The Potential of Immunotherapy for SMARCA4-Deficient Undifferentiated Uterine Sarcoma (SDUS)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.2. Multiplex Immunofluorescence (mIF)
2.3. Tissue Imaging, Phenotyping, and Image Analysis
3. Results
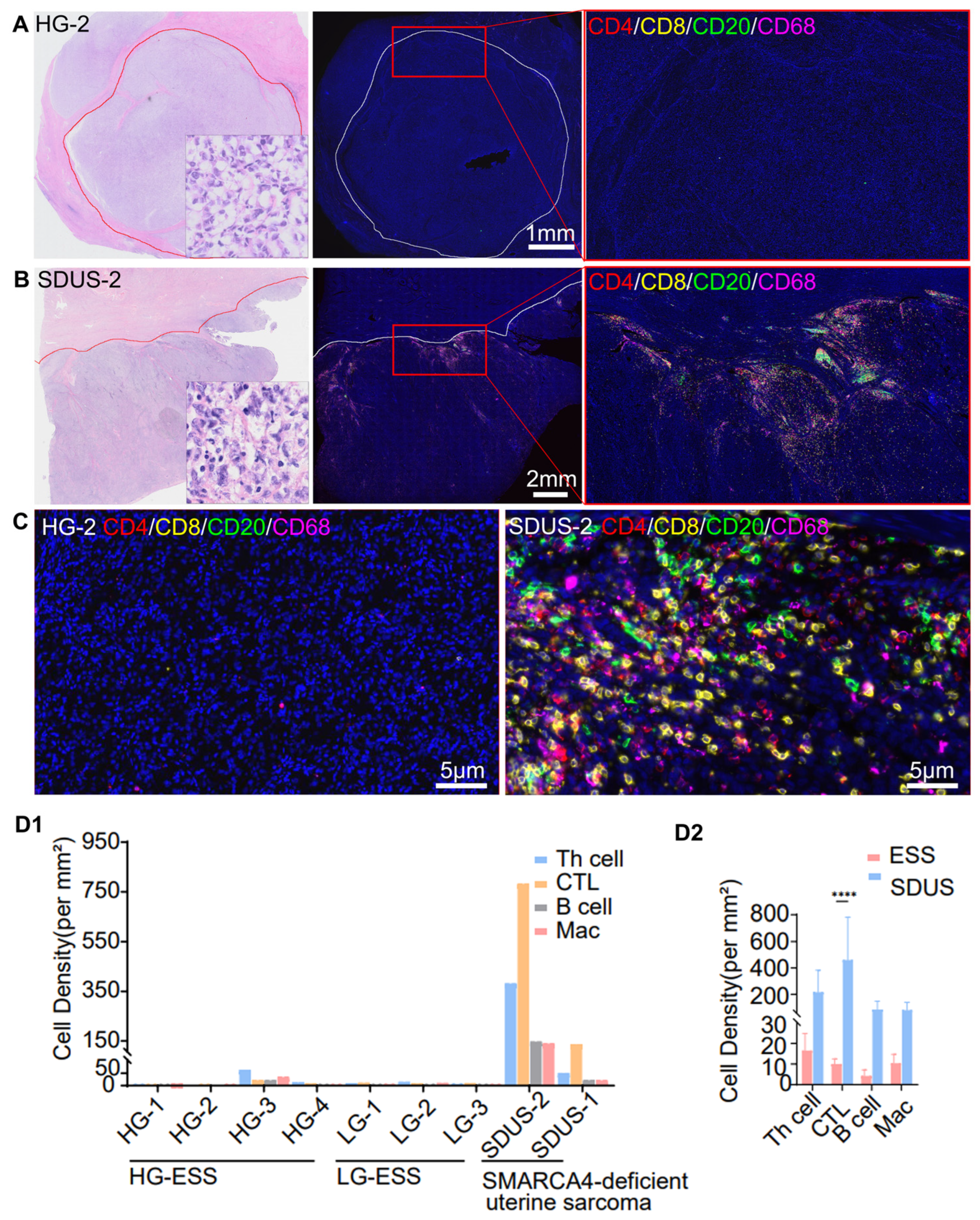
3.1. Comparative Analysis of Immune Infiltration in ESS vs. SDUS
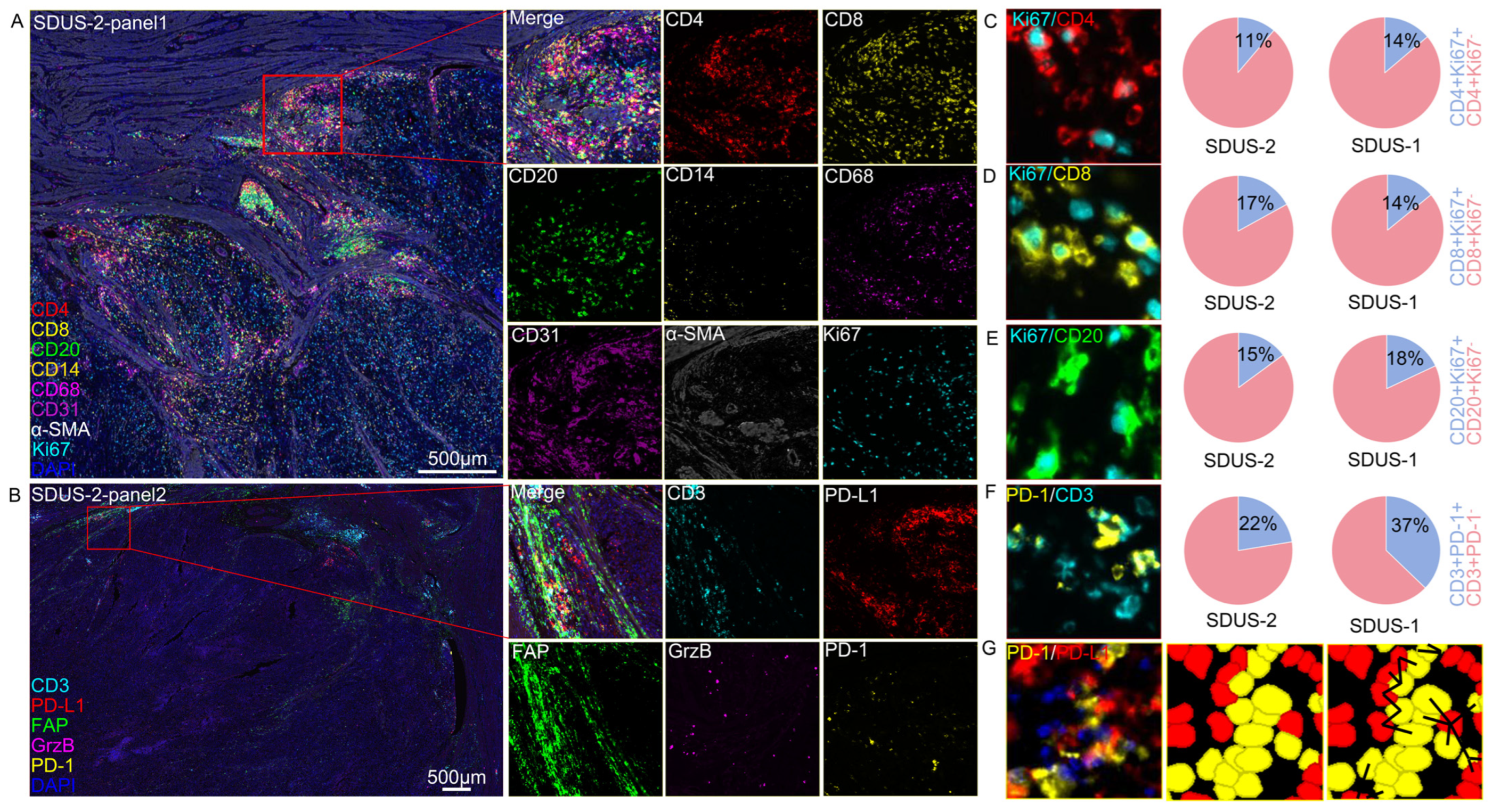
3.2. A Spatial Atlas of the SDUS TiME: A Comprehensive Analysis
Characterization of SDUS Immune Infiltration
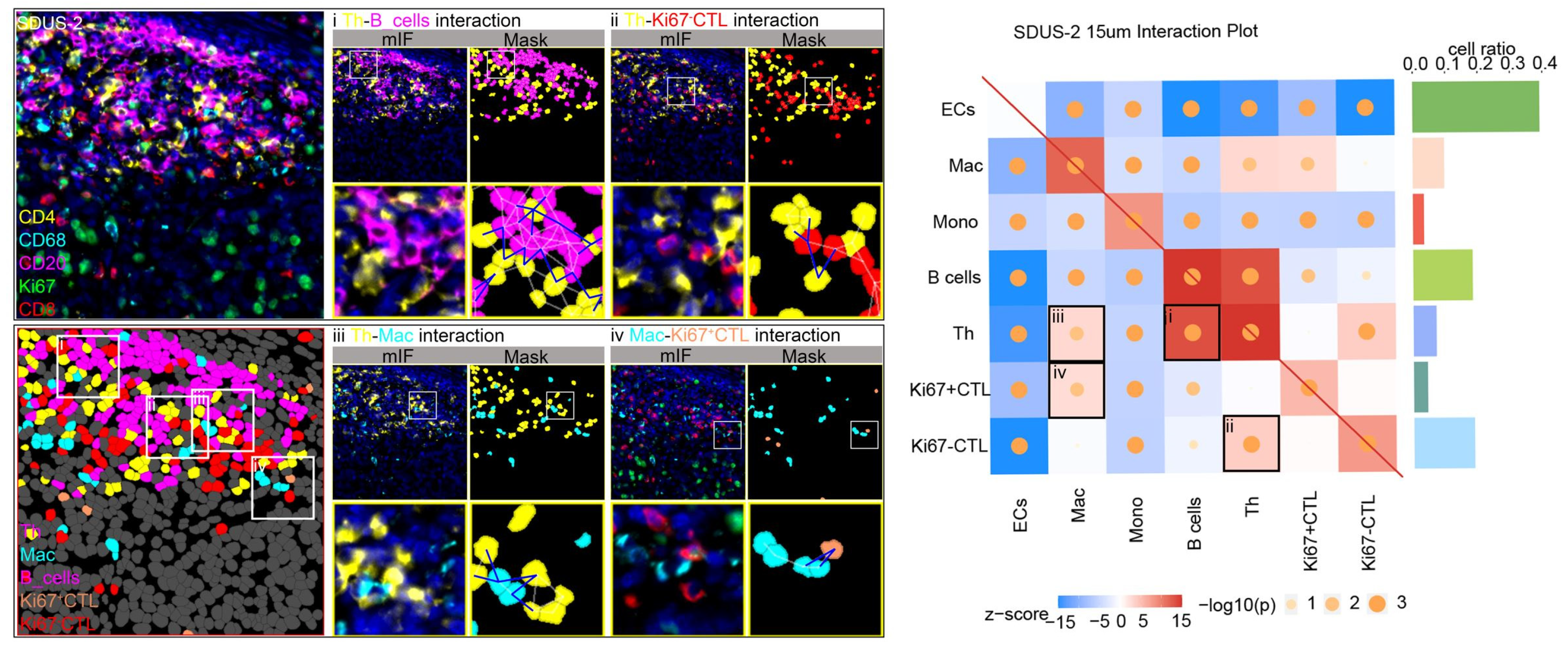
3.3. Exploring Cellular Interactions in the SDUS TiME
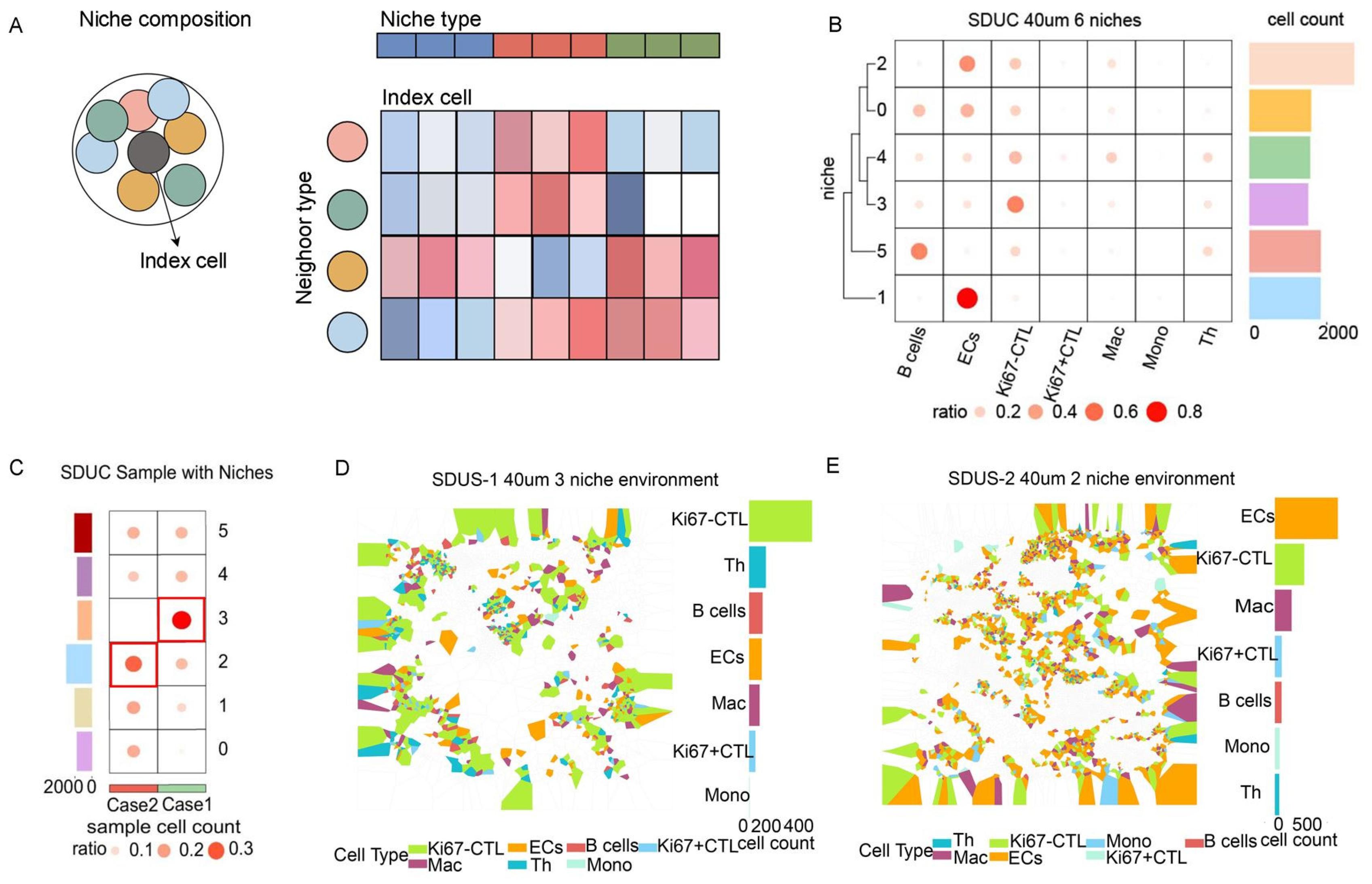
3.4. Unveiling the Intricate Cellular Niches within SDUS
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gadducci, A.; Multinu, F.; De Vitis, L.A.; Cosio, S.; Carinelli, S.; Aletti, G.D. Endometrial stromal tumors of the uterus: Epidemiology, pathological and biological features, treatment options and clinical outcomes. Gynecol. Oncol. 2023, 171, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Hosh, M.; Antar, S.; Nazzal, A.; Warda, M.; Gibreel, A.; Refky, B. Uterine Sarcoma: Analysis of 13,089 Cases Based on Surveillance, Epidemiology, and End Results Database. Int. J. Gynecol. Cancer 2016, 26, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.I.; Allen, J.M.; Hecht, J.L.; Killian, J.K.; Ngo, N.T.; Edgerly, C.; Severson, E.A.; Ali, S.M.; Erlich, R.L.; Ramkissoon, S.H.; et al. SMARCA4 inactivation defines a subset of undifferentiated uterine sarcomas with rhabdoid and small cell features and germline mutation association. Mod. Pathol. 2019, 32, 1675–1687. [Google Scholar] [CrossRef] [PubMed]
