Inflammation: Is It a Healer, Confounder, or a Promoter of Cardiometabolic Risks?
Abstract
1. Introduction
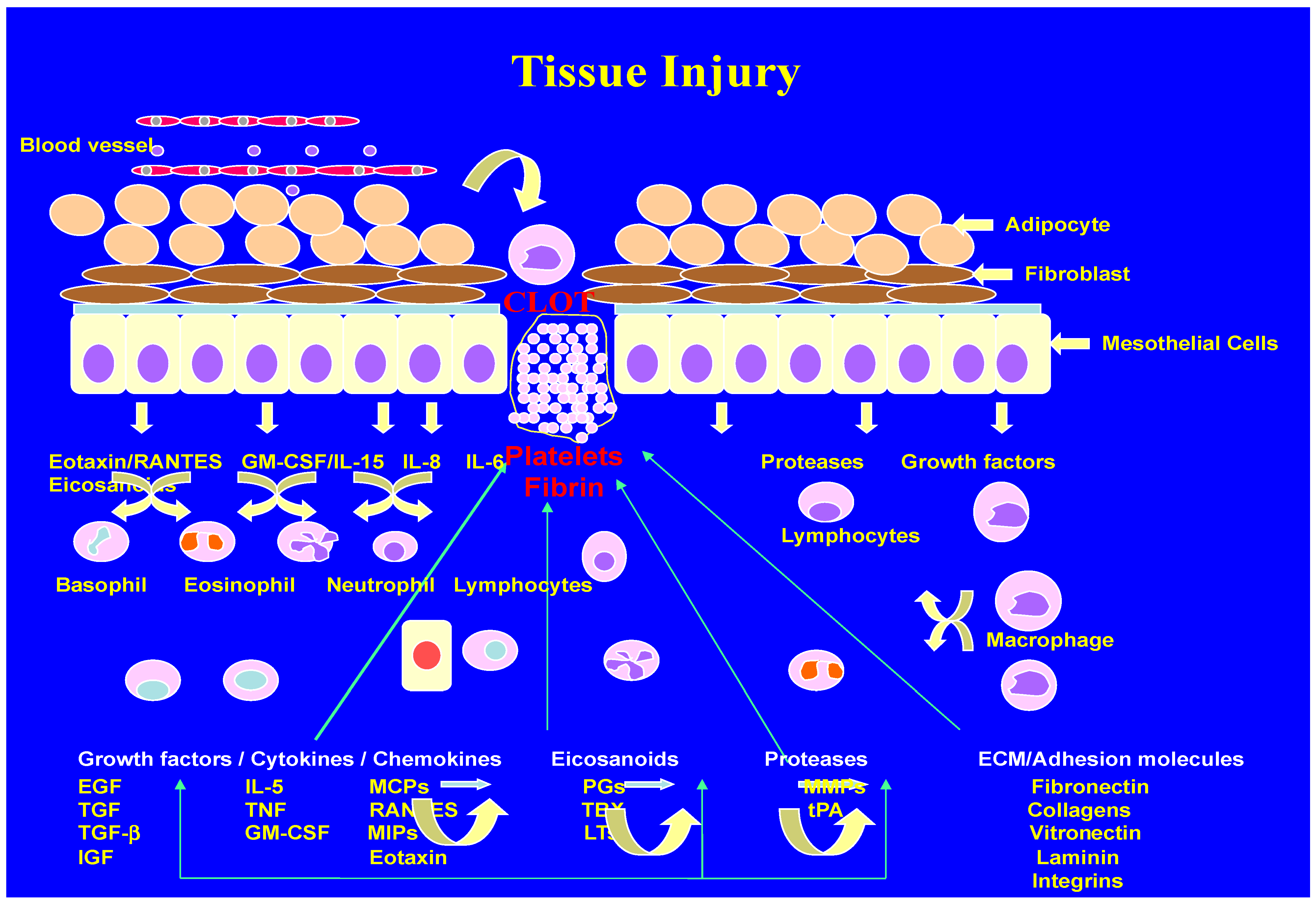
2. Inflammation and Wound Healing
3. Inflammation as a Promoter of Cardiometabolic Diseases
4. Oxidative Stress and Reactive Nitrogen Intermediates (RNIs) in Inflammation
5. Endothelial Dysfunction and Inflammation
6. Atherosclerosis and Inflammation
7. Hypertension and Inflammation
8. Obesity and Inflammation
9. Type-2 Diabetes and Inflammation
10. Arterial Occlusive Events and Inflammation
11. Discussion
12. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases and organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targa, S.; Francheschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Netea, M.G.; Balkwill, F.; Chonchol, M.; Cominelli, F.; Donath, M.Y.; Giamarellos-Bourboulis, E.J.; Golenbock, D.; Gresnigt, M.S.; Heneka, M.T.; Hoffman, H.M. A guiding map for inflammation. Nat. Immunol. 2017, 18, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Huber-Lang, M.; Lambris, J.D.; Ward, P.A. Innate immune response to trauma. Nat. Immunol. 2018, 19, 327–341. [Google Scholar] [CrossRef]
- Oberyszyn, T.M. Inflammation and wound healing. Front. Biosci. 2007, 12, 2993–2999. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Kriegt, T.; Davidson, J.M. Inflammation in wound repair: Molecular and Cellular Mechanisms. J. Investig. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef]
- Lee, Y.S.; Olefsky, J. Chronic tissue inflammation and metabolic disease. Genes Dev. 2021, 35, 307–328. [Google Scholar] [CrossRef] [PubMed]
- Hunter, P. The inflammation theory of disease. EMBO Rep. 2012, 13, 968–970. [Google Scholar] [CrossRef]
- Weavers, H.; Martin, P. The cell biology of inflammation: From common traits to remarkable immunological adaptations. J. Cell Biol. 2020, 219, e202004003. [Google Scholar] [CrossRef]
- Boyapati, R.; Satsangi, J.; Ho, G.T. Pathogenesis of Crohn’s disease. F1000Prime Rep. 2015, 7, 44. [Google Scholar] [CrossRef]
- Schottenfield, D.; Beebe-Dimmer, J. Chronic inflammation: A common and important factor in the pathogenesis of neoplasia. CA 2006, 56, 63–130. [Google Scholar] [CrossRef] [PubMed]
- Ansar, W.; Ghosh, S. Inflammation and inflammatory diseases, markers, and mediators: Role of CRP in some inflammatory diseases. In Biol of C Reactive Protein in Health and Disease; Springer: Berlin/Heidelberg, Germany, 2016; pp. 67–107. [Google Scholar] [CrossRef]
- Presscott, J.A.; Mitchell, J.P.; Cook, S.J. Inhibitory feedback control of NF-kB signaling in health and disease. Biochem. J. 2021, 478, 2619–2664. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wu, M. Pattern recognition receptors in health and diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef] [PubMed]
