Insight into the Association between Slitrk Protein and Neurodevelopmental and Neuropsychiatric Conditions
Abstract
:1. Introduction
2. LRR-Domain Protein Family
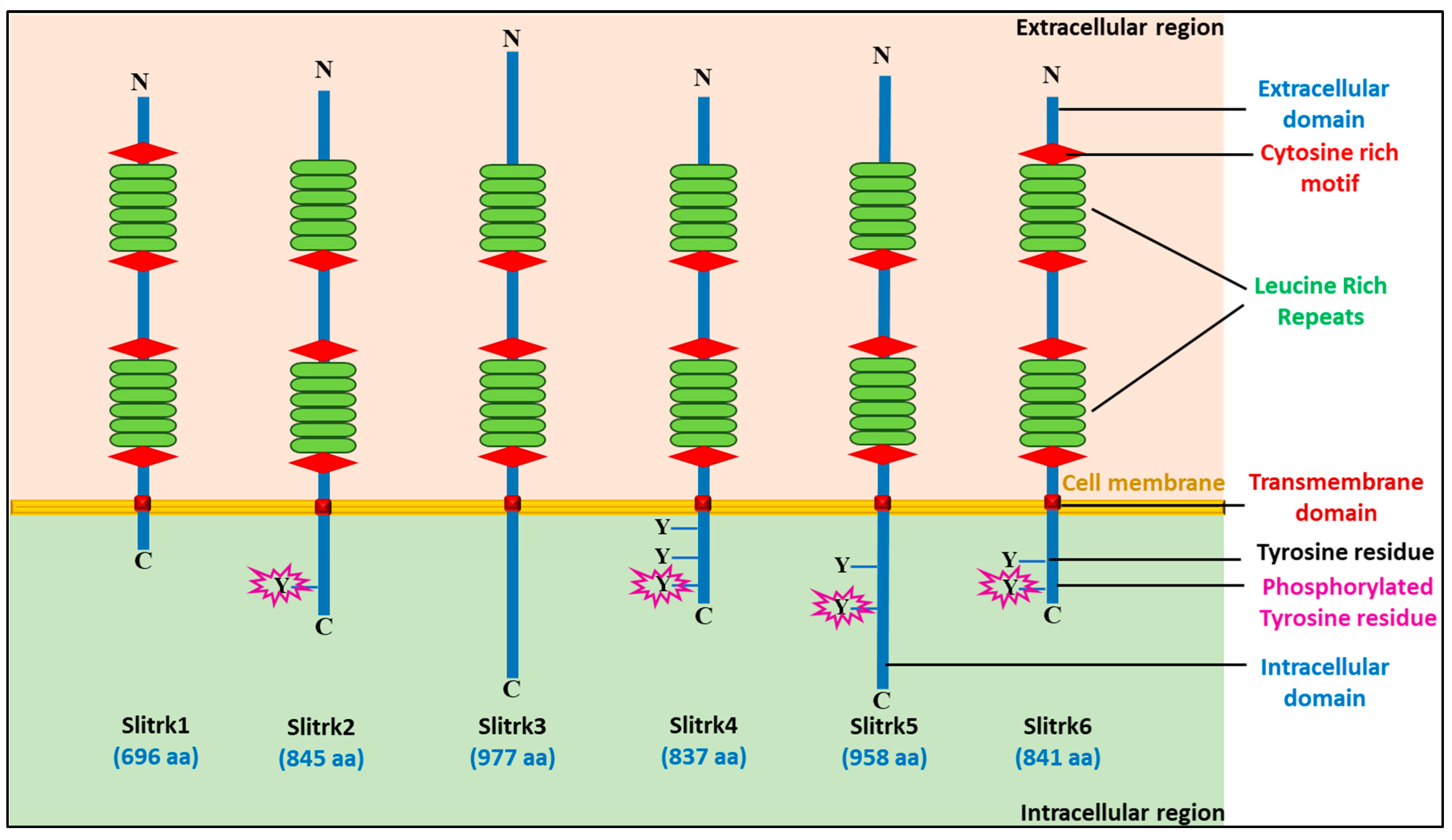
3. Structural Organization of SLITRK Proteins
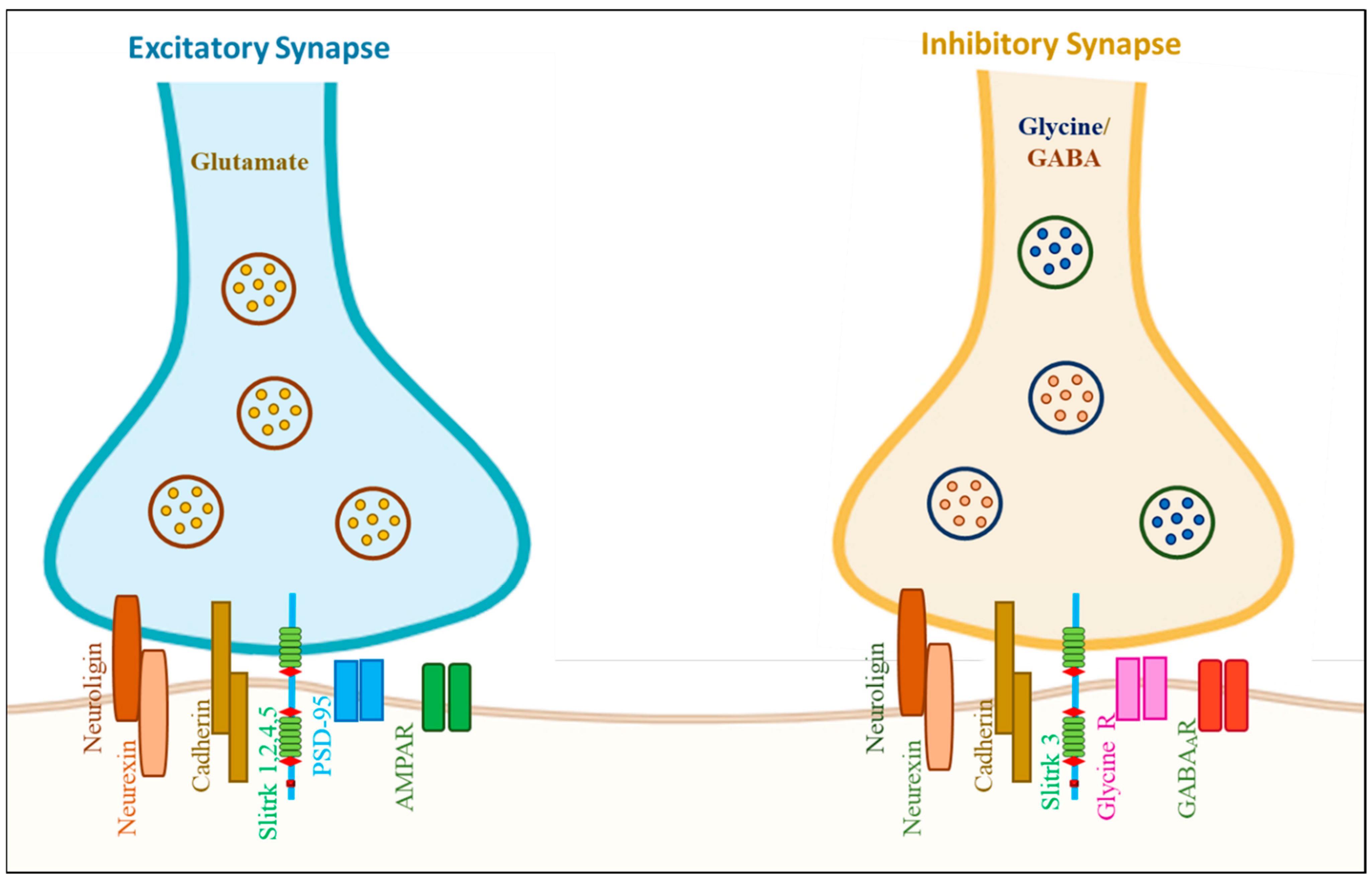
4. Slitrk and Neuro-Disease
4.1. Slitrk1
4.2. Slitrk2
4.3. Slitrk3
4.4. Slitrk4
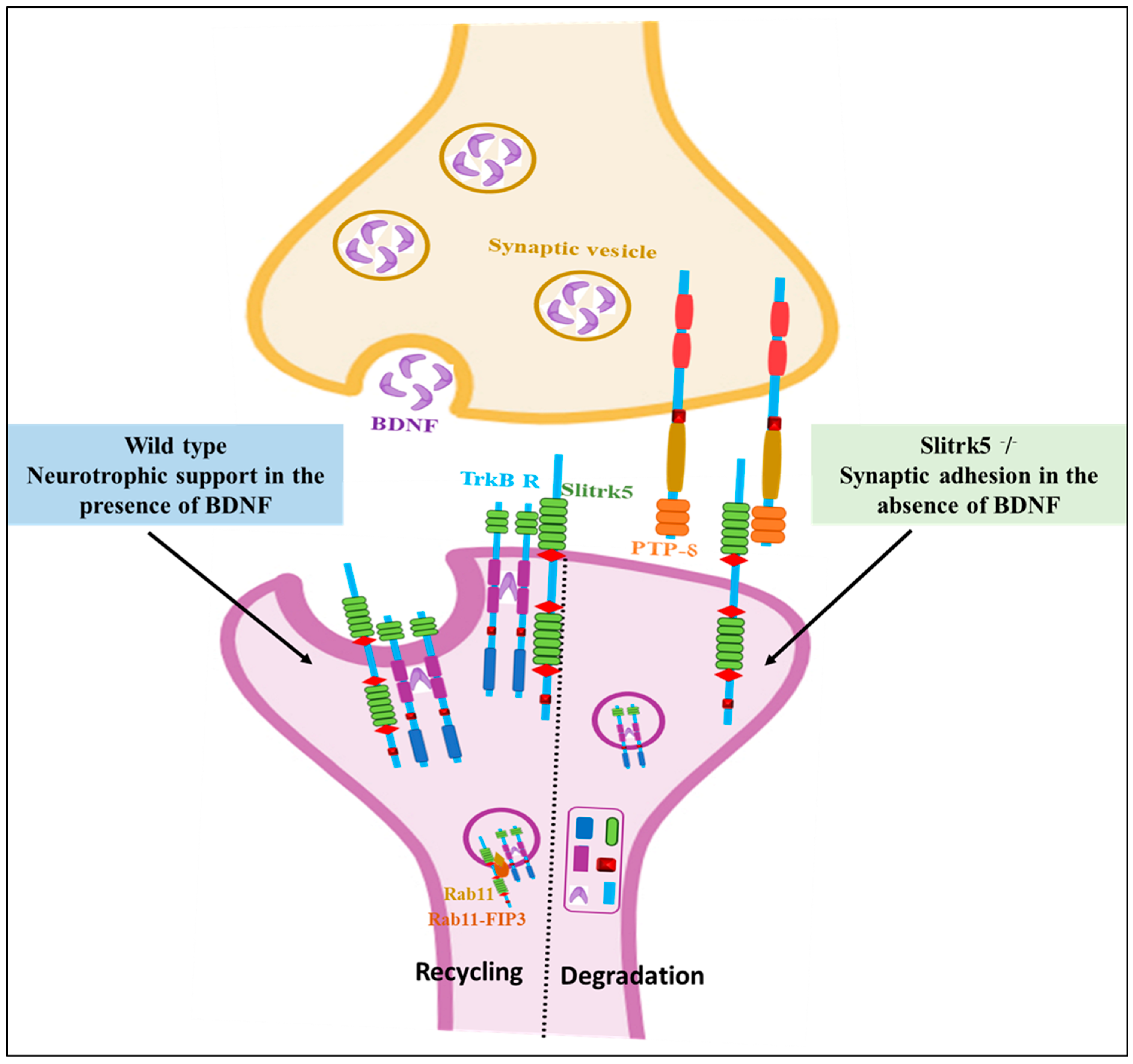
4.5. Slitrk5
4.6. Slitrk6
5. Slitrk Protein-Related Study in Humans and Other Experimental Models
5.1. In Humans
5.2. In Mice
5.3. In Zebrafish
5.4. In Pigs
6. Slitrk and Other Diseases
7. Expert Comment and Future Prospects
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Hu, W.F.; Chahrour, M.H.; Walsh, C.A. The Diverse Genetic Landscape of Neurodevelopmental Disorders. Annu. Rev. Genom. Hum. Genet. 2014, 15, 195–213. [Google Scholar] [CrossRef]
- Parenti, I.; Rabaneda, L.G.; Schoen, H.; Novarino, G. Neurodevelopmental Disorders: From Genetics to Functional Pathways. Trends Neurosci. 2020, 43, 608–621. [Google Scholar] [CrossRef] [PubMed]
- Bray, N.J.; O’Donovan, M.C. The genetics of neuropsychiatric disorders. Brain Neurosci. Adv. 2018, 2, 239821281879927. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, K.J. The genetics of neurodevelopmental disease. Curr. Opin. Neurobiol. 2011, 21, 197–203. [Google Scholar] [CrossRef]
- Walhovd, K.B.; Krogsrud, S.K.; Amlien, I.K.; Bartsch, H.; Bjørnerud, A.; Due-Tønnessen, P.; Grydeland, H.; Hagler, D.J.; Håberg, A.K.; Kremen, W.S.; et al. Neurodevelopmental origins of lifespan changes in brain and cognition. Proc. Natl. Acad. Sci. USA 2016, 113, 9357–9362. [Google Scholar] [CrossRef] [PubMed]
- Michetti, C.; Falace, A.; Benfenati, F.; Fassio, A. Synaptic genes and neurodevelopmental disorders: From molecular mechanisms to developmental strategies of behavioral testing. Neurobiol. Dis. 2022, 173, 105856. [Google Scholar] [CrossRef]
- Lee, K.-M.; Hwang, S.-K.; Lee, J.-A. Neuronal autophagy and neurodevelopmental disorders. Exp. Neurobiol. 2013, 22, 133–142. [Google Scholar] [CrossRef]
- Takumi, T.; Tamada, K. CNV biology in neurodevelopmental disorders. Curr. Opin. Neurobiol. 2018, 48, 183–192. [Google Scholar] [CrossRef]
- Guazzini, A.; Gursesli, M.C.; Serritella, E.; Tani, M.; Duradoni, M. Obsessive-Compulsive Disorder (OCD) Types and Social Media: Are Social Media Important and Impactful for OCD People? Eur. J. Investig. Health Psychol. Educ. 2022, 12, 1108–1120. [Google Scholar] [CrossRef]
- Pedley, R.; Bee, P.; Wearden, A.; Berry, K. Illness perceptions in people with obsessive-compulsive disorder; A qualitative study. PLoS ONE 2019, 14, e0213495. [Google Scholar] [CrossRef]
- Lu, W.; Jiang, J.; Wang, S.; Lu, W.; Jiang, J.; Wang, S. Progress on the Pathogenesis and Treatment of OCD, AD and Depression. J. Biosci. Med. 2022, 10, 224–241. [Google Scholar] [CrossRef]
- Suzuki, K. Neuropathology of developmental abnormalities. Brain Dev. 2007, 29, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Batool, S.; Raza, H.; Zaidi, J.; Riaz, S.; Hasan, S.; Syed, N.I. Synapse formation: From cellular and molecular mechanisms to neurodevelopmental and neurodegenerative disorders. J. Neurophysiol. 2019, 121, 1381–1397. [Google Scholar] [CrossRef]
- Südhof, T.C. Towards an Understanding of Synapse Formation. Neuron 2018, 100, 276–293. [Google Scholar] [CrossRef]
- Kobe, B.; Kajava, A. The leucine-rich repeat as a protein recognition motif. Curr. Opin. Struct. Biol. 2001, 11, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, N.; Takatsuka, S.; Miyashita, H.; Kretsinger, R.H. Leucine Rich Repeat Proteins: Sequences, Mutations, Structures and Diseases. Protein Pept. Lett. 2018, 26, 108–131. [Google Scholar] [CrossRef]
- Ko, J. The leucine-rich repeat superfamily of synaptic adhesion molecules: LRRTMs and Slitrks. Mol. Cells 2012, 34, 335–340. [Google Scholar] [CrossRef]