- Kolin, D.L.; Quick, C.; Dong, F.; Fletcher, C.; Stewart, C.; Soma, A.; Hornick, J.; Nucci, M.; Howitt, B.E. SMARCA4-deficient Uterine Sarcoma and Undifferentiated Endometrial Carcinoma Are Distinct Clinicopathologic Entities. Am. J. Surg. Pathol. 2020, 44, 263–270. [Google Scholar] [CrossRef]
- Lin, Y.; Yu, B.; Sun, H.; Zhang, H.; Hu, Z.; Zhang, Y.; Wu, Z.; Sun, S.; Zhao, X.; Yu, H.; et al. Promising efficacy of immune checkpoint inhibitor plus chemotherapy for thoracic SMARCA4-deficient undifferentiated tumor. J. Cancer Res. Clin. Oncol. 2023, 149, 8663–8671. [Google Scholar] [CrossRef]
- Tan, S.; Li, D.; Zhu, X. Cancer immunotherapy: Pros, cons and beyond. Biomed. Pharmacother. 2020, 124, 109821. [Google Scholar] [CrossRef]
- Marei, H.E.; Hasan, A.; Pozzoli, G.; Cenciarelli, C. Cancer immunotherapy with immune checkpoint inhibitors (ICIs): Potential, mechanisms of resistance, and strategies for reinvigorating T cell responsiveness when resistance is acquired. Cancer Cell Int. 2023, 23, 64. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Dai, L.-J.; Wu, S.-Y.; Xiao, Y.; Ma, D.; Jiang, Y.-Z.; Shao, Z.-M. Spatial architecture of the immune microenvironment orchestrates tumor immunity and therapeutic response. J. Hematol. Oncol. 2021, 14, 98. [Google Scholar] [CrossRef]
- Elhanani, O.; Ben-Uri, R.; Keren, L. Spatial profiling technologies illuminate the tumor microenvironment. Cancer Cell 2023, 41, 404–420. [Google Scholar] [CrossRef]
- De Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef]
