CPT2 Deficiency Modeled in Zebrafish: Abnormal Neural Development, Electrical Activity, Behavior, and Schizophrenia-Related Gene Expression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Zebrafish Husbandry
2.2. Morpholino Injection of Zebrafish Embryos
2.3. Assessment of Morpholino-Injected Zebrafish
2.4. RNA Isolation and cDNA Synthesis
2.5. RT-PCR
2.6. Mitochondrial Isolation
2.7. Larval Protein Extraction
2.8. Western Blot
2.9. Liquid Chromatography in Tandem with Mass Spectrometry
2.10. Morphological Evaluation
2.11. Oil Red O Whole-Mount Stain
2.12. Alcian Blue Whole-Mount Stain and Cartilage Measurement
2.13. Whole Brain Morphology and Immunofluorescence Labeling
2.14. Viewpoint ZebraBox Behavioral Analysis
2.15. Electrophysiology Seizure Assay
2.16. RT-qPCR
2.17. Statistical Analysis
3. Results
3.1. Development of a CPT2 Deficiency Model System in Zebrafish without Toxicity
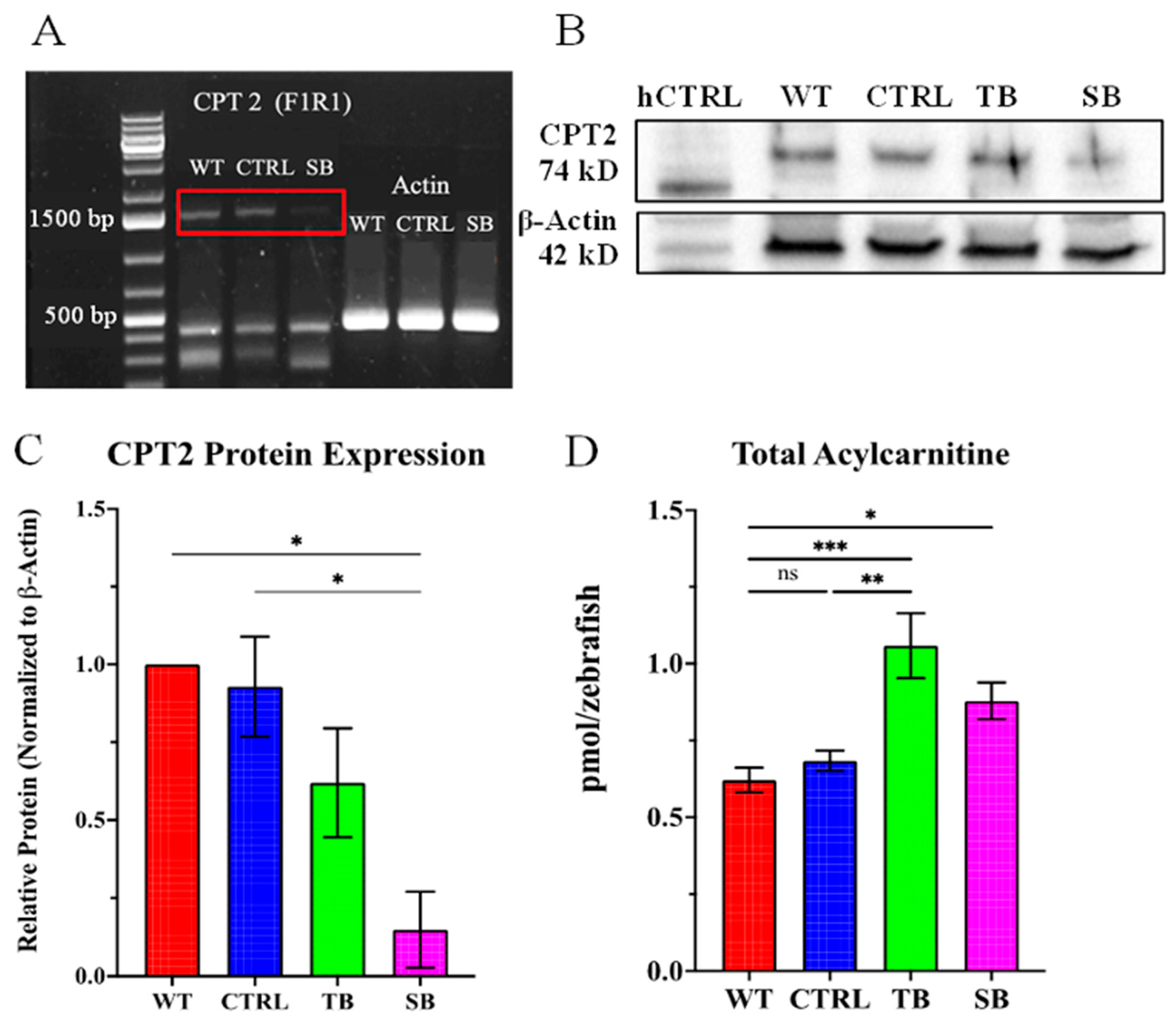
3.2. Evaluation of CPT2 Knockdown in Zebrafish Using Morpholinos
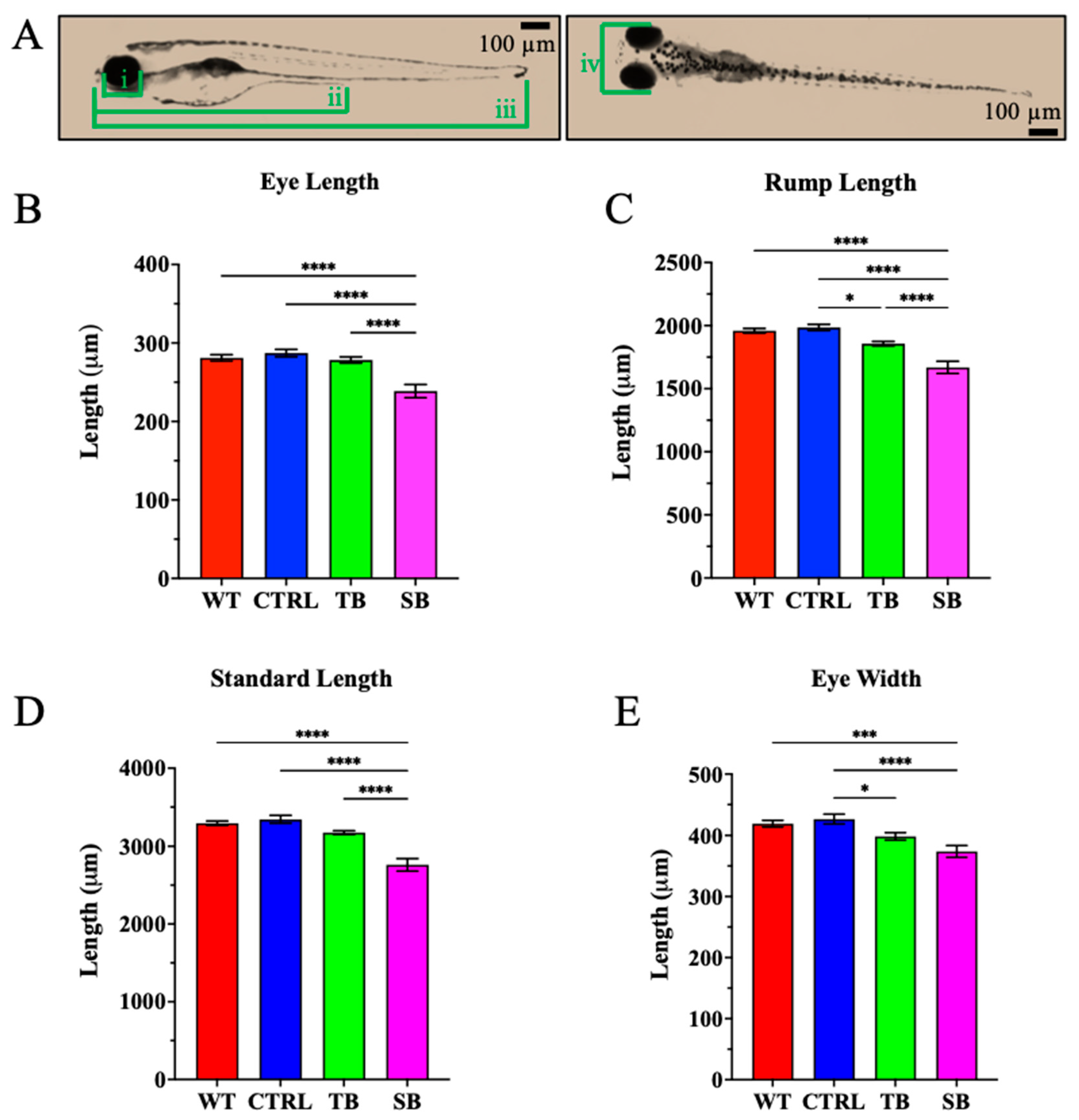
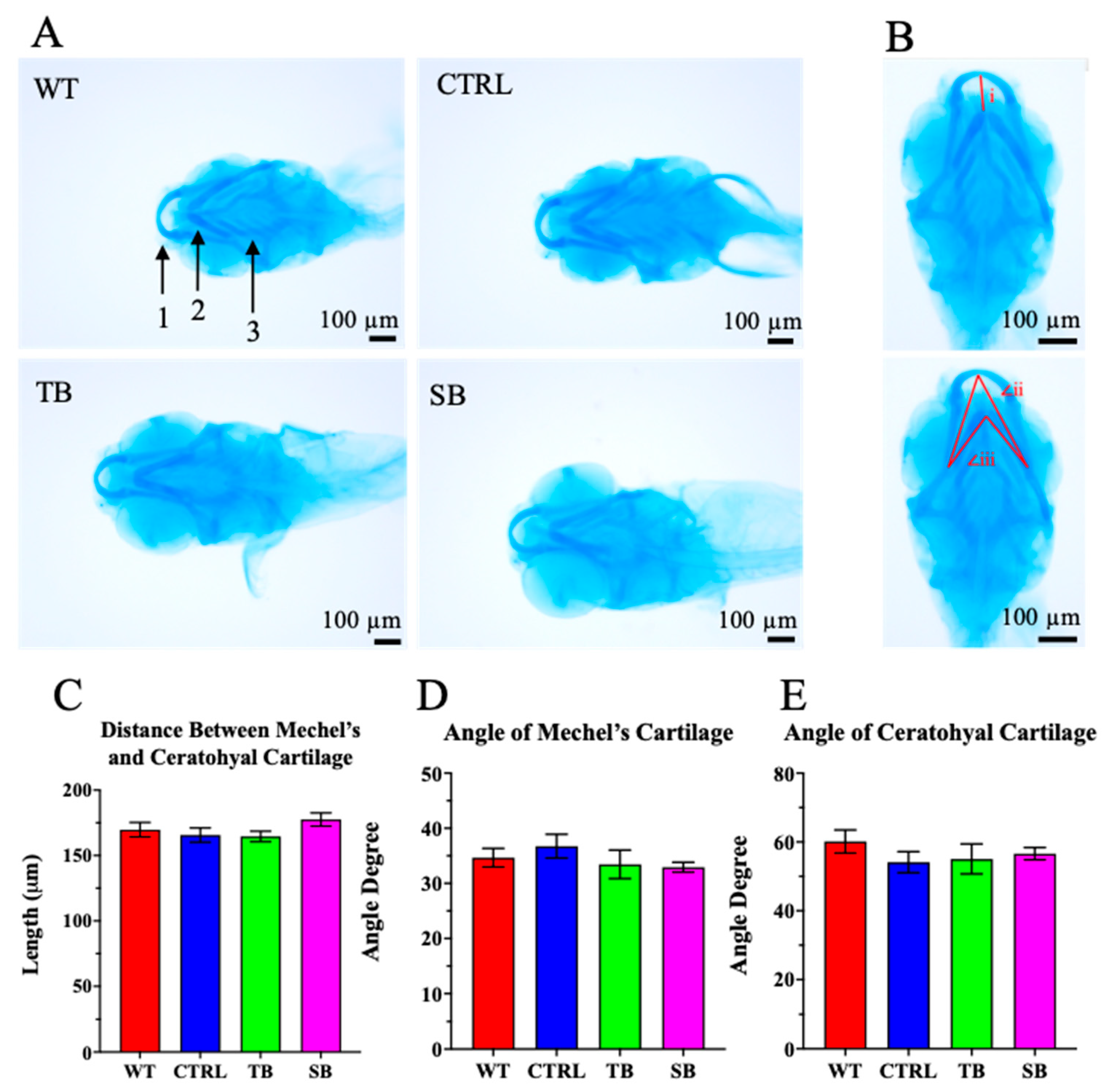
3.3. Effect of CPT2 Knockdown on Zebrafish Morphology
3.4. Effect of CPT2 Knockdown on the Lipid Deposition in Zebrafish
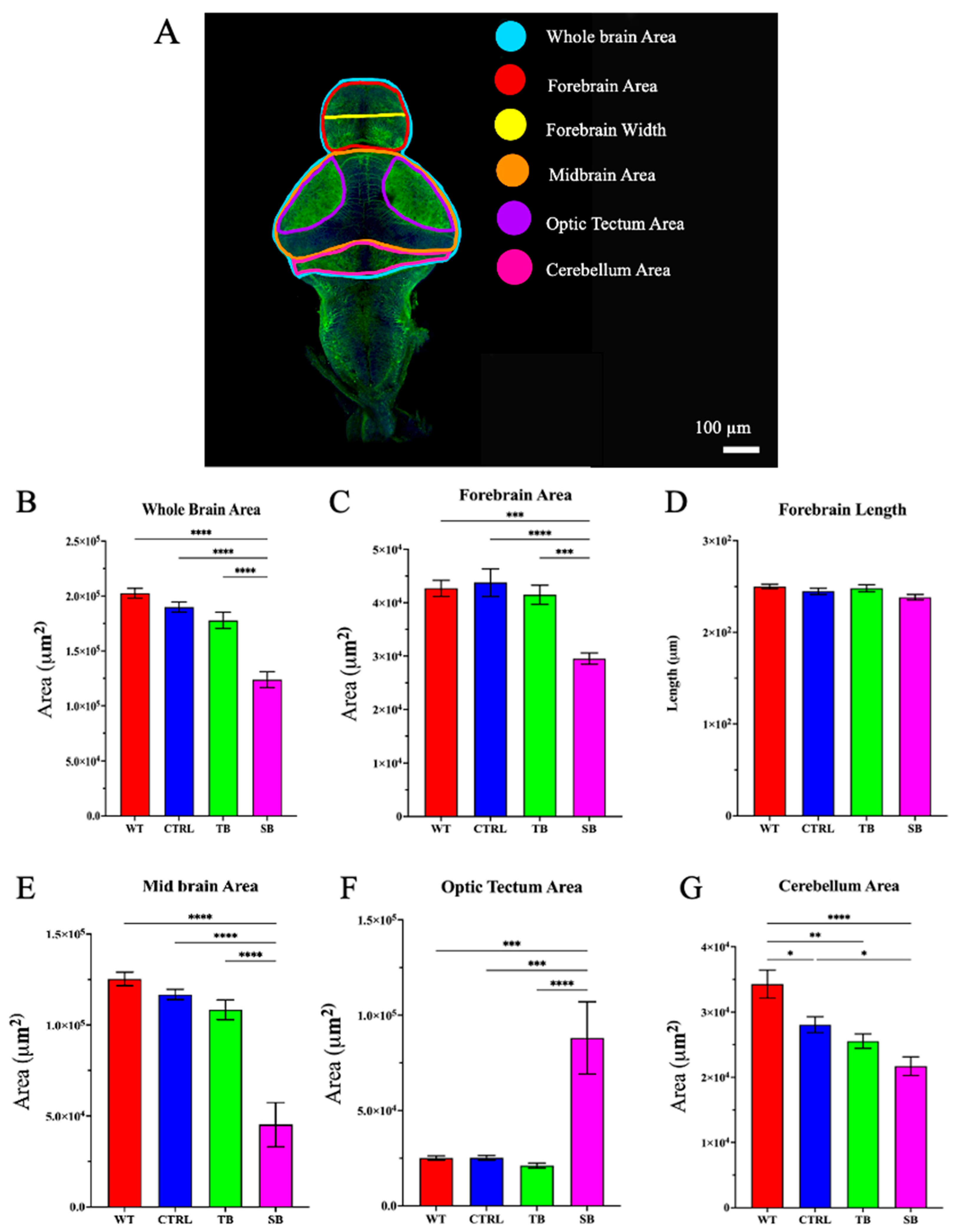
3.5. Effect of CPT2 Knockdown on the Developing Nervous System in Zebrafish
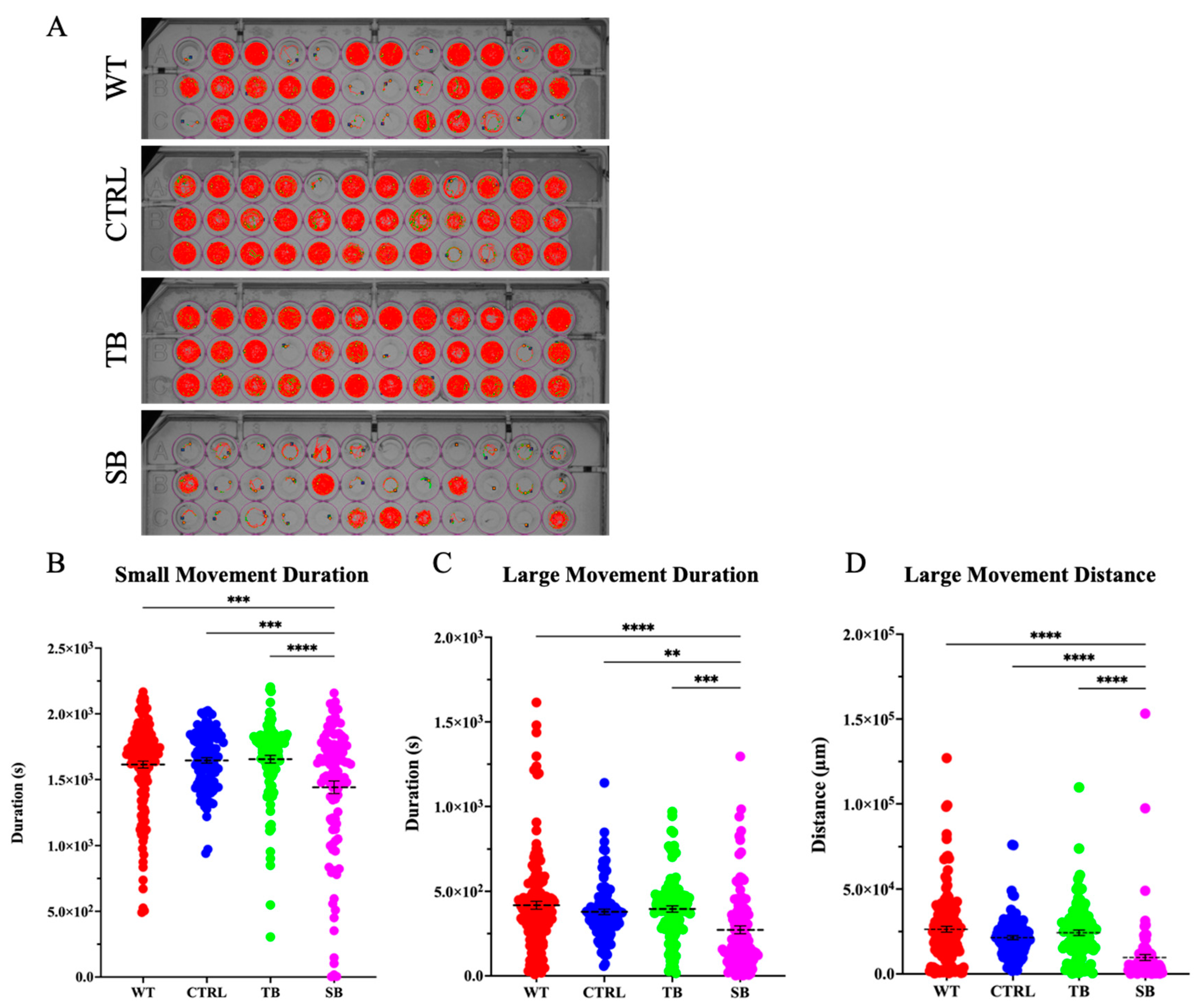
3.6. Effect of CPT2 Knockdown on Behavior and Brain Function
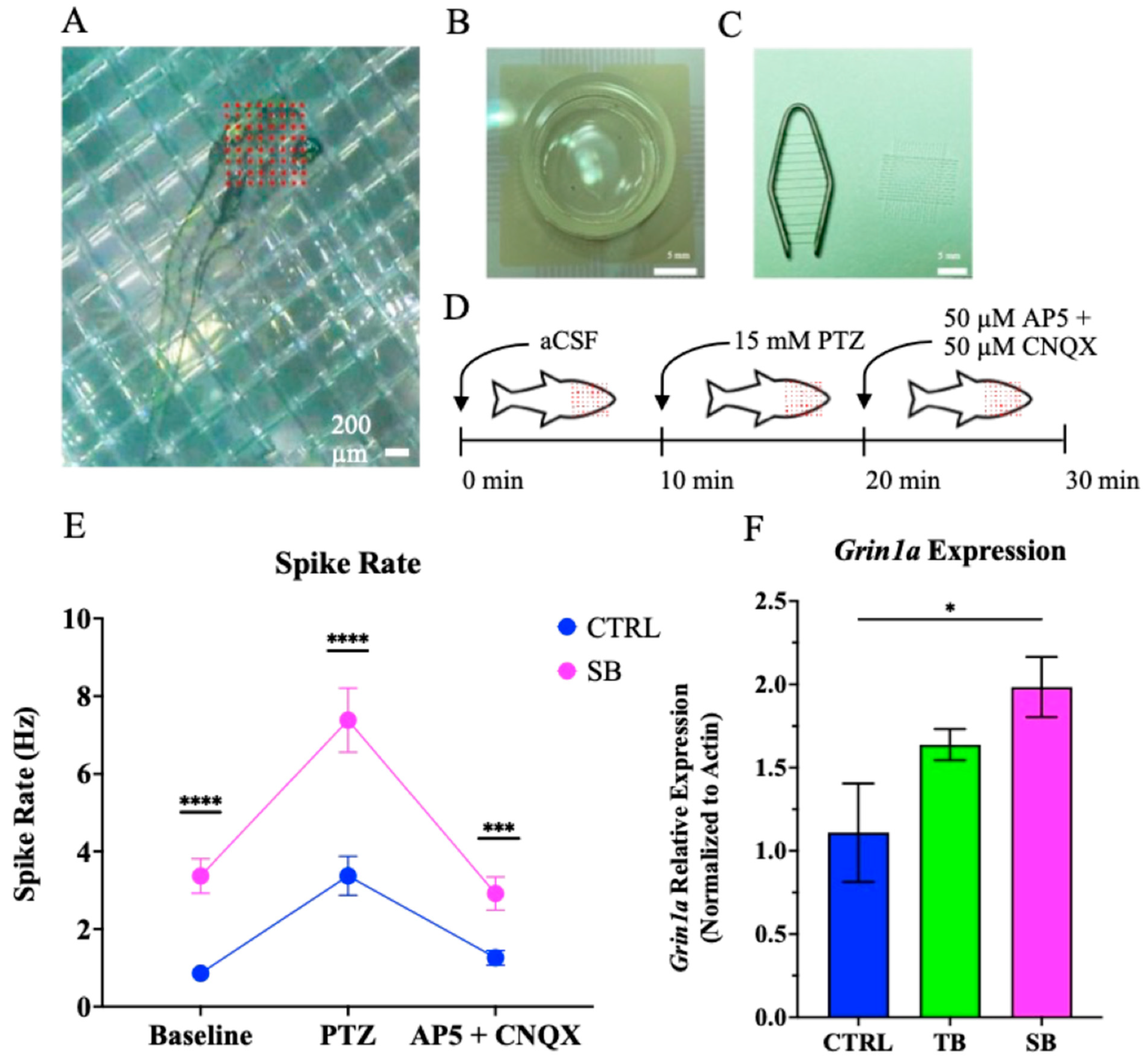
3.7. Effect of CPT2 Knockdown on Neural Network Activity
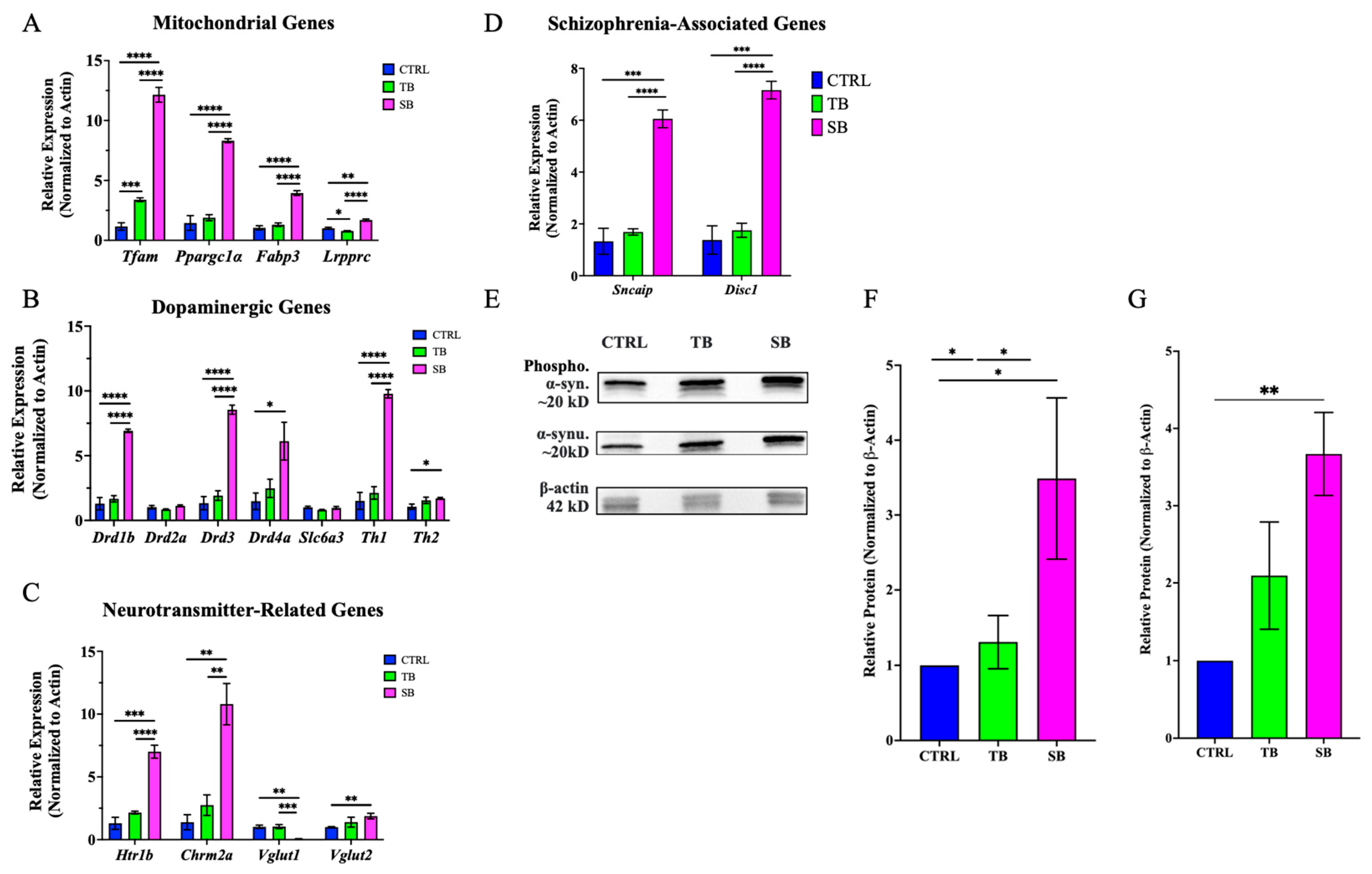
3.8. Effect of CPT2 Knockdown on Mitochondrial and Neuronal Gene Expression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Reagent | Product Information | Product Number |
|---|---|---|
| 2X Laemmli Sample Buffer | Bio-Rad (Bio-Rad Laboratories, Hercules, CA, USA) | 1610737 |
| 4X Laemmli SDS Sample Buffer | ThermoFisher (Thermo Fisher Scientific, Waltham, MA, USA) | J60015 |
| Alcian Blue 8GX | ThermoFisher (Thermo Fisher Scientific, MA, USA) | J60122-14 |
| Anatech Ltd. Prefer Fixative | ThermoFisher (Thermo Fisher Scientific, MA, USA) | NC9053360 |
| Anti-Acetylated Tubulin Monoclonal Antibody | Millipore Sigma (Merck KGaA, Darmstadt, Germany) | T7451 |
| Anti-Tyrosine Hydroxylase Polyclonal Antibody | Millipore Sigma (Merck KGaA, Darmstadt, Germany) | AB152 |
| AP-5 | Sigma-Aldrich (Merck KGaA, Darmstadt, Germany) | 165304 |
| Bovine Serum Albumin | ThermoFisher (Thermo Fisher Scientific, MA, USA) | BP1600-100 |
| Calcium Chloride | Sigma Aldrich (Merck KGaA, Darmstadt, Germany) | C5670 |
| Chloroform | ThermoFisher (Thermo Fisher Scientific, MA, USA) | J67241.AP |
| CNQX Disodium Salt Hydrate | Sigma-Aldrich (Merck KGaA, Darmstadt, Germany) | C239 |
| CPT2 (3B10) Monoclonal Antibody | Bioss (Bioss Inc., Woburn, MA, USA) | Bsm-52621R |
| CPT2 293 Cell Transient Overexpression Lysate | Abnova (Abnova Manufacturer, Taipei, Taiwan) | H00001376-T01 |
| DAPI | ThermoFisher (Thermo Fisher Scientific, MA, USA) | D1306 |
| DISC1 Polyclonal Antibody | Invitrogen (Thermo Fisher Scientific, MA, USA) | 40-6800 |
| Dithiothreitol (DTT) | ThermoFisher (Thermo Fisher Scientific, MA, USA) | D9750 |
| DRD1 Polyclonal Antibody | Invitrogen (Thermo Fisher Scientific, MA, USA) | 720276 |
| EDTA | Sigma Aldrich (Merck KGaA, Darmstadt, Germany) | E6758 |
| EveryBlot Blocking Buffer | Bio-Rad (Bio-Rad Laboratories, CA, USA) | 12010020 |
| GAPDH (14C10) Rabbit Monoclonal Antibody | Cell Signaling (Cell Signaling Technology, Beverly, MA, USA) | 2118S |
| Glucose | Sigma Aldrich (Merck KGaA, Darmstadt, Germany) | G8270 |
| Glycerol | ThermoFisher (Thermo Fisher Scientific, MA, USA) | G33-500 |
| Goat Anti-Mouse IgG (H + L) Cross-Adsorbed Secondary Antibody, Alexa Fluor™ 488 | Invitrogen (Thermo Fisher Scientific, MA, USA) | A-11001 |
