Oxidative Stress and Cataract Formation: Evaluating the Efficacy of Antioxidant Therapies
Abstract
:1. Introduction
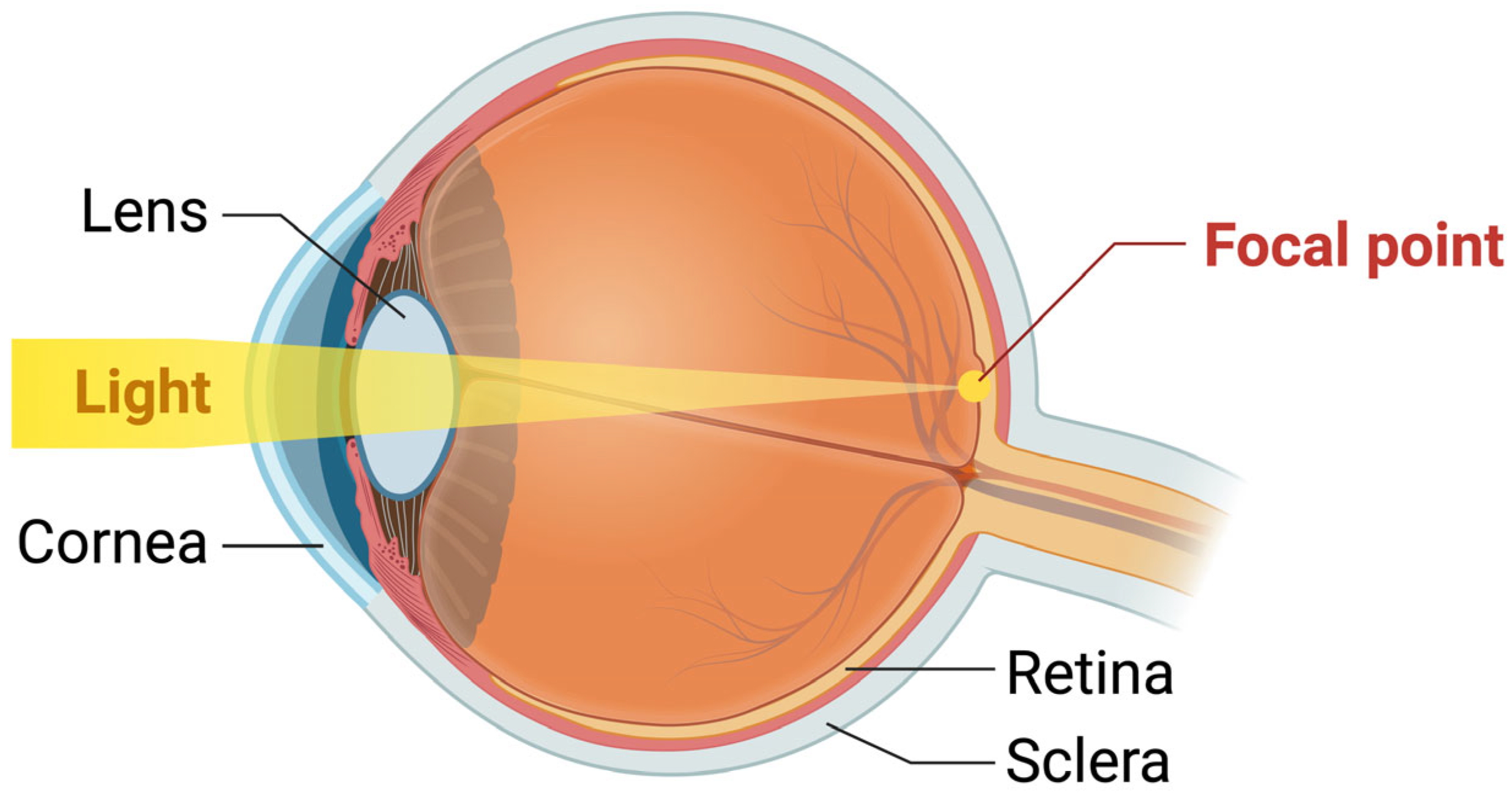
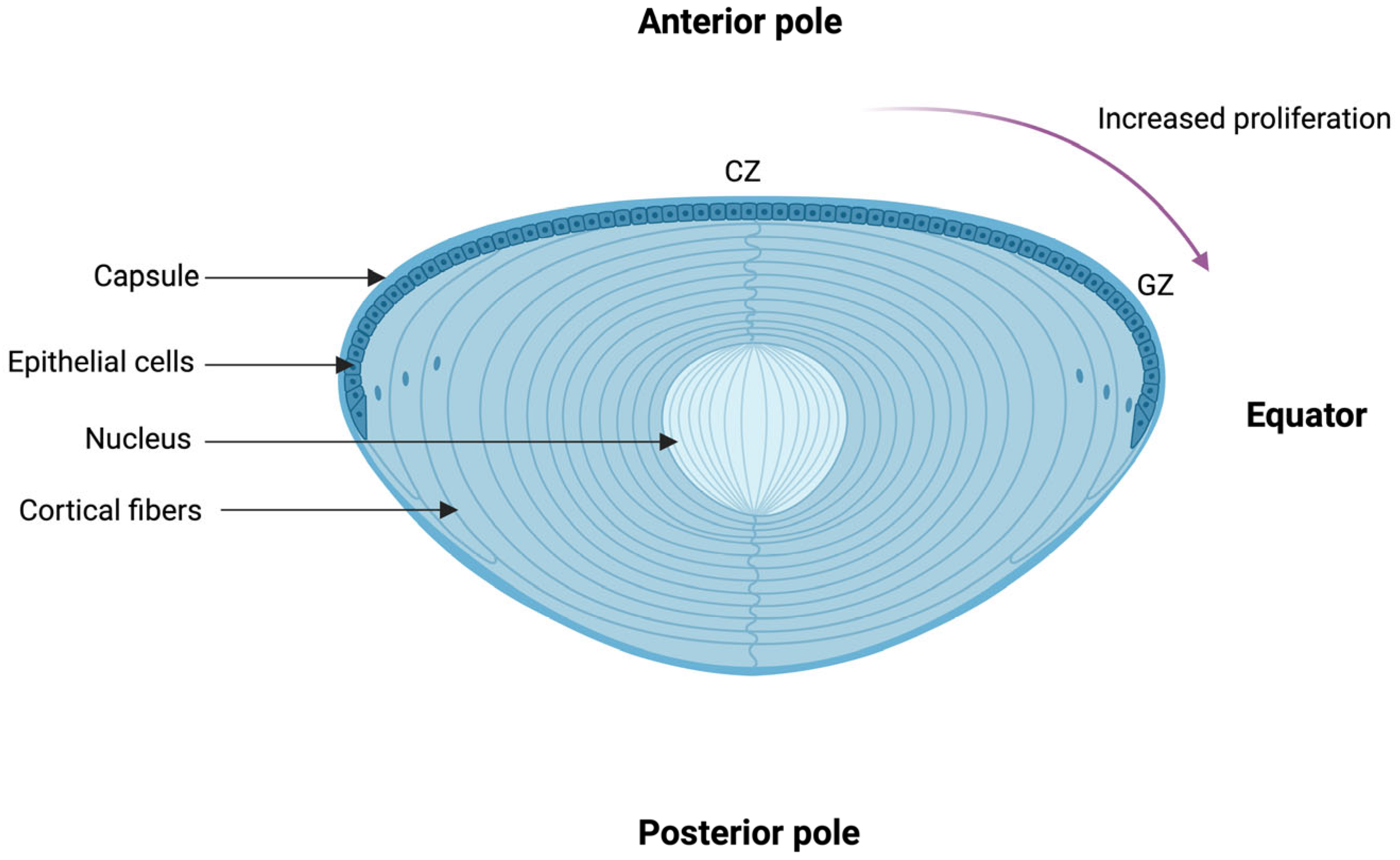
2. The Structure and Physiology of the Lens
Physiology of the Lens and Its Redox Regulatory Mechanisms
3. Ocular Damages Induced by Reactive Oxygen Species: Cataracto-genesis
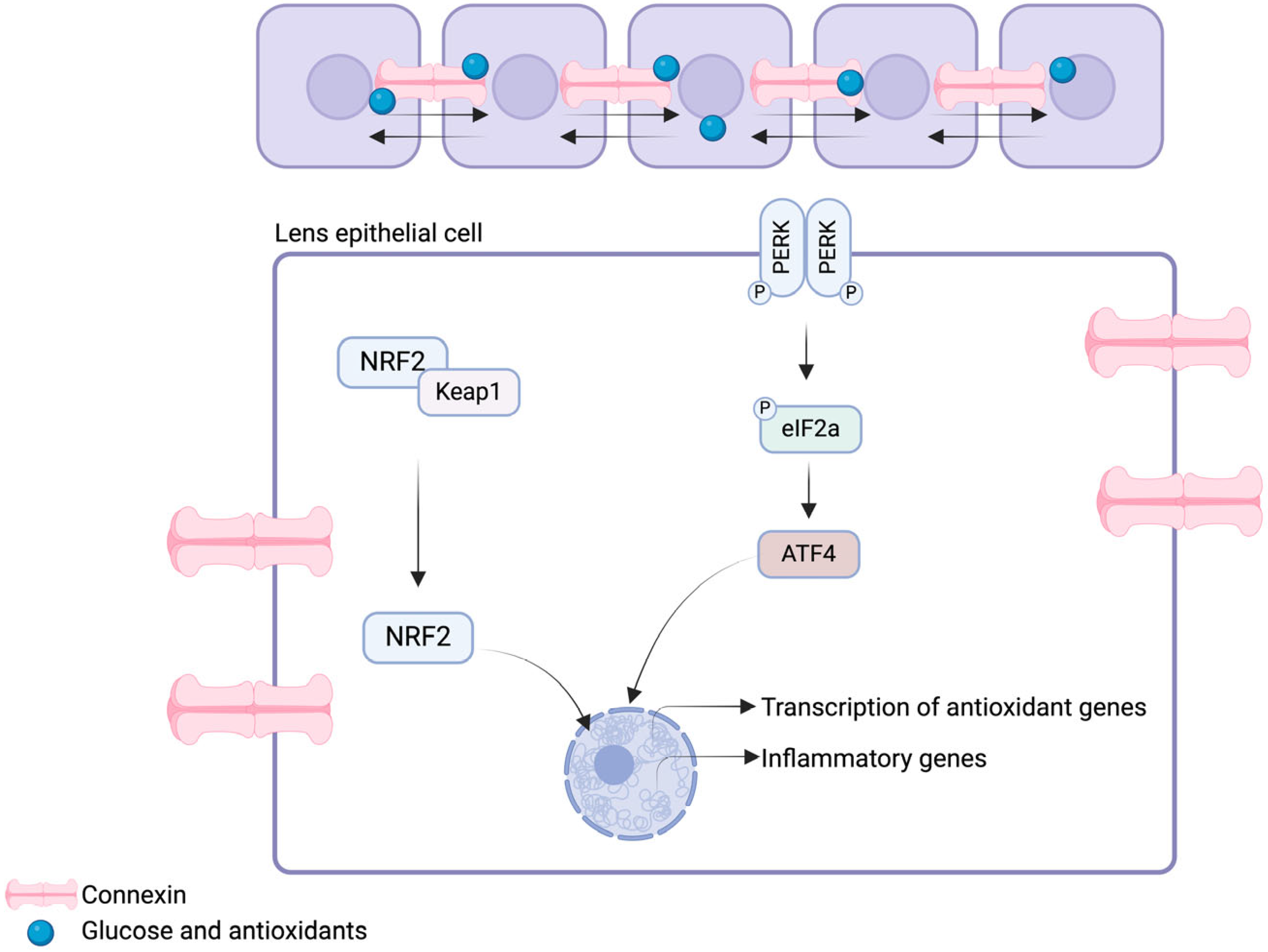
3.1. Antioxidative Systems
3.2. Protein Aggregation, Cross-Linking, and Light-Scattering
3.3. Lipid Peroxidation and Loss of Membrane Integrity
4. Antioxidant Strategies for the Prevention and Management of Cataracts
4.1. Dietary Nutrients and Supplements
4.1.1. Vitamins C and E
4.1.2. Lutein and Zeaxanthin
4.2. Potential Pharmacological Agents with Antioxidative Properties for Cataract Prevention and Treatment
4.2.1. N-acetyl-carnosine
4.2.2. N-acetylcysteine Amide
4.2.3. Resveratrol
4.2.4. Baicalein
4.2.5. Metformin
4.3. Nanotechnology-Based Drug Delivery Systems for Cataract Prevention and Treatment
4.3.1. N-acetylcarnosine Nanoparticles
4.3.2. Resveratrol Nanoparticles and Nanovesicles
4.3.3. Baicalin
4.3.4. Cerium Oxide
4.4. Gene Therapy for Cataract Prevention and Treatment
4.4.1. Suicide Gene Therapy
4.4.2. RNA Interference
4.4.3. CRISPR-Cas9
5. Challenges and Limitations of Antioxidants and Novel Therapeutic Approaches
6. Recommendations and Future Directions
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pesudovs, K.; Lansingh, V.C.; Kempen, J.H.; Tapply, I.; Fernandes, A.G.; Cicinelli, M.V.; Arrigo, A.; Leveziel, N.; Briant, P.S.; Vos, T.; et al. Global estimates on the number of people blind or visually impaired by cataract: A meta-analysis from 2000 to 2020. Eye 2024, 38, 2156–2172. [Google Scholar] [CrossRef]
- Fang, R.; Yu, Y.F.; Li, E.J.; Lv, N.X.; Liu, Z.C.; Zhou, H.G.; Song, X.D. Global, regional, national burden and gender disparity of cataract: Findings from the global burden of disease study 2019. BMC Public Health 2022, 22, 2068. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.P.; Ramke, J.; Cairns, J.; Butt, T.; Zhang, J.H.; Jones, I.; Jovic, M.; Nandakumar, A.; Faal, H.; Taylor, H.; et al. The economics of vision impairment and its leading causes: A systematic review. eClinicalMedicine 2022, 46, 101354. [Google Scholar] [CrossRef] [PubMed]
- Buscho, S.E.; Sharifi, A.; Cayenne, S.; Zhang, Y.; Merkley, K.H.; Gupta, P.K. Racial Disparities in Cataract Surgery Timeline and Intraocular Lens Selection: A Retrospective Study. Transl. Vis. Sci. Technol. 2023, 12, 20. [Google Scholar] [CrossRef]
- Lee, C.S.; Su, G.L.; Baughman, D.M.; Wu, Y.; Lee, A.Y. Disparities in delivery of ophthalmic care; An exploration of public Medicare data. PLoS ONE 2017, 12, e0182598. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Chen, X.Y.; Lou, L.X.; Yao, K. Socio-economic disparity in visual impairment from cataract. Int. J. Ophthalmol. 2021, 14, 1310–1314. [Google Scholar] [CrossRef]
- Nizami, A.A.; Gulani, A.C. Cataract. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Ho, M.C.; Peng, Y.J.; Chen, S.J.; Chiou, S.H. Senile cataracts and oxidative stress. J. Clin. Gerontol. Geriatr. 2010, 1, 17–21. [Google Scholar] [CrossRef]
- Shui, Y.-B.; Fu, J.-J.; Garcia, C.; Dattilo, L.K.; Rajagopal, R.; McMillan, S.; Mak, G.; Holekamp, N.M.; Lewis, A.; Beebe, D.C. Oxygen Distribution in the Rabbit Eye and Oxygen Consumption by the Lens. Investig. Opthalmology Vis. Sci. 2006, 47, 1571–1580. [Google Scholar] [CrossRef]
- Wu, S.Y.; Leske, M.C. Antioxidants and cataract formation: A summary review. Int. Ophthalmol. Clin. 2000, 40, 71–81. [Google Scholar] [CrossRef]
- Danysh, B.P.; Duncan, M.K. The lens capsule. Exp. Eye Res. 2009, 88, 151–164. [Google Scholar] [CrossRef]
- Griep, A.E. Cell cycle regulation in the developing lens. Semin. Cell Dev. Biol. 2006, 17, 686–697. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, Z.; Zhang, H. Research progress of lens zonules. Adv. Ophthalmol. Pract. Res. 2023, 3, 80–85. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, B.; Lal, K.; Liu, H.; Tran, M.; Zhou, M.; Ezugwu, C.; Gao, X.; Dang, T.; Au, M.-L.; et al. Antioxidant System and Endoplasmic Reticulum Stress in Cataracts. Cell. Mol. Neurobiol. 2023, 43, 4041–4058. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.K.; Nahomi, R.B.; Rankenberg, J.; Glomb, M.A.; Nagaraj, R.H. Glycation-mediated inter-protein cross-linking is promoted by chaperone-client complexes of α-crystallin: Implications for lens aging and presbyopia. J. Biol. Chem. 2020, 295, 5701–5716. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.B.; Kumar, P.A.; Kumar, M.S. Chaperone-like activity and hydrophobicity of alpha-crystallin. IUBMB Life 2006, 58, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, J. Alpha-crystallin can function as a molecular chaperone. Proc. Natl. Acad. Sci. USA 1992, 89, 10449–10453. [Google Scholar] [CrossRef]
- Shang, F.; Zetterberg, M.; Zhang, X.; Dudek, E.J.; Taylor, A. Ubiquitin–Proteasome Pathway Is an Important Protein Quality Control Mechanism in the Lens. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1520. [Google Scholar]