- Jackson, H.W.; Fischer, J.R.; Zanotelli, V.R.T.; Ali, H.R.; Mechera, R.; Soysal, S.D.; Moch, H.; Muenst, S.; Varga, Z.; Weber, W.P.; et al. The single-cell pathology landscape of breast cancer. Nature 2020, 578, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Sorin, M.; Rezanejad, M.; Karimi, E.; Fiset, B.; Desharnais, L.; Perus, L.J.M.; Milette, S.; Yu, M.W.; Maritan, S.M.; Doré, S.; et al. Single-cell spatial landscapes of the lung tumour immune microenvironment. Nature 2023, 614, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Karimi, E.; Yu, M.W.; Maritan, S.M.; Perus, L.J.M.; Rezanejad, M.; Sorin, M.; Dankner, M.; Fallah, P.; Doré, S.; Zuo, D.; et al. Single-cell spatial immune landscapes of primary and metastatic brain tumours. Nature 2023, 614, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Moldoveanu, D.; Ramsay, L.; Lajoie, M.; Anderson-Trocme, L.; Lingrand, M.; Berry, D.; Perus, L.J.; Wei, Y.; Moraes, C.; Alkallas, R.; et al. Spatially mapping the immune landscape of melanoma using imaging mass cytometry. Sci. Immunol. 2022, 7, eabi5072. [Google Scholar] [CrossRef] [PubMed]
- Petitprez, F.; de Reyniès, A.; Keung, E.Z.; Chen, T.W.-W.; Sun, C.-M.; Calderaro, J.; Jeng, Y.-M.; Hsiao, L.-P.; Lacroix, L.; Bougoüin, A.; et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature 2020, 577, 556–560. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Jing, S.; Lin, P.; Li, Y.; Zhang, H.; Chen, Y.; Wang, Z.; Li, H. SOAPy: A Python package to dissect spatial architecture, dynamics and communication. bioRxiv 2023. [Google Scholar] [CrossRef]
- Borst, J.; Ahrends, T.; Bąbała, N.; Melief, C.J.M.; Kastenmüller, W. CD4+ T cell help in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2018, 18, 635–647. [Google Scholar] [CrossRef]
- Laumont, C.M.; Banville, A.C.; Gilardi, M.; Hollern, D.P.; Nelson, B.H. Tumour-infiltrating B cells: Immunological mechanisms, clinical impact and therapeutic opportunities. Nat. Rev. Cancer 2022, 22, 414–430. [Google Scholar] [CrossRef]
