Perforin 1 in Cancer: Mechanisms, Therapy, and Outlook
Abstract
:1. Introduction
2. Biological Functions of PRF1
3. PRF1 Deficiency Leads to Tumor Immune Escape and Tumor Growth and Invasion
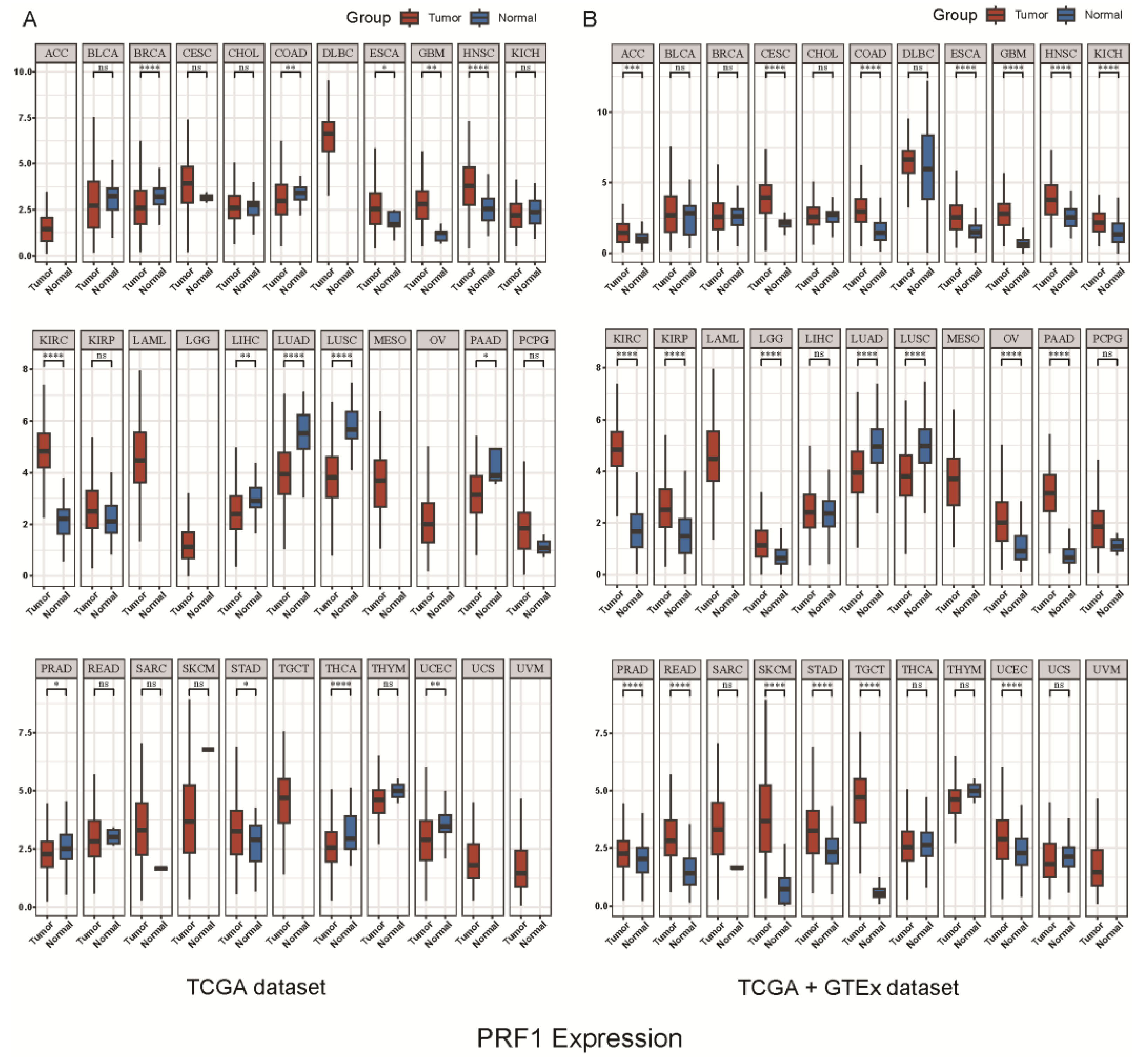
4. Role of PRF1 in Different Cancers
5. Cancer Treatment Modalities
5.1. Conventional Cancer Treatments
5.2. Emerging Technologies for Cancer Treatment
6. The Role of PRF1 in Cancer Therapy
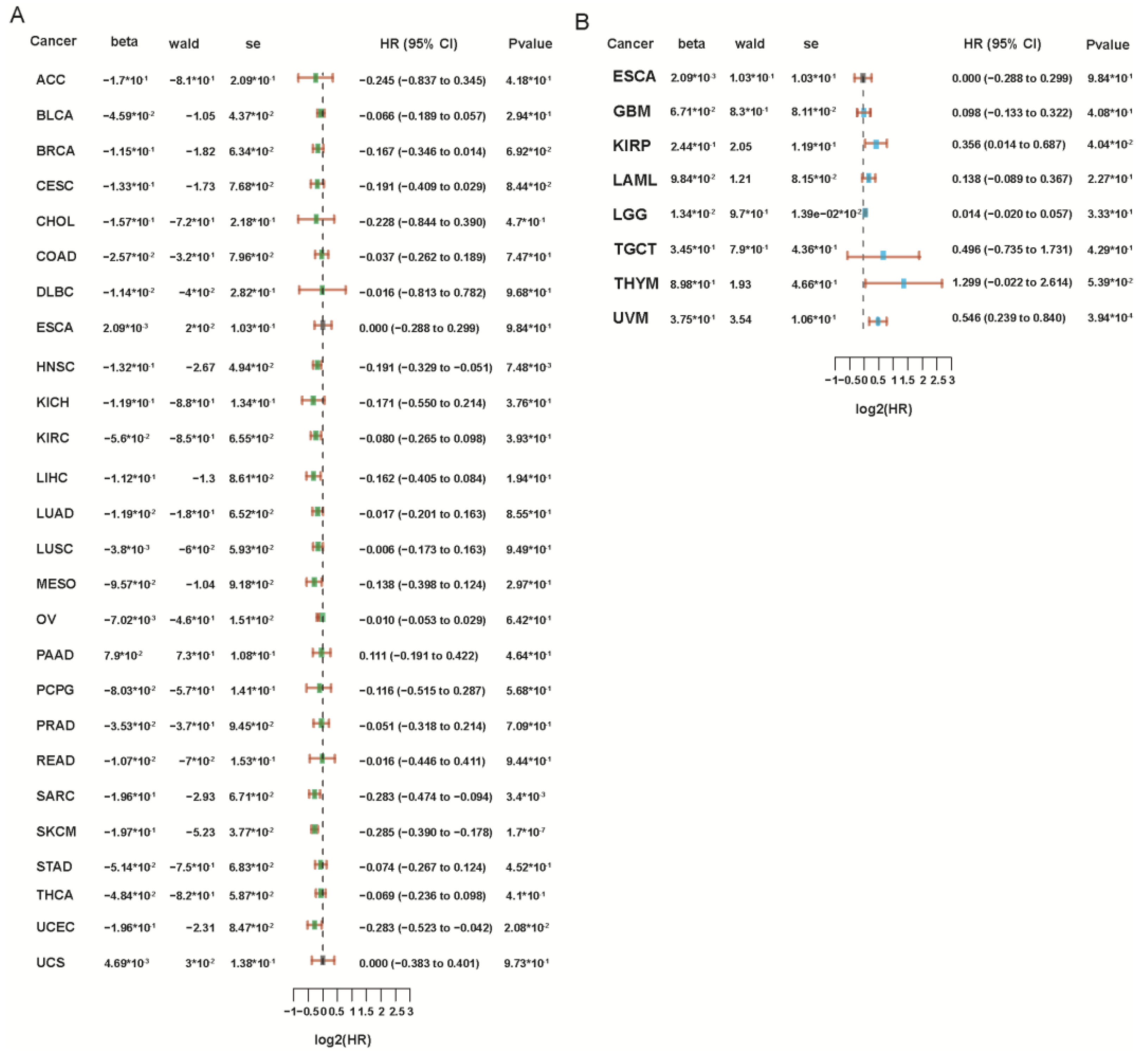
7. The Role of PRF1 in Cancer Prognosis
8. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| perforin 1 | PRF1 |
| natural killer | NK |
| cytotoxic T lymphocytes | CTLs |
| cytokine-induced killer | CIK |
| tumor-infiltrating lymphocytes | TILs |
| granzyme B | GZMB |
| Fas ligands | FASLs |
| CD40 ligands | CD40Ls |
| immune checkpoint inhibitors | ICIs |
| N6 methyladenosine | M6A |
| granzyme A | GZMA |
| immune-related genes | IRGs |
| the tumor microenvironment | TME |
| hemophagocytic lymphohistiocytosis | HLH |
| familial hemophagocytic lymphohistiocytosis type 2 | FHL2 |
| acute lymphoblastic leukemia | ALL |
| high-fat diet | HFD |
| blood–brain barrier | BBB |
| central nervous system | CNS |
| methylation-sensitive region | MSR |
| myeloid-derived suppressor cells | MDSCs |
| complementary and alternative medicine | CAM |
| chimeric antigen receptor T cells | CAR-T |
| immunogenic cell death | ICD |
| oncolytic viruses | OVs |
| interleukin-2 | IL-2 |
| tumor-draining lymph nodes | TDLNs |
| colorectal cancer | CRC |
| renal papillary cell carcinoma | KIRP |
| low-grade glioma of the brain | LGG |
| ovaral melanoma | UVM |
| adrenocortical carcinoma | ACC |
| breast invasive carcinoma | BRCA |
| head and neck carcinoma | HNSC |
| sarcoma | SARC |
| skin melanoma | SKCM |
| endometrioid carcinoma | UCEC |
References
- Roy, P.S.; Saikia, B.J. Cancer and Cure: A Critical Analysis. Indian J. Cancer 2016, 53, 441. [Google Scholar] [CrossRef]
- Dychangco, M.A. Reduce Global Cancer Burden via Cancer Prevention and Early Detection of Cancer. Asia Pac. J. Oncol. Nurs. 2022, 9, 100059. [Google Scholar] [CrossRef]
- Law, R.H.P.; Lukoyanova, N.; Voskoboinik, I.; Caradoc-Davies, T.T.; Baran, K.; Dunstone, M.A.; D’Angelo, M.E.; Orlova, E.V.; Coulibaly, F.; Verschoor, S.; et al. The Structural Basis for Membrane Binding and Pore Formation by Lymphocyte Perforin. Nature 2010, 468, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Voskoboinik, I.; Dunstone, M.A.; Baran, K.; Whisstock, J.C.; Trapani, J.A. Perforin: Structure, Function, and Role in Human Immunopathology. Immunol. Rev. 2010, 235, 35–54. [Google Scholar] [CrossRef]