- Li, M.; Liu, S.; Huang, W.; Zhang, J. Physiological and pathological functions of βB2-crystallins in multiple organs: A systematic review. Aging 2021, 13, 15674–15687. [Google Scholar] [CrossRef]
- Myers, J.B.; Haddad, B.G.; O’neill, S.E.; Chorev, D.S.; Yoshioka, C.C.; Robinson, C.V.; Zuckerman, D.M.; Reichow, S.L. Structure of native lens connexin 46/50 intercellular channels by cryo-EM. Nature 2018, 564, 372–377. [Google Scholar] [CrossRef]
- Berthoud, V.M.; Beyer, E.C. Oxidative Stress, Lens Gap Junctions, and Cataracts. Antioxid. Redox Signal 2009, 11, 339–353. [Google Scholar] [CrossRef]
- Mathias, R.T.; White, T.W.; Gong, X. Lens gap junctions in growth, differentiation, and homeostasis. Physiol. Rev. 2010, 90, 179–206. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xu, S.; Eisenberg, R.S.; Huang, H. A Bidomain Model for Lens Microcirculation. Biophys. J. 2019, 116, 1171–1184. [Google Scholar] [CrossRef]
- Liu, J.; Riquelme, M.A.; Li, Z.; Li, Y.; Tong, Y.; Quan, Y.; Pei, C.; Gu, S.; Jiang, J.X. Mechanosensitive collaboration between integrins and connexins allows nutrient and antioxidant transport into the lens. J. Cell Biol. 2020, 219, e202002154. [Google Scholar] [CrossRef]
- Liu, X.; Hao, J.; Xie, T.; Malik, T.H.; Liu, C.; Shu, C.; Lu, C.; Zhou, D. Nrf2 as a target for prevention of age-related and diabetic cataracts by against oxidative stress. Aging Cell. 2017, 16, 934–942. [Google Scholar] [CrossRef]
- Periyasamy, P.; Shinohara, T. Age-related cataracts: Role of unfolded protein response, Ca2+ mobilization, epigenetic DNA modifications, and loss of Nrf2/Keap1 dependent cytoprotection. Prog. Retin. Eye Res. 2017, 60, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yu, S.; Zhang, C.; Kong, A.N.T. Epigenetic regulation of Keap1-Nrf2 signaling. Free Radic. Biol. Med. 2015, 88 (Pt B), 337–349. [Google Scholar] [CrossRef]
- Ma, T.J.; Lan, D.H.; He, S.Z.; Ye, Z.; Li, P.; Zhai, W.; Chen, W.Q.; Huang, Y.; Fu, Y.; Sun, A.; et al. Nrf2 protects human lens epithelial cells against H2O2-induced oxidative and ER stress: The ATF4 may be involved. Exp. Eye Res. 2018, 169, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C.; Zhang, K.; Kaufman, R.J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol. 2020, 21, 421–438. [Google Scholar] [CrossRef]
- Fukai, T.; Ushio-Fukai, M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid. Redox Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef]
- Reddy, V.N.; Kasahara, E.; Hiraoka, M.; Lin, L.R.; Ho, Y.S. Effects of variation in superoxide dismutases (SOD) on oxidative stress and apoptosis in lens epithelium. Exp. Eye Res. 2004, 79, 859–868. [Google Scholar] [CrossRef]
- Nandi, A.; Yan, L.J.; Jana, C.K.; Das, N. Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Oxid. Med. Cell Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef]
- Chamberlain, C.G.; Mansfield, K.J.; Cerra, A. Glutathione and catalase suppress TGFbeta-induced cataract-related changes in cultured rat lenses and lens epithelial explants. Mol. Vis. 2009, 15, 895–905. [Google Scholar] [PubMed]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione peroxidase-1 in health and disease: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011, 15, 1957–1997. [Google Scholar] [CrossRef]
- Varadaraj, K.; Gao, J.; Mathias, R.T.; Kumari, S.S. GPX1 knockout, not catalase knockout, causes accelerated abnormal optical aberrations and cataract in the aging lens. Mol. Vis. 2022, 28, 11–20. [Google Scholar] [PubMed]
- Huynh, T.P.N.; Bowater, R.P.; Bernuzzi, F.; Saha, S.; Wormstone, I.M. GSH Levels Serve as a Biological Redox Switch Regulating Sulforaphane-Induced Cell Fate in Human Lens Cells. Investig. Opthalmology Vis. Sci. 2021, 62, 2. [Google Scholar] [CrossRef]
- Li, B.; Kim, J.Y.; Martis, R.M.; Donaldson, P.J.; Lim, J.C. Characterisation of Glutathione Export from Human Donor Lenses. Transl. Vis. Sci. Technol. 2020, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.N. Glutathione and its function in the lens—An overview. Exp. Eye Res. 1990, 50, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Giblin, F.J. Glutathione: A vital lens antioxidant. J. Ocul. Pharmacol. Ther. 2000, 16, 121–135. [Google Scholar] [CrossRef]
- Pfaff, A.; Chernatynskaya, A.; Vineyard, H.; Ercal, N. Thiol antioxidants protect human lens epithelial (HLE B-3) cells against tert-butyl hydroperoxide-induced oxidative damage and cytotoxicity. Biochem. Biophys. Rep. 2022, 29, 101213. [Google Scholar] [CrossRef]
- Goyal, M.M.; Gajjar, D.U.; Patel, D.B.; Sune, P.; Vasavda, A.R. Effect of vitamin C and E activity on surgically removed cataractous human lens epithelium cells. Indian J. Clin. Biochem. IJCB 2009, 24, 375–380. [Google Scholar] [CrossRef]
- Hammond Christopher, J.; Snieder Harold Spector Tim, D.; Gilbert Clare, E. Genetic and Environmental Factors in Age-Related Nuclear Cataracts in Monozygotic and Dizygotic Twins. N. Engl. J. Med. 2000, 342, 1786–1790. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Li, D.; Du, Y.; He, W.; Lu, Y. DNA hypermethylation-mediated downregulation of antioxidant genes contributes to the early onset of cataracts in highly myopic eyes. Redox Biol. 2018, 19, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Xing, Y. Effects of UV on apoptotic factors in lens epithelial cells of an animal model. Exp. Ther. Med. 2018, 16, 2309–2312. [Google Scholar] [CrossRef] [PubMed]
