Neglected Tropical Diseases: A Chemoinformatics Approach for the Use of Biodiversity in Anti-Trypanosomatid Drug Discovery
Abstract
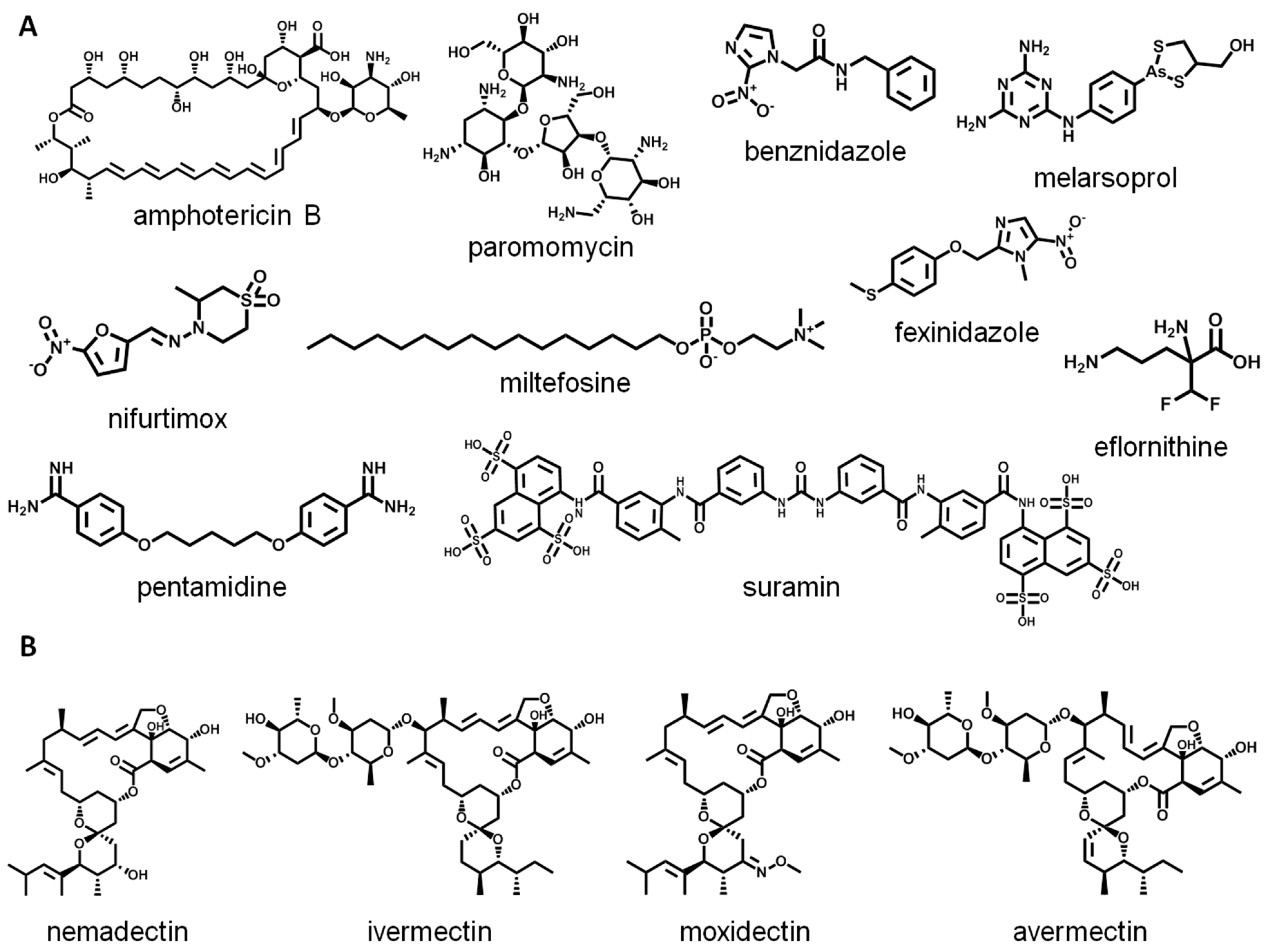
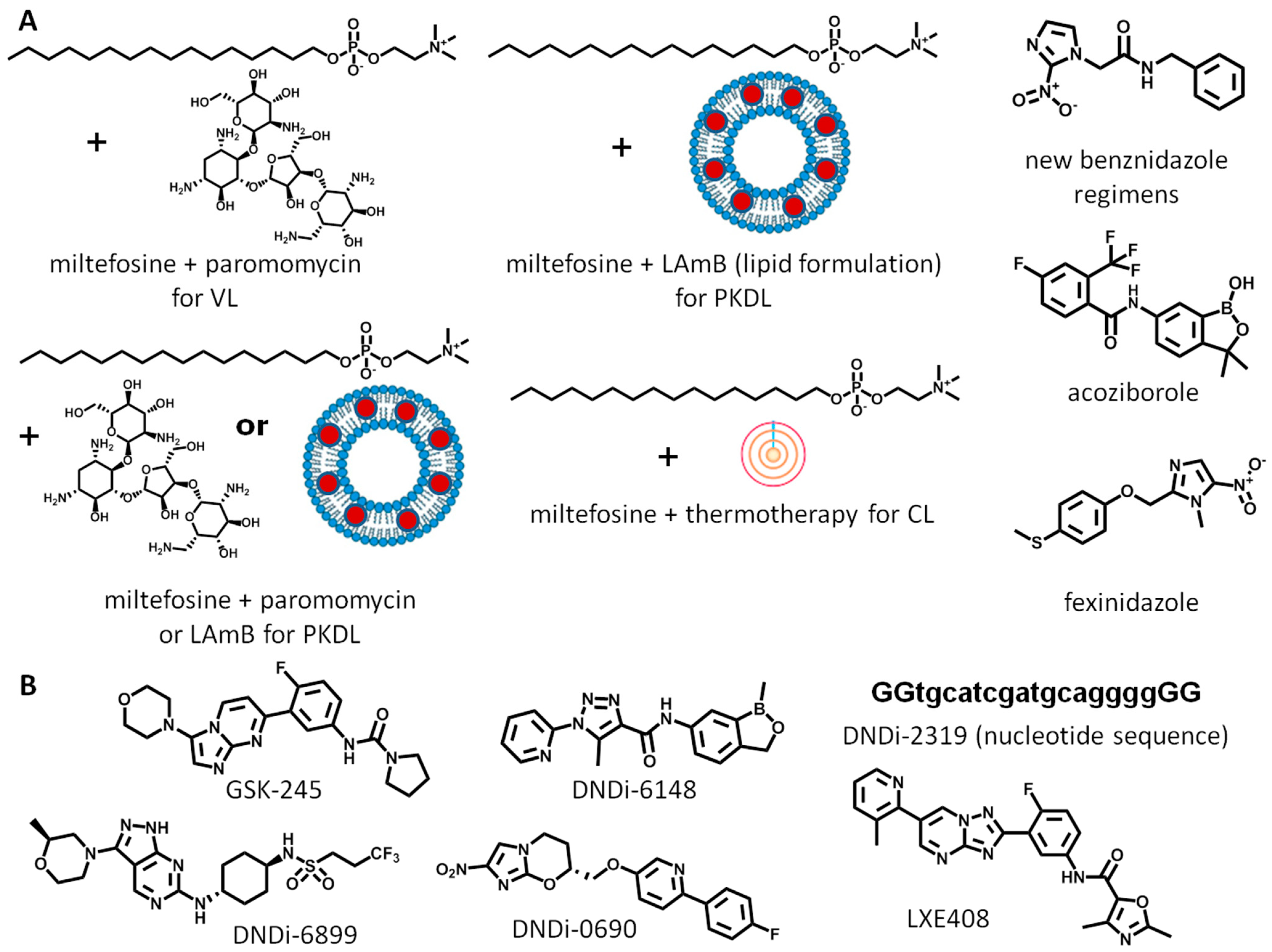