| Goat Anti-Rabbit IgG (H + L) Superclonal™ | Invitrogen (Thermo Fisher Scientific, MA, USA) | 2343633 |
| Goat Anti-Rabbit IgG H&L (Alexa Fluor® 488) Polyclonal Antibody | Abcam (Celgene Corporation, Cambridge, UK) | ab150077 |
| Halt™ Protease and Phosphatase Inhibitor Cocktail (100X) | ThermoFisher (Thermo Fisher Scientific, MA, USA) | 1861284 |
| HEPES | Sigma Aldrich (Merck KGaA, Darmstadt, Germany) | H4034 |
| Hydrogen Peroxide Solution | Millipore Sigma (Merck KGaA, Darmstadt, Germany) | H1009 |
| iScript Reverse Transcription Supermix for RT-qPCR | Bio-Rad (Bio-Rad Laboratories, CA, USA) | 1708841 |
| Isopropanol | ThermoFisher (Thermo Fisher Scientific, MA, USA) | T036181000 |
| Magnesium Sulfate | Sigma Aldrich (Merck KGaA, Darmstadt, Germany) | M2643 |
| Mannitol | Sigma Aldrich (Merck KGaA, Darmstadt, Germany) | M4125 |
| Negative Control Morpholino | Gene Tools (Gene Tools LLC, Philomath, OR, USA) | PCO-Standard Control-100-F |
| Oil Red O Dye | Sigma-Aldrich (Merck KGaA, Darmstadt, Germany) | O0625-25G |
| Paraformaldehyde | ThermoFisher (Thermo Fisher Scientific, MA, USA) | 416785000 |
| Pentylenetetrazol (PTZ) | Sigma-Aldrich (Merck KGaA, Darmstadt, Germany) | P6500 |
| Phosphate-Buffered Saline | ThermoFisher (Thermo Fisher Scientific, MA, USA) | BP399-1 |
| Phospho-α Synuclein (Ser129) Polyclonal Antibody | Invitrogen (Thermo Fisher Scientific, MA, USA) | PA5-37740 |
| Pierce™ BCA Protein Assay Kit | ThermoFisher (Thermo Fisher Scientific, MA, USA) | 23225 |
| Pierce® RIPA Buffer | ThermoFisher (Thermo Fisher Scientific, MA, USA) | 89901 |
| Potassium Chloride | Sigma Aldrich (Merck KGaA, Darmstadt, Germany) | P9333 |
| Potassium Hydroxide | Millipore Sigma (Merck KGaA, Darmstadt, Germany) | 221473 |
| Precision Plus Protein™ WesternC™ Blotting Standards | Bio-Rad (Bio-Rad Laboratories, CA, USA) | 161-0376 |
| Precision Protein StrepTactin HRP Conjugate | Bio-Rad (Bio-Rad Laboratories, CA, USA) | 1610381 |
| Restore™ Western Blot Stripping Buffer | ThermoFisher (Thermo Fisher Scientific, MA, USA) | 21059 |
| Sodium Bicarbonate | Sigma Aldrich (Merck KGaA, Darmstadt, Germany) | S5761 |
| Sodium Chloride | ThermoFisher (Thermo Fisher Scientific, MA, USA) | S642-212 |
| Sodium Phosphate Monobasic | ThermoFisher (Thermo Fisher Scientific, MA, USA) | BP329-1 |
| Splice Blocking Morpholino | Gene Tools (Gene Tools LLC, OR, USA) | Custom Designed |
| SsoAdvanced® Universal SYBR Green Supermix | Bio-Rad (Bio-Rad Laboratories, CA, USA) | 1725271 |
| Sucrose | ThermoFisher (Thermo Fisher Scientific, MA, USA) | S5-500 |
| SuperSignal™ West Pico PLUS Chemiluminescent | ThermoFisher (Thermo Fisher Scientific, MA, USA) | 34577 |
| SYBR Safe DNA Gel Stain | ThermoFisher (Thermo Fisher Scientific, MA, USA) | S33102 |
| TrackIt™ 1Kb Plus DNA Ladder | Invitrogen (Thermo Fisher Scientific, MA, USA) | 10488085 |
| Translation Blocking Morpholino | Gene Tools (Gene Tools LLC, OR, USA) | Custom Designed |
| TRI Reagent™ Solution | Invitrogen (Thermo Fisher Scientific, MA, USA) | AM9738 |
| Tris Base | ThermoFisher (Thermo Fisher Scientific, MA, USA) | BP152-500 |
| Triton X-100 | Cayman Chemical (Cayman Chemical Company, Ann Arbor, MI, USA) | 600217 |
| Tween 20 | ThermoFisher (Thermo Fisher Scientific, MA, USA) | BP337-500 |
| UltraPure Agarose 1000 | Invitrogen (Thermo Fisher Scientific, MA, USA) | 16550-100 |
| α-Synuclein Polyclonal Antibody | Invitrogen (Thermo Fisher Scientific, MA, USA) | PA5-13401 |
| β-Actin Polyclonal Antibody | Invitrogen (Thermo Fisher Scientific, MA, USA) | PA5-98583 |
| Morpholino Type | Sequence |
|---|---|
| Negative Control Morpholino | 5′ CCTCTTACCTCAGTTACAATTTATA 3′ |
| Splice Blocking Morpholino | 5′ TATTAAAGTGCTGTCCTCACCCATG 3′ |
| Translation Blocking Morpholino | 5′ AAAGCAAACCTGCCATCATGCAGCT 3′ |
