Biomembrane-Modified Biomimetic Nanodrug Delivery Systems: Frontier Platforms for Cardiovascular Disease Treatment
Abstract
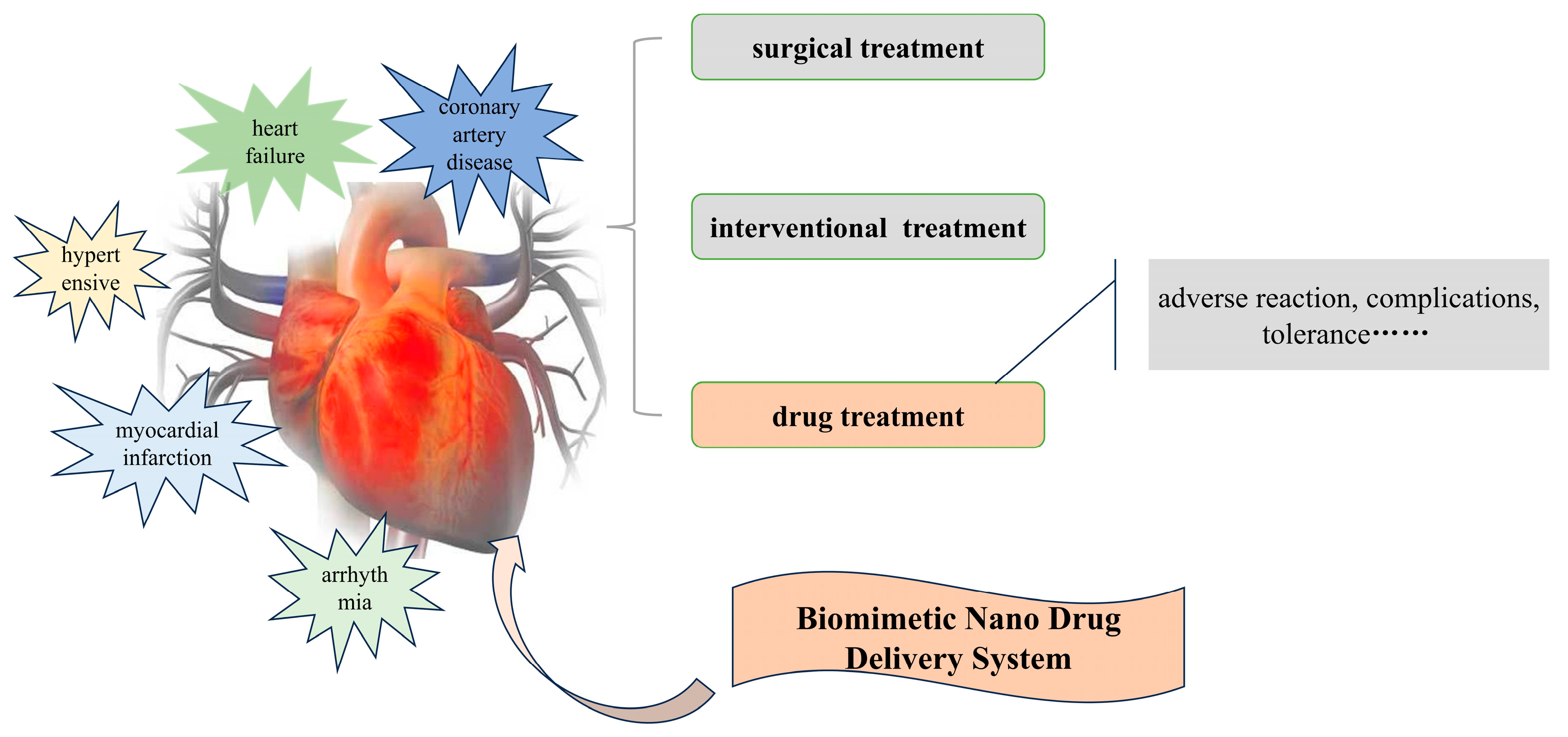
:1. Introduction
2. Overview of CVD Classification and Pathological Mechanisms
2.1. Coronary Artery Disease
2.2. Myocardial Infarction
2.3. Hypertension
2.4. Arrhythmia
2.5. Heart Failure
3. Construction Principles of BNDSs
3.1. Source of Biomembranes
3.2. Biomimetic Principles of Biomembranes
4. Extraction of Biomembranes and Construction of BNDSs
4.1. Extraction of Biomembranes
4.2. Integration of Biomembranes with Drugs
4.2.1. Drug Adsorption on Biomembranes
4.2.2. Drug Encapsulation in Biomembranes
4.3. Construction of BNDSs
5. Applications of BNDSs in the Treatment of CVDs
5.1. Drug Delivery, Release, and Targeted Therapy
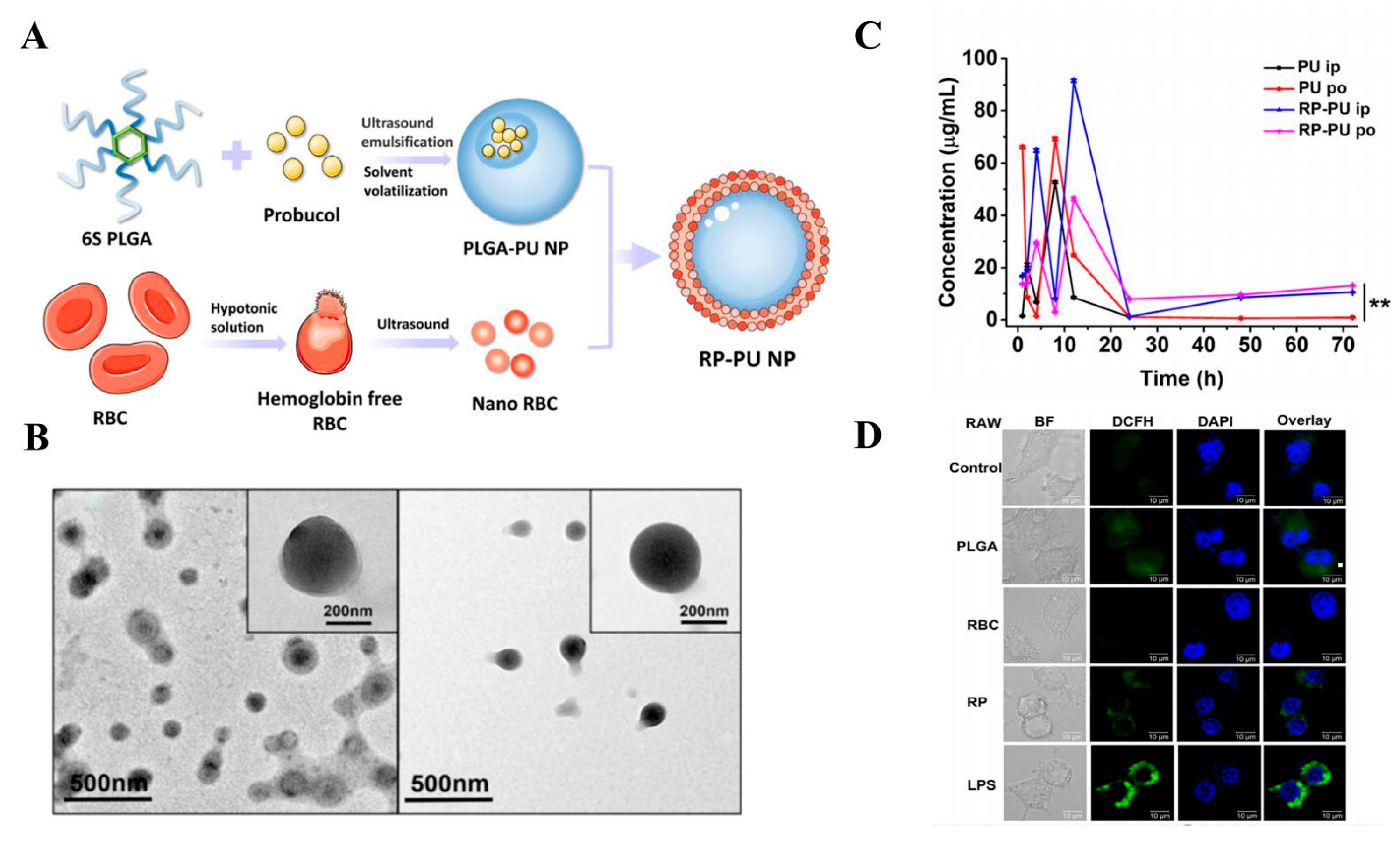
5.1.1. Erythrocyte Membranes
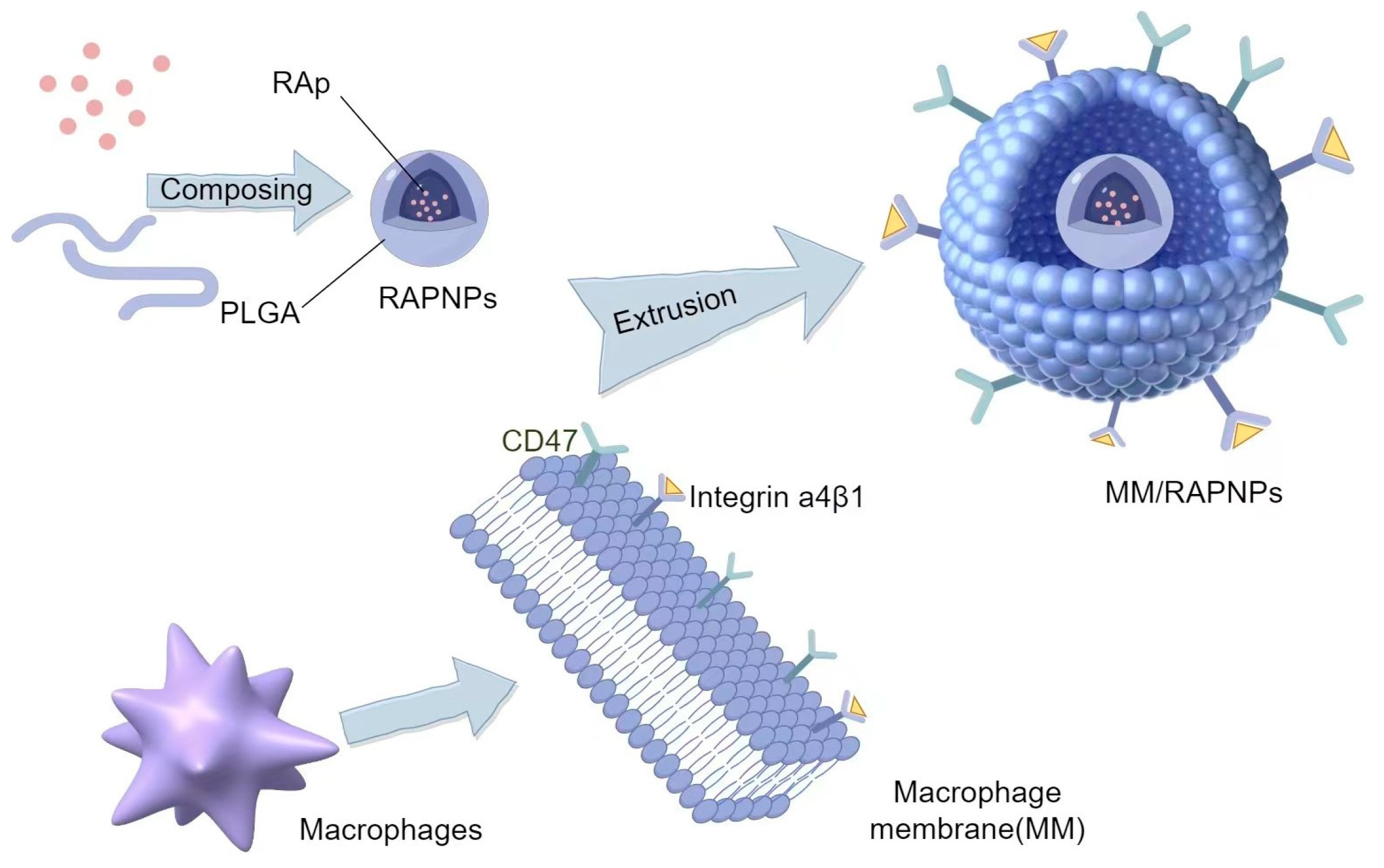
5.1.2. Macrophage Membranes
5.1.3. Platelet Membranes
5.1.4. Stem Cell Membranes
5.1.5. Tumor Cell Membranes
5.1.6. Extracellular Vesicles
5.1.7. Composite Hybrid Membranes
5.2. Imaging and Diagnosis
6. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Soppert, J.; Lehrke, M.; Marx, N.; Jankowski, J.; Noels, H. Lipoproteins and lipids in cardiovascular disease: From mechanistic insights to therapeutic targeting. Adv. Drug Deliv. Rev. 2020, 159, 4–33. [Google Scholar] [CrossRef] [PubMed]
- Nitsa, A.; Toutouza, M.; Machairas, N.; Mariolis, A.; Philippou, A.; Koutsilieris, M. Vitamin D in Cardiovascular Disease. In Vivo 2018, 32, 977–981. [Google Scholar] [CrossRef] [PubMed]
- Leong, D.P.; Joseph, P.G.; McKee, M.; Anand, S.S.; Teo, K.K.; Schwalm, J.D.; Yusuf, S. Reducing the Global Burden of Cardiovascular Disease, Part 2: Prevention and Treatment of Cardiovascular Disease. Circ. Res. 2017, 121, 695–710. [Google Scholar] [CrossRef] [PubMed]
- Leopold, J.A.; Loscalzo, J. Emerging Role of Precision Medicine in Cardiovascular Disease. Circ. Res. 2018, 122, 1302–1315. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Mesquita, F.C.P.; Hochman-Mendez, C. New Biomarkers for Cardiovascular Disease. Tex. Heart Inst. J. 2023, 50, e238178. [Google Scholar] [CrossRef] [PubMed]
- Heidary Moghaddam, R.; Samimi, Z.; Moradi, S.Z.; Little, P.J.; Xu, S.; Farzaei, M.H. Naringenin and naringin in cardiovascular disease prevention: A preclinical review. Eur. J. Pharmacol. 2020, 887, 173535. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.F.; Liu, X.Y.; Deng, N.H.; Ren, Z.; Jiang, Z.S. Outlook of Ferroptosis-Targeted Lipid Peroxidation in Cardiovascular Disease. Curr. Med. Chem. 2023, 30, 3550–3561. [Google Scholar] [CrossRef] [PubMed]
- Doughty, K.N.; Del Pilar, N.X.; Audette, A.; Katz, D.L. Lifestyle Medicine and the Management of Cardiovascular Disease. Curr. Cardiol. Rep. 2017, 19, 116. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Chen, Y.Y.; Lin, Y.H.; Chueh, J.S. Composite Cardiovascular Outcomes in Patients with Primary Aldosteronism Undergoing Medical Versus Surgical Treatment: A Meta-Analysis. Front. Endocrinol. 2021, 12, 644260. [Google Scholar] [CrossRef]
- Paz Ocaranza, M.; Riquelme, J.A.; García, L.; Jalil, J.E.; Chiong, M.; Santos, R.A.; Lavandero, S. Counter-regulatory renin-angiotensin system in cardiovascular disease. Nat. Rev. Cardiol. 2020, 17, 116–129. [Google Scholar] [CrossRef]
- Adhami, M.; Martin, N.K.; Maguire, C.; Courtenay, A.J.; Donnelly, R.F.; Domínguez-Robles, J.; Larrañeta, E. Drug loaded implantable devices to treat cardiovascular disease. Expert Opin. Drug Deliv. 2023, 20, 507–522. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.T.; Kini, V.; Levy, A.E.; Ho, P.M. Medication adherence in cardiovascular medicine. BMJ 2021, 374, n1493. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Shao, H.; Gao, L.; Li, H.; Sheng, H.; Zhu, L. Nano-drug co-delivery system of natural active ingredients and chemotherapy drugs for cancer treatment: A review. Drug Delivery 2022, 29, 2130–2161, Correction in Drug Deliv. 2022, 29, 3051. [Google Scholar] [CrossRef] [PubMed]
- Prakash, S. Nano-based drug delivery system for therapeutics: A comprehensive review. Biomed. Phys. Eng. Express 2023, 9, 52002. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Yu, Z.; Xu, T.; Wang, L.; Meng, N.; Jin, H.; Xu, B. Novel Nano-Drug Delivery System for Brain Tumor Treatment. Cells 2022, 11, 3761. [Google Scholar] [CrossRef] [PubMed]
- Malik, J.A.; Ansari, J.A.; Ahmed, S.; Khan, A.; Ahemad, N.; Anwar, S. Nano-drug delivery system: A promising approach against breast cancer. Ther. Deliv. 2023, 14, 357–381. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, Z.; Guo, C.; Guo, H.; Su, Y.; Chen, Q.; Sun, C.; Liu, Q.; Chen, D.; Mu, H. Hypoxia responsive nano-drug delivery system based on angelica polysaccharide for liver cancer therapy. Drug Deliv. 2022, 29, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Zhou, L.; Li, Y.; Liu, J.; Liu, Y. Nano drug delivery system reconstruct tumour vasculature for the tumour vascular normalisation. J. Drug Target. 2022, 30, 119–130. [Google Scholar] [CrossRef]
- Lv, W.; Liu, Y.; Li, S.; Lv, L.; Lu, H.; Xin, H. Advances of nano drug delivery system for the theranostics of ischemic stroke. J. Nanobiotechnol. 2022, 20, 248. [Google Scholar] [CrossRef]
