The Role of Melatonin in the Inflammatory Process in Patients with Hyperglycemia and Leishmania Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Inclusion and Exclusion Criteria
2.3. Subjects
2.4. Sample Processing
2.5. Glucose Determination
2.6. Melatonin Hormone Dosage
2.7. C-Reactive Protein Determination
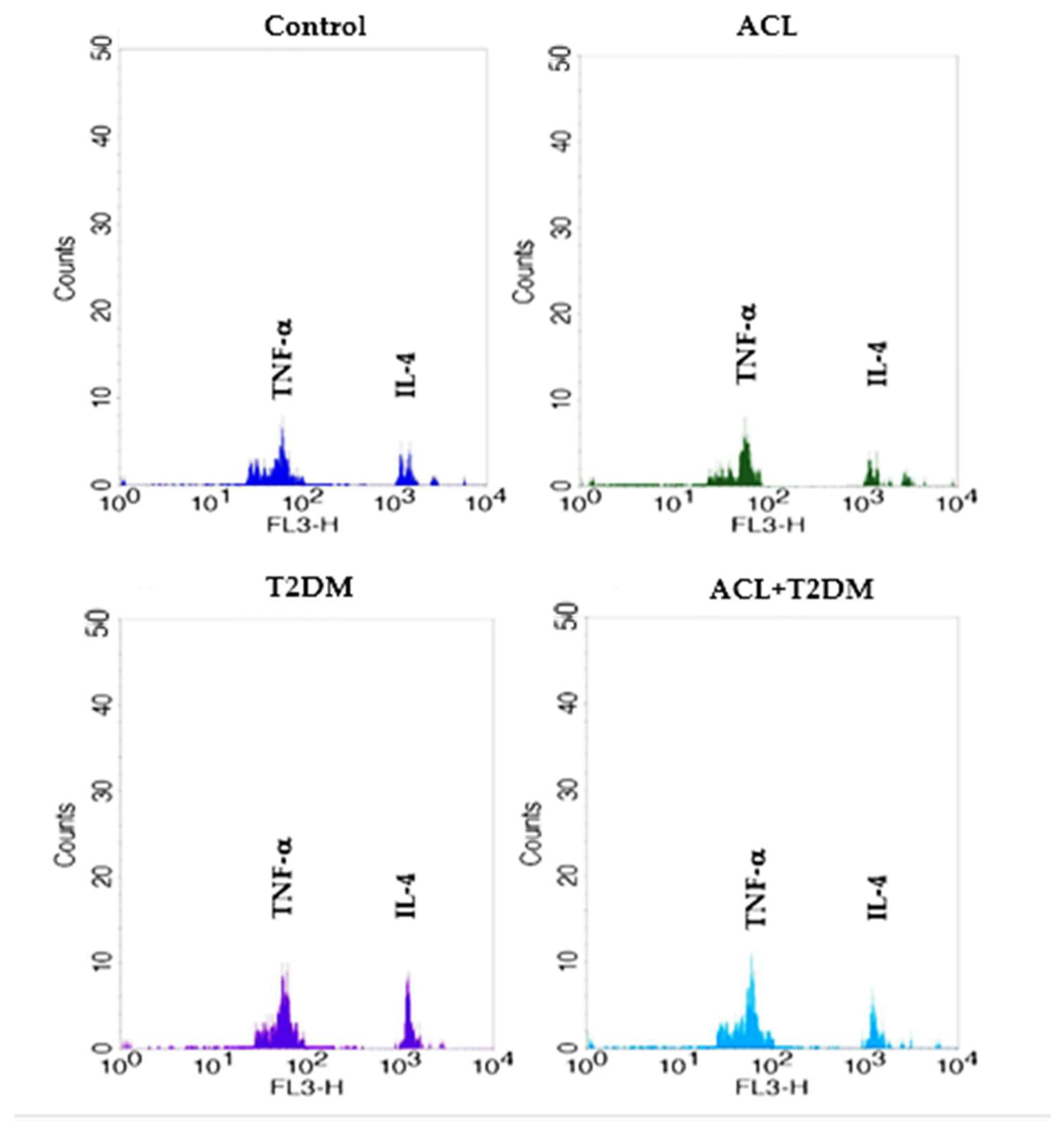
2.8. Cytokine Detection by Flow Cytometry
2.9. Statistical Analysis
3. Results
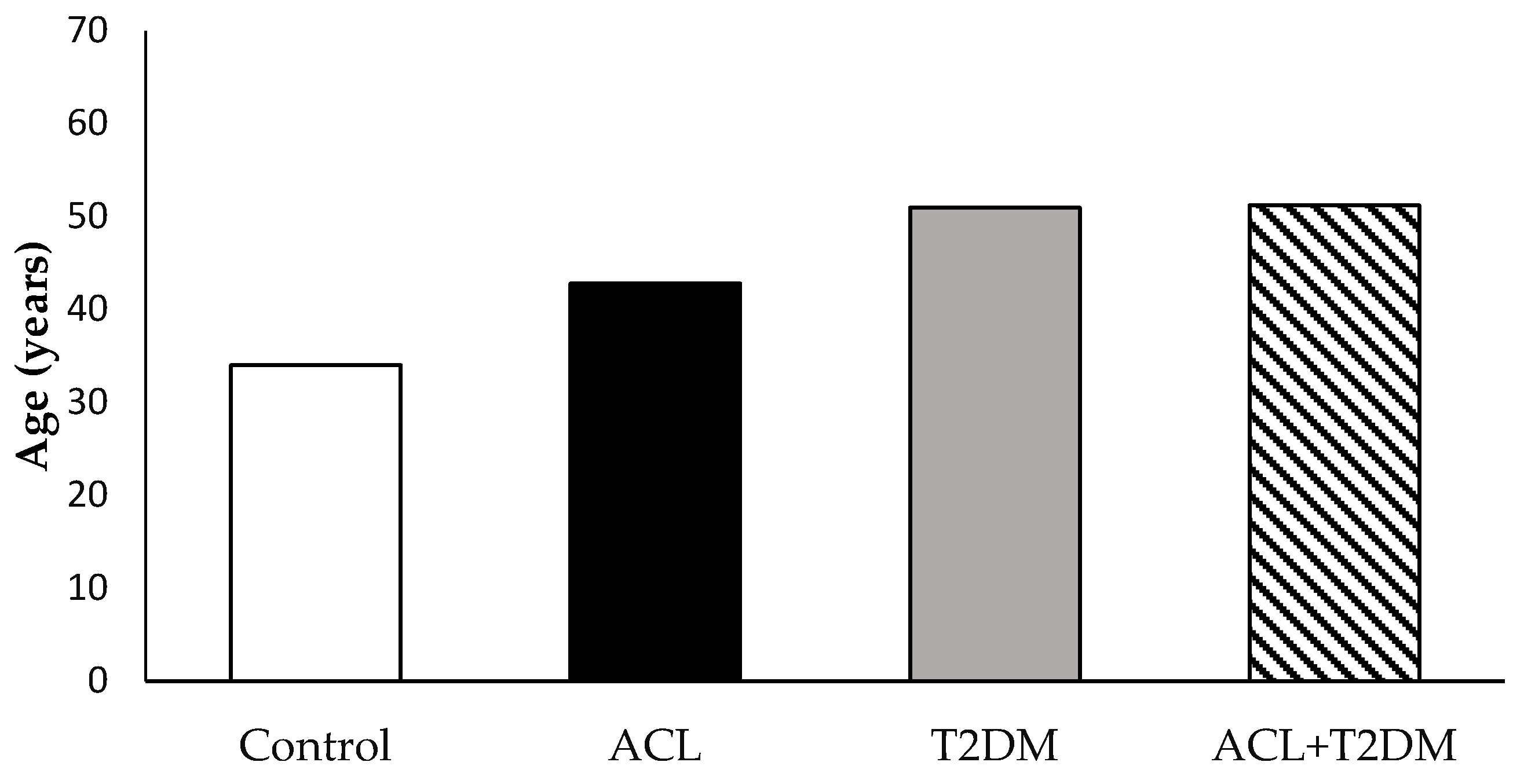
3.1. The Characterization of the Population Studied
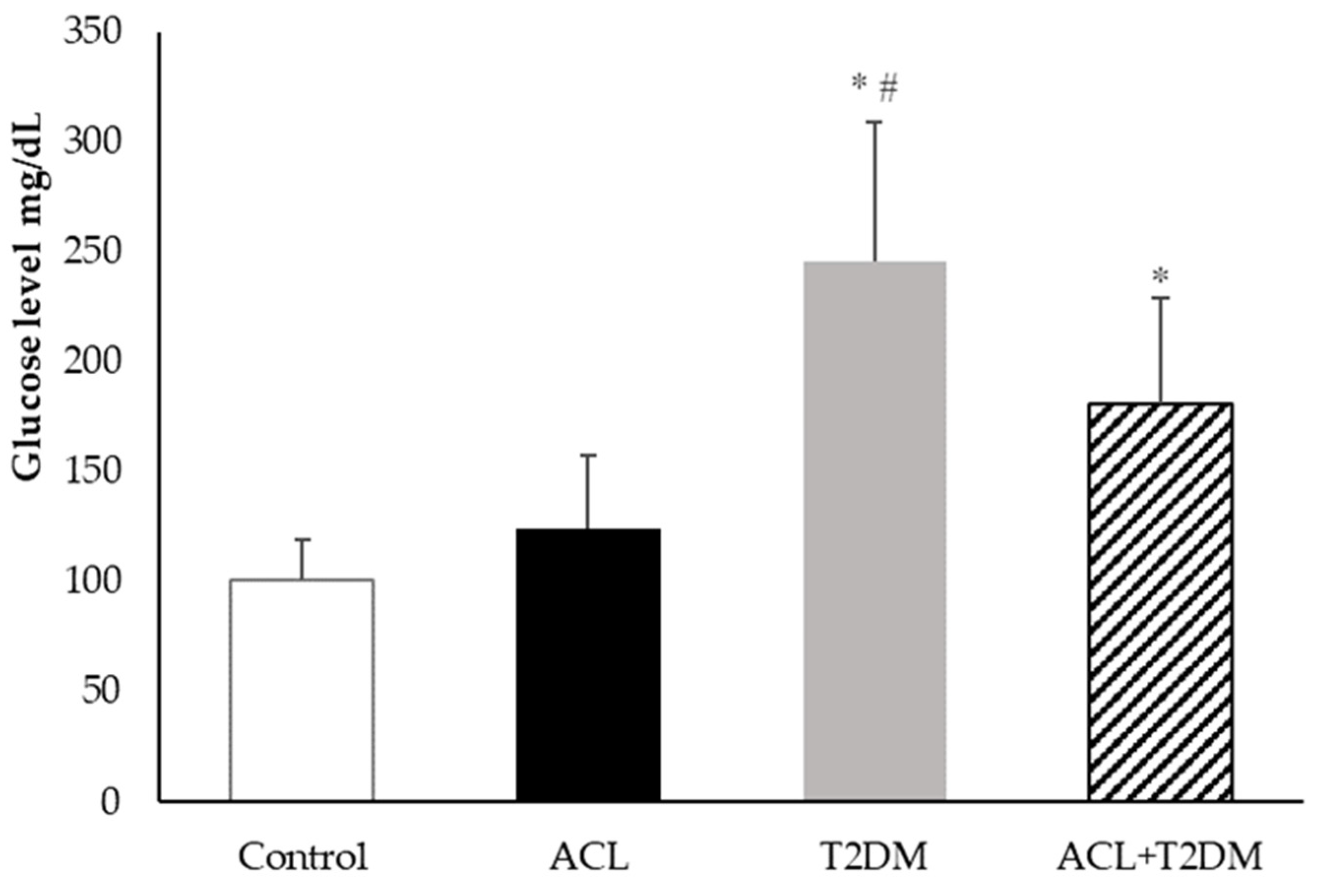
3.2. Plasma Blood Glucose Measurement
3.3. Melatonin Hormone Concentration
3.4. C-Reactive Protein Level
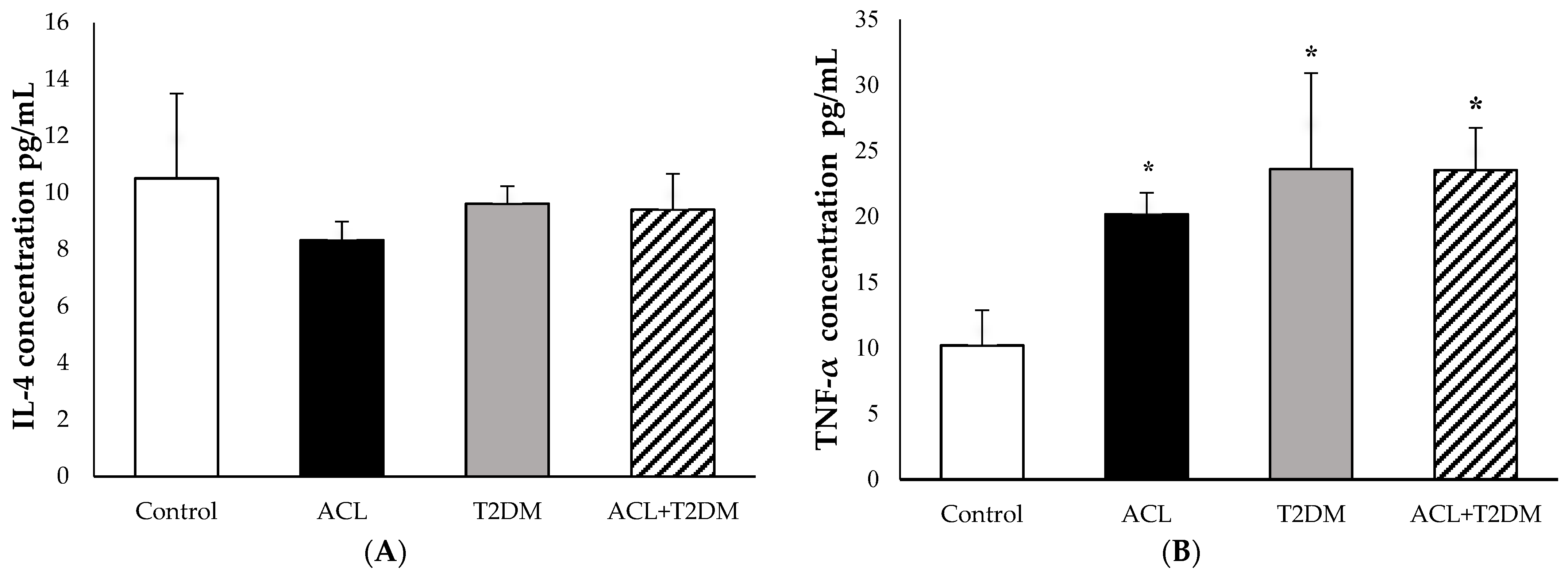
3.5. The Concentration of IL-4 and TNF-α Cytokines
3.6. Correlation of Melatonin Hormone and Inflammatory Mediators
