Plectin: Dual Participation in Tumor Progression
Abstract
:1. Introduction
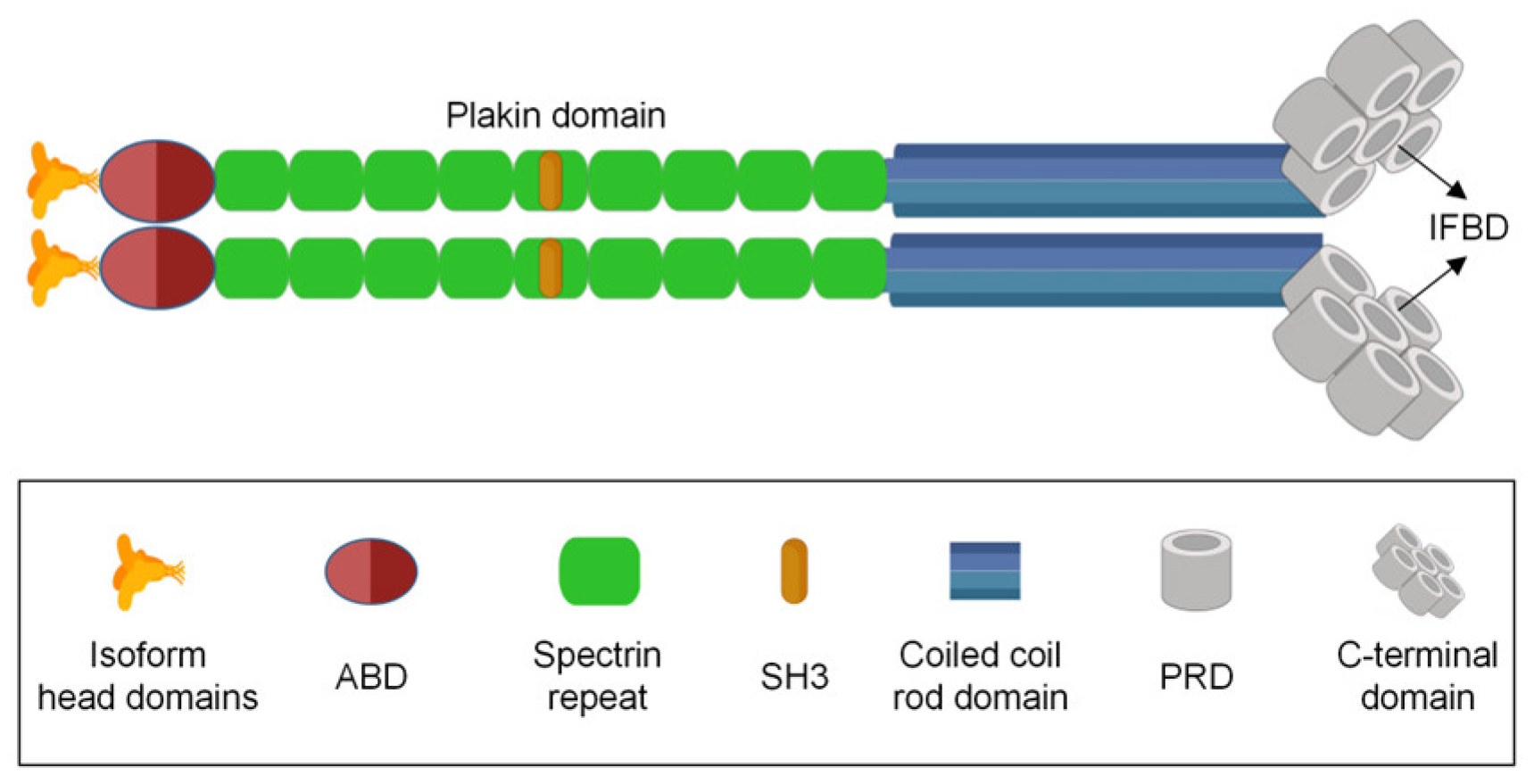
2. Molecular Structure and Function of Plectin
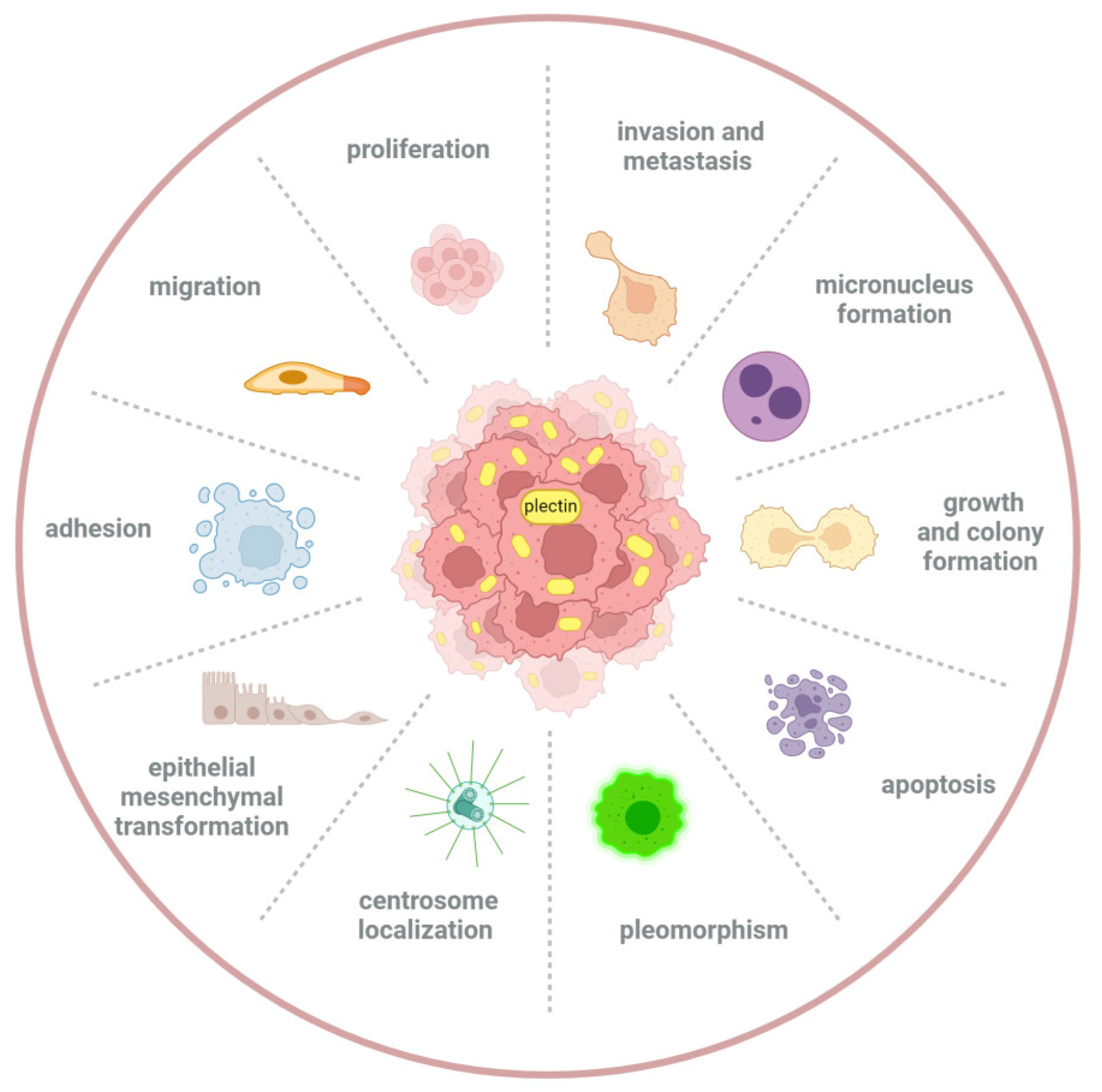
3. The Role of Plectin in Tumor Initiation and Progression
3.1. Plectin Promotes Cancer Development
3.2. Plectin Inhibits Cancer Development
4. Summary and Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Wiche, G. Role of plectin in cytoskeleton organization and dynamics. J. Cell Sci. 1998, 111 Pt 17, 2477–2486. [Google Scholar] [CrossRef] [PubMed]
- Wiche, G.; Winter, L. Plectin isoforms as organizers of intermediate filament cytoarchitecture. Bioarchitecture 2011, 1, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Castañón, M.J.; Walko, G.; Winter, L.; Wiche, G. Plectin-intermediate filament partnership in skin, skeletal muscle, and peripheral nerve. Histochem. Cell Biol. 2013, 140, 33–53. [Google Scholar] [CrossRef]
- Walko, G.; Castañón, M.J.; Wiche, G. Molecular architecture and function of the hemidesmosome. Cell Tissue Res. 2015, 360, 363–378. [Google Scholar] [CrossRef] [PubMed]
- Wiche, G.; Osmanagic-Myers, S.; Castañón, M.J. Networking and anchoring through plectin: A key to IF functionality and mechanotransduction. Curr. Opin. Cell Biol. 2015, 32, 21–29. [Google Scholar] [CrossRef]
- Buckup, M.; Rice, M.A.; Hsu, E.C.; Garcia-Marques, F.; Liu, S.; Aslan, M.; Bermudez, A.; Huang, J.; Pitteri, S.J.; Stoyanova, T. Plectin is a regulator of prostate cancer growth and metastasis. Oncogene 2021, 40, 663–676. [Google Scholar] [CrossRef]
- Gao, K.; Gao, Z.; Xia, M.; Li, H.; Di, J. Role of plectin and its interacting molecules in cancer. Med. Oncol. 2023, 40, 280. [Google Scholar] [CrossRef]
- Katada, K.; Tomonaga, T.; Satoh, M.; Matsushita, K.; Tonoike, Y.; Kodera, Y.; Hanazawa, T.; Nomura, F.; Okamoto, Y. Plectin promotes migration and invasion of cancer cells and is a novel prognostic marker for head and neck squamous cell carcinoma. J. Proteom. 2012, 75, 1803–1815. [Google Scholar] [CrossRef]
- Shin, S.J.; Smith, J.A.; Rezniczek, G.A.; Pan, S.; Chen, R.; Brentnall, T.A.; Wiche, G.; Kelly, K.A. Unexpected gain of function for the scaffolding protein plectin due to mislocalization in pancreatic cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 19414–19419. [Google Scholar] [CrossRef]
- Raymond, A.C.; Gao, B.; Girard, L.; Minna, J.D.; Gomika Udugamasooriya, D. Unbiased peptoid combinatorial cell screen identifies plectin protein as a potential biomarker for lung cancer stem cells. Sci. Rep. 2019, 9, 14954. [Google Scholar] [CrossRef]
- Bausch, D.; Thomas, S.; Mino-Kenudson, M.; Fernández-del, C.C.; Bauer, T.W.; Williams, M.; Warshaw, A.L.; Thayer, S.P.; Kelly, K.A. Plectin-1 as a novel biomarker for pancreatic cancer. Clin. Cancer Res. 2011, 17, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Ho, C.C.; Cheng, C.C.; Pei, R.J.; Hsu, Y.H.; Yeh, K.T.; Tsai, M.C.; Lai, Y.S. Pleomorphism of cancer cells with the expression of plectin and concept of filament bundles in human hepatocellular carcinoma. Res. Commun. Mol. Pathol. Pharmacol. 2007, 120–121, 43–54. [Google Scholar]
- Cheng, C.C.; Lai, Y.C.; Lai, Y.S.; Hsu, Y.H.; Chao, W.T.; Sia, K.C.; Tseng, Y.H.; Liu, Y.H. Transient knockdown-mediated deficiency in plectin alters hepatocellular motility in association with activated FAK and Rac1-GTPase. Cancer Cell Int. 2015, 15, 29. [Google Scholar] [CrossRef] [PubMed]
