Bioactive Peptides in Dairy Milk: Highlighting the Role of Melatonin
Abstract
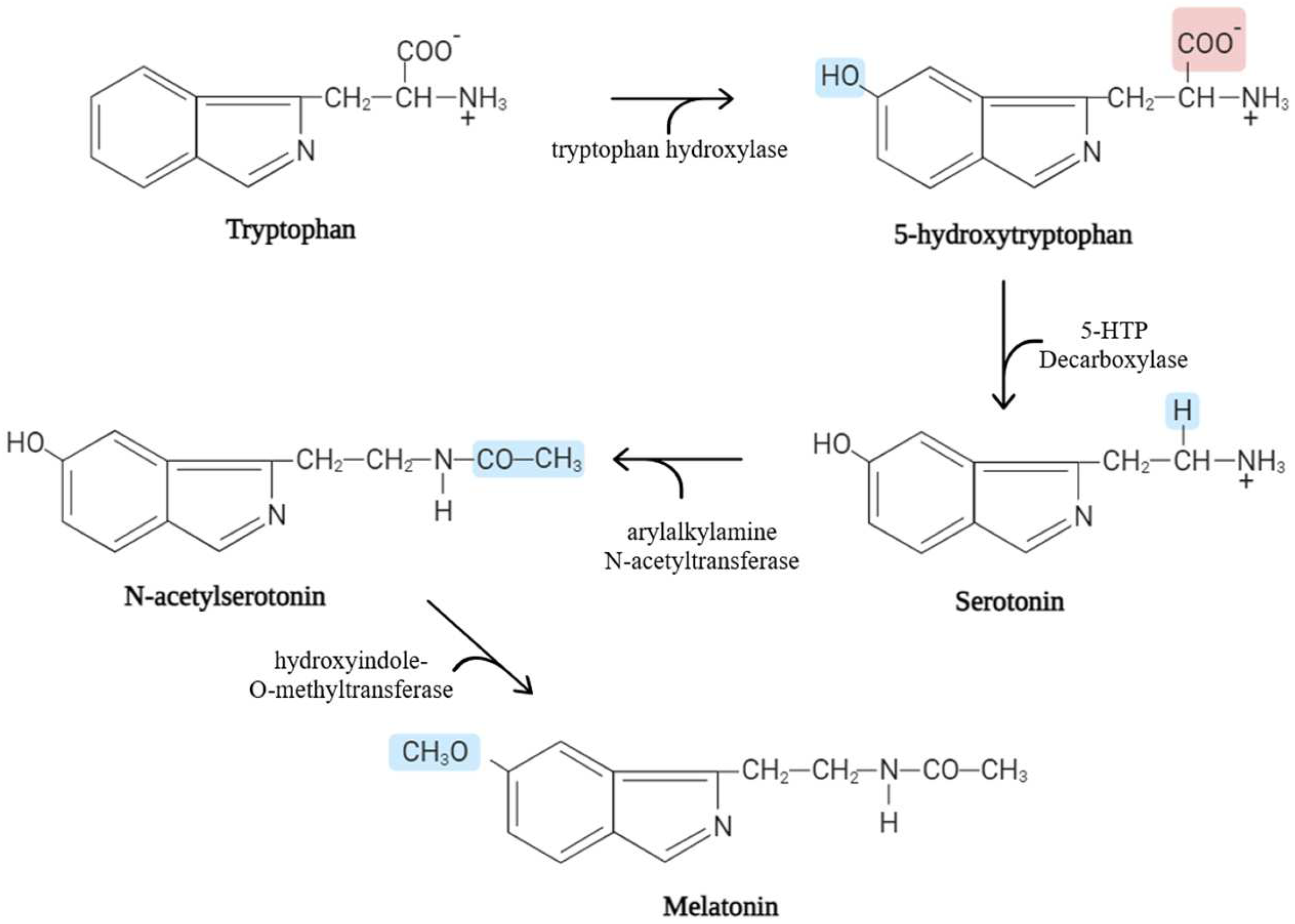
1. Biological Characteristics
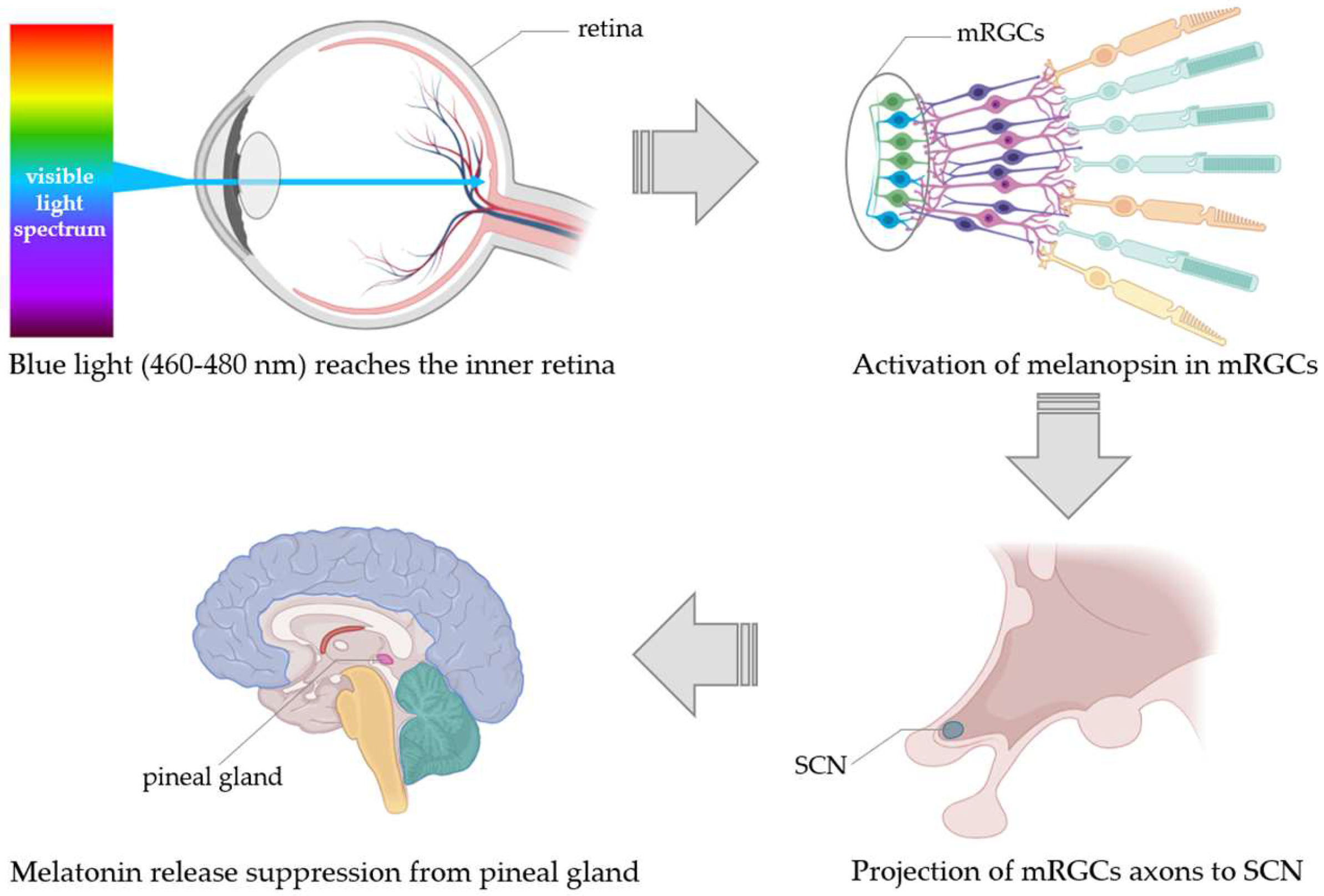
Regulation of Melatonin Release
2. Roles and Mechanisms of Action
2.1. Receptor-Mediated Action
2.2. Non-Receptor-Mediated Actions
3. Melatonin in Bovine Milk
| Breed | Lactation Stage | Milk | Technique for Melatonin Detection | Melatonin (pg/mL) | Lighting (h) | Place | References | ||
|---|---|---|---|---|---|---|---|---|---|
| Animals (n°) | Days after Calving | kg/day | Night Milk | Day Milk | Light | Dark | Maps Coordinate | ||
| Holstein (30) | / | 23 | ELISA KIT RE54041 (IBL International, Hamburg, Germany) | 14.9 ± 7.7 | 6.9 ± 3.1 | 10.30 winter 13.30 summer | Cycle of 2 h light on, 5 h light off, 5 h light on | Castro, Brazil (24°49′06.7″ S 50°00′54.1″ W) | [62] |
| Holstein (10) | 100–150 | 25 | ELISA KIT RE54041 (IBL International, Hamburg, Germany) | 39.4 | 4.0 | / | / | Viçosa, Brazil (37°52′21.0″ N 32°28′28.1″ E) | [52] |
| Holstein (40) | / | / | ELISA KIT MBS743340 (MyBiosource, California, USA) | 163.1 ± 8.9 | 103.7 ± 6.6 | / | 1-week total darkness | Konya, Turkey (37°52′18.5″ N 32°29′32.5″ E) | [54] |
| Holstein (10) | 150 ± 20 | 25 ± 5 | UHPLC | 120.1 | 90.2 | 13 (August) | 11 (August) | China (34°48′08.5″ N 113°41′13.1″ E) | [53] |
| Holstein (28) | 135 | 34.5 | ELISA KIT RE54041 (IBL International, Hamburg, Germany) | 30.7 ± 1.8 (ND) 17.8 ± 0.3 (NI) | 5.4 ± 0.3 (ND) 3.3 ± 0.2 (NI) | 10.40 (November) | 13.60 (November) | Israel (32°42′24.0”N 35°10′46.6″ E) | [56] |
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tan, D.X.; Zheng, X.; Kong, J.; Manchester, L.C.; Hardeland, R.; Kim, S.J.; Xu, X.; Reiter, R.J. Fundamental issues related to the origin of melatonin and melatonin isomers during evolution: Relation to their biological functions. Int. J. Mol. Sci. 2014, 15, 15858–15890. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Ding, D.; Bai, D.; Zhu, Y.; Sun, W.; Sun, Y.; Zhang, D. Melatonin biosynthesis pathways in nature and its production in engineered microorganisms. Synth. Syst. Biotechnol. 2022, 7, 544–553. [Google Scholar] [CrossRef]
- Hwang, O.J.; Back, K. Functional Characterization of Arylalkylamine N-Acetyltransferase, a Pivotal Gene in Antioxidant Melatonin Biosynthesis from Chlamydomonas reinhardtii. Antioxidants 2022, 11, 1531. [Google Scholar] [CrossRef] [PubMed]
- Aykan, U.; Güvel, M.C.; Paykal, G.; Uluoglu, C. Neuropharmacologic modulation of the melatonergic system. Explor. Neurosci. 2023, 2, 287–306. [Google Scholar] [CrossRef]
- Von Gall, C. The Effects of Light and the Circadian System on Rhythmic Brain Function. Int. J. Mol. Sci. 2022, 23, 2778. [Google Scholar] [CrossRef]
