Plasma microRNA Environment Linked to Tissue Factor Pathway and Cancer-Associated Thrombosis: Prognostic Significance in Ovarian Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. miRNA Selection
2.3. Blood Sample Collection and Processing
2.4. Total RNA Extraction and cDNA Synthesis
2.5. Relative Quantification of miRNAs
2.6. Statistical Analysis
2.7. In Silico Analysis
3. Results
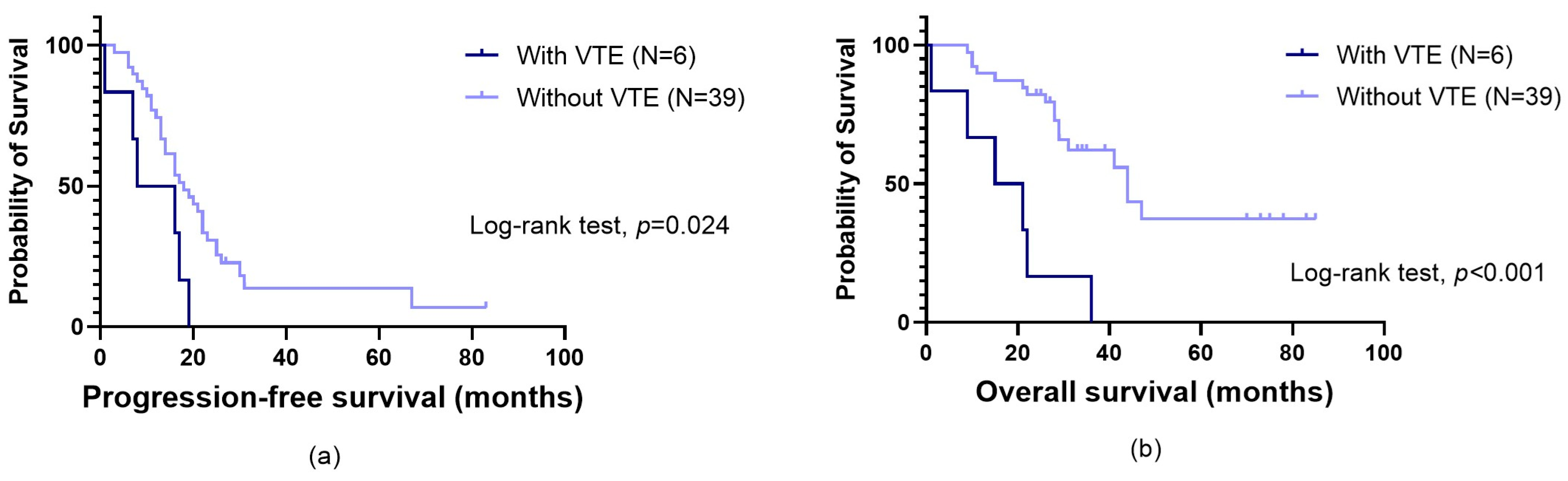
3.1. Impact of VTE on Patients’ Prognosis
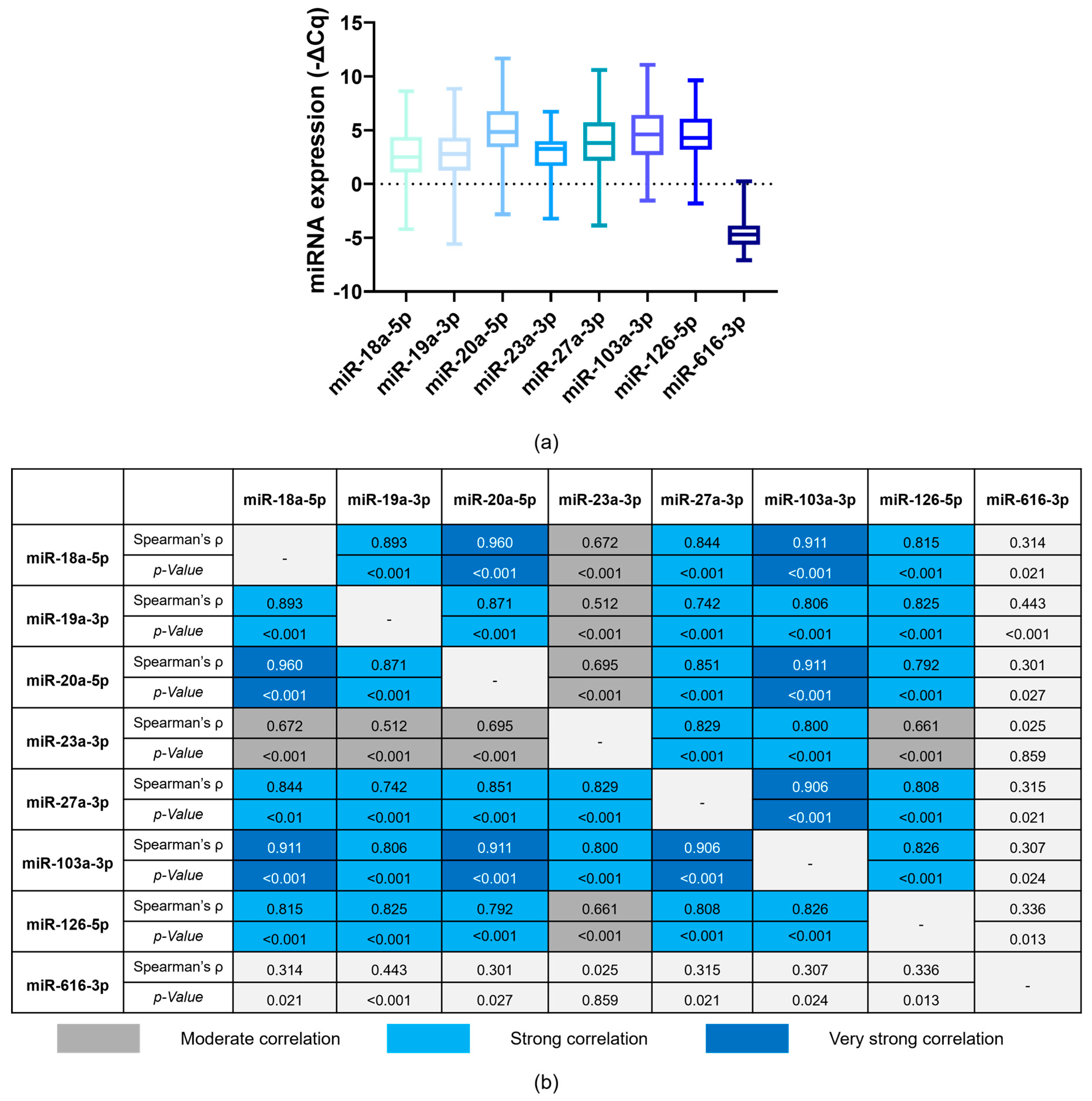
3.2. Correlation between Baseline miRNA Expression
3.3. Baseline miRNA Levels and Patients’ Characteristics
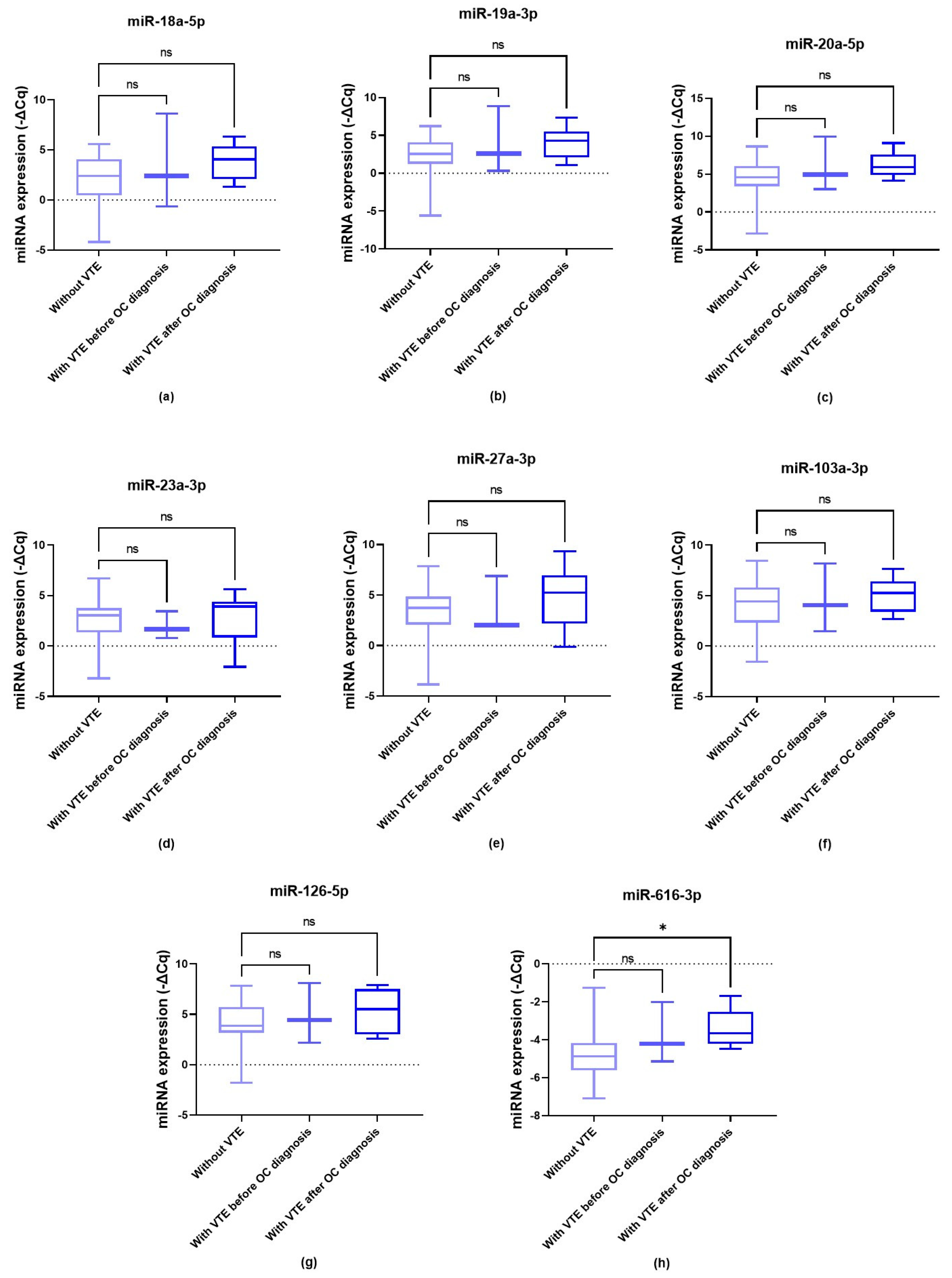
3.4. Baseline miRNA Levels and OC-Related VTE Susceptibility