- Ain, Q.; Sarfraz, M.; Prasesti, G.K.; Dewi, T.I.; Kurniati, N.F. Confounders in identification and analysis of inflammatory biomarkers in cardiovascular diseases. Biomolecules 2021, 11, 1464. [Google Scholar] [CrossRef] [PubMed]
- Geovanini, G.R.; Libby, P. Atherosclerosis, and inflammation: Overview and updates. Clin. Sci. 2018, 132, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Soeki, T.; Sata, M. Inflammatory biomarkers and atherosclerosis. Int. Heart J. 2016, 57, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Tschopp, J. The Inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Sarbijani, H.M.; Khoshnia, M.; Marjani, A. The association between metabolic syndrome and serum levels of lipid peroxidation and interleukin 6 in Gorgan. Diabetes Metab. Syndr. Clin. Res. 2016, 10, S86–S89. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xian, X.; Wang, Z.; Bi, Y.; Chen, Q.; Han, D.T.; Chen, R. Research progress on the relationship between atherosclerosis and inflammation. Biomolecules 2018, 8, 80. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Tiwari, R.K.; Shukla, S.S.; Pandey, R.K. Current and future molecular mechanism of inflammation and arthritis. J. Pharmacopunct. 2020, 23, 54–61. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Inflammasome activation and regulation; toward a better understanding of complex mechanisms. Cell Discov. 2020, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Khare, S. Inflammasomes and Their Activation. Crit. Rev. Immunol. 2010, 30, 463–487. [Google Scholar] [CrossRef] [PubMed]
- Di Micco, A.; Frera, G.; Lugrin, J.; Jamilloux, Y.; Hsu, E.; Tardivel, A.; Gassart, A.D.; Zaffalon, L.; Bujisic, B.; Siegert, S. AIM2 inflammasome is activated by pharmacological disruption of nuclear envelope integrity. Proc. Natl. Acad. Sci. USA 2016, 113, E4671–E4680. [Google Scholar] [CrossRef] [PubMed]
- Yosipovitch, G.; Fast, K.; Bernard, J.D. Noxious heat and scratching decrease histamine induced itch and skin blood flow. J. Investig. Dermatol. 2005, 125, 1268–1272. [Google Scholar] [CrossRef] [PubMed]
- Montazersaheb, S.; Khatibi, S.M.H.; Hejazi, S.; Tahriz, C.; Farjami, A.; Ghasemian, F.; Farahzadi, R.; Ghasemnejad, T. COVID-19 infection: An overview on cytokine storm and related interventions. Virol. J. 2022, 19, 92. [Google Scholar] [CrossRef] [PubMed]
