Identification and Characterization of Extrachromosomal Circular DNA in Slimming Grass Carp
Abstract
:1. Introduction
2. Material and Method
2.1. Samples Collection and DNA Extraction
2.2. Linear DNA Removal and Circular DNA Enrichment
2.3. Illumina Sequencing
2.4. Data Analysis
2.5. Statistical Analysis
3. Results
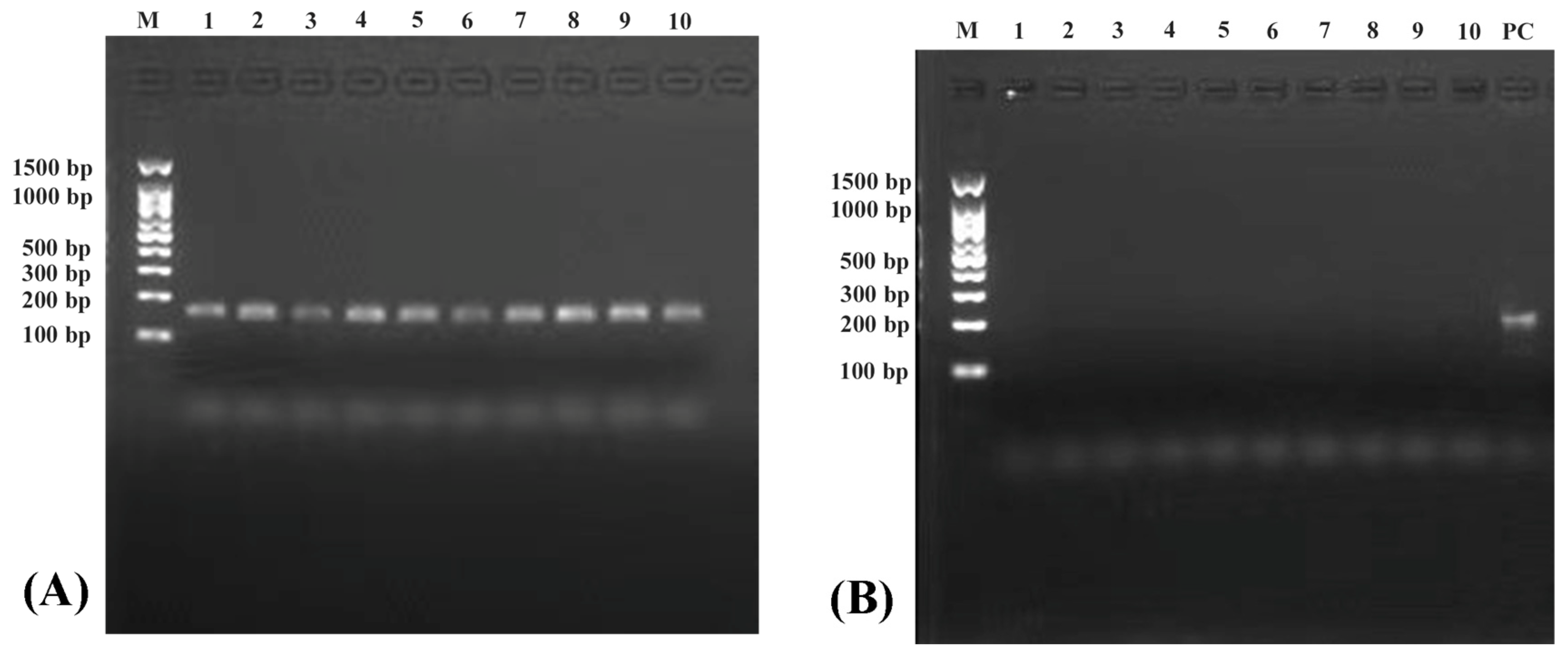
3.1. Detection of Linear DNA Removal and Circular DNA Enrichment
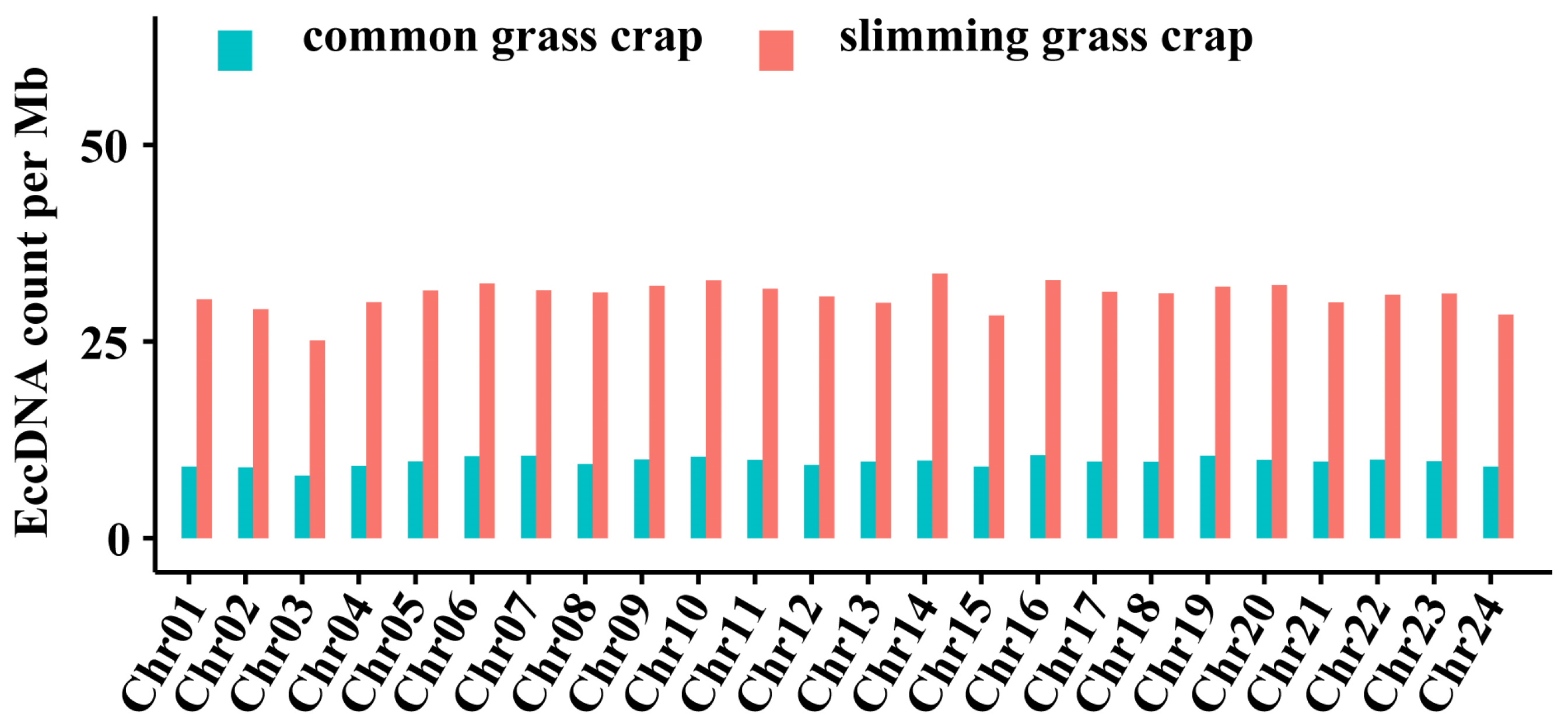
3.2. The Lansacape of eccDNA
3.3. GC Content Distribution near EccDNA Sequence
3.4. GO Analysis
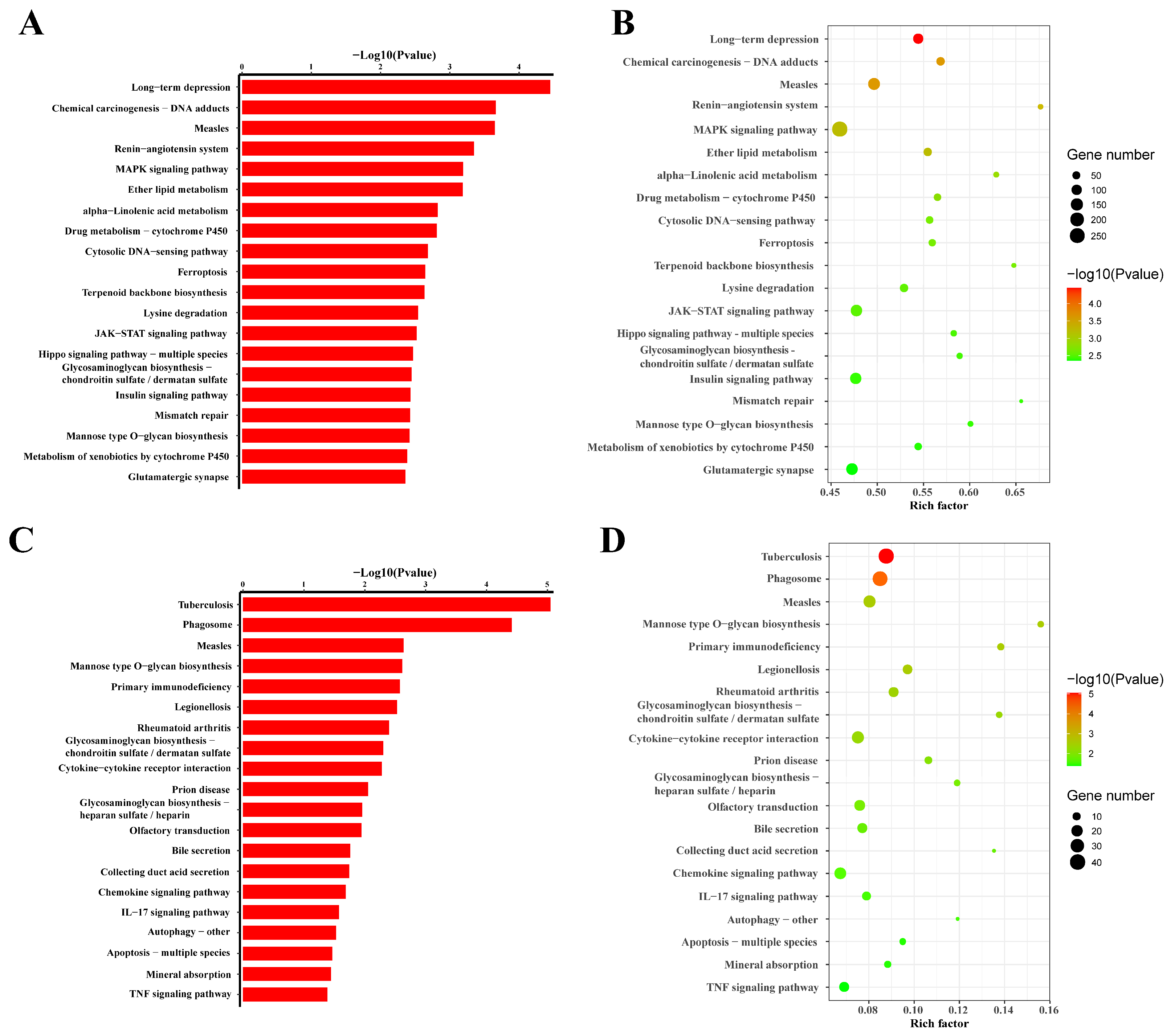
3.5. Pathway Analysis
3.6. Differential Expression Profile of eccDNAs in Slimming Grass Carp
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, X.G.; Shen, H.Y.; Gu, Z.J.; Cheng, G.F.; Wang, J.; Zhu, H. The Environmental Impact and Development Direction of Grass Carp, Ctenopharyngodon Idella, Aquaculture. J. World Aquac. Soc. 2023, 54, 1354–1366. [Google Scholar] [CrossRef]
- National Technology System for Bulk Freshwater Fish Industry. Report on grass carp industry development. China Fish. 2021, 2, 27–37. [Google Scholar]
- Wang, C.; Luo, Y.H.; Guo, Q.H.; Xie, S.L. Slimming Fish’ Industry Development Status and Countermeasures in Qingyuan. Fish. Guide Be Rich. 2018, 16, 22–25. [Google Scholar]
- Chen, C.; Lu, S.H.; Gao, Y.Y. Problems and Key Technologies of Marketable Fish Slimming Processing and Breeding. South China Agric. 2021, 15, 31–35. [Google Scholar]
- Cao, Y.N.; Li, H.; Yang, C.; Ma, L.; Zhang, Y.H. Study of the Changes of Nutrition and Flavor Quality of Grass Carp (Ctenopharyngodon idellus) during Lean Culture. J. Fish. Sci. China 2023, 30, 178–193. [Google Scholar] [CrossRef]
- Lv, H.; Hu, W.; Xiong, S.; You, J.; Fan, Q. Depuration and Starvation Improves Flesh Quality of Grass Carp (Ctenopharyngodon Idella). Aquac. Res. 2018, 49, 3196–3206. [Google Scholar] [CrossRef]
- Zhou, B. Different Culture Models of Grass Carp in Chongqing Area and Their Effects on Muscle Quality; Southwest University: Chongqing, China, 2020. [Google Scholar]