- Song, M.; Tian, J.; Wang, L.; Dong, S.; Fu, K.; Chen, S.; Liu, C. Efficient Delivery of Lomitapide using Hybrid Membrane-Coated Tetrahedral DNA Nanostructures for Glioblastoma Therapy. Adv. Mater. 2024, 36, e2311760. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, J.; Bai, R.; Shi, J.; Zhu, X.; Liu, J.; Guo, J.; Zhang, W.; Liu, H.; Liu, Z. Mitochondria-Inspired Nanoparticles with Microenvironment-Adapting Capacities for On-Demand Drug Delivery after Ischemic Injury. ACS Nano 2020, 14, 11846–11859. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, Y.; Chen, L.; Wang, S.; Liu, C.; Zhao, H.; Jin, M.; Chang, S.; Quan, X.; Cui, M.; et al. Combined Biomimetic MOF-RVG15 Nanoformulation Efficient Over BBB for Effective Anti-Glioblastoma in Mice Model. Int. J. Nanomed. 2022, 17, 6377–6398. [Google Scholar] [CrossRef] [PubMed]
- Sang, Z.; Xu, L.; Ding, R.; Wang, M.; Yang, X.; Li, X.; Zhou, B.; Gou, K.; Han, Y.; Liu, T.; et al. Nanoparticles exhibiting virus-mimic surface topology for enhanced oral delivery. Nat. Commun. 2023, 14, 7694. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, L.; Liu, L.; Qu, X.; Zhao, W.; Ding, J.; Zhao, S.; Xu, B.; Yu, H.; Liu, B.; et al. Acyltransferase zinc finger DHHC-type containing 2 aggravates gastric carcinoma growth by targeting Nrf2 signaling: A mechanism-based multicombination bionic nano-drug therapy. Redox Biol. 2024, 70, 103051. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yao, Q.; Zhu, W.; Yang, Y.; Gao, C.; Han, C.; Chu, X. Biomimetic Antidote Nanoparticles: A Novel Strategy for Chronic Heavy Metal Poisoning. AAPS PharmSciTech 2022, 24, 12. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.S.; Conte, M.S. Specialized pro-resolving lipid mediators in cardiovascular disease, diagnosis, and therapy. Adv. Drug Deliv. Rev. 2020, 159, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Bevan, G.; Pandey, A.; Griggs, S.; Dalton, J.E.; Zidar, D.; Patel, S.; Khan, S.U.; Nasir, K.; Rajagopalan, S.; Al-Kindi, S. Neighborhood-level Social Vulnerability and Prevalence of Cardiovascular Risk Factors and Coronary Heart Disease. Curr. Probl. Cardiol. 2023, 48, 101182. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lin, C.; Cai, X.; Hu, S.; Lv, F.; Yang, W.; Zhu, X.; Ji, L. Anti-inflammatory therapies were associated with reduced risk of myocardial infarction in patients with established cardiovascular disease or high cardiovascular risks: A systematic review and meta-analysis of randomized controlled trials. Atherosclerosis 2023, 379, 117181. [Google Scholar] [CrossRef]
- Perumareddi, P. Prevention of Hypertension Related to Cardiovascular Disease. Prim. Care 2019, 46, 27–39. [Google Scholar] [CrossRef]
- Gasperetti, A.; James, C.A.; Carrick, R.T.; Protonotarios, A.; Te Riele, A.S.; Cadrin-Tourigny, J.; Compagnucci, P.; Duru, F.; Van Tintelen, P.; Elliot, P.M.; et al. Arrhythmic risk stratification in arrhythmogenic right ventricular cardiomyopathy. Europace 2023, 25, euad312. [Google Scholar] [CrossRef]
- Du, L.; Lu, H.; Wang, Z.; Liu, C.; Xiao, Y.; Guo, Z.; Li, Y. Therapeutic Potential of Ginsenoside Rb1-PLGA Nanoparticles for Heart Failure Treatment via the ROS/PPARα/PGC1α Pathway. Molecules 2023, 28, 8118. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Lu, Y.; Chen, S.; Wang, K.; Hu, T.; Cui, H. Sex-specific effect of serum urate levels on coronary heart disease and myocardial infarction prevention: A Mendelian randomization study. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Saleh, J.; Samimi, M.; Al-Bayati, A.; Rasmussen, H.; Kiel, R. Coronary Cameral Fistula: A Rare Case Presenting with Non-ST-Segment Elevation Myocardial Infarction and Pulmonary Arterial Hypertension. Cureus 2024, 16, e61604. [Google Scholar] [CrossRef] [PubMed]
- Jenča, D.; Melenovský, V.; Stehlik, J.; Staněk, V.; Kettner, J.; Kautzner, J.; Adámková, V.; Wohlfahrt, P. Heart failure after myocardial infarction: Incidence and predictors. ESC Heart Fail. 2021, 8, 222–237. [Google Scholar] [CrossRef] [PubMed]
- Lazzeroni, D.; Villatore, A.; Souryal, G.; Pili, G.; Peretto, G. The Aging Heart: A Molecular and Clinical Challenge. Int. J. Mol. Sci. 2022, 23, 16033. [Google Scholar] [CrossRef]
- Zvintzou, E.; Karampela, D.S.; Vakka, A.; Xepapadaki, E.; Karavia, E.A.; Hatziri, A.; Giannopoulou, P.C.; Kypreos, K.E. High density lipoprotein in atherosclerosis and coronary heart disease: Where do we stand today? Vascul. Pharmacol. 2021, 141, 106928. [Google Scholar] [CrossRef] [PubMed]
- Attiq, A.; Afzal, S.; Ahmad, W.; Kandeel, M. Hegemony of inflammation in atherosclerosis and coronary artery disease. Eur. J. Pharmacol. 2024, 966, 176338. [Google Scholar] [CrossRef]
- Welén Schef, K.; Tornvall, P.; Alfredsson, J.; Hagström, E.; Ravn-Fischer, A.; Soderberg, S.; Yndigegn, T.; Jernberg, T. Prevalence of angina pectoris and association with coronary atherosclerosis in a general population. Heart 2023, 109, 1450–1459. [Google Scholar] [CrossRef]
- Wu, Y.T.; Zhang, G.Y.; Li, L.; Liu, B.; Wang, R.Y.; Song, R.Q.; Hua, Y.; Bi, Y.M.; Han, X.; Zhang, F.; et al. Salvia miltiorrhiza suppresses cardiomyocyte ferroptosis after myocardial infarction by activating Nrf2 signaling. J. Ethnopharmacol. 2024, 330, 118214. [Google Scholar] [CrossRef]
- Yang, H.; Zuo, H.; Wu, X.; Jia, S.; Song, X. Association between control of cardiovascular risk factors and acute myocardial infarction among re-hospitalized young patients with prior coronary heart disease. Chin. Med. J. 2023, 136, 1364–1366. [Google Scholar] [CrossRef]