- De Wit, J.; Ghosh, A. Control of neural circuit formation by leucine-rich repeat proteins. Trends Neurosci. 2014, 37, 539–550. [Google Scholar] [CrossRef]
- De Wit, J.; Hong, W.; Luo, L.; Ghosh, A. Role of leucine-rich repeat proteins in the development and function of neural circuits. Annu. Rev. Cell Dev. Biol. 2011, 27, 697–729. [Google Scholar] [CrossRef]
- Kluss, J.H.; Bonet-Ponce, L.; Lewis, P.A.; Cookson, M.R. Directing LRRK2 to membranes of the endolysosomal pathway triggers RAB phosphorylation and JIP4 recruitment. Neurobiol. Dis. 2022, 170, 105769. [Google Scholar] [CrossRef]
- Winther, M.; Walmod, P.S. Neural cell adhesion molecules belonging to the family of leucine-rich repeat proteins. Adv. Neurobiol. 2014, 8, 315–395. [Google Scholar] [CrossRef]
- O’Sullivan, M.L.; de Wit, J.; Savas, J.N.; Comoletti, D.; Otto-Hitt, S.; Yates, J.R., 3rd; Ghosh, A. FLRT proteins are endogenous latrophilin ligands and regulate excitatory synapse development. Neuron 2012, 73, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Arstikaitis, P.; Prasad, T.; Bartlett, T.E.; Wang, Y.T.; Murphy, T.H.; Craig, A.M. Postsynaptic TrkC and presynaptic PTPσ function as a bidirectional excitatory synaptic organizing complex. Neuron 2011, 69, 287–303. [Google Scholar] [CrossRef]
- Hondermarck, H.; Demont, Y.; Bradshaw, R.A. The TrK Receptor Family. Receptor Tyrosine Kinases: Family and Subfamilies; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 777–820. [Google Scholar] [CrossRef]
- Woo, J.; Kwon, S.K.; Kim, E. The NGL family of leucinerich repeat-containing synaptic adhesion molecules. Mol. Cell Neurosci. 2009, 42, 1–10. [Google Scholar] [CrossRef]
- Kim, S.; Burette, A.; Chung, H.S.; Kwon, S.-K.; Woo, J.; Lee, H.W.; Kim, K.; Kim, H.; Weinberg, R.J.; Kim, E. NGL family of PSD-95-interacting adhesion molecules regulate excitatory synapse formation. Nat. Neurosci. 2006, 9, 1294–1301. [Google Scholar] [CrossRef]
- Schroeder, A.; De Wit, J. Leucine-rich repeat-containing synaptic adhesion molecules as organizers of synaptic specificity and diversity. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mah, W.; Ko, J.; Nam, J.; Han, K.; Chung, W.S.; Kim, E. Selected SALM (synaptic adhesion-like molecule) family proteins regulate synapse formation. J. Neurosci. 2010, 30, 5559–5568. [Google Scholar] [CrossRef]
- Kim, M.M.; Kim, J.S.; Moon, S.M.; Choi, M.S.; Park, B.R.; Lee, D.S.; Mo, S.-Y.; Cho, S.-H.; Kim, C.S. Regulation of SLITRK1 gene by neuron restrictive silencer factor in NMB cells. Oral Biol. Res. 2013, 37, 88–97. [Google Scholar]
- Proenca, C.C.; Gao, K.P.; Shmelkov, S.V.; Rafii, S.; Lee, F.S. Slitrks as emerging candidate genes involved in neuropsychiatric disorders. Trends Neurosci. 2011, 34, 143–153. [Google Scholar] [CrossRef]
- Aruga, J.; Mikoshiba, K. Identification and characterization of Slitrk, a novel neuronal transmembrane protein family controlling neurite outgrowth. Mol. Cell Neurosci. 2003, 24, 117–129. [Google Scholar] [CrossRef]
- Round, J.; Ross, B.; Angel, M.; Shields, K.; Lom, B. Slitrk gene duplication and expression in the developing zebrafish nervous system. Dev. Dyn. 2014, 243, 339–349. [Google Scholar] [CrossRef]
- Yim, Y.S.; Kwon, Y.; Nam, J.; Yoon, H.I.; Lee, K.; Kim, D.G.; Kim, E.; Kim, C.H.; Ko, J. Slitrks control excitatory and inhibitory synapse formation with LAR receptor protein tyrosine phosphatases. Proc. Natl. Acad. Sci. USA 2013, 110, 4057–4062. [Google Scholar] [CrossRef] [PubMed]
- Salesse, C.; Charest, J.; Doucet-Beaupré, H.; Castonguay, A.-M.; Labrecque, S.; De Koninck, P.; Lévesque, M. Opposite Control of Excitatory and Inhibitory Synapse Formation by Slitrk2 and Slitrk5 on Dopamine Neurons Modulates Hyperactivity Behavior. Cell Rep. 2020, 30, 2374–2386.e5. [Google Scholar] [CrossRef]
