Molecular Mimicry between Toxoplasma gondii B-Cell Epitopes and Neurodevelopmental Proteins: An Immunoinformatic Approach
Abstract
1. Introduction
2. Materials and Methods
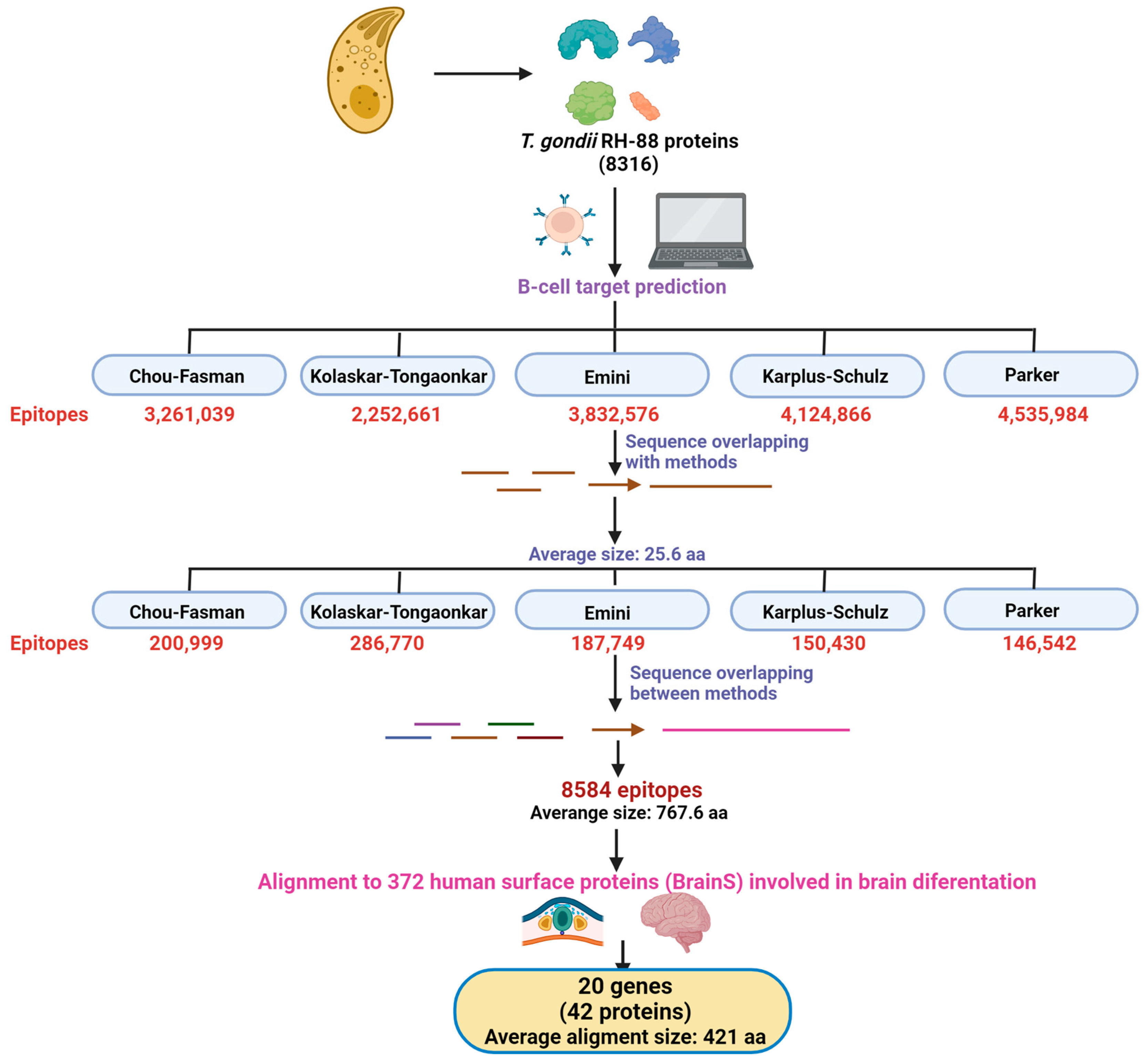
2.1. T. gondii Epitope Prediction
2.2. List of Human Surface Proteins Involved in Brain Development and Differentiation
2.3. Similarity between T. gondii Epitopes and Human Surface Proteins Involved in Brain Development
2.4. Similarity between T. gondii Epitopes and Human Surface Proteins Not Involved in Brain Development
2.5. Protein Network of Human Proteins with the Potential for Molecular Mimicry
2.6. Expression of Genes Identified as Potential Targets of Molecular Mimicry in Mouse Development
2.7. Prediction of Protein Topology
2.8. Overlapping of Protein Structures
3. Results
3.1. Prediction of T. gondii B-Cell Epitopes
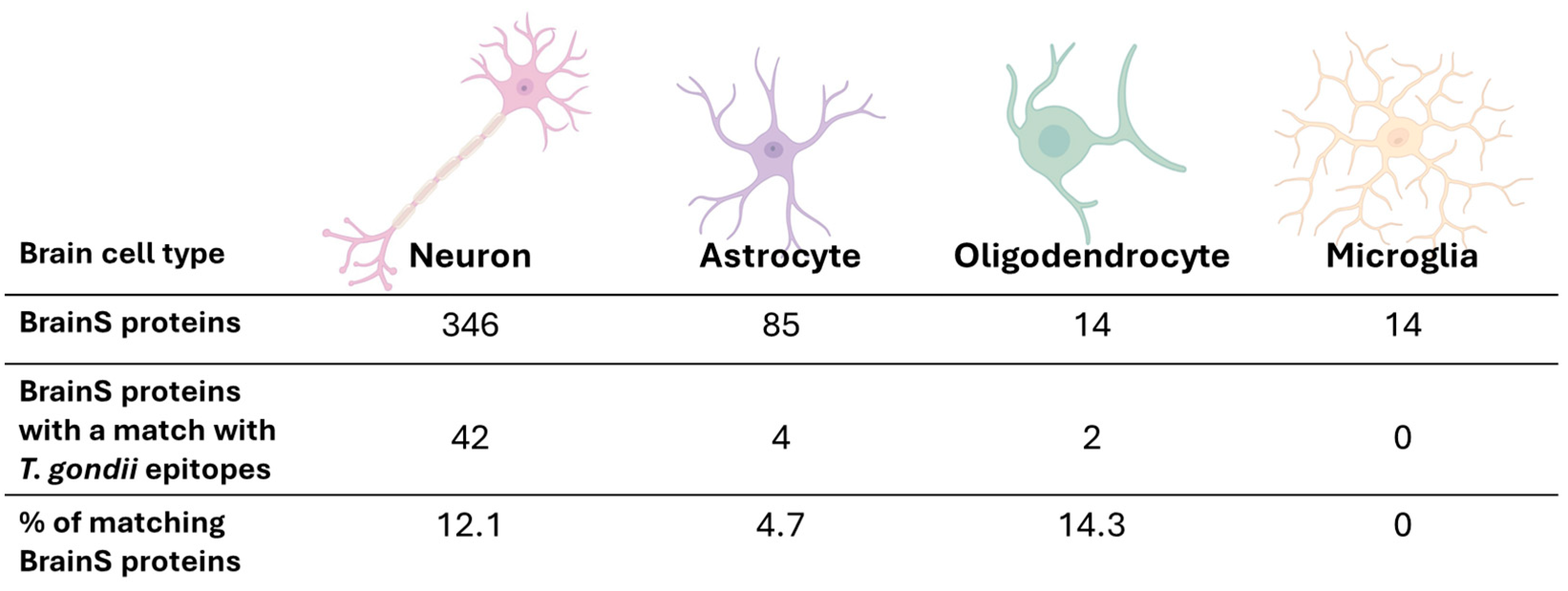
3.2. Prediction of Human Proteins with Potential for Molecular Mimicry by T. gondii B-Cell Epitopes
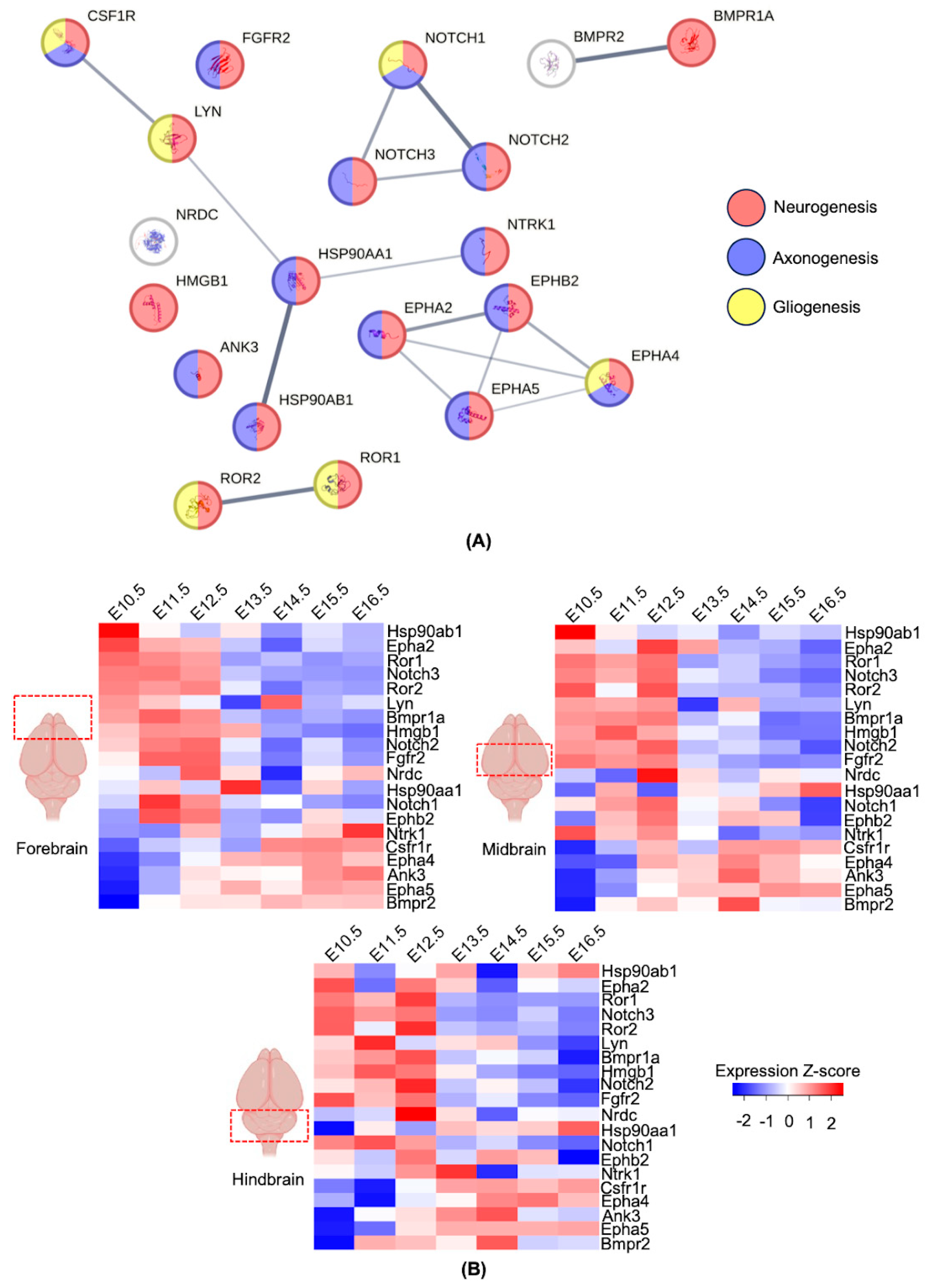
3.3. Network Prediction of Human Proteins with the Potential for Molecular Mimicry
3.4. Expression Profiles of the Genes with the Potential for Molecular Mimicry during Development
3.5. Structural Overlap in Extracellular Regions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tyebji, S.; Seizova, S.; Hannan, A.J.; Tonkin, C.J. Toxoplasmosis: A pathway to neuropsychiatric disorders. Neurosci. Biobehav. Rev. 2019, 96, 72–92. [Google Scholar] [CrossRef] [PubMed]
- Firouzeh, N.; Borj, H.F.; Ziaali, N.; Kareshk, A.T.; Ahmadinejad, M.; Shafiei, R. Genetic Diversity of Toxoplasma gondii by Serological and Molecular Analyzes in Different Sheep and Goat Tissues in Northeastern Iran. Iran. J. Parasitol. 2023, 18, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Chavez, F.; Canedo-Solares, I.; Ortiz-Alegria, L.B.; Flores-Garcia, Y.; Luna-Pasten, H.; Figueroa-Damian, R.; Mora-Gonzalez, J.C.; Correa, D. Maternal Immune Response During Pregnancy and Vertical Transmission in Human Toxoplasmosis. Front. Immunol. 2019, 10, 285. [Google Scholar] [CrossRef] [PubMed]
- Sheikhshoaee, S.; Taheri, F.; Esmaeilpour, K.; Firouzeh, N.; Fard, S.R.N. Aggravation of cognitive impairments in the valproic acid-induced animal model of autism in BALB/c mice infected with Toxoplasma gondii. Int. J. Dev. Neurosci. 2024, 84, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Estes, M.L.; McAllister, A.K. Maternal immune activation: Implications for neuropsychiatric disorders. Science 2016, 353, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Contopoulos-Ioannidis, D.G.; Gianniki, M.; Ai-Nhi Truong, A.; Montoya, J.G. Toxoplasmosis and Schizophrenia: A Systematic Review and Meta-Analysis of Prevalence and Associations and Future Directions. Psychiatr. Res. Clin. Pract. 2022, 4, 48–60. [Google Scholar] [CrossRef]