- Frantz, S.; Hundertmark, M.J.; Schulz-Menger, J.; Bengel, F.M.; Bauersachs, J. Left ventricular remodelling post-myocardial infarction: Pathophysiology, imaging, and novel therapies. Eur. Heart J. 2022, 43, 2549–2561. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Yang, N.; Feng, S.; Guo, J.; Liu, Q.B.; Hu, M. Cost-effectiveness analysis of combining traditional Chinese medicine in the treatment of hypertension: Compound Apocynum tablets combined with Nifedipine sustained-release tablets vs Nifedipine sustained-release tablets alone. BMC Complement. Med. Ther. 2020, 20, 330. [Google Scholar] [CrossRef] [PubMed]
- Cobos-Segarra, L.; Lopez-Jaramillo, P.; Ponte-Negretti, C.I.C.; Villar, R.; Penaherrera, E. Pharmacological Treatment of Hypertension: Effects in Endothelial Function. Curr. Hypertens. Rev. 2018, 14, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Han, T.; Fan, Y.; Wu, S.; Wang, F.; Wang, C. Quercetin improves vascular endothelial function through promotion of autophagy in hypertensive rats. Life Sci. 2020, 258, 118106. [Google Scholar] [CrossRef] [PubMed]
- Leo, C.H.; Ng, H.H.; Marshall, S.A.; Jelinic, M.; Rupasinghe, T.; Qin, C.; Roessner, U.; Ritchie, R.H.; Tare, M.; Parry, L.J. Relaxin reduces endothelium-derived vasoconstriction in hypertension: Revealing new therapeutic insights. Br. J. Pharmacol. 2020, 177, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Shen, A.; Wu, X.; Shen, Z.; Chen, X.; Li, J.; Liu, L.; Lin, X.; Wu, M.; Chen, Y.; et al. Qingda granule attenuates angiotensin II-induced cardiac hypertrophy and apoptosis and modulates the PI3K/AKT pathway. Biomed. Pharmacother. 2021, 133, 111022. [Google Scholar] [CrossRef] [PubMed]
- Esposito, A.; Gatti, M.; Trivieri, M.G.; Agricola, E.; Peretto, G.; Gallone, G.; Catapano, F.; Pradella, S.; Devesa, A.; Bruno, E.; et al. Imaging for the assessment of the arrhythmogenic potential of mitral valve prolapse. Eur. Radiol. 2024, 34, 4243–4260. [Google Scholar] [CrossRef] [PubMed]
- Peretto, G.; Sala, S.; Rizzo, S.; De Luca, G.; Campochiaro, C.; Sartorelli, S.; Benedetti, G.; Palmisano, A.; Esposito, A.; Tresoldi, M.; et al. Arrhythmias in myocarditis: State of the art. Heart Rhythm. 2019, 16, 793–801. [Google Scholar] [CrossRef]
- Dong, R.; Zhang, Y.; Chen, S.; Wang, H.; Hu, K.; Zhao, H.; Tian, Q.; Zeng, K.; Wang, S.; Han, L. Identification of key pharmacodynamic markers of American ginseng against heart failure based on metabolomics and zebrafish model. Front. Pharmacol. 2022, 13, 909084. [Google Scholar] [CrossRef]
- Lai, Q.; Zhu, X.; Zhang, L.; Kou, J.; Liu, F.; Yu, B.; Li, F. Inhibition of OAT1/3 and CMPF uptake attenuates myocardial ischemia-induced chronic heart failure via decreasing fatty acid oxidation and the therapeutic effects of ruscogenin. Transl. Res. 2023, 261, 1–15. [Google Scholar] [CrossRef]
- Halade, G.V.; Lee, D.H. Inflammation and resolution signaling in cardiac repair and heart failure. EBioMedicine 2022, 79, 103992. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Tian, R. Mitochondrial dysfunction in pathophysiology of heart failure. J. Clin. Investig. 2018, 128, 3716–3726. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhu, R.; Zhou, M.; Liu, J.; Dong, K.; Zhao, S.; Cao, J.; Wang, W.; Sun, C.; Wu, S.; et al. Homologous cancer cell membrane-camouflaged nanoparticles target drug delivery and enhance the chemotherapy efficacy of hepatocellular carcinoma. Cancer Lett. 2023, 558, 216106. [Google Scholar] [CrossRef]
- Yao, Q.; Yang, G.; Wang, H.; Liu, J.; Zheng, J.; Lv, B.; Yang, M.; Yang, Y.; Gao, C.; Guo, Y. Aging erythrocyte membranes as biomimetic nanometer carriers of liver-targeting chromium poisoning treatment. Drug Deliv. 2021, 28, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Dong, S.; An, X.; Zhang, W.; Shen, N.; Li, Y.; Guo, C.; Liu, C.; Li, X.; Chen, S. Erythrocyte-biomimetic nanosystems to improve antitumor effects of paclitaxel on epithelial cancers. J. Control. Release 2022, 345, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Daniyal, M.; Jian, Y.; Xiao, F.; Sheng, W.; Fan, J.; Xiao, C.; Wang, Z.; Liu, B.; Peng, C.; Yuhui, Q.; et al. Development of a nanodrug-delivery system camouflaged by erythrocyte membranes for the chemo/phototherapy of cancer. Nanomedicine 2020, 15, 691–709. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Zhang, J.; Pan, H.; Shi, J.; Wang, J.; Chen, L. Preparation and evaluation of long circulating erythrocyte membrane-cloaked anti-cancer drug delivery system. Drug Deliv. Transl. Res. 2020, 10, 1278–1287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Long, Y.; Wan, J.; Liu, S.; Shi, A.; Li, D.; Yu, S.; Li, X.; Wen, J.; Deng, J.; et al. Macrophage membrane biomimetic drug delivery system: For inflammation targeted therapy. J. Drug Target. 2023, 31, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wu, J.; Williams, G.R.; Fan, Q.; Niu, S.; Wu, J.; Xie, X.; Zhu, L.M. Platelet-membrane-biomimetic nanoparticles for targeted antitumor drug delivery. J. Nanobiotechnol. 2019, 17, 60. [Google Scholar] [CrossRef]
- Huang, D.; Wang, Q.; Cao, Y.; Yang, H.; Li, M.; Wu, F.; Zhang, Y.; Chen, G.; Wang, Q. Multiscale NIR-II Imaging-Guided Brain-Targeted Drug Delivery Using Engineered Cell Membrane Nanoformulation for Alzheimer’s Disease Therapy. ACS Nano 2023, 17, 5033–5046. [Google Scholar] [CrossRef]