- O’ Halloran, J.A.; Ko, E.R.; Anstrom, K.J.; Kedar, E.; McCarthy, M.W.; Panettieri, R.A.; Maillo, M.; Numez, P.S.; Lachiewicz, A.M.; Gonzales, C. Abatacept, Cenicriviroc, or infliximab for treatment of adults hospitalized with COVID-19 Pneumonia. JAMA 2023, 330, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Bester, J.; Matshailwe, C.; Pretorius, E.J.C. Simultaneous presence of coagulation and increase clot lysis time due to IL-1B, IL-6 and IL-8. Cytokine 2018, 110, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Chu, J.; Feng, S. Immunological mechanisms of inflammatory diseases caused by gut microbiotat dysbiosis: A review. Biomed. Pharmacother. 2023, 164, 114985. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Islam, F.; Or-Rashid, M.H.; Mamun, A.L.; Rahaman, M.S.; Islam, M.M.; Meem, A.F.K.; Sutradhar, P.R.; Mitra, S.; Mimi, A.A. The gut microbiota (Microbiome) in cardiovascular disease and its therapeutic regulation. Front. Cell. Infect. Microbiol. 2022, 12, 903570. [Google Scholar] [CrossRef]
- Saylos-Baixeras, S.; Dekkers, K.F.; Baldanzi, G.; Jonsson, D.; Hammar, U.; Ahmad, S.; Nguyen, D.; Varotisis, G.; Nielsen, N.; Ekulnd, A.C. Streptococcus Species abundance in the gut is linked to subclinical coronary artery atherosclerosis in 8973 participants from the SCAPIS Cohort. Circulation 2023, 148, 459–472. [Google Scholar] [CrossRef]
- Jebasingh, F.; Thomas, N. Barker Hypothesis and Hypertension. Front. Public Health 2022, 9, 767545. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.B.; Misyak, A.S.; Hontecillas, R.; Bassagabay-Riera, J. Dietary modulation of inflammation-induced colorectal cancer through PPARγ. PPAR Res. 2009, 1, 498352. [Google Scholar] [CrossRef] [PubMed]
- Torday, J.S. Homeostasis as the Mechanism of Evolution. Biology 2015, 4, 573–590. [Google Scholar] [CrossRef] [PubMed]
- Almond, D.; Currie, J. Killing me Softly: The Fetal Origins Hypothesis. J. Econ. Prospect. 2011, 25, 153–172. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, S.C.; Nadler, E.P.; Pillai, D.K.; Hubal, M.J.; Wang, Z.; Wang, J.M.; Gordish-Dressman, H.; Koeck, E.; Sevilla, S.; Wiles, A.A. Adipocyte-derived exosomal miRNAs: A novel mechanism for obesity-related disease. Pediatr. Res. 2015, 77, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.H.R. Fetal Origin of Adult Diseases: Micronutrient and microRNA Interventions. Endocrinol. Metab. Res. 2019, 4.3, 7–16. [Google Scholar]
- Rao, G.H.R. Developmental origin of cardiometabolic diseases: Role of free fatty acids.EC Clin and Med. Case Rep. 2022, 5, 25–37. [Google Scholar]
- Yudkin, J.S.; Yajnik, C.S.; Mohamed-Ali, V.; Bulmer, K. High levels of circulating proinflammatory cytokines and leptin in urban, but not rural, Indians, A potential explanation for increased risk of diabetes and coronary heart disease. Diab. Care 1999, 22, 363–364. [Google Scholar] [CrossRef]
- Schulz, G.S.; Chin, G.A.; Moldawer, L.; Diegelmann, R. Principles of wound healing. In Mechanisms of Vascular Disease; Fitridge, R., Thompson, M., Eds.; University of Adelaide Press: Adelaide, Australia, 2011. Available online: https://www.ncbi.nlm.nih.gov/books/NBK534261/ (accessed on 30 July 2024).