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Magliano, D.J.; Boyko, E.J. Committee International Diabetes Federation. In IDF Diabetes Atlas; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Czech, M.P. Mechanisms of insulin resistance related to white, beige, and brown adipocytes. Mol. Metab. 2020, 34, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.O.; Olvera, A.C.; Marti, A.; Fang, S.; White, J.R.; Westphal, M.; Hewezi, R.; AshShareef, S.T.; García-Peña, L.M.; Koneru, J.; et al. OPA1 Regulates Lipid Metabolism and Cold-Induced Browning of White Adipose Tissue in Mice. Diabetes 2022, 71, 2572–2583. [Google Scholar] [CrossRef] [PubMed]
- Alshaya, O.A.; Korayem, G.B.; Alghwainm, M.; Alyami, W.; Alotaibi, A.; Alyami, M.S.; Almohammed, O.A. The prevalence of cardiovascular diseases, chronic kidney disease, and obesity in patients with type 2 diabetes mellitus and the description of concurrent treatments: A two-center retrospective cross-sectional study in Saudi Arabia. Saudi Pharm. J. 2024, 32, 102054. [Google Scholar] [CrossRef] [PubMed]
- Dhar, A.K.; Anitha, K.; Bhatt, S. Immune system and diabetes mellitus. In Biochemical Immunology of Diabetes and Associated Complications; Academic Press: Cambridge, MA, USA, 2024; pp. 19–27. [Google Scholar] [CrossRef]
- Rocha, R.; Pereira, A.; Maia, C. Non-Endemic Leishmaniases Reported Globally in Humans between 2000 and 2021—A Comprehensive Review. Pathogens 2022, 11, 921. [Google Scholar] [CrossRef] [PubMed]
- Morceli, G.; Honorio-França, A.C.; Fagundes, D.L.G.; Calderon, I.M.P.; Franca, E.L. Antioxidant effect of melatonin on the functional activity of colostral phagocytes in diabetic women. PLoS ONE 2013, 8, e56915. [Google Scholar] [CrossRef] [PubMed]