- Kang, H.; Han, K.A.; Won, S.Y.; Kim, H.M.; Lee, Y.-H.; Ko, J.; Um, J.W. Slitrk Missense Mutations Associated with Neuropsychiatric Disorders Distinctively Impair Slitrk Trafficking and Synapse Formation. Front. Mol. Neurosci. 2016, 9, 104. [Google Scholar] [CrossRef]
- Taoufik, E.; Kouroupi, G.; Zygogianni, O.; Matsas, R. Synaptic dysfunction in neurodegenerative and neurodevelopmental diseases: An overview of induced pluripotent stem-cell-based disease models. Open Biol. 2018, 8, 180138. [Google Scholar] [CrossRef]
- Ozomaro, U.; Cai, G.; Kajiwara, Y.; Yoon, S.; Makarov, V.; Delorme, R.; Betancur, C.; Ruhrmann, S.; Falkai, P.; Grabe, H.J.; et al. Characterization of SLITRK1 variation in obsessive-compulsive disorder. PLoS ONE 2013, 8, e70376. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Liu, J.; Song, Q.; Zhang, Q.; Tian, P.; Nan, Z. Comprehensive analysis of codon usage bias in seven Epichloë species and their peramine-coding genes. Front. Microbiol. 2017, 8, 1419. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Le, W.D.; Xie, W.J.; Jankovic, J. Examination of the SLITRK1 gene in Caucasian patients with Tourette syndrome. Acta Neurol. Scand 2006, 114, 400–402. [Google Scholar] [CrossRef]
- Scharf, J.M.; Moorjani, P.; Fagerness, J.; Platko, J.V.; Illmann, C.; Galloway, B.; Jenike, E.; Stewart, S.E.; Pauls, D.L.; The Tourette Syndrome International Consortium for Genetics. Lack of association between SLITRK1var321 and Tourette syndrome in a large family-based sample. Neurology 2008, 70, 1495–1496. [Google Scholar] [CrossRef]
- El Chehadeh, S.; Han, K.A.; Kim, D.; Jang, G.; Bakhtiari, S.; Lim, D.; Kim, H.Y.; Kim, J.; Kim, H.; Wynn, J.; et al. SLITRK2 variants associated with neurodevelopmental disorders impair excitatory synaptic function and cognition in mice. Nat. Commun. 2022, 13, 4112. [Google Scholar] [CrossRef]
- Gerges, P.; Bitar, T.; Laumonnier, F.; Marouillat, S.; Nemer, G.; Andres, C.R.; Hleihel, W. Identification of Novel Gene Variants for Autism Spectrum Disorders in the Lebanese Population Using Whole-Exome Sequencing. Genes 2022, 13, 186. [Google Scholar] [CrossRef] [PubMed]
- Zimprich, A.; Hatala, K.; Riederer, F.; Stogmann, E.; Aschauer, H.N.; Stamenkovic, M. Sequence analysis of the complete SLITRK1 gene in Austrian patients with Tourette’s disorder. Psychiatr. Genet. 2008, 18, 308–309. [Google Scholar] [CrossRef] [PubMed]
- Depienne, C.; Ciura, S.; Trouillard, O.; Bouteiller, D.; Leitao, E.; Nava, C.; Keren, B.; Marie, Y.; Guegan, J.; Forlani, S.; et al. Association of Rare Genetic Variants in Opioid Receptors with Tourette Syndrome. Tremor Other Hyperkinetic Mov. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Kajiwara, Y.; Buxbaum, J.D.; Grice, D.E. SLITRK1 binds 14-3-3 and regulates neurite outgrowth in a phosphorylation-dependent manner. Biol. Psychiatry 2009, 66, 918–925. [Google Scholar] [CrossRef]
- Zuchner, S.; Cuccaro, M.L.; Tran-Viet, K.N.; Cope, H.; Krishnan, R.R.; A Pericak-Vance, M.; Wright, H.H.; Ashley-Koch, A. SLITRK1 mutations in trichotillomania. Mol. Psychiatry 2006, 11, 887. [Google Scholar] [CrossRef]
- Stillman, A.A.; Krsnik, Ž.; Sun, J.; Rašin, M.; State, M.W.; Šestan, N.; Louvi, A. Developmentally regulated and evolutionarily conserved expression of SLITRK1 in brain circuits implicated in Tourette syndrome. J. Comp. Neurol. 2009, 513, 21–37. [Google Scholar] [CrossRef]
- Beaubien, F.; Raja, R.; Kennedy, T.E.; Fournier, A.E.; Cloutier, J.F. Slitrk1 is localized to excitatory synapses and promotes their development. Sci. Rep. 2016, 6, 27343. [Google Scholar] [CrossRef]
- Miranda, D.M.; Wigg, K.; Kabia, E.M.; Feng, Y.; Sandor, P.; Barr, C.L. Association of SLITRK1 to Gilles de la Tourette Syndrome. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2009, 150, 483–486. [Google Scholar] [CrossRef]
