Omics Science and Social Aspects in Detecting Biomarkers for Diagnosis, Risk Prediction, and Outcomes of Carotid Stenosis
Abstract
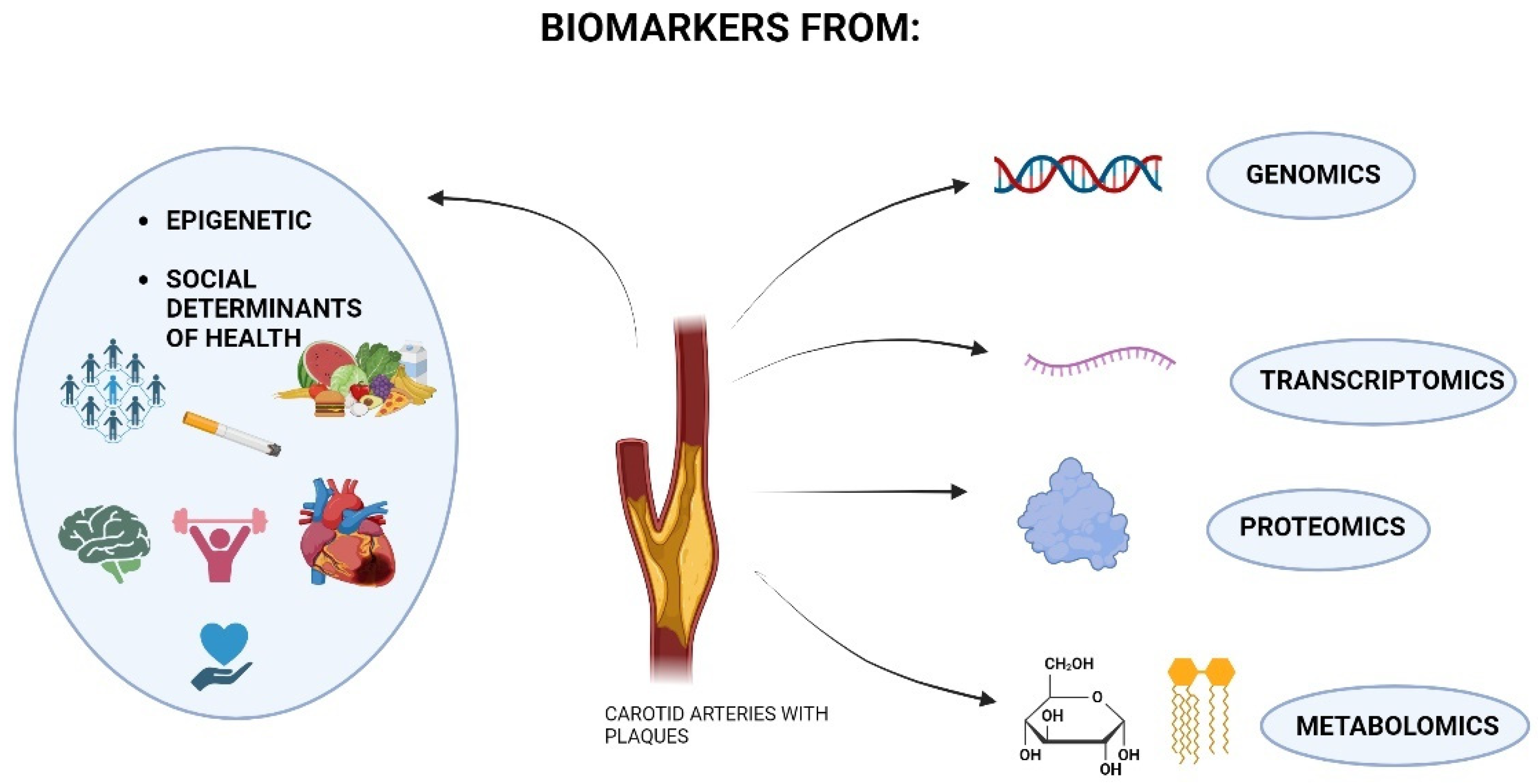
1. Introduction
2. Methods
3. Results
3.1. Quality Assessment of the Included Studies
3.2. Genomics and Carotid Stenosis
3.3. Transcriptomics and Carotid Stenosis
3.4. Proteomics and Carotid Stenosis
3.5. Metabolomics and Carotid Stenosis
3.6. Social Issues and Carotid Stenosis
4. Discussion
4.1. The Role of Genomics in the Onset, Progression, and Outcome of Carotid Stenosis
4.2. The Role of Transcriptomics in the Onset, Progression, and Outcome of Carotid Stenosis
4.3. The Role of Proteomics in the Onset, Progression, and Outcome of Carotid Stenosis
4.4. The Role of Metabolomics in the Onset, Progression, and Outcome of Carotid Stenosis
4.5. The Role of Social Aspects in Carotid Stenosis
4.6. Circulating Biomarkers and Omics Biomarkers in Current Clinical Practice
4.7. “Omics Science” and the Paradigm of Complexity in the Research on New Biomarkers
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- de Weerd, M.; Greving, J.P.; Hedblad, B.; Lorenz, M.W.; Mathiesen, E.B.; O’Leary, D.H.; Rosvall, M.; Sitzer, M.; Buskens, E.; Bots, M.L. Prevalence of asymptomatic carotid artery stenosis in the general population: An individual participant data meta-analysis. Stroke 2010, 41, 1294–1297. [Google Scholar] [CrossRef]
- Dossabhoy, S.; Arya, S. Epidemiology of atherosclerotic carotid artery disease. Semin. Vasc. Surg. 2021, 34, 3–9. [Google Scholar] [CrossRef]
- Woo, S.Y.; Joh, J.H.; Han, S.A.; Park, H.C. Prevalence and risk factors for atherosclerotic carotid stenosis and plaque: A population-based screening study. Medicine 2017, 96, e5999. [Google Scholar] [CrossRef]
- Columbo, J.A.; Stone, D.H. Appropriateness of care: Asymptomatic carotid stenosis including transcarotid artery revascularization. Semin. Vasc. Surg. 2024, in press. [Google Scholar] [CrossRef]
- Harish, K.B.; Speranza, G.; Rockman, C.B.; Sadek, M.; Jacobowitz, G.R.; Garg, K.; Teter, K.A.; Maldonado, T.S. Natural history of internal carotid artery stenosis progression. J. Vasc. Surg. 2024, 79, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Ielapi, N.; Andreucci, M.; Licastro, N.; Faga, T.; Grande, R.; Buffone, G.; Mellace, S.; Sapienza, P.; Serra, R. Precision Medicine and Precision Nursing: The Era of Biomarkers and Precision Health. Int. J. Gen. Med. 2020, 13, 1705–1711. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Zamzam, A.; Shaikh, F.; Saposnik, G.; Mamdani, M.; Qadura, M. Predicting Major Adverse Carotid Cerebrovascular Events in Patients with Carotid Stenosis: Integrating a Panel of Plasma Protein Biomarkers and Clinical Features—A Pilot Study. J. Clin. Med. 2024, 13, 3382. [Google Scholar] [CrossRef]
- Naylor, R.; Rantner, B.; Ancetti, S.; de Borst, G.J.; De Carlo, M.; Halliday, A.; Kakkos, S.K.; Markus, H.S.; McCabe, D.J.H.; Sillesen, H.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2023 Clinical Practice Guidelines on the Management of Atherosclerotic Carotid and Vertebral Artery Disease. Eur. J. Vasc. Endovasc. Surg. 2023, 65, 7–111. [Google Scholar] [CrossRef] [PubMed]
- Stilo, F.; Montelione, N.; Calandrelli, R.; Distefano, M.; Spinelli, F.; Di Lazzaro, V.; Pilato, F. The management of carotid restenosis: A comprehensive review. Ann. Transl. Med. 2020, 8, 1272. [Google Scholar] [CrossRef]
- Serra, R.; Ielapi, N.; Barbetta, A.; Andreucci, M.; de Franciscis, S. Novel biomarkers for cardiovascular risk. Biomark. Med. 2018, 12, 1015–1024. [Google Scholar] [CrossRef]
- de Franciscis, S.; Metzinger, L.; Serra, R. The Discovery of Novel Genomic, Transcriptomic, and Proteomic Biomarkers in Cardiovascular and Peripheral Vascular Disease: The State of the Art. BioMed Res. Int. 2016, 2016, 7829174. [Google Scholar] [CrossRef] [PubMed]
- Metzinger, L.; de Franciscis, S.; Serra, R. The Management of Cardiovascular Risk through Epigenetic Biomarkers. BioMed Res. Int. 2017, 2017, 9158572. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.; Scalise, E.; Ielapi, N.; Bracale, U.M.; Andreucci, M.; Serra, R. Metalloproteinases as Biomarkers and Sociomarkers in Human Health and Disease. Biomolecules 2024, 14, 96. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.; Ielapi, N.; Minici, R.; Bevacqua, E.; Ciranni, S.; Cristodoro, L.; Torcia, G.; Taranto, M.D.D.; Bracale, U.M.; Andreucci, M. Metalloproteinases between History, Health, Disease, and the Complex Dimension of Social Determinants of Health. J. Vasc. Dis. 2023, 2, 282–298. [Google Scholar] [CrossRef]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA-a scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Liao, D.; Wu, L.; Chen, H.; Li, J.; Wang, C. CYP Genetic Variants, CYP Metabolite Levels and Symptomatic Carotid Stenosis in Ischemic Stroke Patients. J. Atheroscler. Thromb. 2016, 23, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, D.; Zhang, H. Investigation of the underlying genes and mechanism of macrophage-enriched ruptured atherosclerotic plaques using bioinformatics method. J. Atheroscler. Thromb. 2019, 26, 636–658. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.K.; Butt, H.Z.; Choke, E.; Moore, D.; West, K.; Robinson, T.G.; Sayers, R.D.; Naylor, A.R.; Bown, M.J. Gene and protein expression of chemokine (C-C-motif) ligand 19 is upregulated in unstable carotid atherosclerotic plaques. Eur. J. Vasc. Endovasc. Surg. 2016, 52, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Vasuri, F.; de Biase, D.; Vacirca, A.; Acquaviva, G.; Sanza, V.; Gargiulo, M.; Pasquinelli, G. Gene polymorphism in tissue epidermal growth factor receptor (EGFR) influences clinical and histological vulnerability of carotid plaques. Pathol. Res. Pract. 2022, 229, 153721. [Google Scholar] [CrossRef]
- Li, Z.; Liu, J.; Liu, Z.; Zhu, X.; Geng, R.; Ding, R.; Xu, H.; Huang, S. Comprehensive analysis identifies crucial genes associated with immune cells mediating progression of carotid atherosclerotic plaque. Aging 2024, 16, 3880–3895. [Google Scholar] [CrossRef]
- Straface, G.; Biscetti, F.; Pitocco, D.; Bertoletti, G.; Misuraca, M.; Vincenzoni, C.; Snider, F.; Arena, V.; Stigliano, E.; Angelini, F.; et al. Assessment of the genetic effects of polymorphisms in the osteoprotegerin gene, TNFRSF11B, on serum osteoprotegerin levels and carotid plaque vulnerability. Stroke 2011, 42, 3022–3028. [Google Scholar] [CrossRef]
- Kostulas, K.; Brophy, V.H.; Moraitis, K.; Manolescu, A.; Kostulas, V.; Gretarsdottir, S.; Cheng, S.; Hillert, J. Genetic profile of ischemic cerebrovascular disease and carotid stenosis. Acta Neurol. Scand. 2008, 118, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Lin, J.; Zhou, Q.; Huang, R.; Chai, Z. The txa2r rs1131882, p2y1 rs1371097 and gpiiia rs2317676 three-loci interactions may increase the risk of carotid stenosis in patients with ischemic stroke. BMC Neurol. 2019, 19, 44. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.W.; Chou, C.L.; Cheng, C.F.; Lu, S.-X.; Wu, Y.-J.; Wang, L.-Y. Associations of genetic markers of diabetes mellitus with carotid atherosclerosis: A community-based case–control study. Cardiovasc. Diabetol. 2023, 22, 51. [Google Scholar] [CrossRef] [PubMed]
- Yocum, G.T.; Gaudet, J.G.; Lee, S.S.; Stern, Y.; Teverbaugh, L.A.; Sciacca, R.R.; Emala, C.W.; Quest, D.O.; McCormick, P.C.; McKinsey, J.F.; et al. Inducible nitric oxide synthase promoter polymorphism affords protection against cognitive dysfunction after carotid endarterectomy. Stroke 2009, 40, 1597–1603. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Ng, H.; Chan, E.; Mahabeleshwar, G. Macrophage–hypoxia-inducible factor-1α signaling in carotid artery stenosis. Am. J. Pathol. 2021, 191, 1118–1134. [Google Scholar] [CrossRef] [PubMed]