- Martinez, V.O.; de Mendonca Lima, F.W.; de Carvalho, C.F.; Menezes-Filho, J.A. Toxoplasma gondii infection and behavioral outcomes in humans: A systematic review. Parasitol. Res. 2018, 117, 3059–3065. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Esquivel, C.; Urbina-Alvarez, J.D.; Estrada-Martinez, S.; Torres-Castorena, A.; Molotla-de-Leon, G.; Liesenfeld, O.; Dubey, J.P. Toxoplasma gondii infection and schizophrenia: A case control study in a low Toxoplasma seroprevalence Mexican population. Parasitol. Int. 2011, 60, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Kezai, A.M.; Lecoeur, C.; Hot, D.; Bounechada, M.; Alouani, M.L.; Marion, S. Association between schizophrenia and Toxoplasma gondii infection in Algeria. Psychiatry Res. 2020, 291, 113293. [Google Scholar] [CrossRef]
- Oncu-Oner, T.; Can, S. Meta-analysis of the relationship between Toxoplasma gondii and schizophrenia. Ann. Parasitol. 2022, 68, 103–110. [Google Scholar]
- Freedman, D.; Bao, Y.; Shen, L.; Schaefer, C.A.; Brown, A.S. Maternal T. gondii, offspring bipolar disorder and neurocognition. Psychiatry Res. 2016, 243, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.S.; Schaefer, C.A.; Quesenberry, C.P., Jr.; Liu, L.; Babulas, V.P.; Susser, E.S. Maternal exposure to toxoplasmosis and risk of schizophrenia in adult offspring. Am. J. Psychiatry 2005, 162, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Cheslack-Postava, K.; Brown, A.S. Prenatal infection and schizophrenia: A decade of further progress. Schizophr. Res. 2022, 247, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Romero Nunez, E.; Blanco Ayala, T.; Vazquez Cervantes, G.I.; Roldan-Roldan, G.; Gonzalez Esquivel, D.F.; Muniz-Hernandez, S.; Salazar, A.; Mendez Armenta, M.; Gomez-Manzo, S.; Gonzalez-Conchillos, H.; et al. Pregestational Exposure to T. gondii Produces Maternal Antibodies That Recognize Fetal Brain Mimotopes and Induces Neurochemical and Behavioral Dysfunction in the Offspring. Cells 2022, 11, 3819. [Google Scholar] [CrossRef] [PubMed]
- McEwan, F.; Glazier, J.D.; Hager, R. The impact of maternal immune activation on embryonic brain development. Front. Neurosci. 2023, 17, 1146710. [Google Scholar] [CrossRef] [PubMed]
- Chou, P.Y.; Fasman, G.D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv. Enzymol. Relat. Areas Mol. Biol. 1978, 47, 45–148. [Google Scholar] [CrossRef]
- Emini, E.A.; Hughes, J.V.; Perlow, D.S.; Boger, J. Induction of hepatitis A virus-neutralizing antibody by a virus-specific synthetic peptide. J. Virol. 1985, 55, 836–839. [Google Scholar] [CrossRef] [PubMed]
- Karplus, P.A.; Schulz, G.E. Prediction of chain flexibility in proteins. Naturwissenschaften 1985, 72, 212–213. [Google Scholar] [CrossRef]
- Kolaskar, A.S.; Tongaonkar, P.C. A semi-empirical method for prediction of antigenic determinants on protein antigens. FEBS Lett. 1990, 276, 172–174. [Google Scholar] [CrossRef]
- Parker, J.M.; Guo, D.; Hodges, R.S. New hydrophilicity scale derived from high-performance liquid chromatography peptide retention data: Correlation of predicted surface residues with antigenicity and X-ray-derived accessible sites. Biochemistry 1986, 25, 5425–5432. [Google Scholar] [CrossRef]
- Jeppe, H.; Konstantinos, D.T.; Mads Damgaard, P.; José Juan Almagro, A.; Paolo, M.; Henrik, N.; Anders, K.; Ole, W. DeepTMHMM predicts alpha and beta transmembrane proteins using deep neural networks. bioRxiv, 2022. [Google Scholar] [CrossRef]