- Katayama, K.; Yamada, K.; Ornthanalai, V.; Inoue, T.; Ota, M. Slitrk1-deficient mice display elevated anxiety-like behavior and noradrenergic abnormalities. Mol. Psychiatry 2010, 15, 177–184. [Google Scholar] [CrossRef]
- Melo-Felippe, F.B.; Fontenelle, L.F.; Kohlrausch, F.B. Gene variations in PBX1, LMX1A and SLITRK1 are associated with obsessive-compulsive disorder and its clinical features. J. Clin. Neurosci. 2019, 61, 180–185. [Google Scholar] [CrossRef]
- Hatayama, M.; Aruga, J. Developmental control of noradrenergic system by SLITRK1 and its implications in the pathophysiology of neuropsychiatric disorders. Front. Mol. Neurosci. 2023, 15, 1080739. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, A.; Sato, Y.; Goto-Ito, S.; Uemura, T.; Maeda, A.; Shiroshima, T.; Yoshida, T.; Fukai, S. Structure of Slitrk2-PTPδ complex reveals mechanisms for splicing-dependent trans-synaptic adhesion. Sci. Rep. 2015, 5, 9686. [Google Scholar] [CrossRef]
- Loomis, C.; Stephens, A.; Janicot, R.; Baqai, U.; Drebushenko, L.; Round, J. Identification of MAGUK scaffold proteins as intracellular binding partners of synaptic adhesion protein Slitrk2. Mol. Cell. Neurosci. 2020, 103, 103465. [Google Scholar] [CrossRef] [PubMed]
- Han, K.A.; Kim, J.; Kim, H.; Kim, D.; Lim, D.; Ko, J.; Um, J.W. Slitrk2 controls excitatory synapse development via PDZ-mediated protein interactions. Sci. Rep. 2019, 9, 17094. [Google Scholar] [CrossRef]
- Katayama, K.-I.; Morimura, N.; Kobayashi, K.; Corbett, D.; Okamoto, T.; Ornthanalai, V.G.; Matsunaga, H.; Fujita, W.; Matsumoto, Y.; Akagi, T.; et al. Slitrk2 deficiency causes hyperactivity with altered vestibular function and serotonergic dysregulation. iScience 2022, 25, 104604. [Google Scholar] [CrossRef] [PubMed]
- Won, S.Y.; Lee, P.; Kim, H.M. Synaptic organizer: Slitrks and type IIa receptor protein tyrosine phosphatases. Curr. Opin. Struct. Biol. 2019, 54, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Katayama, K.-I.; Sohya, K.; Miyamoto, H.; Prasad, T.; Matsumoto, Y.; Ota, M.; Yasuda, H.; Tsumoto, T.; Aruga, J.; et al. Selective control of inhibitory synapse development by Slitrk3-PTPδ trans-synaptic interaction. Nat. Neurosci. 2012, 15, 389–398. [Google Scholar] [CrossRef]
- Li, J.; Han, W.; Pelkey, K.A.; Duan, J.; Mao, X.; Wang, Y.-X.; Craig, M.T.; Dong, L.; Petralia, R.S.; McBain, C.J.; et al. Molecular Dissection of Neuroligin 2 and Slitrk3 Reveals an Essential Framework for GABAergic Synapse Development. Neuron 2017, 96, 808–826.e8. [Google Scholar] [CrossRef]
- Efthymiou, S.; Han, W.; Ilyas, M.; Li, J.; Yu, Y.; Scala, M.; Malintan, N.T.; Ilyas, M.; Vavouraki, N.; Mankad, K.; et al. Human mutations in SLITRK3 implicated in GABAergic synapse development in mice. bioRxiv 2022. [Google Scholar] [CrossRef]
- Marteyn, A.; Maury, Y.; Gauthier, M.M.; Lecuyer, C.; Vernet, R.; Denis, J.A.; Pietu, G.; Peschanski, M.; Martinat, C. Mutant human embryonic stem cells reveal neurite and synapse formation defects in type 1 myotonic dystrophy. Cell Stem Cell 2011, 8, 434–444. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Mei, R.; Ai, M.; Pang, R.; Xia, D.; Chen, L.; Zhong, L. The Role of SliTrk5 in Central Nervous System. BioMed Res. Int. 2022, 2022, 4678026. [Google Scholar] [CrossRef]
- Song, M.; Giza, J.; Proenca, C.C.; Jing, D.; Elliott, M.; Dincheva, I.; Shmelkov, S.V.; Kim, J.; Schreiner, R.; Huang, S.-H.; et al. Slitrk5 Mediates BDNF-Dependent TrkB Receptor Trafficking and Signaling. Dev. Cell 2015, 33, 690–702. [Google Scholar] [CrossRef]
- Song, M.; Mathews, C.A.; Stewart, S.E.; Shmelkov, S.V.; Mezey, J.G.; Rodriguez-Flores, J.L.; Rasmussen, S.A.; Britton, J.C.; Oh, Y.-S.; Walkup, J.T.; et al. Rare Synaptogenesis-Impairing Mutations in SLITRK5 Are Associated with Obsessive Compulsive Disorder. PLoS ONE 2017, 12, e0169994. [Google Scholar] [CrossRef] [PubMed]
