3D Models Currently Proposed to Investigate Human Skin Aging and Explore Preventive and Reparative Approaches: A Descriptive Review
Abstract
:1. Introduction
2. From Basic 2D to Complex 3D Models
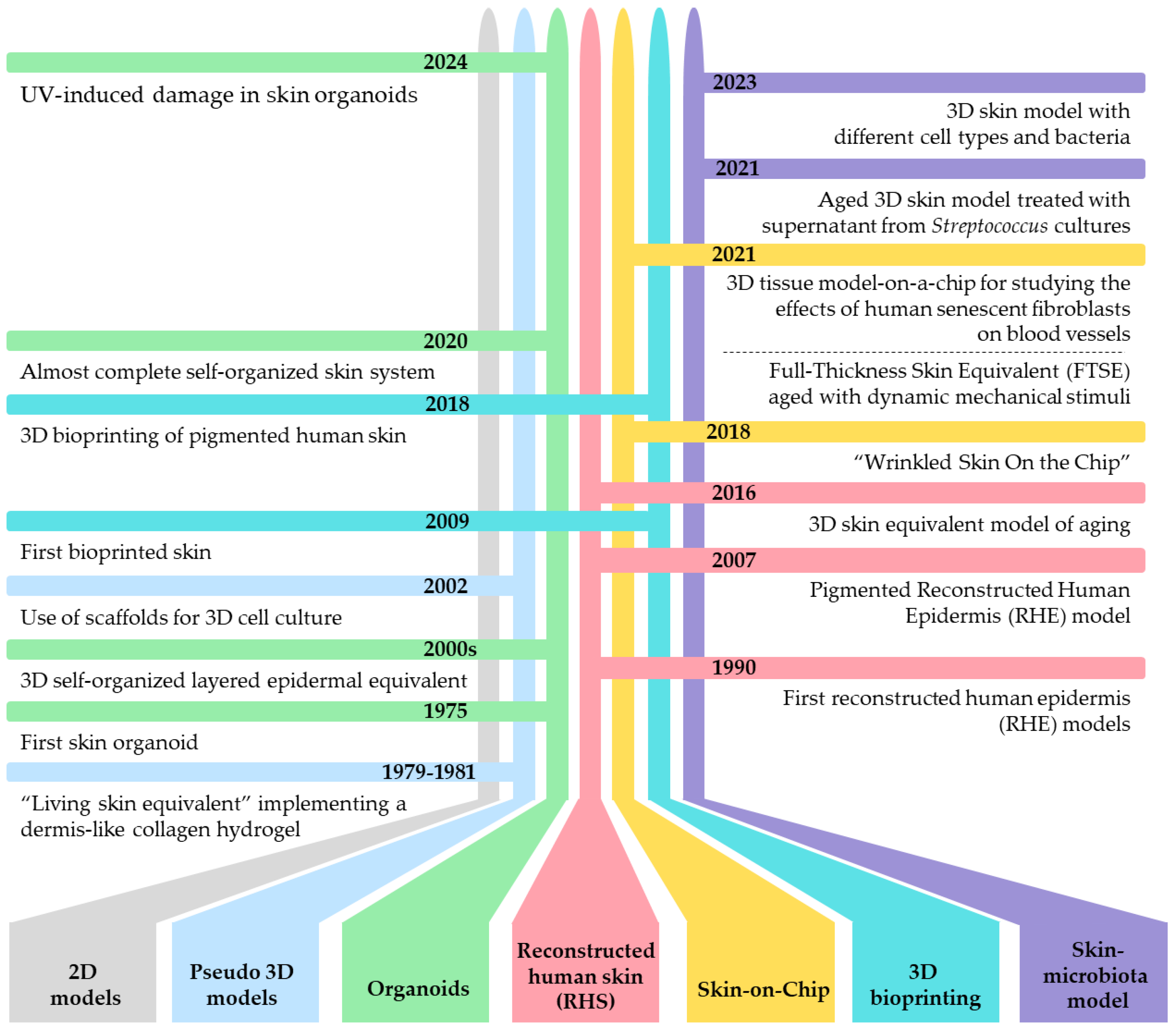
3. 3D Models as Innovative Tool for Studying Skin Aging
4. Overview of 3D Aged Skin Models
4.1. Pseudo-3D Systems
4.2. Organoid Cultures
4.3. Reconstructed Human Skin (RHS)
4.4. The Microfluidic Culture Device Called “Skin-on-Chip”
4.5. 3D Bioprinting Models for Skin Aging as a Viable Alternative to Traditional Animal Testing
4.6. Future Directions for 3D Aged Skin Model Research: The Crucial Role of Microbiota in Enhancing the Realism and Functionality of 3D Skin Models
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Russell, W.M. The development of the three Rs concept. Altern. Lab. Anim. 1995, 23, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Hubrecht, R.C.; Carter, E. The 3Rs and Humane Experimental Technique: Implementing Change. Animals 2019, 9, 754. [Google Scholar] [CrossRef] [PubMed]
- Henkler, F.; Tralau, T.; Tentschert, J.; Kneuer, C.; Haase, A.; Platzek, T.; Luch, A.; Gotz, M.E. Risk assessment of nanomaterials in cosmetics: A European union perspective. Arch. Toxicol. 2012, 86, 1641–1646. [Google Scholar] [CrossRef]
- Sanchez, M.M.; Bagdasarian, I.A.; Darch, W.; Morgan, J.T. Organotypic cultures as aging associated disease models. Aging-Us 2022, 14, 9338–9383. [Google Scholar] [CrossRef]
- Augello, F.R.; Lombardi, F.; Artone, S.; Ciafarone, A.; Altamura, S.; Di Marzio, L.; Cifone, M.G.; Palumbo, P.; Giuliani, M.; Cinque, B. Evaluation of the Effectiveness of an Innovative Polycomponent Formulation on Adult and Aged Human Dermal Fibroblasts. Biomedicines 2023, 11, 2410. [Google Scholar] [CrossRef]
- Quiles, J.; Cabrera, M.; Jones, J.; Tsapekos, M.; Caturla, N. In Vitro Determination of the Skin Anti-Aging Potential of Four-Component Plant-Based Ingredient. Molecules 2022, 27, 8101. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, I.C.; Meng, S.; Xu, J. LincRNA-EPS Promotes Proliferation of Aged Dermal Fibroblast by Inducing CCND1. Int. J. Mol. Sci. 2024, 25, 7677. [Google Scholar] [CrossRef] [PubMed]
- Maione, A.G.; Brudno, Y.; Stojadinovic, O.; Park, L.K.; Smith, A.; Tellechea, A.; Leal, E.C.; Kearney, C.J.; Veves, A.; Tomic-Canic, M.; et al. Three-dimensional human tissue models that incorporate diabetic foot ulcer-derived fibroblasts mimic in vivo features of chronic wounds. Tissue Eng. Part C Methods 2015, 21, 499–508. [Google Scholar] [CrossRef]
- Suarez-Martinez, E.; Suazo-Sanchez, I.; Celis-Romero, M.; Carnero, A. 3D and organoid culture in research: Physiology, hereditary genetic diseases and cancer. Cell Biosci. 2022, 12, 39. [Google Scholar] [CrossRef]
- Baker, P.; Huang, C.; Radi, R.; Moll, S.B.; Jules, E.; Arbiser, J.L. Skin Barrier Function: The Interplay of Physical, Chemical, and Immunologic Properties. Cells 2023, 12, 2745. [Google Scholar] [CrossRef]
- Ueck, C.; Volksdorf, T.; Houdek, P.; Vidal, Y.S.S.; Sehner, S.; Ellinger, B.; Lobmann, R.; Larena-Avellaneda, A.; Reinshagen, K.; Ridderbusch, I.; et al. Comparison of In-Vitro and Ex-Vivo Wound Healing Assays for the Investigation of Diabetic Wound Healing and Demonstration of a Beneficial Effect of a Triterpene Extract. PLoS ONE 2017, 12, e0169028. [Google Scholar] [CrossRef] [PubMed]
- Zoio, P.; Oliva, A. Skin-on-a-Chip Technology: Microengineering Physiologically Relevant In Vitro Skin Models. Pharmaceutics 2022, 14, 682. [Google Scholar] [CrossRef] [PubMed]
- Hayden, P.J.; Harbell, J.W. Special review series on 3D organotypic culture models: Introduction and historical perspective. In Vitro Cell. Dev. Biol. Anim. 2021, 57, 95–103. [Google Scholar] [CrossRef]