- Zhang, W.; Gong, C.; Chen, Z.; Li, M.; Li, Y.; Gao, J. Tumor microenvironment-activated cancer cell membrane-liposome hybrid nanoparticle-mediated synergistic metabolic therapy and chemotherapy for non-small cell lung cancer. J. Nanobiotechnol. 2021, 19, 339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Yang, Q.; Tang, S.; Jiang, S.; Zhao, Q.; Li, J.; Xu, C.; Liu, J.; Fu, Y. CD38-targeted and erythrocyte membrane camouflaged nanodrug delivery system for photothermal and chemotherapy in multiple myeloma. Int. J. Pharm. 2023, 643, 123241. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wan, S.; Yang, S.; Hu, H.; Zhang, C.; Lai, J.; Zhou, J.; Chen, W.; Tang, X.; Luo, J.; et al. Macrophage cell membrane-based nanoparticles: A new promising biomimetic platform for targeted delivery and treatment. J. Nanobiotechnol. 2022, 20, 542. [Google Scholar] [CrossRef] [PubMed]
- Mehdi-Alamdarlou, S.; Ahmadi, F.; Azadi, A.; Shahbazi, M.A.; Heidari, R.; Ashrafi, H. A cell-mimicking platelet-based drug delivery system as a potential carrier of dimethyl fumarate for multiple sclerosis. Int. J. Pharm. 2022, 625, 122084. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Faria, I.; Yousefiasl, S.; Macário-Soares, A.; Pereira-Silva, M.; Peixoto, D.; Zafar, H.; Raza, F.; Faneca, H.; Veiga, F.; Hamblin, M.R.; et al. Stem cell membrane-coated abiotic nanomaterials for biomedical applications. J. Control. Release 2022, 351, 174–197. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Sun, X.; Wu, B.; Shang, Y.; Huang, X.; Dong, H.; Liu, H.; Chen, W.; Gui, R.; Li, J. Construction of homologous cancer cell membrane camouflage in a nano-drug delivery system for the treatment of lymphoma. J. Nanobiotechnol. 2021, 19, 8. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Wang, X.; Li, J.; Zhu, A.; Du, Y.; Zeng, W.; Guo, Y.; Di, L.; Wang, R. Immune Extracellular vesicles Loading Self-Assembled Nanomicelles Traverse the Blood-Brain Barrier for Chemo-immunotherapy against Glioblastoma. ACS Nano 2023, 17, 1464–1484. [Google Scholar] [CrossRef]
- Chen, H.Y.; Deng, J.; Wang, Y.; Wu, C.Q.; Li, X.; Dai, H.W. Hybrid cell membrane-coated nanoparticles: A multifunctional biomimetic platform for cancer diagnosis and therapy. Acta Biomater. 2020, 112, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Yang, Y.; Zhu, J.; Jia, W.; Zhang, T.; Liu, Z.; Chen, X.; Lin, Y. Biomimetic Nanoerythrosome-Coated Aptamer-DNA Tetrahedron/Maytansine Conjugates: pH-Responsive and Targeted Cytotoxicity for HER2-Positive Breast Cancer. Adv. Mater. 2022, 34, e2109609. [Google Scholar] [CrossRef]
- Fukuta, T.; Kogure, K. Biomimetic Nanoparticle Drug Delivery Systems to Overcome Biological Barriers for Therapeutic Applications. Chem. Pharm. Bull. 2022, 70, 334–340. [Google Scholar] [CrossRef]
- Huang, R.; Cai, G.Q.; Li, J.; Li, X.S.; Liu, H.T.; Shang, X.L.; Zhou, J.D.; Nie, X.M.; Gui, R. Platelet membrane-camouflaged silver metal-organic framework drug system against infections caused by methicillin-resistant Staphylococcus aureus. J. Nanobiotechnol. 2021, 19, 229. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yan, R.; Chen, Q.; Wang, Y.; Zhong, X.; Liu, S.; Xie, R.; Ren, L. Functional nano drug delivery system with dual lubrication and immune escape for treating osteoarthritis. J. Colloid Interface Sci. 2023, 652, 2167–2179. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Z.; Guo, Q.; Tang, H.; Wang, Z.; Yang, C.; Fan, H.; Zhang, W.; Ren, C.; Liu, J. Mitochondrion-targeted supramolecular “nano-boat” simultaneously inhibiting dual energy metabolism for tumor selective and synergistic chemo-radiotherapy. Theranostics 2022, 12, 1286–1302. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, Y.; He, R.; Xu, D.; Zang, J.; Weeranoppanant, N.; Dong, H.; Li, Y. Cell membrane biomimetic nanoparticles for inflammation and cancer targeting in drug delivery. Biomater. Sci. 2020, 8, 552–568. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Cui, Y.; Fan, Y.; Chen, M.; Yang, G.; Wang, Y.; Yang, M.; Li, Z.; Gong, W.; Yang, Y.; et al. Hybrid membrane-coated nanosuspensions for multi-modal anti-glioma therapy via drug and antigen delivery. J. Nanobiotechnol. 2021, 19, 378. [Google Scholar] [CrossRef]
- Li, Y.; Ke, J.; Jia, H.; Ren, J.; Wang, L.; Zhang, Z.; Wang, C. Cancer cell membrane coated PLGA nanoparticles as biomimetic drug delivery system for improved cancer therapy. Colloids Surf. B Biointerfaces 2023, 222, 113131. [Google Scholar] [CrossRef]
- Galatage, S.T.; Manjappa, A.S.; Bhagwat, D.A.; Trivedi, R.; Salawi, A.; Sabei, F.Y.; Alsalhi, A. Oral self-nanoemulsifying drug delivery systems for enhancing bioavailability and anticancer potential of fosfestrol: In vitro and in vivo characterization. Eur. J. Pharm. Biopharm. 2023, 193, 28–43. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, K.; Li, C.; Guo, Q.; Chen, Q.; He, X.; Liu, L.; Zhang, Y.; Lu, Y.; Chen, X.; et al. Macrophage-Membrane-Coated Nanoparticles for Tumor-Targeted Chemotherapy. Nano Lett. 2018, 18, 1908–1915. [Google Scholar] [CrossRef]
- Wang, R.; Tong, H. Preparation Methods and Functional Characteristics of Regenerated Keratin-Based Biofilms. Polymers 2022, 14, 4723. [Google Scholar] [CrossRef]
- Xia, Q.; Zhang, Y.; Li, Z.; Hou, X.; Feng, N. Red blood cell membrane-camouflaged nanoparticles: A novel drug delivery system for antitumor application. Acta Pharm. Sin. B 2019, 9, 675–689. [Google Scholar] [CrossRef]
- Ivanov, I.T.; Paarvanova, B.K.; Ivanov, V.; Smuda, K.; Bäumler, H.; Georgieva, R. Effects of heat and freeze on isolated erythrocyte submembrane skeletons. Gen. Physiol. Biophys. 2017, 36, 155–165. [Google Scholar] [CrossRef]
