Loss-of-Imprinting of HM13 Leads to Poor Prognosis in Clear Cell Renal Cell Carcinoma
Abstract
1. Background
2. Materials and Methods
2.1. Samples
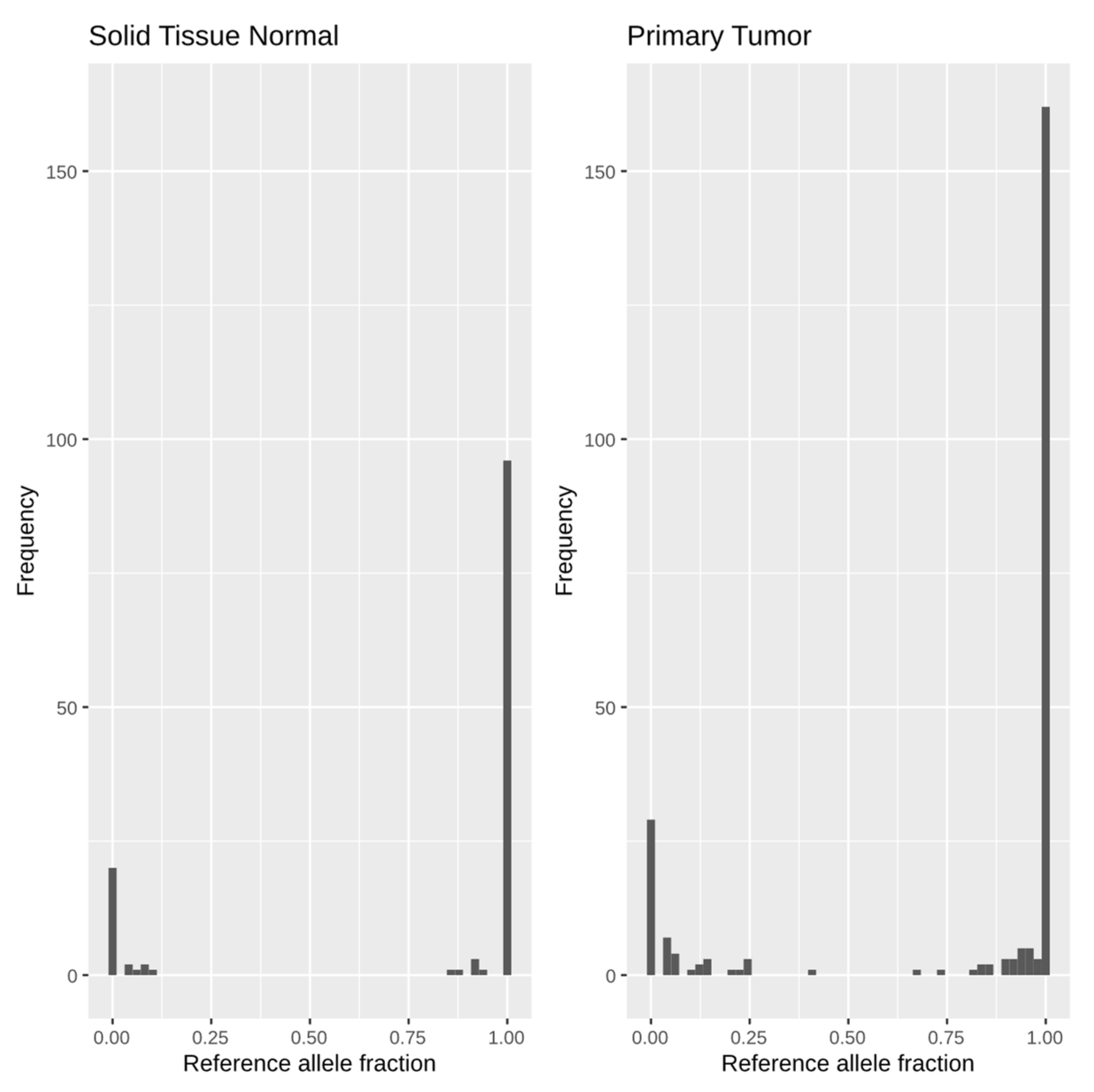
2.2. Imprinting Detection