- Bell, E.; Ivarsson, B.; Merrill, C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc. Natl. Acad. Sci. USA 1979, 76, 1274–1278. [Google Scholar] [CrossRef]
- Bell, E.; Ehrlich, H.P.; Buttle, D.J.; Nakatsuji, T. Living tissue formed in vitro and accepted as skin-equivalent tissue of full thickness. Science 1981, 211, 1052–1054. [Google Scholar] [CrossRef] [PubMed]
- Bell, E.; Ehrlich, H.P.; Sher, S.; Merrill, C.; Sarber, R.; Hull, B.; Nakatsuji, T.; Church, D.; Buttle, D.J. Development and use of a living skin equivalent. Plast. Reconstr. Surg. 1981, 67, 386–392. [Google Scholar] [CrossRef]
- Li, W.J.; Laurencin, C.T.; Caterson, E.J.; Tuan, R.S.; Ko, F.K. Electrospun nanofibrous structure: A novel scaffold for tissue engineering. J. Biomed. Mater. Res. 2002, 60, 613–621. [Google Scholar] [CrossRef]
- Rheinwald, J.G.; Green, H. Serial cultivation of strains of human epidermal keratinocytes: The formation of keratinizing colonies from single cells. Cell 1975, 6, 331–343. [Google Scholar] [CrossRef]
- Itoh, M.; Kiuru, M.; Cairo, M.S.; Christiano, A.M. Generation of keratinocytes from normal and recessive dystrophic epidermolysis bullosa-induced pluripotent stem cells. Proc. Natl. Acad. Sci. USA 2011, 108, 8797–8802. [Google Scholar] [CrossRef]
- Itoh, M.; Umegaki-Arao, N.; Guo, Z.; Liu, L.; Higgins, C.A.; Christiano, A.M. Generation of 3D skin equivalents fully reconstituted from human induced pluripotent stem cells (iPSCs). PLoS ONE 2013, 8, e77673. [Google Scholar] [CrossRef]
- Guenou, H.; Nissan, X.; Larcher, F.; Feteira, J.; Lemaitre, G.; Saidani, M.; Del Rio, M.; Barrault, C.C.; Bernard, F.X.; Peschanski, M.; et al. Human embryonic stem-cell derivatives for full reconstruction of the pluristratified epidermis: A preclinical study. Lancet 2009, 374, 1745–1753. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Rabbani, C.C.; Gao, H.; Steinhart, M.R.; Woodruff, B.M.; Pflum, Z.E.; Kim, A.; Heller, S.; Liu, Y.; Shipchandler, T.Z.; et al. Hair-bearing human skin generated entirely from pluripotent stem cells. Nature 2020, 582, 399–404. [Google Scholar] [CrossRef]
- Fentem, J.H.; Botham, P.A. ECVAM’s activities in validating alternative tests for skin corrosion and irritation. Altern. Lab. Anim. 2002, 30 (Suppl. 2), 61–67. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Suwa, F.; Wang, X.; Takemura, A.; Fang, Y.R.; Li, Y.; Zhao, Y.; Jin, Y. Reconstruction of a tissue-engineered skin containing melanocytes. Cell Biol. Int. 2007, 31, 985–990. [Google Scholar] [CrossRef]
- Diekmann, J.; Alili, L.; Scholz, O.; Giesen, M.; Holtkotter, O.; Brenneisen, P. A three-dimensional skin equivalent reflecting some aspects of in vivo aged skin. Exp. Dermatol. 2016, 25, 56–61. [Google Scholar] [CrossRef]
- Lim, H.Y.; Kim, J.; Song, H.J.; Kim, K.; Choi, K.C.; Park, S.; Sung, G.Y. Development of wrinkled skin-on-a-chip (WSOC) by cyclic uniaxial stretching. J. Ind. Eng. Chem. 2018, 68, 238–245. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, J.; Jeon, H.M.; Kim, K.; Sung, G.Y. Development of an Aged Full-Thickness Skin Model Using Flexible Skin-on-a-Chip Subjected to Mechanical Stimulus Reflecting the Circadian Rhythm. Int. J. Mol. Sci. 2021, 22, 2788. [Google Scholar] [CrossRef] [PubMed]
- Pauty, J.; Nakano, S.; Usuba, R.; Nakajima, T.; Johmura, Y.; Omori, S.; Sakamoto, N.; Kikuchi, A.; Nakanishi, M.; Matsunaga, Y.T. A 3D tissue model-on-a-chip for studying the effects of human senescent fibroblasts on blood vessels. Biomater. Sci. 2021, 9, 199–211. [Google Scholar] [CrossRef]
- Lee, W.; Debasitis, J.C.; Lee, V.K.; Lee, J.H.; Fischer, K.; Edminster, K.; Park, J.K.; Yoo, S.S. Multi-layered culture of human skin fibroblasts and keratinocytes through three-dimensional freeform fabrication. Biomaterials 2009, 30, 1587–1595. [Google Scholar] [CrossRef]