- Young, J.W. Recent advances in membrane mimetics for membrane protein research. Biochem. Soc. Trans. 2023, 51, 1405–1416. [Google Scholar] [CrossRef] [PubMed]
- Qi, R.; Cui, Y.; Liu, J.; Wang, X.; Yuan, H. Recent Advances of Composite Nanomaterials for Antibiofilm Application. Nanomaterials 2023, 13, 2725. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Hensel, A.; Goycoolea, F.M. Chitosan/cyclodextrin surface-adsorbed naringenin-loaded nanocapsules enhance bacterial quorum quenching and anti-biofilm activities. Colloids Surf. B Biointerfaces 2022, 211, 112281. [Google Scholar] [CrossRef]
- Maurya, S.; Gaur, M.; Akhtar, M.S.; Yadav, A.B. Evaluation of Drug-Loaded and Surface-Adsorbed DNase/Tween-80 Solid Lipid Nanoparticles against Staphylococcus aureus Biofilms. ACS Appl. Bio Mater. 2024, 7, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yu, M.; Song, Z.F.; Wei, Z.Y.; Huang, J.; Qian, H.Y. Targeted Delivery of Mesenchymal Stem Cell-Derived Bioinspired Exosome-Mimetic Nanovesicles with Platelet Membrane Fusion for Atherosclerotic Treatment. Int. J. Nanomed. 2024, 19, 2553–2571. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, Z.; Luo, Y.; Ning, T.; Liu, P.; Chen, Q.; Chu, Y.; Guo, Q.; Zhang, Y.; Zhou, W.; et al. Macrophage-Disguised Manganese Dioxide Nanoparticles for Neuroprotection by Reducing Oxidative Stress and Modulating Inflammatory Microenvironment in Acute Ischemic Stroke. Adv. Sci. 2021, 8, e2101526. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Su, Y.Y.; Jiang, X.C.; Gao, J.Q. Cell membrane-coated nanoparticles: A novel multifunctional biomimetic drug delivery system. Drug Deliv. Transl. Res. 2023, 13, 716–737. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.; Tagami, T.; Kishi, T.; Ozeki, T. Curcumin marinosomes as promising nano-drug delivery system for lung cancer. Int. J. Pharm. 2018, 540, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zang, G.; Li, J.; Li, X.; Li, Y.; Zhao, Y. Cell-derived biomimetic nanoparticles as a novel drug delivery system for atherosclerosis: Predecessors and perspectives. Regen. Biomater. 2020, 7, 349–358. [Google Scholar] [CrossRef]
- Yang, M.Y.; Tu, Y.F.; Feng, K.K.; Yin, M.D.; Fang, Y.F.; Le, J.Q.; Luo, B.Y.; Tan, X.R.; Shao, J.W. A erythrocyte-platelet hybrid membrane coated biomimetic nanosystem based on ginsenosides and PFH combined with ultrasound for targeted delivery in thrombus therapy. Colloids Surf. B Biointerfaces 2023, 229, 113468. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Huang, X.H.; Yang, X.; Hu, J.Q.; Zhu, Y.Z.; Yan, P.Y.; Xie, Y. Novel nano-drug delivery system for natural products and their application. Pharmacol. Res. 2024, 201, 107100. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Lee, D.; Lee, S.W.; Lee, J.H.; Lee, G.; Yoon, D.S. Coagulation-Inspired Direct Fibrinogen Assay Using Plasmonic Nanoparticles Functionalized with Red Blood Cell Membranes. ACS Nano 2021, 15, 6386–6394. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, Y.; Zhang, M.; Zhang, J.; Kang, N.; Zheng, L.; Ding, Z. The Optimization Design of Macrophage Membrane Camouflaging Liposomes for Alleviating Ischemic Stroke Injury through Intranasal Delivery. Int. J. Mol. Sci. 2024, 25, 2927. [Google Scholar] [CrossRef]
- Shi, Y.; Jiang, M.; Zhang, Y.; Diao, Y.; Li, N.; Liu, W.; Qiu, Z.; Qiu, Y.; Jia, A. Hyperoside Nanomicelles Alleviate Atherosclerosis by Modulating the Lipid Profile and Intestinal Flora Structure in High-Fat-Diet-Fed Apolipoprotein-E-Deficient Mice. Molecules 2023, 28, 5088. [Google Scholar] [CrossRef] [PubMed]
- Anghelache, M.; Voicu, G.; Deleanu, M.; Turtoi, M.; Safciuc, F.; Anton, R.; Boteanu, D.; Fenyo, I.M.; Manduteanu, I.; Simionescu, M.; et al. Biomimetic Nanocarriers of Pro-Resolving Lipid Mediators for Resolution of Inflammation in Atherosclerosis. Adv. Healthc. Mater. 2024, 13, e2302238. [Google Scholar] [CrossRef]
- Liao, B.; Ying, H.; Yu, C.; Fan, Z.; Zhang, W.; Shi, J.; Ying, H.; Ravichandran, N.; Xu, Y.; Yin, J.; et al. (-)-Epigallocatechin gallate (EGCG)-nanoethosomes as a transdermal delivery system for docetaxel to treat implanted human melanoma cell tumors in mice. Int. J. Pharm. 2016, 512, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Li, H.; Zhang, A.; Tian, X.; Guo, H.; Zhang, H.; Yang, J.; Zeng, Y. Red blood cell biomimetic nanoparticle with anti-inflammatory, anti-oxidative and hypolipidemia effect ameliorated atherosclerosis therapy. Nanomedicine 2022, 41, 102519. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Jiang, H.; Zhao, Y.; Wu, L.; Yang, H.; Yao, Y.; Meng, H.; Yang, Q.; Liu, L.; Li, Y. Shear Stress and ROS Dual-Responsive RBC-Hitchhiking Nanoparticles for Atherosclerosis Therapy. ACS Appl. Mater. Interfaces 2023, 15, 43374–43386. [Google Scholar] [CrossRef]
- Li, J.D.; Yin, J. Interleukin-10-alveolar macrophage cell membrane-coated nanoparticles alleviate airway inflammation and regulate Th17/regulatory T cell balance in a mouse model. Front. Immunol. 2023, 14, 1186393. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, K.; Li, T.; Maruf, A.; Qin, X.; Luo, L.; Zhong, Y.; Qiu, J.; McGinty, S.; Pontrelli, G.; et al. Macrophage membrane functionalized biomimetic nanoparticles for targeted anti-atherosclerosis applications. Theranostics 2021, 11, 164–180. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zeng, G.; Cheng, J.; Hu, J.; Zhang, M.; Li, Y. Engineered macrophage membrane-enveloped nanomedicine for ameliorating myocardial infarction in a mouse model. Bioeng. Transl. Med. 2020, 6, e10197. [Google Scholar] [CrossRef] [PubMed]