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Actin | 5′-TCACACCTTCTACAACGAGCTGCG-3′ | 5′-GAAGCTGTAGCCTCTCTCGGTCAG-3′ |
| Chrm2a | 5′-ACGCCTTACAACGTGATGGT-3′ | 5′-TCTTGTACTGGCAGAGCAGC-3′ |
| Cpt2 | 5′-GCTGGCAAGCAGTTACATGA-3′ | 5′-ACTCCAAAACCATCAGGCAC-3′ |
| Disc1 | 5′-CGGTCTGAGCCGATTAACCA-3′ | 5′-CGAAACCCTCTGTCAGCCAT-3′ |
| Drd1b | 5′-ATCTCGGCGCTAGAAAGAGC-3′ | 5′- CACCCATGATGACCGAGAGG-3′ |
| Drd2 | 5′-TGTGATTGCGAATCCTGCCT-3′ | 5′-CATCATCGGGATGGGTGCAT-3′ |
| Drd3 | 5′-ATCTGTCCACCCTCTCCAGAT-3′ | 5′-CCCAGGGGTGAATGAATGCT-3′ |
| Drd4a | 5′-AGATTGTCCTGCTCTCCGCCA-3′ | 5′-CAGCTCCTCCAAGGTAATGTC-3′ |
| Fabp3 | 5′-ACATCGTCTCCACACGACAC-3′ | 5′-GAAACGGTGCAAAGGAGAGC-3′ |
| Grin1a | 5′-TGGCGGTGAAACAGAGAGAC-3′ | 5′-CAATTGGGCGTCCTGGGAT-3′ |
| Htrlb | 5′-TCACTTGGCTTGGCTACGTC-3′ | 5′-AGCATTCAACAGTGCCGTGA-3′ |
| Lrpprc | 5′-GTCTGCGGAGGACATAGTCG-3′ | 5′-GTGCAAACAGGGGCAAGAAG-3′ |
| Ppargcla | 5′-CCTGCTAACTCCCAGCTCAG-3′ | 5′-GATGCAAGAAGTGCGGTGAG-3′ |
| Slc6a3 | 5′-GCCTGGTTTTACGGAGTGGA-3′ | 5′-GGAGGATTGAAGGTGGCGAA-3′ |
| Sncaip | 5′-CATCTCCCAGCTGTCGTGTT-3′ | 5′-GTGTCTCTGGGATGGGTTCG-3′ |
| Tfam | 5′-AATCTTGGCAAGCCCAAACG-3′ | 5′-TTGCCTGCGTAGTAGTTCCC-3′ |
| Th | 5′-TTGATCGAGGATGCGCGTAA-3′ | 5′-TCAAACACGATCTGCTCGCT-3′ |
| Th2 | 5′-GCCAAACAGAAGTTGAGGCG-3′ | 5′-ATGCTGCAAGTGTAGGGGTC-3′ |
| Vglut1 | 5′-GAGGAGGTGTTCGGCTTTGA-3′ | 5′-GTCCTCAGGTAATCGGCCAG-3′ |
| Vglut2 | 5′-CATATGAGAGCCCGGCTGAA-3′ | 5′-GATTGCATAGACGGGCATGG-3′ |
References
- D’Amico, A.; Bertini, E. Metabolic neuropathies and myopathies. Handb. Clin. Neurol. 2013, 113, 1437–1455. [Google Scholar] [PubMed]
- Ferreira, C.R.; van Karnebeek, C.D.M. Inborn errors of metabolism. Handb. Clin. Neurol. 2019, 162, 449–481. [Google Scholar] [PubMed]
- Gaffar, S.; Aathirah, A.S. Fatty-Acid-Binding Proteins: From Lipid Transporters to Disease Biomarkers. Biomolecules 2023, 13, 1753. [Google Scholar] [CrossRef] [PubMed]
- Vaz, F.M.; Ferdinandusse, S.; Salomons, G.S.; Wanders, R.J.A. Disorders of fatty acid homeostasis. J. Inherit. Metab. Dis. 2024. [Google Scholar] [CrossRef] [PubMed]
- Vianey-Saban, C.; Guffon, N.; Fouilhoux, A.; Acquaviva, C. Fifty years of research on mitochondrial fatty acid oxidation disorders: The remaining challenges. J. Inherit. Metab. Dis. 2023, 46, 848–873. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.P.; Yang, H.; Wu, J.W.; Gauthier, N.; Fukao, T.; Mitchell, G.A. Metabolism as a tool for understanding human brain evolution: Lipid energy metabolism as an example. J. Hum. Evol. 2014, 77, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Schonfeld, P.; Reiser, G. Brain energy metabolism spurns fatty acids as fuel due to their inherent mitotoxicity and potential capacity to unleash neurodegeneration. Neurochem. Int. 2017, 109, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.L.; McDonald, D.A.; Borum, P.R. Acylcarnitines: Role in brain. Prog. Lipid Res. 2010, 49, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Adeva-Andany, M.M.; Carneiro-Freire, N.; Seco-Filgueira, M.; Fernandez-Fernandez, C.; Mourino-Bayolo, D. Mitochondrial β-oxidation of saturated fatty acids in humans. Mitochondrion 2019, 46, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Pan, D.; Liu, Q.; Chen, X.; Wang, S. L-Carnitine in the Treatment of Psychiatric and Neurological Manifestations: A Systematic Review. Nutrients 2024, 16, 1232. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.C.; McKenna, M.C. L-Carnitine and Acetyl-L-carnitine Roles and Neuroprotection in Developing Brain. Neurochem. Res. 2017, 42, 1661–1675. [Google Scholar] [CrossRef] [PubMed]
- Wieser, T. Carnitine Palmitoyltransferase II Deficiency. In GeneReviews® [Internet]; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2004. [Google Scholar] [PubMed]