- Rao, G.H.R. Manual of Blood Platelet Morphology. In Physiology and Pharmacology; Jaypee Medical Publishers: New Delhi, India, 2019; ISBN 978-93-270-202-2. [Google Scholar]
- Koh, T.J.; DiPietro, L.A. Inflammation and Wound Healing: The Role of the macrophage. Expert Rev. Mol. Med. 2011, 13, e23. [Google Scholar] [CrossRef]
- Martin, P.; Leibovich, S.J. Inflammatory cells during wound repair: The good, the bad and the ugly. Trends Cell Biol. 2005, 15, 599–607. [Google Scholar] [CrossRef]
- Tan, P.; Luscinskas, F.W.; Homer-Vanniasinkam, S. Cellular and molecular mechanisms of inflammation and thrombosis. Endovasc. Surg. 1999, 17, 373–389. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rao, G.; Bhat, P.; Nagesh, K.S.; Rao, G.H.R.; Mirle, B.; Kharbahri, L.; Gangapprasad, B. Bone regeneration in extraction sockets with autologous platelet rich fibrin gel. J. Oral Maxillofac. Surg. 2013, 12, 11–16. [Google Scholar]
- Roth, G.A.; Menash, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Neaton, A.; Benjamin, E.J.; Benzinger, C.P. Global burden of cardiovascular disease and risk factors. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.H.R. Prevention or reversal of cardiometabolic diseases. J. Clin. Prev. Cardiol. 2018, 7, 22–28. [Google Scholar] [CrossRef]
- Rao, G.H.R. COVID-19 and Cardiometabolic Diseases. EC Cardiol. 2020, 7, 8–12. [Google Scholar]
- Rao, G.H.R.; Gandhi, P.G.; Sharma, V. Clinical complications of type-2 diabetes mellitus in South Asians and Chinese Populations: An Overview. J. Diab. Metab. 2014, 5, 420. [Google Scholar] [CrossRef]
- Rao, G.H.R.; Thethi, I.; Fareed, J. Vascular disease: Obesity and excess weight as modulators of risk. Expert Rev. Cardiovasc. Ther. 2011, 994, 525–534. [Google Scholar] [CrossRef]
- Rao, G.H.R. Twindemic of coronavirus disease (COVID-19) and cardiometabolic diseases. Int. J. Bio Med. 2021, 11, 11–122. [Google Scholar] [CrossRef]
- Rao, G.H.R. Global syndemic of metabolic disease; Editorial Comments. J. Diab. Clin. Res. 2019, 1, 2–4. [Google Scholar] [CrossRef]
- Rao, G.H.R. Diabetes and cardiovascular disease in South Asians: A global perspective. J. Clin. Prev. Cardiol. 2018, 7, 161–167. [Google Scholar] [CrossRef]
- Rao, G.H.R. Coronavirus disease and acute vascular events. Clin. Appl. Thromb. Hemost. Editor. 2020, 26, 1076029620929091. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.H.R. Number one Killer: Vascular Disease. Ann. Clin. Diab. Endocrinol. 2018, 1, 1008. [Google Scholar]
- Rao, G.H.R. Risk prediction, assessment, and management of type-2 diabetes. EC Endocrinol. Metab. Res. 2018, 3, 30–41. [Google Scholar] [CrossRef]
- Agrawal, A.; Rao, G.H.R. Novel approaches for early diagnosis and prevention of cardiometabolic diseases. J. Clin. Prev. Cardiol. 2023, 12, 23–36. [Google Scholar]
- Rao, G.H.R. Obesity is a unique metabolic disease: An Update. EC Clin. Med. Case Rep. 2023, 6, 1–11. [Google Scholar]
- Xu, B.; Wu, Q.; La, L. Is systemic inflammation a missing link between cardiometabolic index with mortality? Evidence from a large population-based study. Cardiovasc. Diabetol. 2024, 23, 212. [Google Scholar] [PubMed]
- Kwon, D.H.; Cha, H.; Lee, H.; Hong, S.; Park, C.; Park, S.; Kim, S.; Kim, H.; Hwang, H.; Choi, Y.H. Protective effect of glutathione against stress-induced cytotoxicity in RAW264.7 macrophages through activating the nuclear factor erythroid 2-related factor-2/heme oxygenase-1 pathway. Antioxidants 2019, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.B.; Jasso, G.J.; Mok, A.; Clish, C.B.; Carr, S.A.; Xavier, R.J. Nitric oxide engages as anti-inflammatory feedback loop mediated by peroxiredoxin 5 in phagocytes. Cell Rep. 2018, 24, P838–P850. [Google Scholar] [CrossRef] [PubMed]
- Dubois-Deruy, E.; Peugnet, C.; Turkieh, A.; Pinet, F. Oxidative stress in cardiovascular diseases. Antioxidants 2020, 9, 864. [Google Scholar] [CrossRef]