- DeNardo, D.G.; Ruffell, B. Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol. 2019, 19, 369–382. [Google Scholar] [CrossRef]
- Tuyaerts, S.; Amant, F. Endometrial Stromal Sarcomas: A Revision of Their Potential as Targets for Immunotherapy. Vaccines 2018, 6, 56. [Google Scholar] [CrossRef]
- Jelinic, P.; Ricca, J.; Van Oudenhove, E.; Olvera, N.; Merghoub, T.; A Levine, D.; Zamarin, D. Immune-Active Microenvironment in Small Cell Carcinoma of the Ovary, Hypercalcemic Type: Rationale for Immune Checkpoint Blockade. J. Natl. Cancer Inst. 2018, 110, 787–790. [Google Scholar] [CrossRef]
- Lazarus, J.; Maj, T.; Smith, J.J.; Lanfranca, M.P.; Rao, A.; D’angelica, M.I.; Delrosario, L.; Girgis, A.; Schukow, C.; Shia, J.; et al. Spatial and phenotypic immune profiling of metastatic colon cancer. JCI Insight 2018, 3, e121932. [Google Scholar] [CrossRef]
- Church, S.E.; Galon, J. Regulation of CTL Infiltration within the Tumor Microenvironment. Adv. Exp. Med. Biol. 2017, 1036, 33–49. [Google Scholar]
- Dahlin, A.M.; Henriksson, M.L.; Van Guelpen, B.; Stenling, R.; Öberg, Å.; Rutegård, J.; Palmqvist, R. Colorectal cancer prognosis depends on T-cell infiltration and molecular characteristics of the tumor. Mod. Pathol. 2011, 24, 671–682. [Google Scholar] [CrossRef]
- Katsuta, E.; Yan, L.; Opyrchal, M.; Kalinski, P.; Takabe, K. Cytotoxic T-lymphocyte infiltration and chemokine predict long-term patient survival independently of tumor mutational burden in triple-negative breast cancer. Ther. Adv. Med. Oncol. 2021, 13, 17588359211006680. [Google Scholar] [CrossRef]