2.3. Loss-of-Imprinting, Differential Expression, DNA Methylation, Copy Number Variation, and Mutation Analyses
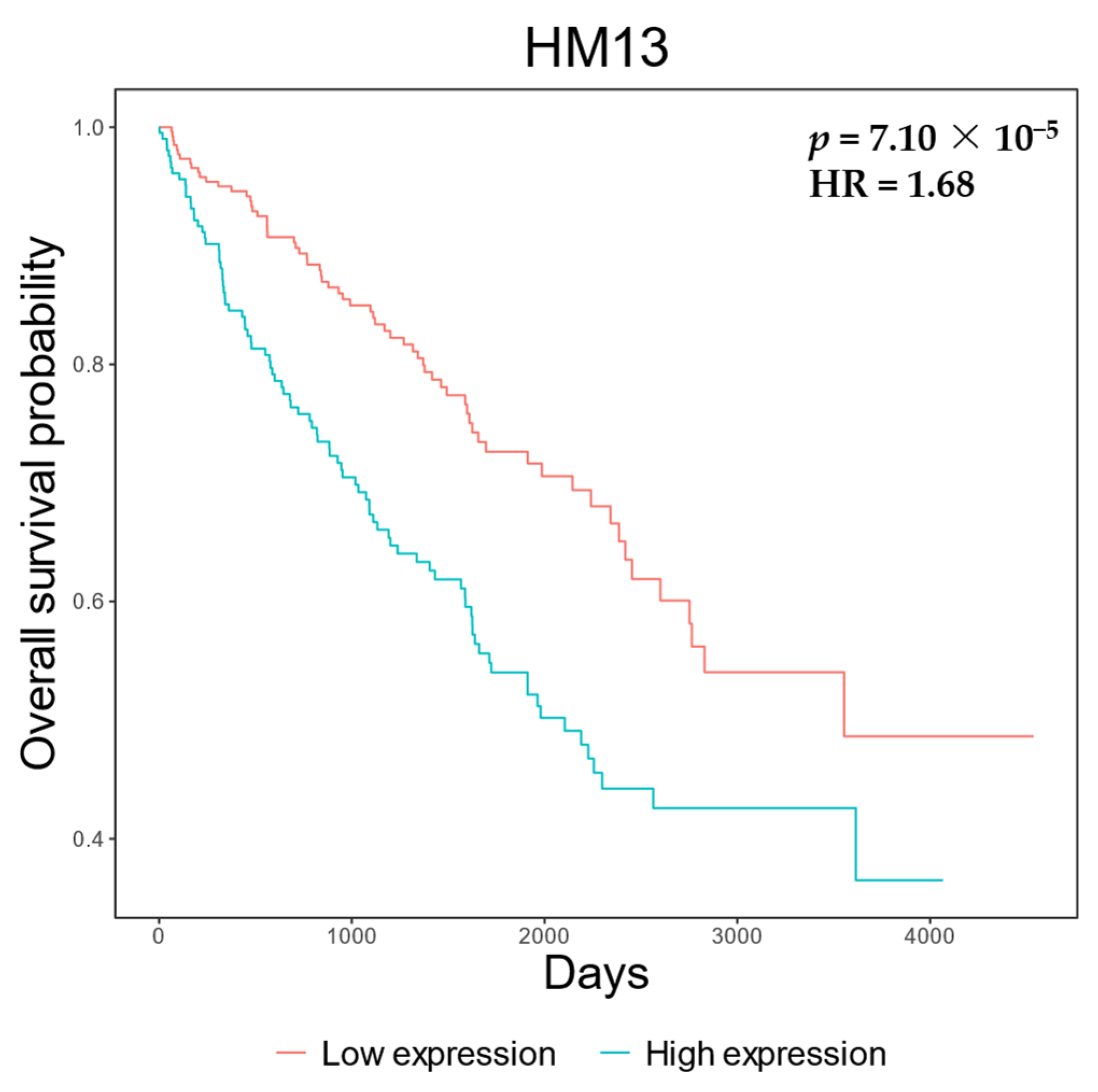
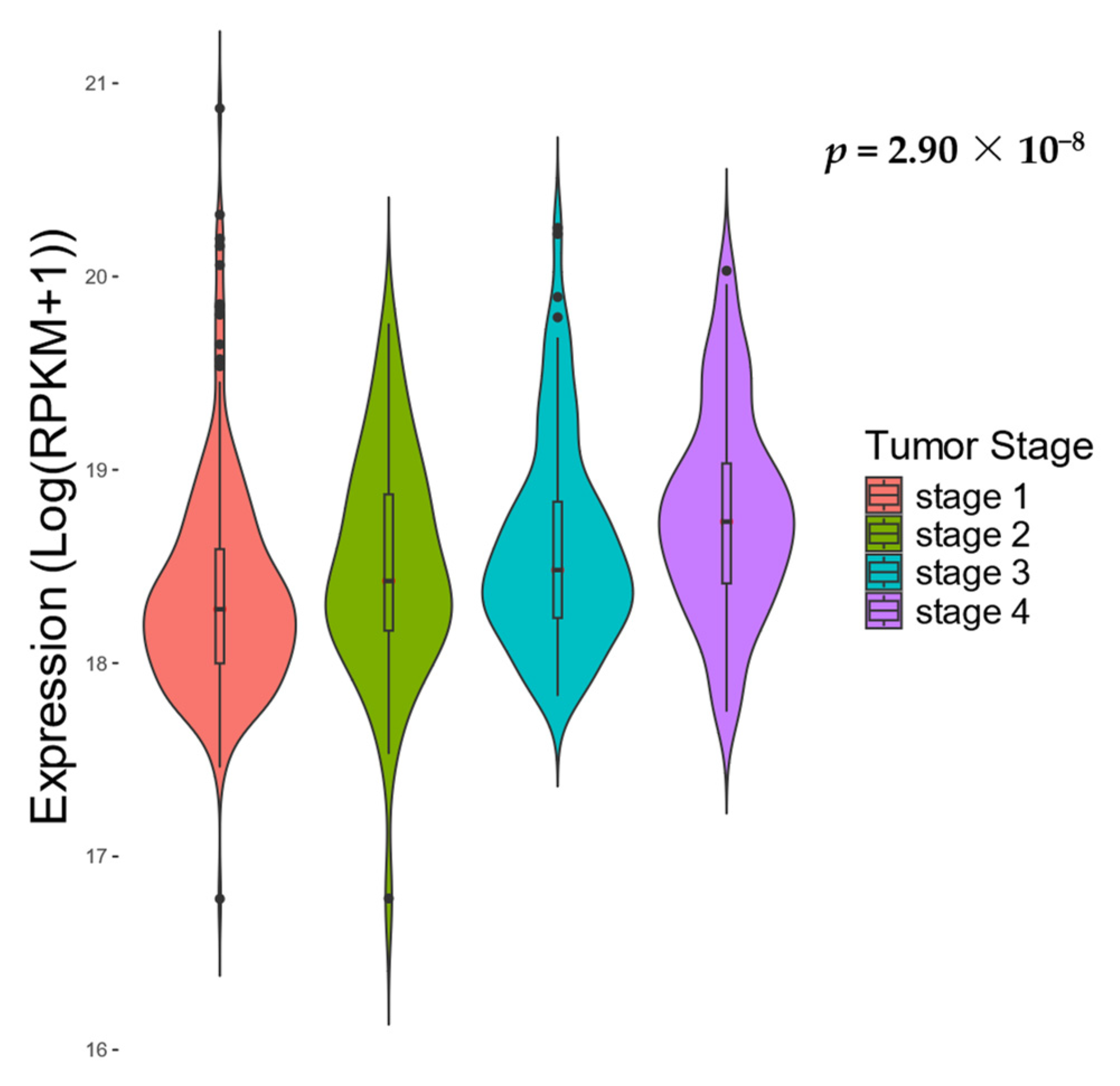
2.4. Clinical Analyses
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Cancer Institute. Clear Cell Renal Cell Carcinoma Study. Available online: https://www.cancer.gov/ccg/research/genome-sequencing/tcga/studied-cancers/clear-cell-renal-carcinoma-study (accessed on 31 July 2024).
