Cannabis sativa L. Extract Alleviates Neuropathic Pain and Modulates CB1 and CB2 Receptor Expression in Rat
Abstract
1. Introduction
2. Methods
2.1. Cannabis sativa L. (CSL) Extract Preparation
2.2. Characteristics of the CSL Extracts
| Component | CBD-A | CBD | Δ9-THC-A | Δ9-THC |
| Concentration [mg/g] | 1.2 | 215.2 | 0.15 | 13.3 |
| Component | CBD-A | CBD | Δ9-THC-A | Δ9-THC |
| Concentration [mg/g] | 1.2 | 220.2 | 0.1 | 15.5 |
2.3. Cannabinoid Identification and Quantification
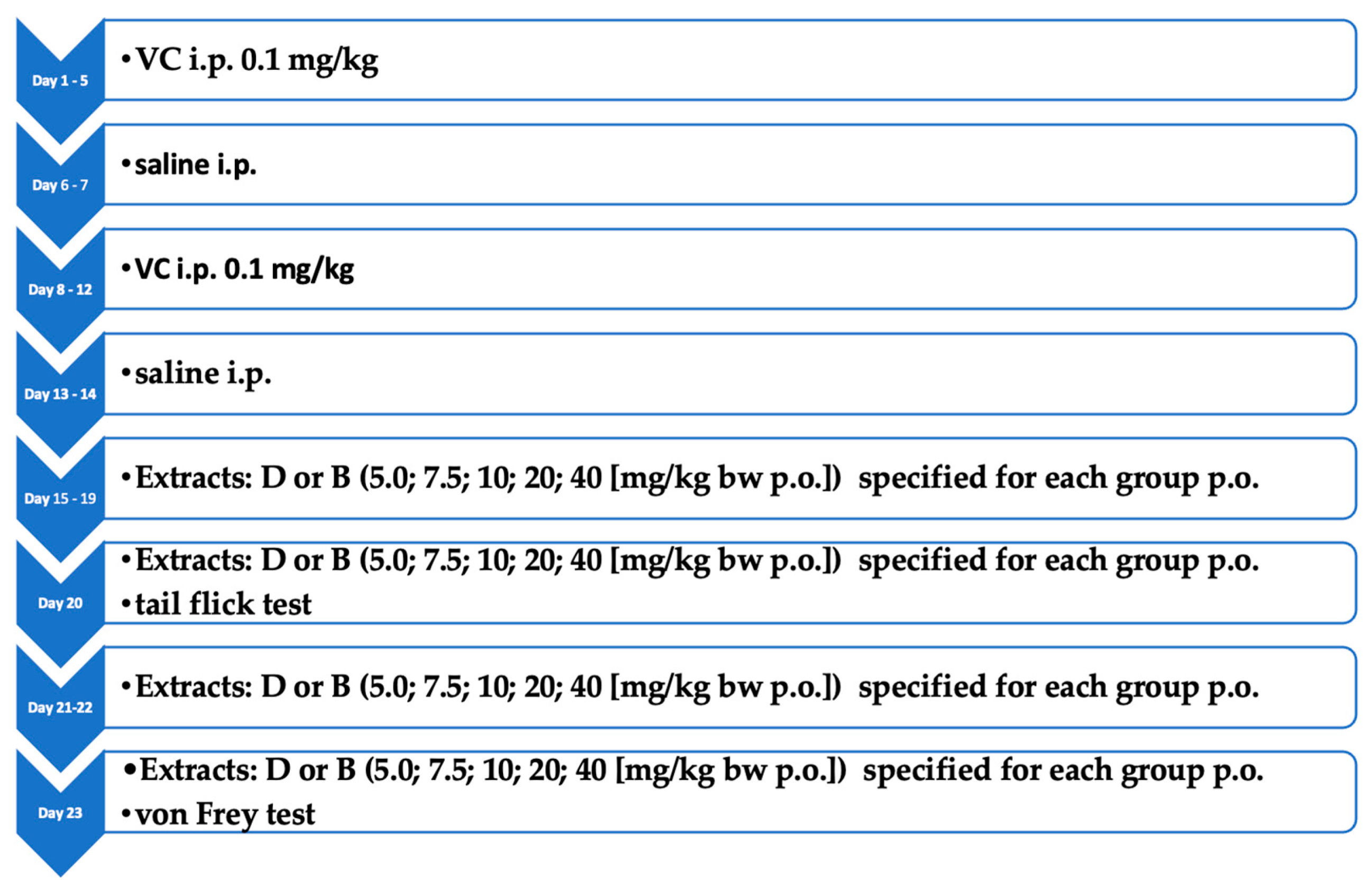
2.4. Animal Study
2.4.1. Induction of Neuropathic Pain
2.4.2. Tail-Flick Test
2.4.3. The von Frey Test
2.5. Real-Time PCR
2.6. CNRI and CNR2 ELISA
2.7. Statistical Analysis
3. Results
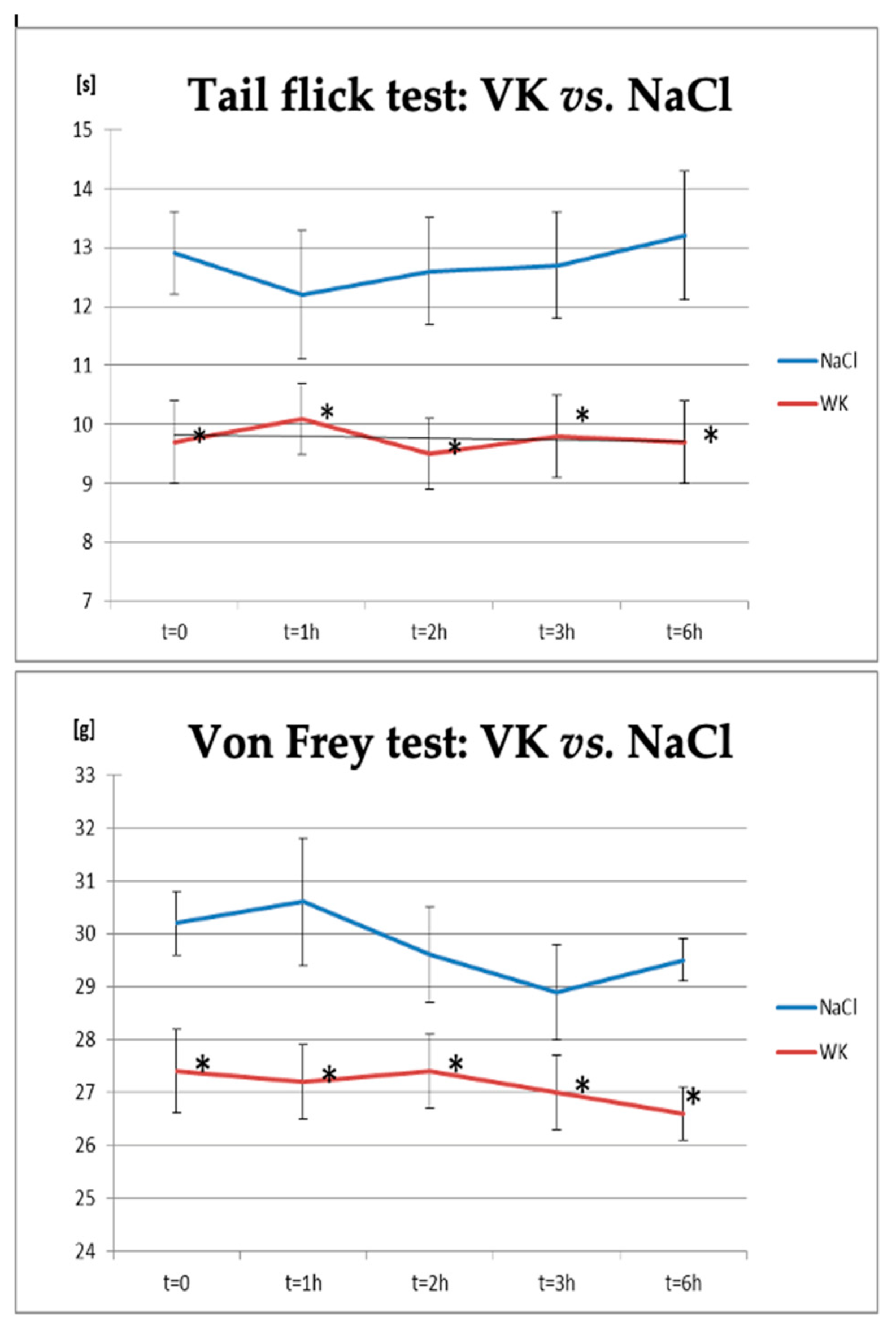
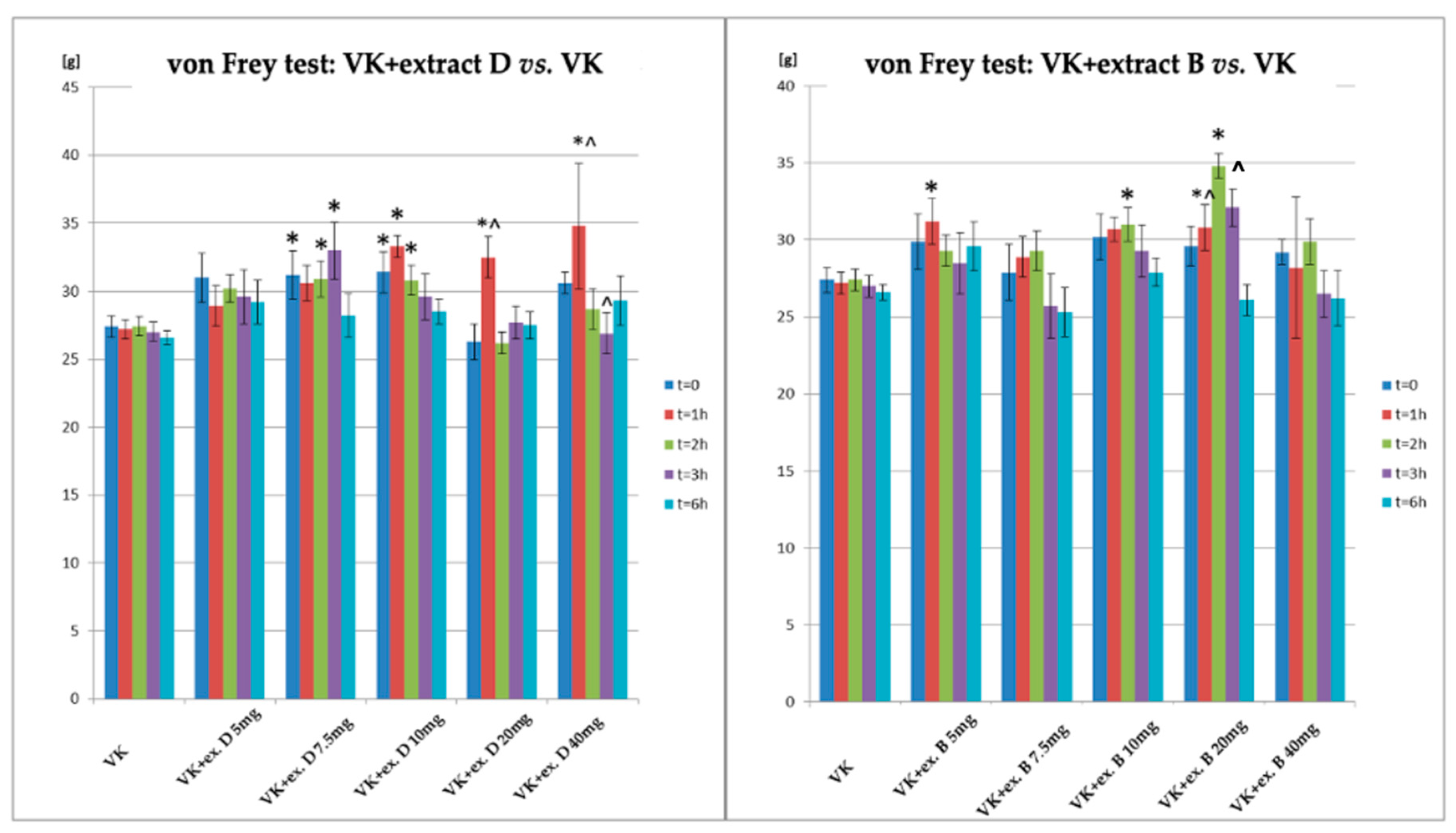
3.1. Neuropathic Pain Induction and Treatment
3.2. The Influence of Neuropathic Pain Induction and Treatment on CB1R and CB2R Protein and mRNA Expression
3.2.1. Vincristine and Gabapentin Treatment on CB1R and CB2R Expression
3.2.2. CB1R Expression in the Rat Brain and Blood Lymphocytes
3.3. CB2R Expression in the Rat Brain and Blood Lymphocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CBD | Cannabidiol |
| CBD-A | Cannabinoid acid |
| CB1R | Cannabinoid receptor 1 |
| CNRI | Cannabinoid receptor 1 gene |
| CB2R | Cannabinoid receptor 2 |
| CNR2 | Cannabinoid receptor 2gene |
| RT-PCR | Real-Time PCR reaction |
| Δ9-THC | THC-Δ9-Tetrahydrocannabinol, Tetrahydrocannabinol |
| Δ9-THC-A | Δ9-Tetrahydrocannabinoid acid |
References
- Finnerup, N.B.; Haroutounian, S.; Kamerman, P.; Baron, R.; Bennett, D.L.; Bouhassira, D.; Cruccu, G.; Freeman, R.; Hansson, P.; Nurmikko, T.; et al. Neuropathic pain: An updated grading system for research and clinical practice. Pain 2016, 157, 1599–1606. [Google Scholar] [CrossRef]
- Cohen, S.P.; Mao, J. Neuropathic pain: Mechanisms and their clinical implications. BMJ 2014, 348, f7656. [Google Scholar] [CrossRef] [PubMed]
- Colvin, L.; Dougherty, P. Peripheral neuropathic pain: Signs, symptoms, mechanisms, and causes: Are they linked? Br. J. Anaesth. 2015, 114, 361–363. [Google Scholar] [CrossRef] [PubMed]
- Ellis, A.; Bennett, D.H.L. Neuroinflammation and the generation of neuropathic pain. Br. J. Anaesth. 2013, 111, 26–37. [Google Scholar] [CrossRef]
- Harriott, B.S.; Gold, M.S. Contribution of primary afferent channels to neuropathic pain. Curr. Pain Headache Rep. 2009, 13, 197–207. [Google Scholar] [CrossRef]
- Paul, J.A.; Moalem-Taylor, G. The neuro-immune balance in neuropathic pain: Involvement of inflammatory immune cells, immune-like glial cells and cytokines. J. Neuroimmunol. 2010, 229, 26–50. [Google Scholar]
- Moore, A.; Derry, S.; Wiffen, P. Gabapentin for Chronic Neuropathic Pain. JAMA 2018, 319, 818–819. [Google Scholar] [CrossRef]
- Lafaye, G.; Karila, L.; Blecha, L.; Benyamina, A. Cannabis, cannabinoids, and health. Dialog. Clin. Neurosci. 2017, 19, 309–316. [Google Scholar] [CrossRef]
- ElSohly, M.A.; Mehmedic, Z.; Foster, S.; Gon, C.; Chandra, S.; Church, J.C. Changes in Cannabis Potency Over the Last 2 Decades (1995–2014): Analysis of Current Data in the United States. Biol. Psychiatry 2016, 79, 613–619. [Google Scholar] [CrossRef]
- Hange, N.; Poudel, S.; Ozair, S.; Paul, T.; Nambakkam, M.; Shrestha, R.; Greye, F.; Shah, S.; Adhikari, Y.R.; Thapa, S.; et al. Managing Chronic Neuropathic Pain: Recent Advances and New Challenges. Neurol. Res. Int. 2022, 2022, 1–14. [Google Scholar] [CrossRef]
- Taneja, A.; Della Pasqua, O.; Danhof, M. Challenges in translational drug research in neuropathic and inflammatory pain: The prerequisites for a new paradigm. Eur. J. Clin. Pharmacol. 2017, 73, 1219–1236. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.E.B.; Ouyang, L.; Kandasamy, R. Antinociceptive effects of minor cannabinoids, terpenes and flavonoids in Cannabis. Behav. Pharmacol. 2021, 33, 130–157. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.K.-S.; Rice, A.S.; Arendt-Nielsen, L. The use of cannabidiol (CBD) as an analgesic component. Lancet Reg. Health Eur. 2023, 35, 100791. [Google Scholar] [CrossRef]