- Perisic, L.; Hedin, E.; Razuvaev, A.; Lengquist, M.; Osterholm, C.; Folkersen, L.; Gillgren, P.; Paulsson-Berne, G.; Ponten, F.; Odeberg, J.; et al. Profiling of atherosclerotic lesions by gene and tissue microarrays reveals PCSK6 as a novel protease in unstable carotid atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2432–2443. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhang, X.; Li, W.; Wang, T.; Cui, S.; Li, T.; Wang, Y.; Xu, W.; Ma, Y.; Yang, B.; et al. Eif2α mediated integrated stress response connects multiple intracellular signaling to reprogram vascular smooth muscle cell fate in carotid plaques. Helyon 2024, 10, e26904. [Google Scholar] [CrossRef] [PubMed]
- Karlöf, E.; Seime, T.; Dias, N.; Lengquist, M.; Witasp, A.; Almqvist, H.; Kronqvist, M.; Gådin, J.R.; Odeberg, J.; Maegdefessel, L.; et al. Correlation of computed tomography with carotid plaque transcriptomes associates calcification with lesion-stabilization. Atherosclerosis 2019, 288, 175–185. [Google Scholar] [CrossRef]
- Tan, J.; Liang, Y.; Yang, Z.; He, Q.; Tong, J.; Deng, Y.; Guo, W.; Liang, K.; Tang, J.; Shi, W.; et al. Single-cell transcriptomics reveals crucial cell subsets and functional heterogeneity associated with carotid atherosclerosis and cerebrovascular events. Arteriosclerosis. Thromb. Vasc. Biol. 2023, 43, 2312–2332. [Google Scholar] [CrossRef]
- Perisic, L.; Aldi, S.; Sun, Y.; Folkersen, L.; Razuvaev, A.; Roy, J.; Lengquist, M.; Åkesson, S.; Wheelock, C.E.; Maegdefessel, L.; et al. Gene expression signatures, pathways and networks in carotid atherosclerosis. J. Intern. Med. 2016, 279, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Forte, A.; Finicelli, M.; De Luca, P.; Quarto, C.; Onorati, F.; Santè, P.; Renzulli, A.; Galderisi, U.; Berrino, L.; De Feo, M.; et al. Expression profiles in surgically—Induced carotid stenosis: A combined transcriptomic and proteomic investigation. J. Cell. Mol. Med. 2008, 12, 1956–1973. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Jiang, S.; Lin, X.; Wang, B.; Chen, B.; Tong, J.; Shi, W.; Yu, B.; Tang, J. Differential expression profile of miRNAs between stable and vulnerable plaques of carotid artery stenosis patients. Genes Genet. Syst. 2023, 98, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Sheu, J.J.; Sun, C.K.; Huang, T.H.; Lin, Y.P.; Yip, H.K. MicroRNA-214 modulates the senescence of vascular smooth muscle cells in carotid artery stenosis. Mol. Med. 2020, 26, 46. [Google Scholar] [CrossRef] [PubMed]
- Theofilatos, K. Proteomic atlas of atherosclerosis: The contribution of proteoglycans to sex differences, plaque phenotypes, and outcomes. Circ. Res. 2023, 133, 542–558. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Ward, L.J.; Karlsson, H.; Ljunggren, S.A.; Li, W.; Lindahl, M.; Yuan, X.M. Distinctive proteomic profiles among different regions of human carotid plaques in men and women. Sci. Rep. 2016, 6, 26231. [Google Scholar] [CrossRef] [PubMed]
- Porcelli, B.; Ciari, I.; Felici, C.; Pagani, R.; Banfi, C.; Brioschi, M.; Giubbolini, M.; de Donato, G.; Setacci, C.; Terzuoli, L. Proteomic analysis of atherosclerotic plaque. Biomed. Pharmacother. 2010, 64, 369–372. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, J.; Liu, P.; Tang, X.; Pang, H.; Xie, T.; Xu, F.; Shao, J.; Chen, Y.; Liu, B.; et al. Urinary Proteomics Identifying Novel Biomarkers for the Diagnosis and Phenotyping of Carotid Artery Stenosis. Front. Mol. Biosci. 2021, 8, 714706. [Google Scholar] [CrossRef] [PubMed]
- Langley, S.R.; Willeit, K.; Didangelos, A.; Matic, L.P.; Skroblin, P.; Barallobre-Barreiro, J.; Lengquist, M.; Rungger, G.; Kapustin, A.; Kedenko, L.; et al. Extracellular matrix proteomics identifies molecular signature of symptomatic carotid plaques. J. Clin. Investig. 2017, 127, 1546–1560. [Google Scholar] [CrossRef]
- Hao, P.; Ren, Y.; Pasterkamp, G.; Moll, F.L.; de Kleijn, D.P.V.; Sze, S.K. Deep proteomic profiling of human carotid atherosclerotic plaques using multidimensional LC-MS/MS. Proteom. Clin. Appl. 2014, 8, 631–635. [Google Scholar] [CrossRef]
- Baragetti, A.; Mattavelli, E.; Grigore, L.; Pellegatta, F.; Magni, P.; Catapano, A.L. Targeted Plasma Proteomics to Predict the Development of Carotid Plaques. Stroke 2022, 53, e411–e414. [Google Scholar] [CrossRef] [PubMed]
- Lorentzen, L.G.; Yeung, K.; Eldrup, N.; Eiberg, J.P.; Sillesen, H.H.; Davies, M.J. Proteomic analysis of the extracellular matrix of human atherosclerotic plaques shows marked changes between plaque types. Matrix Biol. Plus 2024, 21, 100141. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.; Wang, C.; Liu, X.; Sun, H.; Guo, Z.; Shao, J.; Li, K.; Chen, J.; Wang, J.; Lei, X.; et al. Characterization of the proteome of stable and unstable carotid atherosclerotic plaques using data-independent acquisition mass spectrometry. J. Transl. Med. 2024, 22, 247. [Google Scholar] [CrossRef] [PubMed]
- Lepedda, A.J.; Cigliano, A.; Cherchi, G.M.; Spirito, R.; Maggioni, M.; Carta, F.; Turrini, F.; Edelstein, C.; Scanu, A.M.; Formato, M. A proteomic approach to differentiate histologically classified stable and unstable plaques from human carotid arteries. Atherosclerosis 2009, 203, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Cheng, M.L.; Shiao, M.S.; Lin, C.N. Metabolomics study in severe extracranial carotid artery stenosis. BMC Neurol. 2019, 19, 138. [Google Scholar] [CrossRef] [PubMed]
- Azzini, E.; Ruggeri, S.; Polito, A. Homocysteine: Its possible emerging role in at-risk population groups. Int. J. Mol. Sci. 2020, 21, 1421. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.N.; Hsu, K.C.; Huang, K.L.; Huang, W.C.; Hung, Y.L.; Lee, T.H. Identification of Metabolomics Biomarkers in Extracranial Carotid Artery Stenosis. Cells 2022, 11, 3022. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, Y.; Jia, W.; Sun, D.; Zhao, L.; Zhang, C.; Wang, C.; Chen, G.; Fu, S.; Bo, Y.; et al. Association between lipid profiles and presence of carotid plaque. Sci. Rep. 2019, 9, 18011. [Google Scholar] [CrossRef]
- Mas, S.; Martínez-Pinna, R.; Martín-Ventura, J.L.; Pérez, R.; Gomez-Garre, D.; Ortiz, A.; Fernandez-Cruz, A.; Vivanco, F.; Egido, J. Local non-esterified fatty acids correlate with inflammation in atheroma plaques of patients with type 2 diabetes. Diabetes 2010, 59, 1292–1301. [Google Scholar] [CrossRef]
- Vorkas, P.A.; Shalhoub, J.; Isaac, G.; Want, E.J.; Nicholson, J.K.; Holmes, E.; Davies, A.H. Metabolic phenotyping of atherosclerotic plaques reveals latent associations between free cholesterol and ceramide metabolism in atherogenesis. J. Proteome Res. 2015, 14, 1389–1399. [Google Scholar] [CrossRef]
- Vorkas, P.A.; Shalhoub, J.; Lewis, M.R.; Spagou, K.; Want, E.J.; Nicholson, J.K.; Davies, A.H.; Holmes, E. Metabolic Phenotypes of Carotid Atherosclerotic Plaques Relate to Stroke Risk: An Exploratory Study. Eur. J. Vasc. Endovasc. Surg. 2016, 52, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Stegemann, C.; Drozdov, I.; Shalhoub, J.; Humphries, J.; Ladroue, C.; Didangelos, A.; Baumert, M.; Allen, M.; Davies, A.H.; Monaco, C.; et al. Comparative lipidomics profiling of human atherosclerotic plaques. Circ. Cardiovasc. Genet. 2011, 4, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Peters, B.A.; Bryant, M.; Hanna, D.B.; Schwartz, T.; Wang, T.; Sollecito, C.C.; Usyk, M.; Grassi, E.; Wiek, F.; et al. Gut microbiota, circulating inflammatory markers and metabolites, and carotid artery atherosclerosis in HIV infection. Microbiome 2023, 11, 119. [Google Scholar] [CrossRef] [PubMed]