- Bender, M.; Palankar, R. Platelet Shape Changes during Thrombus Formation: Role of Actin-Based Protrusions. Hamostaseologie 2021, 41, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xie, R.; Yodsanit, N.; Ye, M.; Wang, Y.; Wang, B.; Guo, L.W.; Kent, K.C.; Gong, S. Hydrogen peroxide-responsive platelet membrane-coated nanoparticles for thrombus therapy. Biomater. Sci. 2021, 9, 2696–2708. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, M.; Yu, F.; Wei, Q.; Liu, Y.; Tong, J.; Yang, F. Platelet Membrane Nanocarriers Cascade Targeting Delivery System to Improve Myocardial Remodeling Post Myocardial Ischemia-Reperfusion Injury. Adv. Sci. 2024, 11, e2308727. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, J.; Liu, B.; Shao, C.; Shi, Y. Reciprocal regulation of mesenchymal stem cells and immune responses. Cell Stem Cell 2022, 29, 1515–1530. [Google Scholar] [CrossRef] [PubMed]
- Bagno, L.; Hatzistergos, K.E.; Balkan, W.; Hare, J.M. Mesenchymal Stem Cell-Based Therapy for Cardiovascular Disease: Progress and Challenges. Mol. Ther. 2018, 26, 1610–1623. [Google Scholar] [CrossRef]
- Luo, L.; Tang, J.; Nishi, K.; Yan, C.; Dinh, P.U.; Cores, J.; Kudo, T.; Zhang, J.; Li, T.S.; Cheng, K. Fabrication of Synthetic Mesenchymal Stem Cells for the Treatment of Acute Myocardial Infarction in Mice. Circ. Res. 2017, 120, 1768–1775. [Google Scholar] [CrossRef] [PubMed]
- Baxter, A.A.; Lay, F.T.; Poon, I.K.; Kvansakul, M.; Hulett, M.D. Tumor cell membrane-targeting cationic antimicrobial peptides: Novel insights into mechanisms of action and therapeutic prospects. Cell. Mol. Life Sci. 2017, 74, 3809–3825. [Google Scholar] [CrossRef]
- He, W.; Mei, Q.; Li, J.; Zhai, Y.; Chen, Y.; Wang, R.; Lu, E.; Zhang, X.Y.; Zhang, Z.; Sha, X. Preferential Targeting Cerebral Ischemic Lesions with Cancer Cell-Inspired Nanovehicle for Ischemic Stroke Treatment. Nano Lett. 2021, 21, 3033–3043. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. Int. J. Nanomed. 2020, 15, 6917–6934. [Google Scholar] [CrossRef]
- Deng, D.; Li, X.; Zhang, J.J.; Yin, Y.; Tian, Y.; Gan, D.; Wu, R.; Wang, J.; Tian, B.M.; Chen, F.M.; et al. Biotin-Avidin System-Based Delivery Enhances the Therapeutic Performance of MSC-Derived Extracellular vesicles. ACS Nano 2023, 17, 8530–8550. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, S.; Chen, Y.; Shen, H.; Chen, L.; Ding, L.; Tang, Q.; Yang, Z.; Chen, W.; Shen, Z. Precision cardiac targeting: Empowering curcumin therapy through smart exosome-mediated drug delivery in myocardial infarction. Regen. Biomater. 2023, 11, rbad108. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, X.; Hu, J.; Chen, F.; Qiao, S.; Sun, X.; Gao, L.; Xie, J.; Xu, B. Mesenchymal stromal cell-derived extracellular vesicles attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovasc. Res. 2019, 115, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xia, J.; Zhu, Y.; Dong, M.; Wang, J. Establishing Salvia miltiorrhiza-Derived Exosome-like Nanoparticles and Elucidating Their Role in Angiogenesis. Molecules 2024, 29, 1599. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.; Heydarkhan-Hagvall, S.; Tangruksa, B.; González-King Garibotti, H.; Jing, Y.; Maugeri, M.; Kohl, F.; Hultin, L.; Reyahi, A.; Camponeschi, A.; et al. Lipid Nanoparticles Deliver the Therapeutic VEGFA mRNA In Vitro and In Vivo and Transform Extracellular Vesicles for Their Functional Extensions. Adv. Sci. (Weinh.) 2023, 10, e2206187. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, R.; Wang, A.; Li, Y.; Zhang, M.; Kim, J.; Zhu, Y.; Wang, Q.; Zhang, Y.; Wei, Y.; et al. Panax notoginseng: Derived exosome-like nanoparticles attenuate ischemia reperfusion injury via altering microglia polarization. J. Nanobiotechnol. 2023, 21, 416. [Google Scholar] [CrossRef]
- Huang, R.; Zhang, L.; Li, X.; Liu, F.; Cheng, X.; Ran, H.; Wang, Z.; Li, Y.; Feng, Y.; Liang, L.; et al. Anti-CXCR2 antibody-coated nanoparticles with an erythrocyte-platelet hybrid membrane layer for atherosclerosis therapy. J. Control. Release 2023, 356, 610–622. [Google Scholar] [CrossRef] [PubMed]
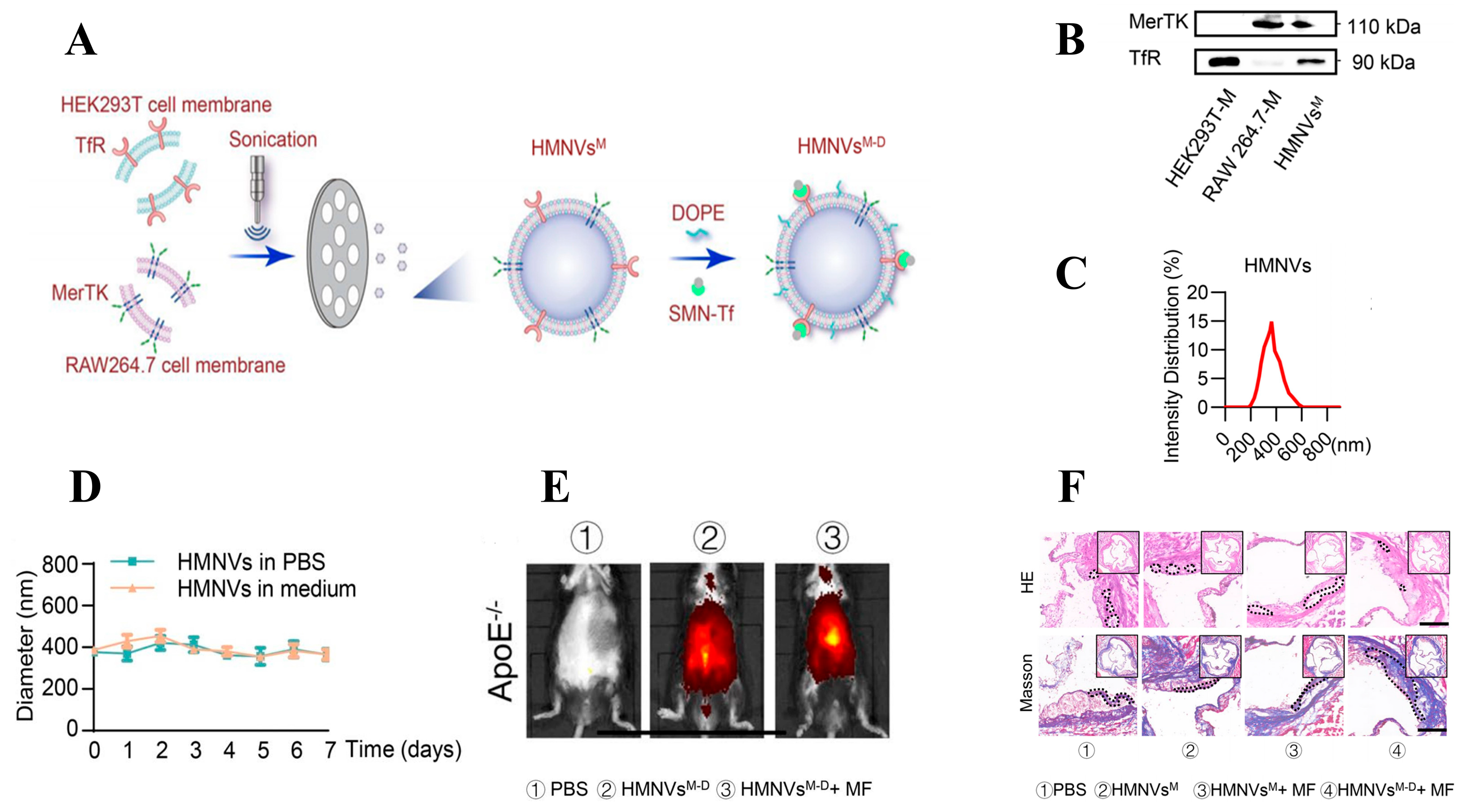
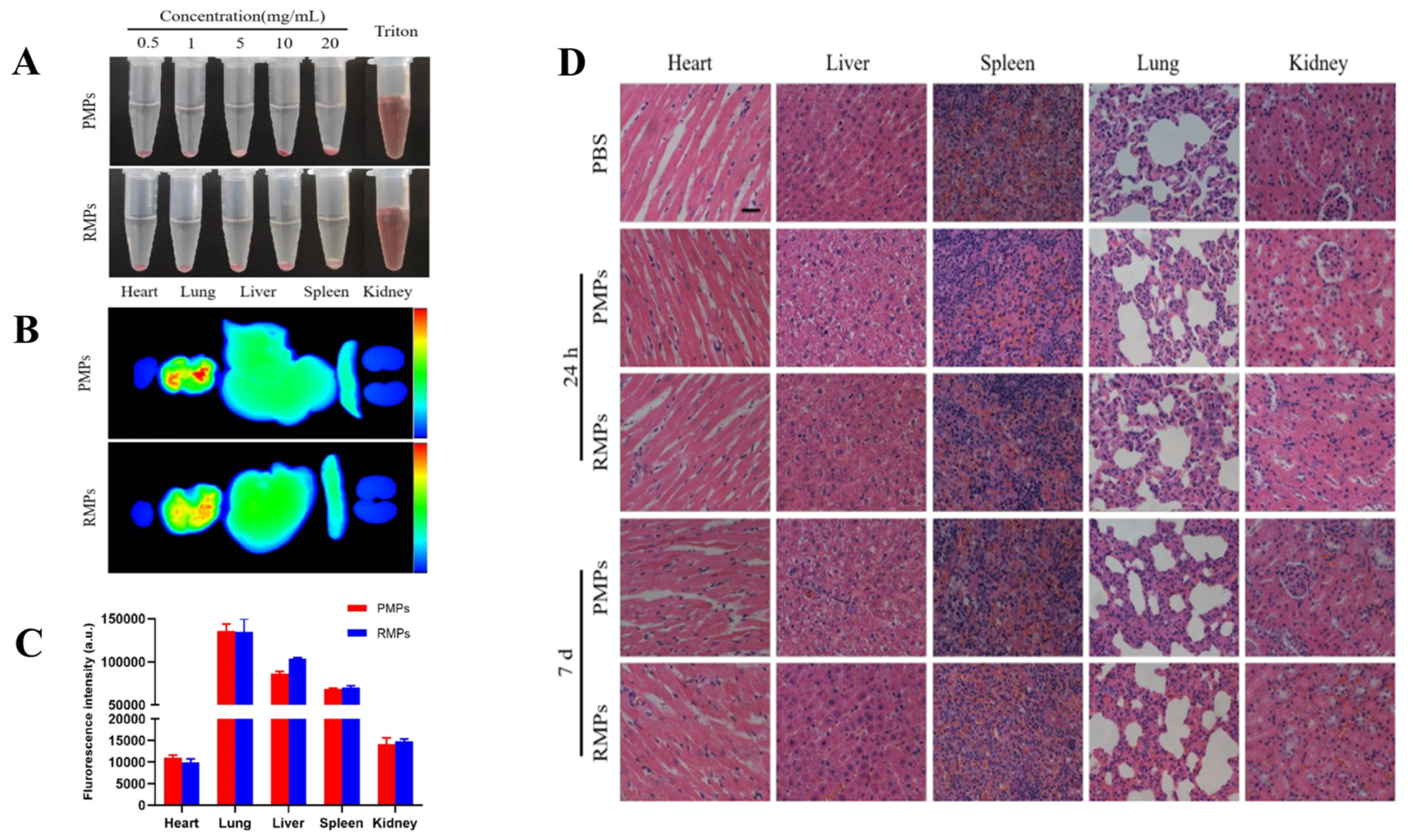
- Qiu, S.; Liu, J.; Chen, J.; Li, Y.; Bu, T.; Li, Z.; Zhang, L.; Sun, W.; Zhou, T.; Hu, W.; et al. Targeted delivery of MerTK protein via cell membrane engineered nanoparticle enhances efferocytosis and attenuates atherosclerosis in diabetic ApoE−/− Mice. J. Nanobiotechnol. 2024, 22, 178. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.R.; Yang, K.; Wen, Y.; Wang, P.; Hu, Y.; Lai, Y.; Wang, Y.; Zhao, K.; Tang, S.; Zhang, A.; et al. Screening and diagnosis of cardiovascular disease using artificial intelligence-enabled cardiac magnetic resonance imaging. Nat. Med. 2024, 30, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Golemati, S.; Cokkinos, D.D. Recent advances in vascular ultrasound imaging technology and their clinical implications. Ultrasonics 2022, 119, 106599. [Google Scholar] [CrossRef] [PubMed]
- Varna, M.; Xuan, H.V.; Fort, E. Gold nanoparticles in cardiovascular imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 10, e1470. [Google Scholar] [CrossRef]
