Activity of Membrane-Permeabilizing Lpt Peptides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bioinformatics
2.2. Lpt-like Cloning, Growth Assay, and Fluorescence Microscopy
2.3. Liposome Preparation
2.4. Lipid Quantification
2.5. Leakage Experiments
2.6. Hemolysis Experiments
2.7. CD Spectroscopy
2.8. DLS Experiments
3. Results
3.1. Structural Comparison between Lpt and Lpt-like
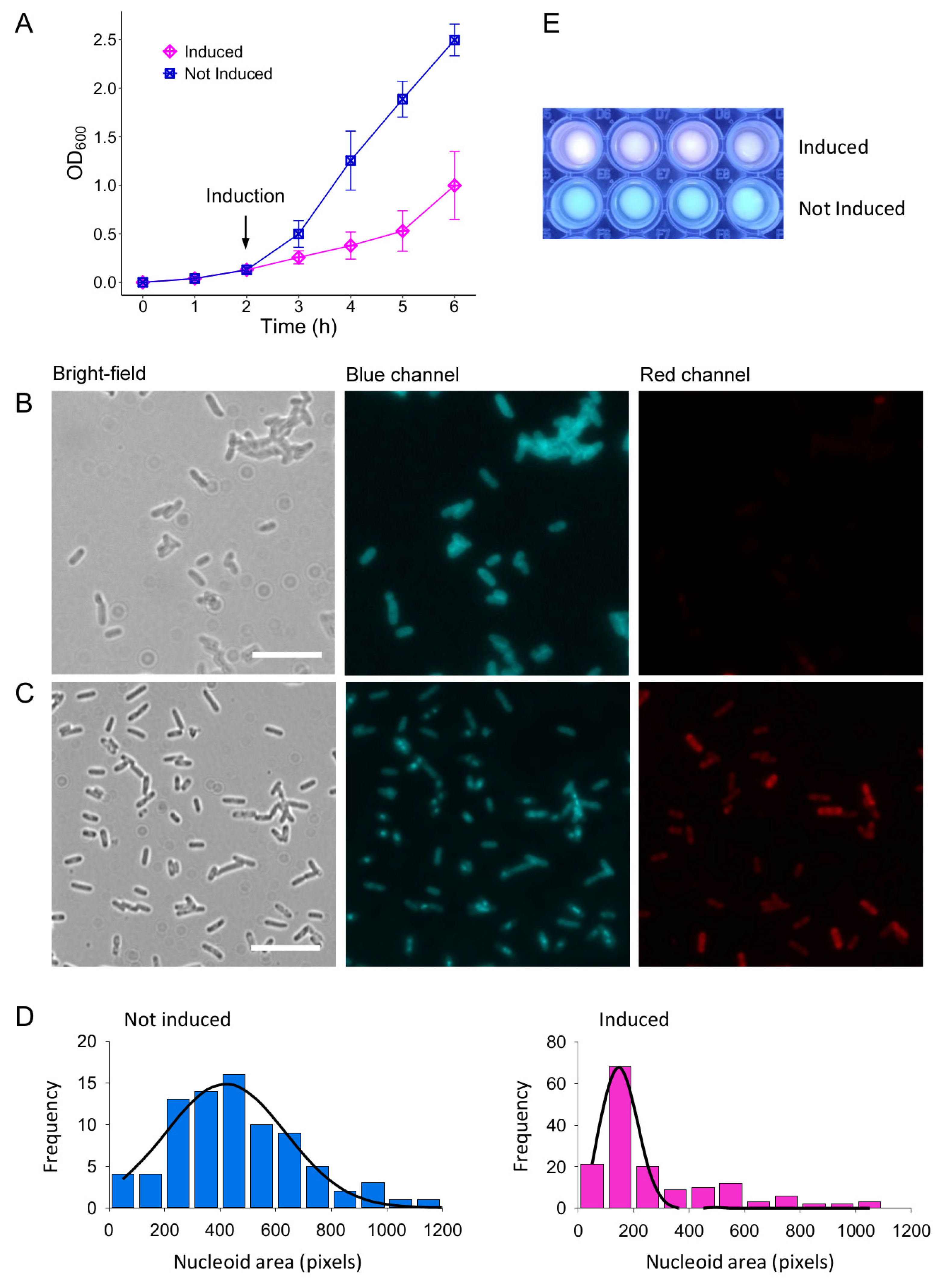
3.2. Toxicity of Lpt-like Peptide in E. coli
3.3. Liposome Leakage Assays
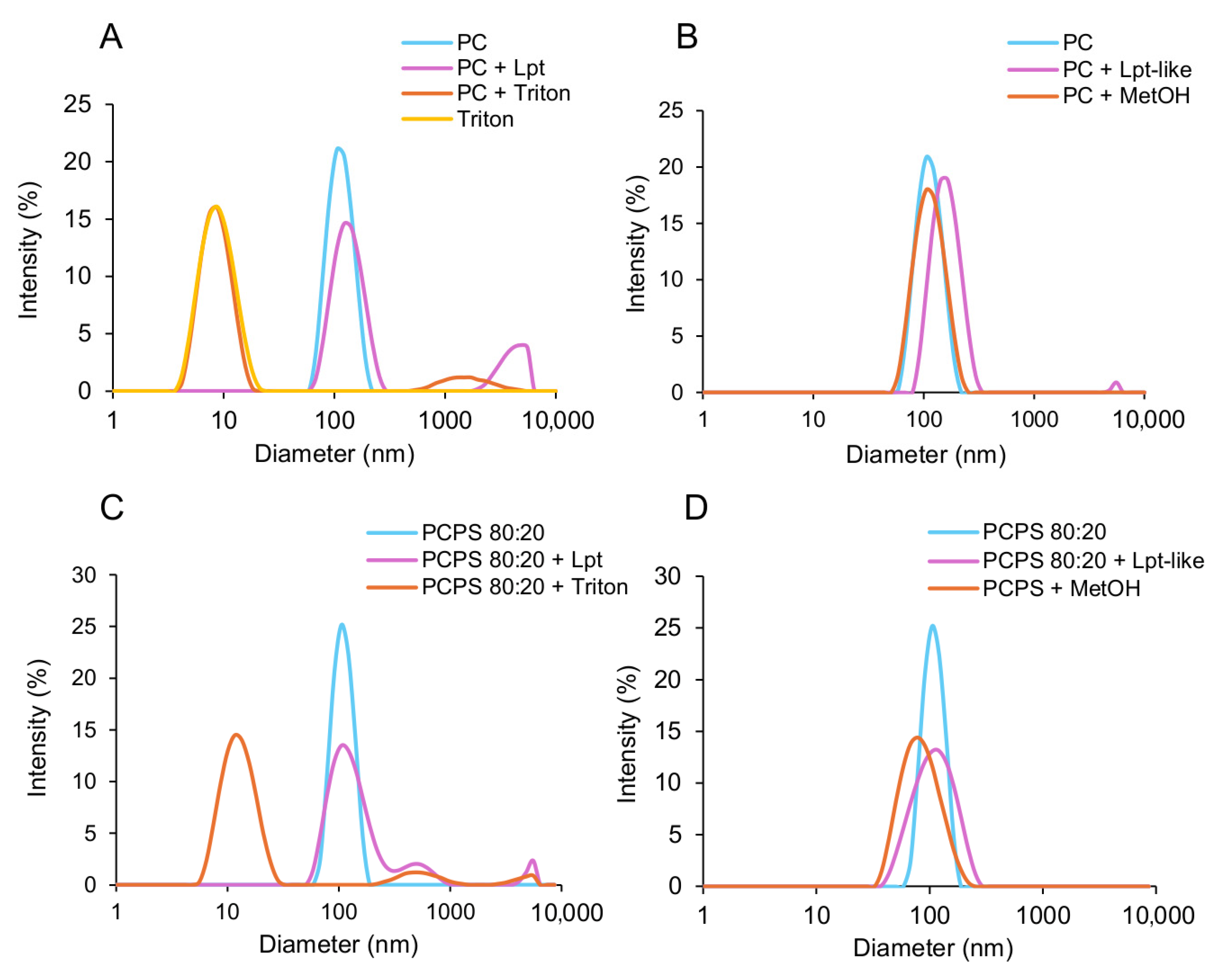
3.4. Lpt and Lpt-like Do Not Disrupt Liposomes
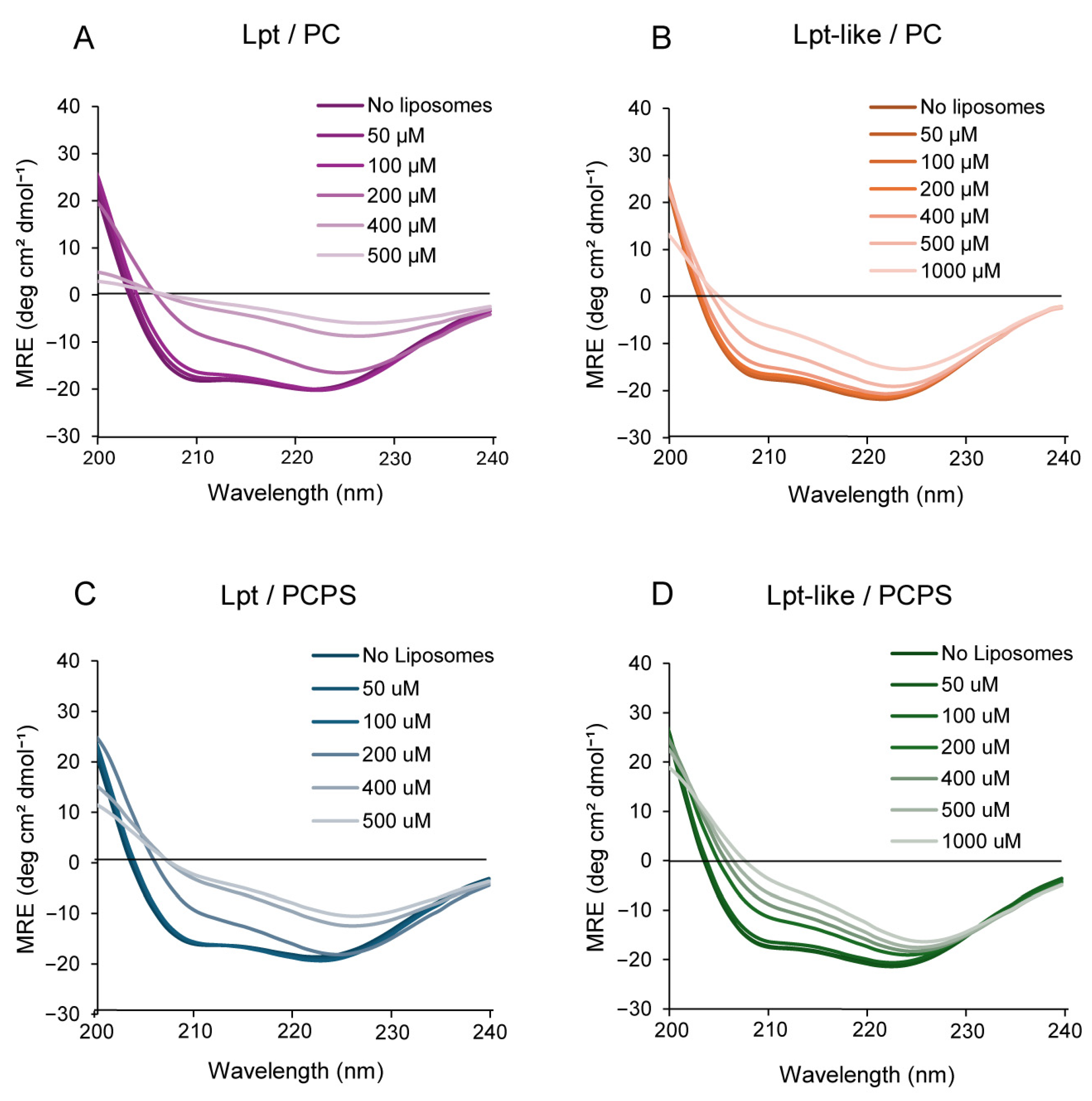
3.5. CD Spectroscopy of Lpt and Lpt-like in the Presence of Liposomes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef]
- Rausch, J.M.; Marks, J.R.; Rathinakumar, R.; Wimley, W.C. β-Sheet Pore-Forming Peptides Selected from a Rational Combinatorial Library: Mechanism of Pore Formation in Lipid Vesicles and Activity in Biological Membranes. Biochemistry 2007, 46, 12124–12139. [Google Scholar] [CrossRef]
- Koller, D.; Lohner, K. The Role of Spontaneous Lipid Curvature in the Interaction of Interfacially Active Peptides with Membranes. Biochim. Biophys. Acta (BBA)—Biomembr. 2014, 1838, 2250–2259. [Google Scholar] [CrossRef]
- Gazit, E.; Miller, I.R.; Biggin, P.C.; Sansom, M.S.; Shai, Y. Structure and Orientation of the Mammalian Antibacterial Peptide Cecropin P1 within Phospholipid Membranes. J. Mol. Biol. 1996, 258, 860–870. [Google Scholar] [CrossRef]