1. Introduction
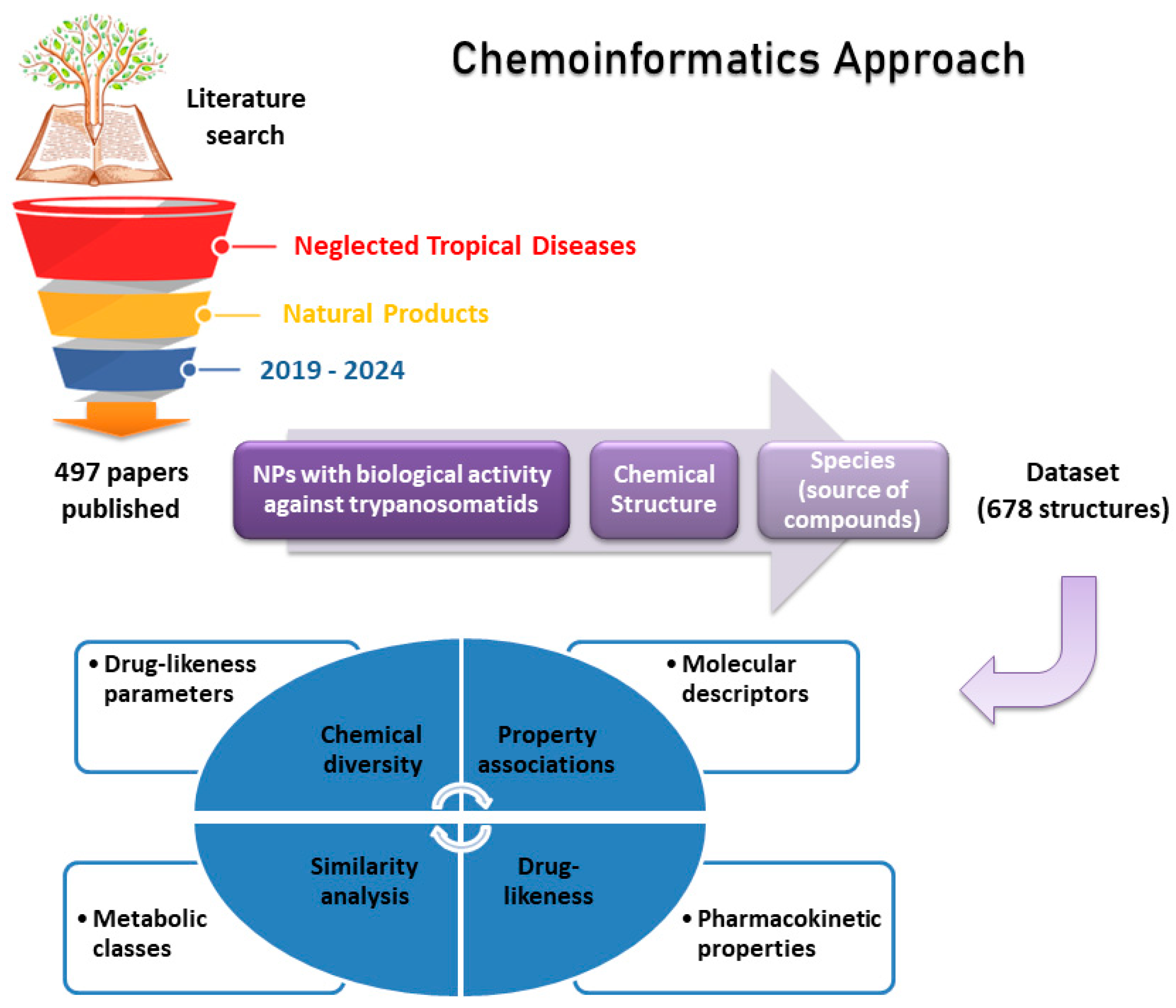
2. Materials and Methods
3. Results and Discussion
3.1. Annotated Compound Database
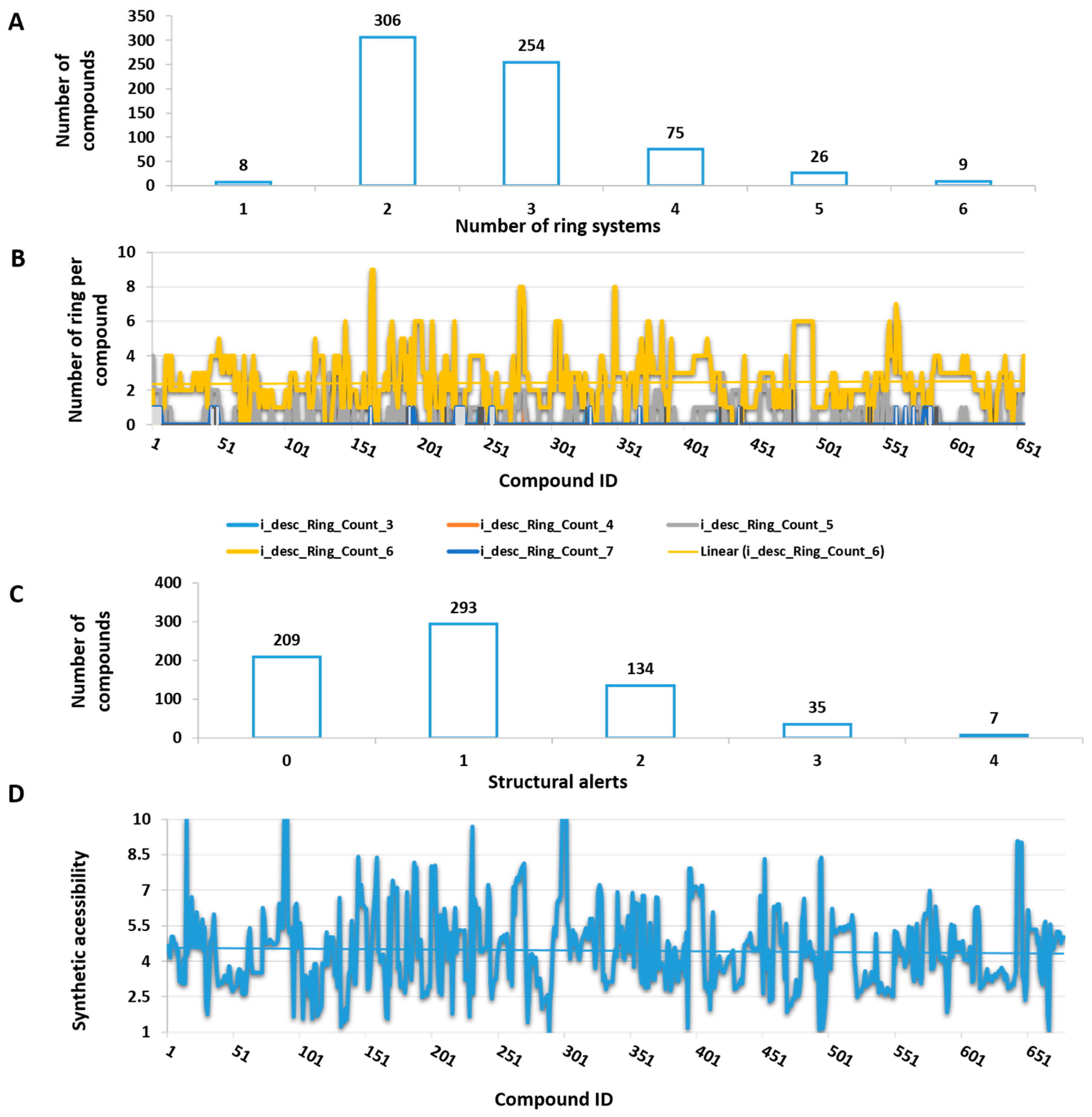
3.2. Ring Content, Structural Alerts, and Synthetic Accessibility