- Shmelkov, S.V.; Hormigo, A.; Jing, D.; Proenca, C.C.; Bath, K.G.; Milde, T.; Shmelkov, E.; Kushner, J.S.; Baljevic, M.; Dincheva, I.; et al. Slitrk5 deficiency impairs corticostriatal circuitry and leads to obsessive-compulsive-like behaviors in mice. Nat. Med. 2010, 16, 598–602. [Google Scholar] [CrossRef]
- Zhang, K.; Feng, Y.; Wigg, K.G.; Sandor, P.; Barr, C.L. Association study of the SLITRK5 gene and Tourette syndrome. Psychiatr. Genet. 2015, 25, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.A. Highly Expressed Genes within Hippocampal Sector CA1: Implications for the Physiology of Memory. Neurol. Int. 2014, 6, 32–35. [Google Scholar] [CrossRef]
- Aruga, J. Slitrk6 expression profile in the mouse embryo and its relationship to that of Nlrr3. Gene Expr. Patterns 2003, 3, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, X.; Deng, Z.; Li, G.; Zhang, R.; Yang, Z.; Che, F.; Liu, S.; Li, H. The role of SLITRK6 in the pathogenesis of Tourette syndrome: From the conclusion of a family-based study in the Chinese Han population. J. Gene Med. 2020, 22, e3173. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Katayama, K.-I.; Okamoto, T.; Yamada, K.; Takashima, N.; Nagao, S.; Aruga, J. Impaired auditory-vestibular functions and behavioral abnormalities of Slitrk6-deficient mice. PLoS ONE 2011, 6, e16497. [Google Scholar] [CrossRef]
- Katayama, K.-I.; Zine, A.; Ota, M.; Matsumoto, Y.; Inoue, T.; Fritzsch, B.; Aruga, J. Disorganized innervations and neuronal loss in the inner ear of Slitrk6-deficient mice. PLoS ONE 2009, 4, e7786. [Google Scholar] [CrossRef]
- Morlet, T.; Rabinowitz, M.R.; Looney, L.R.; Riegner, T.; Greenwood, L.A.; Sherman, E.A.; Achilly, N.; Zhu, A.; Yoo, E.; O’Reilly, R.C.; et al. A homozygous SLITRK6 nonsense mutation is associated with progressive auditory neuropathy in humans. Laryngoscope 2014, 124, E95–E103. [Google Scholar] [CrossRef] [PubMed]
- Abelson, J.F.; Kwan, K.Y.; O’Roak, B.J.; Baek, D.Y.; Stillman, A.A.; Morgan, T.M.; Mathews, C.A.; Pauls, D.L.; Rašin, M.-R.; Gunel, M.; et al. Sequence variants in SLITRK1 are associated with Tourette’s syndrome. Science 2005, 310, 317–320. [Google Scholar] [CrossRef]
- Wendland, J.R.; Kruse, M.R.; Murphy, D.L. Functional SLITRK1 var321, varCDfs and SLC6A4 G56A variants and susceptibility to obsessive-compulsive disorder. Mol. Psychiatry 2006, 11, 802–804. [Google Scholar] [CrossRef]
- Aruga, J.; Yokota, N.; Mikoshiba, K. Human SLITRK family genes: Genomic organization and expression profiling in normal brain and brain tumor tissue. Gene 2003, 315, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Beaubien, F.; Cloutier, J.F. Differential expression of Slitrk family members in the mouse nervous system. Dev. Dyn. 2009, 238, 3285–3296. [Google Scholar] [CrossRef]
- Larsen, K.; Momeni, J.; Farajzadeh, L.; Bendixen, C. Porcine SLITRK1: Molecular cloning and characterization. FEBS Open Bio 2014, 4, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Bollig-Fischer, A.; Bao, B.; Manning, M.; Dyson, G.; Michelhaugh, S.K.; Mittal, S.; Bepler, G.; Mamdani, H. Role of novel cancer gene SLITRK3 to activate NTRK3 in squamous cell lung cancer. Mol. Biomed. 2021, 2, 26. [Google Scholar] [CrossRef]
- Yamazaki, S.; Taoka, K.; Arai, S.; Miyauchi, M.; Kataoka, K.; Yoshimi, A.; Kurokawa, M. Patient-Derived Induced Pluripotent Stem Cells Identified SLITRK4 as a Causative Gene of Chronic Myelomonocytic Leukemia. Blood 2016, 128, 1134. [Google Scholar] [CrossRef]
- Zhou, Y.-Q.; Bao, T.-S.; Xie, J.-X.; Yao, L.-L.; Yu, S.-T.; Li, Q.; Huang, P.-Q.; Zhou, W.-Z.; Wang, Y.-Y.; Chen, S.-Y.; et al. The SLITRK4-CNPY3 axis promotes liver metastasis of gastric cancer by enhancing the endocytosis and recycling of TrkB in tumour cells. Cell. Oncol. 2023, 46, 1049–1067. [Google Scholar] [CrossRef]