- Milanick, W.J.; Polo-Parada, L.; Dantzler, H.A.; Kline, D.D. Activation of alpha-1 adrenergic receptors increases cytosolic calcium in neurones of the paraventricular nucleus of the hypothalamus. J. Neuroendocrinol. 2019, 31, e12791. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Reiter, R.J.; Zimmerman, S.; Hardeland, R. Melatonin: Both a Messenger of Darkness and a Participant in the Cellular Actions of Non-Visible Solar Radiation of Near Infrared Light. Biology 2023, 12, 89. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Xu, B.; Zhou, X.; Reiter, R.J. Pineal Calcification, Melatonin Production, Aging, Associated Health Consequences and Rejuvenation of the Pineal Gland. Molecules 2018, 23, 301. [Google Scholar] [CrossRef] [PubMed]
- Peruri, A.; Morgan, A.; D’Souza, A.; Mellon, B.; Hung, C.W.; Kayal, G.; Shin, H.; Nguyen, K.; Zahed, M.; Yount, M.; et al. Pineal Gland from the Cell Culture to Animal Models: A Review. Life 2022, 12, 1057. [Google Scholar] [CrossRef]
- Masters, A.; Pandi-Perumal, S.R.; Seixas, A.; Girardin, J.L.; McFarlane, S.I. Melatonin, the Hormone of Darkness: From Sleep Promotion to Ebola Treatment. Brain Disord. Ther. 2014, 4, 1000151. [Google Scholar]
- Amaral, F.G.; Cipolla-Neto, J. A brief review about melatonin, a pineal hormone. Arch. Endocrinol. Metab. 2018, 62, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Mannino, G.; Pernici, C.; Serio, G.; Gentile, C.; Bertea, C.M. Melatonin and Phytomelatonin: Chemistry, Biosynthesis, Metabolism, Distribution and Bioactivity in Plants and Animals-An Overview. Int. J. Mol. Sci. 2021, 22, 9996. [Google Scholar] [CrossRef]
- Berthelot, X.; Laurentie, M.; Ravault, J.P.; Ferney, J.; Toutain, P.L. Circadian profile and production rate of melatonin in the cow. Domest. Anim. Endocrinol. 1990, 7, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Skene, D.J.; Papagiannidou, E.; Hashemi, E.; Snelling, J.; Lewis, D.F.; Fernandez, M.; Ioannides, C. Contribution of CYP1A2 in the hepatic metabolism of melatonin: Studies with isolated microsomal preparations and liver slices. J. Pineal Res. 2001, 31, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Cipolla-Neto, J.; Amaral, F.G.D. Melatonin as a Hormone: New Physiological and Clinical Insights. Endocr. Rev. 2018, 39, 990–1028. [Google Scholar] [CrossRef] [PubMed]
- Hastings, M.H.; Maywood, E.S.; Reddy, A.B. Two decades of circadian time. J. Neuroendocrinol. 2008, 20, 812–819. [Google Scholar] [CrossRef]
- Jasser, S.A.; Blask, D.E.; Brainard, G.C. Light during darkness and cancer: Relationships in circadian photoreception and tumor biology. Cancer Causes Control 2006, 17, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Brainard, G.C.; Sliney, D.; Hanifin, J.P.; Glickman, G.; Byrne, B.; Greeson, J.M.; Jasser, S.; Gerner, E.; Rollag, M.D. Sensitivity of the human circadian system to short-wavelength (420-nm) light. J. Biol. Rhythms 2008, 23, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Kumbalasiri, T.; Provencio, I. Melanopsin and other novel mammalian opsins. Exp. Eye Res. 2005, 81, 368–375. [Google Scholar] [CrossRef]