- Fagundes, D.L.G.; França, E.L.; Morceli, G.; Rudge, M.V.C.; Calderon, I.M.P.; Honorio-França, A.C. The Role of Cytokines in the Functional Activity of Phagocytes in Blood and Colostrum of Diabetic Mothers. Clin. Dev. Immunol. 2013, 2013, 590190. [Google Scholar] [CrossRef] [PubMed]
- Fagundes, D.L.G.; França, E.L.; Fernandes, R.T.S.; Hara, C.C.P.; Morceli, G.; Honorio-França, A.C.; Calderon, I.M.P. Changes in T cell phenotype and cytokines profile in maternal blood, cord blood and colostrum of diabetic mothers. J. Matern. Fetal Med. Neonatal. 2016, 29, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Souza, E.G.; Hara, C.C.P.; Fagundes, D.L.G.; Queiroz, A.A.; Morceli, G.; Calderon, I.M.P.; França, E.L.; Honorio-França, A.C. Maternal–Foetal Diabetes Modifies Neonatal Fc Receptor Expression on Human Leucocytes. Scand. J. Immunol. 2016, 84, 237–244. [Google Scholar] [CrossRef]
- WHO. Leishmaniasis. 2023. Available online: http://www.who.int/mediacentre/factsheets/fs375/en/ (accessed on 18 May 2024).