- Dumas, V.; Kanitakis, J.; Charvat, S.; Euvrard, S.; Faure, M.; Claudy, A. Expression of basement membrane antigens and matrix metalloproteinases 2 and 9 in cutaneous basal and squamous cell carcinomas. Anticancer Res. 1999, 19, 2929–2938. [Google Scholar]
- Perez, S.M.; Brinton, L.T.; Kelly, K.A. Plectin in Cancer: From Biomarker to Therapeutic Target. Cells 2021, 10, 2246. [Google Scholar] [CrossRef]
- McInroy, L.; Määttä, A. Plectin regulates invasiveness of SW480 colon carcinoma cells and is targeted to podosome-like adhesions in an isoform-specific manner. Exp. Cell Res. 2011, 317, 2468–2478. [Google Scholar] [CrossRef]
- Winter, L.; Wiche, G. The many faces of plectin and plectinopathies: Pathology and mechanisms. Acta Neuropathol. 2013, 125, 77–93. [Google Scholar] [CrossRef]
- Castañón, M.J.; Wiche, G. Identifying Plectin Isoform Functions through Animal Models. Cells 2021, 10, 2453. [Google Scholar] [CrossRef]
- Chaudhari, P.R.; Vaidya, M.M. Versatile hemidesmosomal linker proteins: Structure and function. Histol. Histopathol. 2015, 30, 425–434. [Google Scholar] [CrossRef]
- Sonnenberg, A.; Liem, R.K. Plakins in development and disease. Exp. Cell Res. 2007, 313, 2189–2203. [Google Scholar] [CrossRef]
- Liu, C.G.; Maercker, C.; Castañon, M.J.; Hauptmann, R.; Wiche, G. Human plectin: Organization of the gene, sequence analysis, and chromosome localization (8q24). Proc. Natl. Acad. Sci. USA 1996, 93, 4278–4283. [Google Scholar] [CrossRef]
- McLean, W.H.; Pulkkinen, L.; Smith, F.J.; Rugg, E.L.; Lane, E.B.; Bullrich, F.; Burgeson, R.E.; Amano, S.; Hudson, D.L.; Owaribe, K.; et al. Loss of plectin causes epidermolysis bullosa with muscular dystrophy: cDNA cloning and genomic organization. Genes Dev. 1996, 10, 1724–1735. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, P.; Zörer, M.; Rezniczek, G.A.; Spazierer, D.; Oehler, S.; Castañón, M.J.; Hauptmann, R.; Wiche, G. Unusual 5′ transcript complexity of plectin isoforms: Novel tissue-specific exons modulate actin binding activity. Hum. Mol. Genet. 1999, 8, 2461–2472. [Google Scholar] [CrossRef] [PubMed]
- Rezniczek, G.A.; Abrahamsberg, C.; Fuchs, P.; Spazierer, D.; Wiche, G. Plectin 5′-transcript diversity: Short alternative sequences determine stability of gene products, initiation of translation and subcellular localization of isoforms. Hum. Mol. Genet. 2003, 12, 3181–3194. [Google Scholar] [CrossRef]
- Foisner, R.; Wiche, G. Structure and hydrodynamic properties of plectin molecules. J. Mol. Biol. 1987, 198, 515–531. [Google Scholar] [CrossRef]
- Wiche, G.; Becker, B.; Luber, K.; Weitzer, G.; Castañon, M.J.; Hauptmann, R.; Stratowa, C.; Stewart, M. Cloning and sequencing of rat plectin indicates a 466-kD polypeptide chain with a three-domain structure based on a central alpha-helical coiled coil. J. Cell Biol. 1991, 114, 83–99. [Google Scholar] [CrossRef]
- Hu, L.; Huang, Z.; Wu, Z.; Ali, A.; Qian, A. Mammalian Plakins, Giant Cytolinkers: Versatile Biological Functions and Roles in Cancer. Int. J. Mol. Sci. 2018, 19, 974. [Google Scholar] [CrossRef]
- Bouameur, J.E.; Favre, B.; Borradori, L. Plakins, a versatile family of cytolinkers: Roles in skin integrity and in human diseases. J. Investig. Dermatol. 2014, 134, 885–894. [Google Scholar] [CrossRef]
