Subtype-Specific Ligand Binding and Activation Gating in Homomeric and Heteromeric P2X Receptors
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Molecular Biology and Cell Culture
2.3. Electrophysiology
2.4. Confocal Microscopy
2.5. Quantification and Statistical Analysis
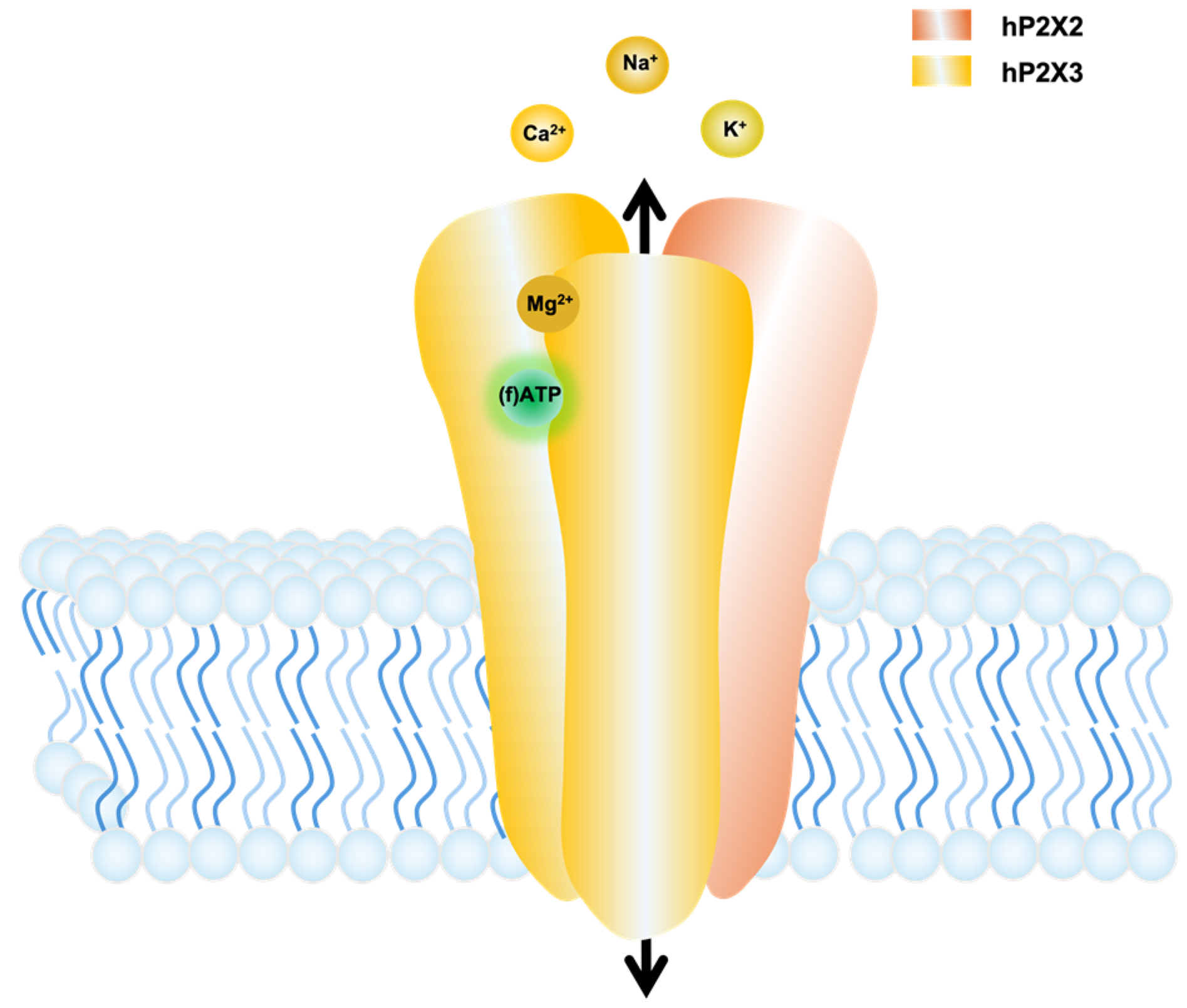
3. Results
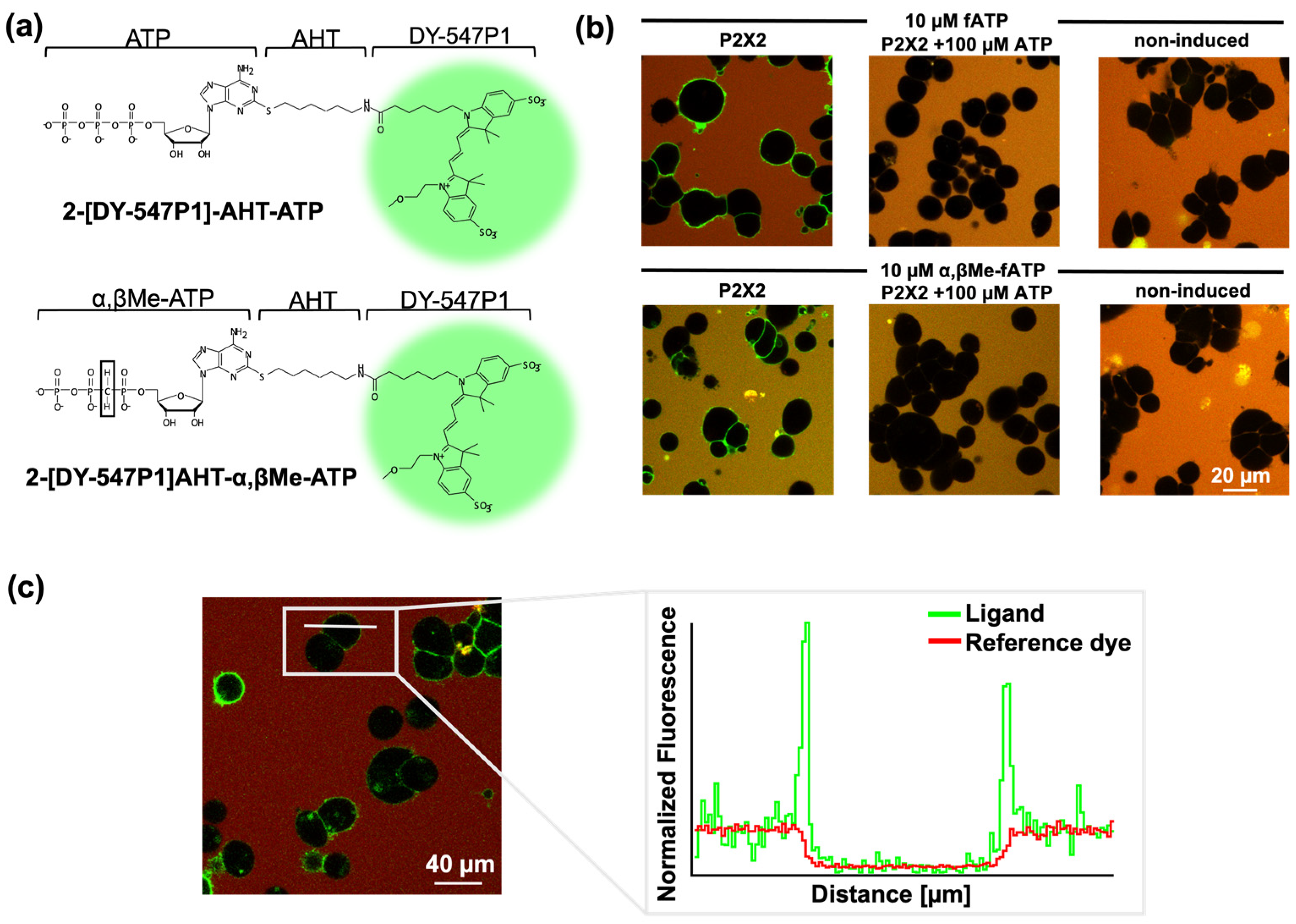
3.1. Novel Fluorescent ATP Derivatives and Visualisation of Ligand Binding
3.2. Subtype-Specific Ligand Response in P2X Receptor Proteins
3.3. P2X Receptor Subtypes Exhibit Unique Ligand Binding Characteristics
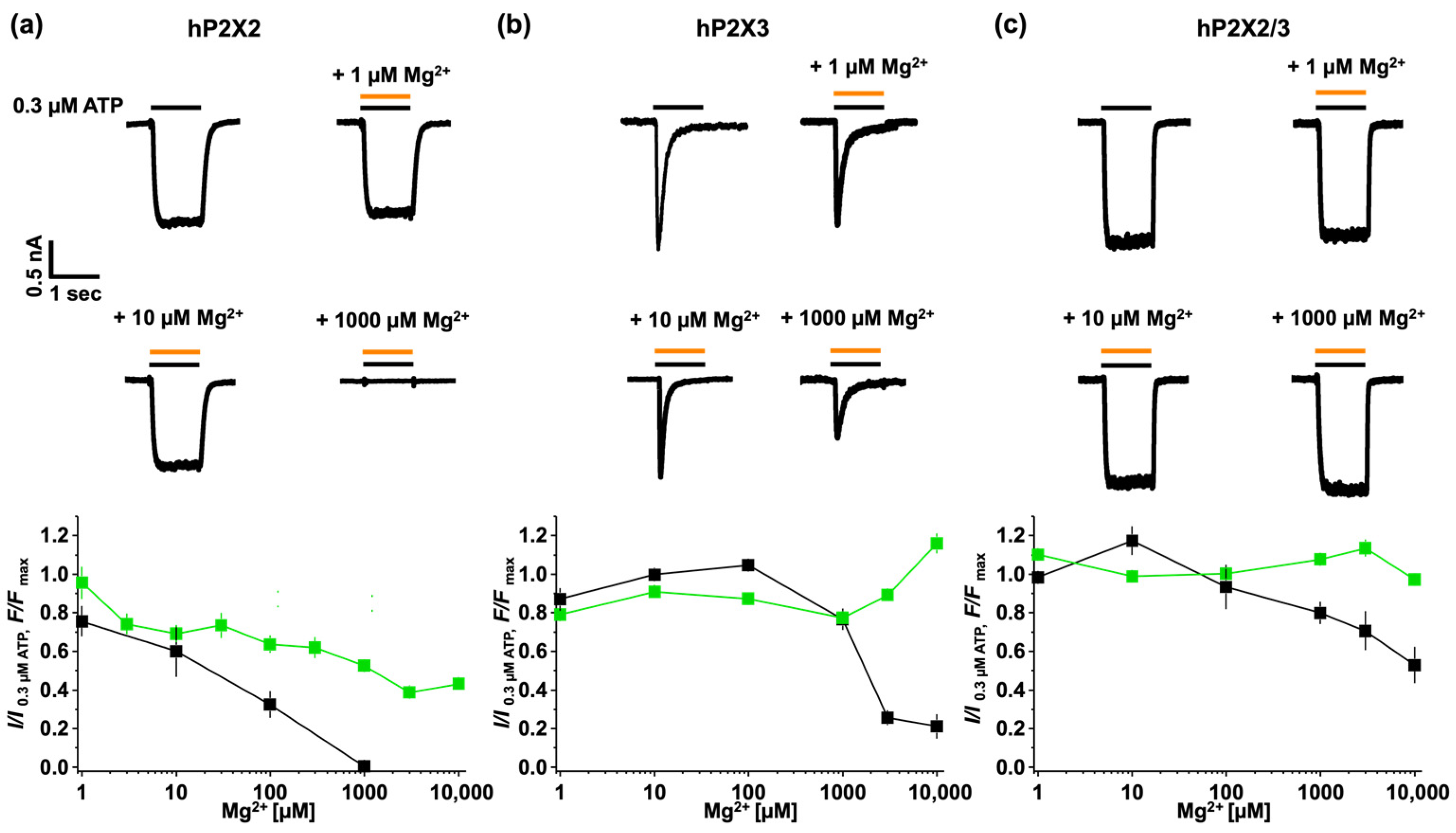
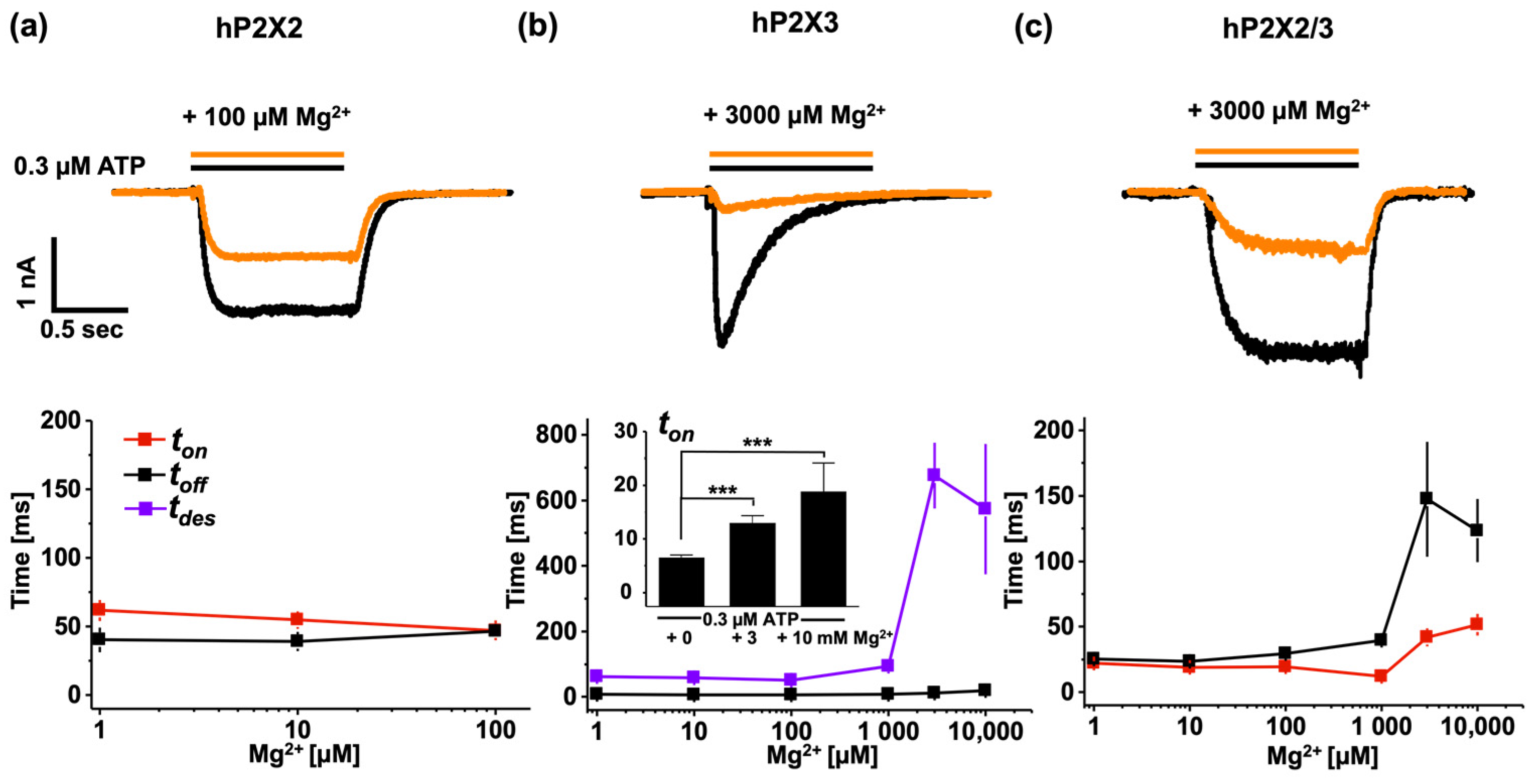
3.4. Modulating P2X Receptor Channel Signalling by Mg2+
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coddou, C.; Yan, Z.; Obsil, T.; Pablo Huidobro-Toro, J.; Stojilkovic, S.S. Activation and Regulation of Purinergic P2X Receptor Channels. Pharmacol. Rev. 2011, 63, 641–683. [Google Scholar] [CrossRef] [PubMed]
- Schmid, R.; Evans, R.J. ATP-Gated P2X Receptor Channels: Molecular Insights into Functional Roles. Annu. Rev. Physiol. 2019, 81, 43–62. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.H. P2X Receptor-Mediated ATP Purinergic Signaling in Health and Disease. Cell Health Cytoskelet. 2012, 4, 83–101. [Google Scholar] [CrossRef]
- North, R.A. Molecular Physiology of P2X Receptors. Physiol. Rev. 2002, 82, 1013–1067. [Google Scholar] [CrossRef] [PubMed]
- Sattler, C.; Benndorf, K. Enlightening Activation Gating in P2X Receptors. Purinergic Signal. 2022, 18, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, E.; Skorinkin, A.; Moiseev, I.; Agrache, A.; Nistri, A.; Giniatullin, R. Experimental and Modeling Studies of Desensitization of P2X3 Receptors. Mol. Pharmacol. 