- Wu, S.; Bafna, V.; Chang, H.Y.; Mischel, P.S. Extrachromosomal DNA: An Emerging Hallmark in Human Cancer. Annu. Rev. Pathol. Mech. Dis. 2021, 17, 367–386. [Google Scholar] [CrossRef]
- Cox, D.; Yuncken, C.; Spriggs, A.I. Minute Chromatin Bodies in Malignant Tumours of Childhood. Lancet 1965, 286, 55–58. [Google Scholar] [CrossRef]
- Møller, H.D.; Parsons, L.; Jørgensen, T.S.; Botstein, D.; Regenberg, B. Extrachromosomal Circular DNA Is Common in Yeast. Proc. Natl. Acad. Sci. USA 2015, 112, E3114–E3122. [Google Scholar] [CrossRef] [PubMed]
- Møller, H.D.; Bojsen, R.; Tachibana, C.; Parsons, L.; Botstein, D.; Regenberg, B. Genome-Wide Purification of Extrachromosomal Circular DNA from Eukaryotic Cells. J. Vis. Exp. 2016, 2016, e54239. [Google Scholar] [CrossRef]
- Kumar, P.; Dillon, L.W.; Shibata, Y.; Jazaeri, A.A.; Jones, D.R.; Dutta, A. Normal and Cancerous Tissues Release Extrachromosomal Circular DNA (EccDNA) into the Circulation. Mol. Cancer Res. 2017, 15, 1197–1205. [Google Scholar] [CrossRef] [PubMed]
- Yerlici, V.T.; Lu, M.W.; Hoge, C.R.; Miller, R.V.; Neme, R.; Khurana, J.S.; Bracht, J.R.; Landweber, L.F. Programmed Genome Rearrangements in Oxytricha Produce Transcriptionally Active Extrachromosomal Circular DNA. Nucleic Acids Res. 2019, 47, 9741–9760. [Google Scholar] [CrossRef] [PubMed]
- Nathanson, D.A.; Gini, B.; Mottahedeh, J.; Visnyei, K.; Koga, T.; Gomez, G.; Eskin, A.; Hwang, K.; Wang, J.; Masui, K.; et al. Targeted Therapy Resistance Mediated by Dynamic Regulation of Extrachromosomal Mutant EGFR DNA. Science 2013, 343, 72–76. [Google Scholar] [CrossRef]
- Paulsen, T.; Kumar, P.; Koseoglu, M.M.; Dutta, A. Discoveries of Extrachromosomal Circles of DNA in Normal and Tumor Cells. Trends Genet. 2018, 34, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Dillon, L.W.; Kumar, P.; Shibata, Y.; Wang, Y.H.; Willcox, S.; Griffith, J.D.; Pommier, Y.; Takeda, S.; Dutta, A. Production of Extrachromosomal MicroDNAs Is Linked to Mismatch Repair Pathways and Transcriptional Activity. Cell Rep. 2015, 11, 1749–1759. [Google Scholar] [CrossRef]