- Sato, H.; Feix, J.B. Peptide–Membrane Interactions and Mechanisms of Membrane Destruction by Amphipathic α-Helical Antimicrobial Peptides. Biochim. Biophys. Acta (BBA)—Biomembr. 2006, 1758, 1245–1256. [Google Scholar] [CrossRef]
- Ladokhin, A.S.; White, S.H. ‘Detergent-like’ Permeabilization of Anionic Lipid Vesicles by Melittin. Biochim. Biophys. Acta (BBA)—Biomembr. 2001, 1514, 253–260. [Google Scholar] [CrossRef]
- Wimley, W.C. Describing the Mechanism of Antimicrobial Peptide Action with the Interfacial Activity Model. ACS Chem. Biol. 2010, 5, 905–917. [Google Scholar] [CrossRef]
- Folli, C.; Levante, A.; Percudani, R.; Amidani, D.; Bottazzi, S.; Ferrari, A.; Rivetti, C.; Neviani, E.; Lazzi, C. Toward the Identification of a Type I Toxin-Antitoxin System in the Plasmid DNA of Dairy Lactobacillus rhamnosus. Sci. Rep. 2017, 7, 12051. [Google Scholar] [CrossRef]
- Levante, A.; Lazzi, C.; Vatsellas, G.; Chatzopoulos, D.; Dionellis, V.S.; Makrythanasis, P.; Neviani, E.; Folli, C. Genome Sequencing of Five Lacticaseibacillus Strains and Analysis of Type I and II Toxin-Antitoxin System Distribution. Microorganisms 2021, 9, 648. [Google Scholar] [CrossRef]
- Masachis, S.; Darfeuille, F. Type I Toxin-Antitoxin Systems: Regulating Toxin Expression via Shine-Dalgarno Sequence Sequestration and Small RNA Binding. Microbiol. Spectr. 2018, 6, RWR-0030-2018. [Google Scholar] [CrossRef]
- Nonin-Lecomte, S.; Fermon, L.; Felden, B.; Pinel-Marie, M.-L. Bacterial Type I Toxins: Folding and Membrane Interactions. Toxins 2021, 13, 490. [Google Scholar] [CrossRef]