- Biffani, S.; Morandi, N.; Locatelli, V.; Pravettoni, D.; Boccardo, A.; Stella, A.; Nicolazzi, E.L.; Biscarini, F. Adding evidence for a role of the SLITRK gene family in the pathogenesis of left displacement of the abomasum in Holstein-Friesian dairy cows. Livest Sci. 2014, 167, 104–109. [Google Scholar] [CrossRef]
- Heiliger, K.-J.; Hess, J.; Vitagliano, D.; Salerno, P.; Braselmann, H.; Salvatore, G.; Ugolini, C.; Summerer, I.; Bogdanova, T.; Unger, K.; et al. Novel candidate genes of thyroid tumourigenesis identified in Trk-T1 transgenic mice. Endocr. Relat. Cancer 2012, 19, 409–421. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Liu, Z.; Zhang, Y.; Ma, Y.; Bai, J.; Yao, H.; Wang, Y.; Zhao, X.; Li, R.; et al. Identification of SLITRK6 as a Novel Biomarker in hepatocellular carcinoma by comprehensive bioinformatic analysis. Biochem. Biophys. Rep. 2021, 28, 101157. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.A.; Pandith, A.A.; Mansoor, S.; Baba, S.M.; Makhdoomi, R.; Ain, Q.-U.; Anwar, I.; Para, S.A.; Bhat, A.H.; Koul, A.M.; et al. Differential expression of SLITRK6 gene as a potential therapeutic target for urothelial carcinoma in particular upper tract cancer. Gene 2023, 878, 147583. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Zhao, X.; Li, M.; Meng, M. SLITRK6 promotes the progression of lung adenocarcinoma by regulating PI3K/AKT/mTOR signaling and Warburg effect. Apoptosis 2023, 28, 1216–1225. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Shin, D.Y.; Eiseman, M.; Yallowitz, A.R.; Li, N.; Lalani, S.; Li, Z.; Cung, M.; Bok, S.; Debnath, S.; et al. SLITRK5 is a negative regulator of hedgehog signaling in osteoblasts. Nat. Commun. 2021, 12, 4611. [Google Scholar] [CrossRef]

| LRR Family Name | Family Members | LRR Domain | Expressed in Human Tissue/Organ | Function | Commonly Associated Disease | References |
|---|---|---|---|---|---|---|
| LRRTM Family | 4 (LRRTM1–4) | Ten LRR motifs | In entire brain | Formation of excitatory synapses; stimulate excitatory synaptogenesis | SZ, ASD, and AD | [21] |
| SLITRK Family | 6 (SLITRK1–6) | Six LRR motifs | Brain and other tissues too | Neuritogenesis | TS, OCD, and ADHD | [21] |
| FLRT (Fibronectin leucine-rich repeat) Family | 3 (FLRT1–3) | Ten LRR motifs | Many tissues, including the brain | Axon guidance and cell migration | ADHD | [22] |
| Trk (Tropomyosin receptor kinase) Family | 3 (TrkA-C) | Three LRRs | Widely expressed throughout the NS | Axonal and dendritic growth and remodeling, synapse maturation and plasticity | AD and PD | [23,24] |
| NGL (Netrin-G ligand; LRRC4) family | 3 (NGL1–3) | Nine LRRs flanked by cysteine-rich domain | Postsynaptic membrane | Regulate the formation of excitatory synapses and; the development of axons, dendrites, and synapses | PD | [25,26] |
| SALMs (Synaptic adhesion-like molecules) Family | 5 (SALM1–5) | Six LRRs motif | Majorly expressed in the brain and various brain regions | Regulate synapse formation | ASD | [27,28] |
| Protein Name (Uniport ID No.) | Gene Location | No of Amino Acid and MW | Protein 3D Structure | Type of PTM (Amino Acid Number) | Majorly Expressed in | Neurological Function | Associated Neurological Disease |
|---|---|---|---|---|---|---|---|
| Slitrk1 (Q96PX8) | Chromosome 13 (13q31.1) | 696 77.7 kDa |  | Phosphoserine (695) | Restricted to brain | Enhances the differentiation of excitatory synapses and is involved in synaptogenesis; Increases the development of neuronal dendrites | Neuropsychiatric disorder (OCD) |