- Morton, A.R.; Dogan-Artun, N.; Faber, Z.J.; MacLeod, G.; Bartels, C.F.; Piazza, M.S.; Allan, K.C.; Mack, S.C.; Wang, X.; Gimple, R.C.; et al. Functional Enhancers Shape Extrachromosomal Oncogene Amplifications. Cell 2019, 179, 1330–1341.e13. [Google Scholar] [CrossRef]
- Hull, R.M.; King, M.; Pizza, G.; Krueger, F.; Vergara, X.; Houseley, J. Transcription-Induced Formation of Extrachromosomal DNA during Yeast Ageing. PLoS Biol. 2019, 17, e3000471. [Google Scholar] [CrossRef]
- Qiu, G.H.; Zheng, X.; Fu, M.; Huang, C.; Yang, X. The Decreased Exclusion of Nuclear EccDNA: From Molecular and Subcellular Levels to Human Aging and Age-Related Diseases. Ageing Res. Rev. 2021, 67, 101306. [Google Scholar] [CrossRef]
- Hotta, Y.; Bassel, A. Molecular Size and Circularity of DNA in Cells of Mammals and Higher Plants. Proc. Natl. Acad. Sci. USA 1965, 53, 356–362. [Google Scholar] [CrossRef]
- Radloff, R.; Bauer, W.; Vinograd, J. A Dye-Buoyant-Density Method for the Detection and Isolation of Closed Circular Duplex DNA: The Closed Circular DNA in Hela Cells. Proc. Natl. Acad. Sci. USA 2014, 57, 1514–1521. [Google Scholar] [CrossRef]
- Durkin, K.; Coppieters, W.; Drögüller, C.; Ahariz, N.; Cambisano, N.; Druet, T.; Fasquelle, C.; Haile, A.; Horin, P.; Huang, L.; et al. Serial Translocation by Means of Circular Intermediates Underlies Colour Sidedness in Cattle. Nature 2012, 482, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Møller, H.D.; Ramos-Madrigal, J.; Prada-Luengo, I.; Gilbert, M.T.P.; Regenberg, B. Near-Random Distribution of Chromosome-Derived Circular DNA in the Condensed Genome of Pigeons and the Larger, More Repeat-Rich Human Genome. Genome Biol. Evol. 2020, 12, 3762–3777. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Arrey, G.; Zole, E.; Lv, W.; Liang, X.; Han, P.; Mohiyuddin, M.; Pilegaard, H.; Regenberg, B. Targeted Removal of Mitochondrial DNA from Mouse and Human Extrachromosomal Circular DNA with CRISPR-Cas9. Comput. Struct. Biotechnol. J. 2022, 20, 3059–3067. [Google Scholar] [CrossRef] [PubMed]