- Holmes, A.; Sadlon, J.; Weaver, K. Charged Residues Flanking the Transmembrane Domain of Two Related Toxin–Antitoxin System Toxins Affect Host Response. Toxins 2021, 13, 329. [Google Scholar] [CrossRef]
- Brielle, R.; Pinel-Marie, M.-L.; Felden, B. Linking Bacterial Type I Toxins with Their Actions. Curr. Opin. Microbiol. 2016, 30, 114–121. [Google Scholar] [CrossRef]
- Guo, Y.; Quiroga, C.; Chen, Q.; McAnulty, M.J.; Benedik, M.J.; Wood, T.K.; Wang, X. RalR (a DNase) and RalA (a Small RNA) Form a Type I Toxin–Antitoxin System in Escherichia coli. Nucleic Acids Res. 2014, 42, 6448–6462. [Google Scholar] [CrossRef]
- Kawano, M.; Aravind, L.; Storz, G. An Antisense RNA Controls Synthesis of an SOS-Induced Toxin Evolved from an Antitoxin. Mol. Microbiol. 2007, 64, 738–754. [Google Scholar] [CrossRef]
- Weaver, K. The Fst/Ldr Family of Type I TA System Toxins: Potential Roles in Stress Response, Metabolism and Pathogenesis. Toxins 2020, 12, 474. [Google Scholar] [CrossRef]
- Maggi, S.; Yabre, K.; Ferrari, A.; Lazzi, C.; Kawano, M.; Rivetti, C.; Folli, C. Functional Characterization of the Type I Toxin Lpt from Lactobacillus rhamnosus by Fluorescence and Atomic Force Microscopy. Sci. Rep. 2019, 9, 15208. [Google Scholar] [CrossRef]
- Maggi, S.; Ferrari, A.; Yabre, K.; Bonini, A.A.; Rivetti, C.; Folli, C. Strategies to Investigate Membrane Damage, Nucleoid Condensation, and RNase Activity of Bacterial Toxin–Antitoxin Systems. Methods Protoc. 2021, 4, 71. [Google Scholar] [CrossRef]