- Min, D.; Lee, W.; Bae, I.H.; Lee, T.R.; Croce, P.; Yoo, S.S. Bioprinting of biomimetic skin containing melanocytes. Exp. Dermatol. 2018, 27, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Kim, M.; Kim, M.; Park, C.; Yoon, Y.; Lim, D.H.; Yeo, H.; Kang, S.; Lee, Y.G.; Beak, N.I.; et al. Spermidine-induced recovery of human dermal structure and barrier function by skin microbiome. Commun. Biol. 2021, 4, 231. [Google Scholar] [CrossRef] [PubMed]
- Rikken, G.; Meesters, L.D.; Jansen, P.A.M.; Rodijk-Olthuis, D.; van Vlijmen-Willems, I.; Niehues, H.; Smits, J.P.H.; Olah, P.; Homey, B.; Schalkwijk, J.; et al. Novel methodologies for host-microbe interactions and microbiome-targeted therapeutics in 3D organotypic skin models. Microbiome 2023, 11, 227. [Google Scholar] [CrossRef]
- Medawar, P.B. The cultivation of adult mammalian skin epithelium in vitro. Q. J. Microsc. Sci. 1948, 89, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Mori, N.; Morimoto, Y.; Takeuchi, S. Skin integrated with perfusable vascular channels on a chip. Biomaterials 2017, 116, 48–56. [Google Scholar] [CrossRef]
- Pitrez, P.R.; Monteiro, L.M.; Borgogno, O.; Nissan, X.; Mertens, J.; Ferreira, L. Cellular reprogramming as a tool to model human aging in a dish. Nat. Commun. 2024, 15, 1816. [Google Scholar] [CrossRef]
- Costello, L.; Dicolandrea, T.; Tasseff, R.; Isfort, R.; Bascom, C.; von Zglinicki, T.; Przyborski, S. Tissue engineering strategies to bioengineer the ageing skin phenotype in vitro. Aging Cell 2022, 21, e13550. [Google Scholar] [CrossRef]
- Dos Santos, M.; Metral, E.; Boher, A.; Rousselle, P.; Thepot, A.; Damour, O. In vitro 3-D model based on extending time of culture for studying chronological epidermis aging. Matrix Biol. 2015, 47, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Lammermann, I.; Terlecki-Zaniewicz, L.; Weinmullner, R.; Schosserer, M.; Dellago, H.; de Matos Branco, A.D.; Autheried, D.; Sevcnikar, B.; Kleissl, L.; Berlin, I.; et al. Blocking negative effects of senescence in human skin fibroblasts with a plant extract. NPJ Aging Mech. Dis. 2018, 4, 4. [Google Scholar] [CrossRef]
- Weinmullner, R.; Zbiral, B.; Becirovic, A.; Stelzer, E.M.; Nagelreiter, F.; Schosserer, M.; Lammermann, I.; Liendl, L.; Lang, M.; Terlecki-Zaniewicz, L.; et al. Organotypic human skin culture models constructed with senescent fibroblasts show hallmarks of skin aging. NPJ Aging Mech. Dis. 2020, 6, 4. [Google Scholar] [CrossRef]
- Victorelli, S.; Lagnado, A.; Halim, J.; Moore, W.; Talbot, D.; Barrett, K.; Chapman, J.; Birch, J.; Ogrodnik, M.; Meves, A.; et al. Senescent human melanocytes drive skin ageing via paracrine telomere dysfunction. EMBO J. 2019, 38, e101982. [Google Scholar] [CrossRef]
- Markiewicz, E.; Jerome, J.; Mammone, T.; Idowu, O.C. Anti-Glycation and Anti-Aging Properties of Resveratrol Derivatives in the in-vitro 3D Models of Human Skin. Clin. Cosmet. Investig. Dermatol. 2022, 15, 911–927. [Google Scholar] [CrossRef]
- Lee, K.H.; Ng, Y.P.; Cheah, P.S.; Lim, C.K.; Toh, M.S. Molecular characterization of glycation-associated skin ageing: An alternative skin model to study in vitro antiglycation activity of topical cosmeceutical and pharmaceutical formulations. Br. J. Dermatol. 2017, 176, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Yang, T.; Yu, D.; Xiong, H.; Zhang, S. Current insights and future perspectives of ultraviolet radiation (UV) exposure: Friends and foes to the skin and beyond the skin. Environ. Int. 2024, 185, 108535. [Google Scholar] [CrossRef]