- Metkar, S.S.; Marchioretto, M.; Antonini, V.; Lunelli, L.; Wang, B.; Gilbert, R.J.; Anderluh, G.; Roth, R.; Pooga, M.; Pardo, J.; et al. Perforin Oligomers Form Arcs in Cellular Membranes: A Locus for Intracellular Delivery of Granzymes. Cell Death Differ. 2015, 22, 74–85. [Google Scholar] [CrossRef]
- Sidore, C.; Orrù, V.; Cocco, E.; Steri, M.; Inshaw, J.R.; Pitzalis, M.; Mulas, A.; McGurnaghan, S.; Frau, J.; Porcu, E.; et al. PRF1 Mutation Alters Immune System Activation, Inflammation, and Risk of Autoimmunity. Mult. Scler. 2021, 27, 1332–1340. [Google Scholar] [CrossRef] [PubMed]
- Qaiyum, Z.; Gracey, E.; Yao, Y.; Inman, R.D. Integrin and Transcriptomic Profiles Identify a Distinctive Synovial CD8+ T Cell Subpopulation in Spondyloarthritis. Ann. Rheum. Dis. 2019, 78, 1566–1575. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, M.S.; Gilmour, K.C.; House, I.G.; Layton, M.; Panoskaltsis, N.; Sohal, M.; Trapani, J.A.; Voskoboinik, I. Missense Mutations in the Perforin (PRF1) Gene as a Cause of Hereditary Cancer Predisposition. OncoImmunology 2016, 5, e1179415. [Google Scholar] [CrossRef]
- Willenbring, R.C.; Johnson, A.J. Finding a Balance between Protection and Pathology: The Dual Role of Perforin in Human Disease. Int. J. Mol. Sci. 2017, 18, 1608. [Google Scholar] [CrossRef]
- Brennan, A.J.; House, I.G.; Oliaro, J.; Ramsbottom, K.M.; Hagn, M.; Yagita, H.; Trapani, J.A.; Voskoboinik, I. A Method for Detecting Intracellular Perforin in Mouse Lymphocytes. J. Immunol. 2014, 193, 5744–5750. [Google Scholar] [CrossRef]
- Hines, M.R.; Knight, T.E.; McNerney, K.O.; Leick, M.B.; Jain, T.; Ahmed, S.; Frigault, M.J.; Hill, J.A.; Jain, M.D.; Johnson, W.T.; et al. Immune Effector Cell-Associated Hemophagocytic Lymphohistiocytosis-Like Syndrome. Transplant. Cell. Ther. 2023, 29, 438.e1–438.e16. [Google Scholar] [CrossRef] [PubMed]
- Alfaraidi, A.T.; Alqarni, A.A.; Aqeel, M.T.; Albalawi, T.A.; Hejazi, A.S. Familial Hemophagocytic Lymphohistiocytosis Secondary to PRF1 Mutation. Case Rep. Hematol. 2021, 2021, e7213939. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Kim, M.; Yun, S.; Doh, J.; Greenberg, P.D.; Kim, T.-D.; Choi, I. MicroRNA-150 Regulates the Cytotoxicity of Natural Killers by Targeting Perforin-1. J. Allergy Clin. Immunol. 2014, 134, 195–203.e4. [Google Scholar] [CrossRef] [PubMed]
- Makaryan, S.Z.; Finley, S.D. An Optimal Control Approach for Enhancing Natural Killer Cells’ Secretion of Cytolytic Molecules. APL Bioeng. 2020, 4, 046107. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Yang, D.; Klement, J.D.; Oh, I.K.; Savage, N.M.; Waller, J.L.; Colby, A.H.; Grinstaff, M.W.; Oberlies, N.H.; Pearce, C.J.; et al. SUV39H1 Represses the Expression of Cytotoxic T-Lymphocyte Effector Genes to Promote Colon Tumor Immune Evasion. Cancer Immunol. Res. 2019, 7, 414–427. [Google Scholar] [CrossRef] [PubMed]