- Wanchai, V.; Jenjaroenpun, P.; Leangapichart, T.; Arrey, G.; Burnham, C.M.; Tümmler, M.C.; Delgado-Calle, J.; Regenberg, B.; Nookaew, I. CReSIL: Accurate Identification of Extrachromosomal Circular DNA from Long-Read Sequences. Brief. Bioinform. 2022, 23. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and Accurate Long-Read Alignment with Burrows-Wheeler Transform. Bioinformatics 2010, 26, 589–595. [Google Scholar] [CrossRef]
- Prada-Luengo, I.; Krogh, A.; Maretty, L.; Regenberg, B. Sensitive Detection of Circular DNAs at Single-Nucleotide Resolution Using Guided Realignment of Partially Aligned Reads. BMC Bioinform. 2019, 20, 663. [Google Scholar] [CrossRef]
- Quinlan, A.R.; Hall, I.M. BEDTools: A Flexible Suite of Utilities for Comparing Genomic Features. Bioinformatics 2010, 26, 841–842. [Google Scholar] [CrossRef] [PubMed]
- Møller, H.D.; Mohiyuddin, M.; Prada-Luengo, I.; Sailani, M.R.; Halling, J.F.; Plomgaard, P.; Maretty, L.; Hansen, A.J.; Snyder, M.P.; Pilegaard, H.; et al. Circular DNA Elements of Chromosomal Origin Are Common in Healthy Human Somatic Tissue. Nat. Commun. 2018, 9, 1069. [Google Scholar] [CrossRef]
- Bailey, C.; Shoura, M.J.; Mischel, P.S.; Swanton, C. Extrachromosomal DNA—Relieving Heredity Constraints, Accelerating Tumour Evolution. Ann. Oncol. 2020, 31, 884–893. [Google Scholar] [CrossRef]
- Harrington, W.F.; Rodgers, M.E. Myosin. Annu. Rev. Biochem. 1983, 53, 35–73. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.; Fu, G.; Bing, S.; Meng, T.; Zhou, R.; Cheng, J.; Zhao, F.; Zhang, H.; Zhang, J. Molecular Cloning and MRNA Expression Analysis of Myosin Heavy Chain (MyHC) from Fast Skeletal Muscle of Grass Carp, Ctenopharyngodon Idella. Chin. J. Oceanol. Limnol. 2010, 28, 239–247. [Google Scholar] [CrossRef]