- Chen, T.; Hou, H.; Fan, Y.; Wang, S.; Chen, Q.; Si, L.; Li, B. Protective effect of gelatin peptides from pacific cod skin against photoaging by inhibiting the expression of MMPs via MAPK signaling pathway. J. Photochem. Photobiol. B. 2016, 165, 34–41. [Google Scholar] [CrossRef]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef] [PubMed]
- Casale, C.; Imparato, G.; Urciuolo, F.; Rescigno, F.; Scamardella, S.; Escolino, M.; Netti, P.A. Engineering a human skin equivalent to study dermis remodelling and epidermis senescence in vitro after UVA exposure. J. Tissue Eng. Regen. Med. 2018, 12, 1658–1669. [Google Scholar] [CrossRef]
- Bouzos, E.; Asuri, P. Sandwich Culture Platforms to Investigate the Roles of Stiffness Gradients and Cell-Matrix Adhesions in Cancer Cell Migration. Cancers 2023, 15, 1729. [Google Scholar] [CrossRef] [PubMed]
- Rebehn, L.; Khalaji, S.; KleinJan, F.; Kleemann, A.; Port, F.; Paul, P.; Huster, C.; Nolte, U.; Singh, K.; Kwapich, L.; et al. The weakness of senescent dermal fibroblasts. Proc. Natl. Acad. Sci. USA 2023, 120, e2301880120. [Google Scholar] [CrossRef]
- Sato, T.; Vries, R.G.; Snippert, H.J.; van de Wetering, M.; Barker, N.; Stange, D.E.; van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef]
- Hu, J.L.; Todhunter, M.E.; LaBarge, M.A.; Gartner, Z.J. Opportunities for organoids as new models of aging. J. Cell Biol. 2018, 217, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Koehler, K.R. Skin organoids: A new human model for developmental and translational research. Exp. Dermatol. 2021, 30, 613–620. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, X.; Dowbaj, A.M.; Sljukic, A.; Bratlie, K.; Lin, L.; Fong, E.L.S.; Balachander, G.M.; Chen, Z.; Soragni, A.; et al. Organoids. Nat. Rev. Methods Primers 2022, 2, 94. [Google Scholar] [CrossRef]
- Hong, Y.J.; Do, J.T. Neural Lineage Differentiation From Pluripotent Stem Cells to Mimic Human Brain Tissues. Front. Bioeng. Biotechnol. 2019, 7, 400. [Google Scholar] [CrossRef]
- Drost, J.; Clevers, H. Organoids in cancer research. Nat. Rev. Cancer 2018, 18, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef] [PubMed]
- Ryu, N.E.; Lee, S.H.; Park, H. Spheroid Culture System Methods and Applications for Mesenchymal Stem Cells. Cells 2019, 8, 1620. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, F.; Augello, F.R.; Artone, S.; Ciafarone, A.; Topi, S.; Cifone, M.G.; Cinque, B.; Palumbo, P. Involvement of Cyclooxygenase-2 in Establishing an Immunosuppressive Microenvironment in Tumorspheres Derived from TMZ-Resistant Glioblastoma Cell Lines and Primary Cultures. Cells 2024, 13, 258. [Google Scholar] [CrossRef]
- Silva-Pedrosa, R.; Salgado, A.J.; Ferreira, P.E. Revolutionizing Disease Modeling: The Emergence of Organoids in Cellular Systems. Cells 2023, 12, 930. [Google Scholar] [CrossRef]
- Qu, S.; Xu, R.; Yi, G.; Li, Z.; Zhang, H.; Qi, S.; Huang, G. Patient-derived organoids in human cancer: A platform for fundamental research and precision medicine. Mol. Biomed. 2024, 5, 6. [Google Scholar] [CrossRef]
- Torrens-Mas, M.; Perello-Reus, C.; Navas-Enamorado, C.; Ibarguen-Gonzalez, L.; Sanchez-Polo, A.; Segura-Sampedro, J.J.; Masmiquel, L.; Barcelo, C.; Gonzalez-Freire, M. Organoids: An Emerging Tool to Study Aging Signature across Human Tissues. Modeling Aging with Patient-Derived Organoids. Int. J. Mol. Sci. 2021, 22, 547. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.; Koehler, K.R.; Shafiee, A. Biofabrication of Human Skin with Its Appendages. Adv. Healthc. Mater. 2022, 11, e2201626. [Google Scholar] [CrossRef] [PubMed]
- Cavallero, S.; Neves Granito, R.; Stockholm, D.; Azzolin, P.; Martin, M.T.; Fortunel, N.O. Exposure of Human Skin Organoids to Low Genotoxic Stress Can Promote Epithelial-to-Mesenchymal Transition in Regenerating Keratinocyte Precursor Cells. Cells 2020, 9, 1912. [Google Scholar] [CrossRef]