2006, 70, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Allsopp, R.C.; Farmer, L.K.; Fryatt, A.G.; Evans, R.J. P2X Receptor Chimeras Highlight Roles of the Amino Terminus to Partial Agonist Efficacy, the Carboxyl Terminus to Recovery from Desensitization, and Independent Regulation of Channel Transitions. J. Biol. Chem. 2013, 288, 21412–21421. [Google Scholar] [CrossRef]
- Rettinger, J.; Schmalzing, G. Desensitization Masks Nanomolar Potency of ATP for the P2X1 Receptor. J. Biol. Chem. 2004, 279, 6426–6433. [Google Scholar] [CrossRef] [PubMed]
- Rettinger, J.; Schmalzing, G. Activation and Desensitization of the Recombinant P2X1 Receptor at Nanomolar ATP Concentrations. J. Gen. Physiol. 2003, 121, 451–461. [Google Scholar] [CrossRef]
- Pratt, E.B.; Brink, T.S.; Bergson, P.; Voigt, M.M.; Cook, S.P. Use-Dependent Inhibition of P2X3 Receptors by Nanomolar Agonist. J. Neurosci. 2005, 25, 7359–7365. [Google Scholar] [CrossRef]
- Li, M.; Banerjee, R.; Marinelli, F.; Silberberg, S.; Faraldo-Gómez, J.D.; Hattori, M.; Swartz, K.J. Molecular Mechanisms of Human P2X3 Receptor Channel Activation and Modulation by Divalent Cation Bound Atp. eLife 2019, 8, 1–20. [Google Scholar] [CrossRef]
- Lewis, C.; Neldhart, S.; Holy, C.; North, R.A.; Buell, G.; Surprenant, A. Coexpression of P2X2 and P2X Receptor Subunits Can Account for ATP-Gated Currents in Sensory Neurons. Nature 1995, 377, 432–435. [Google Scholar] [CrossRef]
- North, R.A.; Surprenant, A. Pharmacology of Cloned P2X Receptors. Annu. Rev. Pharmacol. Toxicol 2000, 40, 563–580. [Google Scholar] [CrossRef] [PubMed]
- Syed, N.i.H.; Kennedy, C. Pharmacology of P2X Receptors. Wiley Interdiscip. Rev. Membr. Transp. Signal. 2012, 1, 16–30. [Google Scholar] [CrossRef]
- Illes, P.; Müller, C.E.; Jacobson, K.A.; Grutter, T.; Nicke, A.; Fountain, S.J.; Kennedy, C.; Schmalzing, G.; Jarvis, M.F.; Stojilkovic, S.S.; et al. Update of P2X Receptor Properties and Their Pharmacology: IUPHAR Review 30. Br. J. Pharmacol. 2021, 178, 489–514. [Google Scholar] [CrossRef]