- Hankins, M.W.; Lucas, R.J. The primary visual pathway in humans is regulated according to long-term light exposure through the action of a nonclassical photopigment. Curr. Biol. 2002, 12, 191–198. [Google Scholar] [CrossRef]
- La Morgia, C.; Carelli, V.; Carbonelli, M. Melanopsin Retinal Ganglion Cells and Pupil: Clinical Implications for Neuro-Ophthalmology. Front. Neurol. 2018, 9, 1047. [Google Scholar] [CrossRef] [PubMed]
- Pilorz, V.; Tam, S.K.; Hughes, S.; Pothecary, C.A.; Jagannath, A.; Hankins, M.W.; Bannerman, D.M.; Lightman, S.L.; Vyazovskiy, V.V.; Nolan, P.M.; et al. Melanopsin Regulates Both Sleep-Promoting and Arousal-Promoting Responses to Light. PLoS Biol. 2016, 14, e1002482. [Google Scholar] [CrossRef] [PubMed]
- Do, M.T.H. Melanopsin and the Intrinsically Photosensitive Retinal Ganglion Cells: Biophysics to Behavior. Neuron 2019, 104, 205–226. [Google Scholar] [CrossRef] [PubMed]
- Münch, M.; Léon, L.; Crippa, S.V.; Kawasaki, A. Circadian and wake-dependent effects on the pupil light reflex in response to narrow-bandwidth light pulses. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4546–4555. [Google Scholar] [CrossRef] [PubMed]
- Skocbat, T.; Haimov, I.; Lavie, P. Melatonin—The key to the gate of sleep. Ann. Med. 1998, 30, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Wahl, S.; Engelhardt, M.; Schaupp, P.; Lappe, C.; Ivanov, I.V. The inner clock-Blue light sets the human rhythm. J. Biophotonics 2019, 12, 201900102. [Google Scholar] [CrossRef] [PubMed]
- Zisapel, N. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br. J. Pharmacol. 2018, 175, 3190–3199. [Google Scholar] [CrossRef]
- Ciani, E.; Haug, T.M.; Maugars, G.; Weltzien, F.A.; Falcón, J.; Fontaine, R. Effects of Melatonin on Anterior Pituitary Plasticity: A Comparison Between Mammals and Teleosts. Front. Endocrinol. 2021, 11, 605111. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Korkmaz, A. The circadian melatonin rhythm and its modulation: Possible impact on hypertension. J. Hypertens. Suppl. 2009, 27, 17–20. [Google Scholar] [CrossRef]
- Boulanger, V.; Zhao, X.; Lacasse, P. Protective effect of melatonin and catalase in bovine neutrophil-induced model of mammary cell damage. J. Dairy Sci. 2002, 85, 562–569. [Google Scholar] [CrossRef]