- Tsirigos, K.D.; Peters, C.; Shu, N.; Kall, L.; Elofsson, A. The TOPCONS web server for consensus prediction of membrane protein topology and signal peptides. Nucleic Acids Res. 2015, 43, W401–W407. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Zidek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef] [PubMed]
- Meng, E.C.; Goddard, T.D.; Pettersen, E.F.; Couch, G.S.; Pearson, Z.J.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Tools for structure building and analysis. Protein Sci. 2023, 32, e4792. [Google Scholar] [CrossRef] [PubMed]
- Akkouh, I.A.; Ueland, T.; Szabo, A.; Hughes, T.; Smeland, O.B.; Andreassen, O.A.; Osete, J.R.; Djurovic, S. Longitudinal Transcriptomic Analysis of Human Cortical Spheroids Identifies Axonal Dysregulation in the Prenatal Brain as a Mediator of Genetic Risk for Schizophrenia. Biol. Psychiatry 2024, 95, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Notaras, M.; Lodhi, A.; Fang, H.; Greening, D.; Colak, D. The proteomic architecture of schizophrenia iPSC-derived cerebral organoids reveals alterations in GWAS and neuronal development factors. Transl. Psychiatry 2021, 11, 541. [Google Scholar] [CrossRef] [PubMed]
- Rizig, M.A.; McQuillin, A.; Ng, A.; Robinson, M.; Harrison, A.; Zvelebil, M.; Hunt, S.P.; Gurling, H.M. A gene expression and systems pathway analysis of the effects of clozapine compared to haloperidol in the mouse brain implicates susceptibility genes for schizophrenia. J. Psychopharmacol. 2012, 26, 1218–1230. [Google Scholar] [CrossRef] [PubMed]
- Kloth, K.; Lozic, B.; Tagoe, J.; Hoffer, M.J.V.; Van der Ven, A.; Thiele, H.; Altmuller, J.; Kubisch, C.; Au, P.Y.B.; Denecke, J.; et al. ANK3 related neurodevelopmental disorders: Expanding the spectrum of heterozygous loss-of-function variants. Neurogenetics 2021, 22, 263–269. [Google Scholar] [CrossRef]
- Nelson, A.D.; Jenkins, P.M. Axonal Membranes and Their Domains: Assembly and Function of the Axon Initial Segment and Node of Ranvier. Front. Cell. Neurosci. 2017, 11, 136. [Google Scholar] [CrossRef]
- Bond, A.M.; Bhalala, O.G.; Kessler, J.A. The dynamic role of bone morphogenetic proteins in neural stem cell fate and maturation. Dev. Neurobiol. 2012, 72, 1068–1084. [Google Scholar] [CrossRef]
- Hu, B.; Duan, S.; Wang, Z.; Li, X.; Zhou, Y.; Zhang, X.; Zhang, Y.W.; Xu, H.; Zheng, H. Insights Into the Role of CSF1R in the Central Nervous System and Neurological Disorders. Front. Aging Neurosci. 2021, 13, 789834. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.; Soriano, E. Functions of ephrin/Eph interactions in the development of the nervous system: Emphasis on the hippocampal system. Brain Res. Brain Res. Rev. 2005, 49, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Szczurkowska, J.; Pischedda, F.; Pinto, B.; Manago, F.; Haas, C.A.; Summa, M.; Bertorelli, R.; Papaleo, F.; Schafer, M.K.; Piccoli, G.; et al. NEGR1 and FGFR2 cooperatively regulate cortical development and core behaviours related to autism disorders in mice. Brain 2018, 141, 2772–2794. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.; Schachner, M.; Shen, Y.Q. HMGB1 in development and diseases of the central nervous system. Mol. Neurobiol. 2012, 45, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.J.; Fort, P.E. Heat Shock Proteins Regulatory Role in Neurodevelopment. Front. Neurosci. 2018, 12, 821. [Google Scholar] [CrossRef] [PubMed]