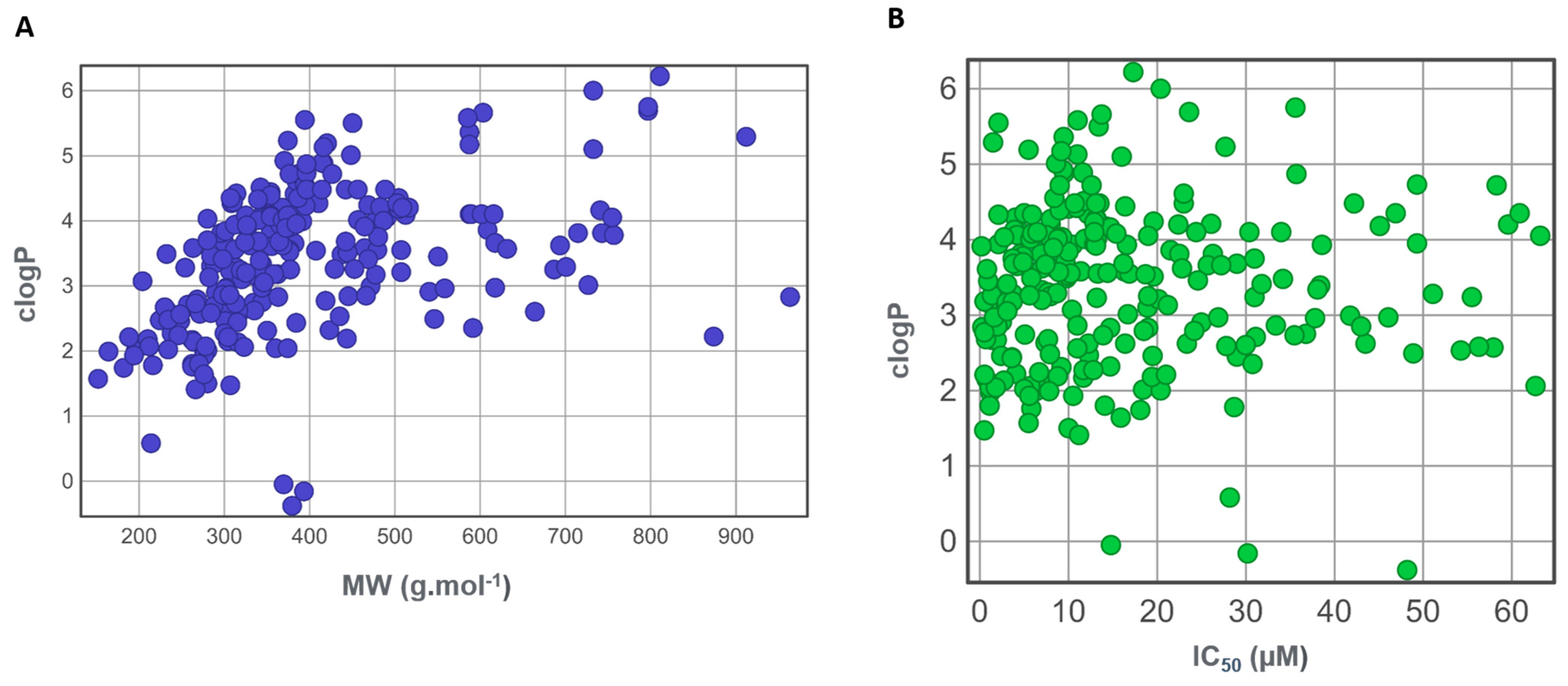
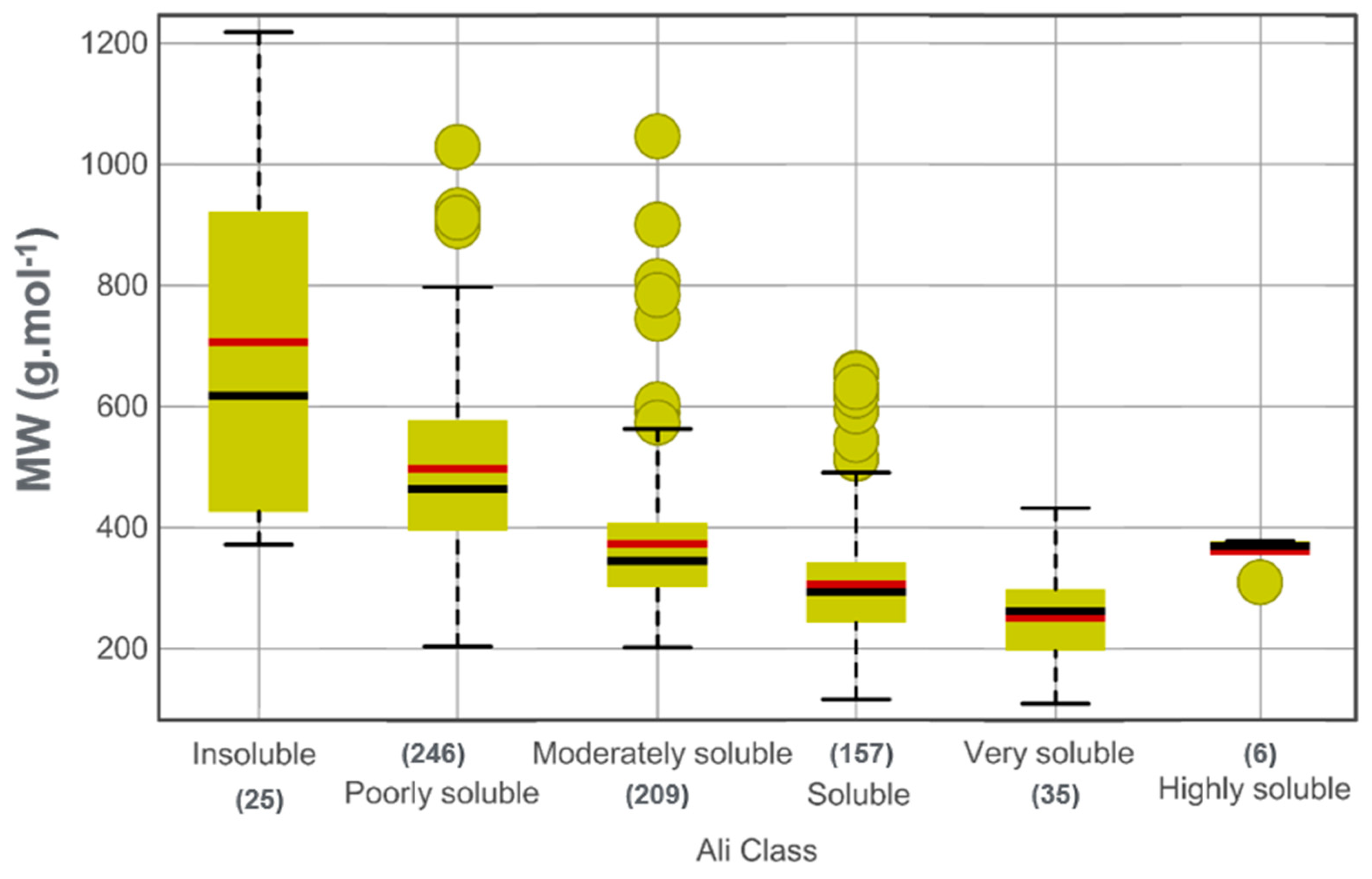
3.3. Drug-Likeness
3.4. Stereogenic Centers
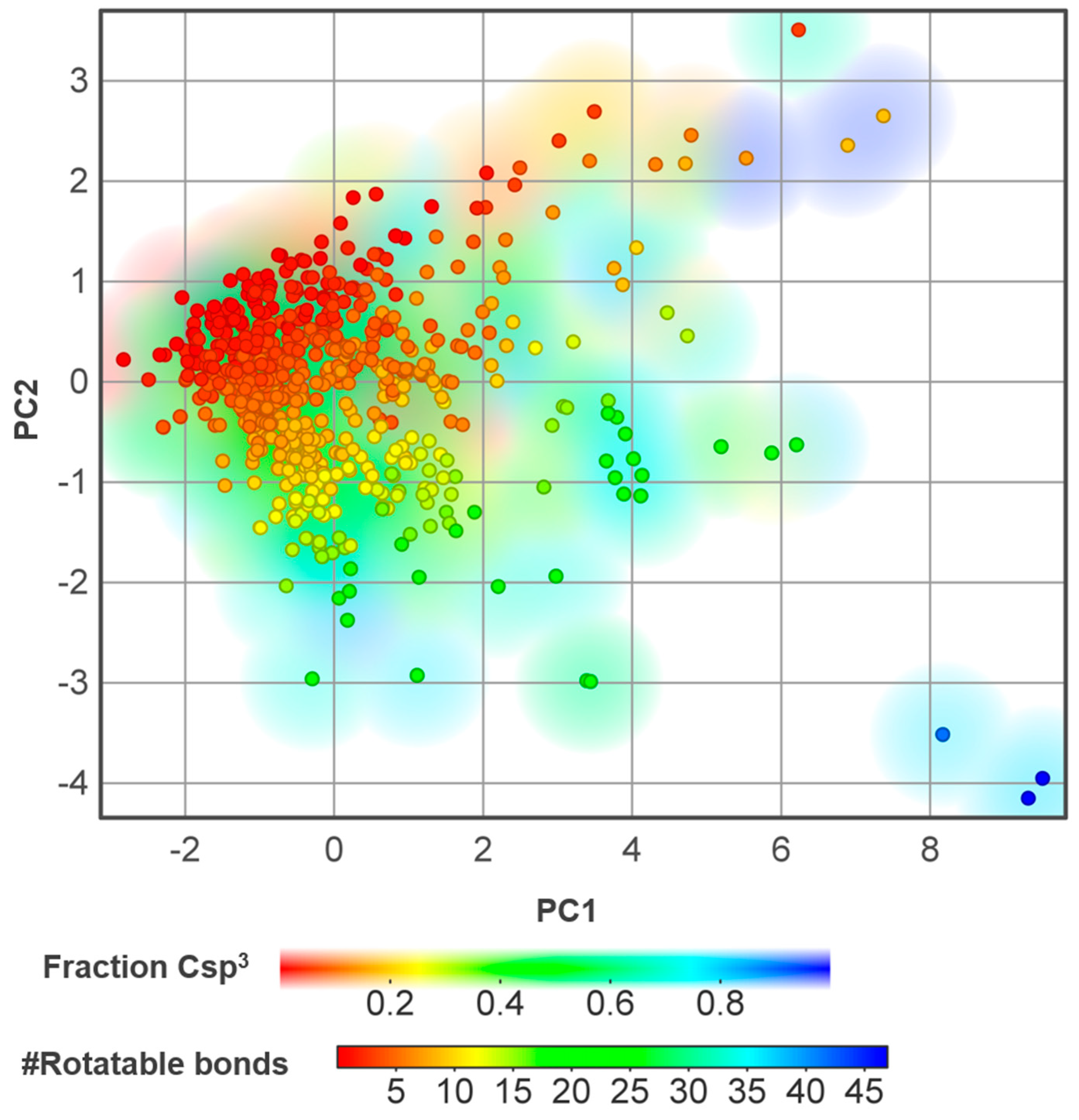
3.5. Chemical Diversity