- Yu, G.M.; Kubota, H.; Okita, M.; Maeda, T. The anti-inflammatory and antioxidant effects of melatonin on LPS-stimulated bovine mammary epithelial cells. PLoS ONE 2017, 12, e0178525. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Shi, J.; Tian, J.; Tao, J.; Chai, M.; Wang, J.; Xu, Z.; Song, Y.; Zhu, K.; Ji, P.; et al. Exogenous melatonin reduces somatic cell count of milk in Holstein cows. Sci. Rep. 2017, 7, 43280. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Gut microbiota-derived metabolites mediate the neuroprotective effect of melatonin in cognitive impairment induced by sleep deprivation. Microbiome 2023, 11, 17. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Fuentes-Broto, L. Melatonin: A multitasking molecule. Prog. Brain Res. 2010, 181, 127–151. [Google Scholar] [PubMed]
- Li, Y.; Zhang, J.; Wan, J.; Liu, A.; Sun, J. Melatonin regulates Aβ production/clearance balance and Aβ neurotoxicity: A potential therapeutic molecule for Alzheimer’s disease. Biomed. Pharmacother. 2020, 132, 110887. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Manchester, L.C.; Pilar Terron, M.; Flores, L.J.; Koppisepi, S. Medical implications of melatonin: Receptor-mediated and receptor-independent actions. Adv. Med. Sci. 2007, 52, 11–28. [Google Scholar] [PubMed]
- Kopustinskiene, D.M.; Bernatoniene, J. Molecular Mechanisms of Melatonin-Mediated Cell Protection and Signaling in Health and Disease. Pharmaceutics 2021, 13, 129. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Clough, S.J.; Hutchinson, A.J.; Adamah-Biassi, E.B.; Popovska-Gorevski, M.; Dubocovich, M.L. MT1 and MT2 Melatonin Receptors: A Therapeutic Perspective. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 361–383. [Google Scholar] [CrossRef] [PubMed]
- Dubocovich, M.L.; Hudson, R.L.; Sumaya, I.C.; Masana, M.I.; Manna, E. Effect of MT1 melatonin receptor deletion on melatonin-mediated phase shift of circadian rhythms in the C57BL/6 mouse. J. Pineal Res. 2005, 39, 113–120. [Google Scholar] [CrossRef]
- Nikolaev, G.; Robeva, R.; Konakchieva, R. Membrane Melatonin Receptors Activated Cell Signaling in Physiology and Disease. Int. J. Mol. Sci. 2021, 23, 471. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, S.; Zhang, Y.; Zhang, Q. Melatonin Receptors: A Key Mediator in Animal Reproduction. Vet. Sci. 2022, 9, 309. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Tamura, H.; Reiter, R.J. Melatonin as a naturally occurring co-substrate of quinone reductase-2, the putative MT3 melatonin membrane receptor: Hypothesis and significance. J. Pineal Res. 2007, 43, 317–320. [Google Scholar] [CrossRef]
- Xu, L.; Su, Y.; Zhao, Y.; Sheng, X.; Tong, R.; Ying, X.; Gao, L.; Ji, Q.; Gao, Y.; Yan, Y.; et al. Melatonin differentially regulates pathological and physiological cardiac hypertrophy: Crucial role of circadian nuclear receptor RORα signaling. J. Pineal Res. 2019, 67, 12579. [Google Scholar] [CrossRef]
- Carrillo-Vico, A.; Lardone, P.J.; Alvarez-Sánchez, N.; Rodríguez-Rodríguez, A.; Guerrero, J.M. Melatonin: Buffering the immune system. Int. J. Mol. Sci. 2013, 14, 8638–8683. [Google Scholar] [CrossRef] [PubMed]