- Foisner, R.; Wiche, G. Intermediate filament-associated proteins. Curr. Opin. Cell Biol. 1991, 3, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Starr, D.A.; Fridolfsson, H.N. Interactions between nuclei and the cytoskeleton are mediated by SUN-KASH nuclear-envelope bridges. Annu. Rev. Cell Dev. Biol. 2010, 26, 421–444. [Google Scholar] [CrossRef] [PubMed]
- De Pascalis, C.; Pérez-González, C.; Seetharaman, S.; Boëda, B.; Vianay, B.; Burute, M.; Leduc, C.; Borghi, N.; Trepat, X.; Etienne-Manneville, S. Intermediate filaments control collective migration by restricting traction forces and sustaining cell-cell contacts. J. Cell Biol. 2018, 217, 3031–3044. [Google Scholar] [CrossRef] [PubMed]
- de Pereda, J.M.; Lillo, M.P.; Sonnenberg, A. Structural basis of the interaction between integrin alpha6beta4 and plectin at the hemidesmosomes. EMBO J. 2009, 28, 1180–1190. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmsen, K.; Litjens, S.H.; Kuikman, I.; Tshimbalanga, N.; Janssen, H.; van den Bout, I.; Raymond, K.; Sonnenberg, A. Nesprin-3, a novel outer nuclear membrane protein, associates with the cytoskeletal linker protein plectin. J. Cell Biol. 2005, 171, 799–810. [Google Scholar] [CrossRef]
- Ketema, M.; Wilhelmsen, K.; Kuikman, I.; Janssen, H.; Hodzic, D.; Sonnenberg, A. Requirements for the localization of nesprin-3 at the nuclear envelope and its interaction with plectin. J. Cell Sci. 2007, 120, 3384–3394. [Google Scholar] [CrossRef] [PubMed]
- García-Alvarez, B.; Bobkov, A.; Sonnenberg, A.; de Pereda, J.M. Structural and functional analysis of the actin binding domain of plectin suggests alternative mechanisms for binding to F-actin and integrin beta4. Structure 2003, 11, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Rezniczek, G.A.; Konieczny, P.; Nikolic, B.; Reipert, S.; Schneller, D.; Abrahamsberg, C.; Davies, K.E.; Winder, S.J.; Wiche, G. Plectin 1f scaffolding at the sarcolemma of dystrophic (mdx) muscle fibers through multiple interactions with beta-dystroglycan. J. Cell Biol. 2007, 176, 965–977. [Google Scholar] [CrossRef]
- Wang, W.; Zuidema, A.; Te Molder, L.; Nahidiazar, L.; Hoekman, L.; Schmidt, T.; Coppola, S.; Sonnenberg, A. Hemidesmosomes modulate force generation via focal adhesions. J. Cell Biol. 2020, 219, e201904137. [Google Scholar] [CrossRef]
- Koster, J.; van Wilpe, S.; Kuikman, I.; Litjens, S.H.; Sonnenberg, A. Role of binding of plectin to the integrin beta4 subunit in the assembly of hemidesmosomes. Mol. Biol. Cell 2004, 15, 1211–1223. [Google Scholar] [CrossRef]
- Djinovic-Carugo, K.; Gautel, M.; Ylänne, J.; Young, P. The spectrin repeat: A structural platform for cytoskeletal protein assemblies. FEBS Lett. 2002, 513, 119–123. [Google Scholar] [CrossRef]
- Matsubara, T.; Yaginuma, T.; Addison, W.N.; Fujita, Y.; Watanabe, K.; Yoshioka, I.; Hikiji, H.; Maki, K.; Baron, R.; Kokabu, S. Plectin stabilizes microtubules during osteoclastic bone resorption by acting as a scaffold for Src and Pyk2. Bone 2020, 132, 115209. [Google Scholar] [CrossRef]
- Wenta, T.; Schmidt, A.; Zhang, Q.; Devarajan, R.; Singh, P.; Yang, X.; Ahtikoski, A.; Vaarala, M.; Wei, G.H.; Manninen, A. Disassembly of α6β4-mediated hemidesmosomal adhesions promotes tumorigenesis in PTEN-negative prostate cancer by targeting plectin to focal adhesions. Oncogene 2022, 41, 3804–3820. [Google Scholar] [CrossRef] [PubMed]