3.5. Impact of Baseline miRNA Levels on Patients’ Prognosis
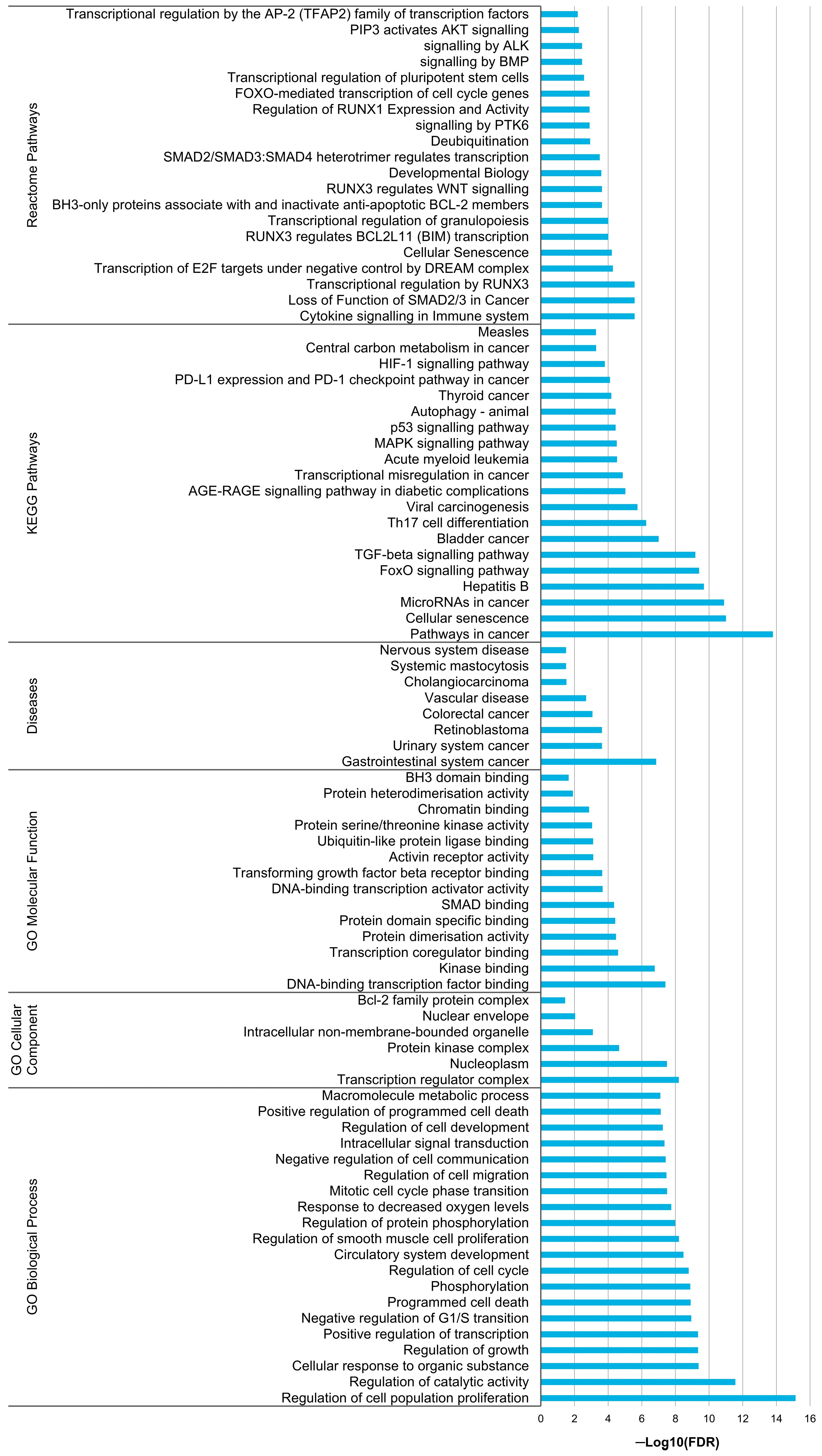
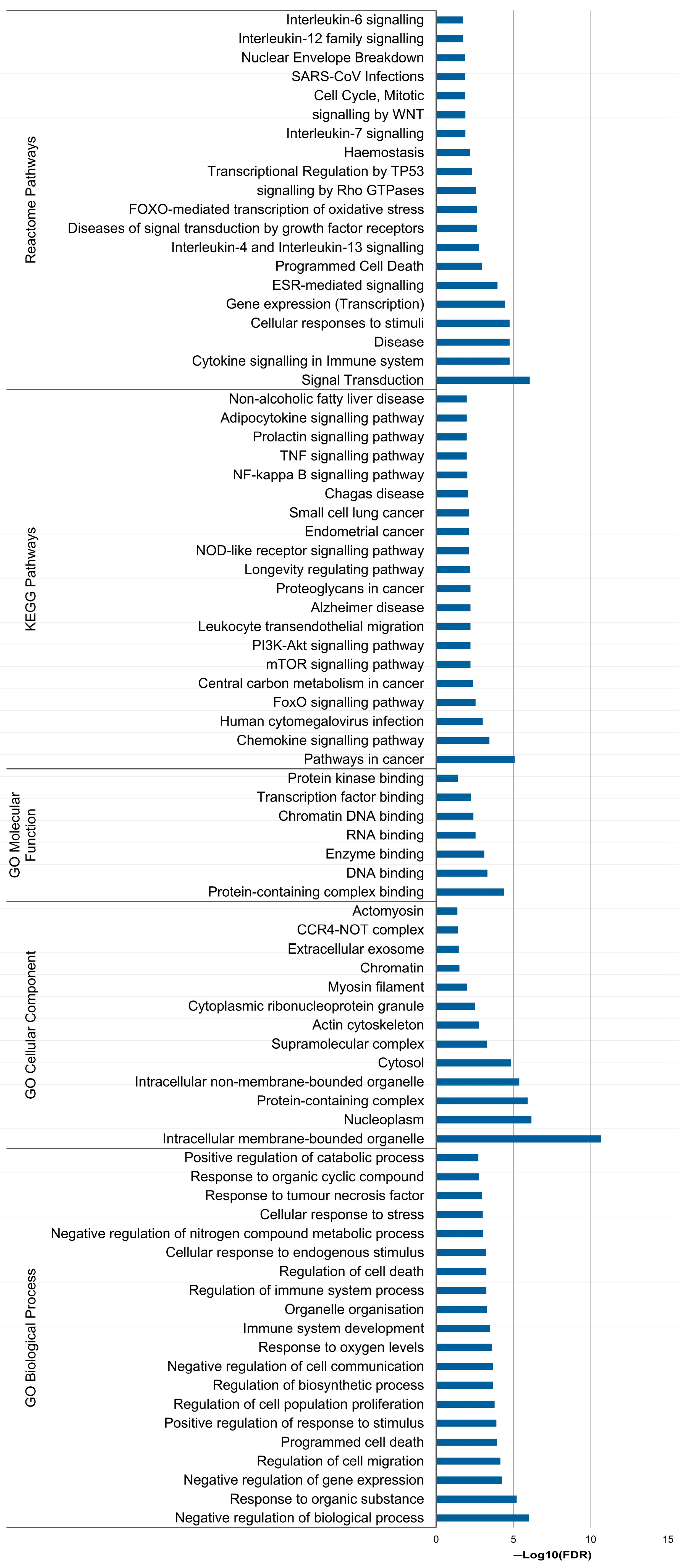
3.6. In Silico Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.E.M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France; Available online: https://gco.iarc.fr/today (accessed on 5 February 2024).