- Brück, O.; Lee, M.H.; Turkki, R.; Uski, I.; Penttilä, P.; Paavolainen, L.; Kovanen, P.; Järvinen, P.; Bono, P.; Pellinen, T.; et al. Spatial immunoprofiling of the intratumoral and peritumoral tissue of renal cell carcinoma patients. Mod. Pathol. 2021, 34, 2229–2241. [Google Scholar] [CrossRef]
- Kim, H.J.; Ji, Y.R.; Lee, Y.M. Crosstalk between angiogenesis and immune regulation in the tumor microenvironment. Arch. Pharmacal Res. 2022, 45, 401–416. [Google Scholar] [CrossRef]
- Fridman, W.H.; Galon, J.; Pagès, F.; Tartour, E.; Sautès-Fridman, C.; Kroemer, G. Prognostic and predictive impact of intra- and peritumoral immune infiltrates. Cancer Res. 2011, 71, 5601–5605. [Google Scholar] [CrossRef]
- Kamphorst, A.O.; Pillai, R.N.; Yang, S.; Nasti, T.H.; Akondy, R.S.; Wieland, A.; Sica, G.L.; Yu, K.; Koenig, L.; Patel, N.T.; et al. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc. Natl. Acad. Sci. USA 2017, 114, 4993–4998. [Google Scholar] [CrossRef]
- Sasaki, T.; Bracero, S.; Keegan, J.; Chen, L.; Cao, Y.; Stevens, E.; Qu, Y.; Wang, G.; Nguyen, J.; Sparks, J.A.; et al. Longitudinal Immune Cell Profiling in Patients with Early Systemic Lupus erythematosus. Arthritis Rheumatol. 2022, 74, 1808–1821. [Google Scholar] [CrossRef]
- Imaizumi, K.; Suzuki, T.; Kojima, M.; Shimomura, M.; Sakuyama, N.; Tsukada, Y.; Sasaki, T.; Nishizawa, Y.; Taketomi, A.; Ito, M.; et al. Ki67 expression and localization of T cells after neoadjuvant therapies as reliable predictive markers in rectal cancer. Cancer Sci. 2020, 111, 23–35. [Google Scholar] [CrossRef]
- Tumeh, P.; Harview, C.; Yearly, J.; Shintaku, I.; Taylor, E.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014, 515, 568–571. [Google Scholar] [CrossRef]