- Hoter, A.; El-Sabban, M.E.; Naim, H.Y. The HSP90 Family: Structure, Regulation, Function, and Implications in Health and Disease. Int. J. Mol. Sci. 2018, 19, 2560. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Zhao, T.; Huang, X.; Liu, Z.H.; Zhao, H.; Li, M.M.; Wu, L.Y.; Shu, H.B.; Zhu, L.L.; Fan, M. Heat shock protein 90 is involved in regulation of hypoxia-driven proliferation of embryonic neural stem/progenitor cells. Cell Stress Chaperones 2009, 14, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Umemori, H.; Wanaka, A.; Kato, H.; Takeuchi, M.; Tohyama, M.; Yamamoto, T. Specific expressions of Fyn and Lyn, lymphocyte antigen receptor-associated tyrosine kinases, in the central nervous system. Brain Res. Mol. Brain Res. 1992, 16, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, K.; Watanabe, Y.; Yamamoto, T.; Sanai, Y. Association of Src family tyrosine kinase Lyn with ganglioside GD3 in rat brain. Possible regulation of Lyn by glycosphingolipid in caveolae-like domains. J. Biol. Chem. 1997, 272, 29947–29953. [Google Scholar] [CrossRef]
- Lathia, J.D.; Mattson, M.P.; Cheng, A. Notch: From neural development to neurological disorders. J. Neurochem. 2008, 107, 1471–1481. [Google Scholar] [CrossRef]
- Ohno, M.; Hiraoka, Y.; Matsuoka, T.; Tomimoto, H.; Takao, K.; Miyakawa, T.; Oshima, N.; Kiyonari, H.; Kimura, T.; Kita, T.; et al. Nardilysin regulates axonal maturation and myelination in the central and peripheral nervous system. Nat. Neurosci. 2009, 12, 1506–1513. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Xu, Y.C.; Hu, H.Y.; Li, Y.Z.; Li, Q.; Luan, Y.Y.; Liu, Y.; Sun, Y.Q.; Feng, Z.K.; Yan, Y.S.; et al. Investigation of a Novel NTRK1 Variation Causing Congenital Insensitivity to Pain With Anhidrosis. Front. Genet. 2021, 12, 763467. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.; Doi, R.; Nishita, M.; Minami, Y. Ror family receptor tyrosine kinases regulate the maintenance of neural progenitor cells in the developing neocortex. J. Cell Sci. 2012, 125, 2017–2029. [Google Scholar] [CrossRef] [PubMed]
- Lima, T.S.; Lodoen, M.B. Mechanisms of Human Innate Immune Evasion by Toxoplasma gondii. Front. Cell. Infect. Microbiol. 2019, 9, 103. [Google Scholar] [CrossRef]
- Montoya, J.G.; Liesenfeld, O. Toxoplasmosis. Lancet 2004, 363, 1965–1976. [Google Scholar] [CrossRef] [PubMed]
- Flegr, J. How and why Toxoplasma makes us crazy. Trends Parasitol. 2013, 29, 156–163. [Google Scholar] [CrossRef]