- Frijns, E.; Kuikman, I.; Litjens, S.; Raspe, M.; Jalink, K.; Ports, M.; Wilhelmsen, K.; Sonnenberg, A. Phosphorylation of threonine 1736 in the C-terminal tail of integrin β4 contributes to hemidesmosome disassembly. Mol. Biol. Cell 2012, 23, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Jang, T.H.; Huang, W.C.; Tung, S.L.; Lin, S.C.; Chen, P.M.; Cho, C.Y.; Yang, Y.Y.; Yen, T.C.; Lo, G.H.; Chuang, S.E.; et al. MicroRNA-485-5p targets keratin 17 to regulate oral cancer stemness and chemoresistance via the integrin/FAK/Src/ERK/β-catenin pathway. J. Biomed. Sci. 2022, 29, 42. [Google Scholar] [CrossRef] [PubMed]
- Wiche, G. Plectin-Mediated Intermediate Filament Functions: Why Isoforms Matter. Cells 2021, 10, 2154. [Google Scholar] [CrossRef]
- Gundesli, H.; Kori, M.; Arga, K.Y. The Versatility of Plectin in Cancer: A Pan-Cancer Analysis on Potential Diagnostic and Prognostic Impacts of Plectin Isoforms. Omics 2023, 27, 281–296. [Google Scholar] [CrossRef]
- Flores, I.L.; Kawahara, R.; Miguel, M.C.; Granato, D.C.; Domingues, R.R.; Macedo, C.C.; Carnielli, C.M.; Yokoo, S.; Rodrigues, P.C.; Monteiro, B.V.; et al. EEF1D modulates proliferation and epithelial-mesenchymal transition in oral squamous cell carcinoma. Clin. Sci. 2016, 130, 785–799. [Google Scholar] [CrossRef]
- Rodrigues, P.C.; Sawazaki-Calone, I.; Ervolino de Oliveira, C.; Soares Macedo, C.C.; Dourado, M.R.; Cervigne, N.K.; Miguel, M.C.; Ferreira do Carmo, A.; Lambert, D.W.; Graner, E.; et al. Fascin promotes migration and invasion and is a prognostic marker for oral squamous cell carcinoma. Oncotarget 2017, 8, 74736–74754. [Google Scholar] [CrossRef]
- Rikardsen, O.G.; Magnussen, S.N.; Svineng, G.; Hadler-Olsen, E.; Uhlin-Hansen, L.; Steigen, S.E. Plectin as a prognostic marker in non-metastatic oral squamous cell carcinoma. BMC Oral Health 2015, 15, 98. [Google Scholar] [CrossRef]
- Chaudhari, P.R.; Charles, S.E.; D’Souza, Z.C.; Vaidya, M.M. Hemidesmosomal linker proteins regulate cell motility, invasion and tumorigenicity in oral squamous cell carcinoma derived cells. Exp. Cell Res. 2017, 360, 125–137. [Google Scholar] [CrossRef]
- Schreurs, O.; Balta, M.G.; Karatsaidis, A.; Schenck, K. Composition of hemidesmosomes in basal keratinocytes of normal buccal mucosa and oral lichen planus. Eur. J. Oral Sci. 2020, 128, 369–378. [Google Scholar] [CrossRef]
- Dmello, C.; Sawant, S.; Alam, H.; Gangadaran, P.; Tiwari, R.; Dongre, H.; Rana, N.; Barve, S.; Costea, D.E.; Chaukar, D.; et al. Vimentin-mediated regulation of cell motility through modulation of beta4 integrin protein levels in oral tumor derived cells. Int. J. Biochem. Cell Biol. 2016, 70, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Liu, Y.H.; Ho, C.C.; Pei, R.J.; Yeh, K.T.; Cheng, C.C.; Lai, Y.S. An early evaluation of malignant tendency with plectin expression in human colorectal adenoma and adenocarcinoma. J. Med. 2004, 35, 141–149. [Google Scholar] [PubMed]
- Zheng, S.; Qin, F.; Yin, J.; Li, D.; Huang, Y.; Hu, L.; He, L.; Lv, C.; Li, X.; Li, S.; et al. Role and mechanism of actin-related protein 2/3 complex signaling in cancer invasion and metastasis: A review. Medicine 2023, 102, e33158. [Google Scholar] [CrossRef] [PubMed]