| Slitrk2 (Q9H156) | Chromosome X (Xq27.3) | 845 95.4 kDa | 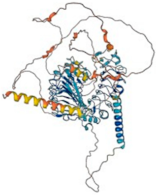 | N-linked-Glycosylation (84 and 421) Disulfide bond (220–243 and 222–263) Phosphotyrosine (756) | Brain and spleen | It contributes to excitatory synapse differentiation during synaptogenesis and inhibits neurite proliferation | Found in a patient with SZ (a serious mental disorder) |
| Slitrk3 (O94933) | Chromosome 3 (3q26.1) | 977 108.9 kDa | 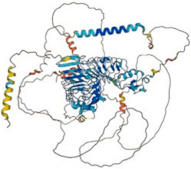 | N-linked-Glycosylation (68 and 596) | Brain and esophagus | Suppresses neurite outgrowth | NA |
| Slitrk4 (Q8IW52) | Chromosome X (Xq27.3) | 837 94.3 kDa |  | N-linked-Glycosylation (81 and 325) Phosphoserine (663 and 741) Phosphotyrosine (742, 769 and 811) | Adrenal gland and brain | It contributes to synapse differentiation and synaptogenesis, and it inhibits neurite outgrowth | Found in a patient with SZ |
| Slitrk5 (O94991) | Chromosome 13 (13q31.2) | 958 107.4 kDa |  | N-linked-Glycosylation (103 and 644) Phosphoserine (846) Phosphotyrosine (932 and 945) | Brain and thyroid gland | Suppresses neurite outgrowth | OCD, ADHD, glioma, ASDs, PD |
| Slitrk6 (Q9H5Y7) | Chromosome 13 (13q31.1) | 841 95.1 kDa | 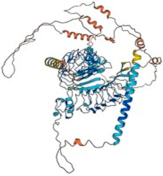 | Phosphoserine (652 and 728) Phosphotyrosine (820 and 833) | Brain, urinary bladder, and salivary gland | Regulator of neurite outgrowth | Hearing loss; associated with high myopia |
| Protein | Interaction with Slitrk Members | Other Interactive Proteins | Function in Neuron |
|---|---|---|---|
| PTPRD | Slitrk 1–6 | PPFIA1, PPFIA2, PPFIA3 IL1RAP and IL1RAPL1 | Induce both pre- and postsynaptic differentiation of neurons; by mediating interaction with transsynaptically |
| PTPRS | Slitrk 1,2,3 and 5 | NTRK3, NTRK1, PPFIA1, PPFIA2 and PPFIA3 | Inhibition of neurite and axonal outgrowth; essential for normal brain development |
| DLGAP3 | Slitrk 1 and 5 | DLG4/PSD-95 | Molecular organization of synapses and neuronal cell signaling |
| PTPRF | Slitrk 1 and 2 | GRIP1, PPFIA1, PPFIA2 and PPFIA3 | Involved in presynaptic differentiation |
| IL1RAPL1 | Slitrk 1,2 and 3 | NCS1, PTPRD | Neurite outgrowth |
| NTRK3 | Slitrk 1 and 3 | PTPRS, QSTM1 and KIDINS220 | Involved in the NS development |
| NLGN1 | Slitrk 1,2 and 3 | NRXN1, NRXN2 and NRXN3 | Synapse function and signal transmission |
| NRXN2 | Slitrk3 | NLGN1, NLGN2 and NLGN3 | Involved in cell recognition and cell adhesion |
| Slitrk Member | Approach | Mutation Type and Amino Acid Substitution | Associated Disease | Summary | References |
|---|---|---|---|---|---|
| Slitrk1 Slitrk4 Slitrk2 | In silico study | Missense N400I, T418S Missense V206I, I578V Missense V89M | NSDs- OCD and SZ | Invitro study—impaired glycosylation of Slitrks, impaired trafficking in neurons; eliminated Slitrk binding to PTP⸹ leads to inhibition of their function on synapse density; impaired synapse formation in cultured neurons | [35] |
| Slitrk1 | Genome sequencing | Synonymous L63L Missense N400I and T418S | OCD | N400I mutation abolishes induced neurite outgrowth | [37] |
| Slitrk5 | Genome sequencing | Non-synonymous mutations N99K, Q118H, E609K, G722∆, A851V, and P891L | OCD | All mutations cause impaired synaptogenic activity in OCD | [38] |
| Slitrk1 | Genome sequencing | Deletion (frameshift mutation) var321 | TS | - | [39,40] |
| Slitrk2 | Genome sequencing | Missense mutation L74S, V201I, and E210K, P374R, R426C, R484Q, V511M, and E555D Nonsense mutation E461 | ID | In vitro study, variations inhibit the functional activity of Slitrk2 and reduce its binding to TrkB in neurons | [41] |
| Slitrk4 | Whole-exome sequencing | Missense mutation c.1860A>C p.Leu620Phe | ASD | Potential ASD candidate genes | [42] |
| Slitrk1 | Sequence analysis | Missense mutation 383g>a | TS | Slitrk1 associated with TS pathophysiology | [43] |
| Slitrk6 | Microarray and pyrosequencing | c.1232C>G, p.Thr411Arg | TS | Alter the opioid pathway | [44] |
| Slitrk | Disease | Model | Target Gene | Method | Outcome | Reference |
