Revealing the Mechanism of Esculin in Treating Renal Cell Carcinoma Based on Network Pharmacology and Experimental Validation
Abstract
1. Introduction
2. Results
2.1. Network Pharmacology Predicts the Mechanisms of Esculin Treats RCC
2.2. Molecular Docking of Core Target Proteins with Esculin
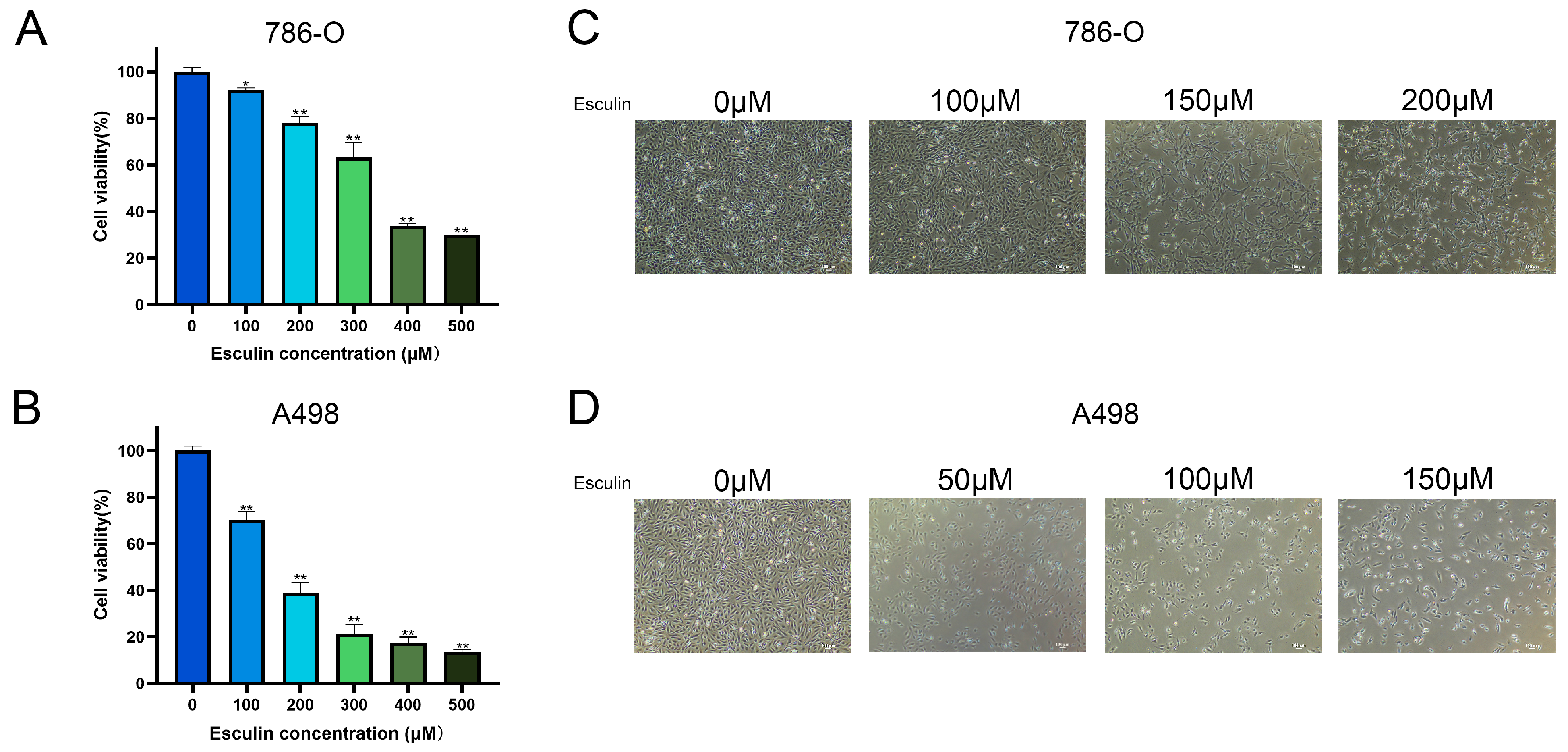
2.3. Esculin Inhibits RCC Cell Viability
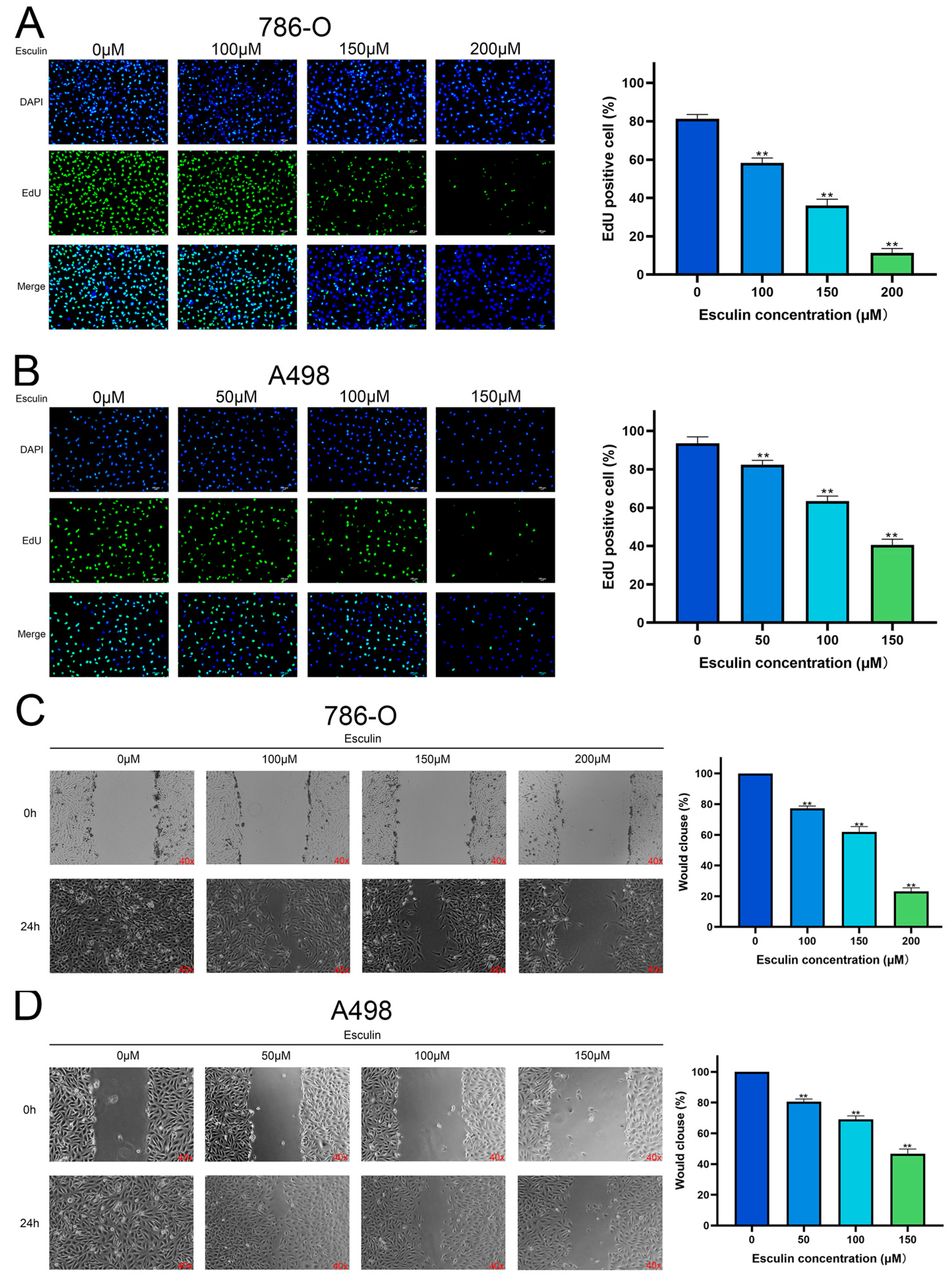
2.4. Esculin Inhibits RCC Cell Proliferation and Migration
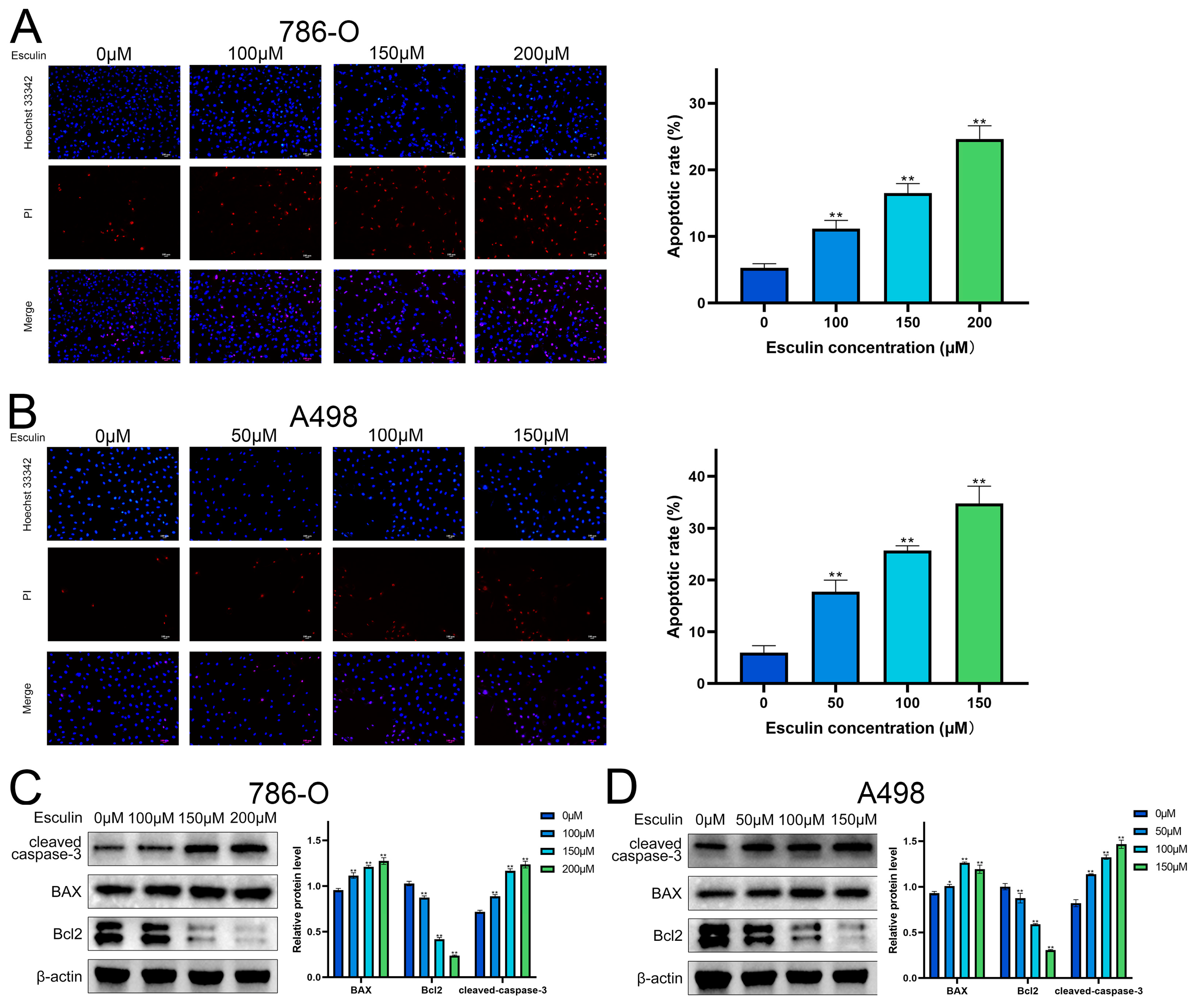
2.5. Esculin Induces Apoptosis in RCC Cells
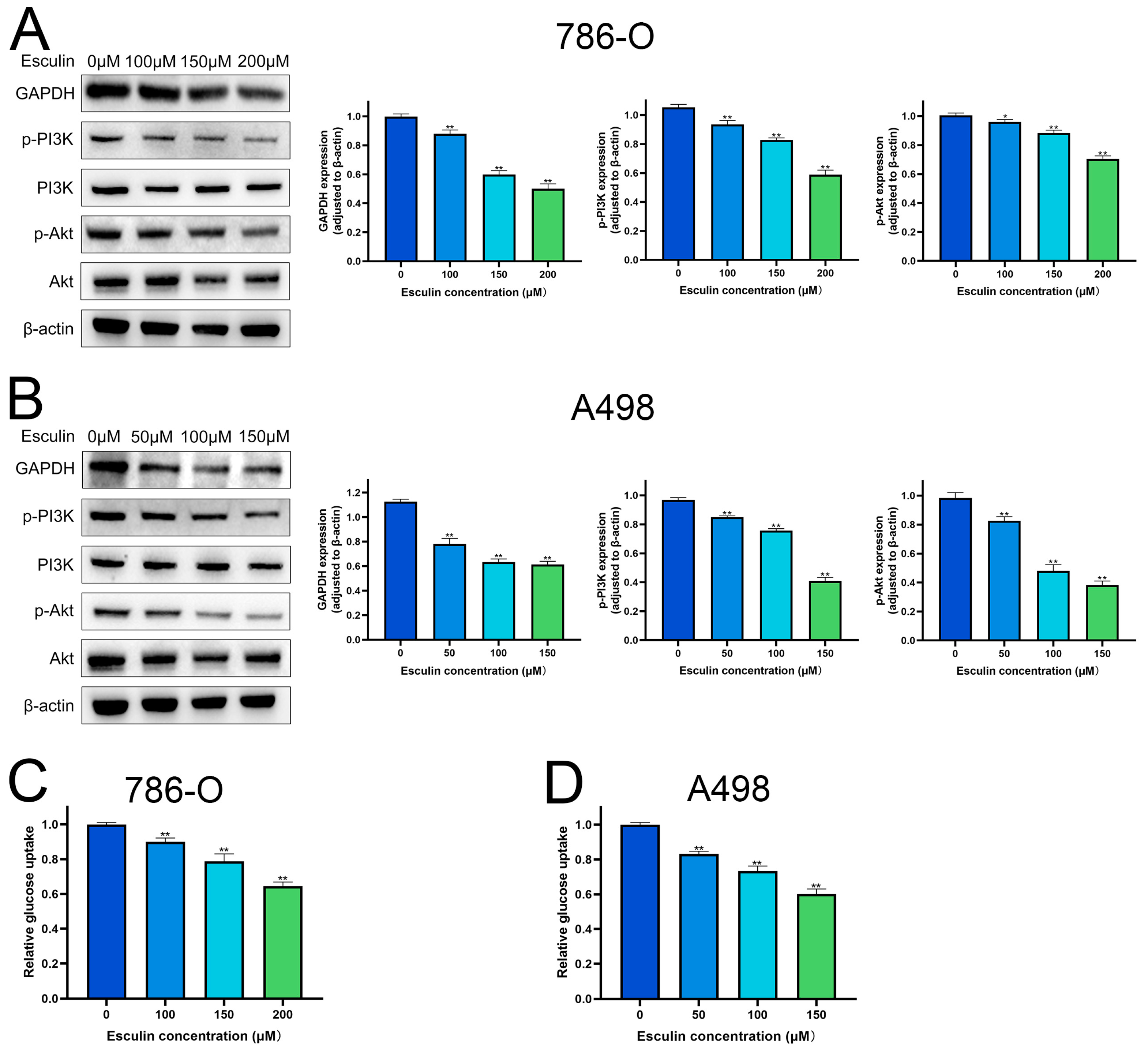
2.6. Esculin Inhibits RCC Cells via PI3K/Akt Pathway
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Targets Acquisition and Analysis
4.3. PPI Network Construction
4.4. Analysis of GO and KEGG Pathway
4.5. Molecular Docking
4.6. Cell Lines
4.7. CCK-8 Assay
4.8. EdU Assay
4.9. Wound Healing Assay
4.10. Apoptosis Assay
4.11. Glucose Uptake Assay
4.12. Western Blot
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.W.; Wang, L.; Panian, J.; Dhanji, S.; Derweesh, I.; Rose, B.; Bagrodia, A.; McKay, R.R. Treatment Landscape of Renal Cell Carcinoma. Curr. Treat. Opt. Oncol. 2023, 24, 1889–1916. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, L.M.; Sigurdson, S.; Wallis, C.J.D.; Lalani, A.K.; Swaminath, A. Advances in the management of renal cell carcinoma. CMAJ 2024, 196, E235–E240. [Google Scholar] [CrossRef] [PubMed]
- Navani, V.; Heng, D.Y.C. Immunotherapy in renal cell carcinoma. Lancet Oncol. 2023, 24, 1164–1166. [Google Scholar] [CrossRef] [PubMed]
- Makhov, P.; Joshi, S.; Ghatalia, P.; Kutikov, A.; Uzzo, R.G.; Kolenko, V.M. Resistance to Systemic Therapies in Clear Cell Renal Cell Carcinoma: Mechanisms and Management Strategies. Mol. Cancer Ther. 2018, 17, 1355–1364. [Google Scholar] [CrossRef]