- Teng, M.W.; Ngiow, S.F.; Ribas, A.; Smyth, M.J. Classifying Cancers Based on T-cell Infiltration and PD-L1. Cancer Res. 2015, 75, 2139–2145. [Google Scholar] [CrossRef] [PubMed]
- Rao, D.A. T Cells That Help B Cells in Chronically Inflamed Tissues. Front. Immunol. 2018, 9, 1924. [Google Scholar] [CrossRef] [PubMed]
- Lund, F.E.; Randall, T.D. Effector and regulatory B cells: Modulators of CD4+ T cell immunity. Nat. Rev. Immunol. 2010, 10, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Hong, Z.; Zhang, C.; Wang, L.; Han, Z.; Ma, D. Immune checkpoint therapy for solid tumours: Clinical dilemmas and future trends. Signal Transduct. Target. Ther. 2023, 8, 320. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, R.; Celis, E. T helper lymphocytes rescue CTL from activation-induced cell death. J. Immunol. 2006, 177, 2862–2872. [Google Scholar] [CrossRef]
- Giuntoli, R.L.; Lu, J.; Kobayashi, H.; Kennedy, R.; Celis, E. Direct costimulation of tumor-reactive CTL by helper T cells potentiate their proliferation, survival, and effector function. Clin. Cancer Res. 2002, 8, 922–931. [Google Scholar]
- Zhang, E.; Ding, C.; Li, S.; Zhou, X.; Aikemu, B.; Fan, X.; Sun, J.; Zheng, M.; Yang, X. Roles and mechanisms of tumour-infiltrating B cells in human cancer: A new force in immunotherapy. Biomark. Res. 2023, 11, 28. [Google Scholar] [CrossRef]
- Luo, P.; Wang, P.; Xu, J.; Hou, W.; Xu, P.; Xu, K.; Liu, L. Immunomodulatory role of T helper cells in rheumatoid arthritis: A comprehensive research review. Bone Jt. Res. 2022, 11, 426–438. [Google Scholar] [CrossRef]
- Laumont, C.M.; Nelson, B.H. B cells in the tumor microenvironment: Multi-faceted organizers, regulators, and effectors of anti-tumor immunity. Cancer Cell 2023, 41, 466–489. [Google Scholar] [CrossRef] [PubMed]