- Ma, B.; Xu, H.; Wang, Y.; Yang, L.; Zhuang, W.; Li, G.; Wang, Y. Biomimetic-Coated Nanoplatform with Lipid-Specific Imaging and ROS Responsiveness for Atherosclerosis-Targeted Theranostics. ACS Appl. Mater. Interfaces 2021, 13, 35410–35421. [Google Scholar] [CrossRef] [PubMed]
- Chi, G.; Lv, Y.; Chao, S.; Hou, C.; Pei, Y.; Pei, Z. Glyconanoparticles with Activatable Near-Infrared Probes for Tumor-Cell Imaging and Targeted Drug Delivery. Int. J. Nanomed. 2022, 17, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.M.; Zhang, D.D.; Fang, G.Z.; Wang, S. Erythrocyte membrane bioinspired near-infrared persistent luminescence nanocarriers for in vivo long-circulating bioimaging and drug delivery. Biomaterials 2018, 165, 39–47. [Google Scholar] [CrossRef]
- Dong, L.; Shen, Z.; Chi, H.; Wang, Y.; Shi, Z.; Fang, H.; Yang, Y.; Rong, J. Research Progress of Chinese Medicine in the Treatment of Myocardial Ischemia-Reperfusion Injury. Am. J. Chin. Med. 2023, 51, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Chen, Y.; Jin, Q.; Wu, Y.; Deng, C.; Zhong, Y.; Lin, L.; Chen, L.; Fu, W.; Yi, L.; et al. Biomimetic PLGA Microbubbles Coated with Platelet Membranes for Early Detection of Myocardial Ischaemia-Reperfusion Injury. Mol. Pharm. 2021, 18, 2974–2985. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; He, T.; Tang, R.; Li, Q.; Wu, N.; Zhou, Y.; He, H.; Wan, L.; Huang, J.; Jiang, Q.; et al. Biomimetic nanoprobe-augmented triple therapy with photothermal, sonodynamic and checkpoint blockade inhibits tumor growth and metastasis. J. Nanobiotechnol. 2022, 20, 80. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Wang, J.; Wan, Z.; Xiong, Y.; Man, J.; Wang, Y.; Mao, G.; Yu, F. Biomimetic-compartmented nanoprobe for in-situ imaging of iron storage and release from ferritin in cells. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 286, 121967. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Shen, A.; Peng, R.; Chen, S.; Lin, S.; Ding, S.; Li, H.; Zhou, D. A Novel Biomimetic Nanoprobe as a Photoacoustic Contrast Agent. Front. Chem. 2021, 9, 721799. [Google Scholar] [CrossRef]
- Ma, X.; Mao, M.; He, J.; Liang, C.; Xie, H.Y. Nanoprobe-based molecular imaging for tumor stratification. Chem. Soc. Rev. 2023, 52, 6447–6496. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Wang, Q.; Li, K.; Li, X.; Wang, C.; Xue, L.; Ju, C.; Zhang, C. A neutrophil-mimetic magnetic nanoprobe for molecular magnetic resonance imaging of stroke-induced neuroinflammation. Biomater. Sci. 2021, 9, 5247–5258. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, H.; Shi, H.; Wang, M.; Huang, C.; Jia, N. A novel multifunctional biomimetic Au@BSA nanocarrier as a potential siRNA theranostic nanoplatform. J. Mater. Chem. B 2016, 4, 2519–2526. [Google Scholar] [CrossRef]
- Yang, Z.; Du, Y.; Sun, Q.; Peng, Y.; Wang, R.; Zhou, Y.; Wang, Y.; Zhang, C.; Qi, X. Albumin-Based Nanotheranostic Probe with Hypoxia Alleviating Potentiates Synchronous Multimodal Imaging and Phototherapy for Glioma. ACS Nano 2020, 14, 6191–6212. [Google Scholar] [CrossRef]
- Wang, Z.H.; Liu, J.M.; Yang, F.E.; Hu, Y.; Lv, H.; Wang, S. Tailor-Made Cell-Based Biomimetic Nanoprobes for Fluorescence Imaging Guided Colorectal Cancer Chemo-immunotherapy. ACS Appl. Bio Mater. 2021, 4, 1920–1931. [Google Scholar] [CrossRef] [PubMed]
- Fracassi, A.; Cao, J.; Yoshizawa-Sugata, N.; Tóth, É.; Archer, C.; Gröninger, O.; Ricciotti, E.; Tang, S.Y.; Handschin, S.; Bourgeois, J.P.; et al. LDL-mimetic lipid nanoparticles prepared by surface KAT ligation for in vivo MRI of atherosclerosis. Chem. Sci. 2020, 11, 11998–12008. [Google Scholar] [CrossRef]
- Ma, Y.; Sun, W.; Ye, Z.; Liu, L.; Li, M.; Shang, J.; Xu, X.; Cao, H.; Xu, L.; Liu, Y.; et al. Oxidative stress biomarker triggered multiplexed tool for auxiliary diagnosis of atherosclerosis. Sci. Adv. 2023, 9, eadh1037. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Wang, T.Y.; Rousseau, J.; Orlando, M.; Mungaray, M.; Michaud, C.; Plaisier, C.; Chen, Z.B.; Wang, K.C. Biomimetic nanodrug targets inflammation and suppresses YAP/TAZ to ameliorate atherosclerosis. Biomaterials 2024, 306, 122505. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Chen, L.; Li, F.; Cao, Y.; Li, D.; Xiong, Q.; Ling, Z. Biomimetic nanoparticles loaded lutein functionalized by macrophage membrane for targeted amelioration pressure overload-induced cardiac fibrosis. Biomed. Pharmacother. 2023, 167, 115579. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Sun, Y.; Fan, Y.; Cheng, Z.; Yu, B. Multimodality Molecular Imaging of Cardiovascular Disease Based on Nanoprobes. Cell. Physiol. Biochem. 2018, 48, 1401–1415. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Wei, A.; Gao, Z.; Mu, X. Current progress of mesenchymal stem cell membrane-camouflaged nanoparticles for targeted therapy. Biomed. Pharmacother. 2023, 161, 114451. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhou, M.; Zeng, Y.; Miao, T.; Luo, H.; Tong, Y.; Zhao, M.; Mu, R.; Gu, J.; Yang, S.; et al. Biomimetic Lipopolysaccharide-Free Bacterial Outer Membrane-Functionalized Nanoparticles for Brain-Targeted Drug Delivery. Adv. Sci. 2022, 9, e2105854. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, N.; Jiang, Y.; Zhou, J.; Mohapatra, A.; Peng, F.X.; Duan, Y.; Holay, M.; Chekuri, S.; Guo, Z.; Gao, W.; et al. A modular approach to enhancing cell membrane-coated nanoparticle functionality using genetic engineering. Nat. Nanotechnol. 2024, 19, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, H.; Hu, X.; Chen, L.; Li, J.; Zhang, L. Hyaluronic acid-based multifunctional nanoplatform for glucose deprivation-enhanced chemodynamic/photothermal synergistic cancer therapy. Int. J. Biol. Macromol. 2024, 275, 133428. [Google Scholar] [CrossRef] [PubMed]




