PLoS ONE 2013, 8, e64396. [Google Scholar] [CrossRef]
- Neri, T.; Scalise, V.; Passalacqua, I.; Sanguinetti, C.; Lombardi, S.; Pergoli, L.; Bollati, V.; Pedrinelli, R.; Paggiaro, P.; Celi, A. Tiotropium inhibits proinflammatory microparticle generation by human bronchial and endothelial cells. Sci. Rep. 2019, 9, 11631. [Google Scholar] [CrossRef] [PubMed]
- Wanger, J.; Clausen, J.L.; Coates, A.; Pedersen, O.F.; Brusasco, V.; Burgos, F.; Casaburi, R.; Crapo, R.; Enright, P.; van der Grinten, C.P.M.; et al. Standardisation of the measurement of lung volumes. Eur. Respir. J. 2005, 26, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.L.; Brusasco, V.; Burgos, F.; Cooper, B.G.; Jensen, R.; Kendrick, A.; MacIntyre, N.R.; Thompson, B.R.; Wanger, J. Executive Summary: 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur. Respir. J. 2017, 49, 16E0016. [Google Scholar] [CrossRef]
- Cotton, D.J.; Soparkar, G.R.; Grahan, B.L. Diffusing capacity in the clinical assessment of chronic airflow limitation. Med. Clin. N. Am. 1996, 80, 549–564. [Google Scholar] [CrossRef]
- Krämer, A.; Green, J.; Pollard, J.; Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Barnes, P.J. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2016, 138, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Cellular and molecular mechanisms of asthma and COPD. Clin. Sci. 2017, 131, 1541–1558. [Google Scholar] [CrossRef]
- Vogelmeier, C.; Hederer, B.; Glaab, T.; Schmidt, H.; Rutten-van Mölken, M.P.; Beeh, K.M.; Rabe, K.F.; Fabbri, L.M.; POET-COPD, I. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N. Engl. J. Med. 2011, 364, 1093–1103. [Google Scholar] [CrossRef]
- Agustí, A.; Edwards, L.D.; Rennard, S.I.; MacNee, W.; Tal-Singer, R.; Miller, B.E.; Vestbo, J.; Lomas, D.A.; Calverley, P.M.A.; Wouters, E.; et al. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: A novel phenotype. PLoS ONE 2012, 7, e37483. [Google Scholar] [CrossRef] [PubMed]
- Nieri, D.; Daniele, M.; Lombardi, S.; Bazzan, E.; Santerini, S.; De Cusatis, G.; Vagaggini, B.; Cosio, M.G.; Saetta, M.; Paggiaro, P.; et al. Circulating Extracellular Vesicles Are Associated with Disease Severity and Interleukin-6 Levels in COPD: A Pilot Study. J. Clin. Med. 2021, 10, 5014. [Google Scholar] [CrossRef]
- De-Torres, J.P.; O’Donnell, D.E.; Marín, J.M.; Cabrera, C.; Casanova, C.; Marín, M.; Ezponda, A.; Cosio, B.G.; Martinez, C.; Solanes, I.; et al. Clinical and Prognostic Impact of Low Diffusing Capacity for Carbon Monoxide Values in Patients With Global Initiative for Obstructive Lung Disease I COPD. Chest 2021, 160, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Thomashow, M.A.; Shimbo, D.; Parikh, M.A.; Hoffman, E.A.; Vogel-Claussen, J.; Hueper, K.; Fu, J.; Liu, C.-Y.; Bluemke, D.A.; Ventetuolo, C.E.; et al. Endothelial microparticles in mild chronic obstructive pulmonary disease and emphysema. The Multi-Ethnic Study of Atherosclerosis Chronic Obstructive Pulmonary Disease study. Am. J. Respir. Crit. Care Med. 2013, 188, 60–68. [Google Scholar] [CrossRef]
- Im, E.-J.; Lee, C.-H.; Moon, P.-G.; Rangaswamy, G.G.; Lee, B.; Lee, J.M.; Lee, J.-C.; Jee, J.-G.; Bae, J.-S.; Kwon, T.-K.; et al. Sulfisoxazole inhibits the secretion of small extracellular vesicles by targeting the endothelin receptor A. Nat. Commun. 2019, 10, 1387. [Google Scholar] [CrossRef]
- Catalano, M.; O’Driscoll, L. Inhibiting extracellular vesicles formation and release: A review of EV inhibitors. J. Extracell. Vesicles 2020, 9, 1703244. [Google Scholar] [CrossRef]
- Hao, Y.; Song, H.; Zhou, Z.; Chen, X.; Li, H.; Zhang, Y.; Wang, J.; Ren, X.; Wang, X. Promotion or inhibition of extracellular vesicle release: Emerging therapeutic opportunities. J. Control. Release 2021, 340, 136–148. [Google Scholar] [CrossRef] [PubMed]
| Age, years | 71.0 (6.0) |
| Birth sex, female:male, n | 9:26 |
| Smoking history | |
| 9 |
| 26 |
| Pack-years | 49.5 ± 23.4 |
| Dyspnea, mMRC | 1 (1) |
| BMI, Kg/m2 | 29.6 ± 6.1 |
| FEV1, L | 1.34 ± 0.44 |
| % pred. | 53.9 ± 11.8 |
| FEV1/FVC, % | 48.0 ± 11.3 |
| TLC, L | 6.70 ± 1.49 |
| % pred. | 112.0 ± 19.3 |
| DLCO, mL/min/mmHg | 17.1 ± 5.5 |
| % pred. | 72.8 ± 22.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carpi, S.; Polini, B.; Nieri, D.; Doccini, S.; Conti, M.; Bazzan, E.; Pagnini, M.; Santorelli, F.M.; Cecchini, M.; Nieri, P.; et al. Extracellular Vesicles Induce Nuclear Factor-κB Activation and Interleukin-8 Synthesis through miRNA-191-5p Contributing to Inflammatory Processes: Potential Implications in the Pathogenesis of Chronic Obstructive Pulmonary Disease. Biomolecules 2024, 14, 1030. https://doi.org/10.3390/biom14081030
Carpi S, Polini B, Nieri D, Doccini S, Conti M, Bazzan E, Pagnini M, Santorelli FM, Cecchini M, Nieri P, et al. Extracellular Vesicles Induce Nuclear Factor-κB Activation and Interleukin-8 Synthesis through miRNA-191-5p Contributing to Inflammatory Processes: Potential Implications in the Pathogenesis of Chronic Obstructive Pulmonary Disease. Biomolecules. 2024; 14(8):1030. https://doi.org/10.3390/biom14081030
Chicago/Turabian StyleCarpi, Sara, Beatrice Polini, Dario Nieri, Stefano Doccini, Maria Conti, Erika Bazzan, Marta Pagnini, Filippo Maria Santorelli, Marco Cecchini, Paola Nieri, and et al. 2024. "Extracellular Vesicles Induce Nuclear Factor-κB Activation and Interleukin-8 Synthesis through miRNA-191-5p Contributing to Inflammatory Processes: Potential Implications in the Pathogenesis of Chronic Obstructive Pulmonary Disease" Biomolecules 14, no. 8: 1030. https://doi.org/10.3390/biom14081030