- Ananda, J.F.; Madrigal-Matute, J.; Rotlan, N.; Fernandez-Hermando, C. MicroRNA modulation of lipid metabolism and oxidative stress in cardiometabolic diseases. Free Radic. Biol. Med. 2013, 64, 31–39. [Google Scholar] [CrossRef]
- Gaggini, M.; Ndreu, R.; Micelucci, E.; Rocchicciol, E.; Vassalle, C. Ceramides as mediators of oxidative stress and inflammation in cardiometabolic disease. Int. J. Mol. Sci. 2022, 23, 2719. [Google Scholar] [CrossRef] [PubMed]
- Hla, T.; Danneberg, A.J. Sphingolipid signaling in metabolic disorders. Cell Metab. 2012, 16, 420–434. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Uchida, Y.; Kobayashi, T.; Shirai, T.; Hiruta, N.; Shimoyama, E.; Tabata, T. Detection of ceramide, a risk factor for coronary artery disease, in human coronary plaques by fluorescent angioscopy. Circ. J. 2017, 81, 1886–1893. [Google Scholar] [CrossRef] [PubMed]
- Radha, E.; Hill, T.D.; Rao, G.H.R.; White, J.G. Glutathione levels inhuman platelet display a circadian rhythm in vitro. Thromb. Res. 1985, 40, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Manasa, K.; Vani, R. Influence of oxidative stress on stored platelets. Adv. Hematol. 2016, 1, 4091461. [Google Scholar] [CrossRef] [PubMed]
- Hill, T.D.; White, J.G.; Rao, G.H.R. Platelet hypersensitivity induced by 1-chloro-2,4-dinitrobenzene hydroperoxides and inhibition of lipoxygenase. Thromb. Res. 1989, 53, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Hill, T.D.; White, J.G.; Rao, G.H.R. Role of glutathione and glutathione peroxidase in human platelet arachidonic acid metabolism. Prostaglandins 1989, 38, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Hill, T.D.; White, J.G.; Rao, G.H.R. The influence of glutathione depleting agents on human platelet function. Thromb. Res. 1989, 53, 457–467. [Google Scholar] [CrossRef]
- Calzada, C.; Vericel, E.; Lagarde, M. Low concentration of lipid hydroperoxides prime human platelet aggregation specifically via cyclooxygenase activation. Biochem. J. 1997, 325 Pt 2, 495–500. [Google Scholar] [CrossRef]
- Yeungm, J.; Hawley, M.; Holinstat, M. The expansive role of oxylipins on platelet biology. J. Mol. Med. 2017, 95, 575–588. [Google Scholar] [CrossRef]
- Tennant, M.; McGeachie, J.K. Blood vessel structure and function: A brief update on recent advances. ANZ J. Surg. 1990, 60, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, C.; Tousoulis, D. Inflammatory mechanisms contributing to endothelial dysfunction. Biomedicines 2021, 9, 781. [Google Scholar] [CrossRef] [PubMed]
- Lerman, A.; Zehler, A.M. Endothelial Function: Cardiac Events. Circulation 2005, 111, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Hays, A.G.; Schar, M.; Bonanno, G.; Lai, S.; Meyer, J.; Afework, Y.; Steinberg, A.; Stradley, S.; Gerstenblith, G.; Weiss, R.G. Randomized trial of anti-inflammatory medications and coronary endothelial dysfunction in patients with stable coronary artery disease. Front. Cardiovasc. Med. 2021, 8, 728654. [Google Scholar] [CrossRef] [PubMed]
- Gounari, P.; Tousoulis, D.; Antoniades, C.; Kampoli, A.M.; Stougiannos, P.; Papageorgiou, N.; Roulia, G.; Stefanad, E.; Siasos, G.; Tsioufis, C. Rosuvastatin but not ezetimide improves endothelial function in patients with heart failure, by mechanisms independent of lipid lowering. Int. J. Cardiol. 2010, 142, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.H.R. Influence of anti-platelet drugs on platelet-vessel wall interactions. Prostag. Leukot. Med. 1987, 30, 133–145. [Google Scholar]
- Rao, G.H.R. Inflammation: Need for the Development of Novel Immunomodulators. Acta Sci. Pharmacol. 2021, 2, 05–09. [Google Scholar]
- Shah, B.; Mayer, L. Current status of monoclonal antibody therapy for the treatment of inflammatory bowel disease. Expert Rev. Clin. Immunol. 2010, 6, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Ross, R. Atherosclerosis- An inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef]