- Pearce, B.D.; Hubbard, S.; Rivera, H.N.; Wilkins, P.P.; Fisch, M.C.; Hopkins, M.H.; Hasenkamp, W.; Gross, R.; Bliwise, N.; Jones, J.L.; et al. Toxoplasma gondii exposure affects neural processing speed as measured by acoustic startle latency in schizophrenia and controls. Schizophr. Res. 2013, 150, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Sutterland, A.L.; Fond, G.; Kuin, A.; Koeter, M.W.; Lutter, R.; van Gool, T.; Yolken, R.; Szoke, A.; Leboyer, M.; de Haan, L. Beyond the association. Toxoplasma gondii in schizophrenia, bipolar disorder, and addiction: Systematic review and meta-analysis. Acta Psychiatr. Scand. 2015, 132, 161–179. [Google Scholar] [CrossRef]
- de Barros, J.; Barbosa, I.G.; Salem, H.; Rocha, N.P.; Kummer, A.; Okusaga, O.O.; Soares, J.C.; Teixeira, A.L. Is there any association between Toxoplasma gondii infection and bipolar disorder? A systematic review and meta-analysis. J. Affect. Disord. 2017, 209, 59–65. [Google Scholar] [CrossRef]
- Milne, G.; Webster, J.P.; Walker, M. Toxoplasma gondii: AnUnderestimated Threat? Trends Parasitol. 2020, 36, 959–969. [Google Scholar] [CrossRef]
- Mortensen, P.B.; Norgaard-Pedersen, B.; Waltoft, B.L.; Sorensen, T.L.; Hougaard, D.; Torrey, E.F.; Yolken, R.H. Toxoplasma gondii as a risk factor for early-onset schizophrenia: Analysis of filter paper blood samples obtained at birth. Biol. Psychiatry 2007, 61, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Goldberg-Murow, M.; Cedillo-Pelaez, C.; Concha-Del-Rio, L.E.; Cheja-Kalb, R.; Salgar-Henao, M.J.; Orozco-Velasco, E.; Luna-Pasten, H.; Gomez-Chavez, F.; Ibarra, A.; Correa, D. Autoantibodies against Ubiquitous and Confined Antigens in Patients with Ocular, Neuro-Ophthalmic and Congenital Cerebral Toxoplasmosis. Front. Immunol. 2021, 12, 606963. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, M.J.; Mysiak, K.S.; Becker, T.; Becker, C.G. Reduce, reuse, recycle-Developmental signals in spinal cord regeneration. Dev. Biol. 2017, 432, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Nagao, M.; Sugimori, M.; Kosako, H.; Nakatomi, H.; Yamamoto, N.; Takebayashi, H.; Nabeshima, Y.; Kitamura, T.; Weinmaster, G.; et al. Transcription factor expression and Notch-dependent regulation of neural progenitors in the adult rat spinal cord. J. Neurosci. 2001, 21, 9814–9823. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Jiang, M.M.; Weinmaster, G.; Jan, L.Y.; Jan, Y.N. Differential expression of mammalian Numb, Numblike and Notch1 suggests distinct roles during mouse cortical neurogenesis. Development 1997, 124, 1887–1897. [Google Scholar] [CrossRef]
- Hansen, D.V.; Lui, J.H.; Parker, P.R.; Kriegstein, A.R. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature 2010, 464, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Lui, J.H.; Hansen, D.V.; Kriegstein, A.R. Development and evolution of the human neocortex. Cell 2011, 146, 18–36. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.K.; Yeager, K.; Morrison, S.J. Physiological Notch signaling promotes gliogenesis in the developing peripheral and central nervous systems. Development 2007, 134, 2435–2447. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Engler, A.; Taylor, V. Notch: An interactive player in neurogenesis and disease. Cell Tissue Res. 2018, 371, 73–89. [Google Scholar] [CrossRef]