- Taube, J.M.; Young, G.D.; McMiller, T.L.; Chen, S.; Salas, J.T.; Pritchard, T.S.; Xu, H.; Meeker, A.K.; Fan, J.; Cheadle, C.; et al. Differential Expression of Immune-Regulatory Genes Associated with PD-L1 Display in Melanoma: Implications for PD-1 Pathway Blockade. Clin. Cancer Res. 2015, 21, 3969–3976. [Google Scholar] [CrossRef]
- Hartana, C.A.; Bergman, E.A.; Zirakzadeh, A.A.; Krantz, D.; Winerdal, M.E.; Winerdal, M.; Johansson, M.; Alamdari, F.; Jakubczyk, T.; Glise, H.; et al. Urothelial Bladder Cancer May Suppress Perforin Expression in CD8+ T Cells by an ICAM-1/TGFβ2 Mediated Pathway. PLoS ONE 2018, 13, e0200079. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, S.; Cao, M.; Fan, X.; Yang, T.; Huang, Y.; Song, X.; Li, Y.; Ye, L.; Shen, N.; et al. Antigen-Specific CD8+ T Cell Feedback Activates NLRP3 Inflammasome in Antigen-Presenting Cells through Perforin. Nat. Commun. 2017, 8, 15402. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Togi, M.; Koya, T.; Taniguchi, M.; Sakamoto, T.; Iwabuchi, K.; Kato, T., Jr.; Shimodaira, S. Identification of CD56dim Subpopulation Marked with High Expression of GZMB/PRF1/PI-9 in CD56+ Interferon-α-Induced Dendritic Cells. Genes Cells 2021, 26, 313–327. [Google Scholar] [CrossRef]
- Meng, M.; Li, L.; Li, R.; Wang, W.; Chen, Y.; Xie, Y.; Han, R.; Zhu, K.; Huang, W.; Yang, L.; et al. A Dynamic Transcriptomic Atlas of Cytokine-Induced Killer Cells. J. Biol. Chem. 2018, 293, 19600–19612. [Google Scholar] [CrossRef]
- Zhu, J.; Xiao, J.; Wang, M.; Hu, D. Pan-Cancer Molecular Characterization of m6A Regulators and Immunogenomic Perspective on the Tumor Microenvironment. Front. Oncol. 2020, 10, 618374. [Google Scholar] [CrossRef]
- Lu, H.; Wang, H.; Yan, L.; Shao, H.; Zhang, W.; Shen, H.; Bo, H.; Tao, C.; Xia, S.; Wu, F. Overexpression of Early T Cell Differentiation-Specific Transcription Factors Transforms the Terminally Differentiated Effector T Cells into Less Differentiated State. Cell. Immunol. 2020, 353, 104118. [Google Scholar] [CrossRef] [PubMed]
- Al-Samkari, H.; Snyder, G.D.; Nikiforow, S.; Tolaney, S.M.; Freedman, R.A.; Losman, J.-A. Haemophagocytic Lymphohistiocytosis Complicating Pembrolizumab Treatment for Metastatic Breast Cancer in a Patient with the PRF1A91V Gene Polymorphism. J. Med. Genet. 2019, 56, 39–42. [Google Scholar] [CrossRef]
- Tesi, B.; Chiang, S.C.C.; El-Ghoneimy, D.; Hussein, A.A.; Langenskiöld, C.; Wali, R.; Fadoo, Z.; Silva, J.P.; Lecumberri, R.; Unal, S.; et al. Spectrum of Atypical Clinical Presentations in Patients with Biallelic PRF1 Missense Mutations. Pediatr. Blood Cancer 2015, 62, 2094–2100. [Google Scholar] [CrossRef]