- Cason, C.A.; Dolan, K.T.; Sharma, G.; Tao, M.; Kulkarni, R.; Helenowski, I.B.; Doane, B.M.; Avram, M.J.; McDermott, M.M.; Chang, E.B.; et al. Plasma microbiome-modulated indole- and phenyl-derived metabolites associate with advanced atherosclerosis and postoperative outcomes. J. Vasc. Surg. 2018, 68, 1552–1562.e7. [Google Scholar] [CrossRef]
- Hsu, H.; Lu, T.; Hansraj, N.; Russeau, A.; Kougias, P.; Barshes, N.R. Gender, racial and ethnic disparities in index hospitalization operations for symptomatic carotid stenosis in Texas hospitals. Ann. Vasc. Surg. 2022, 80, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.W.; Mota, L.; Marcaccio, C.; Liang, P.; Moreira, C.C.; Hughes, K.; Schermerhorn, M.L. Impact of neighborhood social disadvantage on carotid artery disease presentation, management, and discharge outcomes. J. Vasc. Surg. 2023, 77, 1700–1709. [Google Scholar] [CrossRef] [PubMed]
- Baxi, J.; Chao, J.C.; Dewan, K.; Yang, N.K.; Pepe, R.J.; Deng, X.; Soliman, F.K.; Volk, L.; Rahimi, S.; Russo, M.J.; et al. Socioeconomic status as a predictor of post-operative mortality and outcomes in carotid artery stenting vs. carotid endarterectomy. Front. Cardiovasc. Med. 2024, 11, 1286100. [Google Scholar] [CrossRef] [PubMed]
- Aber, A.; Howard, A.; Woods, H.B.; Jones, G.; Michaels, J. Impact of Carotid Artery Stenosis on Quality of Life: A Systematic Review. Patient 2019, 12, 213–222. [Google Scholar] [CrossRef]
- Frazier, L.; Johnson, R.L.; Sparks, E. Genomics and cardiovascular disease. J. Nurs. Sch. 2005, 37, 315–321. [Google Scholar] [CrossRef]
- Zeller, T.; Blankenberg, S.; Diemert, P. Genomewide association studies in cardiovascular disease—An update 2011. Clin. Chem. 2012, 58, 92–103. [Google Scholar] [CrossRef]
- McDonough, C.W. Pharmacogenomics in Cardiovascular Diseases. Curr. Protoc. 2021, 1, e189. [Google Scholar] [CrossRef] [PubMed]
- Pasipoularides, A. Implementing genome-driven personalized cardiology in clinical practice. J. Mol. Cell. Cardiol. 2018, 115, 142–157. [Google Scholar] [CrossRef]
- Abraham, G.; Rutten-Jacobs, L.; Inouye, M. Risk Prediction Using Polygenic Risk Scores for Prevention of Stroke and Other Cardiovascular Diseases. Stroke 2021, 52, 2983–2991. [Google Scholar] [CrossRef] [PubMed]
- Moxon, J.V.; Padula, M.P.; Herbert, B.R.; Golledge, J. Challenges, current status and future perspectives of proteomics in improving understanding, diagnosis and treatment of vascular disease. Eur. J. Vasc. Endovasc. Surg. 2009, 38, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Quertermous, T.; Li, D.Y.; Weldy, C.S.; Ramste, M.; Sharma, D.; Monteiro, J.P.; Gu, W.; Worssam, M.D.; Palmisano, B.T.; Park, C.Y.; et al. Genome-Wide Genetic Associations Prioritize Evaluation of Causal Mechanisms of Atherosclerotic Disease Risk. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Debette, S.; Lambert, J.-C.; Gariépy, J.; Fievet, N.; Tzourio, C.; Dartigues, J.-F.; Ritchie, K.; Dupuy, A.-M.; Alpérovitch, A.; Ducimetière, P.; et al. New insight into the association of apolipoprotein E genetic variants with carotid plaques and intima-media thickness. Stroke 2006, 37, 2917–2923. [Google Scholar] [CrossRef] [PubMed]
- Ou, M.; Liu, S.; Ma, X.; Xing, X.; He, W.; Gao, H. IL-6 promoter polymorphism increased risks of recurrent stroke in the young patients with moderate internal carotid artery stenosis. J. Cell. Biochem. 2018, 119, 2886–2890. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yao, Y.; Wang, Y.; Ji, L.; Zhu, K.; Hu, H.; Chen, J.; Yang, J.; Cui, Q.; Geng, B.; et al. Association between high-sensitivity C-reactive protein, lipoprotein-associated phospholipase A2 and carotid atherosclerosis: A cross-sectional study. J. Cell. Mol. Med. 2018, 22, 5145–5150. [Google Scholar] [CrossRef] [PubMed]
- Vivanco, F.; Martín-Ventura, J.L.; Duran, M.C.; Barderas, M.G.; Blanco-Colio, L.; Dardé, V.M.; Mas, S.; Meilhac, O.; Michel, J.B.; Tuñón, J.; et al. Quest for novel cardiovascular biomarkers by proteomic analysis. J. Proteome Res. 2005, 4, 1181–1191. [Google Scholar] [CrossRef]
- Chiorescu, R.M.; Mocan, M.; Inceu, A.I.; Buda, A.P.; Blendea, D.; Vlaicu, S.I. Vulnerable Atherosclerotic Plaque: Is There a Molecular Signature? Int. J. Mol. Sci. 2022, 23, 13638. [Google Scholar] [CrossRef]
- Mushenkova, N.V.; Summerhill, V.I.; Zhang, D.; Romanenko, E.B.; Grechko, A.V.; Orekhov, A.N. Current Advances in the Diagnostic Imaging of Atherosclerosis: Insights into the Pathophysiology of Vulnerable Plaque. Int J Mol Sci. 2020, 21, 2992. [Google Scholar] [CrossRef] [PubMed]
- Pleskovič, A.; Letonja, M.Š.; Vujkovac, A.C.; Starčević, J.N.; Caprnda, M.; Curilla, E.; Mozos, I.; Kruzliak, P.; Prosecky, R.; Petrovič, D. Matrix metalloproteinase-3 gene polymorphism (rs3025058) affects markers atherosclerosis in type 2 diabetes mellitus. VASA 2017, 46, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Guzman, L.A.; Costa, M.A.; Angiolillo, D.J.; Zenni, M.; Wludyka, P.; Silliman, S.; Bass, T.A. A systematic review of outcomes in patients with staged carotid artery stenting and coronary artery bypass graft surgery. Stroke 2008, 39, 361–365. [Google Scholar] [CrossRef]
- Rugonfalvi-Kiss, S.; Dósa, E.; Madsen, H.O.; Endrész, V.; Prohászka, Z.; Laki, J.; Karádi, I.; Gönczöl, E.; Selmeci, L.; Romics, L.; et al. High rate of early restenosis after carotid eversion endarterectomy in homozygous carriers of the normal mannose-binding lectin genotype. Stroke 2005, 36, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.M.; Hadaya, J.; Trehan, A.; Zekavat, S.M.; Roselli, C.; Klarin, D.; Emdin, C.A.; Hilvering, C.R.; Bianchi, V.; Mueller, C.; et al. A Genetic Variant Associated with Five Vascular Diseases Is a Distal Regulator of Endothelin-1 Gene Expression. Cell 2017, 170, 522–533. [Google Scholar] [CrossRef]
- Houston, M. New concepts in cardiovascular disease. J. Restor. Med. 2013, 2, 30–44. [Google Scholar] [CrossRef]
- Kumanayake, P. Genome-wide SNP discovery in associating with human diseases phenotypes. Sri Lanka J. Bio-Med. Inform. 2013, 3, 25. [Google Scholar] [CrossRef]
- Carballo-Perich, L.; Puigoriol-Illamola, D.; Bashir, S.; Terceño, M.; Silva, Y.; Gubern-Mérida, C.; Serena, J. Clinical Parameters and Epigenetic Biomarkers of Plaque Vulnerability in Patients with Carotid Stenosis. Int. J. Mol. Sci. 2022, 23, 5149. [Google Scholar] [CrossRef] [PubMed]
- Weakley, S.M.; Jiang, J.; Kougias, P.; Lin, P.H.; Yao, Q.; Brunicardi, F.C.; Gibbs, R.A.; Chen, C. Role of somatic mutations in vascular disease formation. Expert Rev. Mol. Diagn. 2010, 10, 173–185. [Google Scholar] [CrossRef]
- Gerritsen, M. Genetic variations in vascular endothelial growth factor and endothelial nitric oxide synthase and their contributions to human disease. Microcirculation 2005, 12, 129–140. [Google Scholar] [CrossRef]
- Gao, C.; Wang, Y. Transcriptome complexity in cardiac development and diseases—An expanding universe between genome and phenome. Circ. J. 2014, 78, 1038–1047. [Google Scholar] [CrossRef]
- Xu, S. Transcriptome Profiling in Systems Vascular Medicine. Front. Pharmacol. 2017, 8, 563. [Google Scholar] [CrossRef] [PubMed]
- Shan, K.; Jiang, Q.; Wang, X.Q.; Wang, Y.N.Z.; Yang, H.; Yao, M.D.; Liu, C.; Li, X.M.; Yao, J.; Liu, B.; et al. Role of long non-coding RNA-RNCR3 in atherosclerosis-related vascular dysfunction. Cell Death Dis. 2016, 7, e2248. [Google Scholar] [CrossRef] [PubMed]