|---|---|---|---|---|---|---|
| Slitrk1 | TS | Human clinical sample | Slitrk1 var321 | Var321 genotyping | Slitrk1 var321 might be associated with TS; however, it is not associated with either TS or OCD within this sample | [40] |
| Slitrk1 | TS | TS patient | Slitrk1 mRNA and hsa-miR-189 | DNA mapping | Frameshift mutation (deletion) resulted in a truncated protein that led to TS | [73] |
| Slitrk1 | OCD | Human | 322 OCD probands for Slitrk1 var321 and varCDfs | Genotyping assays | var321, varCDfs, and SLC6A4 G56A have shown no direct connection with OCD | [74] |
| Slitrk1 | OCD | OCD patient | Slitrk1 gene mutation | Genetic screening | A synonymous L63L change and a missense mutation T418S were identified in OCD individual | [37] |
| Slitrk2 | ADHD | Slitrk2-KO mice | Slitrk2 null mutation | Electrophysiological analysis and behavioral study | Shows hyperactivity with altered vestibular function and serotonergic dysregulation | [56] |
| Slitrk2 and Slitrk5 | ADHD | Lmx1a/b mice | Lmx1a/b target genes | Electrophysiological analysis and behavioral study | Mutation or Slitrk2 KO causes hyperactivity behavior | [34] |
| Slitrk4 | ASD | 23 male Lebanese ASD subjects | Single nucleotide variations in the Slitrk4 gene | Whole-Exome Sequencing | p.Leu620Phe variation in Slitrk4 is as potential ASD candidate genes | [42] |
| Slitrk4 | DM1 | Neural cells and DM1 brain biopsies | Slitrk4 gene | RT-PCR (gene expression) | Downregulation of Slitrk4 leads to change in neurite outgrowth and synaptogenesis | [61] |
| Slitrk5 | TS | 377 affected children | Slitrk5 gene | SNP | No direct correlation between TS and Slitrk5 | [66] |
| Slitrk5 | TLE and | TLE patients and epilepsy rat model | Slitrk5 Gene and protein | Gene expression | Change in Slitrk5 expression leads to epilepsy | [62] |
| Slitrk5 | OCD | OCD mouse | Slitrk5 gene | Behavioral study | This shows up as heightened anxiety-like behaviors and obsessive self-care | [65] |
| Slitrk5 | OCD | Slitrk5-KO mice | Slitrk5 gene | Genomes database-based study and functional study in mice | Mutations in the coding gene of Slitrk5 associated with OCD | [38] |
| Slitrk6 | TS | Human samples | Slitrk6 gene | SNP | No statistically significant allele transfer | [69] |
| Slitrk6 | Ear development | Slitrk6-KO mice | mRNA profiling and Histological examination | Associated with auditory and vestibular sensory organ functions in experimental mice | Slitrk6 regulates the production of tropic factors, such as neurotrophins responsible for the survival of inner ear sensory neurons | [71] |
| Slitrk6 | Auditory-Vestibular functioning | Slitrk6-KO mice | Auditory function and vertical vestibular function test | Systematic behavioral and auditory-vestibular functioning | Head-dip behavior increased and no other clear abnormalities were noted | [70] |
| Slitrk6 | Myopia | 9 patients with myopia and Slitrk6−/−) mice | Slitrk6 | SNP, genotyping, and genetic mapping and Auditory and Vestibular Testing | Slitrk6 homozygote c.1240C>T High myopia, cochlear dysfunction, and progressive auditory neuropathy | [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puranik, N.; Song, M. Insight into the Association between Slitrk Protein and Neurodevelopmental and Neuropsychiatric Conditions. Biomolecules 2024, 14, 1060. https://doi.org/10.3390/biom14091060
Puranik N, Song M. Insight into the Association between Slitrk Protein and Neurodevelopmental and Neuropsychiatric Conditions. Biomolecules. 2024; 14(9):1060. https://doi.org/10.3390/biom14091060
Chicago/Turabian StylePuranik, Nidhi, and Minseok Song. 2024. "Insight into the Association between Slitrk Protein and Neurodevelopmental and Neuropsychiatric Conditions" Biomolecules 14, no. 9: 1060. https://doi.org/10.3390/biom14091060