- Wang, A.W.; Avramopoulos, D.; Lori, A.; Mulle, J.; Conneely, K.; Powers, A.; Duncan, E.; Almli, L.; Massa, N.; McGrath, J.; et al. Genome-wide association study in two populations to determine genetic variants associated with Toxoplasma gondii infection and relationship to schizophrenia risk. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 92, 133–147. [Google Scholar] [CrossRef]
- Feldheim, D.A.; Nakamoto, M.; Osterfield, M.; Gale, N.W.; DeChiara, T.M.; Rohatgi, R.; Yancopoulos, G.D.; Flanagan, J.G. Loss-of-function analysis of EphA receptors in retinotectal mapping. J. Neurosci. 2004, 24, 2542–2550. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Galvao, J.; Beach, K.M.; Luo, W.; Urrutia, R.A.; Goldberg, J.L.; Otteson, D.C. Novel Roles and Mechanism for Kruppel-like Factor 16 (KLF16) Regulation of Neurite Outgrowth and Ephrin Receptor A5 (EphA5) Expression in Retinal Ganglion Cells. J. Biol. Chem. 2016, 291, 18084–18095. [Google Scholar] [CrossRef] [PubMed]
- St John, J.A.; Key, B. EphB2 and two of its ligands have dynamic protein expression patterns in the developing olfactory system. Brain Res. Dev. Brain Res. 2001, 126, 43–56. [Google Scholar] [CrossRef]
- Quinta, H.R.; Galigniana, M.D. The neuroregenerative mechanism mediated by the Hsp90-binding immunophilin FKBP52 resembles the early steps of neuronal differentiation. Br. J. Pharmacol. 2012, 166, 637–649. [Google Scholar] [CrossRef] [PubMed]


| Alignment Positions | Extracellular Region Positions | |||||
|---|---|---|---|---|---|---|
| Protein Name | Start (aa) | End (aa) | Alignment Length | e-Value | Start (aa) | End (aa) |
| ANK3 | 363 | 527 | 168 | 9.09 × 10−16 | φ | φ |
| BMPR1A | 240 | 529 | 321 | 6.20 × 10−11 | 24 | 153 |
| BMPR2 | 206 | 534 | 354 | 3.78 × 10−7 | 27 | 149 |
| CSF1R | 704 | 908 | 213 | 3.48 × 10−9 | 22 | 515 |
| EPHA2 | 615 | 870 | 264 | 3.01 × 10−17 | 24 | 535 |
| EPHA4 | 622 | 876 | 263 | 6.30 × 10−15 | 22 | 547 |
| EPHA5 | 694 | 971 | 327 | 3.75 × 10−13 | 22 | 573 |
| EPHB2 | 625 | 877 | 261 | 6.40 × 10−17 | 22 | 544 |
| FGFR2 | 399 | 666 | 272 | 3.64 × 10−14 | 23 | 377 |
| HMGB1 | 83 | 165 | 85 | 2.70 × 10−12 | φ | φ |
| HSP90AA1 | 18 | 703 | 721 | 0.0 | φ | φ |
| HSP90AB1 | 17 | 717 | 739 | 0.0 | φ | φ |
| LYN | 248 | 508 | 284 | 2.65 × 10−8 | φ | φ |
| NOTCH1 receptor | 707 | 944 | 283 | 1.28 × 10−4 | 22 | 1735 |
| NOTCH2 receptor | 651 | 922 | 281 | 6.31 × 10−8 | 26 | 1677 |
| NOTCH3 receptor | 1079 | 1373 | 295 | 7.30 × 10−7 | 22 | 1643 |
| NRDC | 205 | 1089 | 916 | 2.52 × 10−65 | φ | φ |
| NTRK1 | 509 | 777 | 273 | 6.65 × 10−11 | 31 | 417 |
| ROR1 | 476 | 736 | 262 | 9.72 × 10−15 | 30 | 406 |
| ROR2 | 476 | 738 | 264 | 1.59 × 10−20 | 31 | 405 |
| Candidate | Function | References |
|---|---|---|
| ANK3 | Ankyrin-3 (ANK3) regulates dendrites morphology and N-methyl-D-aspartate (NMDA) receptor trafficking. ANK3 participates in the formation and maintenance of the axon initial segment (AIS) and the nodes of Ranvier. Some mutant variants of the ANK3 gene have been associated with neurological disorders such as schizophrenia and autism. | [29,30] |