- Ghosh, S.; Garige, M.; Haggerty, P.R.; Norris, A.; Chou, C.K.; Wu, W.W.; Shen, R.F.; Sourbier, C. Impact of sunitinib resistance on clear cell renal cell carcinoma therapeutic sensitivity in vitro. Cell Cycle 2024, 23, 43–55. [Google Scholar] [CrossRef]
- Zhao, P.; Qiu, J.; Pan, C.; Tang, Y.; Chen, M.; Song, H.; Yang, J.; Hao, X. Potential roles and molecular mechanisms of bioactive ingredients in Curcumae Rhizoma against breast cancer. Phytomedicine 2023, 114, 154810. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Su, Z.; Gao, M.; Weng, L.; Xu, T. Esculin targets TLR4 to protect against LPS-induced septic cardiomyopathy. Int. Immunopharmacol. 2024, 131, 111897. [Google Scholar] [CrossRef]
- Cheng, Z.; Liu, Z.; Liu, C.; Yang, A.; Miao, H.; Bai, X. Esculin suppresses the PERK-eIF2α-CHOP pathway by enhancing SIRT1 expression in oxidative stress-induced rat chondrocytes, mitigating osteoarthritis progression in a rat model. Int. Immunopharmacol. 2024, 132, 112061. [Google Scholar] [CrossRef]
- Ni, J.; Li, G.; Dai, N.; Quan, Z.; Tong, H.; Liu, Y. Esculin alleviates LPS-induced acute lung injury via inhibiting neutrophil recruitment and migration. Int. Immunopharmacol. 2023, 119, 110177. [Google Scholar] [CrossRef]
- Xu, S.; Chen, Y.; Miao, J.; Li, Y.; Liu, J.; Zhang, J.; Liang, J.; Chen, S.; Hou, S. Esculin inhibits hepatic stellate cell activation and CCl(4)-induced liver fibrosis by activating the Nrf2/GPX4 signaling pathway. Phytomedicine 2024, 128, 155465. [Google Scholar] [CrossRef]
- Ji, X.; Chen, Z.; Lin, W.; Wu, Q.; Wu, Y.; Hong, Y.; Tong, H.; Wang, C.; Zhang, Y. Esculin induces endoplasmic reticulum stress and drives apoptosis and ferroptosis in colorectal cancer via PERK regulating eIF2α/CHOP and Nrf2/HO-1 cascades. J. Ethnopharmacol. 2024, 328, 118139. [Google Scholar] [CrossRef]