- Rahn, E.J.; Hohmann, A.G. Cannabinoids as Pharmacotherapies for Neuropathic Pain: From the Bench to the Bedside. Neurotherapeutics 2009, 6, 713–737. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Chang, M.C. Effect of Repetitive Transcranial Magnetic Stimulation on Pain Management: A Systematic Narrative Review. Front. Neurol. 2020, 11, 114. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Mizrahi, D.; Goldstein, D.; Kiernan, M.C.; Park, S.B. Chemotherapy and peripheral neuropathy. Neurol. Sci. 2021, 42, 4109–4121. [Google Scholar] [CrossRef]
- Liampas, A.; Rekatsina, M.; Vadalouca, A.; Paladini, A.; Varrassi, G.; Zis, P. Pharmacological Management of Painful Peripheral Neuropathies: A Systematic Review. Pain Ther. 2021, 10, 55–68. [Google Scholar] [CrossRef]
- Rekatsina, M.; Paladini, A.; Piroli, A.; Zis, P.; Pergolizzi, J.V.; Varrassi, G. Pathophysiologic Approach to Pain Therapy for Complex Pain Entities: A Narrative Review. Pain Ther. 2020, 9, 7–21. [Google Scholar] [CrossRef]
- Monti, M.C.; Frei, P.; Weber, S.; Scheurer, E.; Mercer-Chalmers-Bender, K. Beyond Δ9-tetrahydrocannabinol and cannabidiol: Chemical differentiation of cannabis varieties applying targeted and untargeted analysis. Anal. Bioanal. Chem. 2022, 414, 3847–3862. [Google Scholar] [CrossRef]
- Salehi, A.; Puchalski, K.; Shokoohinia, Y.; Zolfaghari, B.; Asgary, S. Differentiating Cannabis Products: Drugs, Food, and Supplements. Front. Pharmacol. 2022, 13, 906038. [Google Scholar] [CrossRef]
- Mackie, K. Distribution of Cannabinoid Receptors in the Central and Peripheral Nervous System. Handb. Exp. Pharmacol. 2005, 168, 299–325. [Google Scholar] [CrossRef]
- Clayton, N.; Marshall, F.H.; Bountra, C.; O’Shaughnessy, C.T. CB1 and CB2 cannabinoid receptors are implicated in inflammatory pain. Pain 2002, 96, 253–260. [Google Scholar] [CrossRef]
- Malan, P.T., Jr.; Ibrahim, M.M.; Deng, H.; Liu, Q.; Mata, H.P.; Vanderah, T.; Porreca, F.; Makriyannis, A. CB2 cannabinoid receptor-mediated peripheral antinociception. Pain 2001, 93, 239–245. [Google Scholar] [CrossRef]
- Hill, K.P.; Palastro, M.D.; Johnson, B.; Ditre, J.W. Cannabis and Pain: A Clinical Review. Cannabis Cannabinoid Res. 2017, 2, 96–104. [Google Scholar] [CrossRef]
- Bisogno, T.; Hanuš, L.; De Petrocellis, L.; Tchilibon, S.; Ponde, D.E.; Brandi, I.; Moriello, A.S.; Davis, J.B.; Mechoulam, R.; Di Marzo, V. Molecular targets for cannabidiol and its synthetic analogues: Effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol. 2001, 134, 845–852. [Google Scholar] [CrossRef]
- Starkus, J.; Jansen, C.; Shimoda, L.M.N.; Stokes, A.J.; Small-Howard, A.L.; Turner, H. Diverse TRPV1 responses to cannabinoids. Channels 2019, 13, 172–191. [Google Scholar] [CrossRef] [PubMed]
- Rice, A.S. Cannabinoids and pain. Curr. Opin. Investig. Drugs 2001, 2, 399–414. [Google Scholar] [PubMed]
- Bridges, D.; Ahmad, K.; Rice, A.S.C. The synthetic cannabinoid WIN55,212-2 attenuates hyperalgesia and allodynia in a rat model of neuropathic pain. Br. J. Pharmacol. 2001, 133, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Fox, A.; Kesingland, A.; Gentry, C.; McNair, K.; Patel, S.; Urban, L.; James, I. The role of central and peripheral Cannabinoid1 receptors in the antihyperalgesic activity of cannabinoids in a model of neuropathic pain. Pain 2001, 92, 91–100. [Google Scholar] [CrossRef]
- Hamann, W.; di Vadi, P. Analgesic effect of the cannabinoid analogue nabilone is not mediated by opioid receptors. Lancet 1999, 353, 560. [Google Scholar] [CrossRef]
- Herzberg, U.; Eliav, E.; Bennett, G.; Kopin, I.J. The analgesic effects of R(+)-WIN 55,212–2 mesylate, a high affinity cannabinoid agonist, in a rat model of neuropathic pain. Neurosci. Lett. 1997, 221, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Price, D.D.; Mayer, D.J. Mechanisms of hyperalgesian and morphine tolerance: A current view of their possible interactions. Pain 1995, 62, 259–274. [Google Scholar] [CrossRef]