| BMPR1A/BMPR2 | Bone morphogenetic protein receptor type 1A (BMPR1A) and type 2 (BMPR2) are receptors for the bone morphogenetic proteins (BMPs). The BMP signaling pathway induces the formation of the dorso-ventral axis of the developing spinal cord and brain, neurogenesis, and later astrogliogenesis, and it participates in neurite outgrowth from immature neurons. Inhibition of BMP signaling during early development promotes neuroectoderm-from-ectoderm differentiation. | [31] |
| CSF1R | Colony-stimulating factor 1 receptor (CSF1R) is the receptor for the colony-stimulating factor 1 (CSF1) cytokine, which controls the generation, differentiation, and function of macrophages. In the central nervous system (CNS), CSF1 is a major regulator of microglial development and maintenance. Meanwhile, CSF1R is expressed in neural progenitor cells (NPCs). Mice lacking CSF1R exhibit global defects in brain development, including atrophy of the olfactory bulb, expansion of the lateral ventricle, thinning of the neocortex, and functional abnormalities of the sensory nervous system. | [32] |
| EPHA2-5/EPHB2 | The erythropoietin-producing hepatocellular (EPH) family of tyrosine kinases receptors (RTKs) includes several members. EPH-B2 is expressed in hippocampal astrocytes, and it regulates neurogenesis and promotes neuronal differentiation of neural stem cells (NSCs). These receptors regulate cell migration by promoting changes in cellular adhesion to the extracellular matrix. They are also involved in the remodeling of efferent axons to ensure the correct and precise innervations to their target cells, in dendritic spine morphology, in synaptogenesis, and in synapse stabilization. | [33] |
| FGFR2 | Fibroblast growth factor receptor 2 (FGFR2) controls neuronal morphological maturation, neuronal migration, and spine density during cortical development by interacting with the neuronal growth regulator 1 (NEGR1) protein. | [34] |
| HHMGB1 | High mobility group box 1 (HHGB1) is required for the differentiation and proliferation of NSCs and NPCs. It also promotes neurite outgrowth during early forebrain development. | [35] |
| HSP90AA1 (HSP90α) | Heat shock protein 90 alpha family class A member 1 (HSP90AA1) is induced by stress and can be expressed in the brain, retina, and spinal cord. It is highly expressed between the G0 and G1 cell cycle phases during embryonic neurodevelopment which correlate with neuronal differentiation and polarization. It also promotes neurite growth. | [36,37] |
| HSP90AB1 (HSP90β) | Heat shock protein 90 alpha family class B member 1 (HSP90AB1) is highly and constitutively expressed in the brain, the retina, and the spinal cord between the G0 and the G1 phases of the cell cycle during embryonic neurodevelopment. It stabilizes and controls the activity of hypoxia inducible factor-1 (HIF-1) to promote NPC proliferation. | [38] |
| LYN | Lyn expression is relatively low in the brains of embryonic and neonatal mice. However, at the protein level, LYN has been suggested to participate in the formation and function of synapses between granule and Purkinje cells in the rat cerebellum, resulting in motor learning. | [39,40] |