- Nag, S.; Aggarwal, S.; Rauthan, A.; Warrier, N. Maintenance therapy for newly diagnosed epithelial ovarian cancer—A review. J. Ovarian Res. 2022, 15, 88. [Google Scholar] [CrossRef] [PubMed]
- Tavares, V.; Marques, I.S.; Melo, I.G.d.; Assis, J.; Pereira, D.; Medeiros, R. Paradigm Shift: A Comprehensive Review of Ovarian Cancer Management in an Era of Advancements. Int. J. Mol. Sci. 2024, 25, 1845. [Google Scholar] [CrossRef] [PubMed]
- Liz-Pimenta, J.; Tavares, V.; Neto, B.V.; Santos, J.M.; Guedes, C.B.; Araújo, A.; Khorana, A.A.; Medeiros, R. Thrombosis and cachexia in cancer: Two partners in crime? Crit. Rev. Oncol./Hematol. 2023, 186, 103989. [Google Scholar] [CrossRef] [PubMed]
- Tavares, V.; Neto, B.V.; Marques, I.S.; Assis, J.; Pereira, D.; Medeiros, R. Cancer-associated thrombosis: What about microRNAs targeting the tissue factor coagulation pathway? Biochim. Biophys. Acta (BBA) Rev. Cancer 2023, 1879, 189053. [Google Scholar] [CrossRef] [PubMed]
- Oncul, S.; Cho, M.S. Interactions between platelets and tumor microenvironment components in ovarian cancer and their implications for treatment and clinical outcomes. Cancers 2023, 15, 1282. [Google Scholar] [CrossRef] [PubMed]
- Toden, S.; Zumwalt, T.J.; Goel, A. Non-coding RNAs and potential therapeutic targeting in cancer. Biochim. Biophys. Acta (BBA) Rev. Cancer 2021, 1875, 188491. [Google Scholar] [CrossRef] [PubMed]
- Correia de Sousa, M.; Gjorgjieva, M.; Dolicka, D.; Sobolewski, C.; Foti, M. Deciphering miRNAs’ action through miRNA editing. Int. J. Mol. Sci. 2019, 20, 6249. [Google Scholar] [CrossRef] [PubMed]
- de Bono, J.S.; Harris, J.R.; Burm, S.M.; Vanderstichele, A.; Houtkamp, M.A.; Aarass, S.; Riisnaes, R.; Figueiredo, I.; Nava Rodrigues, D.; Christova, R. Systematic study of tissue factor expression in solid tumors. Cancer Rep. 2023, 6, e1699. [Google Scholar] [CrossRef] [PubMed]
- Saadeh, F.A.; Norris, L.; O’Toole, S.; Mohamed, B.M.; Langhe, R.; O’Leary, J.; Gleeson, N. Tumour expresion of tissue factor and tissue factor pathway inhibitor in ovarian cancer-relationship with venous thrombosis risk. Thromb. Res. 2013, 132, 627–634. [Google Scholar] [CrossRef]
- Uno, K.; Homma, S.; Satoh, T.; Nakanishi, K.; Abe, D.; Matsumoto, K.; Oki, A.; Tsunoda, H.; Yamaguchi, I.; Nagasawa, T. Tissue factor expression as a possible determinant of thromboembolism in ovarian cancer. Br. J. Cancer 2007, 96, 290–295. [Google Scholar] [CrossRef]
- Sakurai, M.; Matsumoto, K.; Gosho, M.; Sakata, A.; Hosokawa, Y.; Tenjimbayashi, Y.; Katoh, T.; Shikama, A.; Komiya, H.; Michikami, H. Expression of tissue factor in epithelial ovarian carcinoma is involved in the development of venous thromboembolism. Int. J. Gynecol. Cancer 2017, 27, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zhang, T.; Wang, R.; Cheng, Z.; Xu, H.; Li, W.; Wang, Y.; Wang, X. Tissue factor-factor VIIa complex induces epithelial ovarian cancer cell invasion and metastasis through a monocytes-dependent mechanism. Int. J. Gynecol. Cancer 2011, 21, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Steidel, C.; Ender, F.; Rody, A.; von Bubnoff, N.; Gieseler, F. Biologically active tissue factor-bearing larger Ectosome-like extracellular vesicles in malignant effusions from ovarian cancer patients: Correlation with incidence of thrombosis. Int. J. Mol. Sci. 2021, 22, 790. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Matsubara, S.; Yoshimoto, C.; Shigetomi, H.; Imanaka, S. Tissue factor pathway inhibitor 2: Current understanding, challenges, and future perspectives. J. Obstet. Gynaecol. Res. 2023, 49, 2575–2583. [Google Scholar] [CrossRef] [PubMed]
- Hembrough, T.A.; Swartz, G.M.; Papathanassiu, A.; Vlasuk, G.P.; Rote, W.E.; Green, S.J.; Pribluda, V.S. Tissue factor/factor VIIa inhibitors block angiogenesis and tumor growth through a nonhemostatic mechanism. Cancer Res. 2003, 63, 2997–3000. [Google Scholar] [PubMed]
- Marques, I.S.; Tavares, V.; Savva-Bordalo, J.; Rei, M.; Liz-Pimenta, J.; de Melo, I.G.; Assis, J.; Pereira, D.; Medeiros, R. Long Non-Coding RNAs: Bridging Cancer-Associated Thrombosis and Clinical Outcome of Ovarian Cancer Patients. Int. J. Mol. Sci. 2023, 25, 140. [Google Scholar] [CrossRef] [PubMed]
- Tavares, V.; Savva-Bordalo, J.; Rei, M.; Liz-Pimenta, J.; Assis, J.; Pereira, D.; Medeiros, R. Haemostatic Gene Expression in Cancer-Related Immunothrombosis: Contribution for Venous Thromboembolism and Ovarian Tumour Behaviour. Cancers 2024, 16, 2356. [Google Scholar] [CrossRef]
- Berek, J.S.; Renz, M.; Kehoe, S.; Kumar, L.; Friedlander, M. Cancer of the ovary, fallopian tube, and peritoneum: 2021 update. Int. J. Gynecol. Obstet. 2021, 155, 61–85. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; Kuderer, N.M.; Culakova, E.; Lyman, G.H.; Francis, C.W. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood J. Am. Soc. Hematol. 2008, 111, 4902–4907. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, H.; Lou, J.R.; Zheng, J.; Zhu, H.; Popescu, N.-I.; Lupu, F.; Lind, S.E.; Ding, W.-Q. MicroRNA-19 (miR-19) regulates tissue factor expression in breast cancer cells. J. Biol. Chem. 2011, 286, 1429–1435. [Google Scholar] [CrossRef]
- Eisenreich, A.; Rauch, U. Regulation of the tissue factor isoform expression and thrombogenicity of HMEC-1 by miR-126 and miR-19a. Cell Biol. Res. Ther. 2013, 2, 1. [Google Scholar]