- Rudd, E.; Göransdotter Ericson, K.; Zheng, C.; Uysal, Z.; Ozkan, A.; Gürgey, A.; Fadeel, B.; Nordenskjöld, M.; Henter, J.-I. Spectrum and Clinical Implications of Syntaxin 11 Gene Mutations in Familial Haemophagocytic Lymphohistiocytosis: Association with Disease-Free Remissions and Haematopoietic Malignancies. J. Med. Genet. 2006, 43, e14. [Google Scholar] [CrossRef]
- Hu, L.-Y.; Wan, L.; Wang, Q.-H.; Shi, X.-Y.; Meng, Y.; Yang, X.-F.; Yang, G.; Zou, L.-P. Case Report: Chronic Inflammatory Demyelinating Polyradiculoneuropathy Rather than Hemophagocytic Lymphohistiocytosis-the Initial Phenotype of PRF1 Gene Mutation. Front. Immunol. 2023, 14, 1306338. [Google Scholar] [CrossRef] [PubMed]
- House, I.G.; Thia, K.; Brennan, A.J.; Tothill, R.; Dobrovic, A.; Yeh, W.Z.; Saffery, R.; Chatterton, Z.; Trapani, J.A.; Voskoboinik, I. Heterozygosity for the Common Perforin Mutation, p.A91V, Impairs the Cytotoxicity of Primary Natural Killer Cells from Healthy Individuals. Immunol. Cell Biol. 2015, 93, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Cetica, V.; Sieni, E.; Pende, D.; Danesino, C.; Fusco, C.D.; Locatelli, F.; Micalizzi, C.; Putti, M.C.; Biondi, A.; Fagioli, F.; et al. Genetic Predisposition to Hemophagocytic Lymphohistiocytosis: Report on 500 Patients from the Italian Registry. J. Allergy Clin. Immunol. 2016, 137, 188–196.e4. [Google Scholar] [CrossRef]
- Jaworowska, A.; Pastorczak, A.; Trelinska, J.; Wypyszczak, K.; Borowiec, M.; Fendler, W.; Sedek, L.; Szczepanski, T.; Ploski, R.; Młynarski, W. Perforin Gene Variation Influences Survival in Childhood Acute Lymphoblastic Leukemia. Leuk. Res. 2018, 65, 29–33. [Google Scholar] [CrossRef]
- Yang, L.; Liu, H.; Zhao, J.; Da, W.; Zheng, J.; Wang, L.; Li, G.; Zhu, P. Mutations of Perforin Gene in Chinese Patients with Acute Lymphoblastic Leukemia. Leuk. Res. 2011, 35, 196–199. [Google Scholar] [CrossRef]
- Revelo, X.S.; Tsai, S.; Lei, H.; Luck, H.; Ghazarian, M.; Tsui, H.; Shi, S.Y.; Schroer, S.; Luk, C.T.; Lin, G.H.Y.; et al. Perforin Is a Novel Immune Regulator of Obesity-Related Insulin Resistance. Diabetes 2015, 64, 90–103. [Google Scholar] [CrossRef]
- Elgizouli, M.; Logan, C.; Nieters, A.; Brenner, H.; Rothenbacher, D. Cord Blood PRF1 Methylation Patterns and Risk of Lower Respiratory Tract Infections in Infants: Findings From the Ulm Birth Cohort. Medicine 2015, 94, e332. [Google Scholar] [CrossRef] [PubMed]
- Bacolod, M.D.; Barany, F.; Fisher, P.B. Can CpG Methylation Serve as Surrogate Markers for Immune Infiltration in Cancer? Adv. Cancer Res. 2019, 143, 351–384. [Google Scholar] [CrossRef] [PubMed]