- Chow, W.H.; Dong, L.M.; Devesa, S.S. Epidemiology and risk factors for kidney cancer. Nat. Rev. Urol. 2010, 7, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Padala, S.A.; Barsouk, A.; Thandra, K.C.; Saginala, K.; Mohammed, A.; Vakiti, A.; Rawla, P.; Barsouk, A. Epidemiology of renal cell carcinoma. World J. Oncol. 2020, 11, 79–87. [Google Scholar] [CrossRef]
- Muglia, V.F.; Prando, A. Renal cell carcinoma: Histological classification and correlation with imaging findings. Radiol. Bras. 2015, 48, 166–174. [Google Scholar] [CrossRef]
- Kerr, D.J.; Yang, L. Personalising cancer medicine with prognostic markers. EBioMedicine 2021, 72, 103577. [Google Scholar] [CrossRef] [PubMed]
- Jirtle, R.L. Genomic imprinting and cancer. Exp. Cell Res. 1999, 248, 18–24. [Google Scholar] [CrossRef]
- Kim, K.S.; Lee, Y.I. Biallelic expression of the H19 and IGF2 genes in hepatocellular carcinoma. Cancer Lett. 1997, 119, 143–148. [Google Scholar] [CrossRef]
- Sakatani, T.; Kaneda, A.; Iacobuzio-Donahue, C.A.; Carter, M.G.; de Boom Witzel, S.; Okano, H.; Ko, M.S.H.; Ohlsson, R.; Longo, D.L.; Feinberg, A.P. Loss of imprinting of Igf2 alters intestinal maturation and tumorigenesis in mice. Science 2005, 307, 1976–1978. [Google Scholar] [CrossRef] [PubMed]
- Nonomura, N.; Nishimura, K.; Miki, T.; Kanno, N.; Kojima, Y.; Yokoyama, M.; Okuyama, A. Loss of imprinting of the insulin-like growth factor II gene in renal cell carcinoma. Cancer Res. 1997, 57, 2575–2577. [Google Scholar]
- Vu, T.H.; Chuyen, N.V.; Li, T.; Hoffman, A.R. Loss of imprinting of IGF2 sense and antisense transcripts in Wilms’ tumor. Cancer Res. 2003, 63, 1900–1905. [Google Scholar]
- Cui, H.; Cruz-Correa, M.; Giardiello, F.M.; Hutcheon, D.F.; Kafonek, D.R.; Brandenburg, S.; Wu, Y.; He, X.; Powe, N.R.; Feinberg, A.P. Loss of IGF2 imprinting: A potential marker of colorectal cancer risk. Science 2003, 299, 1753–1755. [Google Scholar] [CrossRef]
- Fu, V.X.; Dobosy, J.R.; Desotelle, J.A.; Almassi, N.; Ewald, J.A.; Srinivasan, R.; Berres, M.; Svaren, J.; Weindruch, R.; Jarrard, D.F. Aging and Cancer-Related Loss of Insulin-like Growth Factor 2 Imprinting in the Mouse and Human Prostate. Cancer Res. 2008, 68, 6797–6802. [Google Scholar] [CrossRef] [PubMed]
- Damaschke, N.A.; Yang, B.; Bhusari, S.; Avilla, M.; Zhong, W.; Blute, M.L.; Huang, W.; Jarrard, D.F. Loss of Igf2 Gene Imprinting in Murine Prostate Promotes Widespread Neoplastic Growth. Cancer Res. 2017, 77, 5236–5247. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, J.N.; Collisson, E.A.; Mills, G.B.; Shaw, K.R.M.; Ozenberger, B.A.; Ellrott, K.; Shmulevich, I.; Sander, C.; Stuart, J.M. The Cancer Genome Atlas Pan-Cancer analysis project. Nat. Genet. 2013, 45, 1113–1120. [Google Scholar] [CrossRef]