- Yu, G.; Wang, X.; Wu, T.; Zhu, J.; Huang, S.; Wan, Y.; Tang, J. MicroRNA-19a targets tissue factor to inhibit colon cancer cells migration and invasion. Mol. Cell. Biochem. 2013, 380, 239–247. [Google Scholar] [CrossRef]
- Balia, C.; Giordano, M.; Scalise, V.; Neri, T.; Fontanini, G.; Basolo, F.; Celi, A.; Pedrinelli, R. miR-19a and miR-20a and tissue factor expression in activated human peripheral blood mononuclear cells. Thrombosis 2017, 2017, 1076397. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, M.; Tabaraie, T.; Steffens, D.; Friebel, J.; Dörner, A.; Skurk, C.; Witkowski, M.; Stratmann, B.; Tschoepe, D.; Landmesser, U. MicroRNA-19a contributes to the epigenetic regulation of tissue factor in diabetes. Cardiovasc. Diabetol. 2018, 17, 34. [Google Scholar] [CrossRef]
- Chen, Q.-Q.; Shi, J.-M.; Ding, Z.; Xia, Q.; Zheng, T.-S.; Ren, Y.-B.; Li, M.; Fan, L.-H. Berberine induces apoptosis in non-small-cell lung cancer cells by upregulating miR-19a targeting tissue factor. Cancer Manag. Res. 2019, 11, 9005. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Lu, S.; Yang, X.; Li, M.; Jia, H.; Liao, J.; Jing, Q.; Wu, Y.; Wang, H.; Xiao, F. miR-19a-3p downregulates tissue factor and functions as a potential therapeutic target for sepsis-induced disseminated intravascular coagulation. Biochem. Pharmacol. 2021, 192, 114671. [Google Scholar] [CrossRef] [PubMed]
- Oto, J.; Navarro, S.; Larsen, A.C.; Solmoirago, M.J.; Plana, E.; Hervás, D.; Fernández-Pardo, Á.; España, F.; Kristensen, S.R.; Thorlacius-Ussing, O. MicroRNAs and neutrophil activation markers predict venous thrombosis in pancreatic ductal adenocarcinoma and distal extrahepatic cholangiocarcinoma. Int. J. Mol. Sci. 2020, 21, 840. [Google Scholar] [CrossRef]
- Teruel, R.; Perez-Sanchez, C.; Corral, J.; Herranz, M.; Perez-Andreu, V.; Saiz, E.; Garcia-Barbera, N.; Martinez-Martinez, I.; Roldan, V.; Vicente, V. Identification of miRNAs as potential modulators of tissue factor expression in patients with systemic lupus erythematosus and antiphospholipid syndrome. J. Thromb. Haemost. 2011, 9, 1985–1992. [Google Scholar] [CrossRef]
- Yu, Y.-H.; Wu, D.-S.; Huang, F.-F.; Zhang, Z.; Liu, L.-X.; Zhang, J.; Zhan, H.-E.; Peng, M.-Y.; Zeng, H.; Chen, F.-P. MicroRNA-20b and ERK1/2 pathway independently regulate the expression of tissue factor in hematopoietic and trophoblastic differentiation of human embryonic stem cells. Stem Cell Res. Ther. 2013, 4, 121. [Google Scholar] [CrossRef]
- Wang, W.; Ning, J.Z.; Tang, Z.G.; He, Y.; Yao, L.-C.; Ye, L.; Wu, L. MicroRNA-23a acts as an oncogene in pancreatic carcinoma by targeting TFPI-2. Exp. Ther. Med. 2020, 20, 53. [Google Scholar] [CrossRef]
- Oto, J.; Plana, E.; Solmoirago, M.J.; Fernández-Pardo, Á.; Hervás, D.; Cana, F.; España, F.; Artoni, A.; Bucciarelli, P.; Carrabba, G. microRNAs and markers of neutrophil activation as predictors of early incidental post-surgical pulmonary embolism in patients with intracranial tumors. Cancers 2020, 12, 1536. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, J.; Jiang, Z.; Wu, F.; Ping, J.; Ming, L. Diagnostic value of circulating microRNA-27a/b in patients with acute pulmonary embolism. Int. Angiol. J. Int. Union Angiol. 2017, 37, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Arroyo, A.; González-Conejero, R.; Stavik, B.; Iversen, N.; Sandset, P.; Martínez, C.; Skretting, G. The role of micro RNA-27a/b and micro RNA-494 in estrogen-mediated downregulation of tissue factor pathway inhibitor α. J. Thromb. Haemost. 2016, 14, 1226–1237. [Google Scholar] [CrossRef]
- Geng, G.; Liu, X.; Xu, A.; Lu, Z.; Chen, K.; He, J.; Qi, D.; Yuan, X. Low abundance of TFPI-2 by both promoter methylation and miR-27a-3p regulation is linked with poor clinical outcome in gastric cancer. J. Gene Med. 2020, 22, e3166. [Google Scholar] [CrossRef]
- Sun, S.; Chai, S.; Zhang, F.; Lu, L. Overexpressed microRNA-103a-3p inhibits acute lower-extremity deep venous thrombosis via inhibition of CXCL12. IUBMB Life 2020, 72, 492–504. [Google Scholar] [CrossRef]
- Wang, X.; Sundquist, K.; Svensson, P.J.; Rastkhani, H.; Palmér, K.; Memon, A.A.; Sundquist, J.; Zöller, B. Association of recurrent venous thromboembolism and circulating microRNAs. Clin. Epigenet. 2019, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rius, A.; Lopez, S.; Martinez-Perez, A.; Souto, J.C.; Soria, J.M. Identification of a plasma MicroRNA profile associated with venous thrombosis. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1392–1399. [Google Scholar] [CrossRef]
- Rossetti, P.; Goldoni, M.; Pengo, V.; Vescovini, R.; Mozzoni, P.; Tassoni, M.I.; Lombardi, M.; Rubino, P.; Bernuzzi, G.; Verzicco, I. MiRNA 126 as a new predictor biomarker in venous thromboembolism of persistent residual vein obstruction: A review of the literature plus a pilot study. Semin. Thromb. Hemost. 2021, 47, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Chan, Y.P.; Kwan, P.S.; Lee, T.K.; Yan, M.; Tang, K.H.; Ling, M.T.; Vielkind, J.R.; Guan, X.-Y.; Chan, K.W. MicroRNA-616 induces androgen-independent growth of prostate cancer cells by suppressing expression of tissue factor pathway inhibitor TFPI-2. Cancer Res. 2011, 71, 583–592. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, D.; Jiang, Z.; Zhang, Y.; Wang, S.; Ma, Z.; Hui, B.; Wang, J.; Qian, W.; Ge, Z. MiR-616-3p modulates cell proliferation and migration through targeting tissue factor pathway inhibitor 2 in preeclampsia. Cell Prolif. 2018, 51, e12490. [Google Scholar] [CrossRef]