- Haddad, H.; Mejri, R.; de Araujo, A.R.; Zaïri, A. Evaluation of the Antibacterial Activity of New Dermaseptin Derivatives against Acinetobacter baumannii. Pharmaceuticals 2024, 17, 171. [Google Scholar] [CrossRef]
- Fernandez, D.I.; Brun, A.P.L.; Whitwell, T.C.; Sani, M.-A.; James, M.; Separovic, F. The Antimicrobial Peptide Aurein 1.2 Disrupts Model Membranes via the Carpet Mechanism. Phys. Chem. Chem. Phys. 2012, 14, 15739–15751. [Google Scholar] [CrossRef]
- Madeira, F.; Pearce, M.; Tivey, A.R.N.; Basutkar, P.; Lee, J.; Edbali, O.; Madhusoodanan, N.; Kolesnikov, A.; Lopez, R. Search and Sequence Analysis Tools Services from EMBL-EBI in 2022. Nucleic Acids Res. 2022, 50, W276–W279. [Google Scholar] [CrossRef]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.A.; Clamp, M.; Barton, G.J. Jalview Version 2—A Multiple Sequence Alignment Editor and Analysis Workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef]
- Stewart, J.C.M. Colorimetric Determination of Phospholipids with Ammonium Ferrothiocyanate. Anal. Biochem. 1980, 104, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Wessman, P.; Morin, M.; Reijmar, K.; Edwards, K. Effect of α-Helical Peptides on Liposome Structure: A Comparative Study of Melittin and Alamethicin. J. Colloid Interface Sci. 2010, 346, 127–135. [Google Scholar] [CrossRef]
- Weaver, K.E.; Chen, Y.; Miiller, E.M.; Johnson, J.N.; Dangler, A.A.; Manias, D.A.; Clem, A.M.; Schjodt, D.J.; Dunny, G.M. Examination of Enterococcus Faecalis Toxin-Antitoxin System Toxin Fst Function Utilizing a Pheromone-Inducible Expression Vector with Tight Repression and Broad Dynamic Range. J. Bacteriol. 2017, 199, e00065-17. [Google Scholar] [CrossRef] [PubMed]