- Prosseda, P.P.; Alvarado, J.A.; Wang, B.; Kowal, T.J.; Ning, K.; Stamer, W.D.; Hu, Y.; Sun, Y. Optogenetic stimulation of phosphoinositides reveals a critical role of primary cilia in eye pressure regulation. Sci. Adv. 2020, 6, eaay8699. [Google Scholar] [CrossRef]
- Jiu, Y.; Lehtimäki, J.; Tojkander, S.; Cheng, F.; Jäälinoja, H.; Liu, X.; Varjosalo, M.; Eriksson, J.E.; Lappalainen, P. Bidirectional Interplay between Vimentin Intermediate Filaments and Contractile Actin Stress Fibers. Cell Rep. 2015, 11, 1511–1518. [Google Scholar] [CrossRef]
- Pawar, H.; Kashyap, M.K.; Sahasrabuddhe, N.A.; Renuse, S.; Harsha, H.C.; Kumar, P.; Sharma, J.; Kandasamy, K.; Marimuthu, A.; Nair, B.; et al. Quantitative tissue proteomics of esophageal squamous cell carcinoma for novel biomarker discovery. Cancer Biol. Ther. 2011, 12, 510–522. [Google Scholar] [CrossRef]
- Knox, J.D.; Cress, A.E.; Clark, V.; Manriquez, L.; Affinito, K.S.; Dalkin, B.L.; Nagle, R.B. Differential expression of extracellular matrix molecules and the alpha 6-integrins in the normal and neoplastic prostate. Am. J. Pathol. 1994, 145, 167–174. [Google Scholar]
- Davis, T.L.; Cress, A.E.; Dalkin, B.L.; Nagle, R.B. Unique expression pattern of the alpha6beta4 integrin and laminin-5 in human prostate carcinoma. Prostate 2001, 46, 240–248. [Google Scholar] [CrossRef]
- Stanzani, E.; Pedrosa, L.; Bourmeau, G.; Anezo, O.; Noguera-Castells, A.; Esteve-Codina, A.; Passoni, L.; Matteoli, M.; de la Iglesia, N.; Seano, G.; et al. Dual Role of Integrin Alpha-6 in Glioblastoma: Supporting Stemness in Proneural Stem-Like Cells While Inducing Radioresistance in Mesenchymal Stem-Like Cells. Cancers 2021, 13, 3055. [Google Scholar] [CrossRef]
- Xu, R.; He, S.; Ma, D.; Liang, R.; Luo, Q.; Song, G. Plectin Downregulation Inhibits Migration and Suppresses Epithelial Mesenchymal Transformation of Hepatocellular Carcinoma Cells via ERK1/2 Signaling. Int. J. Mol. Sci. 2022, 24, 73. [Google Scholar] [CrossRef]
- Bausch, D.; Mino-Kenudson, M.; Fernández-Del Castillo, C.; Warshaw, A.L.; Kelly, K.A.; Thayer, S.P. Plectin-1 is a biomarker of malignant pancreatic intraductal papillary mucinous neoplasms. J. Gastrointest. Surg. 2009, 13, 1948–1954. [Google Scholar] [CrossRef]
- Moris, M.; Dawson, D.W.; Jiang, J.; Lewis, J.; Nassar, A.; Takeuchi, K.K.; Lay, A.R.; Zhai, Q.; Donahue, T.R.; Kelly, K.A.; et al. Plectin-1 as a Biomarker of Malignant Progression in Intraductal Papillary Mucinous Neoplasms: A Multicenter Study. Pancreas 2016, 45, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Mizuta, K.; Matsubara, T.; Goto, A.; Addison, W.N.; Nakatomi, M.; Matsuo, K.; Tada-Shigeyama, Y.; Yaginuma, T.; Honda, H.; Yoshioka, I.; et al. Plectin promotes tumor formation by B16 mouse melanoma cells via regulation of Rous sarcoma oncogene activity. BMC Cancer 2022, 22, 936. [Google Scholar] [CrossRef] [PubMed]
- Sutoh Yoneyama, M.; Hatakeyama, S.; Habuchi, T.; Inoue, T.; Nakamura, T.; Funyu, T.; Wiche, G.; Ohyama, C.; Tsuboi, S. Vimentin intermediate filament and plectin provide a scaffold for invadopodia, facilitating cancer cell invasion and extravasation for metastasis. Eur. J. Cell Biol. 2014, 93, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, F.; Panneer, N.; Tutino, C.M.; Wu, M.; Skrabal, W.R.; Moskaluk, C.; Kelly, K.A. A functional proteomic method for biomarker discovery. PLoS ONE 2011, 6, e22471. [Google Scholar] [CrossRef]