- Bazan, H.A.; Brooks, A.J.; Vongbunyong, K.; Tee, C.; Douglas, H.F.; Klingenberg, N.C.; Woods, T.C. A pro-inflammatory and fibrous cap thinning transcriptome profile accompanies carotid plaque rupture leading to stroke. Sci. Rep. 2022, 12, 13499. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Xie, W.L.; Kong, W.W.; Chen, D.; Qu, P. Expression of the NLRP3 Inflammasome in Carotid Atherosclerosis. J. Stroke Cerebrovasc. Dis. 2015, 24, 2455–2466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Li, X.-K.; Lv, S.-J.; Wang, H.-P.; Liu, Y.; Zhou, J.; Gong, H.; Chen, X.-F.; Ren, S.-C.; et al. Sirtuin 2 deficiency aggravates ageing-induced vascular remodelling in humans and mice. Eur. Heart J. 2023, 44, 2746–2759. [Google Scholar] [CrossRef] [PubMed]
- Forte, A.; Rinaldi, B.; Berrino, L.; Rossi, F.; Galderisi, U.; Cipollaro, M. Novel potential targets for prevention of arterial restenosis: Insights from the pre-clinical research. Clin. Sci. 2014, 127, 615–634. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Horie, T.; Baba, O.; Kimura, M.; Tsuji, S.; Rodriguez, R.R.; Miyagawa, S.; Kimura, T. Functional non—Coding RNAs in vascular diseases. FEBS J. 2022, 288, 6315–6330. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Song, J. Single-Cell Transcriptomics Reveals the Cellular Heterogeneity of Cardiovascular Diseases. Front. Cardiovasc. Med. 2021, 8, 643519. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Nelson, P.R.; Odgren, P.R.; Nelson, J.D.; Vasiliu, C.; Park, J.; Morris, M.; Lian, J.; Cutler, B.S.; et al. Temporal evolution of gene expression in rat carotid artery following balloon angioplasty. J. Cell. Biochem. 2007, 101, 399–410. [Google Scholar] [CrossRef]
- Sopić, M.; Karaduzovic-Hadziabdic, K.; Kardassis, D.; Maegdefessel, L.; Martelli, F.; Meerson, A.; Munjas, J.; Niculescu, L.S.; Stoll, M.; Magni, P.; et al. Transcriptomic research in atherosclerosis: Unravelling plaque phenotype and overcoming methodological challenges. J. Mol. Cell. Cardiol. Plus 2023, 6, 100048. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, S.; Yan, P.; Ren, J.; Song, M.; Li, J.; Lei, J.; Pan, H.; Wang, S.; Ma, X.; et al. A single-cell transcriptomic landscape of primate arterial aging. Nat. Commun. 2020, 11, 2202. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.; Barallobre-Barreiro, J.; Jahangiri, M.; Mayr, M. Vascular proteomics in metabolic and cardiovascular diseases. J. Intern. Med. 2016, 280, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Collins, P. HDL-C in post-menopausal women: An important therapeutic target. Int. J. Cardiol. 2008, 124, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Mercuro, G.; Deidda, M.; Bina, A.; Manconi, E.; Rosano, G.M. Gender-specific aspects in primary and secondary prevention of cardiovascular disease. Curr. Pharm. Des. 2011, 17, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Kiechl, S.; Willeit, J.; Egger, G.; Poewe, W.; Oberhollenzer, F. Body iron stores and the risk of carotid atherosclerosis: Prospective results from the Bruneck study. Circulation 1997, 96, 3300–3307. [Google Scholar] [CrossRef] [PubMed]
- Wendorff, C.; Wendorff, H.; Pelisek, J.; Tsantilas, P.; Zimmermann, A.; Zernecke, A.; Kuehnl, A.; Eckstein, H.H. Carotid Plaque Morphology Is Significantly Associated with Sex, Age, and History of Neurological Symptoms. Stroke 2015, 46, 3213–3219. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Colio, L.M.; Martín-Ventura, J.L.; Vivanco, F.; Michel, J.B.; Meilhac, O.; Egido, J. Biology of atherosclerotic plaques: What we are learning from proteomic analysis. Cardiovasc. Res. 2006, 72, 18–29. [Google Scholar] [CrossRef]
- Nordon, I.; Brar, R.; Hinchliffe, R.; Cockerill, G.; Loftus, I.; Thompson, M. The role of proteomic research in vascular disease. J. Vasc. Surg. 2009, 49, 1602–1612. [Google Scholar] [CrossRef]
- Boccardi, C.; Cecchettini, A.; Caselli, A.; Camici, G.; Evangelista, M.; Mercatanti, A.; Rainaldi, G.; Citti, L. A proteomic approach to the investigation of early events involved in the activation of vascular smooth muscle cells. Cell Tissue Res. 2007, 329, 119–128. [Google Scholar] [CrossRef]
- Romuk, E.; Wojciechowska, C.; Jacheć, W.; Nowak, J.; Niedziela, J.; Malinowska-Borowska, J.; Głogowska-Gruszka, A.; Birkner, E.; Rozentryt, P. Comparison of Oxidative Stress Parameters in Heart Failure Patients Depending on Ischaemic or Nonischaemic Aetiology. Oxidative Med. Cell. Longev. 2019, 2019, 7156038. [Google Scholar] [CrossRef] [PubMed]
- Eilenberg, W.; Stojkovic, S.; Kaider, A.; Kozakowski, N.; Domenig, C.M.; Burghuber, C.; Nanobachvili, J.; Huber, K.; Klinger, M.; Neumayer, C.; et al. NGAL and MMP-9/NGAL as biomarkers of plaque vulnerability and targets of statins in patients with carotid atherosclerosis. Clin. Chem. Lab. Med. 2017, 56, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.; Andreucci, M.; Ielapi, N.; Serraino, G.F.; Mastroroberto, P.; Bracale, U.M.; Serra, R. Vascular Biology of Arterial Aneurysms. Ann. Vasc. Surg. 2023, 94, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Serra, R.; Jiritano, F.; Bracale, U.M.; Ielapi, N.; Licastro, N.; Provenzano, M.; Andreucci, M.; Rizzuto, A.; Mastroroberto, P.; Serraino, G.F. Novel Biomarkers in Cardiovascular Surgery. Biomark. Med. 2021, 15, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Busceti, M.T.; Grande, R.; Amato, B.; Gasbarro, V.; Buffone, G.; Amato, M.; Gallelli, L.; Serra, R.; De Franciscis, S. Pulmonary embolism, metalloproteinases and neutrophil gelatinase associated lipocalin. Acta Phlebol. 2013, 14, 115–121. [Google Scholar]
- Rocchiccioli, S.; Pelosi, G.; Rosini, S.; Marconi, M.; Viglione, F.; Citti, L.; Ferrari, M.; Trivella, M.G.; Cecchettini, A. Secreted proteins from carotid endarterectomy: An untargeted approach to disclose molecular clues of plaque progression. J. Transl. Med. 2013, 11, 260. [Google Scholar] [CrossRef] [PubMed]
- Surendran, A.; Atefi, N.; Zhang, H.; Aliani, M.; Ravandi, A. Defining Acute Coronary Syndrome through Metabolomics. Metabolites 2021, 11, 685. [Google Scholar] [CrossRef] [PubMed]
- Vojinovic, D.; van der Lee, S.J.; van Duijn, C.M.; Vernooij, M.W.; Kavousi, M.; Amin, N.; Demirkan, A.; Ikram, M.A.; van der Lugt, A.; Bos, D. Metabolic profiling of intra- and extracranial carotid artery atherosclerosis. Atherosclerosis 2018, 272, 60–65. [Google Scholar] [CrossRef]
- Jin, H.; Zhang, C.; Nagenborg, J.; Juhasz, P.; Ruder, A.V.; Sikkink, C.J.J.M.; Mees, B.M.E.; Waring, O.; Sluimer, J.C.; Neumann, D.; et al. Genome-scale metabolic network of human carotid plaque reveals the pivotal role of glutamine/glutamate metabolism in macrophage modulating plaque inflammation and vulnerability. Cardiovasc. Diabetol. 2024, 23, 240. [Google Scholar] [CrossRef]
- Gao, S.; Wang, X.; Huang, J.; Zhu, Y.; Zhang, R.; He, J.; Abliz, Z. Development and validation of a sensitive and reliable targeted metabolomics method for the quantification of cardiovascular disease-related biomarkers in plasma using ultrahigh-performance liquid chromatography-tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2022, 36, e9292. [Google Scholar] [CrossRef]
- Cheng, S.; Shah, S.H.; Corwin, E.J.; Fiehn, O.; Fitzgerald, R.L.; Gerszten, R.E.; Illig, T.; Rhee, E.P.; Srinivas, P.R.; Wang, T.J.; et al. Potential Impact and Study Considerations of Metabolomics in Cardiovascular Health and Disease: A Scientific Statement From the American Heart Association. Circ. Cardiovasc. Genet. 2017, 10, e000032. [Google Scholar] [CrossRef] [PubMed]