- Metallo, C.M.; Ji, L.; de Pablo, J.J.; Palecek, S.P. Retinoic acid and bone morphogenetic protein signaling synergize to efficiently direct epithelial differentiation of human embryonic stem cells. Stem Cells 2008, 26, 372–380. [Google Scholar] [CrossRef]
- Miyake, T.; Shimada, M.; Matsumoto, Y.; Okino, A. DNA Damage Response After Ionizing Radiation Exposure in Skin Keratinocytes Derived from Human-Induced Pluripotent Stem Cells. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Feldman, A.; Mukha, D.; Maor, I.I.; Sedov, E.; Koren, E.; Yosefzon, Y.; Shlomi, T.; Fuchs, Y. Blimp1(+) cells generate functional mouse sebaceous gland organoids in vitro. Nat. Commun. 2019, 10, 2348. [Google Scholar] [CrossRef] [PubMed]
- Diao, J.; Liu, J.; Wang, S.; Chang, M.; Wang, X.; Guo, B.; Yu, Q.; Yan, F.; Su, Y.; Wang, Y. Sweat gland organoids contribute to cutaneous wound healing and sweat gland regeneration. Cell Death Dis. 2019, 10, 238. [Google Scholar] [CrossRef]
- Rossi, G.; Manfrin, A.; Lutolf, M.P. Progress and potential in organoid research. Nat. Rev. Genet. 2018, 19, 671–687. [Google Scholar] [CrossRef]
- Sun, X.; Sun, F.; Zhang, Y.; Qu, J.; Zhang, W.; Liu, G.-H. A narrative review of organoids for investigating organ aging: Opportunities and challenges. J. Bio-XResearch 2023, 6, 3–14. [Google Scholar] [CrossRef]
- Kim, M.J.; Ahn, H.J.; Kong, D.; Lee, S.; Kim, D.H.; Kang, K.S. Modeling of solar UV-induced photodamage on the hair follicles in human skin organoids. J. Tissue Eng. 2024, 15, 20417314241248753. [Google Scholar] [CrossRef]
- Ding, X.; Kakanj, P.; Leptin, M.; Eming, S.A. Regulation of the Wound Healing Response during Aging. J. Investig. Dermatol. 2021, 141, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Ju, J.H. Generation of 3D Skin Organoid from Cord Blood-derived Induced Pluripotent Stem Cells. J. Vis. Exp. 2019, 146, e59297. [Google Scholar] [CrossRef]
- Oceguera-Yanez, F.; Avila-Robinson, A.; Woltjen, K. Differentiation of pluripotent stem cells for modeling human skin development and potential applications. Front. Cell Dev. Biol. 2022, 10, 1030339. [Google Scholar] [CrossRef] [PubMed]
- Dash, B.C.; Korutla, L.; Vallabhajosyula, P.; Hsia, H.C. Unlocking the Potential of Induced Pluripotent Stem Cells for Wound Healing: The Next Frontier of Regenerative Medicine. Adv. Wound Care 2022, 11, 622–638. [Google Scholar] [CrossRef]
- Lo Sardo, V.; Ferguson, W.; Erikson, G.A.; Topol, E.J.; Baldwin, K.K.; Torkamani, A. Influence of donor age on induced pluripotent stem cells. Nat. Biotechnol. 2017, 35, 69–74. [Google Scholar] [CrossRef]
- Mahmoudi, S.; Brunet, A. Aging and reprogramming: A two-way street. Curr. Opin. Cell Biol. 2012, 24, 744–756. [Google Scholar] [CrossRef]
- Kim, J.; Koo, B.K.; Knoblich, J.A. Human organoids: Model systems for human biology and medicine. Nat. Rev. Mol. Cell Biol. 2020, 21, 571–584. [Google Scholar] [CrossRef]
- Asselineau, D.; Bernard, B.A.; Bailly, C.; Darmon, M.; Prunieras, M. Human epidermis reconstructed by culture: Is it “normal”? J. Investig. Dermatol. 1986, 86, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Prunieras, M.; Regnier, M.; Woodley, D. Methods for cultivation of keratinocytes with an air-liquid interface. J. Investig. Dermatol. 1983, 81, 28s–33s. [Google Scholar] [CrossRef]
- Hofmann, E.; Schwarz, A.; Fink, J.; Kamolz, L.P.; Kotzbeck, P. Modelling the Complexity of Human Skin In Vitro. Biomedicines 2023, 11, 794. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, J.E.; Kim, B.J.; Cho, K.H. In vitro phototoxicity test using artificial skin with melanocytes. Photodermatol. Photoimmunol. Photomed. 2007, 23, 73–80. [Google Scholar] [CrossRef]