- Noz, P.M.; Bekkering, S.; Groh, L.; Nielen, T.M.J.; Lamfers, E.J.P.; Schlitzer, A.; Messaoudi, S.E.L.; Royen, N.; Huys, E.H.J.P.G.; Preijers, F.W.M.B. Reprogramming of bonemarrow myeloid progenitor cells in patients with severe coronary artery disease. eLife 2020, 9, e60939. [Google Scholar] [CrossRef]
- Keener, A.B. Immune cells that remember inflammation could offer treatment targets for atherosclerosis. Nature 2021, 594, S10–S11. [Google Scholar] [CrossRef]
- Engelen, S.E.; Robinson, A.J.B.; Zurke, Y.; Monaco, C. Therapeutic strategies targeting inflammation and immunity in atherosclerosis: How to proceed. Nat. Rev. Cardiol. 2022, 19, 522–542. [Google Scholar] [CrossRef] [PubMed]
- Sauter, M.; Langer, H.F. Targeting Cell-specific molecular mechanisms of innate immunity in atherosclerosis. Front. Physiol. 2022, 13, 802990. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, Y.; Macfadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Ankler, S.D. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Spence, J.D. Measurement of Carotid plaque burden. JAMA Neurol. 2015, 72, 383–384. [Google Scholar] [CrossRef] [PubMed]
- Spence, J.D.; Hackam, D.G. Treating arteries instead of risk factors: A paradigm change in management of atherosclerosis. Stroke 2010, 41, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Orekhov, A.N.; Ivanova, E.A. Introcuction of the special issue “Atherosclerosis and Related Diseases”. Vessel Plus. 2017, 1, 163–165. [Google Scholar]
- Mandraffino, G.; Mattina, A.; Scicali, R. Emerging Circulating Biomarkers in Atherosclerosis: From Molecular Mechanisms to Therapeutic Strategies. Biomolecules 2022, 12, 809. [Google Scholar] [CrossRef]
- Komatsu, T.; Abe, S.; Nakashima, S.; Sasaki, K.; Higaki, Y.; Saku, K.; Miura, S.-i.; Uehara, Y. Dipeptidyl Peptidase-4 Inhibitor Sitagliptin Phosphate Accelerates Cellular Cholesterol Efflux in THP-1 Cells. Biomolecules 2023, 13, 228. [Google Scholar] [CrossRef]
- Mills, K.T.; Stefanescu, A.; He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef]
- Patrick, D.M.; van Beusecum, J.P.; Kirabo, A. The role of inflammation in hypertension: Novel concepts. Curr. Opin. Physiol. 2021, 19, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.S.; May-Whang, L.S. Isolevuglandins and Cardiovascular Disease. Prostag. Other Lipid Mediat. 2018, 139, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.S.; May-Zhang, L.S.; Boutaud, O.; Amarnath, V.; Kirabo, A.; Harrison, D.G. Isolevuglandins as mediators of diseases and the development of dicarbonyl scavengers as pharmaceutical interventions. Pharmocol. Ther. 2020, 205, 107418. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, J.; Visitacion, N.; Hennen, E.M.; Harrison, D.G.; Patrick, D.M. IsoLG drive neutrophil migration in hypertension and are essential for the formation of neutrophil traps. Hypertension 2022, 79, 1644–1655. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.H.R.; Mahadevappa, V.G.; Hill, T.D.; White, J.G. Arachidonic acid oxidation and platelet function. In Biological Oxidation Systems; Reddy, C.C., Hamilton, G.A., Madyasta, K.M., Eds.; Academic Press: Cambridge, MA, USA, 1990; pp. 770–790. [Google Scholar]
- Prinsen, J.K.; Kannankerl, P.J.; Sidorova, T.N.; Yermalitskaya, L.V.; Boutaud, O.; Zagol-Ikapittew, I.; Barnett, J.V.; Murphy, M.B.; Subati, T.; Stark, J.M.; et al. Highly reactive isolevuglandin promote atrial fibrillation caused by hypertension. J. Am. Coll. Cardiol. 2020, 5, 602–615. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Chen, J.L. Inflammation may be a bridge connecting hypertension and atherosclerosis. Med. Hypoth. 2005, 64, 925–929. [Google Scholar] [CrossRef] [PubMed]