- Kraya, A.A.; Maxwell, K.N.; Eiva, M.A.; Wubbenhorst, B.; Pluta, J.; Feldman, M.; Nayak, A.; Powell, D.J.; Domchek, S.M.; Vonderheide, R.H.; et al. PTEN Loss and BRCA1 Promoter Hypermethylation Negatively Predict for Immunogenicity in BRCA-Deficient Ovarian Cancer. JCO Precis. Oncol. 2022, 6, e2100159. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-F.; Tulahong, A.; Uddin, M.N.; Zhao, H.; Zhang, H. Meta-Analysis Identifying Epithelial-Derived Transcriptomes Predicts Poor Clinical Outcome and Immune Infiltrations in Ovarian Cancer. Math. Biosci. Eng. 2021, 18, 6527–6551. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz-Sanabria, A.; Baliu-Piqué, M.; Saiz-Ladera, C.; Rojas, K.; Manzano, A.; Marquina, G.; Casado, A.; Cimas, F.J.; Pérez-Segura, P.; Pandiella, A.; et al. Genomic Signatures of Immune Activation Predict Outcome in Advanced Stages of Ovarian Cancer and Basal-Like Breast Tumors. Front. Oncol. 2019, 9, 1486. [Google Scholar] [CrossRef] [PubMed]
- Widowati, W.; Jasaputra, D.K.; Sumitro, S.B.; Widodo, M.A.; Mozef, T.; Rizal, R.; Kusuma, H.S.W.; Laksmitawati, D.R.; Murti, H.; Bachtiar, I.; et al. Effect of Interleukins (IL-2, IL-15, IL-18) on Receptors Activation and Cytotoxic Activity of Natural Killer Cells in Breast Cancer Cell. Afr. Health Sci. 2020, 20, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Romero, J.M.; Grünwald, B.; Jang, G.-H.; Bavi, P.P.; Jhaveri, A.; Masoomian, M.; Fischer, S.E.; Zhang, A.; Denroche, R.E.; Lungu, I.M.; et al. A Four-Chemokine Signature Is Associated with a T-Cell–Inflamed Phenotype in Primary and Metastatic Pancreatic Cancer. Clin. Cancer Res. 2020, 26, 1997–2010. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhou, L.; Yang, H.-C.; Yu, C.-W. Chemokine CCL5 Immune Subtypes of Human Liver Cancer with Prognostic Significance. Int. Immunopharmacol. 2022, 113, 109372. [Google Scholar] [CrossRef]
- Tosolini, M.; Kirilovsky, A.; Mlecnik, B.; Fredriksen, T.; Mauger, S.; Bindea, G.; Berger, A.; Bruneval, P.; Fridman, W.-H.; Pagès, F.; et al. Clinical Impact of Different Classes of Infiltrating T Cytotoxic and Helper Cells (Th1, Th2, Treg, Th17) in Patients with Colorectal Cancer. Cancer Res. 2011, 71, 1263–1271. [Google Scholar] [CrossRef]
- Qu, Z. Investigating Conventional and Novel Methods for Treatment of Cancer. In Proceedings of the 2023 13th International Conference on Bioscience, Biochemistry and Bioinformatics, Association for Computing Machinery, New York, NY, USA, 9 August 2023; pp. 103–111. [Google Scholar]