- Mittal, P.; Klingler-Hoffmann, M.; Arentz, G.; Winderbaum, L.; Lokman, N.A.; Zhang, C.; Anderson, L.; Scurry, J.; Leung, Y.; Stewart, C.J.; et al. Lymph node metastasis of primary endometrial cancers: Associated proteins revealed by MALDI imaging. Proteomics 2016, 16, 1793–1801. [Google Scholar] [CrossRef]
- Perez, S.M.; Dimastromatteo, J.; Landen, C.N., Jr.; Kelly, K.A. A Novel Monoclonal Antibody Targeting Cancer-Specific Plectin Has Potent Antitumor Activity in Ovarian Cancer. Cells 2021, 10, 2218. [Google Scholar] [CrossRef]
- Puiffe, M.L.; Le Page, C.; Filali-Mouhim, A.; Zietarska, M.; Ouellet, V.; Tonin, P.N.; Chevrette, M.; Provencher, D.M.; Mes-Masson, A.M. Characterization of ovarian cancer ascites on cell invasion, proliferation, spheroid formation, and gene expression in an in vitro model of epithelial ovarian cancer. Neoplasia 2007, 9, 820–829. [Google Scholar] [CrossRef]
- Oto, A.; Eltorky, M.A.; Dave, A.; Ernst, R.D.; Chen, K.; Rampy, B.; Chaljub, G.; Nealon, W. Mimicks of pancreatic malignancy in patients with chronic pancreatitis: Correlation of computed tomography imaging features with histopathologic findings. Curr. Probl. Diagn. Radiol. 2006, 35, 199–205. [Google Scholar] [CrossRef]
- Wang, C.I.; Wang, C.L.; Wu, Y.C.; Feng, H.P.; Liu, P.J.; Chang, Y.S.; Yu, J.S.; Yu, C.J. Quantitative proteomics reveals a novel role of karyopherin alpha 2 in cell migration through the regulation of vimentin-pErk protein complex levels in lung cancer. J. Proteome Res. 2015, 14, 1739–1751. [Google Scholar] [CrossRef]
- Niwa, T.; Saito, H.; Imajoh-ohmi, S.; Kaminishi, M.; Seto, Y.; Miki, Y.; Nakanishi, A. BRCA2 interacts with the cytoskeletal linker protein plectin to form a complex controlling centrosome localization. Cancer Sci. 2009, 100, 2115–2125. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.C.; Liu, Y.H.; Ho, C.C.; Chao, W.T.; Pei, R.J.; Hsu, Y.H.; Yeh, K.T.; Ho, L.C.; Tsai, M.C.; Lai, Y.S. The influence of plectin deficiency on stability of cytokeratin18 in hepatocellular carcinoma. J. Mol. Histol. 2008, 39, 209–216. [Google Scholar] [CrossRef]
- Liu, Y.H.; Cheng, C.C.; Ho, C.C.; Chao, W.T.; Pei, R.J.; Hsu, Y.H.; Yeh, K.T.; Ho, L.C.; Tsai, M.C.; Lai, Y.S. Degradation of plectin with modulation of cytokeratin 18 in human liver cells during staurosporine-induced apoptosis. In Vivo 2008, 22, 543–548. [Google Scholar]
- Liu, Y.H.; Ho, C.C.; Cheng, C.C.; Chao, W.T.; Pei, R.J.; Hsu, Y.H.; Lai, Y.S. Cytokeratin 18-mediated disorganization of intermediate filaments is induced by degradation of plectin in human liver cells. Biochem. Biophys. Res. Commun. 2011, 407, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.C.; Chao, W.T.; Liao, C.C.; Tseng, Y.H.; Lai, Y.C.; Lai, Y.S.; Hsu, Y.H.; Liu, Y.H. Plectin deficiency in liver cancer cells promotes cell migration and sensitivity to sorafenib treatment. Cell Adhes. Migr. 2018, 12, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Raymond, K.; Kreft, M.; Song, J.Y.; Janssen, H.; Sonnenberg, A. Dual Role of alpha6beta4 integrin in epidermal tumor growth: Tumor-suppressive versus tumor-promoting function. Mol. Biol. Cell 2007, 18, 4210–4221. [Google Scholar] [CrossRef]