- Weel-Sneve, R.; Kristiansen, K.I.; Odsbu, I.; Dalhus, B.; Booth, J.; Rognes, T.; Skarstad, K.; Bjørås, M. Single Transmembrane Peptide DinQ Modulates Membrane-Dependent Activities. PLoS Genet. 2013, 9, e1003260. [Google Scholar] [CrossRef]
- Lambert, B.; Le Pecq, J.B. Effect of Mutation, Electric Membrane Potential, and Metabolic Inhibitors on the Accessibility of Nucleic Acids to Ethidium Bromide in Escherichia Coli Cells. Biochemistry 1984, 23, 166–176. [Google Scholar] [CrossRef]
- Jimah, J.R.; Schlesinger, P.H.; Tolia, N.H. Liposome Disruption Assay to Examine Lytic Properties of Biomolecules. Bio. Protoc. 2017, 7, e2433. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Korsmeyer, S.J.; Schlesinger, P.H. BAX-Dependent Transport of Cytochrome c Reconstituted in Pure Liposomes. Nat. Cell Biol. 2000, 2, 553–555. [Google Scholar] [CrossRef]
- Cho, N.-J.; Dvory-Sobol, H.; Xiong, A.; Cho, S.-J.; Frank, C.W.; Glenn, J.S. Mechanism of an Amphipathic α-Helical Peptide’s Antiviral Activity Involves Size-Dependent Virus Particle Lysis. ACS Chem. Biol. 2009, 4, 1061–1067. [Google Scholar] [CrossRef]
- Nakano, K.; Tozuka, Y.; Yamamoto, H.; Kawashima, Y.; Takeuchi, H. A Novel Method for Measuring Rigidity of Submicron-Size Liposomes with Atomic Force Microscopy. Int. J. Pharm. 2008, 355, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Jackman, J.A.; Cho, N.-J. Comparing the Membrane-Interaction Profiles of Two Antiviral Peptides: Insights into Structure-Function Relationship. Langmuir 2019, 35, 9934–9943. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-S.; Schantz, A.B.; Song, S.; Kumar, M.; Wood, T.K. GhoT of the GhoT/GhoS Toxin/Antitoxin System Damages Lipid Membranes by Forming Transient Pores. Biochem. Biophys. Res. Commun. 2018, 497, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Mosbahi, K.; Walker, D.; James, R.; Moore, G.R.; Kleanthous, C. Global structural rearrangement of the cell penetrating ribonuclease colicin E3 on interaction with phospholipid membranes. Protein Sci. 2006, 15, 620–627. [Google Scholar] [CrossRef]
- Battista, F.; Oliva, R.; Del Vecchio, P.; Winter, R.; Petraccone, L. Insights into the action mechanism of the antimicrobial peptide lasioglossin III. Int. J. Mol. Sci. 2021, 22, 2857. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maggi, S.; Mori, G.; Maglie, L.; Carnuccio, D.; Delfino, D.; Della Monica, E.; Rivetti, C.; Folli, C. Activity of Membrane-Permeabilizing Lpt Peptides. Biomolecules 2024, 14, 994. https://doi.org/10.3390/biom14080994
Maggi S, Mori G, Maglie L, Carnuccio D, Delfino D, Della Monica E, Rivetti C, Folli C. Activity of Membrane-Permeabilizing Lpt Peptides. Biomolecules. 2024; 14(8):994. https://doi.org/10.3390/biom14080994
Chicago/Turabian StyleMaggi, Stefano, Giulia Mori, Luigi Maglie, Dario Carnuccio, Danila Delfino, Emanuele Della Monica, Claudio Rivetti, and Claudia Folli. 2024. "Activity of Membrane-Permeabilizing Lpt Peptides" Biomolecules 14, no. 8: 994. https://doi.org/10.3390/biom14080994