- Li, M.; Silberberg, S.D.; Swartz, K.J. Subtype-Specific Control of P2X Receptor Channel Signaling by ATP and Mg2+. Proc. Natl. Acad. Sci. USA 2013, 110, E3455–E3463. [Google Scholar] [CrossRef]
- Kasuya, G.; Fujiwara, Y.; Takemoto, M.; Dohmae, N.; Nakada-Nakura, Y.; Ishitani, R.; Hattori, M.; Nureki, O. Structural Insights into Divalent Cation Modulations of ATP-Gated P2X Receptor Channels. Cell Rep. 2016, 14, 932–944. [Google Scholar] [CrossRef] [PubMed]
- Lazarowski, E.R.; Boucher, R.C.; Harden, T.K. Mechanisms of Release of Nucleotides and Integration of Their Action as P2X- and P2Y-Receptor Activating Molecules. Mol. Pharmacol. 2003, 64, 785–795. [Google Scholar] [CrossRef]
- Tittle, R.K.; Hume, R.I. Opposite Effects of Zinc on Human and Rat P2X2 Receptors. J. Neurosci. 2008, 28, 11131–11140. [Google Scholar] [CrossRef]
- Rokic, M.B.; Castro, P.; Leiva-Salcedo, E.; Tomic, M.; Stojilkovic, S.S.; Coddou, C. Opposing Roles of Calcium and Intracellular ATP on Gating of the Purinergic P2X2 Receptor Channel. Int. J. Mol. Sci. 2018, 19, 1161. [Google Scholar] [CrossRef]
- Giniatullin, R.; Sokolova, E.; Nistri, A. Modulation of P2X3 Receptors by Mg2+ on Rat DRG Neurons in Culture. Neuropharmacology 2003, 44, 132–140. [Google Scholar] [CrossRef]
- Grimes, L.; Young, M. Purinergic P2X Receptors: Structural and Functional Features Depicted by X-Ray and Molecular Modelling Studies. Curr. Med. Chem. 2015, 22, 783–798. [Google Scholar] [CrossRef] [PubMed]