- McGuigan, K.; Phillips, P.C.; Postlethwait, J.H. Evolution of Sarcomeric Myosin Heavy Chain Genes: Evidence from Fish. Mol. Biol. Evol. 2004, 21, 1042–1056. [Google Scholar] [CrossRef] [PubMed]
- Wilborn, C.D.; Taylor, L.W.; Greenwood, M.; Kreider, R.B.; Willoughby, D.S. Effects of Different Intensities of Resistance Exercise on Regulators of Myogenesis. J. Strength. Cond. Res. 2009, 23, 2179–2187. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, D.S.; Nelson, M.J. Myosin Heavy-Chain MRNA Expression after a Single Session of Heavy-Resistance Exercise. Med. Sci. Sports Exerc. 2002, 34, 1262–1269. [Google Scholar] [CrossRef] [PubMed]
- Puhke, R.; Aunola, S.; Ailanto, P.; Alev, K.; Venojärvi, M.; Rusko, H.; Seene, T. Adaptive Changes of Myosin Isoforms in Response to Long-Term Strength and Power Training in Middle-Aged Men. J. Sports Sci. Med. 2006, 5, 349–358. [Google Scholar]
- Nose, A.; Isshiki, T.; Takeichi, M. Regional Specification of Muscle Progenitors in Drosophila: The Role of the Msh Homeobox Gene. Development 1998, 125, 215–223. [Google Scholar] [CrossRef]
- Lv, Y.P.; Yao, W.J.; Chen, J.; Bao, B.L. Newly Identified Gene Muscle Segment Homeobox C May Play a Role in Intermuscular Bone Development of Hemibarbus labeo. Genet. Mol. Res. 2015, 14, 11324–11334. [Google Scholar] [CrossRef]
- Pang, T.S.; Che, J.Y.; Fan, C.X.; Bao, B.L. The Role of MsxC Gene in the Development of Intermuscular Spines and Large Lateral Muscles in Zebrafish. J. Shanghai Ocean. Univ. 2022, 31, 328–335. [Google Scholar]
- Li, L.; Mirza, S.; Richardson, S.J.; Gallant, E.M.; Thekkedam, C.; Pace, S.M.; Zorzato, F.; Liu, D.; Beard, N.A.; Dulhunty, A.F. A New Cytoplasmic Interaction between Junctin and Ryanodine Receptor Ca2+ Release Channels. J. Cell Sci. 2015, 128, 951–963. [Google Scholar] [CrossRef]
- Beard, N.A.; Sakowska, M.M.; Dulhunty, A.F.; Laver, D.R. Calsequestrin Is an Inhibitor of Skeletal Muscle Ryanodine Receptor Calcium Release Channels. Biophys. J. 2002, 82, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Ikemoto, N.; Ronjat, M.; Mészáros, L.G.; Koshita, M. Postulated Role of Calsequestrin in the Regulation of Calcium Release from Sarcoplasmic Reticulum. Biochemistry 1989, 28, 6764–6771. [Google Scholar] [CrossRef]