- Katoh, N.; Jonasson, F.; Sasaki, H.; Kojima, M.; Ono, M.; Takahashi, N.; Sasaki, K.; Reykjavik Eye Study Group Cortical lens opacification in Iceland. Cortical lens opacification in Iceland. Risk factor analysis—Reykjavik Eye Study. Acta Ophthalmol. Scand. 2001, 79, 154–159. [Google Scholar] [CrossRef]
- Masuda, T.; Shimazawa, M.; Hara, H. Retinal Diseases Associated with Oxidative Stress and the Effects of a Free Radical Scavenger (Edaravone). Oxid. Med. Cell Longev. 2017, 2017, 9208489. [Google Scholar] [CrossRef]
- Ahmad, A.; Ahsan, H. Biomarkers of inflammation and oxidative stress in ophthalmic disorders. J. Immunoass. Immunochem. 2020, 41, 257–271. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, R.; Zhao, X.; Ma, Z.; Xin, J.; Xu, S.; Guo, D. The role of oxidative stress in the pathogenesis of ocular diseases: An overview. Mol. Biol. Rep. 2024, 51, 454. [Google Scholar] [CrossRef]
- Atalay, E.; Oğurel, T.; Derici, M.K. The role of oxidative damage in cataract etiopathogenesis. Ther. Adv. Ophthalmol. 2023, 15, 25158414231168813. [Google Scholar] [CrossRef]
- Quan, Y.; Du, Y.; Wu, C.; Gu, S.; Jiang, J.X. Connexin hemichannels regulate redox potential via metabolite exchange and protect lens against cellular oxidative damage. Redox Biol. 2021, 46, 102102. [Google Scholar] [CrossRef]
- Wang, G.; Quan, Y.; Ma, B.; Gu, S.; Jiang, J.X. Functional Interplay of Connexin Hemichannels in Glutathione Export from Lens Epithelial Cells and in Fiber Cell protection against Oxidative Stress. Investig. Ophthalmol. Vis. Sci. 2023, 64, 5118. [Google Scholar]
- Lasmaini, O.; Hidayat, M.; Wati, R. The Effect of Topical Glutathione on Malondialdehyde Levels in Rat with Cataract-Induced Sodium Selenite. Biosci. Med. J. Biomed. Transl. Res. 2023, 7, 3356–3361. [Google Scholar] [CrossRef]
- Schmid, P.W.N.; Lim, N.C.H.; Peters, C.; Back, K.C.; Bourgeois, B.; Pirolt, F.; Richter, B.; Peschek, J.; Puk, O.; Amarie, O.V.; et al. Imbalances in the eye lens proteome are linked to cataract formation. Nat. Struct. Mol. Biol. 2021, 28, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.P.; O’Neill, S.E.; Lampi, K.J.; Reichow, S.L. The α-crystallin Chaperones Undergo a Quasi-ordered Co-aggregation Process in Response to Saturating Client Interaction. J. Mol. Biol. 2024, 436, 168499. [Google Scholar] [CrossRef] [PubMed]
- Moosavi-Movahedi, F.; Saboury, A.A.; Ghasemi, A.; Pirhaghi, M.; Mamashli, F.; Mohammad-Zaheri, M.; Arghavani, P.; Yousefi, R.; Moosavi-Movahedi, A.A. Exploring the significance of potassium homeostasis in copper ion binding to human αB-Crystallin. Int. J. Biol. Macromol. 2024, 263, 130261. [Google Scholar] [CrossRef]
- Michiel, M.; Duprat, E.; Skouri-Panet, F.; Lampi, J.A.; Tardieu, A.; Lampi, K.J.; Finet, S. Aggregation of deamidated human βB2-crystallin and incomplete rescue by α-crystallin chaperone. Exp. Eye Res. 2010, 90, 688–698. [Google Scholar] [CrossRef]
- Ren, L.; Hu, L.; Zhang, Y.; Liu, J.; Xu, W.; Wu, W.; Xu, J.; Chen, X.; Yao, K.; Yu, Y. Cataract-causing S93R mutant destabilized structural conformation of βB1 crystallin linking with aggregates formation and cellular viability. Front. Mol. Biosci. 2022, 9, 844719. [Google Scholar] [CrossRef]
- Hill, J.A.; Nyathi, Y.; Horrell, S.; von Stetten, D.; Axford, D.; Owen, R.L.; Beddard, G.S.; Pearson, A.R.; Ginn, H.M.; Yorke, B.A. An ultraviolet-driven rescue pathway for oxidative stress to eye lens protein human gamma-D crystallin. Commun. Chem. 2024, 7, 81. [Google Scholar] [CrossRef]
- Budnar, P.; Tangirala, R.; Bakthisaran, R.; Rao, C.M. Protein Aggregation and Cataract: Role of Age-Related Modifications and Mutations in α-Crystallins. Biochem. Mosc. 2022, 87, 225–241. [Google Scholar] [CrossRef]
- Bellmaine, S.; Schnellbaecher, A.; Zimmer, A. Reactivity and degradation products of tryptophan in solution and proteins. Free Radic. Biol. Med. 2020, 160, 696–718. [Google Scholar] [CrossRef]
- Truscott, R.J.W.; Friedrich, M.G. Molecular Processes Implicated in Human Age-Related Nuclear Cataract. Investig. Ophthalmol. Vis. Sci. 2019, 60, 5007–5021. [Google Scholar] [CrossRef]
- Taylor, A.; Davies, K.J.A. Protein oxidation and loss of protease activity may lead to cataract formation in the aged lens. Free Radic. Biol. Med. 1987, 3, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.A.; Sprague-Piercy, M.A.; Kwok, A.O.; Roskamp, K.W.; Martin, R.W. Chemical Properties Determine Solubility and Stability in βγ-Crystallins of the Eye Lens. ChemBioChem 2021, 22, 1329–1346. [Google Scholar] [CrossRef]
- Nandwani, N.; Surana, P.; Negi, H.; Mascarenhas, N.M.; Udgaonkar, J.B.; Das, R.; Gosavi, S. A five-residue motif for the design of domain swapping in proteins. Nat. Commun. 2019, 10, 452. [Google Scholar] [CrossRef]
- Anbaraki, A.; Ghahramani, M.; Muranov, K.O.; Kurganov, B.I.; Yousefi, R. Structural and functional alteration of human αA-crystallin after exposure to full spectrum solar radiation and preventive role of lens antioxidants. Int. J. Biol. Macromol. 2018, 118 (Pt A), 1120–1130. [Google Scholar] [CrossRef]