- Caseley, E.A.; Muench, S.P.; Jiang, L.H. Tyrosine 288 in the Extracellular Domain of the Human P2X7 Receptor Is Critical for Receptor Function Revealed by Structural Modeling and Site-Directed Mutagenesis. Proteins Struct. Funct. Bioinforma. 2022, 90, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, R.; Kless, A.; Schmalzing, G. Key Sites for P2X Receptor Function and Multimerization: Overview of Mutagenesis Studies on a Structural Basis. Curr. Med. Chem. 2014, 22, 799–818. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McCarthya, A.E.; Yoshiok, C.; Mansoor, S.E. Full-Length P2X7 Structures Reveal How Palmitoylation Prevents Channel Desensitization. Cell 2019, 197, 659–670. [Google Scholar] [CrossRef]
- Hattori, M.; Gouaux, E. Molecular Mechanism of ATP Binding and Ion Channel Activation in P2X Receptors. Nature 2012, 485, 207–212. [Google Scholar] [CrossRef]
- Sattler, C.; Schmauder, R.; Schwabe, T.; Schweinitz, A.; Unzeitig, C.; Schwede, F.; Otte, M.; Benndorf, K. Relating Ligand Binding to Activation Gating in P2X2 Receptors Using a Novel Fluorescent ATP Derivative. J. Neurochem. 2020, 154, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, Y.; Nicke, A.; Rettinger, J. Validation of Alexa-647-ATP as a Powerful Tool to Study P2X Receptor Ligand Binding and Desensitization. Biochem. Biophys. Res. Commun. 2013, 438, 295–300. [Google Scholar] [CrossRef]
- Kowalski, M.; Hausmann, R.; Dopychai, A.; Grohmann, M.; Franke, H.; Nieber, K.; Schmalzing, G.; Illes, P.; Riedel, T. Conformational Flexibility of the Agonist Binding Jaw of the Human P2X3 Receptor Is a Prerequisite for Channel Opening. Br. J. Pharmacol. 2014, 171, 5093–5112. [Google Scholar] [CrossRef]
- Hamill, O.P.; Marty, A.; Neher, E.; Sakmann, B.; Sigworth, F.J. Improved Patch-Clamp Techniques for High-Resolution Current Recording from Cells and Cell-Free Membrane Patches. Pflügers Arch. Eur. J. Physiol. 1981, 391, 85–100. [Google Scholar] [CrossRef]
- Sobel, I. An Isotropic 3x3 Image Gradient Operator; Academic Press: Cambridge, MA, USA, 1990. [Google Scholar]