- Silva, J.; Tavares, V.; Afonso, A.; Garcia, J.; Cerqueira, F.; Medeiros, R. Plasmatic MicroRNAs and Treatment Outcomes of Patients with Metastatic Castration-Resistant Prostate Cancer: A Hospital-Based Cohort Study and In Silico Analysis. Int. J. Mol. Sci. 2023, 24, 9101. [Google Scholar] [CrossRef]
- Hu, J.; Wang, Z.; Liao, B.Y.; Yu, L.; Gao, X.; Lu, S.; Wang, S.; Dai, Z.; Zhang, X.; Chen, Q. Human miR-1228 as a stable endogenous control for the quantification of circulating microRNAs in cancer patients. Int. J. Cancer 2014, 135, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Blondal, T.; Nielsen, S.J.; Baker, A.; Andreasen, D.; Mouritzen, P.; Teilum, M.W.; Dahlsveen, I.K. Assessing sample and miRNA profile quality in serum and plasma or other biofluids. Methods 2013, 59, S1–S6. [Google Scholar] [CrossRef]
- Shah, J.S.; Soon, P.S.; Marsh, D.J. Comparison of methodologies to detect low levels of hemolysis in serum for accurate assessment of serum microRNAs. PLoS ONE 2016, 11, e0153200. [Google Scholar] [CrossRef] [PubMed]
- Prajzlerová, K.; Šenolt, L.; Filková, M. Is there a potential of circulating miRNAs as biomarkers in rheumatic diseases? Genes Dis. 2023, 10, 1263–1278. [Google Scholar] [CrossRef]
- Galmiche, A.; Rak, J.; Roumenina, L.T.; Saidak, Z. Coagulome and the tumor microenvironment: An actionable interplay. Trends Cancer 2022, 8, 369–383. [Google Scholar] [CrossRef]
- Li, A.; Garcia, D.A.; Lyman, G.H.; Carrier, M. Direct oral anticoagulant (DOAC) versus low-molecular-weight heparin (LMWH) for treatment of cancer associated thrombosis (CAT): A systematic review and meta-analysis. Thromb. Res. 2019, 173, 158–163. [Google Scholar] [CrossRef]
- Lattuca, F.A.; Moore, J.; Treptow, C.; Delibert, K.; Baran, A.; Akwaa, F. Bleeding and venous thromboembolism events in cancer patients taking direct oral anticoagulants vs. low molecular weight heparin. Thromb. Update 2023, 10, 100129. [Google Scholar] [CrossRef]
- Wang, J.; Peng, X.; Li, R.; Liu, K.; Zhang, C.; Chen, X.; Huang, G.; Zhao, L.; Chen, Z.; Lai, Y. Evaluation of serum miR-17-92 cluster as noninvasive biomarkers for bladder cancer diagnosis. Front. Oncol. 2021, 11, 795837. [Google Scholar] [CrossRef]
- Zhang, P.; Zhao, Q.; Gong, K.; Long, Y.; Zhang, J.; Li, Y.; Guo, X. Downregulation of miR-103a-3p contributes to endothelial progenitor cell dysfunction in deep vein thrombosis through PTEN targeting. Ann. Vasc. Surg. 2020, 64, 339–346. [Google Scholar] [CrossRef]
- Cao, G.; Zhou, H.; Wang, D.; Xu, L. Knockdown of lncRNA XIST Ameliorates IL-1β-Induced Apoptosis of HUVECs and Change of Tissue Factor Level via miR-103a-3p/HMGB1 Axis in Deep Venous Thrombosis by Regulating the ROS/NF-κB Signaling Pathway. Cardiovasc. Ther. 2022, 2022, 6256384. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Yao, X.; Lin, Y.; Zhang, D.; Cui, R.; Zhang, X. Interactive Functions of microRNAs in the miR-23a-27a-24-2 Cluster and the Potential for Targeted Therapy in Cancer. J. Cell. Physiol. 2020, 235, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lei, Y.; Li, S.; Li, F.; Lei, J. MicroRNA-20a-5p inhibits the autophagy and cisplatin resistance in ovarian cancer via regulating DNMT3B-mediated DNA methylation of RBP1. Reprod. Toxicol. 2022, 109, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Liu, Y.; Jiang, J.; Ma, Z.; Wu, H.; Liu, T.; Liu, M.; Li, X.; Tang, H. miR-20a promotes proliferation and invasion by targeting APP in human ovarian cancer cells. Acta Biochim. Biophys. Sin. 2010, 42, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Han, S.; Li, Y.; Liu, Y.; Zhang, D.; Li, Y.; Zhang, J. MicroRNA-20a contributes to cisplatin-resistance and migration of OVCAR3 ovarian cancer cell line. Oncol. Lett. 2017, 14, 1780–1786. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Liu, M.; Li, Y.; Nie, Y.; Mi, Q.; Zhao, S. Ovarian tumor-associated microRNA-20a decreases natural killer cell cytotoxicity by downregulating MICA/B expression. Cell. Mol. Immunol. 2014, 11, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, S.; Miyake, R.; Yamada, Y.; Kawaguchi, R.; Ootake, N.; Myoba, S.; Kobayashi, H. Tissue factor pathway inhibitor 2: A novel biomarker for predicting asymptomatic venous thromboembolism in patients with epithelial ovarian cancer. Gynecol. Obstet. Investig. 2022, 87, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Miyake, R.; Yamada, Y.; Yamanaka, S.; Kawaguchi, R.; Ootake, N.; Myoba, S.; Kobayashi, H. Tissue factor pathway inhibitor 2 as a serum marker for diagnosing asymptomatic venous thromboembolism in patients with epithelial ovarian cancer and positive D-dimer results. Mol. Clin. Oncol. 2022, 16, 46. [Google Scholar] [CrossRef] [PubMed]
- Maehana, T.; Kawaguchi, R.; Nishikawa, K.; Kawahara, N.; Yamada, Y.; Kimura, F. Investigating the efficacy of tissue factor pathway inhibitor-2 as a promising prognostic marker for ovarian cancer. Oncol. Lett. 2024, 28, 302. [Google Scholar] [CrossRef]