3.6. Property Associations
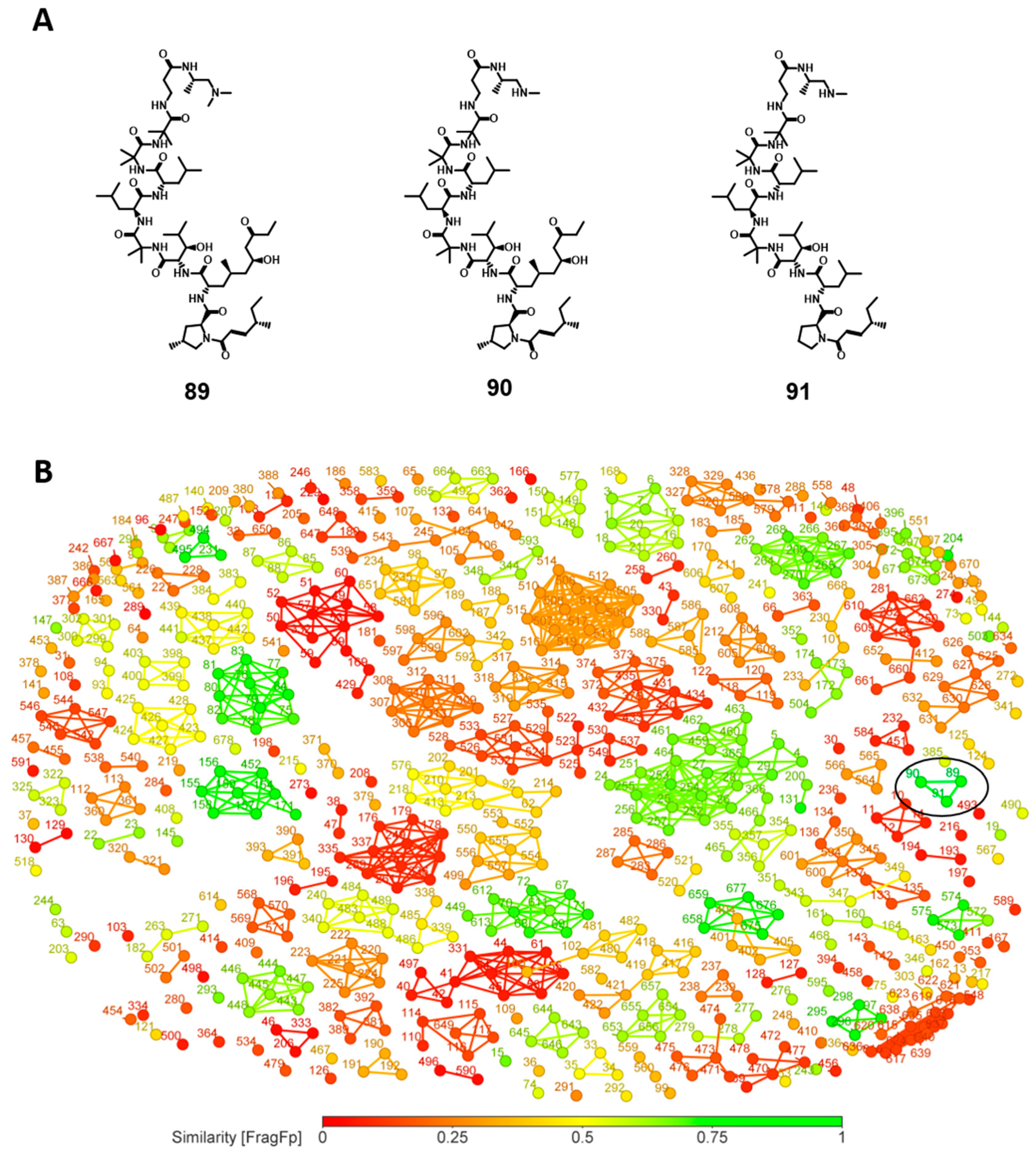
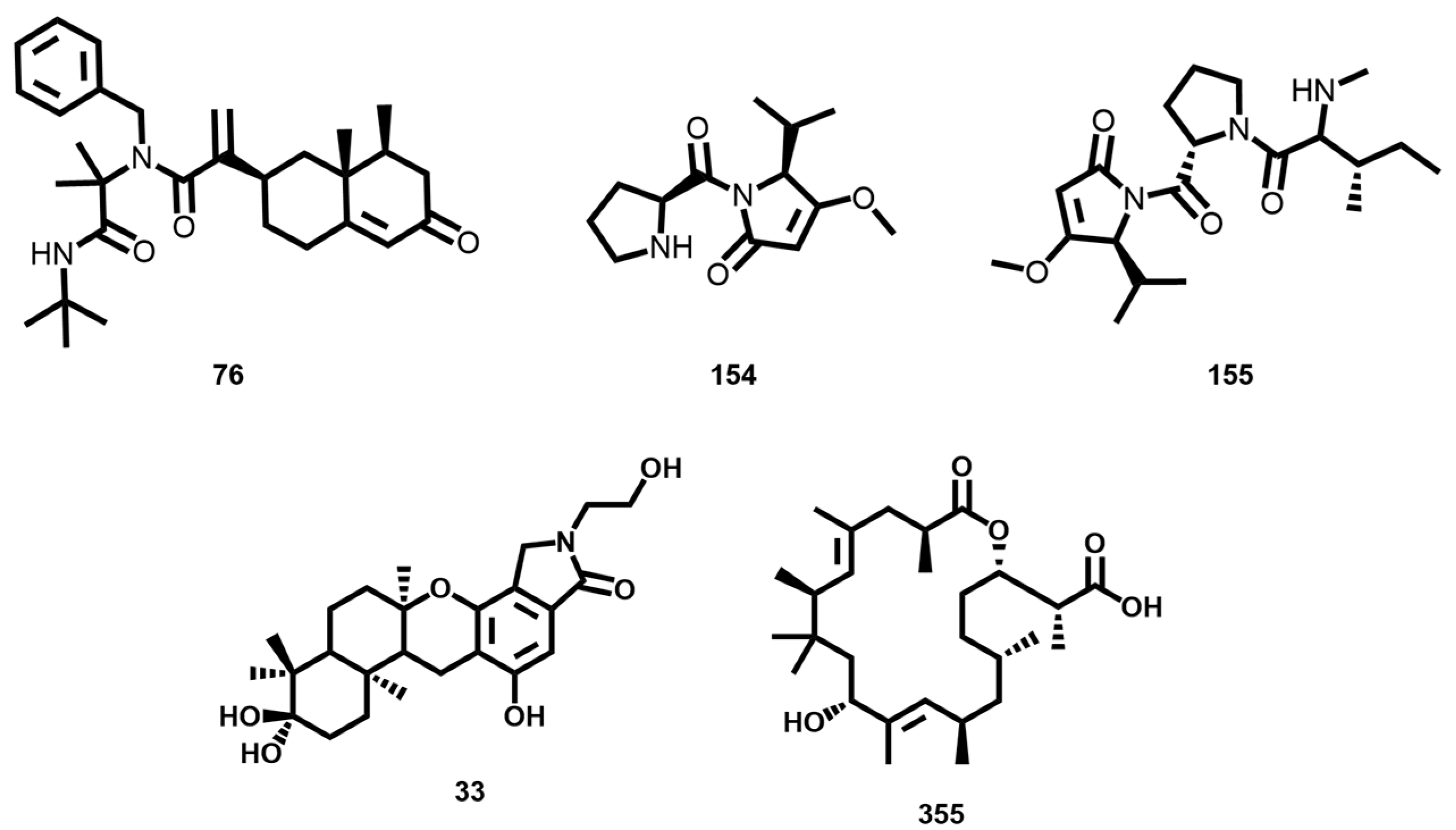
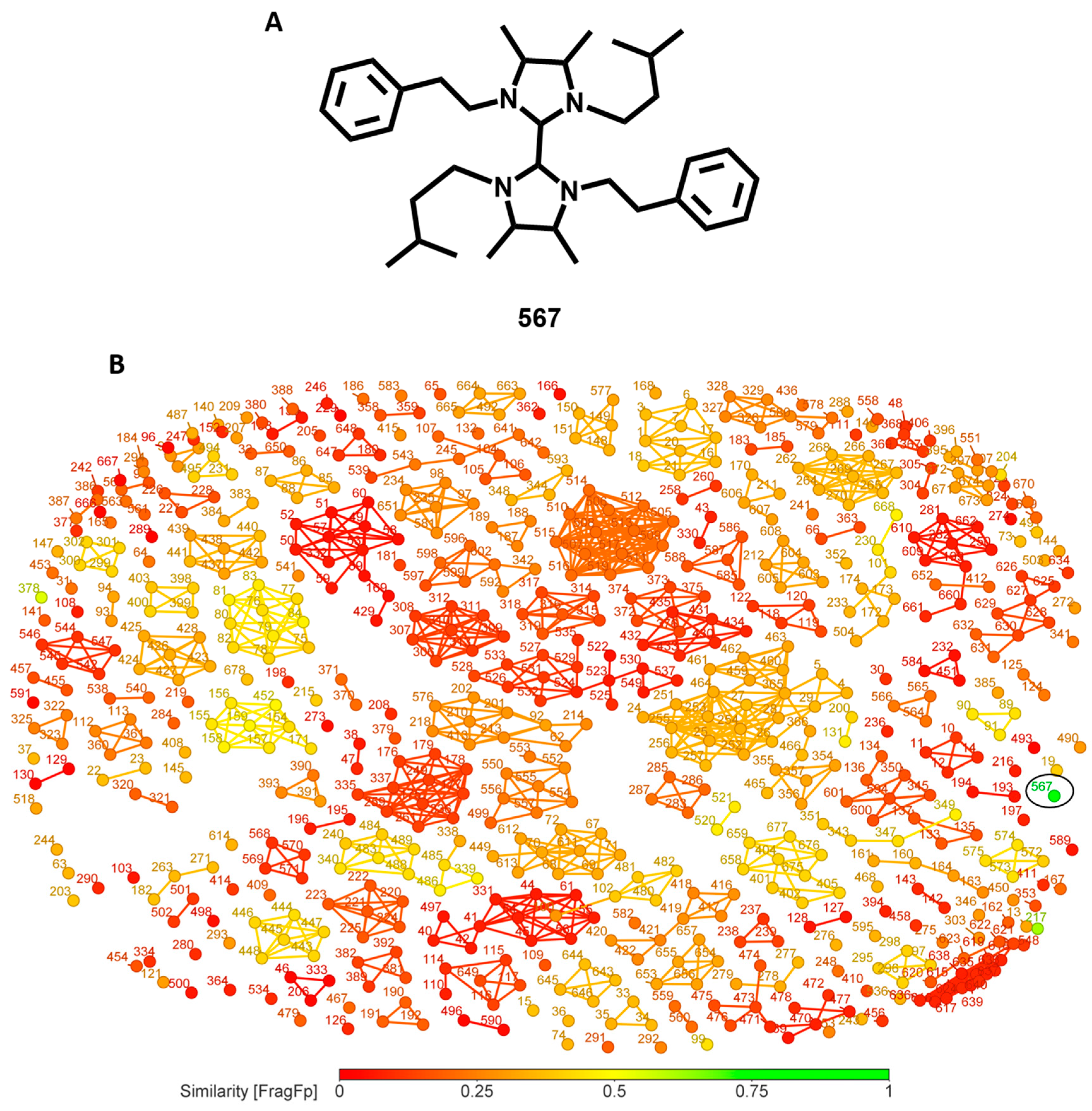
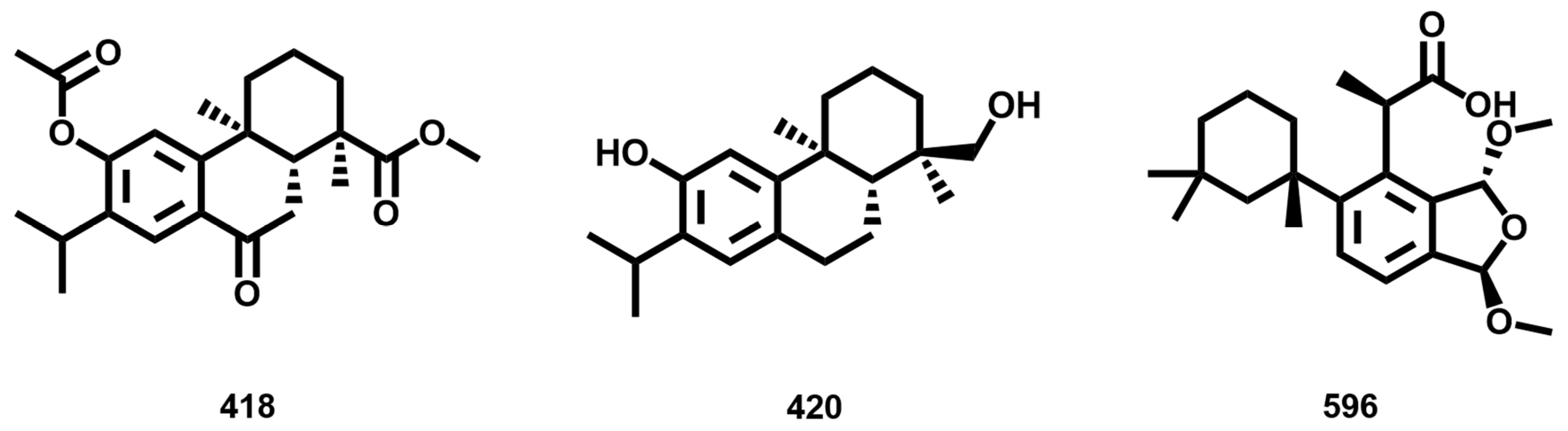
3.7. Similarity Analysis of Potent Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ferreira, L.L.G.; Andricopulo, A.D. Drugs and vaccines in the 21st century for neglected diseases. Lancet Infect. Dis. 2019, 19, 125–127. [Google Scholar] [CrossRef]
- World Health Organization. Ending the Neglect to Attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases, 2021–2030. Available online: https://www.who.int/publications/i/item/9789240010352 (accessed on 1 June 2024).