- Szalata, M.; Dreger, M.; Zielińska, A.; Banach, J.; Szalata, M.; Wielgus, K. Simple Extraction of Cannabinoids from Female Inflorescences of Hemp (Cannabis sativa L.). Molecules 2022, 27, 5868. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; da Ana, R.; Fonseca, J.; Szalata, M.; Wielgus, K.; Fathi, F.; Oliveira, M.B.P.P.; Staszewski, R.; Karczewski, J.; Souto, E.B. Phytocannabinoids: Chromatographic Screening of Cannabinoids and Loading into Lipid Nanoparticles. Molecules 2023, 28, 2875. [Google Scholar] [CrossRef] [PubMed]
- Authier, N.; Gillet, J.-P.; Fialip, J.; Eschalier, A.; Coudore, F. A New Animal Model of Vincristine-Induced Nociceptive Peripheral Neuropathy. NeuroToxicology 2003, 24, 797–805. [Google Scholar] [CrossRef]
- Mulder, G.B.; Pritchett, K. Rodent analgesiometry: The hot plate, tail flick and Von Frey hairs. Contemp. Top Lab. Anim. Sci. 2004, 43, 54–55. [Google Scholar]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Piomelli, D.; Giuffrida, A.; Calignano, A.; de Fonseca, F.R. The endocannabinoid system as a target for therapeutic drugs. Trends Pharmacol. Sci. 2000, 21, 218–224. [Google Scholar] [CrossRef]
- Wood, J.N.; Abrahamsen, B.; Baker, M.D.; Boorman, J.D.; Donier, E.; Drew, L.J.; Nassar, M.A.; Okuse, K.; Seereeram, A.; Stirling, C.L.; et al. Ion channel activities implicated in pathological pain. Novartis Found. Symp. 2004, 261, 32–40. [Google Scholar]
- Hossain, M.Z.; Ando, H.; Unno, S.; Kitagawa, J. Targeting Peripherally Restricted Cannabinoid Receptor 1, Cannabinoid Receptor 2, and Endocannabinoid-Degrading Enzymes for the Treatment of Neuropathic Pain Including Neuropathic Orofacial Pain. Int. J. Mol. Sci. 2020, 21, 1423. [Google Scholar] [CrossRef]
- Segat, G.C.; Manjavachi, M.N.; Matias, D.O.; Passos, G.F.; Freitas, C.S.; Costa, R.; Calixto, J.B. Antiallodynic effect of β-caryophyllene on paclitaxel-induced peripheral neuropathy in mice. Neuropharmacology 2017, 125, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Elmes, S.J.R.; Jhaveri, M.D.; Smart, D.; Kendall, D.A.; Chapman, V. Cannabinoid CB2 receptor activation inhibits mechanically evoked responses of wide dynamic range dorsal horn neurons in naïve rats and in rat models of inflammatory and neuropathic pain. Eur. J. Neurosci. 2004, 20, 2311–2320. [Google Scholar] [CrossRef]
- Leichsenring, A.; Andriske, M.; Bäcker, I.; Stichel, C.C.; Lübbert, H. Analgesic and antiinflammatory effects of cannabinoid receptor agonists in a rat model of neuropathic pain. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2009, 379, 627–636. [Google Scholar] [CrossRef]
- Folkesson, A.; Honoré, P.H.; Bjerrum, O.J. Co-administered gabapentin and venlafaxine in nerve injured rats: Effect on mechanical hypersensitivity, motor function and pharmacokinetics. Scand. J. Pain 2010, 1, 91–97. [Google Scholar] [CrossRef]
- Mangaiarkkarasi, A.; Rameshkannan, S.; Ali, R.M. Effect of Gabapentin and Pregabalin in Rat Model of Taxol Induced Neuropathic Pain. J. Clin. Diagn. Res. 2015, 9, FF11–FF14. [Google Scholar] [CrossRef]
- Chiba, T.; Oka, Y.; Sashida, H.; Kanbe, T.; Abe, K.; Utsunomiya, I.; Taguchi, K. Vincristine-induced peripheral neuropathic pain and expression of transient receptor potential vanilloid 1 in rat. J. Pharmacol. Sci. 2017, 133, 254–260. [Google Scholar] [CrossRef]
- Ja’afer, F.M.H.; Hamdan, F.B.; Mohammed, F.H. Vincristine-induced neuropathy in rat: Electrophysiological and histological study. Exp. Brain Res. 2006, 173, 334–345. [Google Scholar] [CrossRef]
- Jaggi, A.S.; Singh, N. Mechanisms in cancer-chemotherapeutic drugs-induced peripheral neuropathy. Toxicology 2012, 291, 1–9. [Google Scholar] [CrossRef]
- Han, Y.; Smith, M.T. Pathobiology of cancer chemotherapy-induced peripheral neuropathy (CIPN). Front. Pharmacol. 2013, 4, 156. [Google Scholar] [CrossRef]
- Li, G.-Z.; Hu, Y.-H.; Li, D.-Y.; Zhang, Y.; Guo, H.-L.; Li, Y.-M.; Chen, F.; Xu, J. Vincristine-induced peripheral neuropathy: A mini-review. NeuroToxicology 2020, 81, 161–171. [Google Scholar] [CrossRef]