- Ricketts, C.J.; De Cubas, A.A.; Fan, H.; Smith, C.C.; Lang, M.; Reznik, E.; Bowlby, R.; Gibb, E.A.; Akbani, R.; Beroukhim, R.; et al. The Cancer Genome Atlas Comprehensive Molecular Characterization of Renal Cell Carcinoma. Cell Rep. 2018, 23, 313–326.e5. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.; Jeschke, J.; Van Criekinge, W.; van Engeland, M.; De Meyer, T. MEXPRESS update 2019. Nucleic Acids Res. 2019, 47, W561–W565. [Google Scholar] [CrossRef] [PubMed]
- Goovaerts, T.; Steyaert, S.; Vandenbussche, C.A.; Galle, J.; Thas, O.; Van Criekinge, W.; De Meyer, T. A comprehensive overview of genomic imprinting in breast and its deregulation in cancer. Nat. Commun. 2018, 9, 4120. [Google Scholar] [CrossRef] [PubMed]
- Lorenzi, L.; Chiu, H.S.; Avila Cobos, F.; Gross, S.; Volders, P.J.; Cannoodt, R.; Nuytens, J.; Vanderheyden, K.; Anckaert, J.; Lefever, S.; et al. The RNA Atlas expands the catalog of human non-coding RNAs. Nat. Biotechnol. 2021, 39, 1453–1465. [Google Scholar] [CrossRef] [PubMed]
- Sillars-Hardebol, A.H.; Carvalho, B.; Tijssen, M.; Beliën, J.A.M.; de Wit, M.; van Diemen, P.M.D.; Pontén, F.; van de Wiel, M.A.; Fijneman, R.J.; Meijer, G.A. TPX2 and AURKA promote 20q amplicon-driven colorectal adenoma to carcinoma progression. Gut 2012, 61, 1568. [Google Scholar] [CrossRef] [PubMed]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Cheng, T.; Li, X.; Hu, J.; Li, E.; Ding, M.; Shen, R.; Pineda, J.P.; Li, C.; Lu, S.; et al. Epigenetic imprinting alterations as effective diagnostic biomarkers for early-stage lung cancer and small pulmonary nodules. Clin. Epigenetics 2021, 13, 220. [Google Scholar] [CrossRef]
- Liu, J.; Li, W.; Wu, L. Pan-cancer analysis suggests histocompatibility minor 13 is an unfavorable prognostic biomarker promoting cell proliferation, migration, and invasion in hepatocellular carcinoma. Front. Pharmacol. 2022, 13, 950156. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, Z.; Wang, Z.; Zhang, X.; Dai, X.; Zhou, G.; Ding, Q. Histocompatibility Minor 13 (HM13), targeted by miR-760, exerts oncogenic role in breast cancer by suppressing autophagy and activating PI3K-AKT-mTOR pathway. Cell Death Dis. 2022, 13, 728. [Google Scholar] [CrossRef] [PubMed]