- Arakawa, N.; Kobayashi, H.; Yonemoto, N.; Masuishi, Y.; Ino, Y.; Shigetomi, H.; Furukawa, N.; Ohtake, N.; Miyagi, Y.; Hirahara, F. Clinical significance of tissue factor pathway inhibitor 2, a serum biomarker candidate for ovarian clear cell carcinoma. PLoS ONE 2016, 11, e0165609. [Google Scholar] [CrossRef]
- Miyagi, E.; Arakawa, N.; Sakamaki, K.; Yokota, N.R.; Yamanaka, T.; Yamada, Y.; Yamaguchi, S.; Nagao, S.; Hirashima, Y.; Kasamatsu, Y. Validation of tissue factor pathway inhibitor 2 as a specific biomarker for preoperative prediction of clear cell carcinoma of the ovary. Int. J. Clin. Oncol. 2021, 26, 1336–1344. [Google Scholar] [CrossRef] [PubMed]
- Didar, H.; Farzaneh, F.; Najafiarab, H.; Namakin, K.; Gohari, K.; Sheidaei, A.; Ramezani, S. Clear cell carcinoma of the ovary and venous thromboembolism: A systematic review and meta-analysis. Curr. Med. Res. Opin. 2023, 39, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Tavares, V.; Pinto, R.; Assis, J.; Coelho, S.; Brandao, M.; Alves, S.; Pereira, D.; Medeiros, R. Implications of venous thromboembolism GWAS reported genetic makeup in the clinical outcome of ovarian cancer patients. Pharmacogenom. J. 2021, 21, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Neto, B.V.; Tavares, V.; da Silva, J.B.; Liz-Pimenta, J.; Marques, I.S.; Carvalho, L.; Salgado, L.; Pereira, D.; Medeiros, R. Thrombogenesis-associated genetic determinants as predictors of thromboembolism and prognosis in cervical cancer. Sci. Rep. 2023, 13, 9519. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liu, Y.; Lin, S.; Shang, J.; Liu, J.; Li, J.; Yuan, S.; Zhang, L. Early growth response gene-1 and hypoxia-inducible factor-1α affect tumor metastasis via regulation of tissue factor. Acta Oncol. 2013, 52, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.E.; Bendahl, P.-O.; Belting, M.; Branco, C.; Johnson, R.S. Diverse roles of cell-specific hypoxia-inducible factor 1 in cancer-associated hypercoagulation. Blood J. Am. Soc. Hematol. 2016, 127, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chang, Y.; Yang, X.; Han, Z. Deep sequencing of circulating miRNAs and target mRNAs level in deep venous thrombosis patients. IET Syst. Biol. 2023, 17, 212–227. [Google Scholar] [CrossRef] [PubMed]
- Bochenek, M.L.; Leidinger, C.; Rosinus, N.S.; Gogiraju, R.; Guth, S.; Hobohm, L.; Jurk, K.; Mayer, E.; Münzel, T.; Lankeit, M. Activated endothelial TGFβ1 signaling promotes venous thrombus nonresolution in mice via endothelin-1: Potential role for chronic thromboembolic pulmonary hypertension. Circ. Res. 2020, 126, 162–181. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Quintero, B. Cell-free microRNAs in blood and other body fluids, as cancer biomarkers. Cell Prolif. 2016, 49, 281–303. [Google Scholar] [CrossRef]
- Fu, Y.; Sun, H. The molecular mechanism of circRHOBTB3 inhibits the proliferation and invasion of epithelial ovarian cancer by serving as the ceRNA of miR-23a-3p. J. Ovarian Res. 2022, 15, 66. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, Y.; Xu, H.; Shi, Y.; Shen, R.; Teng, F.; Xu, J.; Jia, X. Circular RNA hsa_circ_0007444 inhibits ovarian cancer progression through miR-23a-3p/DICER1 axis: Hsa_circ_0007444/miR-23a-3p/DICER1 in ovarian cancer. Acta Biochim. Biophys. Sin. 2023, 55, 574. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Jiang, H.; Lin, L.; Li, Y.; Li, J. lncRNA GAS5 suppression of the malignant phenotype of ovarian cancer via the miR-23a-WT1 axis. Ann. Transl. Med. 2023, 11, 119. [Google Scholar] [CrossRef]
- Todeschini, P.; Salviato, E.; Romani, C.; Raimondi, V.; Ciccarese, F.; Ferrari, F.; Tognon, G.; Marchini, S.; D’Incalci, M.; Zanotti, L. Comprehensive profiling of hypoxia-related miRNAs identifies miR-23a-3p overexpression as a marker of platinum resistance and poor prognosis in high-grade serous ovarian cancer. Cancers 2021, 13, 3358. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Shen, L.; Lin, Q.; Dong, C.; Maswela, B.; Illahi, G.S.; Wu, X. SNHG5 enhances Paclitaxel sensitivity of ovarian cancer cells through sponging miR-23a. Biomed. Pharmacother. 2020, 123, 109711. [Google Scholar] [CrossRef] [PubMed]
- Andikyan, V.; Mullokandov, G.; Agudo, J.; Sachidanandam, R.; Fishman, D.; Baccarini, A.; Brown, B.D. MicroRNA activity profile in the ovarian cancer cell line OVCAR3 identifies a proapoptotic effect of miR-23a. Adv. Genom. Genet. 2015, 2015, 355–364. [Google Scholar] [CrossRef]
- Huang, S.-L.; Xin, H.-Y.; Wang, X.-Y.; Feng, G.-G.; Wu, F.-Q.; Feng, Z.-P.; Xing, Z.; Zhang, X.-H.; Xin, H.-W.; Luo, W.-Y. Recent Advances on the Molecular Mechanism and Clinical Trials of Venous Thromboembolism. J. Inflamm. Res. 2023, 16, 6167–6178. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-H.; Lin, C.; Liu, C.-C.; Jiang, W.-W.; Huang, M.-Z.; Liu, X.; Guo, W.-J. MiR-616-3p promotes angiogenesis and EMT in gastric cancer via the PTEN/AKT/mTOR pathway. Biochem. Biophys. Res. Commun. 2018, 501, 1068–1073. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhu, J.; Zhu, Y.; Wang, J. MicroRNA-616 promotes the progression of ovarian cancer by targeting TIMP2. Oncol. Rep. 2018, 39, 2960–2968. [Google Scholar] [CrossRef]
- Yuan, C. miR-616 promotes breast cancer migration and invasion by targeting TIMP2 and regulating MMP signaling. Oncol. Lett. 2019, 18, 2348–2355. [Google Scholar] [CrossRef]