- Mokdad-Bzeouich, I.; Kovacic, H.; Ghedira, K.; Chebil, L.; Ghoul, M.; Chekir-Ghedira, L.; Luis, J. Esculin and its oligomer fractions inhibit adhesion and migration of U87 glioblastoma cells and in vitro angiogenesis. Tumour Biol. 2016, 37, 3657–3664. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, C.; Zhou, J.; Zhou, F.; Gui, A.; Chu, H.; Shao, Q. Chrysanthemum morifolium as a traditional herb: A review of historical development, classification, phytochemistry, pharmacology and application. J. Ethnopharmacol. 2024, 330, 118198. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Shan, X.; Songru, Y.; Fu, M.; Zhao, P.; Guo, W.; Xu, M.; Chen, H.; Lu, R.; Zhang, C. Network pharmacology integrated with experimental validation to elucidate the mechanisms of action of the Guizhi-Gancao Decoction in the treatment of phenylephrine-induced cardiac hypertrophy. Pharm. Biol. 2024, 62, 456–471. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, D.; Yang, X.; Liu, C.; Li, X.; Zhang, X.; Liu, K.; Zhang, Y.; Zhang, M.; Wang, C.; et al. Anti-Tumor Activity and Mechanism of Silibinin Based on Network Pharmacology and Experimental Verification. Molecules 2024, 29, 1901. [Google Scholar] [CrossRef]
- Peng, Y.; Zhu, G.; Ma, Y.; Huang, K.; Chen, G.; Liu, C.; Tao, Y. Network Pharmacology-Based Prediction and Pharmacological Validation of Effects of Astragali Radix on Acetaminophen-Induced Liver Injury. Front. Med. 2022, 9, 697644. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.J.; Zhang, Y.F.; Bai, X.H.; Zhou, M.Q.; Zhang, Z.Y.; Zhang, S.X.; Cao, Z.J.; Wang, L.; Ding, S.W.; Zheng, H.J.; et al. Integrated Network Pharmacology Analysis and Experimental Validation to Elucidate the Mechanism of Acteoside in Treating Diabetic Kidney Disease. Drug Des. Dev. Ther. 2024, 18, 1439–1457. [Google Scholar] [CrossRef]
- Wei, X.; Wang, D.; Liu, J.; Zhu, Q.; Xu, Z.; Niu, J.; Xu, W. Interpreting the Mechanism of Active Ingredients in Polygonati Rhizoma in Treating Depression by Combining Systemic Pharmacology and In Vitro Experiments. Nutrients 2024, 16, 1167. [Google Scholar] [CrossRef]