- Won, E.; Na, K.S.; Kim, Y.K. Associations between Melatonin, Neuroinflammation, and Brain Alterations in Depression. Int. J. Mol. Sci. 2022, 23, 305. [Google Scholar] [CrossRef] [PubMed]
- Bonmatí-Carrión, M.Á.; Rol, M.A. Melatonin as a Mediator of the Gut Microbiota–Host Interaction: Implications for Health and Disease. Antioxidants 2024, 13, 34. [Google Scholar] [CrossRef]
- Eriksson, L.; Valtonen, M.; Laitinen, J.T.; Paananen, M.; Kaikkonen, M. Diurnal rhythm of melatonin in bovine milk: Pharmacokinetics of exogenous melatonin in lactating cows and goats. Acta Vet. Scand. 1998, 39, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, A.; Andrés, S.; Sanchez, J. Effect of melatonin implants on somatic cell counts in dairy goats. Small Rum. Res. 2009, 84, 116–120. [Google Scholar] [CrossRef]
- Hattar, S.; Liao, H.W.; Takao, M.; Berson, D.M.; Yau, K.W. Melanopsin-containing retinal ganglion cells: Architecture, projections, and intrinsic photosensitivity. Science 2002, 295, 1065–1070. [Google Scholar] [CrossRef]
- Kollmann, M.T.; Locher, M.; Hirche, F.; Eder, K.; Meyer, H.H.; Bruckmaier, R.M. Effects of tryptophan supplementation on plasma tryptophan and related hormone levels in heifers and dairy cows. Domest. Anim. Endocrinol. 2008, 34, 14–24. [Google Scholar] [CrossRef]
- Dahl, G.E.; Tao, S.; Thompson, I.M. Lactation Biology Symposium: Effects of photoperiod on mammary gland development and lactation. J. Anim. Sci. 2012, 90, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Milagres, M.P.; Minim, V.P.; Minim, L.A.; Simiqueli, A.A.; Moraes, L.E.; Martino, H.S. Night milking adds value to cow’s milk. J. Sci. Food Agric. 2014, 94, 1688–1692. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.W.; Yang, G.Q.; Wang, I.F.; Fu, T.; Lian, H.X.; Sun, Y.; Han, L.Q.; Zhang, L.Y.; Gao, T.Y. Effects of The Circadian Rhythm On Milk Composition In Dairy Cows: Does Day Milk Differ From Night Milk? J. Dairy. Sci. 2021, 104, 8301–8313. [Google Scholar] [CrossRef] [PubMed]
- Sahin, O.; Akiurek, F.; Boztepe, S.; Aytekin, I.; Keskin, I. Detemination of melatonin in differences between day and night milk in dairy cattle. J. Agric. Sci. 2021, 27, 449–453. [Google Scholar]
- Castro, N.; Spengler, M.; Lollivier, V.; Wellnitz, O.; Meyer, H.H.D.; Bruckmaier, R.M. Diurnal pattern of melatonin in blood and milk of dairy Cows. Milk Sci. Int. 2011, 66, 2. [Google Scholar]
- Asher, A.; Shabtay, A.; Brosh, A.; Eitam, H.; Agmon, R.; Cohen-Zinder, M.; Zubidat, A.E.; Haim, A. “Chrono-functional milk”: The difference between melatonin concentrations in night-milk versus day-milk under different night illumination conditions. Chronobiol. Int. 2015, 32, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Boztepe, S.; Keskin, I.; Semacan, A.; Akyürek, F.; Aytekin, I.; Sahin, Ö. Melatonin differences between day and night milk in primiparous Holstein Friesian and Jersey dairy cattle. Selcuk. J. Agric. Food Sci. 2022, 36, 27–30. [Google Scholar] [CrossRef]