- Hall, M.J.; Lopes-Ventura, S.; Neto, M.V.; Charneca, J.; Zoio, P.; Seabra, M.C.; Oliva, A.; Barral, D.C. Reconstructed human pigmented skin/epidermis models achieve epidermal pigmentation through melanocore transfer. Pigment. Cell Melanoma Res. 2022, 35, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Nedachi, T.; Bonod, C.; Rorteau, J.; Chinoune, W.; Ishiuchi, Y.; Hughes, S.; Gillet, B.; Bechetoille, N.; Sigaudo-Roussel, D.; Lamartine, J. Chronological aging impacts abundance, function and microRNA content of extracellular vesicles produced by human epidermal keratinocytes. Aging 2023, 15, 12702–12722. [Google Scholar] [CrossRef]
- Cho, S.W.; Malick, H.; Kim, S.J.; Grattoni, A. Advances in Skin-on-a-Chip Technologies for Dermatological Disease Modeling. J. Investig. Dermatol. 2024, 144, 1707–1715. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Sito, L.; Mao, M.; He, J.; Zhang, Y.S.; Zhao, X. Current advances in skin-on-a-chip models for drug testing. Microphysiol. Syst. 2018, 2, 4. [Google Scholar] [CrossRef]
- Abaci, H.E.; Gledhill, K.; Guo, Z.Y.; Christiano, A.M.; Shuler, M.L. Pumpless microfluidic platform for drug testing on human skin equivalents. Lab Chip 2015, 15, 882–888. [Google Scholar] [CrossRef]
- Sutterby, E.; Thurgood, P.; Baratchi, S.; Khoshmanesh, K.; Pirogova, E. Microfluidic Skin-on-a-Chip Models: Toward Biomimetic Artificial Skin. Small 2020, 16, e2002515. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Getschman, A.E.; Hwang, S.; Volkman, B.F.; Klonisch, T.; Levin, D.; Zhao, M.; Santos, S.; Liu, S.; Cheng, J.; et al. Investigations on T cell transmigration in a human skin-on-chip (SoC) model. Lab Chip 2021, 21, 1527–1539. [Google Scholar] [CrossRef]
- Risueno, I.; Valencia, L.; Jorcano, J.L.; Velasco, D. Skin-on-a-chip models: General overview and future perspectives. APL Bioeng. 2021, 5, 030901. [Google Scholar] [CrossRef]
- Liu, H.; Xing, F.; Yu, P.; Zhe, M.; Duan, X.; Liu, M.; Xiang, Z.; Ritz, U. A review of biomacromolecule-based 3D bioprinting strategies for structure-function integrated repair of skin tissues. Int. J. Biol. Macromol. 2024, 268, 131623. [Google Scholar] [CrossRef]
- Ong, C.S.; Yesantharao, P.; Huang, C.Y.; Mattson, G.; Boktor, J.; Fukunishi, T.; Zhang, H.; Hibino, N. 3D bioprinting using stem cells. Pediatr. Res. 2018, 83, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.L.; Qi, J.T.Z.; Yeong, W.Y.; Naing, M.W. Proof-of-concept: 3D bioprinting of pigmented human skin constructs. Biofabrication 2018, 10, 025005. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Luo, Y.; Ma, Y.; Wang, P.; Yao, R. Converging bioprinting and organoids to better recapitulate the tumor microenvironment. Trends Biotechnol. 2024, 42, 648–663. [Google Scholar] [CrossRef] [PubMed]
- Arslan-Yildiz, A.; El Assal, R.; Chen, P.; Guven, S.; Inci, F.; Demirci, U. Towards artificial tissue models: Past, present, and future of 3D bioprinting. Biofabrication 2016, 8, 014103. [Google Scholar] [CrossRef]
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2020, 226, 119536. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Xu, S.; Wang, J. 3D Bioprinting: An Important Tool for Tumor Microenvironment Research. Int. J. Nanomedicine 2023, 18, 8039–8057. [Google Scholar] [CrossRef]
- Ribezzi, D.; Barbaglio, F.; Pinos, R.; Bonetti, L.; Farè, S.; Scielzo, C. Design of a Novel Bioink Suitable for the 3d Printing of Lymphoid Cells. Front. Biomater. Sci. 2022, 28, S545. [Google Scholar] [CrossRef]
- Puistola, P.; Miettinen, S.; Skottman, H.; Moro, A. Novel strategy for multi-material 3D bioprinting of human stem cell based corneal stroma with heterogenous design. Mater. Today Bio 2024, 24, 100924. [Google Scholar] [CrossRef]
- Kang, M.S.; Jang, J.; Jo, H.J.; Kim, W.H.; Kim, B.; Chun, H.J.; Lim, D.; Han, D.W. Advances and Innovations of 3D Bioprinting Skin. Biomolecules 2022, 13, 55. [Google Scholar] [CrossRef]