- Aley, K.; Reichling, D.; Levine, J. Vincristine hyperalgesia in the rat: A model of painful vincristine neuropathy in humans. Neuroscience 1996, 73, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, S.; Singh, N.; Jaggi, A.S. Dose-related neuropathic and anti-neuropathic effects of simvastatin in vincristine-induced neuropathic pain in rats. Food Chem. Toxicol. 2015, 80, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Amaya, F.; Shimosato, G.; Kawasaki, Y.; Hashimoto, S.; Tanaka, Y.; Ji, R.-R.; Tanaka, M. Induction of CB1 cannabinoid receptor by inflammation in primary afferent neurons facilitates antihyperalgesic effect of peripheral CB1 agonist. Pain 2006, 124, 175–183. [Google Scholar] [CrossRef]
- Lim, G.; Sung, B.; Ji, R.-R.; Mao, J. Upregulation of spinal cannabinoid-1-receptors following nerve injury enhances the effects of Win 55,212-2 on neuropathic pain behaviors in rats. Pain 2003, 105, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Chogtu, B.; Bairy, K.; Smitha, D.; Dhar, S.; Himabindu, P. Comparison of the efficacy of carbamazepine, gabapentin and lamotrigine for neuropathic pain in rats. Indian J. Pharmacol. 2011, 43, 596–598. [Google Scholar] [CrossRef]
- Andolfo, I.; Alper, S.L.; Iolascon, A. Nobel prize in physiology or medicine 2021, receptors for temperature and touch: Implications for hematology. Am. J. Hematol. 2021, 97, 168–170. [Google Scholar] [CrossRef]
- Munawar, N.; Oriowo, M.A.; Masocha, W. Antihyperalgesic Activities of Endocannabinoids in a Mouse Model of Antiretroviral-Induced Neuropathic Pain. Front. Pharmacol. 2017, 8, 136. [Google Scholar] [CrossRef]
- Castañé, A.; Célérier, E.; Martín, M.; Ledent, C.; Parmentier, M.; Maldonado, R.; Valverde, O. Development and expression of neuropathic pain in CB1 knockout mice. Neuropharmacology 2006, 50, 111–122. [Google Scholar] [CrossRef]
- Siegling, A.; A Hofmann, H.; Denzer, D.; Mauler, F.; De Vry, J. Cannabinoid CB1 receptor upregulation in a rat model of chronic neuropathic pain. Eur. J. Pharmacol. 2001, 415, R5–R7. [Google Scholar] [CrossRef]
- Tanner, K.D.; Reichling, D.B.; Levine, J.D. Nociceptor hyper-responsiveness during vincristine-induced painful peripheral neuropathy in the rat. J. Neurosci. 1998, 18, 6480–6491. [Google Scholar] [CrossRef]
- Costa, B.; Trovato, A.E.; Comelli, F.; Giagnoni, G.; Colleoni, M. The non-psychoactive cannabis constituent cannabidiol is an orally effective therapeutic agent in rat chronic inflammatory and neuropathic pain. Eur. J. Pharmacol. 2007, 556, 75–83. [Google Scholar] [CrossRef]
- Finn, D.P.; Haroutounian, S.; Hohmann, A.G.; Krane, E.; Soliman, N.; Rice, A.S. Cannabinoids, the endocannabinoid system, and pain: A review of preclinical studies. Pain 2021, 162, S5–S25. [Google Scholar] [CrossRef]
- Tyrtyshnaia, A.; Manzhulo, I.; Kipryushina, Y.; Ermolenko, E. Neuroinflammation and adult hippocampal neurogenesis in neuropathic pain and alkyl glycerol ethers treatment in aged mice. Int. J. Mol. Med. 2019, 43, 2153–2163. [Google Scholar] [CrossRef]
- Maresz, K.; Pryce, G.; Ponomarev, E.D.; Marsicano, G.; Croxford, J.L.; Shriver, L.P.; Ledent, C.; Cheng, X.; Carrier, E.J.; Mann, M.K.; et al. Direct suppression of CNS autoimmune inflammation via the cannabinoid receptor CB1 on neurons and CB2 on autoreactive T cells. Nat. Med. 2007, 13, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Fernández-López, D.; Martínez-Orgado, J.; Nuñez, E.; Romero, J.; Lorenzo, P.; Moro, M.; Lizasoain, I. Characterization of the Neuroprotective Effect of the Cannabinoid Agonist WIN-55212 in an In Vitro Model of Hypoxic-Ischemic Brain Damage in Newborn Rats. Pediatr. Res. 2006, 60, 169–173. [Google Scholar] [CrossRef]
- Marchalant, Y.; Brothers, H.M.; Norman, G.J.; Karelina, K.; DeVries, A.C.; Wenk, G.L. Cannabinoids attenuate the effects of aging upon neuroinflammation and neurogenesis. Neurobiol. Dis. 2009, 34, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Taylor, B.K. Activation of cannabinoid CB 2receptors reduces hyperalgesia in an experimental autoimmune encephalomyelitis mouse model ofmultiple sclerosis. Neurosci. Lett. 2015, 595, 1–6. [Google Scholar] [CrossRef]