- Barrow, M.A.; Martin, M.E.; Coffey, A.; Andrews, P.L.; Jones, G.S.; Reaves, D.K.; Parker, J.S.; Troester, M.A.; Fleming, J.M. A functional role for the cancer disparity-linked genes, CRYβB2 and CRYβB2P1, in the promotion of breast cancer. Breast Cancer Res. 2019, 21, 105. [Google Scholar] [CrossRef] [PubMed]
| Gene | N | Î | p (LOI) | p (DE) | Log2FC |
|---|---|---|---|---|---|
| ZDBF2 | 6 | 0.98 | 4.05 × 10−1 | 9.90 × 10−16 | −1.36 |
| COPG2IT1 $ | 5 | 0.95 | 2.75 × 10−1 | 9.16 × 10−25 | −1.65 |
| MEST | 2 | 0.9 | 6.30 × 10−5 | 1.05 × 10−14 | −1.77 |
| H19 | 2 | 0.93 | 1.89 × 10−1 | 8.68 × 10−1 | 0.05 |
| MEG3 | 7 | 0.95 | 9.09 × 10−1 | 8.68 × 10−1 | −0.05 |
| PWAR6 $ | 4 | 0.99 | 3.71 × 10−1 | 4.84 × 10−29 | −1.16 |
| SNHG14 | 14 | 0.99 | 2.95 × 10−1 | 9.04 × 10−12 | −0.73 |
| IPW $ | 2 | 0.98 | 4.82 × 10−1 | 1.36 × 10−18 | −0.76 |
| ZNF597 | 5 | 0.95 | 3.49 × 10−1 | 1.30 × 10−2 | 0.22 |
| PEG3 | 6 | 0.97 | 1.59 × 10−1 | 9.90 × 10−16 | −2.66 |
| ZNF331 | 3 | 0.97 | 5.01 × 10−25 | 4.32 × 10−6 | −1.12 |
| HM13 | 3 | 0.97 | 2.74 × 10−3 | 9.90 × 10−16 | 0.60 |
| PCBD2 | 2 | 0.95 | 3.25 × 10−5 | 6.53 × 10−1 | 0.04 |
| CRYBB2P1 ** | 2 | 0.82 | 6.33 × 10−2 | 2.02 × 10−10 | 0.98 |
| LLGL2 ** | 2 | 0.85 | 2.99 × 10−1 | 9.90 × 10−16 | −1.35 |
| SLC9A3R2 | 1 | 0.88 | 5.71 × 10−8 | 8.68 × 10−1 | −0.02 |
| PEG10 | 1 | 0.98 | 5.93 × 10−1 | 1.15 × 10−4 | −0.52 |
| NDN | 1 | 0.99 | 9.71 × 10−3 | 5.03 × 10−1 | 0.10 |
| GNAS | 1 | 0.99 | 8.05 × 10−1 | 6.53 × 10−1 | −0.05 |
| RPH3AL | 1 | 0.97 | 3.91 × 10−12 | 1.17 × 10−2 | −0.29 |
| HLA–DQA2 $* | 2 | 0.82 | 5.64 × 10−14 | 1.82 × 10−15 | 1.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voorthuijzen, F.; Stroobandt, C.; Van Criekinge, W.; Goovaerts, T.; De Meyer, T. Loss-of-Imprinting of HM13 Leads to Poor Prognosis in Clear Cell Renal Cell Carcinoma. Biomolecules 2024, 14, 936. https://doi.org/10.3390/biom14080936
Voorthuijzen F, Stroobandt C, Van Criekinge W, Goovaerts T, De Meyer T. Loss-of-Imprinting of HM13 Leads to Poor Prognosis in Clear Cell Renal Cell Carcinoma. Biomolecules. 2024; 14(8):936. https://doi.org/10.3390/biom14080936
Chicago/Turabian StyleVoorthuijzen, Floris, Cedric Stroobandt, Wim Van Criekinge, Tine Goovaerts, and Tim De Meyer. 2024. "Loss-of-Imprinting of HM13 Leads to Poor Prognosis in Clear Cell Renal Cell Carcinoma" Biomolecules 14, no. 8: 936. https://doi.org/10.3390/biom14080936
APA StyleVoorthuijzen, F., Stroobandt, C., Van Criekinge, W., Goovaerts, T., & De Meyer, T. (2024). Loss-of-Imprinting of HM13 Leads to Poor Prognosis in Clear Cell Renal Cell Carcinoma. Biomolecules, 14(8), 936. https://doi.org/10.3390/biom14080936