- Zhang, Y.; Enhejirigala; Yao, B.; Li, Z.; Song, W.; Li, J.; Zhu, D.; Wang, Y.; Duan, X.; Yuan, X.; et al. Using bioprinting and spheroid culture to create a skin model with sweat glands and hair follicles. Burns Trauma. 2021, 9, tkab013. [Google Scholar] [CrossRef]
- Weng, T.T.; Zhang, W.; Xia, Y.L.; Wu, P.; Yang, M.; Jin, R.H.; Xia, S.Z.; Wang, J.L.; You, C.G.; Han, C.M.; et al. 3D bioprinting for skin tissue engineering: Current status and perspectives. J. Tissue Eng. 2021, 12, 20417314211028574. [Google Scholar] [CrossRef]
- Ansaf, R.B.; Ziebart, R.; Gudapati, H.; Simoes Torigoe, R.M.; Victorelli, S.; Passos, J.; Wyles, S.P. 3D bioprinting-a model for skin aging. Regen. Biomater. 2023, 10, rbad060. [Google Scholar] [CrossRef] [PubMed]
- Bhar, B.; Das, E.; Manikumar, K.; Mandal, B.B. 3D Bioprinted Human Skin Model Recapitulating Native-Like Tissue Maturation and Immunocompetence as an Advanced Platform for Skin Sensitization Assessment. Adv. Healthc. Mater. 2024, 13, e2303312. [Google Scholar] [CrossRef] [PubMed]
- Olejnik, A.; Semba, J.A.; Kulpa, A.; Danczak-Pazdrowska, A.; Rybka, J.D.; Gornowicz-Porowska, J. 3D Bioprinting in Skin Related Research: Recent Achievements and Application Perspectives. ACS Synth. Biol. 2022, 11, 26–38. [Google Scholar] [CrossRef]
- Sun, W.; Wang, B.; Yang, T.; Yin, R.; Wang, F.; Zhang, H.; Zhang, W. Three-Dimensional Bioprinted Skin Microrelief and Its Role in Skin Aging. Biomimetics 2024, 9, 366. [Google Scholar] [CrossRef] [PubMed]
- Shboul, S.A.; DeLuca, V.J.; Dweiri, Y.A.; Saleh, T. Can 3D bioprinting solve the mystery of senescence in cancer therapy? Ageing Res. Rev. 2022, 81, 101732. [Google Scholar] [CrossRef]
- Prasanna, P.G.; Citrin, D.E.; Hildesheim, J.; Ahmed, M.M.; Venkatachalam, S.; Riscuta, G.; Xi, D.; Zheng, G.; Deursen, J.V.; Goronzy, J.; et al. Therapy-Induced Senescence: Opportunities to Improve Anticancer Therapy. J. Natl. Cancer Inst. 2021, 113, 1285–1298. [Google Scholar] [CrossRef]
- Szabo, K.; Bolla, B.S.; Erdei, L.; Balogh, F.; Kemeny, L. Are the Cutaneous Microbiota a Guardian of the Skin’s Physical Barrier? The Intricate Relationship between Skin Microbes and Barrier Integrity. Int. J. Mol. Sci. 2023, 24, 5962. [Google Scholar] [CrossRef]
- Boxberger, M.; Cenizo, V.; Cassir, N.; La Scola, B. Challenges in exploring and manipulating the human skin microbiome. Microbiome 2021, 9, 125. [Google Scholar] [CrossRef]
- Meisel, J.S.; Sfyroera, G.; Bartow-McKenney, C.; Gimblet, C.; Bugayev, J.; Horwinski, J.; Kim, B.; Brestoff, J.R.; Tyldsley, A.S.; Zheng, Q.; et al. Commensal microbiota modulate gene expression in the skin. Microbiome 2018, 6, 20. [Google Scholar] [CrossRef]
- Bolla, B.S.; Erdei, L.; Urban, E.; Burian, K.; Kemeny, L.; Szabo, K. Cutibacterium acnes regulates the epidermal barrier properties of HPV-KER human immortalized keratinocyte cultures. Sci. Rep. 2020, 10, 12815. [Google Scholar] [CrossRef] [PubMed]
- Pivarcsi, A.; Bodai, L.; Rethi, B.; Kenderessy-Szabo, A.; Koreck, A.; Szell, M.; Beer, Z.; Bata-Csorgoo, Z.; Magocsi, M.; Rajnavolgyi, E.; et al. Expression and function of Toll-like receptors 2 and 4 in human keratinocytes. Int. Immunol. 2003, 15, 721–730. [Google Scholar] [CrossRef]
- Larson, P.J.; Chong, D.; Fleming, E.; Oh, J. Challenges in Developing a Human Model System for Skin Microbiome Research. J. Investig. Dermatol. 2021, 141, 228–231.e4. [Google Scholar] [CrossRef]
- Emmert, H.; Rademacher, F.; Glaser, R.; Harder, J. Skin microbiota analysis in human 3D skin models-”Free your mice”. Exp. Dermatol. 2020, 29, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Landemaine, L.; Cenizo, V.; Lemaire, G.J.; Portes, P. 961 Colonization of a 3D skin model with a complete microbiota is more beneficial to the skin barrier than with Staphylococcus epidermidis alone. J. Investig. Dermatol. 2018, 138, S163. [Google Scholar] [CrossRef]