- Abdullaev, A.; Abdullaeva, G.; Yusupova, K. Metabolomic approaches in studying of cardiovascular diseases. Eurasian Heart J. 2021, 1, 106–117. [Google Scholar] [CrossRef]
- Goonewardena, S.N.; Prevette, L.E.; Desai, A.A. Metabolomics and atherosclerosis. Curr. Atheroscler. Rep. 2010, 12, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.; Ielapi, N.; Bevacqua, E.; Ciranni, S.; Cristodoro, L.; Torcia, G.; Serra, R. Social Determinants of Health and Vascular Diseases: A Systematic Review and Call for Action. Soc. Sci. 2023, 12, 214. [Google Scholar] [CrossRef]
- Joo, W.T.; Lee, C.J.; Oh, J.; Kim, I.C.; Lee, S.H.; Kang, S.M.; Kim, H.C.; Park, S.; Youm, Y. The Association between Social Network Betweenness and Coronary Calcium: A Baseline Study of Patients with a High Risk of Cardiovascular Disease. J. Atheroscler. Thromb. 2018, 25, 131–141. [Google Scholar] [CrossRef]
- Nishi, N. Social Network Structure and Atherosclerotic Cardiovascular Disease. J. Atheroscler. Thromb. 2018, 25, 124–125. [Google Scholar] [CrossRef] [PubMed]
- Wang, M. Research and solutions to cardiovascular disease in China. Theor. Nat. Sci. 2024, 33, 258–262. [Google Scholar] [CrossRef]
- Bonomo, J.A.; Luo, K.; Ramallo, J.A. LGBTQ+ cardiovascular health equity: A brief review. Front. Cardiovasc. Med. 2024, 11, 1350603. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, Y.; Whelan, B.; Kenny, R. The differential impact of subjective and objective aspects of social engagement on cardiovascular risk factors. BMC Geriatr. 2010, 10, 81. [Google Scholar] [CrossRef]
- Havranek, E.P.; Mujahid, M.S.; Barr, D.A.; Blair, I.V.; Cohen, M.S.; Cruz-Flores, S.; Smith, G.D.; Himmelfarb, C.D.; Lauer, M.S.; Lockwood, D.W.; et al. Social determinants of risk and outcomes for cardiovascular disease. Circulation 2015, 132, 873–898. [Google Scholar] [CrossRef]
- Inoue, N. Stress and atherosclerotic cardiovascular disease. J. Atheroscler. Thromb. 2014, 21, 391–401. [Google Scholar] [CrossRef]
- Koenig, H.G. Religion and medicine III: Developing a theoretical model. Int. J. Psychiatry Med. 2001, 31, 199–216. [Google Scholar] [CrossRef]
- Dégano, I.R.; Marrugat, J.; Grau, M.; Salvador-González, B.; Ramos, R.; Zamora, A.; Martí, R.; Elosua, R. The association between education and cardiovascular disease incidence is mediated by hypertension, diabetes, and body mass index. Sci. Rep. 2017, 7, 12370. [Google Scholar] [CrossRef]
- Tian, X.; Wang, X.; Shi, Z.; Yu, C.; Li, M.; Chen, L.; Jia, Q.; Liang, G. Tumor necrosis factor-stimulated gene-6 new serum identification marker to identify severe and symptomatic carotid artery stenosis. Pathol. Res. Pract. 2022, 232, 153838. [Google Scholar] [CrossRef]
- Sun, C.; Xi, N.; Sun, Z.; Zhang, X.; Wang, X.; Cao, H.; Jia, X. The Relationship between Intracarotid Plaque Neovascularization and Lp (a) and Lp-PLA2 in Elderly Patients with Carotid Plaque Stenosis. Dis. Markers 2022, 2022, 6154675. [Google Scholar] [CrossRef] [PubMed]
- Debing, E.; Peeters, E.; Demanet, C.; De Waele, M.; Van den Brande, P. Markers of inflammation in patients with symptomatic and asymptomatic carotid artery stenosis: A case-control study. Vasc. Endovasc. Surg. 2008, 42, 122–127. [Google Scholar] [CrossRef]
- Fatemi, S.; Acosta, S.; Zarrouk, M.; Engström, G.; Melander, O.; Gottsäter, A. Pro b-type natriuretic peptide and midregional proadrenomedullin are associated with incident carotid stenosis during long term follow-up. J. Stroke Cerebrovasc. Dis. 2021, 30, 105403. [Google Scholar] [CrossRef]
- Soylu, A.; Cortcu, S.; Uzunkaya, F.; Atalay, Y.; Bekçi, T.; Güngör, L.; Belet, Ü. The correlation of the platelet-to-lymphocyte ratio with the severity of stenosis and stroke in patients with carotid arterial disease. Vascular 2016, 25, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Massiot, N.; Lareyre, F.; Voury-Pons, A.; Pelletier, Y.; Chikande, J.; Carboni, J.; Umbdenstock, E.; Jean-Baptiste, E.; Hassen-Khodja, R.; Raffort, J. High Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Ratio are Associated with Symptomatic Internal Carotid Artery Stenosis. J. Stroke Cerebrovasc. Dis. 2019, 28, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, D.; Hu, Y.; Chen, Y. Oxidative Stress and Inflammation Are Associated with Coexistent Severe Multivessel Coronary Artery Stenosis and Right Carotid Artery Severe Stenosis in Elderly Patients. Oxidative Med. Cell. Longev. 2021, 2021, 2976447. [Google Scholar] [CrossRef] [PubMed]
- Kinsella, J.A.; Tobin, W.O.; Kavanagh, G.F.; O'Donnell, J.S.; McGrath, R.T.; Tierney, S.; Feeley, T.M.; Egan, B.; O'Neill, D.; Collins, R.D.; et al. Increased endothelial activation in recently symptomatic versus asymptomatic carotid artery stenosis and in cerebral microembolic-signal-negative patient subgroups. Eur. J. Neurol. 2014, 21, 969. [Google Scholar] [CrossRef] [PubMed]
- Miceli, G.; Basso, M.G.; Pintus, C.; Pennacchio, A.R.; Cocciola, E.; Cuffaro, M.; Profita, M.; Rizzo, G.; Tuttolomondo, A. Molecular Pathways of Vulnerable Carotid Plaques at Risk of Ischemic Stroke: A Narrative Review. Int. J. Mol. Sci. 2024, 25, 4351. [Google Scholar] [CrossRef] [PubMed]
- Jaroslav, P.; Christian, R.; Stefan, O.; Alexander, Z.; Zepper, P.; Holger, P.; Hans-Henning, E. Evaluation of serum biomarkers for patients at increased risk of stroke. Int. J. Vasc. Med. 2012, 2012, 906954. [Google Scholar] [CrossRef] [PubMed]
- Karczewski, K.J.; Snyder, M.P. Integrative omics for health and disease. Nat. Rev. Genet. 2018, 19, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Li, L.; Zhang, Y.; Yuan, H.; Ye, L.; Zhao, J.; Duan, D.D. Phenomics of Vascular Disease: The Systematic Approach to the Combination Therapy. Curr. Vasc. Pharmacol. 2015, 13, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, H. Omics Approaches Unveiling the Biology of Human Atherosclerotic Plaques. Am. J. Pathol. 2024, 194, 482–498. [Google Scholar] [CrossRef] [PubMed]
- Vuković-Cvetković, V. Microembolus detection by transcranial Doppler sonography: Review of the literature. Stroke Res. Treat. 2012, 2012, 382361. [Google Scholar] [CrossRef] [PubMed]
- Rajeev, V.; Chai, Y.L.; Poh, L.; Selvaraji, S.; Fann, D.Y.; Jo, D.-G.; De Silva, T.M.; Drummond, G.R.; Sobey, C.G.; Arumugam, T.V.; et al. Chronic cerebral hypoperfusion: A critical feature in unravelling the etiology of vascular cognitive impairment. Acta Neuropathol. Commun. 2023, 11, 93. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.; Sali, A.; Baumeister, W. The molecular sociology of the cell. Nature 2007, 450, 973–982. [Google Scholar] [CrossRef]
- Morin, E. Restricted complexity, general complexity. In Worldviews Science and Us: Philosophy and Complexity; Gershenson, C., Aerts, D., Edmonds, B., Eds.; World Scientific: Singapore, 2007; pp. 5–29. [Google Scholar]
- Cabral, M.d.F.C.T.; Viana, A.L.; Gontijo, D.T. Use of the complexity paradigm in the field of health: Scope review. Esc. Anna Nery 2020, 24, e20190235. [Google Scholar] [CrossRef]
- Stringhini, S.; Zaninotto, P.; Kumari, M.; Kivimäki, M.; Lassale, C.; Batty, G.D. Socio-economic trajectories and cardiovascular disease mortality in older people: The English Longitudinal Study of Ageing. Int. J. Epidemiol. 2018, 47, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Acquah, I.; Hagan, K.; Javed, Z.; Taha, M.B.; Valero-Elizondo, J.; Nwana, N.; Yahya, T.; Sharma, G.; Gulati, M.; Hammoud, A.; et al. Social Determinants of Cardiovascular Risk, Subclinical Cardiovascular Disease, and Cardiovascular Events. J. Am. Heart Assoc. 2023, 12, e025581. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.; Stokes, D.; Darley, A. Use of complexity theory in health and social care: A scoping review protocol. BMJ Open 2021, 11, e047633. [Google Scholar] [CrossRef] [PubMed]