- Xian, M.; Xu, J.; Zheng, Y.; Zhang, L.; Zhao, J.; Chen, J.; Li, S.; Lin, L.; Zhong, Y.; Yang, Z.; et al. Network Pharmacology and Experimental Verification Reveal the Regulatory Mechanism of Chuanbeimu in Treating Chronic Obstructive Pulmonary Disease. Int. J. Chronic Obstr. Pulm. Dis. 2024, 19, 799–813. [Google Scholar] [CrossRef]
- Wang, R.; Tang, D.; Ou, L.; Jiang, J.; Wu, Y.N.; Tian, X. β-Sitosterol alleviates the malignant phenotype of hepatocellular carcinoma cells via inhibiting GSK3B expression. Hum. Cell 2024, 37, 1156–1169. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, R.; Chen, X.; Yuan, M.; Wu, J.; Sun, Q.; Miao, C.; Jing, Y. The potential effect and mechanism of Saikosaponin A against gastric cancer. BMC Complement. Med. Ther. 2023, 23, 295. [Google Scholar] [CrossRef] [PubMed]
- Hirai, Y.; Makita, Y.; Asaoka, J.; Aoyagi, Y.; Nomoto, A.; Okamura, H.; Fujiwara, S.I. Boron Clusters Alter the Membrane Permeability of Dicationic Fluorescent DNA-Staining Dyes. ACS Omega 2023, 8, 35321–35327. [Google Scholar] [CrossRef] [PubMed]
- Asadi, M.; Taghizadeh, S.; Kaviani, E.; Vakili, O.; Taheri-Anganeh, M.; Tahamtan, M.; Savardashtaki, A. Caspase-3: Structure, function, and biotechnological aspects. Biotechnol. Appl. Biochem. 2022, 69, 1633–1645. [Google Scholar] [CrossRef]
- Kunjithapatham, R.; Ganapathy-Kanniappan, S. GAPDH with NAD(+)-binding site mutation competitively inhibits the wild-type and affects glucose metabolism in cancer. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2555–2563. [Google Scholar] [CrossRef]
- Cirillo, L.; Innocenti, S.; Becherucci, F. Global epidemiology of kidney cancer. Nephrol. Dial. Transplant. 2024, 39, 920–928. [Google Scholar] [CrossRef]