- Ni, Y.; Wang, X.; Yin, X.; Li, Y.; Liu, X.; Wang, H.; Liu, X.; Zhang, J.; Gao, H.; Shi, B.; et al. Plectin protects podocytes from adriamycin-induced apoptosis and F-actin cytoskeletal disruption through the integrin α6β4/FAK/p38 MAPK pathway. J. Cell. Mol. Med. 2018, 22, 5450–5467. [Google Scholar] [CrossRef]
- Ridley, A.J. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006, 16, 522–529. [Google Scholar] [CrossRef]
- Moch, M.; Windoffer, R.; Schwarz, N.; Pohl, R.; Omenzetter, A.; Schnakenberg, U.; Herb, F.; Chaisaowong, K.; Merhof, D.; Ramms, L.; et al. Effects of Plectin Depletion on Keratin Network Dynamics and Organization. PLoS ONE 2016, 11, e0149106. [Google Scholar] [CrossRef]
- Bahadoran, P.; Perrin, C.; Aberdam, D.; Spadafora-Pisani, A.; Meneguzzi, G.; Ortonne, J.P. Altered expression of the hemidesmosome-anchoring filament complex proteins in basal cell carcinoma: Possible role in the origin of peritumoral lacunae. Br. J. Dermatol. 1997, 136, 35–42. [Google Scholar] [CrossRef]
- Dajee, M.; Lazarov, M.; Zhang, J.Y.; Cai, T.; Green, C.L.; Russell, A.J.; Marinkovich, M.P.; Tao, S.; Lin, Q.; Kubo, Y.; et al. NF-kappaB blockade and oncogenic Ras trigger invasive human epidermal neoplasia. Nature 2003, 421, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Caglar, H.O.; Biray Avci, C. Alterations of cell cycle genes in cancer: Unmasking the role of cancer stem cells. Mol. Biol. Rep. 2020, 47, 3065–3076. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, K.; Van Bockstaele, D.R.; Berneman, Z.N. The cell cycle: A review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003, 36, 131–149. [Google Scholar] [CrossRef]
- Gaiko-Shcherbak, A.; Eschenbruch, J.; Kronenberg, N.M.; Teske, M.; Wolters, B.; Springer, R.; Gather, M.C.; Merkel, R.; Hoffmann, B.; Noetzel, E. Cell Force-Driven Basement Membrane Disruption Fuels EGF- and Stiffness-Induced Invasive Cell Dissemination from Benign Breast Gland Acini. Int. J. Mol. Sci. 2021, 22, 3962. [Google Scholar] [CrossRef] [PubMed]
- Liotta, L.A. Adhere, Degrade, and Move: The Three-Step Model of Invasion. Cancer Res. 2016, 76, 3115–3117. [Google Scholar] [CrossRef]
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 2020, 11, 5120. [Google Scholar] [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef]
- Koivusalo, S.; Schmidt, A.; Manninen, A.; Wenta, T. Regulation of Kinase Signaling Pathways by α6β4-Integrins and Plectin in Prostate Cancer. Cancers 2022, 15, 149. [Google Scholar] [CrossRef]
- Žugec, M.; Furlani, B.; Castañon, M.J.; Rituper, B.; Fischer, I.; Broggi, G.; Caltabiano, R.; Barbagallo, G.M.V.; Di Rosa, M.; Tibullo, D.; et al. Plectin plays a role in the migration and volume regulation of astrocytes: A potential biomarker of glioblastoma. J. Biomed. Sci. 2024, 31, 14. [Google Scholar] [CrossRef]
- Wu, Y.; Tang, Y.; Xie, S.; Zheng, X.; Zhang, S.; Mao, J.; Wang, B.; Hou, Y.; Hu, L.; Chai, K.; et al. Chimeric peptide supramolecular nanoparticles for plectin-1 targeted miRNA-9 delivery in pancreatic cancer. Theranostics 2020, 10, 1151–1165. [Google Scholar] [CrossRef]