- Paviani, V.; Junqueira de Melo, P.; Avakin, A.; Di Mascio, P.; Ronsein, G.E.; Augusto, O. Human cataractous lenses contain cross-links produced by crystallin-derived tryptophanyl and tyrosyl radicals. Free Radic. Biol. Med. 2020, 160, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Wilmarth, P.A.; Tanner, S.; Dasari, S.; Nagalla, S.R.; Riviere, M.A.; Bafna, V.; Pevzner, P.A.; David, L.L. Age-related changes in human crystallins determined from comparative analysis of post-translational modifications in young and aged lens: Does deamidation contribute to crystallin insolubility? J. Proteome Res. 2006, 5, 2554–2566. [Google Scholar] [CrossRef] [PubMed]
- Vetter, C.J.; Thorn, D.C.; Wheeler, S.G.; Mundorff, C.C.; Halverson, K.A.; Wales, T.E.; Shinde, U.P.; Engen, J.R.; David, L.L.; Carver, J.A.; et al. Cumulative deamidations of the major lens protein γS-crystallin increase its aggregation during unfolding and oxidation. Protein Sci. 2020, 29, 1945–1963. [Google Scholar] [CrossRef]
- He, Y.; Kang, J.; Song, J. ATP antagonizes the crowding-induced destabilization of the human eye-lens protein γS-crystallin. Biochem. Biophys. Res. Commun. 2020, 526, 1112–1117. [Google Scholar] [CrossRef] [PubMed]
- Timsina, R.; Khadka, N.K.; Maldonado, D.; Mainali, L. Interaction of alpha-crystallin with four major phospholipids of eye lens membranes. Exp. Eye Res. 2021, 202, 108337. [Google Scholar] [CrossRef]
- Timsina, R.; Hazen, P.; Trossi-Torres, G.; Khadka, N.K.; Kalkat, N.; Mainali, L. Cholesterol Content Regulates the Interaction of αA-, αB-, and α-Crystallin with the Model of Human Lens-Lipid Membranes. Int. J. Mol. Sci. 2024, 25, 1923. [Google Scholar] [CrossRef]
- Yang, X.; Xu, J.; Fu, C.; Jia, Z.; Yao, K.; Chen, X. The cataract-related S39C variant increases γS-crystallin sensitivity to environmental stress by destroying the intermolecular disulfide cross-links. Biochem. Biophys. Res. Commun. 2020, 526, 459–465. [Google Scholar] [CrossRef]
- Chen, S.; Guo, J.; Xu, W.; Song, H.; Xu, J.; Luo, C.; Yao, K.; Hu, L.; Chen, X.; Yu, Y. Cataract-related variant R114C increases βA3-crystallin susceptibility to environmental stresses by disrupting the protein senior structure. Int. J. Biol. Macromol. 2024, 262 Pt 2, 130191. [Google Scholar] [CrossRef] [PubMed]
- Ghahramani, M.; Yousefi, R.; Krivandin, A.; Muranov, K.; Kurganov, B.; Moosavi-Movahedi, A.A. Structural and functional characterization of D109H and R69C mutant versions of human αB-crystallin: The biochemical pathomechanism underlying cataract and myopathy development. Int. J. Biol. Macromol. 2020, 146, 1142–1160. [Google Scholar] [CrossRef] [PubMed]
- Simonelli, F.; Nesti, A.; Pensa, M.; Romano, L.; Savastano, S.; Rinaldi, E.; Auricchio, G. Lipid peroxidation and human cataractogenesis in diabetes and severe myopia. Exp. Eye Res. 1989, 49, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Nam, T.G. Lipid peroxidation and its toxicological implications. Toxicol. Res. 2011, 27, 1–6. [Google Scholar] [CrossRef]
- Babizhayev, M.A. Analysis of Lipid Peroxidation and Electron Microscopic Survey of Maturation Stages during Human Cataractogenesis. Drugs R&D 2005, 6, 345–369. [Google Scholar] [CrossRef]
- Kreuzer, M.; Dučić, T.; Hawlina, M.; Andjelic, S. Synchrotron-based FTIR microspectroscopy of protein aggregation and lipids peroxidation changes in human cataractous lens epithelial cells. Sci. Rep. 2020, 10, 15489. [Google Scholar] [CrossRef]
- Wei, Z.; Hao, C.; Huangfu, J.; Srinivasagan, R.; Zhang, X.; Fan, X. Aging lens epithelium is susceptible to ferroptosis. Free Radic. Biol. Med. 2021, 167, 94–108. [Google Scholar] [CrossRef]
- Fan, X.; Wei, Z.; Hao, C.; Huangfu, J.; Srinivasagan, R.; Zhang, X. Lipid peroxidation induces ferroptosis in the lens epithelium. Investig. Ophthalmol. Vis. Sci. 2022, 63, 1146. [Google Scholar]
- Sardelli, G.; Scali, V.; Signore, G.; Balestri, F.; Cappiello, M.; Mura, U.; Del Corso, A.; Moschini, R. Response of a Human Lens Epithelial Cell Line to Hyperglycemic and Oxidative Stress: The Role of Aldose Reductase. Antioxidants 2023, 12, 829. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jin, Z.; Xia, R.; Zheng, Z.; Zha, Y.; Wang, Q.; Wan, X.; Yang, H.; Cai, J. Protection of Human Lens Epithelial Cells from Oxidative Stress Damage and Cell Apoptosis by KGF-2 through the Akt/Nrf2/HO-1 Pathway. Oxidative Med. Cell. Longev. 2022, 2022, 6933812. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.E.; Ng, H.S.; Yap, W.S.; Goh, H.J.H.; Yim, H.S. Nutrients for Prevention of Macular Degeneration and Eye-Related Diseases. Antioxidants 2019, 8, 85. [Google Scholar] [CrossRef]
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Krčmová, L.K.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P.; et al. Vitamin C-Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef] [PubMed]
- Ringvold, A. The Significance of Ascorbate in the Aqueous Humour Protection Against UV-A and UV-B. Exp. Eye Res. 1996, 62, 261–264. [Google Scholar] [CrossRef]