- Joshi, P.R.; Deschauer, M.; Zierz, S. Clinically symptomatic heterozygous carnitine palmitoyltransferase II (CPT II) deficiency. Wien. Klin. Wochenschr. 2012, 124, 851–854. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Gozalbo, M.E.; Bakker, J.A.; Waterham, H.R.; Wanders, R.J.A. Carnitine-acylcarnitine translocase deficiency, clinical, biochemical and genetic aspects. Acta Paediatr. 2004, 25, 521–532. [Google Scholar]
- Wickramasekara, R.N.; Lookian, P.P.; Ngo, J.; Shibata, A.; Stessman, H.A.F. Schizophrenic Psychosis Symptoms in a Background of Mild-To-Moderate Carnitine Palmitoyltransferase II Deficiency: A Case Report. Reports 2020, 3, 31. [Google Scholar] [CrossRef]
- Virmani, A.; Pinto, L.; Bauermann, O.; Zerelli, S.; Diedenhofen, A.; Binienda, Z.K.; Ali, S.F.; van der Leij, F.R. The Carnitine Palmitoyl Transferase (CPT) System and Possible Relevance for Neuropsychiatric and Neurological Conditions. Mol. Neurobiol. 2015, 52, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Wang, D.; Pan, Z.; Brietzke, E.; McIntyre, R.S.; Musial, N.; Mansur, R.B.; Subramanieapillai, M.; Zeng, J.; Huang, N.; et al. Characterizing acyl-carnitine biosignatures for schizophrenia: A longitudinal pre- and post-treatment study. Transl. Psychiatry 2019, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Mednova, I.A.; Chernonosov, A.A.; Kornetova, E.G.; Semke, A.V.; Bokhan, N.A.; Koval, V.V.; Ivanova, S.A. Levels of Acylcarnitines and Branched-Chain Amino Acids in Antipsychotic-Treated Patients with Paranoid Schizophrenia with Metabolic Syndrome. Metabolites 2022, 12, 850. [Google Scholar] [CrossRef] [PubMed]
- Gut, P.; Reischauer, S.; Stainier, D.Y.R.; Arnaout, R. Little Fish, Big Data: Zebrafish as a Model for Cardiovascular and Metabolic Disease. Physiol. Rev. 2017, 97, 889–938. [Google Scholar] [CrossRef] [PubMed]
- Seth, A.; Stemple, D.L.; Barroso, I. The emerging use of zebrafish to model metabolic disease. Dis. Model. Mech. 2013, 6, 1080–1088. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Teame, T.; Zhang, Z.; Ran, C.; Zhang, H.; Yang, Y.; Ding, Q.; Xie, M.; Gao, C.; Ye, Y.; Duan, M.; et al. The use of zebrafish (Danio rerio) as biomedical models. Anim. Front. 2019, 9, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Adamson, K.I.; Sheridan, E.; Grierson, A.J. Use of zebrafish models to investigate rare human disease. J. Med Genet. 2018, 55, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Steele, S.L.; Prykhozhij, S.V.; Berman, J.N. Zebrafish as a model system for mitochondrial biology and diseases. Transl. Res. 2014, 163, 79–98. [Google Scholar] [CrossRef] [PubMed]
- Blum, M.; De Robertis, E.M.; Wallingford, J.B.; Niehrs, C. Morpholinos: Antisense and Sensibility. Dev. Cell 2015, 35, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Rosen, J.N.; Sweeney, M.F.; Mably, J.D. Microinjection of zebrafish embryos to analyze gene function. J. Vis. Exp. 2009, 25, e1115. [Google Scholar] [CrossRef]
- Prudent, J.; Popgeorgiev, N.; Bonneau, B.; Gillet, G. Subcellular fractionation of zebrafish embryos and mitochondrial calcium uptake application. Protoc. Exch. 2013. [Google Scholar] [CrossRef]
- Smith, E.H.; Matern, D. Acylcarnitine analysis by tandem mass spectrometry. Curr. Protoc. Hum. Genet. 2010, 64, 17.8.1–17.8.20. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wang, C.Q.; Fan, Y.Q.; Xie, Y.S.; Yin, Z.F.; Xu, Z.J.; Zhang, H.L.; Cao, J.T.; Han, Z.H.; Wang, Y.; et al. Optimizing methods for the study of intravascular lipid metabolism in zebrafish. Mol. Med. Rep. 2015, 11, 1871–1876. [Google Scholar] [CrossRef] [PubMed]
- Inohaya, K. Staining of Cartilage and Bone in Whole Embryos, Larvae and Adults by Alcian Blue and Alizarin Red. Available online: https://shigen.nig.ac.jp/medaka/medakabook/index.php?4.4.1+Staining+of+cartilage+and+bone+in+whole+embryos%2C+larvae+and+adults+by+Alcian+blue+and+Alizarin+red (accessed on 1 July 2024).