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development. Available online: https://sustainabledevelopment.un.org/content/documents/21252030%20Agenda%20for%20Sustainable%20Development%20web.pdf (accessed on 1 June 2024).
- World Health Organization. Global Report on Neglected Tropical Diseases. 2024. Available online: https://www.who.int/teams/control-of-neglected-tropical-diseases/global-report-on-neglected-tropical-diseases-2024 (accessed on 1 June 2024).
- Ferreira, L.L.G.; de Moraes, J.; Andricopulo, A.D. Approaches to advance drug discovery for neglected tropical Diseases. Drug Discov. Today 2022, 27, 2278–2287. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S. The United States Food and Drug Administration (FDA) regulatory response to combat neglected tropical diseases (NTDs): A review. PLoS Negl. Trop. Dis. 2023, 17, e0011010. [Google Scholar] [CrossRef] [PubMed]
- De Rycker, M.; Wyllie, S.; Horn, D.; Read, K.D.; Gilbert, I.H. Anti-trypanosomatid drug discovery: Progress and challenges. Nat. Rev. Microbiol. 2023, 21, 35–50. [Google Scholar] [CrossRef]
- World Health Organization. Chagas Disease (Also Known as American Trypanosomiasis). Available online: https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis) (accessed on 1 June 2024).
- Nambala, P.; Mulindwa, J.; Chammudzi, P.; Senga, E.; Lemelani, M.; Zgambo, D.; Matovu, E.; MacLeod, A.; Musaya, J. Persistently High Incidences of Trypanosoma brucei rhodesiense Sleeping Sickness With Contrasting Focus-Dependent Clinical Phenotypes in Malawi. Front. Trop. Dis. 2022, 3, 824484. [Google Scholar] [CrossRef]
- Drugs for Neglected Diseases Initiative. R&D Portfolio June 2023—12 Treatments Delivered. Available online: https://dndi.org/wp-content/uploads/2023/07/DNDi-RD-Portfolio-June-2023.pdf (accessed on 1 June 2024).
- Ntie-Kang, F.; Nyongbela, K.D.; Ayimele, G.A.; Shekfeh, S. “Drug-likeness” properties of natural compounds. Phys. Sci. Rev. 2019, 4, 20180169. [Google Scholar] [CrossRef]
- SciFinder-n. Chemical Abstracts Service. Available online: https://www.cas.org/solutions/cas-scifinder-discovery-platform/cas-scifinder-n?gad_source=1 (accessed on 1 June 2024).
- Clarivate. Web of Science. Available online: https://www.webofscience.com/wos/woscc/smart-search (accessed on 1 June 2024).
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Djoumbou, F.Y.; Eisner, R.; Knox, C.; Chepelev, L.; Hastings, J.; Owen, G.; Fahy, E.; Steinbeck, C.; Subramanian, S.; Bolton, E.; et al. ClassyFire: Automated Chemical Classification With A Comprehensive, Computable Taxonomy. J. Cheminform. 2016, 8, 61. [Google Scholar] [CrossRef]
- Rácz, A.; Bajusz, D.; Héberger, K. Life beyond the Tanimoto coefficient: Similarity measures for interaction fingerprints. J. Cheminform. 2018, 10, 48. [Google Scholar] [CrossRef]
- Sander, T.; Freyss, J.; von Korff, M.; Rufener, C. DataWarrior: An Open-Source Program for Chemistry Aware Data Visualization and Analysis. J. Chem. Inf. Model. 2015, 55, 460–473. [Google Scholar] [CrossRef]
- Artusi, R.; Verderio, P.; Marubini, E. Bravais-Pearson and Spearman correlation coefficients: Meaning, test of hypothesis and confidence interval. Int. J. Biol. Markers 2002, 17, 148–151. [Google Scholar] [CrossRef]
- Brenk, R.; Schipani, A.; James, D.; Krasowski, A.; Gilbert, I.H.; Frearson, J.; Wyatt, P.G. Lessons Learnt from Assembling Screening Libraries for Drug Discovery for Neglected Diseases. ChemMedChem 2008, 3, 435–444. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Méndez-Lucio, O.; Medina-Franco, J.L. The many roles of molecular complexity in drug discovery. Drug Discov. Today 2017, 22, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Greenacre, M.; Groenen, P.J.F.; Hastie, T.; D’Enza, A.I.; Markos, A.; Tuzhilina, E. Principal component analysis. Nat. Rev. Methods Primers 2022, 2, 100. [Google Scholar] [CrossRef]
- Kusaka, Y.; Hasegawa, T.; Kaji, H. Noise Reduction in Solid-State NMR Spectra Using Principal Component Analysis. J. Phys. Chem. A 2019, 123, 10333–10338. [Google Scholar] [CrossRef]
- Miller, R.R.; Madeira, M.; Wood, H.B.; Geissler, W.M.; Raab, C.E.; Martin, I.J. Integrating the Impact of Lipophilicity on Potency and Pharmacokinetic Parameters Enables the Use of Diverse Chemical Space during Small Molecule Drug Optimization. J. Med. Chem. 2020, 63, 12156–12170. [Google Scholar] [CrossRef]
- Júnior, C.d.O.R.; Martinez, P.D.G.; Ferreira, R.A.A.; Koovits, P.J.; Soares, B.M.; Ferreira, L.L.; Michelan-Duarte, S.; Chelucci, R.C.; Andricopulo, A.D.; Matheeussen, A.; et al. Hit-to-lead optimization of a 2-aminobenzimidazole series as new candidates for chagas disease. Eur. J. Med. Chem. 2023, 246, 114925. [Google Scholar] [CrossRef]
- Barrett, J.A.; Yang, W.; Skolnik, S.M.; Belliveau, L.M.; Patros, K.M. Discovery solubility measurement and assessment of small molecules with drug development in mind. Drug Discov. Today 2022, 27, 1315–1325. [Google Scholar] [CrossRef]