- Zarazaga, L.A.; Malpaux, B.; Bodin, L.; Chemineau, P. The large variability in melatonin blood levels in ewes is under strong genetic influence. Am. J. Physiol.—Endocrinol. Metab. 1998, 37, E607–E610. [Google Scholar] [CrossRef] [PubMed]
- Valtonen, M.; Niskanen, L.; Kangas, A.P.; Koskinen, T. Effect of melatonin-rich night-time milk on sleep and activity in elderly institutionalized subjects. Nord. J. Psychiatry 2005, 59, 217–221. [Google Scholar] [CrossRef]
- Dela Peña, I.J.; Hong, E.; de la Peña, J.B.; Kim, H.J.; Botanas, C.J.; Hong, Y.S.; Hwang, Y.S.; Moon, B.S.; Cheong, J.H. Milk Collected at Night Induces Sedative and Anxiolytic-Like Effects and Augments Pentobarbital-Induced Sleeping Behavior in Mice. J. Med. Food 2015, 18, 1255–1261. [Google Scholar] [CrossRef]
- Bae, S.M.; Jeong, J.; Jeon, H.J.; Bang, Y.R.; Yoon, I.Y. Effects of melatonin-rich milk on mild insomnia symptoms. Sleep Med. Res. 2016, 7, 60–67. [Google Scholar] [CrossRef]
- Romanini, E.B.; Marchi Volpato, A.; Dos Santos, J.S.; De Santana, E.H.W.; De Souza, C.H.B.; Ludovico, A. Melatonin concentration in cow’s milk and sources of its variation. J. Appl. Anim. Res. 2019, 47, 140–145. [Google Scholar] [CrossRef]
- Shields, S.L.; Rezamand, P.; Sevier, D.L.; Seo, K.S.; Price, W.; McGuire, M.A. Effects of increased milking frequency for the first 21 days postpartum on selected measures of mammary gland health, milk yield and milk composition. J. Dairy. Res. 2011, 78, 301–307. [Google Scholar] [CrossRef]
- Stelwagen, K.; Phyn, C.V.; Davis, S.R.; Guinard-Flament, J.; Pomiès, D.; Roche, J.R.; Kay, J.K. Invited review: Reduced milking frequency: Milk production and management implications. J. Dairy. Sci. 2013, 96, 3401–3413. [Google Scholar] [CrossRef] [PubMed]
- Wall, E.H.; Bond, J.P.; McFadden, T.B. Milk yield responses to changes in milking frequency during early lactation are associated with coordinated and persistent changes in mammary gene expression. BMC Genom. 2013, 14, 296. [Google Scholar] [CrossRef] [PubMed]
- Hanling, H.H.; McGilliard, M.L.; Corl, B.A. The Enhanced Milk Yield Effect of Early Lactation Increased Milking Frequency and Bovine Somatotropin Is Additive and Not Synergistic. Animals 2023, 13, 2202. [Google Scholar] [CrossRef] [PubMed]
- Helmreich, S.; Wechsler, B.; Hauser, R.; Gygax, L. Effects of milking frequency in automatic milking systems on salivary cortisol, immunoglobulin A, somatic cell count and melatonin. Schweiz. Arch. Tierheilkd. 2016, 158, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Lawson, T.J.; Kennedy, A.D. Inhibition of nighttime melatonin secretion in cattle: Threshold light intensity for dairy heifers. Can. J. Anim. Sci. 2001, 81, 153–156. [Google Scholar] [CrossRef]