- Ibarra-García-Padilla, R.; Howard, A.G.A., 4th; Singleton, E.W.; Uribe, R.A. A protocol for whole-mount immuno-coupled hybridization chain reaction (WICHCR) in zebrafish embryos and larvae. STAR Protoc. 2021, 2, 100709. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Basnet, R.M.; Zizioli, D.; Taweedet, S.; Finazzi, D.; Memo, M. Zebrafish Larvae as a Behavioral Model in Neuropharmacology. Biomedicines 2019, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; Dhamne, S.C.; LaCoursiere, C.M.; Tambunan, D.; Poduri, A.; Rotenberg, A. Microarray Noninvasive Neuronal Seizure Recordings from Intact Larval Zebrafish. PLoS ONE 2016, 11, e0156498. [Google Scholar]
- Eshun-Wilson, L.; Zhang, R.; Portran, D.; Nachury, M.V.; Toso, D.B.; Löhr, T.; Vendruscolo, M.; Bonomi, M.; Fraser, J.S.; Nogales, E. Effects of alpha-tubulin acetylation on microtubule structure and stability. Proc. Natl. Acad. Sci. USA 2019, 116, 10366–10371. [Google Scholar] [CrossRef] [PubMed]
- Nekooki-Machida, Y.; Hagiwara, H. Role of tubulin acetylation in cellular functions and diseases. Med. Mol. Morphol. 2020, 53, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Heap, L.A.; Vanwalleghem, G.C.; Thompson, A.W.; Favre-Bulle, I.; Rubinsztein-Dunlop, H.; Scott, E.K. Hypothalamic Projections to the Optic Tectum in Larval Zebrafish. Front. Neuroanat. 2018, 11, 135. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.M. Catecholaminergic systems in the zebrafish. IV. Organization and projection pattern of dopaminergic neurons in the diencephalon. J. Comp. Neurol. 2003, 460, 13–37. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, A.V.; Latchford, K.J.; Samson, W.K. The paraventricular nucleus of the hypothalamus—A potential target for integrative treatment of autonomic dysfunction. Expert Opin. Ther. Targets 2008, 12, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Li, J.; Tang, K. The Paraventricular Nucleus of the Hypothalamus: Development, Function, and Human Diseases. Endocrinology 2018, 159, 3458–3472. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.M. Catecholaminergic systems in the zebrafish. III. Organization and projection pattern of medullary dopaminergic and noradrenergic neurons. J. Comp. Neurol. 1997, 381, 411–427. [Google Scholar] [CrossRef]
- Perez-Costas, E.; Melendez-Ferro, M.; Rice, M.W.; Conley, R.R.; Roberts, R.C. Dopamine pathology in schizophrenia: Analysis of total and phosphorylated hydroxylase in the substantia nigra. Front. Psychiatry 2012, 3, 31. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Li, S.; Zhong, H.; Lin, S. Zebrafish tyrosine hydroxylase 2 gene encodes tryptophan hydroxylase. J. Biol. Chem. 2013, 288, 22451–22459. [Google Scholar] [CrossRef] [PubMed]
- Rice, M.W.; Roberts, R.C.; Melendez-Ferro, M.; Perez-Costas, E. Mapping dopaminergic deficiencies in the substantia nigra/ventral tegmental area in schizophrenia. Anat. Embryol. 2016, 221, 185–201. [Google Scholar] [CrossRef] [PubMed]
- Kawahata, I.; Fukunaga, K. Pathogenic Impact of Fatty Acid-Binding Proteins in Parkinson’s Disease-Potential Biomarkers and Therapeutic Targets. Int. J. Mol. Sci. 2023, 24, 17037. [Google Scholar] [CrossRef] [PubMed]
- Kawahata, I.; Fukunaga, K. Impact of fatty acid-binding proteins and dopamine receptors on alpha-synucleinopathy. J. Pharmacol. Sci. 2022, 148, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Altburger, C.; Holzhauser, J.; Driever, W. CRISPR/Cas9-based QF2 knock-in at the tyrosine hydroxylase (th) locus reveals novel th-expressing neuron populations in the zebrafish mid- and hindbrain. Front. Neuroanat. 2023, 17, 1196868. [Google Scholar] [CrossRef] [PubMed]