- O’Donovan, D.H.; De Fusco, C.; Kuhnke, L.; Reichel, A. Trends in Molecular Properties, Bioavailability, and Permeability across the Bayer Compound Collection. J. Med. Chem. 2023, 66, 2347–2360. [Google Scholar] [CrossRef]
- Ali, J.; Camilleri, P.; Brown, M.B.; Hutt, A.J.; Kirton, S.B. In silico prediction of aqueous solubility using simple QSPR models: The importance of phenol and phenol-like moieties. J. Chem. Inf. Model. 2012, 52, 2950–2957. [Google Scholar] [CrossRef] [PubMed]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Lovering, F.; Bikker, J.; Humblet, C. Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem. 2009, 52, 6752–6756. [Google Scholar] [CrossRef] [PubMed]
- Durant, J.L.; Leland, B.A.; Henry, D.R.; Nourse, J.G. Reoptimization of MDL Keys for Use in Drug Discovery. J. Chem. Inf. Comput. Sci. 2002, 42, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Bernatchez, J.A.; Kil, Y.S.; da Silva, E.B.; Thomas, D.; McCall, L.I.; Wendt, K.L.; Souza, J.M.; Ackermann, J.; McKerrow, J.H.; Cichewicz, R.H.; et al. Identification of Leucinostatins from Ophiocordyceps sp. as Antiparasitic Agents against Trypanosoma cruzi. ACS Omega 2022, 7, 7675–7682. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, M.; Singha, U.K.; Vanderloop, B.H.; Tripathi, A.; Nes, W.D. Steroidal Antimetabolites Protect Mice against Trypanosoma brucei. Molecules 2022, 27, 4088. [Google Scholar] [CrossRef] [PubMed]
- Amang À Ngnoung, G.A.; Sidjui, L.S.; Leutcha, P.B.; Nganso Ditchou, Y.O.; Tchokouaha, L.R.Y.; Herbette, G.; Baghdikian, B.; Kowa, T.K.; Soh, D.; Kemzeu, R.; et al. Antileishmanial and Antiplasmodial Activities of Secondary Metabolites from the Root of Antrocaryon klaineanum Pierre (Anacardiaceae). Molecules 2023, 28, 2730. [Google Scholar] [CrossRef]
- Lourenço, E.M.G.; Di Iório, J.F.; da Silva, F.; Fialho, F.L.B.; Monteiro, M.M.; Beatriz, A.; Perdomo, R.T.; Barbosa, E.G.; Oses, J.P.; de Arruda, C.C.P.; et al. Flavonoid Derivatives as New Potent Inhibitors of Cysteine Proteases: An Important Step toward the Design of New Compounds for the Treatment of Leishmaniasis. Microorganisms 2023, 11, 225. [Google Scholar] [CrossRef]




| SciFinder-n Search | |||
| Search | Abstract/Keywords | Publication Year | Number of Papers |
| Natural products | Trypanosomatida | 2019–2024 | 20 |
| Natural products | Trypanosoma | 2019–2024 | 240 |
| Natural products | Leishmania | 2019–2024 | 329 |
| Natural products | Leishmaniasis | 2019–2024 | 252 |
| Natural products | Chagas | 2019–2024 | 120 |
| Natural products | Neglected Tropical Diseases | 2019–2024 | 119 |
| Secondary metabolites | Trypanosoma | 2019–2024 | 36 |
| Secondary metabolites | Leishmania | 2019–2024 | 66 |
| Secondary metabolites | Trypanosomatida | 2019–2024 | 2 |
| Secondary metabolites | Leishmaniasis | 2019–2024 | 42 |
| Secondary metabolites | Chagas | 2019–2024 | 9 |
| Secondary metabolites | Neglected Tropical Diseases | 2019–2024 | 25 |
| Web of Science Search | |||
| Topic | Topic | Year Published | Number of Papers |
| Natural products | Trypanosomatida | 2019–2024 | 13 |
| Natural products | Trypanosoma | 2019–2024 | 234 |
| Natural products | Leishmania | 2019–2024 | 311 |
| Natural products | Leishmaniasis | 2019–2024 | 272 |
| Natural products | Chagas | 2019–2024 | 113 |
| Natural products | Neglected Tropical Diseases | 2019–2024 | 89 |
| Secondary metabolites | Trypanosoma | 2019–2024 | 50 |
| Secondary metabolites | Leishmania | 2019–2024 | 65 |
| Secondary metabolites | Trypanosomatida | 2019–2024 | 2 |
| Secondary metabolites | Leishmaniasis | 2019–2024 | 54 |
| Secondary metabolites | Chagas | 2019–2024 | 16 |
| Secondary metabolites | Neglected Tropical Diseases | 2019–2024 | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valli, M.; Döring, T.H.; Marx, E.; Ferreira, L.L.G.; Medina-Franco, J.L.; Andricopulo, A.D. Neglected Tropical Diseases: A Chemoinformatics Approach for the Use of Biodiversity in Anti-Trypanosomatid Drug Discovery. Biomolecules 2024, 14, 1033. https://doi.org/10.3390/biom14081033
Valli M, Döring TH, Marx E, Ferreira LLG, Medina-Franco JL, Andricopulo AD. Neglected Tropical Diseases: A Chemoinformatics Approach for the Use of Biodiversity in Anti-Trypanosomatid Drug Discovery. Biomolecules. 2024; 14(8):1033. https://doi.org/10.3390/biom14081033
Chicago/Turabian StyleValli, Marilia, Thiago H. Döring, Edgard Marx, Leonardo L. G. Ferreira, José L. Medina-Franco, and Adriano D. Andricopulo. 2024. "Neglected Tropical Diseases: A Chemoinformatics Approach for the Use of Biodiversity in Anti-Trypanosomatid Drug Discovery" Biomolecules 14, no. 8: 1033. https://doi.org/10.3390/biom14081033
APA StyleValli, M., Döring, T. H., Marx, E., Ferreira, L. L. G., Medina-Franco, J. L., & Andricopulo, A. D. (2024). Neglected Tropical Diseases: A Chemoinformatics Approach for the Use of Biodiversity in Anti-Trypanosomatid Drug Discovery. Biomolecules, 14(8), 1033. https://doi.org/10.3390/biom14081033