- Maldonado, R.; Banos, J.E.; Cabanero, D. The endocannabinoid system and neuropathic pain. Pain 2016, 157, S23–S32. [Google Scholar] [CrossRef] [PubMed]
- Buffon, A.C.; Javornik, M.A.; Heymanns, A.C.; Salm, D.C.; Horewicz, V.V.; Martins, D.F.; Piovezan, A.P. Role of the endocannabinoid system on the antihyperalgesic action of gabapentin in animal model of neuropathic pain induced by partial sciatic nerve ligation. An. Acad. Bras. Cienc. 2020, 92, e20191155. [Google Scholar] [CrossRef]
- Boorman, E.; Zajkowska, Z.; Ahmed, R.; Pariante, C.M.; Zunszain, P.A. Crosstalk between endocannabinoid and immune systems: A potential dysregulation in depression? Psychopharmacology 2015, 233, 1591–1604. [Google Scholar] [CrossRef] [PubMed]
- Leonard, B.E.; Aricioglu, F. Cannabinoids and neuroinflammation: Therapeutic implications. J. Affect. Disord. Rep. 2023, 12, 100463. [Google Scholar] [CrossRef]
- Walker, J.M.; Krey, J.F.; Chu, C.J.; Huang, S.M. Endocannabinoids and related fatty acid derivatives in pain modulation. Chem. Phys. Lipids 2002, 121, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Comelli, F.; Giagnoni, G.; Bettoni, I.; Colleoni, M.; Costa, B. Antihyperalgesic effect of a Cannabis sativa extract in a rat model of neuropathic pain: Mechanisms involved. Phytotherapy Res. 2008, 22, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B. The Case for the Entourage Effect and Conventional Breeding of Clinical Cannabis: No “Strain,” No Gain. Front. Plant Sci. 2019, 9, 1969. [Google Scholar] [CrossRef]
- Russo, E.B. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef] [PubMed]
- Shustorovich, A.; Corroon, J.; Wallace, M.S.; Sexton, M. Biphasic effects of cannabis and cannabinoid therapy on pain severity, anxiety, and sleep disturbance: A scoping review. Pain Med. 2024, 25, 387–399. [Google Scholar] [CrossRef]
- Lowe, H.; Toyang, N.; Steele, B.; Bryant, J.; Ngwa, W. The Endocannabinoid System: A Potential Target for the Treatment of Various Diseases. Int. J. Mol. Sci. 2021, 22, 9472. [Google Scholar] [CrossRef]
- Juknat, A.; Pietr, M.; Kozela, E.; Rimmerman, N.; Levy, R.; Coppola, G.; Geschwind, D.; Vogel, Z. Differential transcriptional profiles mediated by exposure to the cannabinoids cannabidiol and Δ9-tetrahydrocannabinol in BV-2 microglial cells. Br. J. Pharmacol. 2012, 165, 2512–2528. [Google Scholar] [CrossRef]
- Blando, S.; Raffaele, I.; Chiricosta, L.; Valeri, A.; Gugliandolo, A.; Silvestro, S.; Pollastro, F.; Mazzon, E. Cannabidiol Promotes Neuronal Differentiation Using Akt and Erk Pathways Triggered by Cb1 Signaling. Molecules 2022, 27, 5644. [Google Scholar] [CrossRef]
- Kano, M.; Ohno-Shosaku, T.; Hashimotodani, Y.; Uchigashima, M.; Watanabe, M. Endocannabinoid-Mediated Control of Synaptic Transmission. Physiol. Rev. 2009, 89, 309–380. [Google Scholar] [CrossRef]


| Group | Intervention | Number of Rats |
|---|---|---|
| 1 | NaCl (0.9%, 1 mL i.p.) + Rape oil (1 mL p.o.) | 10 |
| 2 | VK + Rape oil—1 mL p.o. | 10 |
| 3 | VK + Gabapentin (solution 1 mg/mL)—60 mg/kg bw p.o. | 10 |
| 4 | VK + Extract D—5.0 mg/kg bw p.o | 10 |
| 5 | VK + Extract D—7.5 mg/kg bw p.o | 10 |
| 6 | VK + Extract D—10.0 mg/kg bw p.o | 10 |
| 7 | VK + Extract D—20.0 mg/kg bw p.o | 10 |
| 8 | VK + Extract D—40.0 mg/kg bw p.o | 10 |
| 9 | VK + Extract B—5.0 mg/kg bw p.o | 10 |
| 10 | VK + Extract B—7.5 mg/kg bw p.o | 10 |
| 11 | VK + Extract B—10.0 mg/kg bw p.o | 10 |
| 12 | VK + Extract B—20.0 mg/kg bw p.o | 10 |
| 13 | VK + Extract B—40.0 mg/kg bw p.o | 10 |
| Gene | Sequence 5′→ 3′ |
|---|---|
| CB1R | Primer F: TAATATGAAGCAAGATACCAG Primer R: CCATTTACAGAGACAACAAG |
| CB2R | Primer F: CAGTTACAGAGACAGAGGC Primer R: TGTTTCCATTACCCTAGAGC |
| GADPH | Primer F: GATGGTGAAGGTCGGTGTG Primer R: ATGAAGGGGTCGTTGATGG |
| Group | t = 0 | t = 1 h | t = 2 h | t = 3 h | t = 6 h |
|---|---|---|---|---|---|