- Richendrfer, H.; Pelkowski, S.D.; Colwill, R.M.; Creton, R. On the edge: Pharmacological evidence for anxiety-related behavior in zebrafish larvae. Behav. Brain Res. 2012, 228, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Hanada, T. Ionotropic Glutamate Receptors in Epilepsy: A Review Focusing on AMPA and NMDA Receptors. Biomolecules 2020, 10, 464. [Google Scholar] [CrossRef] [PubMed]
- Jewett, B.E.; Thapa, B. Physiology, NMDA Receptor; StatPearls Publishing LLC: Treasure Island, FL, USA, 2024. [Google Scholar]
- Kalev-Zylinska, M.L.; Symes, W.; Young, D.; During, M.J. Knockdown and overexpression of NR1 modulates NMDA receptor function. Mol. Cell. Neurosci. 2009, 41, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Burre, J. The Synaptic Function of alpha-Synuclein. J. Park. Dis. 2015, 5, 699–713. [Google Scholar]
- Mao, Y.; Ge, X.; Frank, C.L.; Madison, J.M.; Koehler, A.N.; Doud, M.K.; Tassa, C.; Berry, E.M.; Soda, T.; Singh, K.K.; et al. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/beta-catenin signaling. Cell 2009, 136, 1017–1031. [Google Scholar] [CrossRef] [PubMed]
- Hennah, W.; Varilo, T.; Kestilä, M.; Paunio, T.; Arajärvi, R.; Haukka, J.; Parker, A.; Martin, R.; Levitzky, S.; Partonen, T.; et al. Haplotype transmission analysis provides evidence of association for DISC1 to schizophrenia and suggests sex-dependent effects. Hum. Mol. Genet. 2003, 12, 3151–3159. [Google Scholar] [CrossRef] [PubMed]
- Sawamura, N.; Sawa, A. Disrupted-in-schizophrenia-1 (DISC1): A key susceptibility factor for major mental illnesses. Ann. N. Y. Acad. Sci. 2006, 1086, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Korth, C. Aggregated proteins in schizophrenia and other chronic mental diseases: DISC1opathies. Prion 2012, 6, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Vidovic, M.; Rikalovic, M.G. Alpha-Synuclein Aggregation Pathway in Parkinson’s Disease: Current Status and Novel Therapeutic Approaches. Cells 2022, 11, 1732. [Google Scholar] [CrossRef] [PubMed]
- Sardar Sinha, M.; Villamil Giraldo, A.M.; Ollinger, K.; Hallbeck, M.; Civitelli, L. Lipid vesicles affect the aggregation of 4-hydroxy-2-nonenal-modified alpha-synuclein oligomers. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2018, 1864 Pt B, 3060–3068. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.R.; Zierz, S. Muscle Carnitine Palmitoyltransferase II (CPT II) Deficiency: A Conceptual Approach. Molecules 2020, 25, 1784. [Google Scholar] [CrossRef] [PubMed]
- Morales Corado, J.A.; Lee, C.U.; Enns, G.M. Carnitine-Acylcarnitine Translocase Deficiency. In GeneReviews® [Internet]; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2004. [Google Scholar] [PubMed]
- Almannai, M.; Alfadhel, M.; El-Hattab, A.W. Carnitine Inborn Errors of Metabolism. Molecules 2019, 24, 3251. [Google Scholar] [CrossRef] [PubMed]
- Longo, N.; Frigeni, M.; Pasquali, M. Carnitine transport and fatty acid oxidation. Biochim. Biophys. Acta 2016, 1863, 2422–2435. Available online: https://www.sciencedirect.com/science/article/pii/S0167488916300131 (accessed on 1 July 2024). [CrossRef] [PubMed]
- McCoin, C.S.; Piccolo, B.D.; Knotts, T.A.; Matern, D.; Vockley, J.; Gillingham, M.B.; Adams, S.H. Unique plasma metabolomic signatures of individuals with inherited disorders of long-chain fatty acid oxidation. J. Inherit. Metab. Dis. 2016, 39, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Elizondo, G.; Matern, D.; Vockley, J.; Harding, C.O.; Gillingham, M.B. Effects of fasting, feeding and exercise on plasma acylcarnitines among subjects with CPT2D, VLCADD and LCHADD/TFPD. Mol. Genet. Metab. 2020, 131, 90–97. [Google Scholar] [CrossRef]
- Yan, L.; Zhou, J.; Wang, D.; Si, D.; Liu, Y.; Zhong, L.; Yin, Y. Unbiased lipidomic profiling reveals metabolomic changes during the onset and antipsychotics treatment of schizophrenia disease. Metabolomics 2018, 14, 80. [Google Scholar] [CrossRef] [PubMed]