- Eremia, I.A.; Serban, B.; Popa, M.; Iancu, A.; Nica, S.; Cirstoiu, C. Practical management of renal cell carcinoma: Integrating current approaches with advances in bone metastasis treatment. EFORT Open Rev. 2024, 9, 488–502. [Google Scholar] [CrossRef]
- Evans, S.T.; Jani, Y.; Jansen, C.S.; Yildirim, A.; Kalemoglu, E.; Bilen, M.A. Understanding and overcoming resistance to immunotherapy in genitourinary cancers. Cancer Biol. Ther. 2024, 25, 2342599. [Google Scholar] [CrossRef]
- Chen, T.; Xiao, Z.; Liu, X.; Wang, T.; Wang, Y.; Ye, F.; Su, J.; Yao, X.; Xiong, L.; Yang, D.H. Natural products for combating multidrug resistance in cancer. Pharmacol. Res. 2024, 202, 107099. [Google Scholar] [CrossRef]
- Ghosh, S.; Das, S.K.; Sinha, K.; Ghosh, B.; Sen, K.; Ghosh, N.; Sil, P.C. The Emerging Role of Natural Products in Cancer Treatment. Arch. Toxicol. 2024, 98, 2353–2391. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Dong, Y.; Li, G.; Wang, S. Thymoquinone: An Effective Natural Compound for Kidney Protection. Am. J. Chin. Med. 2024, 52, 775–797. [Google Scholar] [CrossRef]
- Feng, C.; Gong, L.; Wang, J. Arborinine from Glycosmis parva leaf extract inhibits clear-cell renal cell carcinoma by inhibiting KDM1A/UBE2O signaling. Food Nutr. Res. 2022, 66, 8714. [Google Scholar] [CrossRef]
- Feng, C.; Lyu, Y.; Gong, L.; Wang, J. Therapeutic Potential of Natural Products in the Treatment of Renal Cell Carcinoma: A Review. Nutrients 2022, 14, 2274. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.S.; Lee, W.; Jung, Y.; Lee, J.H.; Lee, S.; Eom, D.W.; Jeon, Y.; Yoo, H.H.; Jin, M.J.; Song, K.I.; et al. Protective effect of esculin on streptozotocin-induced diabetic renal damage in mice. J. Agric. Food Chem. 2014, 62, 2069–2076. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.Y.; Lee, C.Y.; Kim, Y.S.; Kim, C.E. The Methodological Trends of Traditional Herbal Medicine Employing Network Pharmacology. Biomolecules 2019, 9, 362. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Zhang, F.; Hong, C.Q.; Giuliano, A.E.; Cui, X.J.; Zhou, G.J.; Zhang, G.J.; Cui, Y.K. Critical protein GAPDH and its regulatory mechanisms in cancer cells. Cancer Biol. Med. 2015, 12, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, M.; Liu, M.; Xu, Y.; Wu, G. Glycolysis-Related Genes Serve as Potential Prognostic Biomarkers in Clear Cell Renal Cell Carcinoma. Oxid. Med. Cell Longev. 2021, 2021, 6699808. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.; Liu, Y.; Shi, J.; Liu, C.; Fu, S.; Wang, H.; Ren, B.; Mi, D.; Gao, S.; Sun, D. Single-Cell Transcriptome Analysis Reveals Mesenchymal Stem Cells in Cavernous Hemangioma. Front. Cell Dev. Biol. 2022, 10, 916045. [Google Scholar] [CrossRef]
- Cornett, K.; Puderbaugh, A.; Back, O.; Craven, R. GAPDH in neuroblastoma: Functions in metabolism and survival. Front. Oncol. 2022, 12, 979683. [Google Scholar] [CrossRef]
- Ganapathy-Kanniappan, S.; Geschwind, J.F. Tumor glycolysis as a target for cancer therapy: Progress and prospects. Mol. Cancer 2013, 12, 152. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Zhang, W.; Kong, X.T.; Hai, W.X.; Guo, R.; Zhang, M.; Zhang, S.L.; Li, B. The therapeutic effect of a novel GAPDH inhibitor in mouse model of breast cancer and efficacy monitoring by molecular imaging. Cancer Cell Int. 2024, 24, 188. [Google Scholar] [CrossRef] [PubMed]
- Pacchiana, R.; Mullappilly, N.; Pinto, A.; Bova, S.; Forciniti, S.; Cullia, G.; Dalla Pozza, E.; Bottani, E.; Decimo, I.; Dando, I.; et al. 3-Bromo-Isoxazoline Derivatives Inhibit GAPDH Enzyme in PDAC Cells Triggering Autophagy and Apoptotic Cell Death. Cancers 2022, 14, 3153. [Google Scholar] [CrossRef]