| Type | Advantages | Disadvantages | Refs. |
|---|---|---|---|
| Erythrocyte membranes | Easily obtainable, long circulation time, low immunogenicity, high biocompatibility | Considerations for blood type compatibility | [62] |
| Macrophage membranes | High biocompatibility, low toxicity, inflammation targeting | Complex preparation, potential for immune reactions | [63] |
| Platelet membranes | Inflammation targeting | Complex preparation, restricted storage conditions | [64] |
| Stem cell membranes | Low immunogenicity, promotes tissue regeneration and functional recovery | Limited source, potential for heterogeneity | [65] |
| Tumor cell membranes | Tumor targeting | Biological safety remains unclear | [66] |
| Extracellular vesicles | High biocompatibility, low immunogenicity | Complex preparation | [67] |
| Composite hybrid membranes | High biocompatibility, high targeting | Low stability | [68] |
| Method | Principle | Advantages | Disadvantages | Applications | Refs. |
|---|---|---|---|---|---|
| Ultrasonication | Using ultrasonic energy to disrupt cell membrane structure and release cytoplasm containing membranes | Efficient and straightforward | Excessive acoustic power may compromise membrane structure | Wang et al. found that ultrasonic extraction rapidly disrupts keratin biomembranes, demonstrating its efficiency and convenience | [79] |
| Centrifugation | Cell membrane-containing supernatant was separated from other cellular components using varying centrifugation speeds and times | High purity and broad applicability | Significant loss | Qing et al. used centrifugation to extract red blood cell membranes for nanoparticle drug delivery. Hemoglobin was effectively removed through hypotonic centrifugation | [80] |
| Freeze–thaw cycle | By repeatedly freezing and thawing cell samples, the freeze–thaw process induces volume changes in water, disrupting cell membranes and releasing membrane structures | Efficient and straightforward | Low purity | Ivan et al. found that slow freeze–thaw cycles can eliminate residuals in red blood cell membranes, and hemoglobin denatures at 49 °C | [81] |
| Chemical approach | Disruption of lipid bilayer structure of cell membranes using surfactants or solvents to release membrane lipids and proteins | High purity and strong controllability | High cost and residual impurities | John et al. used chemical detergents to rapidly detach proteins and obtain pure cell membranes | [82] |
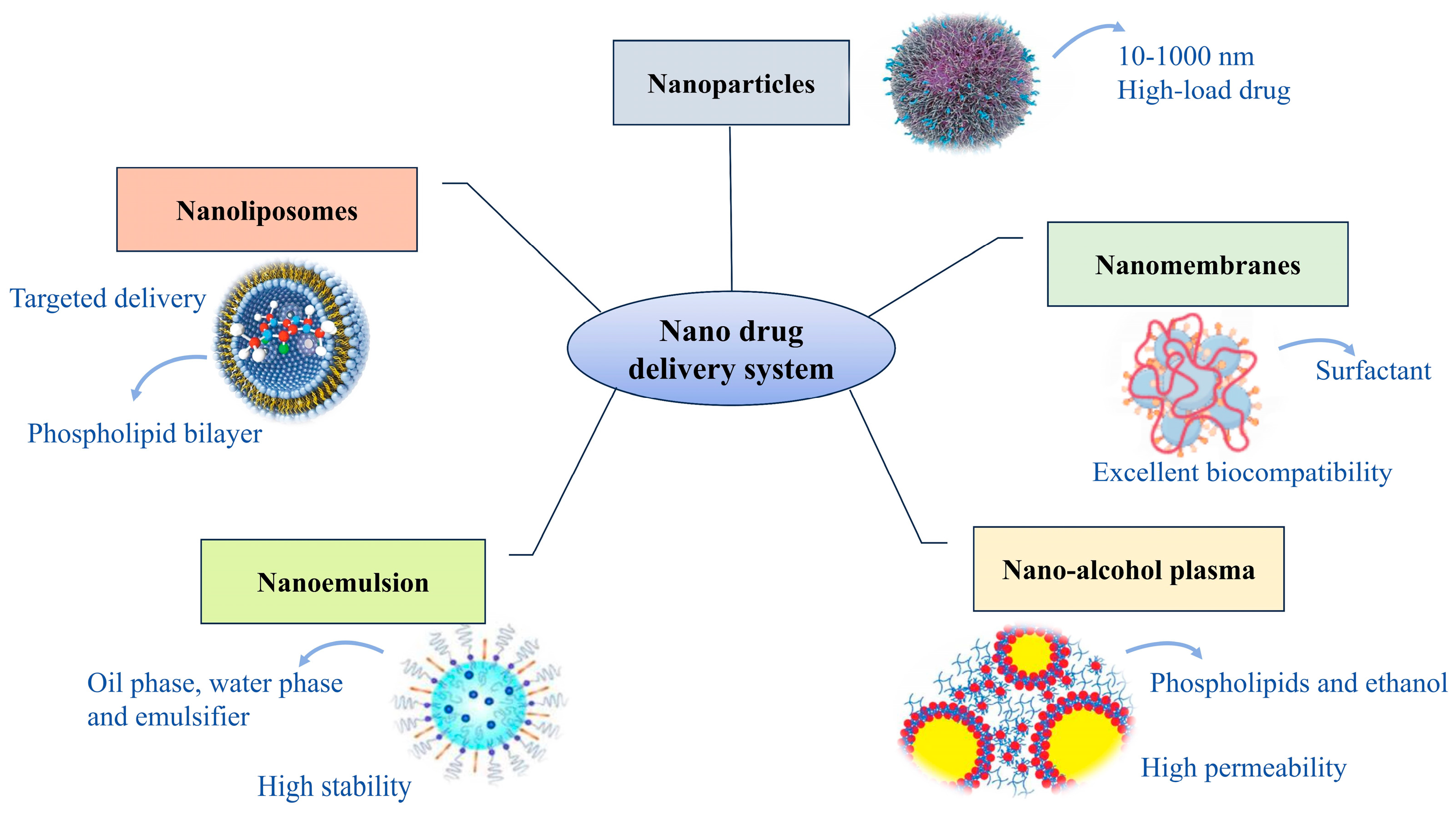
| Type | Characteristics | Applications | Refs. |
|---|---|---|---|
| Nanoparticles | The drug and carrier form solid colloidal substances with particle sizes ranging from 10 to 1000 nm, characterized by high surface area and high drug loading efficiency | Insu Kim et al. prepared erythrocyte membrane-functionalized Au nanoparticles for rapid fibrinogen detection via receptor cross-linking, aimed at CVD diagnosis | [93] |
| Nanoliposomes | Nanoscale vesicles formed by phospholipid bilayers, offering high biocompatibility, drug encapsulation, and targeted delivery advantages | Liu et al. developed a macrophage membrane-coated liposome co-loaded with Panax notoginseng saponins and ginsenoside Rg3 using Box–Behnken design for targeted therapy of ischemic stroke | [94] |
| Nanomembranes | Nanostructures formed by self-assembly of surfactants, exhibiting high drug loading capacity and good biocompatibility | Shi et al. developed nanomicelles using quercetin and polyethylene glycol, finding they can alleviate atherosclerosis by modulating gut microbiota composition | [95] |
| Nanoemulsion | Nano-emulsion composed of oil phase, water phase, and emulsifier, exhibiting high stability and bioavailability | Anghelache et al. developed a novel carrier, Bio-LN/SPMs, by coating macrophage membranes onto lipid emulsions containing lipolytic mediators. Their study demonstrated reduced lipid accumulation and inflammation levels in a mouse model of aortic disease. | [96] |
| Nano-alcohol plasma | Nanodelivery structures composed of phospholipids and high concentrations of ethanol, exhibiting high permeability and drug loading capacity | Liao et al. formulated ellagic acid with 30% ethanol and 1% Tween-80 into nano-cochleates. Transmission electron microscopy revealed that the nano-cochleates exhibited a compact and intact morphology. In vitro experiments also demonstrated their potential for transdermal drug delivery. | [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, Y.; Du, L.; Wu, Y.; Qin, J.; Gu, X.; Guo, Z.; Li, Y. Biomembrane-Modified Biomimetic Nanodrug Delivery Systems: Frontier Platforms for Cardiovascular Disease Treatment. Biomolecules 2024, 14, 960. https://doi.org/10.3390/biom14080960
Gu Y, Du L, Wu Y, Qin J, Gu X, Guo Z, Li Y. Biomembrane-Modified Biomimetic Nanodrug Delivery Systems: Frontier Platforms for Cardiovascular Disease Treatment. Biomolecules. 2024; 14(8):960. https://doi.org/10.3390/biom14080960
Chicago/Turabian StyleGu, Yunan, Lixin Du, Yuxin Wu, Juan Qin, Xiang Gu, Zhihua Guo, and Ya Li. 2024. "Biomembrane-Modified Biomimetic Nanodrug Delivery Systems: Frontier Platforms for Cardiovascular Disease Treatment" Biomolecules 14, no. 8: 960. https://doi.org/10.3390/biom14080960