- Dandona, P.; Dhinsa, S.; Ghanim, H.; Chaudhuri, A. Angiotensin 11, and inflammation: The effect of angiotensin-concerting enzyme inhibition and angiotensin 11 receptor blockade. J. Hum. Hypertens. 2007, 21, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Koh, K.K.; Oh, P.C.; Quon, M.J. Does reversal of oxidative stress and inflammation provide vascular protection? Cardiovasc. Res. 2009, 81, 649–659. [Google Scholar] [CrossRef]
- Hossain, P.; Kawar, B.; Nahas, M. Obesity and diabetes in the developing world-a growing challenge. N. Engl. J. Med. 2007, 356, 213–215. [Google Scholar] [CrossRef]
- Hildebrandt, X.; Ibrahim, M.; Peltzer, N. Cell death and inflammation during obesity: “Know my methods, WAT (son)”. Nat. Cell Death Differ. 2023, 30, 279–292. [Google Scholar] [CrossRef]
- Khanna, D.; Khanna, P.; Kahar, P.; Patel, B.M. Obesity: A chronic low-grade inflammation and its markers. Cereus 2022, 14, e22711. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; An, Y.A.; Kim, M.; Zhang, Z.; Zhao, S.; Zhu, Y.; Asterholm, I.W.; Kusminski, C.M.; Scherer, P.E. Suppressing adipocyte inflammation promotes insulin resistance in mice. Mol. Metab. 2020, 39, 101010. [Google Scholar] [CrossRef] [PubMed]
- da Cruz Nascimento, S.S.; de Queiroz, J.L.C.; de Medeiros, A.F.; de Franca Nunes, A.C.; Piuvezam, G.; Lima Maciel, B.L.; Passos, T.S.; de Araujo Morais, A.H. Anti-inflammatory agents as modulators of the inflammation in adipose tissue: A systematic review. PLoS ONE 2022, 17, eo273942. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wang, M.; Long, Z.; Ning, H.; Li, J.; Cao, Y.; Liao, Y.; Liu, G.; Wang, F.; Pan, A. Global burden of type-2 diabetes in adolescents and young adults, 1990–2019: Systematic analysis of the Global Burden of Disease Study 2019. BMJ 2022, 379, e072385. [Google Scholar] [CrossRef]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Robertson, P. Chronic oxidative stress as a central mechanism of glucose toxicity in pancreatic islet beta cells in diabetes. J. Biol. Chem. 2004, 279, 42351–42354. [Google Scholar] [CrossRef]
- Pathofer, K.G. Interaction between glucose and lipid metabolism: More than diabetic dyslipidemia. Diab. Metab. J. 2015, 3995, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Bevan, P. Insulin signaling. Cell Sci. 2001, 114, 1429–1430. [Google Scholar] [CrossRef]
- de Luca, C.; Olefsky, J.M. Inflammation and Insulin Resistance. FEBS Lett. 2008, 582, 97–105. [Google Scholar] [CrossRef]
- Sun, Q.; Gao, F. New insights into insulin: The anti-inflammatory effect and its clinical relevance. World J. Diab. 2014, 5, 89–96. [Google Scholar] [CrossRef]
- Li, D.; Zhong, J.; Zhang, Q.; Zhang, J. Effects of anti-inflammatory therapies on glycemic control in type 2 diabetes mellitus. Front. Immunol. 2023, 14, 1125116. [Google Scholar] [CrossRef] [PubMed]
- Insull, W. The pathology of atherosclerosis: Plaque development and plaque responses to medical treatment. Am. J. Med. 2009, 122, S3–S14. [Google Scholar] [CrossRef]
- Henein, M.; Vancheri, S.; Longo, G.; Vancheri, F. The role of inflammation in cardiovascular disease. Int. J. Mol. Sci. 2022, 23, 12906. [Google Scholar] [CrossRef] [PubMed]
- Santilli, F.; Simeone, P.; Liani, R. The role of platelets in diabetes mellitus, Chapter 27—The Role of Platelets in Diabetes Mellitus. In Platelets, 4th ed.; Science Direct: Amsterdam, The Netherlands, 2019; pp. 469–503. [Google Scholar] [CrossRef]
- Rao, G.H.R. Hyperglycemia, Dyslipidemia, and Diabetes Mellitus. Diab. Obes. Int. J. 2019, 4, 000198. [Google Scholar]
- Rao, G.H.R. Altered glucose metabolism and acute vascular events. J. Clin. Cardiol. Diagn. 2022, 4, 9–16. [Google Scholar]
- Scherlinger, M.; Richez, C.; Tsokos, G.C.; Bollard, E.; Blanco, P. The role of platelets in immune-mediated inflammatory diseases. Nat. Rev. Immunol. 2023, 23, 495–510. [Google Scholar] [CrossRef] [PubMed]
- Regmi, M.; Siccardi, M.A. Coronary Artery Disease Prevention. In StatPearls; StatPearls: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK547760/ (accessed on 30 July 2024).