- Muthuramalingam, P.; Kennedy, A.D.; Berry, R.J. Plasma melatonin and insulin-like growth factor-1 responses to dim light at night in dairy heifers. J. Pineal Res. 2006, 40, 225–229. [Google Scholar] [CrossRef]
- Bal, M.A.; Penner, G.B.; Oba, M.; Kennedy, A.D. Effects of dim light at night on milk yield, milk composition and endocrine profile of lactating dairy cows. Can. J. Anim. Sci. 2008, 88, 609–612. [Google Scholar] [CrossRef]
- Valtonen, M.; Kangas, A.P.; Voutilainen, M. Method for Producing Melatonin Rich. Milk. Patent WO2001001784A1, 11 January 2001. [Google Scholar]
- Asher, A.; Fialko, M.; Fares, F.; Moallem, U.; Yaacoby, S.; Gutman, R. The Effect of Short-Wavelength White LED Illumination throughout the Night on the Milk Fatty Acid Profile of High-Yielding Dairy Cows. Biology 2022, 11, 1799. [Google Scholar] [CrossRef]
- Pimputkar, S.; Speck, J.S.; DenBaars, S.P.; Nakamura, S. Prospects for LED lighting. Nat. Photonics 2009, 3, 180–182. [Google Scholar] [CrossRef]
- Murphy, B.A.; Herlihy, M.M.; Nolan, M.B.; Butler, S.T. Blue light from light-emitting diodes (LEDs) directed at a single eye elicits a dose-dependent suppression of melatonin in dairy cows. J. Dairy. Sci. 2017, 100, 2011. [Google Scholar]
- Elsabagh, M.; Mon, M.; Takao, Y.; Shinoda, A.; Watanabe, T.; Kushibiki, S.; Obitsu, T.; Sugino, T. Exposure to blue LED light before the onset of darkness under a long-day photoperiod alters melatonin secretion, feeding behaviour and growth in female dairy calves. Anim. Sci. J. 2020, 91, 13353. [Google Scholar] [CrossRef] [PubMed]
- Gnann, T. Method for the Production of Milk or Milk Products with a High Proportion of Melatonin. U.S. Patent 8003130, 23 August 2011. [Google Scholar]
- Haigh, B.S. Method for Producing Milk with an Enhanced Content of Naturally Expressed Melatonin. UK Patent 2 387 009 A, 17 April 2002. [Google Scholar]
- Yao, S.; Wu, H.; Ma, H.; Fu, Y.; Wei, W.; Wang, T.; Guan, S.; Yang, H.; Li, X.; Guo, J.; et al. Effects of rumen bypass melatonin feeding (RBMF) on milk quality and mastitis of Holstein cows. PeerJ 2020, 8, e9147. [Google Scholar] [CrossRef]
- Wu, H.; Yao, S.; Wang, T.; Wang, J.; Ren, K.; Yang, H.; Ma, W.; Ji, P.; Lu, Y.; Ma, H.; et al. Effects of Melatonin on Dairy Herd Improvement (DHI) of Holstein Cow with High SCS. Molecules 2021, 26, 834. [Google Scholar] [CrossRef]
- Garcia-Ispierto, I.; Abdelfatah, A.; López-Gatius, F. Melatonin treatment at dry-off improves reproductive performance postpartum in high-producing dairy cows under heat stress conditions. Reprod. Domest. Anim. 2013, 48, 577–583. [Google Scholar] [CrossRef]
- Morini, G.; Pittella, M.; Poli, A.; De Rensis, F. Effect of melatonin administration prior to calving on milk secretion the next lactation in dairy cows. Vet. Stanica 2018, 49, 85–89. [Google Scholar]