- Murzilli, S.; Serano, M.; Pietrangelo, L.; Protasi, F.; Paolini, C. Structural Adaptation of the Excitation–Contraction Coupling Apparatus in Calsequestrin1-Null Mice during Postnatal Development. Biology 2023, 12, 1064. [Google Scholar] [CrossRef]
- Zhang, M.; Abrams, C.; Wang, L.; Gizzi, A.; He, L.; Lin, R.; Chen, Y.; Loll, P.J.; Pascal, J.M.; Zhang, J.F. Structural Basis for Calmodulin as a Dynamic Calcium Sensor. Structure 2012, 20, 911–923. [Google Scholar] [CrossRef]
- French, R.J.; Zamponi, G.W. Voltage-Gated Sodium and Calcium Channels in Nerve, Muscle, and Heart. IEEE Trans. Nanobioscience 2005, 4, 58–69. [Google Scholar] [CrossRef]
- Wray, D. Structure and Function of Ion Channels. Eur. Biophys. J. 2009, 38, 271–272. [Google Scholar] [CrossRef] [PubMed]
- Melzer, W.; Andronache, Z.; Ursu, D. Functional Roles of the Gamma Subunit of the Skeletal Muscle DHP-Receptor. J. Muscle Res. Cell Motil. 2006, 27, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Lory, P.; Bidaud, I.; Chemin, J. T-Type Calcium Channels in Differentiation and Proliferation. Cell Calcium 2006, 40, 135–146. [Google Scholar] [CrossRef]




| Sample Name | Raw Reads | Clean Reads | Q30 (%) |
|---|---|---|---|
| Slimming grass carp 1 | 149,672,200 | 130,405,698 | 90.11 |
| Slimming grass carp 2 | 185,402,510 | 164,979,168 | 91.25 |
| Slimming grass carp 3 | 146,103,008 | 125,514,344 | 89.46 |
| Common grass carp 1 | 157,244,712 | 139,871,412 | 91.78 |
| Common grass carp 2 | 139,295,000 | 123,377,174 | 91.32 |
| Common grass carp 3 | 153,561,782 | 130,942,792 | 91.82 |
| Gene Description | Annotation | Regulation | log2FC | p-Value |
|---|---|---|---|---|
| Muscle fiber structure and development | ||||
| Myosin heavy chain, fast skeletal muscle | GC01Gene03301 | Up | 21.2875216485768 | 0.000000051702 |