- Carvalho, L.S.; Braga, M.G.; Costa, D.A.S.; Simões, T.C.; Lula, M.D.; Silveira, M.R. Lethality among individuals infected with visceral leishmaniasis in Brazil: A retrospective study (2007–2018). Parasitol. Res. 2022, 121, 725–736. [Google Scholar] [CrossRef]
- de Sousa, J.M.S.; Nunes, T.A.L.; Rodrigues, R.R.L.; de Sousa, J.P.A.; Val, M.C.A.; Coelho, F.A.C.; Santos, A.L.S.; Maciel, N.B.; de Souza, V.M.R.; Machado, Y.A.A.; et al. Cytotoxic and Antileishmanial Effects of the Monoterpene β-Ocimene. Pharmaceuticals 2023, 16, 183. [Google Scholar] [CrossRef]
- Sandoval Pacheco, C.M.; Araujo Flores, G.V.; Ferreira, A.F.; da Matta, V.L.; de Castro Gomes, C.M.; Sosa-Ochoa, W.H.; Zuniga, C.; Silveira, F.T.; Corbett, C.E.P.; Laurenti, M.D. Role of antigen-presenting cells in non-ulcerated skin lesions caused by Leishmania (Leishmania) infantum chagasi. Parasite Immunol. 2023, 45, 12971. [Google Scholar] [CrossRef] [PubMed]
- Osero, B.O.; Arulebac, R.T.; Brombachera, F.; Hurdayal, R. Unravelling the unsolved paradoxes of cytokine families in host resistance and susceptibility to Leishmania infection. Cytokine X 2020, 2, 100043. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.M.; Costa, R.S.; Lago, A.; Bacellar, O.; Beiting, D.P.; Scott, P.; Carvalho, L.P.; Carvalho, E.M. In Situ versus Systemic Immune Response in the Pathogenesis of Cutaneous Leishmaniasis. Pathogens 2024, 13, 199. [Google Scholar] [CrossRef] [PubMed]
- Dubie, T.; Mohammed, Y. Review on the role of host immune response in protection and immunopathogenesis during cutaneous leishmaniasis infection. J. Immunol. Res. 2020, 2020, 2496713. [Google Scholar] [CrossRef] [PubMed]
- Prasad, E.M. Is the C-Reactive Protein (CRP) test a Worthy Indicator of Inflammation? Lond. J. Med. Health Res. 2023, 23, 45–58. [Google Scholar]
- Bhattacharya, S.; Munshi, C. Biological significance of C-reactive protein, the ancient acute phase functionary. Front. Immunol. 2023, 14, 1238411. [Google Scholar] [CrossRef] [PubMed]
- Markozannes, G.; Koutsioumpa, C.; Cividini, S.; Monori, G.; Tsilidis, K.K.; Kretsavos, N.; Theodoratou, E.; Gill, D.; Ioannidis, J.P.; Tzoulaki, I. Global assessment of C-reactive protein and health-related outcomes: An umbrella review of evidence from observational studies and Mendelian randomization studies. Eur. J. Epidemiol. 2021, 36, 11–36. [Google Scholar] [CrossRef] [PubMed]
- Rizo-Téllez, S.A.; Sekheri, M.; Filep, J.G. C-reactive protein: A target for therapy to reduce inflammation. Front. Immunol. 2023, 14, 1237729. [Google Scholar] [CrossRef] [PubMed]
- Zarezadeh, M.; Khorshidi, M.; Emami, M.; Janmohammadi, P.; Kord-Varkaneh, H.; Mousavi, S.M.; Mohammed, S.H.; Saedisomeolia, H.; Alizadeh, S. Melatonin supplementation and pro-inflammatory mediators: A systematic review and meta-analysis of clinical trials. Eur. J. Nutr. 2020, 59, 1803–1813. [Google Scholar] [CrossRef]
- Rathwa, N.; Patel, R.; Palit, S.P.; Parmar, N.; Rana, S.; Ansari, M.I.; Begum, R. β-Cell Replenishment: Possible Curative Approaches for Diabetes Mellitus. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1870–1881. [Google Scholar] [CrossRef]
- Acuña-Castroviejo, D.; Escames, G.; Venegas, C.; Díaz-Casado, M.E.; Lima-Cabello, E.; López, L.C.; Rosales-Corral, S.; Tan, D.; Reiter, R.J. Extrapineal melatonin: Sources, regulation, and potential functions. Cell. Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef]
- Slominski, A.T.; Zmijewski, M.A.; Semak, I.; Kim, T.K.; Janjetovic, Z.; Slominski, R.M.; Zmijewski, J.W. Melatonin, mitochondria, and the skin. Cell. Mol. Life Sci. 2017, 74, 3913–3925. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.-K.; Slominski, R.M.; Song, Y.; Qayyum, S.; Placha, W.; Janjetovic, Z.; Kleszczyński, K.; Atigadda, V.; Song, Y.; et al. Melatonin and Its Metabolites Can Serve as Agonists on the Aryl Hydrocarbon Receptor and Peroxisome Proliferator-Activated Receptor Gamma. Int. J. Mol. Sci. 2023, 24, 15496. [Google Scholar] [CrossRef]
- Slominski, R.M.; Reiter, R.J.; Schlabritz-Loutsevitch, N.; Ostrom, R.S.; Slominski, A.T. Melatonin membrane receptors in peripheral tissues: Distribution and functions. Mol. Cell. Endocrinol. 2012, 351, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin Synthesis and Function: Evolutionary History in Animals and Plants. Front. Endocrinol. 2019, 10, 249. [Google Scholar] [CrossRef]