- Liu, H.; Li, X.; Duan, Y.; Xie, J.B.; Piao, X.L. Mechanism of gypenosides of Gynostemma pentaphyllum inducing apoptosis of renal cell carcinoma by PI3K/AKT/mTOR pathway. J. Ethnopharmacol. 2021, 271, 113907. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, Y.; Ren, B.; Wu, Y.; Qiu, Z.; Cheng, Y.; Qiu, B. Poria acid inhibit the growth and metastasis of renal cell carcinoma by inhibiting the PI3K/akt/NF-κb signaling pathway. Heliyon 2024, 10, e31106. [Google Scholar] [CrossRef]
- Guo, Y.; Jiang, L.; Luo, S.; Hu, D.; Zhao, X.; Zhao, G.; Tang, W. Network Analysis and Basic Experiments on the Inhibition of Renal Cancer Proliferation and Migration by Alpinetin through PI3K/AKT/ mTOR Pathway. Curr. Mol. Med. 2024, 24, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Xi, C.; Zhang, G.; Sun, N.; Liu, M.; Ju, N.; Shen, C.; Song, H.; Luo, Q.; Qiu, Z. Repurposing homoharringtonine for thyroid cancer treatment through TIMP1/FAK/PI3K/AKT signaling pathway. iScience 2024, 27, 109829. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhao, X.; Long, F.; Tian, K.; Wu, L. Lianhua Qingwen exerts anti-liver cancer effects and synergistic efficacy with sorafenib through PI3K/AKT pathway: Integrating network pharmacology, molecular docking, and experimental validation. Gene 2024, 912, 148383. [Google Scholar] [CrossRef] [PubMed]
- Jacquin, M.A.; Chiche, J.; Zunino, B.; Bénéteau, M.; Meynet, O.; Pradelli, L.A.; Marchetti, S.; Cornille, A.; Carles, M.; Ricci, J.E. GAPDH binds to active Akt, leading to Bcl-xL increase and escape from caspase-independent cell death. Cell Death Differ. 2013, 20, 1043–1054. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Tong, L.; Liu, B.; Ai, Z.; Hong, Z.; You, P.; Wu, H.; Yang, Y. Bioactive ingredients obtained from Cortex Fraxini impair interactions between FAS and GPI. Free Radic. Biol. Med. 2020, 152, 504–515. [Google Scholar] [CrossRef]
- Pavlíková, N. Caffeic Acid and Diseases-Mechanisms of Action. Int. J. Mol. Sci. 2022, 24, 588. [Google Scholar] [CrossRef]