| NOTCH1-3 receptor | The NOTCH receptor signaling pathway keeps NSCs in a proliferative state by promoting their survival and self-renewal. This pathway also has a role in defining cell fate (neuronal or glial). Briefly, it induces the expression of HES1 and HES5, which repress the expression of proneural genes and therefore inhibit neural differentiation. | [41] |
| NRDC | Nardilysin convertase (NRDC) is a regulator of axonal maturation and myelination of both the CNS and the peripherical nervous system (PNS). Axon diameter and myelin thickness correlate with NRDC expression levels. It also participates in the proteolysis of NGR1, which is a regulator of myelination. | [42] |
| NTRK1 | Neurotrophic receptor tyrosine kinase type 1 (NTRK1), also known as TRKA, belongs to a family of nerve growth-factor receptors whose ligands include neurotrophins, like the nerve growth factor (NGF), which is involved in the regulation of the development of central and peripheral neurons. | [43] |
| ROR1-2 | Receptor tyrosine kinase-like orphan receptors (RORs) 1 and 2 are expressed exclusively in the developing nervous system, including in the mouse neocortex. It also regulates neurite extension and synapse formation in hippocampal neurons. The ROR1-WNT5a and ROR2-WNT5a signaling pathways regulate the maintenance of the proliferative and neurogenic states of NPCs. | [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meza-Sosa, K.F.; Valle-Garcia, D.; González-Conchillos, H.; Blanco-Ayala, T.; Salazar, A.; Flores, I.; Gómez-Manzo, S.; González Esquivel, D.F.; Pérez de la Cruz, G.; Pineda, B.; et al. Molecular Mimicry between Toxoplasma gondii B-Cell Epitopes and Neurodevelopmental Proteins: An Immunoinformatic Approach. Biomolecules 2024, 14, 933. https://doi.org/10.3390/biom14080933
Meza-Sosa KF, Valle-Garcia D, González-Conchillos H, Blanco-Ayala T, Salazar A, Flores I, Gómez-Manzo S, González Esquivel DF, Pérez de la Cruz G, Pineda B, et al. Molecular Mimicry between Toxoplasma gondii B-Cell Epitopes and Neurodevelopmental Proteins: An Immunoinformatic Approach. Biomolecules. 2024; 14(8):933. https://doi.org/10.3390/biom14080933
Chicago/Turabian StyleMeza-Sosa, Karla F., David Valle-Garcia, Hugo González-Conchillos, Tonali Blanco-Ayala, Alelí Salazar, Itamar Flores, Saúl Gómez-Manzo, Dinora Fabiola González Esquivel, Gonzalo Pérez de la Cruz, Benjamín Pineda, and et al. 2024. "Molecular Mimicry between Toxoplasma gondii B-Cell Epitopes and Neurodevelopmental Proteins: An Immunoinformatic Approach" Biomolecules 14, no. 8: 933. https://doi.org/10.3390/biom14080933
APA StyleMeza-Sosa, K. F., Valle-Garcia, D., González-Conchillos, H., Blanco-Ayala, T., Salazar, A., Flores, I., Gómez-Manzo, S., González Esquivel, D. F., Pérez de la Cruz, G., Pineda, B., & Pérez de la Cruz, V. (2024). Molecular Mimicry between Toxoplasma gondii B-Cell Epitopes and Neurodevelopmental Proteins: An Immunoinformatic Approach. Biomolecules, 14(8), 933. https://doi.org/10.3390/biom14080933