| ITEMS | Justification of the Article’s Importance for the Readership | Statement of Concrete Aims or Formulation of Questions | Description of the Literature Search | Referencing | Scientific Reasoning | Appropriate Presentation of Data | Total Score |
|---|---|---|---|---|---|---|---|
|
|
|
|
|
| ||
| Yi et al. [16] | Score: 2 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | 12 |
| Wang et al. [17] | Score: 1 | Score: 2 | Score: 1 | Score: 2 | Score: 2 | Score: 2 | 10 |
| Li et al. [18] | Score: 2 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | 12 |
| Straface et al. [19] | Score: 2 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | 12 |
| Salem et al. [20] | Score: 1 | Score: 2 | Score: 1 | Score: 2 | Score: 2 | Score: 2 | 10 |
| Vasuri et al. [21] | Score: 1 | Score: 2 | Score: 1 | Score: 2 | Score: 2 | Score: 2 | 10 |
| Kostulas et al. [22] | Score: 2 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | 12 |
| Yi et al. [23] | Score: 1 | Score: 2 | Score: 1 | Score: 2 | Score: 2 | Score: 2 | 10 |
| Wu et al. [24] | Score: 2 | Score: 2 | Score: 2 | Score: 2 | Score: 1 | Score: 1 | 10 |
| Yocum et al. [25] | Score: 2 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | 12 |
| ITEMS | Justification of the Article’s Importance for the Readership | Statement of Concrete Aims or Formulation of Questions | Description of the Literature Search | Referencing | Scientific Reasoning | Appropriate Presentation of Data | Total Score |
|---|---|---|---|---|---|---|---|
|
|
|
|
|
| ||
| Kim et al. [26] | Score: 2 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | 12 |
| Perisic et al. [27] | Score: 2 | Score: 2 | Score: 2 | Score: 1 | Score: 1 | Score: 2 | 10 |
| Luo et al. [28] | Score: 2 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | 12 |
| Karlof et al. [29] | Score: 2 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | 12 |
| Tan et al. [30] | Score: 1 | Score: 2 | Score: 1 | Score: 2 | Score: 2 | Score: 2 | 10 |
| Perisic et al. [31] | Score: 1 | Score: 1 | Score: 2 | Score: 1 | Score: 2 | Score: 2 | 10 |
| Forte et al. [32] | Score: 2 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | 12 |
| Deng et al. [33] | Score: 2 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | 12 |
| Chen et al. [34] | Score: 2 | Score: 2 | Score: 1 | Score: 1 | Score: 2 | Score: 2 | 10 |
| ITEMS | Justification of the Article’s Importance for the Readership | Statement of Concrete Aims or Formulation of Questions | Description of the Literature Search | Referencing | Scientific Reasoning | Appropriate Presentation of Data | Total Score |
|---|---|---|---|---|---|---|---|
|
|
|
|
|
| ||
| Forte et al. [32] | Score: 2 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | 12 |
| Theofilatos et al. [35] | Score: 2 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | 12 |
| Liang et al. [36] | Score: 1 | Score: 1 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | 10 |
| Porcelli et al. [37] | Score: 1 | Score: 2 | Score: 1 | Score: 2 | Score: 2 | Score: 2 | 10 |
| Wang et al. [38] | Score: 1 | Score: 1 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | 10 |
| Langley et al. [39] | Score: 2 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | 12 |
| Hao et al. [40] | Score: 1 | Score: 1 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | 10 |
| Baragetti et al. [41] | Score: 1 | Score: 1 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | 10 |
| Lorentsen et al. [42] | Score: 2 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | 12 |
| Lai et al. [43] | Score: 2 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | 12 |
| Lepedda et al. [44] | Score: 2 | Score: 1 | Score: 1 | Score: 2 | Score: 2 | Score: 2 | 10 |
| AUTHORS | Justification of the Article’s Importance for the Readership | Statement of Concrete Aims or Formulation of Questions | Description of the Literature Search | Referencing | Scientific Reasoning | Appropriate Presentation of Data | Total Score |
|---|---|---|---|---|---|---|---|
|
|
|
|
|
| ||
| Lee et al. [45] | Score: 2 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | 12 |
| Azzini et al. [46] | Score: 2 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | 12 |
| Lin et al. [47] | Score: 1 | Score: 1 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | 10 |
| Liu et al. [48] | Score: 2 | Score: 1 | Score: 1 | Score: 2 | Score: 2 | Score: 2 | 10 |
| Mas et al. [49] | Score: 2 | Score: 1 | Score: 2 | Score: 1 | Score: 2 | Score: 2 | 10 |
| Vorkas et al. [50] | Score: 2 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | 12 |
| Vorkas et al. [51] | Score: 1 | Score: 1 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | 10 |
| Stegemann et al. [52] | Score: 1 | Score: 2 | Score: 2 | Score: 1 | Score: 2 | Score: 2 | 10 |
| Wang et al. [53] | Score: 2 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | 12 |
| Cason et al. [54] | Score: 1 | Score: 1 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | 10 |
| AUTHORS | Justification of the Article’s Importance for the Readership | Statement of Concrete Aims of Formulation of Questions | Description of the Literature Search | Referencing | Scientific Reasoning | Appropriate Presentation of Data | Total Score |
|---|---|---|---|---|---|---|---|
|
|
|
|
|
| ||
| Hsu et al. [55] | Score: 2 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | 12 |
| Wu et al. [56] | Score: 2 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | 12 |
| Baxi et al. [57] | Score: 2 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | Score: 2 | 12 |
| Aber et al. [58] | Score: 2 | Score: 1 | Score: 1 | Score: 2 | Score: 2 | Score: 2 | 10 |
| AUTHORS | TITLE | YEAR OF PUBLICATION | RESEARCH DESIGN | SAMPLE CHARACTERISTICS | MAIN FINDING |
|---|---|---|---|---|---|
| Yi et al. [16] | CYP genetic variants, CYP metabolite levels, and symptomatic carotid stenosis in ischemic stroke patients | 2016 | Experimental study | 136 patients with CS 1 and ischemic stroke and 158 without CS | Analysis of the differences between the cytochrome P 450 genetic polymorphism and cytochrome P metabolites in patients with symptomatic CS |
| Wang et al. [17] | Investigation of the underlying genes and mechanism of macrophage-enriched ruptured atherosclerotic plaques using bioinformatics method | 2019 | Experimental prospective study | 6 stable atherosclerotic samples and 5 ruptured samples from the GEO database | Several differentially expressed genes in ruptured and macrophagic atherosclerotic plaques |
| Li et al. [18] | Comprehensive analysis identifies crucial genes associated with immune cells mediating progression of carotid atherosclerotic plaque | 2024 | Experimental study | Gene datasets with 16 advanced and 13 early atherosclerotic plaques, 32 atheromas, and 32 intact tissues | The gene PLEK 5 was associated with monocyte and macrophage functions in atherosclerotic plaque |
| Straface et al. [19] | Assessment of the genetic effects of polymorphisms in the osteoprotegerin gene, TNFRSF11B, on serum osteoprotegerin levels and carotid plaque vulnerability | 2011 | Experimental study | Carotid plaques from 177 patients who underwent CEA and 303 healthy controls | The gene polymorphism of TNFRSF11B 6 is associated with unstable carotid plaque |
| Salem et al. [20] | Gene and protein expression of chemokine (C-C-Motif) ligand 19 is upregulated in unstable carotid atherosclerotic plaques | 2016 | Experimental prospective study | 24 sample plaques from patients who underwent CEA 2 | Upregulation of the CCL19 3 gene in unstable atherosclerotic plaques |