- Shahidi, F.; Pinaffi-Langley, A.C.C.; Fuentes, J.; Speisky, H.; de Camargo, A.C. Vitamin E as an essential micronutrient for human health: Common, novel, and unexplored dietary sources. Free Radic. Biol. Med. 2021, 176, 312–321. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Hashizume, K.; Kishimoto, S.; Tezuka, Y.; Nishigori, H.; Yamamoto, N.; Kondo, Y.; Maruyama, N.; Ishigami, A.; Kurosaka, D. Effect of vitamin C depletion on UVR-B induced cataract in SMP30/GNL knockout mice. Exp. Eye Res. 2012, 94, 85–89. [Google Scholar] [CrossRef]
- Haque, S.E.; Gilani, K.M.A. Effect of ambroxol, spirulina and vitamin-E in naphthalene induced cataract in female rats. Indian. J. Physiol. Pharmacol. 2005, 49, 57–64. [Google Scholar] [PubMed]
- Mares-Perlman, J.A.; Lyle, B.J.; Klein, R.; Fisher, A.I.; Brady, W.E.; VandenLangenberg, G.M.; Trabulsi, J.N.; Palta, M. Vitamin supplement use and incident cataracts in a population-based study. Arch. Ophthalmol. 2000, 118, 1556–1563. [Google Scholar] [CrossRef]
- Ghazala; Siddiqui, J.A.; Ali, N.; Ali, S.L.; Shaikh, G.S.; Qureshi, A. Serum vitamin A, E & C in cortical & nuclear cataract patients. Prof. Med. J. 2021, 28, 1137–1141. [Google Scholar] [CrossRef]
- Lim, J.C.; Caballero Arredondo, M.; Braakhuis, A.J.; Donaldson, P.J. Vitamin C and the Lens: New Insights into Delaying the Onset of Cataract. Nutrients 2020, 12, 3142. [Google Scholar] [CrossRef]
- Christen, W.G.; Glynn, R.J.; Sesso, H.D.; Kurth, T.; MacFadyen, J.; Bubes, V.; Buring, J.E.; Manson, J.E.; Gaziano, J.M. Age-related cataract in a randomized trial of vitamins E and C in men. Arch. Ophthalmol. 2010, 128, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Gritz, D.C.; Srinivasan, M.; Smith, S.D.; Kim, U.; Lietman, T.M.; Wilkins, J.H.; Priyadharshini, B.; John, R.K.; Aravind, S.; Prajna, N.V.; et al. The Antioxidants in Prevention of Cataracts Study: Effects of antioxidant supplements on cataract progression in South India. Br. J. Ophthalmol. 2006, 90, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Ravindran, R.D.; O’Brien, K.S.; Kim, U.R.; Wilkins, J.H.; Whitcher, J.P.; Lietman, T.M.; Gritz, D.C.; Keenan, J.D. Antioxidant Vitamins for Cataracts: 15-Year Follow-up of a Randomized Trial. Ophthalmology 2020, 127, 986–987. [Google Scholar] [CrossRef]
- Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E and beta carotene for age-related cataract and vision loss: AREDS report no. 9. Arch. Ophthalmol. 2001, 119, 1439–1452. [Google Scholar] [CrossRef]
- Zheng Selin, J.; Rautiainen, S.; Lindblad, B.E.; Morgenstern, R.; Wolk, A. High-Dose Supplements of Vitamins C and E, Low-Dose Multivitamins, and the Risk of Age-related Cataract: A Population-based Prospective Cohort Study of Men. Am. J. Epidemiol. 2013, 177, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Barker, F.M., 2nd; Snodderly, D.M.; Johnson, E.J.; Schalch, W.; Koepcke, W.; Gerss, J.; Neuringer, M. Nutritional manipulation of primate retinas, V: Effects of lutein, zeaxanthin, and n-3 fatty acids on retinal sensitivity to blue-light-induced damage. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3934–3942. [Google Scholar] [CrossRef]
- Varma, S.D.; Kovtun, S.; Hegde, K.R. Role of ultraviolet irradiation and oxidative stress in cataract formation-medical prevention by nutritional antioxidants and metabolic agonists. Eye Contact Lens. 2011, 37, 233–245. [Google Scholar] [CrossRef]
- Bernstein, P.S.; Li, B.; Vachali, P.P.; Gorusupudi, A.; Shyam, R.; Henriksen, B.S.; Nolan, J.M. Lutein, zeaxanthin, and meso-zeaxanthin: The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog. Retin. Eye Res. 2016, 50, 34–66. [Google Scholar] [CrossRef]
- Walchuk, C.; Suh, M. Nutrition and the aging retina: A comprehensive review of the relationship between nutrients and their role in age-related macular degeneration and retina disease prevention. Adv. Food Nutr. Res. 2020, 93, 293–332. [Google Scholar] [CrossRef]
- Vu, H.T.V.; Robman, L.; Hodge, A.; McCarty, C.A.; Taylor, H.R. Lutein and Zeaxanthin and the Risk of Cataract: The Melbourne Visual Impairment Project. Investig. Opthalmology Vis. Sci. 2006, 47, 3783. [Google Scholar] [CrossRef]
- Moeller, S.M. Associations Between Age-Related Nuclear Cataract and Lutein and Zeaxanthin in the Diet and Serum in the Carotenoids in the Age-Related Eye Disease Study (CAREDS), an Ancillary Study of the Women’s Health Initiative. Arch. Ophthalmol. 2008, 126, 354. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Yu, R.B.; Liu, R.; Hao, Z.X.; Han, C.C.; Zhu, Z.H.; Ma, L. Association between Lutein and Zeaxanthin Status and the Risk of Cataract: A Meta-Analysis. Nutrients 2014, 6, 452–465. [Google Scholar] [CrossRef]
- Chew, E.Y.; SanGiovanni, J.P.; Ferris, F.L.; Wong, W.T.; Agron, E.; Clemons, T.E.; Sperduto, R.; Danis, R.; Chandra, S.R.; Blodi, B.A.; et al. Lutein/zeaxanthin for the treatment of age-related cataract: AREDS2 randomized trial report no. 4. JAMA Ophthalmol. 2013, 131, 843–850. [Google Scholar] [CrossRef]
- Zetterberg, M.; Montan, P.; Kugelberg, M.; Nilsson, I.; Lundström, M.; Behndig, A. Cataract Surgery Volumes and Complications per Surgeon and Clinical Unit: Data from the Swedish National Cataract Register 2007 to 2016. Ophthalmology 2020, 127, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.; Duan, P.; Xie, J.; Gao, H.; Chen, M.; Gong, Y.; Li, J.; Xu, H. Recent progress and research trend of anti-cataract pharmacology therapy: A bibliometric analysis and literature review. Eur. J. Pharmacol. 2022, 934, 175299. [Google Scholar] [CrossRef]