- Bers, D.M.; Patton, C.W.; Nuccitelli, R. A Practical Guide to the Preparation of Ca2+ Buffers. Methods Cell Biol. 2010, 99, 1–26. [Google Scholar] [PubMed]
- Kusch, J.; Biskup, C.; Thon, S.; Schulz, E.; Nache, V.; Zimmer, T.; Schwede, F.; Benndorf, K. Interdependence of Receptor Activation and Ligand Binding in HCN2 Pacemaker Channels. Neuron 2010, 67, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Biskup, C.; Kusch, J.; Schulz, E.; Nache, V.; Schwede, F.; Lehmann, F.; Hagen, V.; Benndorf, K. Relating Ligand Binding to Activation Gating in CNGA2 Channels. Nature 2007, 446, 440–443. [Google Scholar] [CrossRef]
- Gusic, M.; Benndorf, K.; Sattler, C. Dissecting Activation Steps in P2X7 Receptors. Biochem. Biophys. Res. Commun. 2021, 569, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Spelta, V.; Jiang, L.H.; Surprenant, A.; North, R.A. Kinetics of Antagonist Actions at Rat P2X2/3 Heteromeric Receptors. Br. J. Pharmacol. 2002, 135, 1524–1530. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; King, B.F.; Dunn, P.M.; Rong, W.; Townsend-Nicholson, A.; Burnstock, G. Coexpression of P2X3 and P2X2 Receptor Subunits in Varying Amounts Generates Heterogeneous Populations of P2X Receptors That Evoke a Spectrum of Agonist Responses Comparable to That Seen in Sensory Neurons. J. Pharmacol. Exp. Ther. 2001, 296, 1043–1050. [Google Scholar] [PubMed]
- Jiang, L.H.; Kim, M.; Spelta, V.; Bo, X.; Surprenant, A.; North, R.A. Subunit Arrangement in P2X Receptors. J. Neurosci. 2003, 23, 8903–8910. [Google Scholar] [CrossRef] [PubMed]
- Gasparri, F.; Wengel, J.; Grutter, T.; Pless, S.A. Molecular Determinants for Agonist Recognition and Discrimination in P2X2 Receptors. J. Gen. Physiol. 2019, 151, 898–911. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Lemoine, D.; Martz, A.; Taly, A.; Gonin, S.; Prado De Carvalho, L.; Specht, A.; Grutter, T. Agonist Trapped in ATP-Binding Sites of the P2X2 Receptor. Proc. Natl. Acad. Sci. USA 2011, 108, 9066–9071. [Google Scholar] [CrossRef]