- Silverio, A.; Cancerro, F.P.; Esposito, L.; Bellino, M.; Verdoia, M.; Vasallo, M.G.; Ciccarelli, M.; Vecchione, C.; Luca, G.D. Secondary cardiovascular prevention of acute coronary syndrome: Emerging risk factors and novel therapeutic targets. J. Clin. Med. 2023, 12, 2161. [Google Scholar] [CrossRef] [PubMed]
- Gerrard, J.; Stuart, M.J.; Rao, G.H.R.; Steffes, M.W.; Brown, D.M.; White, J.G. Alteration in the balance of prostaglandin and thromboxane synthesis in diabetic rats. J. Lab. Clin. Med. 1980, 95, 950–958. [Google Scholar]
- Sharma, P. Inflammation and the Metabolic Syndrome. Ind. J. Clin. Biochem. 2011, 26, 317–318. [Google Scholar] [CrossRef]
- Donath, M.Y.; Meier, D.T.; Boni-Schnetzler, M. Inflammation in the pathophysiology and therapy of cardiometabolic disease. Endocr. Rev. 2019, 40, 1080–1091. [Google Scholar] [CrossRef]
- Banait, T.; Wanjari, A.; Danade, V.; Banait, S.; Jain, J. Role of high sensitivity C-Reactive Protein (Hs-CRP) in non-communicable disease: A Review. Cureus 2022, 14, e30335. [Google Scholar] [CrossRef] [PubMed]
- Burger, P.M.; Pradhan, A.D.; Dorresteijn, J.A.N.; Koudstaal, S.; Teraa, M.; deBorst, G.J.; van der Meer, M.D.; Mosterd, A. C-Reactive Protein and risk of cardiovascular events and mortality in patients with various cardiovascular disease locations. Am. J. Cardiol. 2023, 197, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, E.R.; Toliver-Kinsky, T. Mechanisms of the inflammatory response. Best Pract. Res. Clin. Anaesthesiol. 2004, 18, 385–405. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Jia, M.; Li, L.; Liu, D.; Chu, S.; Li, H. Association of circulating inflammatory proteins with type 2 diabetes mellitus and its complications: A bidirectional Mendelian randomized study. Front. Endocrinol. 2024, 15, 1358311. [Google Scholar] [CrossRef]
- Hotamisligi, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tate, A.R.; Rao, G.H.R. Inflammation: Is It a Healer, Confounder, or a Promoter of Cardiometabolic Risks? Biomolecules 2024, 14, 948. https://doi.org/10.3390/biom14080948
Tate AR, Rao GHR. Inflammation: Is It a Healer, Confounder, or a Promoter of Cardiometabolic Risks? Biomolecules. 2024; 14(8):948. https://doi.org/10.3390/biom14080948
Chicago/Turabian StyleTate, Amit R., and Gundu H. R. Rao. 2024. "Inflammation: Is It a Healer, Confounder, or a Promoter of Cardiometabolic Risks?" Biomolecules 14, no. 8: 948. https://doi.org/10.3390/biom14080948
APA StyleTate, A. R., & Rao, G. H. R. (2024). Inflammation: Is It a Healer, Confounder, or a Promoter of Cardiometabolic Risks? Biomolecules, 14(8), 948. https://doi.org/10.3390/biom14080948