- Maestroni, G.J.M. Role of Melatonin in Viral, Bacterial and Parasitic Infections. Biomolecules 2024, 14, 356. [Google Scholar] [CrossRef] [PubMed]
- Carretero, V.J.; Ramos, E.; Segura-Chama, P.; Hernández, A.; Baraibar, A.M.; Álvarez-Merz, I.; Muñoz, F.L.; Egea, J.; Solís, J.M.; Romero, A.; et al. Non-Excitatory Amino Acids, Melatonin, and Free Radicals: Examining the Role in Stroke and Aging. Antioxidants 2023, 12, 1844. [Google Scholar] [CrossRef] [PubMed]
- Garaulet, M.; Gómez-Abellán, P.; Rubio-Sastre, P.; Madrid, J.A.; Saxena, R.; Scheer, F.A.J.L. Common type 2 diabetes risk variant in MTNR1B worsens the deleterious effect of melatonin on glucose tolerance in humans. Metabolism 2015, 64, 1650–1657. [Google Scholar] [CrossRef]
- Karasek, M. Melatonin, human aging, and age-related diseases. Exp. Gerontol. 2004, 39, 1723–1729. [Google Scholar] [CrossRef]
- Katsarou, A.; Gudbjörnsdottir, S.; Rawshani, A.; Dabelea, D.; Bonifacio, E.; Anderson, B.J.; Jacobsen, L.M.; Desmond, A.S.; Lernmark, Å. Type 1 diabetes mellitus. Nat. Rev. Dis. Primers 2017, 3, 17016. [Google Scholar] [CrossRef]
- Vincent, A.M.; Callaghan, B.C.; Smith, A.L.; Feldman, E.L. Diabetic neuropathy: Cellular mechanisms as therapeutic targets. Nat. Rev. Neurol. 2011, 7, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Quan, Y.; Liu, Y.; Liu, K.; Li, H.; Jiang, Z.; Lai, Y. Hyperglycaemia inhibits REG3A expression to exacerbate TLR3-mediated skin inflammation in diabetes. Nat. Commun. 2016, 7, 13393. [Google Scholar] [CrossRef] [PubMed]
- Goonoo, N.; Laetitia Huët, M.A.; Chummun, I.; Karuri, N.; Badu, K.; Gimié, F.; Bergrath, J.; Schulze, M.; Müller, M.; Bhaw-Luximon, A. Nanomedicine-based strategies to improve treatment of cutaneous leishmaniasis. R. Soc. Open Sci. 2022, 15, 220058. [Google Scholar] [CrossRef] [PubMed]
- Castle, S.C. Relevância clínica da disfunção imunológica relacionada à idade. Clin. Infect. Dis. 2000, 31, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Arlt, W.; Hewison, M. Hormones and immune function: Implications of aging. Aging Cell 2004, 3, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Terzieva, D.D.; Mateva, N.D.; Vladimirova-Kitova, L.G. Melatonin reference limits at 3:00 AM and 8:00 AM in healthy adults. Clin. Lab. 2009, 55, 359–361. [Google Scholar] [PubMed]
- Brun, J.; Chamba, G.; Khalfallah, Y.; Girard, P.; Boissy, I.; Bastuji, H.; Claustrat, B. Effect of modafinil on plasma melatonin, cortisol and growth hormone rhythms, rectal temperature and performance in healthy subjects during a 36 h sleep deprivation. J. Sleep Res. 1998, 7, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Melone, M.A.; Becker, T.C.; Wendt, L.H.; Ten Eyck, P.; Patel, S.B.; Poston, J.; Pohlman, M.; Miller, A.; Nedeltcheva, A.; Hall, J.B.; et al. Disruption of the circadian rhythm of melatonin: A biomarker of critical illness severity. Sleep Med. 2023, 110, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.K.; Khan, M.I.; Ashfaq, F.; Rizvi, S.I. Crosstalk between aging, circadian rhythm, and melatonin. Rejuvenation Res. 2023, 26, 229–241. [Google Scholar] [CrossRef]
- Markus, R.P.; Fernandes, P.A.; Kinker, G.S.; da Silveira Cruz-Machado, S.; Marçola, M. Immune-pineal axis-acute inflammatory responses coordinate melatonin synthesis by pinealocytes and phagocytes. Br. J. Pharmacol. 2018, 175, 3239–3250. [Google Scholar] [CrossRef]
- Silva, T.F.; Detoni, M.B.; Concato-Lopes, V.M.; Tomiotto-Pellissier, F.; Miranda-Sapla, M.M.; da Silva Bortoleti, B.T.; Gonçalves, M.D.; Rodrigues, A.C.J.; Sanfelice, R.A.; Cruz, E.M.S.; et al. Leishmania amazonensis infection regulates oxidate stress in hyperglycemia and diabetes impairing macrophage’s function and immune response. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2024, 1870, 167078. [Google Scholar] [CrossRef] [PubMed]