- Cutress, R.; Gathani, T. Cancer Diagnosis. Annals 2023, 105, 291–292. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.; Sharma, P. Cancer Stem Cells: Future Possibilities for Cancer Therapy. Indian. J. Clin. Biochem. 2023, 38, 149–150. [Google Scholar] [CrossRef] [PubMed]
- Michalczyk, K.; Pawlik, J.; Czekawy, I.; Kozłowski, M.; Cymbaluk-Płoska, A. Complementary Methods in Cancer Treatment-Cure or Curse? Int. J. Environ. Res. Public Health 2021, 18, 356. [Google Scholar] [CrossRef] [PubMed]
- Bidram, E.; Esmaeili, Y.; Ranji-Burachaloo, H.; Al-Zaubai, N.; Zarrabi, A.; Stewart, A.; Dunstan, D.E. A Concise Review on Cancer Treatment Methods and Delivery Systems. J. Drug Deliv. Sci. Technol. 2019, 54, 101350. [Google Scholar] [CrossRef]
- Chen, M.; Hu, S.; Li, Y.; Jiang, T.T.; Jin, H.; Feng, L. Targeting Nuclear Acid-Mediated Immunity in Cancer Immune Checkpoint Inhibitor Therapies. Signal Transduct. Target. Ther. 2020, 5, 270. [Google Scholar] [CrossRef] [PubMed]
- Eisenstein, M. Four Ways Research Aims to Outwit Cancer’s Evasion Tactics. Nature 2023, 621, S8–S11. [Google Scholar] [CrossRef] [PubMed]
- Franciosa, G.; Kverneland, A.H.; Jensen, A.W.P.; Donia, M.; Olsen, J.V. Proteomics to Study Cancer Immunity and Improve Treatment. Semin. Immunopathol. 2023, 45, 241–251. [Google Scholar] [CrossRef]
- Guo, C.-X.; Huang, X.; Xu, J.; Zhang, X.-Z.; Shen, Y.-N.; Liang, T.-B.; Bai, X.-L. Combined Targeted Therapy and Immunotherapy for Cancer Treatment. World J. Clin. Cases 2021, 9, 7643–7652. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, L.H. Cancer Vaccines. BMJ 2015, 350, h988. [Google Scholar] [CrossRef]
- Gunturi, A.; McDermott, D.F. Nivolumab for the Treatment of Cancer. Expert. Opin. Investig. Drugs 2015, 24, 253–260. [Google Scholar] [CrossRef]
- Shah, N.J.; Kelly, W.J.; Liu, S.V.; Choquette, K.; Spira, A. Product Review on the Anti-PD-L1 Antibody Atezolizumab. Hum. Vaccin. Immunother. 2018, 14, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Graziani, G.; Tentori, L.; Navarra, P. Ipilimumab: A Novel Immunostimulatory Monoclonal Antibody for the Treatment of Cancer. Pharmacol. Res. 2012, 65, 9–22. [Google Scholar] [CrossRef]
- Gribben, J.G.; Hallek, M. Rediscovering Alemtuzumab: Current and Emerging Therapeutic Roles. Br. J. Haematol. 2009, 144, 818–831. [Google Scholar] [CrossRef] [PubMed]
- Husain, S.R.; Han, J.; Au, P.; Shannon, K.; Puri, R.K. Gene Therapy for Cancer: Regulatory Considerations for Approval. Cancer Gene Ther. 2015, 22, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Li, Z.; Fu, Y.; Liu, H.; Guo, H.; Guan, X.; Niu, M.; Zhang, C. Analyzing the Research Landscape: Mapping Frontiers and Hot Spots in Anti-Cancer Research Using Bibliometric Analysis and Research Network Pharmacology. Front. Pharmacol. 2023, 14, 1256188. [Google Scholar] [CrossRef]