- Sant, K.E.; Timme-Laragy, A.R. Zebrafish as a Model for Toxicological Perturbation of Yolk and Nutrition in the Early Embryo. Curr. Environ. Health Rep. 2018, 5, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Van Hooijdonk, C.F.; van der Pluijm, M.; Bosch, I.; van Amelsvoort, T.A.; Booij, J.; de Haan, L.; Selten, J.-P.; van de Giessen, E. The substantia nigra in the pathology of schizophrenia: A review on post-mortem and molecular imaging findings. Eur. Neuropsychopharmacol. 2023, 68, 57–77. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.K.; Roche, J.K.; Sinha, V.; Roberts, R.C. Substantia nigra ultrastructural pathology in schizophrenia. Schizophr. Res. 2018, 197, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Brisch, R.; Saniotis, A.; Wolf, R.; Bielau, H.; Bernstein, H.G.; Steiner, J.; Bogerts, B.; Braun, K.; Jankowski, Z.; Kumaratilake, J.; et al. The role of dopamine in schizophrenia from a neurobiological and evolutionary perspective: Old fashioned, but still in vogue. Front. Psychiatry 2014, 5, 47. [Google Scholar]
- Xia, X.; Ding, M.; Xuan, J.-F.; Xing, J.-X.; Pang, H.; Wang, B.-J.; Yao, J. Polymorphisms in the human serotonin receptor 1B (HTR1B) gene are associated with schizophrenia: A case control study. BMC Psychiatry 2018, 18, 303. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Ding, M.; Xuan, J.-F.; Xing, J.-X.; Yao, J.; Wu, X.; Wang, B.-J. Functional polymorphisms and transcriptional analysis in the 5′ region of the human serotonin receptor 1B gene (HTR1B) and their associations with psychiatric disorders. BMC Psychiatry 2020, 20, 499. [Google Scholar] [CrossRef] [PubMed]
- Brasso, C.; Colli, G.; Sgro, R.; Bellino, S.; Bozzatello, P.; Montemagni, C.; Villari, V.; Rocca, P. Efficacy of Serotonin and Dopamine Activity Modulators in the Treatment of Negative Symptoms in Schizophrenia: A Rapid Review. Biomedicines 2023, 11, 921. [Google Scholar] [CrossRef] [PubMed]
- Gosso, F.M.; de Geus, E.J.C.; Polderman, T.J.C.; Boomsma, D.I.; Posthuma, D.; Heutink, P. Exploring the functional role of the CHRM2 gene in human cognition: Results from a dense genotyping and brain expression study. BMC Med. Genet. 2007, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Schnorr, S.J.; Steenbergen, P.J.; Richardson, M.K.; Champagne, D.L. Measuring thigmotaxis in larval zebrafish. Behav. Brain Res. 2012, 228, 367–374. [Google Scholar] [CrossRef] [PubMed]
- De Abreu, M.S.; Demin, K.A.; Kotova, M.M.; Mirzaei, F.; Shariff, S.; Kantawala, B.; Zakharchenko, K.V.; Kolesnikova, T.O.; Dilbaryan, K.; Grigoryan, A.; et al. Developing Novel Experimental Models of m-TORopathic Epilepsy and Related Neuropathologies: Translational Insights from Zebrafish. Int. J. Mol. Sci. 2023, 24, 1530. [Google Scholar] [CrossRef] [PubMed]
- Yaksi, E.; Jamali, A.; Diaz Verdugo, C.; Jurisch-Yaksi, N. Past, present and future of zebrafish in epilepsy research. FEBS J. 2021, 288, 7243–7255. [Google Scholar] [CrossRef] [PubMed]
- Tein, I. Role of carnitine and fatty acid oxidation and its defects in infantile epilepsy. J. Child Neurol. 2002, 17, 3S57–3S82, discussion 3S82-3. [Google Scholar] [PubMed]
- Auvin, S. Fatty acid oxidation and epilepsy. Epilepsy Res. 2012, 100, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Santoro, V.; Hou, M.D.; Premoli, I.; Belardinelli, P.; Biondi, A.; Carobin, A.; Puledda, F.; Michalopoulou, P.; Richardson, M.; Rocchi, L.; et al. Investigating cortical excitability and inhibition in patients with schizophrenia: A TMS-EEG study. Brain Res. Bull. 2024, 212, 110972. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, P. (Ed.) Increased Cortical Excitability as a Critical Element in Schizophrenia Pathophysiology; Cortical Deficits in Schizophrenia; Springer: Boston, MA, USA, 2008; 18p, Available online: http://link.springer.com/10.1007/978-0-387-74351-6_11 (accessed on 1 July 2024).