- Elmahallawy, E.K.; Jiménez-Aranda, A.; Martínez, A.S.; Rodriguez-Granger, J.; Navarro-Alarcón, M.; Gutiérrez-Fernández, J.; Agil, A. Activity of melatonin against Leishmania infantum promastigotes by mitochondrial dependente pathway. Chem.-Biol. Interact. 2014, 220, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Laranjeira-Silva, M.F.; Zampieri, R.A.; Muxel, S.M.; Floeter-Winter, L.M.; Markus, R.P. Melatonin attenuates Leishmania (L.) amazonensis infection by modulating arginine metabolism. J. Pineal Res. 2015, 59, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Liebmann, P.M.; Wölfler, A.; Felsner, P.; Hofer, D.; Schauenstein, K. Melatonin and the immune system. Intern. Arch. Aller. Immunol. 1997, 112, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Calvo, J.R.; Karbownik, M.; Qi, W.; Tan, D.X. Melatonin and its relation to the immune system and inflammation. Ann. N. Y. Acad. Sci. 2000, 917, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Horodincu, L.; Solcan, C. Influence of Different Light Spectra on Melatonin Synthesis by the Pineal Gland and Influence on the Immune System in Chickens. Animals 2023, 13, 2095. [Google Scholar] [CrossRef]
- Cardinali, D.P.; Esquifino, A.I.; Srinivasan, V.; Pandi-Perumal, S.R. Melatonin and the immune system in aging. Neuroimmunomodulation 2008, 15, 272–278. [Google Scholar] [CrossRef]
- Carrillo-Vico, A.; Lardone, P.J.; Alvarez-Sánchez, N.; Rodríguez-Rodríguez, A.; Guerrero, J.M. Melatonin: Buffering the immune system. Int. J. Mol. Sci. 2013, 14, 8638–8683. [Google Scholar] [CrossRef]
- Claustrat, B.; Leston, J. Melatonin: Physiological effects in humans. Neurochirurgie 2015, 61, 77–84. [Google Scholar] [CrossRef]
- Hardeland, R. Aging, Melatonin, and the Pro- and Anti-Inflammatory Networks. Int. J. Mol. Sci. 2019, 20, 1223. [Google Scholar] [CrossRef]
- Fiore, A.; Murray, P.J. Tryptophan and indole metabolism in immune regulation. Curr. Opin. Immunol. 2021, 70, 7–14. [Google Scholar] [CrossRef]
- Moslehi, M.; Moazamiyanfar, R.; Dakkali, M.S.; Rezaei, S.; Rastegar-Pouyani, N.; Jafarzadeh, E.; Mouludi, K.; Khodamoradi, E.; Taeb, S.; Najafi, M. Modulation of the immune system by melatonin; implications for cancer therapy. Int. Immunopharmacol. 2022, 108, 108890. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Fuentes-Broto, L.; Paredes, S.D.; Reiter, R.J. Significance and application of melatonin in regulating brown adipose tissue metabolism: Relation to human obesity. Obes. Rev. 2010, 12, 167–188. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, A.; Agil, A. Changes in Plasma Oxidative Stress and Antioxidant Activity, Measured with Melatonin Levels, and its Relationship to Newborns from Obese and Diabetic Pregnancies. J. Diabete Metab. 2011, 2011, 4. [Google Scholar]
- Bonyek-Silva, I.; Nunes, S.; Santos, R.L.; Lima, F.R.; Lago, A.; Silva, J.; Carvalho, L.P.; Arruda, S.M.; Serezani, H.C.; Carvalho, E.M.; et al. Unbalanced production of LTB4/PGE2 driven by diabetes increases susceptibility to cutaneous leishmaniasis. Emerg. Microbes Infect. 2020, 9, 1275–1286. [Google Scholar] [CrossRef]
- Fernandes, J.C.R.; Aoki, J.I.; Maia Acuña, S.; Zampieri, R.A.; Markus, R.P.; Floeter-Winter, L.M.; Muxel, S.M. Melatonin and Leishmania amazonensis infection altered mir-294, mir-30e, and mir-302d impacting on TNF, MCP-1, and nos2 expression. Front. Cell. Infect. Microbiol. 2019, 9, 60. [Google Scholar] [CrossRef]
- Xia, M.Z.; Liang, Y.L.; Wang, H.; Chen, X.; Huang, Y.Y.; Zhang, Z.H.; Song, L.H. Melatonin modulates TLR4-mediated inflammatory genes through MyD88-and TRIF-dependent signaling pathways in lipopolysaccharide-stimulated RAW264. 7 cells. J. Pineal Res. 2012, 53, 325–334. [Google Scholar] [CrossRef]
- Ahmad, S.B.; Ali, A.; Bilal, M.; Rashid, S.M.; Wani, A.B.; Bhat, R.R.; Rehman, M.U. Melatonin and health: Insights of melatonin action, biological functions, and associated disorders. Cell. Mol. Neurobiol. 2023, 43, 2437–2458. [Google Scholar] [CrossRef]
- Pontes, G.N.; Cardoso, E.C.; Carneiro-Sampaio, M.M.; Markus, R.P. Pineal melatonin and the innate immune response: The increase in TNF alpha after cesarean sections suppresses nocturnal melatonin production. J. Pineal Res. 2007, 43, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, S.; Kireev, R.; Forman, K.; Garcia, C.; Escames, G.; Ariznavarreta, C.; Vara, E.; Tresguerres, J.A. Melatonin improves inflammation processes in the liver of senescence-accelerated prone male mice (samp8). Exp. Gerontol. 2010, 45, 950–956. [Google Scholar] [CrossRef]