- De Palma, M.; Biziato, D.; Petrova, T.V. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer 2017, 17, 457–474. [Google Scholar] [CrossRef] [PubMed]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef]
- Alessi, J.V.; Ricciuti, B.; Spurr, L.F.; Gupta, H.; Li, Y.Y.; Glass, C.; Nishino, M.; Cherniack, A.D.; Lindsay, J.; Sharma, B.; et al. SMARCA4 and Other SWItch/Sucrose NonFermentable Family Genomic Alterations in NSCLC: Clinicopathologic Characteristics and Outcomes to Immune Checkpoint Inhibition. J. Thorac. Oncol. 2021, 16, 1176–1187. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.; Catino, A.; Montrone, M.; Montagna, E.S.; Pesola, F.; Marech, I.; Pizzutilo, P.; Nardone, A.; Perrone, A.; Gesualdo, M.; et al. Treatment of Thoracic SMARCA4-Deficient Undifferentiated Tumors: Where We Are and Where We Will Go. Int. J. Mol. Sci. 2024, 25, 3237. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, X.; He, Y.; Xiao, C.; Zhou, R.; Zhao, C.; Wang, W. The Potential of Immunotherapy for SMARCA4-Deficient Undifferentiated Uterine Sarcoma (SDUS). Biomolecules 2024, 14, 987. https://doi.org/10.3390/biom14080987
Yao X, He Y, Xiao C, Zhou R, Zhao C, Wang W. The Potential of Immunotherapy for SMARCA4-Deficient Undifferentiated Uterine Sarcoma (SDUS). Biomolecules. 2024; 14(8):987. https://doi.org/10.3390/biom14080987
Chicago/Turabian StyleYao, Xiaohong, Ying He, Chaoxin Xiao, Ruihan Zhou, Chengjian Zhao, and Wei Wang. 2024. "The Potential of Immunotherapy for SMARCA4-Deficient Undifferentiated Uterine Sarcoma (SDUS)" Biomolecules 14, no. 8: 987. https://doi.org/10.3390/biom14080987