| Myosin light chain, fast skeletal muscle | GC01Gene04413 | Up | 11.7632038868467 | 0.002605161394 |
| Myosin binding protein C, fast type a | GC01Gene04318 | Up | 11.7632038868467 | 0.002605161394 |
| Myosin, light chain kinase | GC01Gene04351 | Up | 11.7632038868467 | 0.002605161394 |
| Myosin phosphatase | GC01Gene04201 | Up | 11.7632038868467 | 0.002605161394 |
| Actin, alpha | GC01Gene16235 | Up | 21.2677854176002 | 0.00000005320 |
| Capping protein | GC01Gene07223 | Up | 11.2325831506477 | 0.00404054634 |
| Muscle segment homeobox C (MsxC) | GC01Gene17144 | Up | 21.0562052584647 | 0.000000072098 |
| Integrin | GC01Gene09573 | Down | −9.47427441877102 | 0.00925059453 |
| Fibroblast growth factor | GC01Gene14267 | Down | −9.47427441877102 | 0.00925059453 |
| Calcium metabolism | ||||
| Calsequestrin | GC01Gene12461 | Up | 26.0132467521269 | 0.000000000027 |
| Calmodulin | GC01Gene00579 | Up | 21.2677854176002 | 0.00000005320 |
| Voltage-sensitive calcium channels | GC01Gene04287 | Up | 11.7632038868467 | 0.002605161394 |
| Dihydropyridine (DHP) sensitive calcium channel | GC01Gene04863 | Up | 11.7632038868467 | 0.002605161394 |
| Voltage-dependent T-type calcium channel subunit | GC01Gene00457 | UP | 8.55777414188946 | 0.017851975849 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, H.; Gao, Z.; Hu, Z.; Liang, G.; Huang, Y.; Zhou, M.; Liang, R.; Zhang, K. Identification and Characterization of Extrachromosomal Circular DNA in Slimming Grass Carp. Biomolecules 2024, 14, 1045. https://doi.org/10.3390/biom14091045
He H, Gao Z, Hu Z, Liang G, Huang Y, Zhou M, Liang R, Zhang K. Identification and Characterization of Extrachromosomal Circular DNA in Slimming Grass Carp. Biomolecules. 2024; 14(9):1045. https://doi.org/10.3390/biom14091045
Chicago/Turabian StyleHe, Haobin, Zihan Gao, Zehua Hu, Guanyu Liang, Yanhua Huang, Meng Zhou, Rishen Liang, and Kai Zhang. 2024. "Identification and Characterization of Extrachromosomal Circular DNA in Slimming Grass Carp" Biomolecules 14, no. 9: 1045. https://doi.org/10.3390/biom14091045