- Webler, R.D.; Hamady, C.; Molnar, C.; Johnson, K.; Bonilha, L.; Anderson, B.S.; Bruin, C.; Bohning, D.E.; George, M.S.; Nahas, Z. Decreased interhemispheric connectivity and increased cortical excitability in unmedicated schizophrenia: A prefrontal interleaved TMS fMRI study. Brain Stimul. 2020, 13, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Carment, L.; Dupin, L.; Guedj, L.; Térémetz, M.; Krebs, M.-O.; Cuenca, M.; Maier, M.A.; Amado, I.; Lindberg, P.G. Impaired attentional modulation of sensorimotor control and cortical excitability in schizophrenia. Brain 2019, 142, 2149–2164. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, S.; Ghasemi, M.; Schachter, S.C. Targeting NMDA Receptor Complex in Management of Epilepsy. Pharmaceuticals 2022, 15, 1297. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Mahnke, A.H.; Doyle, S.; Fan, N.; Wang, C.-C.; Hall, B.J.; Tang, Y.-P.; Inglis, F.M.; Chen, C.; Erickson, J.D. Neurodevelopmental role for VGLUT2 in pyramidal neuron plasticity, dendritic refinement, and in spatial learning. J. Neurosci. 2012, 32, 15886–15901. [Google Scholar] [CrossRef] [PubMed]
- Balschun, D.; Moechars, D.; Callaerts-Vegh, Z.; Ben Vermaercke, B.; Van Acker, N.; Andries, L.; D’Hooge, R. Vesicular glutamate transporter VGLUT1 has a role in hippocampal long-term potentiation and spatial reversal learning. Cereb. Cortex 2010, 20, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, H.-G.; Keilhoff, G.; Steiner, J. The implications of hypothalamic abnormalities for schizophrenia. Handb. Clin. Neurol. 2021, 182, 107–120. [Google Scholar] [PubMed]
- Kang, I.; Chu, C.T.; Kaufman, B.A. The mitochondrial transcription factor TFAM in neurodegeneration: Emerging evidence and mechanisms. FEBS Lett. 2018, 592, 793–811. [Google Scholar] [CrossRef] [PubMed]
- Frasca, D.; Blomberg, B.B. Adipose tissue, immune aging, and cellular senescence. In Seminars in Immunopathology; Springer: Berlin/Heidelberg, Germany, 2020; Volume 42, pp. 573–587. [Google Scholar]
- Lee, S.M.; Lee, S.H.; Jung, Y.; Lee, Y.; Yoon, J.H.; Choi, J.Y.; Hwang, C.Y.; Son, Y.H.; Park, S.S.; Hwang, G.S.; et al. FABP3-mediated membrane lipid saturation alters fluidity and induces ER stress in skeletal muscle with aging. Nat. Commun. 2020, 11, 5661. [Google Scholar] [CrossRef] [PubMed]
- Shioda, N.; Yabuki, Y.; Kobayashi, Y.; Onozato, M.; Owada, Y.; Fukunaga, K. FABP3 protein promotes alpha-synuclein oligomerization associated with 1-methyl-1,2,3,6-tetrahydropiridine-induced neurotoxicity. J. Biol. Chem. 2014, 289, 18957–18965. [Google Scholar] [CrossRef] [PubMed]
- Sekimori, T.; Fukunaga, K.; Oizumi, H.; Baba, T.; Totsune, T.; Takeda, A.; Sasaki, T.; Kawahata, I. FABP2 is Involved in Intestinal alpha-Synuclein Pathologies. J. Integr. Neurosci. 2024, 23, 44. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Wang, L.; Ren, X.; Zhang, Y.; Zhang, H. LRPPRC: A Multifunctional Protein Involved in Energy Metabolism and Human Disease. Front. Physiol. 2019, 10, 595. [Google Scholar] [CrossRef] [PubMed]
- Panchal, K.; Tiwari, A.K. Mitochondrial dynamics, a key executioner in neurodegenerative diseases. Mitochondrion 2019, 47, 151–173. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, J.-M.; Gainetdinov, R.R. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol. Rev. 2011, 63, 182–217. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, M.; Chung, K.A.; Zabetian, C.P.; Leverenz, J.B.; Berg, D.; Srulijes, K.; Trojanowski, J.Q.; Lee, V.M.Y.; Siderowf, A.D.; et al. Phosphorylated alpha-synuclein in Parkinson’s disease. Sci. Transl. Med. 2012, 4, 121ra20. [Google Scholar] [CrossRef] [PubMed]
- Kawahata, I.; Finkelstein, D.I.; Fukunaga, K. Pathogenic Impact of alpha-Synuclein Phosphorylation and Its Kinases in alpha-Synucleinopathies. Int. J. Mol. Sci. 2022, 23, 6216. [Google Scholar] [CrossRef] [PubMed]
- Sarchione, A.; Marchand, A.; Taymans, J.-M.; Chartier-Harlin, M.-C. Alpha-Synuclein and Lipids: The Elephant in the Room? Cells 2021, 10, 2452. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Jia, W.; Kawahata, I.; Fukunaga, K. Impact of Fatty Acid-Binding Proteins in alpha-Synuclein-Induced Mitochondrial Injury in Synucleinopathy. Cells 2021, 9, 560. [Google Scholar] [CrossRef]
- Lopez-Merino, E.; Cuartero, M.I.; Esteban, J.A.; Briz, V. Perinatal exposure to pesticides alters synaptic plasticity signaling and induces behavioral deficits associated with neurodevelopmental disorders. Cell Biol. Toxicol. 2022, 39, 1573–6822. [Google Scholar] [CrossRef] [PubMed]
- Costas-Ferreira, C.; Durán, R.; Faro, L.R.F. Toxic Effects of Glyphosate on the Nervous System: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 4605. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baker, C.E.; Marta, A.G.; Zimmerman, N.D.; Korade, Z.; Mathy, N.W.; Wilton, D.; Simeone, T.; Kochvar, A.; Kramer, K.L.; Stessman, H.A.F.; et al. CPT2 Deficiency Modeled in Zebrafish: Abnormal Neural Development, Electrical Activity, Behavior, and Schizophrenia-Related Gene Expression. Biomolecules 2024, 14, 914. https://doi.org/10.3390/biom14080914
Baker CE, Marta AG, Zimmerman ND, Korade Z, Mathy NW, Wilton D, Simeone T, Kochvar A, Kramer KL, Stessman HAF, et al. CPT2 Deficiency Modeled in Zebrafish: Abnormal Neural Development, Electrical Activity, Behavior, and Schizophrenia-Related Gene Expression. Biomolecules. 2024; 14(8):914. https://doi.org/10.3390/biom14080914
Chicago/Turabian StyleBaker, Carly E., Aaron G. Marta, Nathan D. Zimmerman, Zeljka Korade, Nicholas W. Mathy, Delaney Wilton, Timothy Simeone, Andrew Kochvar, Kenneth L. Kramer, Holly A. F. Stessman, and et al. 2024. "CPT2 Deficiency Modeled in Zebrafish: Abnormal Neural Development, Electrical Activity, Behavior, and Schizophrenia-Related Gene Expression" Biomolecules 14, no. 8: 914. https://doi.org/10.3390/biom14080914