- Babizhayev, M.A.; Yermakova, V.N.; Semiletov, Y.A.; Deyev, A.I. The natural histidine-containing dipeptide Nalpha-acetylcarnosine as an antioxidant for ophthalmic use. Biochem. Biokhimiia 2000, 65, 588–598. [Google Scholar]
- Williams, D.L.; Munday, P. The effect of a topical antioxidant formulation including N-acetyl carnosine on canine cataract: A preliminary study. Vet. Ophthalmol. 2006, 9, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Babizhayev, M.A. Rejuvenation of visual functions in older adult drivers and drivers with cataract during a short-term administration of N-acetylcarnosine lubricant eye drops. Rejuvenation Res. 2004, 7, 186–198. [Google Scholar] [CrossRef]
- Martis, R.M.; Grey, A.C.; Wu, H.; Wall, G.M.; Donaldson, P.J.; Lim, J.C. N-Acetylcysteine amide (NACA) and diNACA inhibit H(2)O(2)-induced cataract formation ex vivo in pig and rat lenses. Exp. Eye Res. 2023, 234, 109610. [Google Scholar] [CrossRef]
- Maddirala, Y.; Tobwala, S.; Karacal, H.; Ercal, N. Prevention and reversal of selenite-induced cataracts by N-acetylcysteine amide in Wistar rats. BMC Ophthalmol. 2017, 17, 54. [Google Scholar] [CrossRef]
- Higashi, Y.; Higashi, K.; Mori, A.; Sakamoto, K.; Ishii, K.; Nakahara, T. Anti-cataract Effect of Resveratrol in High-Glucose-Treated Streptozotocin-Induced Diabetic Rats. Biol. Pharm. Bull. 2018, 41, 1586–1592. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Bodakhe, S.H. Resveratrol delay the cataract formation against naphthalene-induced experimental cataract in the albino rats. J. Biochem. Mol. Toxicol. 2020, 34, e22420. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Yao, Z.; He, Z. Resveratrol protects against high glucose-induced oxidative damage in human lens epithelial cells by activating autophagy. Exp. Ther. Med. 2021, 21, 440. [Google Scholar] [CrossRef]
- Machado, N.D.; Gutiérrez, G.; Matos, M.; Fernández, M.A. Preservation of the Antioxidant Capacity of Resveratrol via Encapsulation in Niosomes. Foods 2021, 10, 988. [Google Scholar] [CrossRef]
- Hu, N.; Jin, F.Y.; Gao, M.M.; Liu, L.J.; Wang, J.H.; Yang, B.F.; Li, C.L. Baicalein improves Na2SeO3 induced cataract by enhancing the antioxidant capacity of juvenile Sprague Dawley Rat. J. Ethnopharmacol. 2024, 320, 117433. [Google Scholar] [CrossRef]
- Chen, M.; Fu, Y.; Wang, X.; Wu, R.; Su, D.; Zhou, N.; Qi, Y. Metformin protects lens epithelial cells against senescence in a naturally aged mouse model. Cell Death Discov. 2022, 8, 8. [Google Scholar] [CrossRef]
- March, M.E.; Gutierrez-Uzquiza, A.; Snorradottir, A.O.; Matsuoka, L.S.; Balvis, N.F.; Gestsson, T.; Nguyen, K.; Sleiman, P.M.A.; Kao, C.; Isaksson, H.J.; et al. NAC blocks Cystatin C amyloid complex aggregation in a cell system and in skin of HCCAA patients. Nat. Commun. 2021, 12, 1827. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liu, Y.; Ge, J.; Wang, X.; Liu, L.; Bu, Z.; Liu, P. Resveratrol protects human lens epithelial cells against H2O2-induced oxidative stress by increasing catalase, SOD-1, and HO-1 expression. Mol. Vis. 2010, 16, 1467–1474. [Google Scholar]
- Hu, Z.; Guan, Y.; Hu, W.; Xu, Z.; Ishfaq, M. An overview of pharmacological activities of baicalin and its aglycone baicalein: New insights into molecular mechanisms and signaling pathways. Iran. J. Basic. Med. Sci. 2022, 25, 14–26. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, C.; Zhou, N.; Wang, X.; Su, D.; Qi, Y. Metformin alleviates oxidative stress-induced senescence of human lens epithelial cells via AMPK activation and autophagic flux restoration. J. Cell Mol. Med. 2021, 25, 8376–8389. [Google Scholar] [CrossRef]
- Goutham, G.; Manikandan, R.; Beulaja, M.; Thiagarajan, R.; Arulvasu, C.; Arumugam, M.; Setzer, W.N.; Daglia, M.; Nabavi, S.M. A focus on resveratrol and ocular problems, especially cataract: From chemistry to medical uses and clinical relevance. Biomed. Pharmacother. 2017, 86, 232–241. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, R.; Hu, H.; Peng, T. Biosynthesis, characterization and cytotoxicity of gold nanoparticles and their loading with N-acetylcarnosine for cataract treatment. J. Photochem. Photobiol. B. 2018, 187, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Vora, D.; Heruye, S.; Kumari, D.; Opere, C.; Chauhan, H. Preparation, Characterization and Antioxidant Evaluation of Poorly Soluble Polyphenol-Loaded Nanoparticles for Cataract Treatment. AAPS PharmSciTech 2019, 20, 163. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhao, Z.; Ma, H.; Liu, Y.; Nwafor, E.-O.; Zhu, S.; Jia, L.; Pang, X.; Han, Z.; Tian, B.; et al. Optimization and Characterization of Low-Molecular-Weight Chitosan-Coated Baicalin mPEG-PLGA Nanoparticles for the Treatment of Cataract. Mol. Pharm. 2022, 19, 3831–3845. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, L.; Li, S.; Li, S.; Zhao, M.; Zhou, Q.; Gong, X.; Yang, J.; Chang, J. Autoregenerative redox nanoparticles as an antioxidant and glycation inhibitor for palliation of diabetic cataracts. Nanoscale 2019, 11, 13126–13138. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Cha, M.-Y.; Kim, D.; Kim, D.K.; Soh, M.; Shin, K.; Hyeon, T.; Mook-Jung, I. Mitochondria-Targeting Ceria Nanoparticles as Antioxidants for Alzheimer’s Disease. ACS Nano. 2016, 10, 2860–2870. [Google Scholar] [CrossRef]