- Quixabeira, D.C.A.; Zafar, S.; Santos, J.M.; Cervera-Carrascon, V.; Havunen, R.; Kudling, T.V.; Basnet, S.; Anttila, M.; Kanerva, A.; Hemminki, A. Oncolytic Adenovirus Coding for a Variant Interleukin 2 (vIL-2) Cytokine Re-Programs the Tumor Microenvironment and Confers Enhanced Tumor Control. Front. Immunol. 2021, 12, 674400. [Google Scholar] [CrossRef]
- Yang, K.; Zhao, W.; Lou, G.; Rong, Z.; Xu, H.; Wang, W.; Song, W.; Cai, Y.; Hou, Y.; Li, K. An Immunophenotyping of Ovarian Cancer With Clinical and Immunological Significance. Front. Immunol. 2018, 9, 757. [Google Scholar] [CrossRef] [PubMed]
- Roufas, C.; Chasiotis, D.; Makris, A.; Efstathiades, C.; Dimopoulos, C.; Zaravinos, A. The Expression and Prognostic Impact of Immune Cytolytic Activity-Related Markers in Human Malignancies: A Comprehensive Meta-Analysis. Front. Oncol. 2018, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.; Kawaguchi, T.; Yan, L.; Peng, X.; Qi, Q.; Takabe, K. Cytolytic Activity Score to Assess Anticancer Immunity in Colorectal Cancer. Ann. Surg. Oncol. 2018, 25, 2323–2331. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, F.; Yang, K.; Lu, Y.; Zhang, Y.; Wang, W.; Xie, H.; Deng, K.; Yang, C.; Rong, Z.; et al. An Immunophenotyping of Renal Clear Cell Carcinoma with Characteristics and a Potential Therapeutic Target for Patients Insensitive to Immune Checkpoint Blockade. J. Cell Biochem. 2019, 120, 13330–13341. [Google Scholar] [CrossRef]
- Mizutani, K.; Kawakami, K.; Fujita, Y.; Kato, T.; Takai, M.; Kato, D.; Iinuma, K.; Koie, T.; Ito, M. Gene Therapy of Prostate Cancer Using Liposomes Containing Perforin Expression Vector Driven by the Promoter of Prostate-Specific Antigen Gene. Sci. Rep. 2022, 12, 1442. [Google Scholar] [CrossRef] [PubMed]
- Chavez, M.; Silvestrini, M.T.; Ingham, E.S.; Fite, B.Z.; Mahakian, L.M.; Tam, S.M.; Ilovitsh, A.; Monjazeb, A.M.; Murphy, W.J.; Hubbard, N.E.; et al. Distinct Immune Signatures in Directly Treated and Distant Tumors Result from TLR Adjuvants and Focal Ablation. Theranostics 2018, 8, 3611–3628. [Google Scholar] [CrossRef] [PubMed]
- Althaus, J.; Nilius-Eliliwi, V.; Maghnouj, A.; Döring, S.; Schroers, R.; Hudecek, M.; Hahn, S.A.; Mika, T. Cytotoxicity of CD19-CAR-NK92 Cells Is Primarily Mediated via Perforin/Granzyme Pathway. Cancer Immunol. Immunother. 2023, 72, 2573–2583. [Google Scholar] [CrossRef] [PubMed]
- Agioti, S.; Zaravinos, A. Immune Cytolytic Activity and Strategies for Therapeutic Treatment. Int. J. Mol. Sci. 2024, 25, 3624. [Google Scholar] [CrossRef] [PubMed]