| Tail flick test | |||||
| [s] | |||||
| NaCl | 12.9 ± 0.7 | 12.2 ± 1.1 | 12.6 ± 0.9 | 12.7 ± 0.9 | 13.2 ± 1.1 |
| VK | 9.7 ± 0.7 * | 10.1 ± 0.6 * | 9.5 ± 0.6 * | 9.8 ± 0.7 * | 9.7 ± 0.7 * |
| Von Frey test | |||||
| [g] | |||||
| NaCl | 30.2 ± 0.6 | 30.6 ± 1.2 | 29.6 ± 0.9 | 28.9 ± 0.9 | 29.5 ± 0.4 |
| VK | 27.4 ± 0.8 * | 27.2 ± 0.7 * | 27.4 ± 0.7 * | 27 ± 0.7 * | 26.6 ± 0.5 * |
| Group | t = 0 | t = 1 h | t = 2 h | t = 3 h | t = 6 h |
|---|---|---|---|---|---|
| Tail flick test | |||||
| [s] | |||||
| VK | 9.7 ± 0.7 | 10.1 ± 0.6 | 9.5 ± 0.6 | 9.8 ± 0.7 | 9.7 ± 0.7 |
| VK + GP | 8.5 ± 1 | 9.3 ± 1.4 | 9.1 ± 1.2 | 12.1 ± 1.6 | 18.6 ± 3.9 *^ |
| Von Frey test | |||||
| [g] | |||||
| VK | 27.4 ± 0.8 | 27.2 ± 0.7 | 27.4 ± 0.7 | 27 ± 0.7 | 26.6 ± 0.5 |
| VK + GP | 27.2 ± 0.8 | 27.5 ± 0.6 | 27.8 ± 1.8 | 29.8 ± 1.1 *^ | 31.2 ± 0.7 *^ |
| Intervention | t = 0 | t = 1 h | t = 2 h | t = 3 h | t = 6 h |
|---|---|---|---|---|---|
| [s] | |||||
| VK + Gabapentin | 9.7 ± 0.7 | 10.1 ± 0.6 | 9.5 ± 0.6 | 9.8 ± 0.7 | 9.7 ± 0.7 |
| VK + Extract D 5 mg/kg | 7.3 ± 0.9 | 22.8 ± 8.3 *^ | 17.7 ± 6.7 *^ | 22.9 ± 6.6 *^ | 19.3 ± 6.4 *^ |
| VK + Extract D 7.5 mg/kg | 8.9 ± 0.9 | 20.4 ± 5.6 *^ | 21.1 ± 3.3 *^ | 18.2 ± 3.2 ^ | 17.6 ± 4.6 ^ |
| VK + Extract D 10 mg/kg | 8.8 ± 0.9 | 20.5 ± 3.5 *^ | 15.1 ± 4.3 | 11.2 ± 1.9 | 18.5 ± 5.5 |
| VK + Extract D 20 mg/kg | 8.8 ± 1.2 | 17.6 ± 2.4^ | 14.9 ± 2.3 | 12.7 ± 1.4 | 13.4 ± 1.3 |
| VK + Extract D 40 mg | 7.9 ± 0.6 | 12.7 ± 2 | 11.6 ± 1.6 | 13.3 ± 2.1 | 15 ± 1.8 ^ |
| VK + Extract B 5 mg/kg | 9.4 ± 1.1 | 17.2 ± 3.2 *^ | 13.7 ± 1.1 | 10.6 ± 1.0 | 10.7 ± 0.9 |
| VK + Extract B 7.5 mg/kg | 9.0 ± 1.2 | 15.2 ± 4.4 ^ | 10.3 ± 1.0 | 9.2 ± 0.9 | 13.7 ± 3.7 |
| VK + Extract B 10 mg/kg | 7.8 ± 1.0 | 10.3 ± 0.9 | 9.5 ± 0.6 *^ | 11.9 ± 1.2 | 12.1 ± 1.7 |
| VK + Extract B 20 mg/kg | 6.3 ± 0.8 | 16.4 ± 5.5 *^ | 10.6 ± 1.1 | 15.6 ± 5.7 ^ | 15.4 ± 5.7 ^ |
| VK + Extract B 40 mg/kg | 8.1 ± 1.0 | 9.0 ± 0.8 | 11.6 ± 1.0 | 9.8 ± 1.1 | 16.4 ± 3.6 *^ |
| Grupa | t = 0 | t = 1 h | t = 2 h | t = 3 h | t = 6 h |
|---|---|---|---|---|---|
| [g] | |||||
| VK + Gabapentin | 27.4 ± 0.8 | 27.2 ± 0.7 | 27.4 ± 0.7 | 27 ± 0.7 | 26.6 ± 0.5 |
| VK + Extract D 5 mg/kg | 31 ± 1.8 | 28.9 ± 1.5 | 30.2 ± 1.0 | 29.6 ± 2.0 | 29.2 ± 1.6 |
| VK + Extract D 7.5 mg/kg | 31.2 ± 1.8 * | 30.6 ± 1.3 | 30.9 ± 1.3 * | 33 ± 2.1 * | 28.2 ± 1.6 |
| VK + Extract D 10 mg/kg | 31.4 ± 1.5 * | 33.3 ± 0.8 * | 30.8 ± 1.1 * | 29.6 ± 1.7 | 28.5 ± 0.9 |
| VK + Extract D 20 mg/kg | 26.3 ± 1.3 | 32.5 ± 1.5 *^ | 26.2 ± 0.8 | 27.7 ± 1.2 | 27.5 ± 1.0 |
| VK + Extract D 40 mg | 30.6 ± 0.8 | 34.8 ± 4.6 *^ | 28.7 ± 1.5 | 26.9 ± 1.5 ^ | 29.3 ± 1.8 |
| VK + Extract B 5 mg/kg | 29.9 ± 1.3 | 31.2 ± 2.1 * | 29.3 ± 1.9 | 28.5 ± 1.2 | 29.6 ± 2.3 |
| VK + Extract B 7.5 mg/kg | 27.9 ± 1.2 | 28.9 ± 1.2 | 29.3 ± 1.4 | 25.7 ± 2.0 | 25.3 ± 1.8 |
| VK + Extract B 10 mg/kg | 30.2 ± 1.4 | 30.7 ± 2.0 | 31 ± 1.8 * | 29.3 ± 2.0 | 27.9 ± 1.5 |
| VK + Extract B 20 mg/kg | 29.6 ± 1.5 | 30.8 ± 1.7 *^ | 34.8 ± 1.9 * | 32.1 ± 2.3 ^ | 26.1 ± 1.8 |
| VK + Extract B 40 mg/kg | 29.2 ± 1.7 | 28.2 ± 0.8 | 29.9 ± 1.7 | 26.5 ± 0.7 | 26.2 ± 2.2 |
| CB1R (Vincristine Group VK vs. NaCl Group) | |||||
|---|---|---|---|---|---|
| Intervention | Hippocampus | Cortex | Lymphocytes | ||
| Protein [ng/mL] Median (1st; 3rd Quartile) | Gene Expression Median (1st; 3rd Quartile) | Protein [ng/mL] Median (1st; 3rd Quartile) | Gene Expression Median (1st; 3rd Quartile) | Gene Expression Median (1st; 3rd Quartile) | |
| NaCl- 1 mL i.p. + Rape oil -1 mL p.o. | 1.45 (1.14; 2.69) | 3.04 × 10−3 (1.92 × 10−3; 5.25 × 10−3) | 3.99 (3.32; 4.57) | 21.20 × 10−3 (13.10 × 10−3; 84.20 × 10−3) | 0.03 × 10−3 (0.001 × 10−3; 0.87 × 10−3) |
| VK +Rape oil—1 mL p.o. | 0.89 (0.39; 1.03) | 4.36 × 10−3 (2.92 × 10−3; 6.68 × 10−3) | 2.75 (2.36; 3.78) * | 22.90 × 10−3 (14.40 × 10−3; 35.40 × 10−3) | 0.02 × 10−3 (0.05 × 10−3; 0.16 × 10−3) |
| CB2R (vincristine group VK vs. NaCl group) | |||||