- Maccarone, R.; Tisi, A.; Passacantando, M.; Ciancaglini, M. Ophthalmic Applications of Cerium Oxide Nanoparticles. J. Ocul. Pharmacol. Ther. 2020, 36, 376–383. [Google Scholar] [CrossRef]
- Milazzo, S.; Grenot, M.; Benzerroug, M. La cataracte secondaire. J. Fr. Ophtalmol. 2014, 37, 825–830. [Google Scholar] [CrossRef]
- Da, W.J.; Shang, Z.J.; Xia, L.X.; Jie, W.K.; Meng, L.; Yan, M.Y.; Hua, W.X. Knockout of TGF-β receptor II by CRISPR/Cas9 delays mesenchymal transition of Lens epithelium and posterior capsule opacification. Int. J. Biol. Macromol. 2024, 259, 129290. [Google Scholar] [CrossRef]
- Kubo, E.; Shibata, T.; Singh, D.P.; Sasaki, H. Roles of TGF β and FGF Signals in the Lens: Tropomyosin Regulation for Posterior Capsule Opacity. Int. J. Mol. Sci. 2018, 19, 3093. [Google Scholar] [CrossRef]
- Thompson, B.; Davidson, E.A.; Chen, Y.; Orlicky, D.J.; Thompson, D.C.; Vasiliou, V. Oxidative stress induces inflammation of lens cells and triggers immune surveillance of ocular tissues. Chem. Biol. Interact. 2022, 355, 109804. [Google Scholar] [CrossRef] [PubMed]
- Malecaze, F.; Decha, A.; Serre, B.; Penary, M.; Duboue, M.; Berg, D.; Levade, T.; Lubsen, N.H.; Kremer, E.J.; Couderc, B. Prevention of posterior capsule opacification by the induction of therapeutic apoptosis of residual lens cells. Gene Ther. 2006, 13, 440–448. [Google Scholar] [CrossRef]
- Malecaze, F.; Lubsen, N.H.; Serre, B.; Decha, A.; Duboue, M.; Penary, M.; Berg, D.; Arnaud, J.-D.; Titeux, M.; Kremer, E.J.; et al. Lens cell targetting for gene therapy of prevention of posterior capsule opacification. Gene Ther. 2006, 13, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.X.; Lu, Y.; Liu, T.J.; Yang, J.; Chen, Y.; Fang, Y.W. Using HSV-TK/GCV suicide gene therapy to inhibit lens epithelial cell proliferation for treatment of posterior capsular opacification. Mol. Vis. 2011, 17, 291–299. [Google Scholar] [PubMed]
- Huang, W.R.; Fan, X.X.; Tang, X. SiRNA targeting EGFR effectively prevents posterior capsular opacification after cataract surgery. Mol. Vis. 2011, 17, 2349–2355. [Google Scholar]
- Zhang, R.; Wei, Y.-H.; Zhao, C.-Y.; Song, H.-Y.; Shen, N.; Cui, X.; Gao, X.; Qi, Z.-T.; Zhong, M.; Shen, W. EDIL3 depletion suppress epithelial-mesenchymal transition of lens epithelial cells via transforming growth factor β pathway. Int. J. Ophthalmol. 2018, 11, 18–24. [Google Scholar] [CrossRef]
- Zheng, D.; Song, T.; Zhongliu, X.; Wu, M.; Liang, J.; Liu, Y. Downregulation of transforming growth factor-β type II receptor prohibit epithelial-to-mesenchymal transition in lens epithelium. Mol. Vis. 2012, 18, 1238–1246. [Google Scholar]
- Li, P.; Jing, J.; Hu, J.; Li, T.; Sun, Y.; Guan, H. RNA Interference Targeting Snail Inhibits the Transforming Growth Factor β 2-Induced Epithelial-Mesenchymal Transition in Human Lens Epithelial Cells. J. Ophthalmol. 2013, 2013, 869101. [Google Scholar] [CrossRef]
- Sun, J.; Xie, L.; Wang, Y.; Liu, T. Inhibition of human lens epithelial B-3 cell proliferation by adenovirus-mediated transfer of antisense c-myc construct. Graefes Arch. Clin. Exp. Ophthalmol. 2005, 243, 601–606. [Google Scholar] [CrossRef]
- Couderc, B.C.; de Neuville, S.; Douin-Echinard, V.; Serres, B.; Manenti, S.; Darbon, J.-M.; Malecaze, F. Retrovirus-mediated transfer of a suicide gene into lens epithelial cells in vitro and in an experimental model of posterior capsule opacification. Curr. Eye Res. 1999, 19, 472–482. [Google Scholar] [CrossRef]
- Malecaze, F.; Couderc, B.; De Neuville, S.; Serres, B.; Mallet, J.; Douin-Echinard, V.; Manenti, S.; Revah, F.; Darbon, J.-M. Adenovirus-Mediated Suicide Gene Transduction: Feasibility in Lens Epithelium and in Prevention of Posterior Capsule Opacification in Rabbits. Hum. Gene Ther. 1999, 10, 2365–2372. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.-P.; Zhang, S.-B.; Wang, F.; Liu, H.; Zhang, W.; Song, B.; Liu, Z.-Y.; Xiong, L.; Fan, Y.-Z.; Liao, D.-Y. Effects of lentiviral RNA interference-mediated downregulation of integrin-linked kinase on biological behaviors of human lens epithelial cells. Int. J. Ophthalmol. 2016, 9, 21–28. [Google Scholar] [CrossRef]
- Drag, S.; Dotiwala, F.; Upadhyay, A.K. Gene Therapy for Retinal Degenerative Diseases: Progress, Challenges, and Future Directions. Investig. Opthalmology Vis. Sci. 2023, 64, 39. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Cheng, J.; Lu, C.; Li, X.; Li, F.; Liu, C.; Zhang, M.; Zhu, S.; Ma, X. A Novel Mutation in the Connexin 50 Gene (GJA8) Associated with Autosomal Dominant Congenital Nuclear Cataract in a Chinese Family. Curr. Eye Res. 2010, 35, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Li, X.; Yang, J. Mutations of CX46/CX50 and Cataract Development. Front. Mol. Biosci. 2022, 9, 842399. [Google Scholar] [CrossRef]
- Ma, B.; Jing, R.; Liu, J.; Yang, L.; Li, J.; Qin, L.; Cui, L.; Pei, C. CTGF Contributes to the Development of Posterior Capsule Opacification: An in vitro and in vivo study. Int. J. Biol. Sci. 2018, 14, 437–448. [Google Scholar] [CrossRef]