- Wesley, T.; Berzins, S.; Kannourakis, G.; Ahmed, N. The attributes of plakins in cancer and disease: Perspectives on ovarian cancer progression, chemoresistance and recurrence. Cell Commun. Signal. 2021, 19, 55. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.C.; Lai, Y.C.; Lai, Y.S.; Chao, W.T.; Tseng, Y.H.; Hsu, Y.H.; Chen, Y.Y.; Liu, Y.H. Cell Pleomorphism and Cytoskeleton Disorganization in Human Liver Cancer. In Vivo 2016, 30, 549–555. [Google Scholar] [PubMed]
- Jain, R.K.; Martin, J.D.; Stylianopoulos, T. The role of mechanical forces in tumor growth and therapy. Annu. Rev. Biomed. Eng. 2014, 16, 321–346. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, X.; Zhou, J.; Li, W.; Shu, X.; Wu, Y.; Long, M. Multiscale biomechanics and mechanotransduction from liver fibrosis to cancer. Adv. Drug Deliv. Rev. 2022, 188, 114448. [Google Scholar] [CrossRef]
- Gargalionis, A.N.; Papavassiliou, K.A.; Basdra, E.K.; Papavassiliou, A.G. mTOR Signaling Components in Tumor Mechanobiology. Int. J. Mol. Sci. 2022, 23, 1825. [Google Scholar] [CrossRef]
- Passi, M.; Zahler, S. Mechano-Signaling Aspects of Hepatocellular Carcinoma. J. Cancer 2021, 12, 6411–6421. [Google Scholar] [CrossRef]
- Bregenzer, M.E.; Horst, E.N.; Mehta, P.; Novak, C.M.; Repetto, T.; Mehta, G. The Role of Cancer Stem Cells and Mechanical Forces in Ovarian Cancer Metastasis. Cancers 2019, 11, 1008. [Google Scholar] [CrossRef]
| Tumor Types | Outcomes | Signal Molecules | Refs. |
|---|---|---|---|
| HNSCC | Increased proliferation, migration, and invasion | Erk1/2 | [8] |
| OSCC | Increased migration, invasion, tumorigenicity and levels of F-actin | Integrin β4, Cdc42, F-actin, Arp 2/3, MMP-9, NDRG1 | [46,49] |
| Colon cancer | Increased migration, invasion and adhesion | Actin | [16] |
| ESCC | Promotes the occurrence of ESCC | Caspase-8 | [56] |
| IPMN | Enhances the malignance | Unknown | [61] |
| PDAC | Increased migration and invasion; a specific marker | BRCA2 | [11] |
| Prostate cancer | Increased growth, metastasis, invasion and colony formation | Clusterin, NNMT, QARS, RPS2, RPLP0, GRHPR, GlnRS, Actin, Glutamine | [6] |
| HCC | Increased migration, invasion and EMT | Erk1/2 | [60] |
| Melanoma | Increased proliferation, Src activity and cell adhesion | Src | [63] |
| Bladder cancer | Increased migration, invasion and metastases | Vimentin, Cortactin, F-actin, MMPs | [64] |
| Lung adenocarcinoma | Increased migration and invasion | Erk1/2 | [70] |
| Ovarian cancer | Increased invasion | Actin | [68] |
| Tumor types | Outcomes | Signal Molecules | Refs. |
|---|---|---|---|
| HCC | Increases cell motility and causes pleomorphism of cancer cells | FAK, Rac1-GTPase | [12,13] |
| BCC | Increases invasion and metastasis | Integrin α6β4 | [14] |
| SCC | |||
| Situ skin carcinomas | |||
| Breast cancer | Promotes nuclear centrosome dissociation and micronucleus formation | BRCA2, centrosome | [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Wang, W.; Luo, Q.; Song, G. Plectin: Dual Participation in Tumor Progression. Biomolecules 2024, 14, 1050. https://doi.org/10.3390/biom14091050
Wang Z, Wang W, Luo Q, Song G. Plectin: Dual Participation in Tumor Progression. Biomolecules. 2024; 14(9):1050. https://doi.org/10.3390/biom14091050
Chicago/Turabian StyleWang, Zhihui, Wenbin Wang, Qing Luo, and Guanbin Song. 2024. "Plectin: Dual Participation in Tumor Progression" Biomolecules 14, no. 9: 1050. https://doi.org/10.3390/biom14091050