| NaCl- 1 mL i.p. + Rape oil -1 mL p.o. | 0.25 (0.18; 0.32) | 3.90 × 10−3 (2.21 × 10−3; 11.40 × 10−3) | 1.26 (1.02; 1.45) | 50.30 × 10−3 (13.80 × 10−3; 71.40 × 10−3) | 32.10 × 10−3 (14.60 × 10−3; 41.50 × 10−3) |
| VK + Rape oil—1 mL p.o. | 0.29 (0.26; 0.55) | 3.45 × 10−3 (2.71 × 10−3; 4.56 × 10−3) | 0.87 (0.83; 0.97) | 3.18 × 10−3 * (1.67 × 10−3; 7.43 × 10−3) | 23.50 × 10−3 (18.70 × 10−3; 84.30 × 10−3) |
| CB1R (vincristine group VK vs. gabapentin group) | |||||
| VK + Rape oil—1 mL p.o. | 0.89 (0.39; 1.03) | 4.36 × 10−3 (2.92 × 10−3; 6.68 × 10−3) | 2.75 (2.36; 3.78) | 22.90 × 10−3 (14.40 × 10−3; 35.40 × 10−3) | 0.02 × 10−3 (0.05 × 10−3; 0.16 × 10−3) |
| VK + Gabapentin-60 mg/kg p.o. | 2.51 (2.13; 2.8) ^ | 3.02 × 10−3 (1.14 × 10−3; 4.6 × 10−3) | 2.74 (2.09; 3.2) | 31.30 × 10−3 (26.50 × 10−3; 33.30 × 10−3) | 0.01 × 10−3 (0.001 × 10−3; 0.011 × 10−3) |
| CB2R (vincristine group VK vs. gabapentin group) | |||||
| VK +Rape oil—1 mL p.o. | 0.29 (0.26; 0.55) | 3.45 × 10−3 (2.71 × 10−3; 4.56 × 10−3) | 0.87 (0.83; 0.97) | 3.18 × 10−3 (1.67 × 10−3; 7.43 × 10−3) | 23.50 × 10−3 (18.70 × 10−3; 84.30 × 10−3) |
| VK + Gabapentin-60 mg/kg p.o. | 0.42 (0.34; 0.5) | 4.79 × 10−3 (2.94 × 10−3; 13.80 × 10−3) | 1.25 (1.03; 1.40) | 2.35 × 10−3 (0.51 × 10−3; 3.63 × 10−3) | 35.30 × 10−3 (14.70 × 10−3; 57.10 × 10−3) |
| CB1R | |||||
|---|---|---|---|---|---|
| Type of Intervention | Hippocampus | Cortex | Lymphocytes | ||
| Protein [ng/mL] Median (1st; 3rd Quartile) | Gene Expression Median (1st; 3rd Quartile) | Protein [ng/mL] Median (1st; 3rd Quartile) | Gene Expression Median (1st; 3rd Quartile) | Gene Expression Median (1st; 3rd Quartile) | |
| VK + Rape oil | 0.89 (0.39; 1.03) | 4.36 × 10−3 (2.92 × 10−3; 6.68 × 10−3) | 2.75 (2.36; 3.78) | 22.90 × 10−3 (14.40 × 10−3; 35.40 × 10−3) | 0.02 × 10−3 (0.05 × 10−3; 0.16 × 10−3) |
| VK + Gabapentin | 2.51 (2.13; 2.8) | 3.02 × 10−3 (1.14 × 10−3; 4.6 × 10−3) | 2.74 (2.09; 3.2) | 31.30 × 10−3 (26.50 × 10−3; 33.30 × 10−3) | 0.01 × 10−3 (0.001 × 10−3; 0.011 × 10−3) |
| VK + Extract D 5 mg/kg | 1.96 (1.44; 2.09) | 3.15 × 10−3 (1.45 × 10−3; 3.67 × 10−3) | 2.97 (1.74; 3.2) | 2.95 × 10−3 (2.21 × 103; 6.61 × 10−3) | 15 0.07 × 10−3 (0.01 × 10−3; 0.10 × 10−3) |
| VK + Extract D 7.5 mg/kg | 2.4 (2.17; 3.8) | 5 1.73 × 10−3 (0.89 × 10−3; 2.43 × 10−3) | 6 3.4 (3.27; 4.2) | 11 439.90 × 10−3 (2.72 × 10−3; 1957 × 10−3) | 0.11 × 10−3 (0.06 × 10−3; 0.18 × 10−3) |
| VK + Extract D 10 mg/kg | 1 3.26 (3.22; 4.06) | 9.97 × 10−3 (5.51 × 10−3; 14.7 × 10−3) | 7 4.35 (4.11; 5.15) | 5.51 × 10−3 (3.56 × 10−3; 5.51 × 10−3) | 0.64 × 10−3 (0.16 × 10−3; 1.08 × 10−3) |
| VK + Extract D 20 mg/kg | 2.24 (1.71; 2.95) | 6.54 × 10−3 (0.37 × 10−3; 7.35 × 10−3) | 1.72 (1.06; 2.24) | 12 0.24 × 10−3 (0.03 × 10−3; 1.53 × 10−3) | 0.56 × 10−3 (0.03 × 10−3; 2.03 × 10−3) |
| VK + Extract D 40 mg | 2 3.77 (2.78; 4.34) | 2.44 × 10−3 (0.89 × 10−3; 3.8 × 10−3) | 1.5 (1.35; 1.77) | 4.12 × 10−3 (2.34 × 10−3; 5.64 × 10−3) | 0.14 × 10−3 (0.01 × 10−3; 0.21 × 10−3) |
| VK + Extract B 5 mg/kg | 1.24 (0.26; 2.65) | 1.57 × 10−3 (0.70 × 10−3; 5.85 × 10−3) | 1.53 (1.16; 1.64) | 13 1.14 × 10−3 (0.34 × 10−3; 2.22 × 10−3) | 0.26 × 10−3 (0.12 × 10−3; 0.74 × 10−3) |
| VK + Extract B 7.5 mg/kg | 3 5.65 (3.22; 9.02) | 7.57 × 10−3 (5.19 × 10−3; 11.7 × 10−3) | 1.49 (1.19; 1.55) | 14 0.05 × 10−3 (0.001 × 10−3; 0.07 × 10−3) | 0.41 × 10−3 (0.11 × 10−3; 1.29 × 10−3) |
| VK + Extract B 10 mg/kg | 4 3.6 (2.90; 5.00) | 8.12 × 10−3 (4.17 × 10−3; 12.0 × 10−3) | 8 0.6 (0.2; 1.0) | 11.16 × 10−3 (9.82 × 10−3; 19.30 × 10−3) | 0.27 × 10−3 (0.11 × 10−3; 0.54 × 10−3) |
| VK + Extract B 20 mg/kg | 2.88 (2.70; 3.60) | 4.17 × 10−3 (3.41 × 10−3; 5.07 × 10−3) | 9 0.36 (0.34; 0.4) | 2.43 × 10−3 (1.73 × 10−3; 3.55 × 10−3) | 0.37 × 10−3 (0.11 × 10−3; 0.61 × 10−3) |
| VK + Extract B 40 mg/kg | 2.44 (1.43; 3.38) | 7.99 × 10−3 (1.90 × 10−3; 9.59 × 10−3) | 10 0.75 (0.53; 1.01) | 3.77 × 10−3 (1.86 × 10−3; 5.46 × 10−3) | 0.02 × 10−3 (0.01 × 10−3; 0.04 × 10−3) |
| Type of Intervention | CB2R | ||||
|---|---|---|---|---|---|
| Hippocampus | Cortex | Lymphocytes | |||