- Marques, I.S.; Tavares, V.; Neto, B.V.; Mota, I.N.R.; Pereira, D.; Medeiros, R. Long Non-Coding RNAs in Venous Thromboembolism: Where Do We Stand? Int. J. Mol. Sci. 2023, 24, 12103. [Google Scholar] [CrossRef]


| Variable | N (%) |
|---|---|
| Age at OC diagnosis (years) † | 63.8 ± 11.9 |
| ≥64 | 31 (56.4) |
| Hormonal status at OC diagnosis | |
| Post-menopausal | 43 (78.2) |
| Baseline BMI (kg/m2) † | 26.6 ± 4.7 |
| ≥27.0 | 22 (40.0) |
| ECOG PS at OC diagnosis | |
| >1 | 7 (12.7) |
| OC-related germline mutations | 6 (10.9) |
| Histology | |
| Serous | 46 (83.6) |
| Clear cell | 3 (5.5) |
| Endometroid | 2 (3.6) |
| Mixed | 2 (3.6) |
| Unusual | 2 (3.6) |
| Histological grade | |
| High | 51 (92.7) |
| FIGO stage § | |
| I/II | 11 (20.0) |
| III/IV | 44 (80.0) |
| Baseline CA-125 levels (U/mL) ‡ | |
| ≥913.0 | 27 (49.1) |
| Baseline aPTT (s) ‡ | |
| ≥27.2 | 24 (43.6) |
| Baseline PT (s) ‡ | |
| ≥14.2 | 22 (40.0) |
| Baseline INR ‡ | |
| ≥1.06 | 23 (41.8) |
| KS ‡‡ | |
| ≥2 | 21 (38.2) |
| Anticoagulation therapy †† | 3 (5.5) |
| Platelet anti-aggregation therapy | 7 (12.7) |
| First-line treatment | |
| Surgery and adjuvant chemotherapy | 24 (43.6) |
| Neoadjuvant chemotherapy, surgery and adjuvant chemotherapy | 15 (27.3) |
| Chemotherapy only | 12 (21.8) |
| Neoadjuvant chemotherapy and surgery | 4 (7.3) |
| Platinum sensitivity §§ | 39 (70.9) |
| Maintenance therapy | |
| PARP inhibitors | 17 (30.9) |
| bevacizumab | 9 (16.4) |
| miRNAs | Target Gene | miRNA–mRNA Interaction on Physio-Pathological Settings | Predicted miRNA–mRNA Interactions | Experimentally Validated miRNA–mRNA Interactions * | VTE ** | ||||
|---|---|---|---|---|---|---|---|---|---|
| TargetScanHuman | MiRDB | miRmap | miRwalk | DIANA-TarBase | miRTarBase | ||||
| Site Type (Context++ Score Percentile) | Target Score | miRmap Score | Score (Region) | MicroT Score | Level of Evidence | ||||
| miR-18a-5p | F3 | - | 7mer-m8 (98) | 51 | 98.28 | 0.92 (3′ UTR) | 0.71 | Less strong | - |
| miR-19a-3p | F3 | [21,22,23,24,25,26,27] | 8mer (98) | 92 | 85.15 | - | 0.97 | - | B [28] |
| miR-20a-5p | F3 | [24,29,30] | 8mer (99) | 95 | 84.88 | - | 0.25 | Less strong | - |
| TFPI2 | - | 7mer-A1 (88) | - | 87.88 | - | 0.74 | - | ||
| miR-23a-3p | TFPI2 | [31] | 7mer-m8 (92) | - | 52.23 | 0.92 (CDS) | 0.75 | - | B [32] |
| miR-27a-3p | F3 | - | 7mer-m8 (92) | 57 | 79.20 | 0.85 (3′ UTR) | 0.83 | - | A [33] |
| TFPI1 | [34,35] | 8mer (97) | 72 | 84.00 | 0.92 (3′ UTR) | 0.96 | - | ||
| miR-103a-3p | TFPI2 | - | - | - | 52.23 | 1.00 (CDS) | 0.72 | - | C [28,36,37] |
| miR-126-5p | F3 | [22,25] | - | - | - | - | - | - | C [32,38,39] |
| miR-616-3p | TFPI2 | [40,41] | 7mer-m8 (96) | - | 48.40 | - | - | Strong | - |
| Model (N) | Variable | aHR | 95%CI | p-Value | C-Index | Event | |
|---|---|---|---|---|---|---|---|
| Clinic Model | Integrative Model | ||||||
| Integrative model A1 (47) | Surgery (Yes vs. no 1) | 0.16 | 0.07–0.37 | <0.001 | 0.902 | 0.952 | Risk of disease progression |
| miR-19a-3p *** (Low vs. high levels 1) | 4.85 | 1.37–17.16 | 0.014 | ||||
| miR-20a-5p * (High vs. low levels 1) | 6.13 | 1.72–21.83 | 0.005 | ||||
| Integrative model A2 (52) | Primary treatment (Surgery vs. chemotherapy 1) | 0.33 | 0.17–0.64 | 0.001 | 0.671 | 0.898 | |
| miR-20a-5p * (High vs. low levels 1) | 2.17 | 1.14–4.15 | 0.019 | ||||
| Integrative model B1 (46) | Patients’ age at OC diagnosis (≥64 vs. <64 years 1) | 7.43 | 2.24–24.65 | 0.001 | 0.821 | 0.848 | Risk of death |
| Surgery (Yes vs. no 1) | 0.16 | 0.05–0.47 | <0.001 | ||||
| Platinum sensitivity (No vs. yes 1) | 4.90 | 1.96–12.24 | <0.001 | ||||
| miR-616-3p *** (High vs. low levels 1) | 3.72 | 1.23–11.21 | 0.020 | ||||
| Integrative model B2 (52) | Patients’ age at OC diagnosis (≥64 vs. <64 years 1) | 6.03 | 2.15–16.88 | <0.001 | 0.699 | 0.713 | |
| miR-23a-3p ** (High vs. low levels 1) | 2.68 | 1.06–6.77 | 0.037 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavares, V.; Savva-Bordalo, J.; Rei, M.; Liz-Pimenta, J.; Assis, J.; Pereira, D.; Medeiros, R. Plasma microRNA Environment Linked to Tissue Factor Pathway and Cancer-Associated Thrombosis: Prognostic Significance in Ovarian Cancer. Biomolecules 2024, 14, 928. https://doi.org/10.3390/biom14080928
Tavares V, Savva-Bordalo J, Rei M, Liz-Pimenta J, Assis J, Pereira D, Medeiros R. Plasma microRNA Environment Linked to Tissue Factor Pathway and Cancer-Associated Thrombosis: Prognostic Significance in Ovarian Cancer. Biomolecules. 2024; 14(8):928. https://doi.org/10.3390/biom14080928
Chicago/Turabian StyleTavares, Valéria, Joana Savva-Bordalo, Mariana Rei, Joana Liz-Pimenta, Joana Assis, Deolinda Pereira, and Rui Medeiros. 2024. "Plasma microRNA Environment Linked to Tissue Factor Pathway and Cancer-Associated Thrombosis: Prognostic Significance in Ovarian Cancer" Biomolecules 14, no. 8: 928. https://doi.org/10.3390/biom14080928
APA StyleTavares, V., Savva-Bordalo, J., Rei, M., Liz-Pimenta, J., Assis, J., Pereira, D., & Medeiros, R. (2024). Plasma microRNA Environment Linked to Tissue Factor Pathway and Cancer-Associated Thrombosis: Prognostic Significance in Ovarian Cancer. Biomolecules, 14(8), 928. https://doi.org/10.3390/biom14080928