- Abdelkafi-Koubaa, Z.; Aissa, I.; Ben Jannet, H.; Srairi-Abid, N.; Marrakchi, N.; Menif, S. Tyrosol Derivatives, Bearing 3,5-Disubstituted Isoxazole and 1,4-Disubstituted Triazole, as Potential Antileukemia Agents by Promoting Apoptosis. Molecules 2022, 27, 5086. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Wang, M.H.; Wang, F.; Chen, P.Y.; Ke, X.G.; Yu, B.; Yang, Y.F.; You, P.T.; Wu, H.Z. Network pharmacology and molecular docking reveal the mechanism of Scopoletin against non-small cell lung cancer. Life Sci. 2021, 270, 119105. [Google Scholar] [CrossRef] [PubMed]
- Bardou, P.; Mariette, J.; Escudié, F.; Djemiel, C.; Klopp, C. jvenn: An interactive Venn diagram viewer. BMC Bioinform. 2014, 15, 293. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Wang, C.; Cai, Y.; Xu, A.; Han, C.; Tong, Y.; Cheng, S.; Liu, M. Revealing the Mechanism of Esculin in Treating Renal Cell Carcinoma Based on Network Pharmacology and Experimental Validation. Biomolecules 2024, 14, 1043. https://doi.org/10.3390/biom14081043
Chen Z, Wang C, Cai Y, Xu A, Han C, Tong Y, Cheng S, Liu M. Revealing the Mechanism of Esculin in Treating Renal Cell Carcinoma Based on Network Pharmacology and Experimental Validation. Biomolecules. 2024; 14(8):1043. https://doi.org/10.3390/biom14081043
Chicago/Turabian StyleChen, Zixuan, Cunzhou Wang, Yuesong Cai, An Xu, Chengtao Han, Yanjun Tong, Sheng Cheng, and Min Liu. 2024. "Revealing the Mechanism of Esculin in Treating Renal Cell Carcinoma Based on Network Pharmacology and Experimental Validation" Biomolecules 14, no. 8: 1043. https://doi.org/10.3390/biom14081043
APA StyleChen, Z., Wang, C., Cai, Y., Xu, A., Han, C., Tong, Y., Cheng, S., & Liu, M. (2024). Revealing the Mechanism of Esculin in Treating Renal Cell Carcinoma Based on Network Pharmacology and Experimental Validation. Biomolecules, 14(8), 1043. https://doi.org/10.3390/biom14081043