| Vasuri et al. [21] | Gene polymorphism in tissue epidermal growth factor receptor (EGFR) influences clinical and histological vulnerability of carotid plaques | 2022 | Retrospective study | 29 patients who underwent CEA | The polymorphism observed for the EGFR 4 gene is associated with protection against plaque hemorrhage and could, therefore, be a determinant of pre-surgical decision-making |
| Kostulas et al. [22] | Genetic profile of ischemic cerebrovascular disease and carotid stenosis | 2008 | Experimental prospective study | 928 patients with ischemic stroke and CS, and 602 healthy patients | Gene polymorphisms related to the factor VII gene, apolipoprotein E gene, and two renin gene polymorphisms were associated with ischemic stroke and CS |
| Yi et al. [23] | The txa2r rs1131882, p2y1 rs1371097 and gpiiia rs2317676 three-loci interactions may increase the risk of carotid stenosis in patients with ischemic stroke | 2019 | Experimental prospective study | 236 ischemic stroke patients with CS 1 and 378 ischemic stroke patients without CS | Gene interaction among three different loci related to platelet activation and responsible for the higher risk of CS in ischemic stroke patients |
| Wu et al. [24] | Associations of genetic markers of diabetes mellitus with carotid atherosclerosis: A community-based case–control study | 2023 | Case–control study | 309 patients with carotid plaque and 439 healthy controls | The positive association between nine single-nucleotide polymorphisms related to diabetes mellitus and CS |
| Yocum et al. [25] | Inducible nitric oxide synthase promoter polymorphism affords protection against cognitive dysfunction after carotid endarterectomy | 2009 | Experimental prospective study | 185 patients who underwent CEA and 60 in the control group | A polymorphism within the promoter region of iNOS 7 protects from moderate to severe cognitive dysfunction 1 month after CEA |
| AUTHORS | TITLE | YEAR OF PUBLICATION | RESEARCH DESIGN | SAMPLE CHARACTERISTICS | MAIN FINDING |
|---|---|---|---|---|---|
| Kim et al. [26] | Macrophage–hypoxia-inducible factor-1α signaling in carotid artery stenosis. American Journal of Pathology | 2021 | Experimental study | Macrophage cell line culture for in vitro studies and mice with ligated carotid for in vivo studies | Controlling the interactions between macrophages and HIF1α 1 could halt the advancement of CS 2 |
| Perisic et al. [27] | Profiling of atherosclerotic lesions by gene and tissue microarrays reveals PCSK6 as a novel protease in unstable carotid atherosclerosis | 2013 | Experimental study | 127 plaques selected from asymptomatic and symptomatic patients who underwent carotid surgery | Transcriptomic method to identify proteins, like PCFK6 3, differentially expressed in plaques from symptomatic patients |
| Luo et al. [28] | Eif2α mediated integrated stress response connects multiple intracellular signaling to reprogram vascular smooth muscle cell fate in carotid plaques | 2024 | Experimental study | Primary carotid vascular smooth muscle cells derived from carotid atherosclerotic plaques | Morphological and functional modifications in carotid vascular smooth muscle cells could be considerable biomarkers for CS |
| Karlöf et al. [29] | Correlation of computed tomography with carotid plaque transcriptomes associates calcification with lesion-stabilization | 2019 | Experimental study | Plaques calcified from patients who underwent CEA 4 | Transcriptional evaluation of plaque calcification levels. Calcification is indicative of plaque stability |
| Tan et al. [30] | Transcriptomics reveals crucial cell subsets and functional heterogeneity associated with carotid atherosclerosis and cerebrovascular events | 2023 | Experimental study | Atherosclerotic plaque tissue from 20 individuals who underwent CEA | The different types of cells present in atherosclerotic environments may be usable for developing targeted cardiovascular immunotherapies |
| Perisic et al. [31] | Gene expression signatures, pathways and networks in carotid atherosclerosis | 2016 | Experimental study | 127 plaques and 96 blood samples from asymptomatic and symptomatic patients | Transcriptomic analysis reveals the role of inflammation and proteases in plaque instability, enhancing the risk of stroke |
| Forte et al. [32] | Expression profiles in surgically-induced carotid stenosis: A combined transcriptomic and proteomic investigation | 2008 | Experimental study | Rats with carotid arteriotomy | Transcriptional approach to revealing molecular pathways that could be activated after carotid surgery to prevent restenosis |
| Deng et al. [33] | Differential expression profile of miRNAs between stable and vulnerable plaques of carotid artery stenosis patients | 2023 | Experimental study | 3 patients with stable plaque and 3 patients with unstable plaque | Determination of different transcription profiles, between stable and unstable atherosclerotic plaques |
| Chen et al. [34] | MicroRNA-214 modulates the senescence of vascular smooth muscle cells in carotid artery stenosis | 2020 | Experimental study | Blood samples from patients with CS and aortic vascular smooth muscle cells from rats for in vitro studies | miR-214 (microRNA 214) induces senescence of vascular smooth muscle cells and cell death, resulting in CS |
| AUTHORS | TITLE | RESEARCH DESIGN | SAMPLE CHARACTERISTICS | MAIN FINDING | |
|---|---|---|---|---|---|
| Forte et al. [32] | Expression profiles in surgically-induced carotid stenosis: A combined transcriptomic and proteomic investigation | 2008 | Experimental study | Rats with carotid arteriotomy | Differential protein pattern in carotid artery contractility after CEA 1 intervention that may lead to restenosis |
| Theofilatos et al. [35] | Proteomic atlas of atherosclerosis: The contribution of proteoglycans to sex differences, plaque phenotypes, and outcomes | 2023 | Experimental study | Atherosclerotic plaques from 120 patients who underwent CEA | Proteomic signatures to understand how proteoglycans contribute to the differences between sexes, plaque characteristics, and outcomes in CS 2 |
| Liang et al. [36] | Distinctive proteomic profiles among different regions of human carotid plaques in men and women | 2016 | Experimental study | Atherosclerotic plaques from 26 patients who underwent CEA | Proteomic differences between male and female patients with CS |
| Porcelli et al. [37] | Proteomic analysis of atherosclerotic plaque | 2010 | Experimental study | Atherosclerotic plaques from 10 patients who underwent CEA | Analysis of protein profile responsible for genesis and progression of atherosclerotic plaque in CS |
| Wang et al. [38] | Urinary proteomics identifying novel biomarkers for the diagnosis and phenotyping of carotid artery stenosis | 2021 | Experimental study | Urine samples from patients with CS who underwent CEA | Different urinary proteins could differentiate between symptomatic and asymptomatic patients |
| Langley et al. [39] | Extracellular matrix proteomics identifies molecular signature of symptomatic carotid plaques | 2017 | Experimental study | Atherosclerotic plaques from 12 patients who underwent CEA: 6 asymptomatic and 6 symptomatic patients | Proteomic analysis revealed the presence of high levels of MMP-9 3 in atherosclerotic plaques of symptomatic patients with CS |