- Chen, C.C.; Akopian, A.N.; Sivilottit, L.; Colquhount, D.; Burnstock, G.; Woodi, J.N. A P2X Purinoceptor Expressed by a Subset of Sensory Neurons. Nature 1995, 377, 428–431. [Google Scholar] [CrossRef]
- Evans, R.J.; Lewis, C.; Buell, G.; Valera, S.; North, R.A.; Surprenant, A. Pharmacological Characterization of Heterologously Expressed ATP-Gated Cation Channels (P2X Purinoceptors). Mol. Pharmacol. 1995, 48, 178–183. [Google Scholar] [PubMed]
- Eickhorst, A.N.; Berson, A.; Cockayne, D.; Lester, H.A.; Khakh, B.S. Control of P2X2 Channel Permeability by the Cytosolic Domain. J. Gen. Physiol. 2002, 120, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Zemkova, H.; He, M.L.; Koshimizu, T.A.; Stojilkovic, S.S. Identification of Ectodomain Regions Contributing to Gating, Deactivation, and Resensitization of Purinergic P2X Receptors. J. Neurosci. 2004, 24, 6968–6978. [Google Scholar] [CrossRef] [PubMed]
- Allsopp, R.C.; Evans, R.J. Contribution of the Juxtatransmembrane Intracellular Regions to the Time Course and Permeation of ATP-Gated P2X7 Receptor Ion Channels. J. Biol. Chem. 2015, 290, 14556–14566. [Google Scholar] [CrossRef] [PubMed]
- Allsopp, R.C.; Evans, R.J. The Intracellular Amino Terminus Plays a Dominant Role in Desensitization of ATP-Gated P2X Receptor Ion Channels. J. Biol. Chem. 2011, 286, 44691–44701. [Google Scholar] [CrossRef] [PubMed]
- Browne, L.E.; North, R.A. P2X Receptor Intermediate Activation States Have Altered Nucleotide Selectivity. J. Neurosci. 2013, 33, 14801–14808. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Ghansah, E.; Chen, Y.; Ye, J.; Weiss, D.S. Desensitization Mechanism of GABA Receptors Revealed by Single Oocyte Binding and Receptor Function. J. Neurosci. 2002, 22, 7982–7990. [Google Scholar] [CrossRef]