| Protein [ng/mL] Median (1st; 3rd Quartile) | Gene Expression Median (1st; 3rd Quartile) | Protein [ng/mL] Median (1st; 3rd Quartile) | Gene Expression Median (1st; 3rd Quartile) | Gene Expression Median (1st; 3rd Quartile) | |
| VK + Rape oil | 0.29 (0.26; 0.55) | 3.45 × 10−3 (2.71 × 10−3; 4.56 × 10−3) | 0.87 (0.83; 0.97) | 3.18 × 10−3 (1.67 × 10−3; 7.43 × 10−3) | 23.50 × 10−3 (18.70 × 10−3; 84.30 × 10−3) |
| VK + Gabapentin | 0.42 (0.34; 0.5) | 4.79 × 10−3 (2.94 × 10−3; 13.80 × 10−3) | 1.25 (1.03; 1.40) | 2.35 × 10−3 (0.51 × 10−3; 3.63 × 10−3) | 35.30 × 10−3 (14.70 × 10−3; 57.10 × 10−3) |
| VK + Extract D 5 mg/kg | 0.25 (0.22; 0.41) | 2.79 × 10−3 (2.02 × 10−3; 5.33 × 10−3) | 0.97 (0.87; 1.13) | 13.90 × 10−3 (6.15 × 10−3; 29.60 × 10−3) | 43.80 × 10−3 (32.70 × 10−3; 117.30 × 10−3) |
| VK + Extract D 7.5 mg/kg | 0.4 (0.27; 0.50) | 2 7.87 × 10−3 (4.51 × 10−3; 22.9 × 10−3) | 1.00 (0.92; 1.10) | 27.90 × 10−3 (15.00 × 10−3; 49.70 × 10−3) | 59.00 × 10−3 (29.10 × 10−3; 79.60 × 10−3) |
| VK + Extract D 10 mg/kg | 0.32 (0.29 0.41) | 1.49 × 10−3 (1.32 × 10−3; 3.72 × 10−3) | 3 1.06 (0.88; 1.42) | 6 3.24 × 10−3 (2.34 × 10−3; 7.20 × 10−3) | 61.50 × 10−3 (52.40 × 10−3; 91.90 × 10−3) |
| VK + Extract D 20 mg/kg | 0.25 (0.12; 0.29) | 2.39 × 10−3 (0.35 × 10−3; 7.03 × 10−3) | 0.76 (0.71; 0.88) | 45.50 × 10−3 (13.50 × 10−3; 97.20 × 10−3) | 60.10 × 10−3 (39.80 × 10−3; 73.10 × 10−3) |
| VK + Extract D 40 mg | 0.39 (0.34; 0.44) | 5.73 × 10−3 (1.36 × 10−3; 8.47 × 10−3) | 0.34 (0.25; 0.72) | 5.50 × 10−3 (4.00 × 10−3; 15.50 × 10−3) | 38.20 × 10−3 (32.00 × 10−3; 84.60 × 10−3) |
| VK + Extract B 5 mg/kg | 0.32 (0.22; 0.45) | 2.31 × 10−3 (1.06 × 10−3; 3.02 × 10−3) | 0.27 (0.13; 0.41) | 14.50 × 10−3 (6.01 × 10−3; 27.70 × 10−3) | 29.20 × 10−3 (15.40 × 10−3; 73.80 × 10−3) |
| VK + Extract B 7.5 mg/kg | 1 1.39 (0.83; 1.52) | 0.27 × 10−3 (0.20 × 10−3; 0.42 × 10−3) | 0.10 (0.08; 0.17) | 0.32 × 10−3 (0.001 × 10−3; 0.13 × 10−3) | 37.40 × 10−3 (25.30 × 10−3; 47.40 × 10−3) |
| VK + Extract B 10 mg/kg | 0.8 (0.70; 1.00) | 0.30 × 10−3 (0.16 × 10−3; 0.48 × 10−3) | 4 0.10 (0.01; 0.19) | 1.62 × 10−3 (0.99 × 10−3; 1.87 × 10−3) | 63.80 × 10−3 (31.70 × 10−3; 72.50 × 10−3) |
| VK + Extract B 20 mg/kg | 0.94 (0.73; 1.10) | 1.51 × 10−3 (0.53 × 10−3; 3.40 × 10−3) | 5 0.05 (0.04; 0.10) | 1.53 × 10−3 (1.21 × 10−3; 2.89 × 10−3) | 24.00 × 10−3 (19.30 × 10−3; 38.60 × 10−3) |
| VK + Extract B 40 mg/kg | 0.92 (0.54; 1.02) | 5.82 × 10−3 (1.36 × 10−3; 6.96 × 10−3) | 0.09 (0.07; 0.12) | 1.00 × 10−3 (0.65 × 10−3; 1.34 × 10−3) | 48.20 × 10−3 (42.00 × 10−3; 54.90 × 10−3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartkowiak-Wieczorek, J.; Bienert, A.; Czora-Poczwardowska, K.; Kujawski, R.; Szulc, M.; Mikołajczak, P.; Wizner, A.-M.; Jamka, M.; Hołysz, M.; Wielgus, K.; et al. Cannabis sativa L. Extract Alleviates Neuropathic Pain and Modulates CB1 and CB2 Receptor Expression in Rat. Biomolecules 2024, 14, 1065. https://doi.org/10.3390/biom14091065
Bartkowiak-Wieczorek J, Bienert A, Czora-Poczwardowska K, Kujawski R, Szulc M, Mikołajczak P, Wizner A-M, Jamka M, Hołysz M, Wielgus K, et al. Cannabis sativa L. Extract Alleviates Neuropathic Pain and Modulates CB1 and CB2 Receptor Expression in Rat. Biomolecules. 2024; 14(9):1065. https://doi.org/10.3390/biom14091065
Chicago/Turabian StyleBartkowiak-Wieczorek, Joanna, Agnieszka Bienert, Kamila Czora-Poczwardowska, Radosław Kujawski, Michał Szulc, Przemysław Mikołajczak, Anna-Maria Wizner, Małgorzata Jamka, Marcin Hołysz, Karolina Wielgus, and et al. 2024. "Cannabis sativa L. Extract Alleviates Neuropathic Pain and Modulates CB1 and CB2 Receptor Expression in Rat" Biomolecules 14, no. 9: 1065. https://doi.org/10.3390/biom14091065
APA StyleBartkowiak-Wieczorek, J., Bienert, A., Czora-Poczwardowska, K., Kujawski, R., Szulc, M., Mikołajczak, P., Wizner, A.-M., Jamka, M., Hołysz, M., Wielgus, K., Słomski, R., & Mądry, E. (2024). Cannabis sativa L. Extract Alleviates Neuropathic Pain and Modulates CB1 and CB2 Receptor Expression in Rat. Biomolecules, 14(9), 1065. https://doi.org/10.3390/biom14091065