| Hao et al. [40] | Deep proteomic profiling of human carotid atherosclerotic plaques using multidimensional LC-MS/MS | 2014 | Experimental study | Atherosclerotic plaques from 38 patients who underwent CEA | Using LC-MS/MS 4, it was possible to assess the presence of many atherosclerotic plaque-derived proteins, including metalloproteinases and their inhibitors |
| Baragetti et al. [41] | Targeted plasma proteomics to predict the development of carotid plaques | 2022 | Longitudinal observational analysis | Plasma from 586 subjects with carotid atherosclerosis | Variation in the levels of proteins, including a reduction in MMP-10 5, may predict the appearance of subclinical CS |
| Lorentzen et al. [42] | Proteomic analysis of the extracellular matrix of human atherosclerotic plaques shows marked changes between plaque types | 2024 | Experimental study | Plaque sample from 21 patients who underwent CEA | Unstable plaque is rich in proteins associated with inflammation, extracellular matrix (ECM) remodeling, and protein degradation; stable plaque is rich in structural proteins |
| Lai et al. [43] | Characterization of the proteome of stable and unstable carotid atherosclerotic plaques using data-independent acquisition mass spectrometry | 2024 | Experimental cohort study | 182 patients with CS | Different protein identifies stable and unstable plaques |
| Lepedda et al. [44] | A proteomic approach to differentiate histologically classified stable and unstable plaques from human carotid arteries | 2009 | Experimental study | 19 stable plaques and 29 unstable plaques from patients with CS who underwent CEA | Proteomic analysis to differentiate stable plaques from unstable plaques populated by proteins with pro-oxidant and pro-inflammatory activity |
| AUTHORS | TITLE | YEAR OF PUBLICATION | RESEARCH DESIGN | SAMPLE CHARACTERISTICS | MAIN FINDING |
|---|---|---|---|---|---|
| Lee et al. [45] | Metabolomics study in severe extracranial carotid artery stenosis | 2019 | Experimental research | 130 patients with CS 1 | Some metabolites related to diet are associated with the pathological mechanism of CS |
| Azzini et al. [46] | Homocysteine: Its possible emerging role in at-risk population groups | 2020 | Review | None | Highlights homocysteine, a metabolic marker for CS in male patients |
| Lin et al. [47] | Identification of metabolomics biomarkers in extracranial carotid artery stenosis | 2022 | Experimental study | 176 male healthy controls and 173 patients with stroke and CS | Through metabolomic analysis, it is possible to distinguish patients with CS from healthy controls |
| Liu et al. [48] | Association between lipid profiles and presence of carotid plaque | 2019 | Experimental study | 3214 patients with CS | Different lipid profiles are connected to CS, in particular, with the morphology/composition of the plaque |
| Mas et al. [49] | Local non-esterified fatty acids correlate with inflammation in atheroma plaques of patients with type 2 diabetes | 2010 | Experimental study | Carotid plaques from 40 patients who underwent CEA 2 | It is proposed that 3 NEFAs are produced at the carotid site in diabetic subjects with CS, suggesting a link between CS and diabetes through this metabolite |
| Vorkas et al. [50] | Metabolic phenotyping of atherosclerotic plaques reveals latent associations between free cholesterol and ceramide metabolism in atherogenesis | 2015 | Experimental study | 78 patients: 52 underwent carotid endarterectomy, and 26 underwent femoral endarterectomy | Analysis of ceramide, cholesterol, and beta-oxidation pathway to better comprehend the characteristics of atherosclerotic plaques |
| Vorkas et al. [51] | Metabolic phenotypes of carotid atherosclerotic plaques relate to stroke risk: An exploratory study | 2016 | Experimental study | Atherosclerotic plaque samples derived from 5 symptomatic patients and 5 asymptomatic patients who underwent CEA | Thanks to metabolomic technology, it is possible to distinguish between symptomatic and asymptomatic CS patients |
| Stegemann et al. [52] | Comparative lipidomics profiling of human atherosclerotic plaques | 2011 | Experimental study | 26 patients with atherosclerotic disease | Lipidomic analysis of stable and unstable plaques of subjects with atherosclerotic disease |
| Wang et al. [53] | Gut microbiota, circulating inflammatory markers and metabolites, and carotid artery atherosclerosis in HIV infection | 2023 | Experimental study | Atherosclerotic plaque from 320 women with 4 HIV or at high risk of HIV | Association between a gut microbial metabolite, imidazole propionate, and CS |
| Cason et al. [54] | Plasma microbiome-modulated indole- and phenyl-derived metabolites associate with advanced atherosclerosis and postoperative outcomes | 2018 | Experimental, observational study | Plasma from 100 patients who underwent CEA and 22 healthy controls | Specific metabolites produced by gut microbes are connected with severe atherosclerosis and cardiac complications after interventions |
| AUTHORS | TITLE | YEAR OF PUBLICATION | RESEARCH DESIGN | SAMPLE CHARACTERISTICS | MAIN FINDING |
|---|---|---|---|---|---|
| Hsu et al. [55] | Gender, racial and ethnic disparities in index hospitalization operations for symptomatic carotid stenosis in Texas hospitals | 2022 | Experimental study | 153,484 symptomatic patients with CS 1 | Social factors that characterize carotid revascularization during the initial hospital stay for patients with symptomatic CS |
| Wu et al. [56] | Impact of neighborhood social disadvantage on carotid artery disease presentation, management, and discharge outcomes | 2023 | Experimental study | 91,904 patients who underwent CEA 2, tfCAS 3, and TCAR 4 | Social disadvantages like a low socioeconomic status determine severe CS and the need for TCAR |
| Baxi et al. [57] | Socioeconomic status as a predictor of post-operative mortality and outcomes in carotid artery stenting vs. carotid endarterectomy | 2024 | Experimental study | 43,824 patients who underwent CEA and CAS 5 | A low socioeconomic status is associated with stroke in patients who will undergo CEA and with mortality after the CEA intervention |
| Aber et al. [58] | Impact of carotid artery stenosis on quality of life: A systematic review | 2019 | Systematic review | None | Identification of 16 social themes as indicators of HRQoL 6 in patients with CS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, D.; Scalise, E.; Ielapi, N.; Bracale, U.M.; Faga, T.; Michael, A.; Andreucci, M.; Serra, R. Omics Science and Social Aspects in Detecting Biomarkers for Diagnosis, Risk Prediction, and Outcomes of Carotid Stenosis. Biomolecules 2024, 14, 972. https://doi.org/10.3390/biom14080972
Costa D, Scalise E, Ielapi N, Bracale UM, Faga T, Michael A, Andreucci M, Serra R. Omics Science and Social Aspects in Detecting Biomarkers for Diagnosis, Risk Prediction, and Outcomes of Carotid Stenosis. Biomolecules. 2024; 14(8):972. https://doi.org/10.3390/biom14080972
Chicago/Turabian StyleCosta, Davide, Enrica Scalise, Nicola Ielapi, Umberto Marcello Bracale, Teresa Faga, Ashour Michael, Michele Andreucci, and Raffaele Serra. 2024. "Omics Science and Social Aspects in Detecting Biomarkers for Diagnosis, Risk Prediction, and Outcomes of Carotid Stenosis" Biomolecules 14, no. 8: 972. https://doi.org/10.3390/biom14080972
APA StyleCosta, D., Scalise, E., Ielapi, N., Bracale, U. M., Faga, T., Michael, A., Andreucci, M., & Serra, R. (2024). Omics Science and Social Aspects in Detecting Biomarkers for Diagnosis, Risk Prediction, and Outcomes of Carotid Stenosis. Biomolecules, 14(8), 972. https://doi.org/10.3390/biom14080972