- Weber, M.; David Pfeuty, T.; Changeux, J.P. Regulation of Binding Properties of the Nicotinic Receptor Protein by Cholinergic Ligands in Membrane Fragments from Torpedo Marmorata. Proc. Natl. Acad. Sci. USA 1975, 72, 3443–3447. [Google Scholar] [CrossRef] [PubMed]
- Sattler, C.; Eick, T.; Hummert, S.; Schulz, E.; Schmauder, R.; Schweinitz, A.; Unzeitig, C.; Schwede, F.; Benndorf, K. Unravelling the Intricate Cooperativity of Subunit Gating in P2X2 Ion Channels. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Moffatt, L.; Hume, R.I. Responses of Rat P2X2 Receptors to Ultrashort Pulses of ATP Provide Insights into ATP Binding and Channel Gating. J. Gen. Physiol. 2007, 130, 183–201. [Google Scholar] [CrossRef]
- Jiang, R.; Taly, A.; Lemoine, D.; Martz, A.; Specht, A.; Grutter, T. Intermediate Closed Channel State(s) Precede(s) Activation in the ATP-Gated P2X2 Receptor. Channels 2012, 6, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Immadisetty, K.; Alenciks, J.; Kekenes-Huskey, P.M. Modulation of P2X4 Pore Closure by Magnesium, Potassium, and ATP. Biophys. J. 2022, 121, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, S.E.; Lü, W.; Oosterheerta, W.; Shekharc, M.; Tajkhorshid, E.; Gouaux, E. X-Ray Structures Define Human P2X3 Receptor Gating Cycle and Antagonist Action. Nature 2016, 538, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, M.; Hausmann, R.; Schmid, J.; Dopychai, A.; Stephan, G.; Tang, Y.; Schmalzing, G.; Illes, P.; Rubini, P. Flexible Subunit Stoichiometry of Functional Human P2X2/3 Heteromeric Receptors. Neuropharmacology 2015, 99, 115–130. [Google Scholar] [CrossRef]