- Bodhini, D.; Mohan, V. Mediators of insulin resistance & cardiometabolic risk: Newer insights. Indian J. Med. Res. 2018, 148, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Kojta, I.; Chacińska, M.; Błachnio-Zabielska, A. Obesity, bioactive lipids, and adipose tissue inflammation in insulin resistance. Nutrients 2020, 12, 1305. [Google Scholar] [CrossRef] [PubMed]
- Al-Mansoori, L.; Al-Jaber, H.; Prince, M.S.; Elrayess, M.A. Role of inflammatory cytokines, growth factors and adipokines in adipogenesis and insulin resistance. Inflammation 2022, 45, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Peschke, E.; Frese, T.; Chankiewitz, E.; Peschke, D.; Preiss, U.; Schneyer, U.; Mühlbauer, E. Diabetic GotoKakizaki rats as well as type 2 diabetic patients show a decreased diurnal serum melatonin level and an increased pancreatic melatonin-receptor status. J. Pineal Res. 2006, 40, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Espino, J.; Pariente, J.A.; Rodríguez, A.B. Role of melatonin on diabetes-related metabolic disorders. World J. Diabetes 2011, 2, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Mühlbauer, E.; Peschke, E. Evidence for the expression of both the MT1-and, in addition, the MT2-melatonin receptor in the rat pancreas, islet, and beta-cell. J. Pineal Res. 2007, 42, 105–106. [Google Scholar] [CrossRef] [PubMed]
- Mühlbauer, E.; Wolgast, S.; Finckh, U.; Peschke, D.; Peschke, E. Indication of circadian oscillations in the rat pancreas. FEBS Lett. 2004, 564, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Antar, S.A.; Ashour, N.A.; Sharaky, M.; Khattab, M.; Ashour, N.A.; Zaid, R.T.; Roh, E.J.; Elkamhawy, A.; Al-Karmalawy, A.A. Diabetes mellitus: Classification, mediators, and complications; A gate to identify potential targets for the development of new effective treatments Biomed. Pharmacother. 2023, 168, 115734. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ren, J.; Li, Y.; Wu, Q.; Wei, J. Oxidative stress: The nexus of obesity and cognitive dysfunction in diabetes. Front. Endocrinol. 2023, 14, 1134025. [Google Scholar] [CrossRef]
- Paskaloglu, K.; Sener, L.; Ayangolu-Dulger, L. Melatonin treatment protects against diabetes-induced functional and biochemical changes in rat aorta and corpus cavernosum. Eur. J. Pharmacol. 2004, 499, 345–354. [Google Scholar] [CrossRef]


| Participants (n = 25) | |
|---|---|
| Characteristics | n |
| Gender | |
| Male | 10 |
| Female | 15 |
| Locality | |
| Rural | 7 |
| Urban | 18 |
| Injury sites (ACL) | |
| Upper limbs | 4 |
| Lower limbs | 6 |
| Others | 2 |
| Control | ACL | T2DM | ACL + T2DM | |||||
|---|---|---|---|---|---|---|---|---|
| Rs | p | Rs | p | Rs | p | Rs | p | |
| Melatonin/Age | −0.470 | 0.423 | 0.090 | 0.880 | −0.665 | 0.220 | −0.397 | 0.507 |
| Melatonin/glucose | 0.083 | 0.894 | 0.876 | 0.051 | −0.962 | 0.008 * | −0.814 | 0.093 |
| Melatonin/CRP | −0.914 | 0.029 * | 0.278 | 0.650 | 0.583 | 0.349 | 0.155 | 0.802 |
| Melatonin/IL-4 | 0.126 | 0.873 | 0.549 | 0.450 | −0.631 | 0.368 | 0.343 | 0.657 |
| Melatonin/TNF-α | 0.443 | 0.556 | 0.681 | 0.318 | −0.180 | 0.819 | −0.080 | 0.920 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, T.M.d.M.; Ferrari, F.R.; de Queiroz, A.A.; Dalcin, L.D.L.; França, D.C.H.; Honório-França, A.C.; França, E.L.; Fagundes-Triches, D.L.G. The Role of Melatonin in the Inflammatory Process in Patients with Hyperglycemia and Leishmania Infection. Biomolecules 2024, 14, 950. https://doi.org/10.3390/biom14080950
Martins TMdM, Ferrari FR, de Queiroz AA, Dalcin LDL, França DCH, Honório-França AC, França EL, Fagundes-Triches DLG. The Role of Melatonin in the Inflammatory Process in Patients with Hyperglycemia and Leishmania Infection. Biomolecules. 2024; 14(8):950. https://doi.org/10.3390/biom14080950
Chicago/Turabian StyleMartins, Thalissa Mariana de Moraes, Felipe Rubin Ferrari, Adriele Ataides de Queiroz, Letícia Damas Leão Dalcin, Danielle Cristina Honorio França, Adenilda Cristina Honório-França, Eduardo Luzía França, and Danny Laura Gomes Fagundes-Triches. 2024. "The Role of Melatonin in the Inflammatory Process in Patients with Hyperglycemia and Leishmania Infection" Biomolecules 14, no. 8: 950. https://doi.org/10.3390/biom14080950