- Guo, M.; Su, F.; Chen, Y.; Su, B. Interfering Hsa_circRNA_0060640 Suppresses TGF-β2-Induced Proliferation, Motility and EMT in Human Lens Epithelium Cells by Targeting miR-214-3p and Collagen Type I alpha2 Chain. Curr. Eye Res. 2022, 47, 735–746. [Google Scholar] [CrossRef]
| Treatment | Structure and Description | Implications for Cataract Treatment | References |
|---|---|---|---|
| N-acetylcarnosine | Prodrug of l-carnosine. | Reduces lens opacification in canine cataracts, NACS eyedrops improve visual acuity and glare sensitivity in humans with cataracts. | [107,108,109] |
| N-acetylcysteine amide | Analog of NAC, a glutathione prodrug.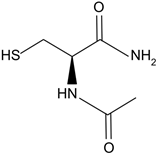 | NACA intraperitoneal injection prevents sodium selenite-induced cataract formation in rats, NACA eye drops reverse sodium selenite-induced cataract grade in rats, NACA and diNACA reduce H2O2-induced lens opacity in pig and rat lenses, with NACA increasing antioxidant levels as well. | [110,111] |
| diNACA | Analog of NACA. | ||
| Resveratrol | Polyphenolic phytoalexin produced in plants, trans isomer is more bioactive.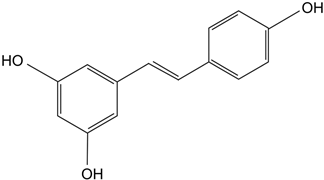 | Delays diabetic cataract formation in rats, mitigates oxygen-mediated protein oxidation in diabetic rats, protects human lens epithelial cells against oxidative damage, increases antioxidant levels and delays lenticular opacity in rats with naphthalene-induced cataracts. | [112,113,114,115] |
| Baicalein | Antioxidant flavonoid.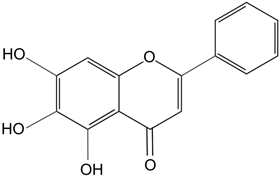 | In rats with sodium-selenite induced cataracts, it decreases dense opacity of the lens, increases soluble protein content, reduces oxidative stress, and prevents damage of lens epithelial cells. | [116] |
| Metformin | 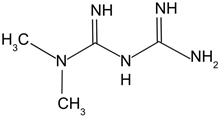 | Chronic low dose of metformin in mice significantly decreased lens opacity and lens epithelial cell senescence, which increasing autophagy. | [117] |
| Drug | Nanotechnology Used | Outcomes | Reference |
|---|---|---|---|
| NACS | Encapsulated NACS into gold nanoparticles. | Attenuated NACS toxicity at high concentrations, increased biocompatibility and bioavailability. | [123] |
| Resveratrol | Encapsulated resveratrol into lipid cyclodextrin-based nanoparticles. | Increased levels of antioxidant markers in bovine lens cultures to a higher degree than resveratrol alone. | [124] |
| Resveratrol | Encapsulated resveratrol into niosomes. | Maintained antioxidant capacity of resveratrol, prevented light irradiation-induced isomer conversion of resveratrol to its less bioactive cis isomer. | [115] |
| Baicalin | Encapsulated baicalin into chitosan-coated mPEG-PLGA nanoparticles. | Increased cellular uptake of baicalin, increased corneal retention of baicalin in rabbits, increased antioxidant levels and decreased oxidative stress markers in rabbits with selenite-induced cataract to a greater degree than baicalin alone. | [125] |
| CeO2 | Encapsulated CeO2 in PEG-PLGA coated nanoparticles. | Allowed for water soluble formation of CeO2 suitable for biological use. Decreased peroxide and superoxide concentrations in lens epithelial cell cultures. Reduced oxidative stress markers, increased antioxidant levels, and attenuated cataract development in rats with diabetic cataracts. | [126] |
| Gene(s) of Interest | Outcomes | Reference |
|---|---|---|
| Suicide Gene Therapy | ||
| Procaspase 3 or Bax | Overexpression of pro-apoptotic molecules was successfully targeted to rabbit residual lens epithelial cells, and sufficiently prevented PCO in rabbits. | [133,134] |
| HSV-tk (plus treatment with GNV) | HSV-tk was successfully expressed in HLECs and, when treated with GNV, was able to cause cell death. | [135] |
| RNA Interference | ||
| EGF | siRNA successfully inhibited cell proliferation of HLECs and significantly reduced PCO in a rat model. | [136] |
| EDIL3 | Knockdown significantly reduced HLEC proliferation and migration in vitro. | [137] |
| TGF-βRII | RNAi significantly reduced LEC migration. | [138] |
| Snail | siRNA successfully inhibited TGF-βII-mediated EMT of human epithelial cells. | [139] |
| ILK | shRNA significantly decreased migration, increased apoptosis, and caused arresting of cells at G1/S transition. | [138] |
| CRISPR-Cas9 | ||
| TGF-βRII | TGF-βRII knockout caused significant decrease in PCO incidence for rabbit PCO model, as well as significant decreased in in vitro HLEC proliferation. | [130] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulbay, M.; Wu, K.Y.; Nirwal, G.K.; Bélanger, P.; Tran, S.D. Oxidative Stress and Cataract Formation: Evaluating the Efficacy of Antioxidant Therapies. Biomolecules 2024, 14, 1055. https://doi.org/10.3390/biom14091055
Kulbay M, Wu KY, Nirwal GK, Bélanger P, Tran SD. Oxidative Stress and Cataract Formation: Evaluating the Efficacy of Antioxidant Therapies. Biomolecules. 2024; 14(9):1055. https://doi.org/10.3390/biom14091055
Chicago/Turabian StyleKulbay, Merve, Kevin Y. Wu, Gurleen K. Nirwal, Paul Bélanger, and Simon D. Tran. 2024. "Oxidative Stress and Cataract Formation: Evaluating the Efficacy of Antioxidant Therapies" Biomolecules 14, no. 9: 1055. https://doi.org/10.3390/biom14091055