- Ding, S.; Sachs, F. Ion Permeation and Block of P2X2 Purinoceptors: Single Channel Recordings. Membr. Biol. 1999, 172, 215–223. [Google Scholar] [CrossRef]



| Receptor | Ligand | EC50 [µM] | Hill | Effect (Fold in ATP Potency) | Effect (Fold in ATP Efficacy) |
|---|---|---|---|---|---|
| hP2X2 | ATP | 0.7 ± 0.1 | 1.1 ± 0.1 | 1 | 1 |
| α,βMe-ATP | 6.8 ± 4.6 | 1.7 ± 1.5 | 0.1 | 0.1 | |
| fATP | 4.6 ± 0.3 | 1.4 ± 0.1 | 0.2 | 1.0 | |
| α,βMe-fATP | n.d. | n.d. | n.d. | n.d. | |
| hP2X3 | ATP | 1.1 ± 0.1 | 1.3 ± 0.2 | 1 | 1 |
| α,βMe-ATP | 9.6 ± 3.3 | 1.6 ± 0.7 | 0.1 | 1.9 | |
| fATP | 1.3 ± 0.1 | 1.6 ± 0.1 | 0.9 | 0.8 | |
| α,βMe-fATP | 8.1 ± 0.0 | 3.5 ± 0.0 | 0.1 | 1.0 | |
| hP2X2/3 | ATP | 2.7 ± 0.4 | 0.9 ± 0.1 | 1 | 1 |
| α,βMe-ATP | n.d. | n.d. | n.d. | n.d. | |
| fATP | 0.7 ± 0.3 | 0.9 ± 0.2 | 4.0 | 0.4 | |
| α,βMe-fATP | 1.5 ± 0.6 | 4.4 ± 3.5 | 1.8 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brünings, X.; Schmauder, R.; Mrowka, R.; Benndorf, K.; Sattler, C. Subtype-Specific Ligand Binding and Activation Gating in Homomeric and Heteromeric P2X Receptors. Biomolecules 2024, 14, 942. https://doi.org/10.3390/biom14080942
Brünings X, Schmauder R, Mrowka R, Benndorf K, Sattler C. Subtype-Specific Ligand Binding and Activation Gating in Homomeric and Heteromeric P2X Receptors. Biomolecules. 2024; 14(8):942. https://doi.org/10.3390/biom14080942
Chicago/Turabian StyleBrünings, Xenia, Ralf Schmauder, Ralf Mrowka, Klaus Benndorf, and Christian Sattler. 2024. "Subtype-Specific Ligand Binding and Activation Gating in Homomeric and Heteromeric P2X Receptors" Biomolecules 14, no. 8: 942. https://doi.org/10.3390/biom14080942
APA StyleBrünings, X., Schmauder, R., Mrowka, R., Benndorf, K., & Sattler, C. (2024). Subtype-Specific Ligand Binding and Activation Gating in Homomeric and Heteromeric P2X Receptors. Biomolecules, 14(8), 942. https://doi.org/10.3390/biom14080942