- Saeedabadi, S.; Abazari-Kia, A.H.; Rajabi, H.; Parivar, K.; Salehi, M. Melatonin Improves the Developmental Competence of Goat Oocytes. Int. J. Fertil. Steril. 2018, 12, 157–163. [Google Scholar]
- Tölü, C.; Yazgan, N.; Akbağ, H.I.; Yurtman, İ.Y.; Savaş, T. Effects of melatonin implants on reproductive performance of dairy sheep and dairy goats. Reprod. Domest. Anim. 2022, 57, 665–672. [Google Scholar] [CrossRef]
- Zarazaga, L.A.; Gatica, M.C.; Gallego-Calvo, L.; Celi, I.; Guzman, J.L. Melatonin improves the reproductive performance of seasonal anoestrus goats exposed to buck effect during early post-partum. Span. J. Agric. Res. 2013, 11, 997–1003. [Google Scholar] [CrossRef][Green Version]
- Zarazaga, L.A.; Gatica, M.C.; Hernandez, H.; Chemineau, P.; Delgadillo, J.A.; Guzman, J.L. Photoperiod-treated bucks are equal to melatonin-treated bucks for inducing reproductive behaviour and physiological functions via the “male effect” in Mediterranean goats. Anim. Reprod. Sci. 2019, 202, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Molik, E.; Bonczar, G.; Zebrowska, A.; Misztal, T.; Pustkowiak, H.; Zieba, D. Effect of day length and exogenous melatonin on chemical composition of sheep milk. Arch. Anim. Breed. 2011, 54, 177–187. [Google Scholar] [CrossRef][Green Version]
- Cosso, G.; Mura, M.C.; Pulinas, L.; Curone, G.; Vigo, D.; Carcangiu, V.; Luridiana, S. Effects of melatonin treatment on milk traits, reproductive performance and immune response in Sarda dairy sheep. Ital. J. Anim. Sci. 2021, 20, 632–639. [Google Scholar] [CrossRef]
- Auldist, M.J.; Turner, S.A.; McMahon, C.D.; Prosser, C.G. Effects of melatonin on the yield and composition of milk from grazing dairy cows in New Zealand. J. Dairy. Res. 2007, 74, 52–57. [Google Scholar] [CrossRef]
- Boland, M. Designer milks: Functional foods from milk. In Improving the Safety and Quality of Milk; Woodhead Publishing Limited: Cambridge, UK, 2010; Volume 2, pp. 74–96. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrani, M.; Dall’Olio, E.; De Rensis, F.; Tummaruk, P.; Saleri, R. Bioactive Peptides in Dairy Milk: Highlighting the Role of Melatonin. Biomolecules 2024, 14, 934. https://doi.org/10.3390/biom14080934
Andrani M, Dall’Olio E, De Rensis F, Tummaruk P, Saleri R. Bioactive Peptides in Dairy Milk: Highlighting the Role of Melatonin. Biomolecules. 2024; 14(8):934. https://doi.org/10.3390/biom14080934
Chicago/Turabian StyleAndrani, Melania, Eleonora Dall’Olio, Fabio De Rensis, Padet Tummaruk, and Roberta Saleri. 2024. "Bioactive Peptides in Dairy Milk: Highlighting the Role of Melatonin" Biomolecules 14, no. 8: 934. https://doi.org/10.3390/biom14080934
APA StyleAndrani, M., Dall’Olio, E., De Rensis, F., Tummaruk, P., & Saleri, R. (2024). Bioactive Peptides in Dairy Milk: Highlighting the Role of Melatonin. Biomolecules, 14(8), 934. https://doi.org/10.3390/biom14080934