- E, J.; Yan, F.; Kang, Z.; Zhu, L.; Xing, J.; Yu, E. CD8+CXCR5+ T Cells in Tumor-Draining Lymph Nodes Are Highly Activated and Predict Better Prognosis in Colorectal Cancer. Hum. Immunol. 2018, 79, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Gui, Q.-F.; Lu, H.-F.; Zhang, C.-X.; Xu, Z.-R.; Yang, Y.-H. Well-Balanced Commensal Microbiota Contributes to Anti-Cancer Response in a Lung Cancer Mouse Model. Genet. Mol. Res. 2015, 14, 5642–5651. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Yuan, X.; Cao, J.; Zhao, X.; Wang, Y.; Liu, W.; Liu, B.; Zeng, Q. RNA-Seq and Immune Repertoire Analysis of Normal and Hepatocellular Carcinoma Relapse After Liver Transplantation. Int. J. Gen. Med. 2023, 16, 4329–4341. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Gao, J.; Lou, W.; Zhang, Y.; Deng, Y.; Wang, C.; Huang, H.; Xu, H.; Guo, S.; Lai, S.; et al. Prognostic Signature for Hepatocellular Carcinoma Based on 4 Pyroptosis-Related Genes. BMC Med. Genom. 2022, 15, 166. [Google Scholar] [CrossRef] [PubMed]
- Ju, M.; Jiang, L.; Wei, Q.; Yu, L.; Chen, L.; Wang, Y.; Hu, B.; Qian, P.; Zhang, M.; Zhou, C.; et al. A Immune-Related Signature Associated with TME Can Serve as a Potential Biomarker for Survival and Sorafenib Resistance in Liver Cancer. Onco Targets Ther. 2021, 14, 5065–5083. [Google Scholar] [CrossRef]
- Fan, C.; Hu, H.; Shen, Y.; Wang, Q.; Mao, Y.; Ye, B.; Xiang, M. PRF1 Is a Prognostic Marker and Correlated with Immune Infiltration in Head and Neck Squamous Cell Carcinoma. Transl. Oncol. 2021, 14, 101042. [Google Scholar] [CrossRef]
- Li, D.; Ma, D.; Hou, Y. Pyroptosis Patterns Influence the Clinical Outcome and Immune Microenvironment Characterization in HPV-Positive Head and Neck Squamous Cell Carcinoma. Infect. Agent. Cancer 2023, 18, 30. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Aoyagi, K.; Minashi, K.; Komatsuzaki, R.; Komatsu, M.; Chiwaki, F.; Tamaoki, M.; Nishimura, T.; Takahashi, N.; Oda, I.; et al. Discovery of a Good Responder Subtype of Esophageal Squamous Cell Carcinoma with Cytotoxic T-Lymphocyte Signatures Activated by Chemoradiotherapy. PLoS ONE 2015, 10, e0143804. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, X.; Guo, H.; Guo, Y.; Han, Q.; Li, Z.; Zhang, C. Perforin 1 in Cancer: Mechanisms, Therapy, and Outlook. Biomolecules 2024, 14, 910. https://doi.org/10.3390/biom14080910
Guan X, Guo H, Guo Y, Han Q, Li Z, Zhang C. Perforin 1 in Cancer: Mechanisms, Therapy, and Outlook. Biomolecules. 2024; 14(8):910. https://doi.org/10.3390/biom14080910
Chicago/Turabian StyleGuan, Xiaoya, Huina Guo, Yujia Guo, Qi Han, Zhongxun Li, and Chunming Zhang. 2024. "Perforin 1 in Cancer: Mechanisms, Therapy, and Outlook" Biomolecules 14, no. 8: 910. https://doi.org/10.3390/biom14080910