- Loomis, K.H.; Wu, S.K.; Ernlund, A.; Zudock, K.; Reno, A.; Blount, K.; Karig, D.K. A mixed community of skin microbiome representatives influences cutaneous processes more than individual members. Microbiome 2021, 9, 22. [Google Scholar] [CrossRef]
- Myers, T.; Bouslimani, A.; Huang, S.; Hansen, S.T.; Clavaud, C.; Azouaoui, A.; Ott, A.; Gueniche, A.; Bouez, C.; Zheng, Q.; et al. A multi-study analysis enables identification of potential microbial features associated with skin aging signs. Front. Aging 2023, 4, 1304705. [Google Scholar] [CrossRef]
- Janson, D.; Rietveld, M.; Willemze, R.; El Ghalbzouri, A. Effects of serially passaged fibroblasts on dermal and epidermal morphogenesis in human skin equivalents. Biogerontology 2013, 14, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Adamus, J.; Aho, S.; Meldrum, H.; Bosko, C.; Lee, J.M. p16INK4A influences the aging phenotype in the living skin equivalent. J. Investig. Dermatol. 2014, 134, 1131–1133. [Google Scholar] [CrossRef]
- Wedel, S.; Martic, I.; Hrapovic, N.; Fabre, S.; Madreiter-Sokolowski, C.T.; Haller, T.; Pierer, G.; Ploner, C.; Jansen-Dürr, P.; Cavinato, M. tBHP treatment as a model for cellular senescence and pollution-induced skin aging. Mech. Ageing Dev. 2020, 190, 111318. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, S.; Luo, D.; Chen, D.; Zhou, H.; Zhang, S.; Chen, X.; Lu, W.; Liu, W. Systematic Study of Resveratrol Nanoliposomes Transdermal Delivery System for Enhancing Anti-Aging and Skin-Brightening Efficacy. Molecules 2023, 28, 2738. [Google Scholar] [CrossRef] [PubMed]
- Wedel, S.; Martic, I.; Navarro, L.G.; Ploner, C.; Pierer, G.; Jansen-Dürr, P.; Cavinato, M. Depletion of growth differentiation factor 15 (GDF15) leads to mitochondrial dysfunction and premature senescence in human dermal fibroblasts. Aging Cell 2023, 22, e13752. [Google Scholar] [CrossRef]
- Klinngam, W.; Rungkamoltip, P.; Wongwanakul, R.; Joothamongkhon, J.; Du, A.M.S.; Khongkow, M.; Asawapirom, U.; Iempridee, T.; Ruktanonchai, U. Skin Rejuvenation Efficacy and Safety Evaluation of Kaempferia parviflora Standardized Extract (BG100) in Human 3D Skin Models and Clinical Trial. Biomolecules 2024, 14, 776. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Jung, Y.S.; Cho, Y.R.; Seo, J.W.; Lim, W.C.; Nam, T.G.; Lim, T.G.; Byun, S. Oral Administration of Prevents UVB-Induced Skin Aging through Targeting the c-Raf Signaling Axis. Antioxidants 2021, 10, 1663. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.O.; Smith, A.; Liu, Y.; Du, P.; Blumberg, J.B.; Garlick, J. Photoprotection by pistachio bioactives in a 3-dimensional human skin equivalent tissue model. Int. J. Food Sci. Nutr. 2017, 68, 712–718. [Google Scholar] [CrossRef]
- Yonezawa, T.; Momota, R.; Iwano, H.; Zhao, S.; Hakozaki, T.; Soh, C.; Sawaki, S.; Toyama, K.; Oohashi, T. Unripe peach (Prunus persica) extract ameliorates damage from UV irradiation and improved collagen XVIII expression in 3D skin model. J. Cosmet. Dermatol. 2019, 18, 1507–1515. [Google Scholar] [CrossRef]
- Fernandez-Carro, E.; Remacha, A.R.; Orera, I.; Lattanzio, G.; Garcia-Barrios, A.; del Barrio, J.; Alcaine, C.; Ciriza, J. Human Dermal Decellularized ECM Hydrogels as Scaffolds for 3D In Vitro Skin Aging Models. Int. J. Mol. Sci. 2024, 25, 4020. [Google Scholar] [CrossRef]
- Min, D.; Ahn, Y.; Lee, H.K.; Jung, W.; Kim, H.J. A novel optical coherence tomography-based in vitro method of anti-aging skin analysis using 3D skin wrinkle mimics. Skin Res. Technol. 2023, 29, e13354. [Google Scholar] [CrossRef]


| SoC Advantages | Ref. | SoC Disadvantages and/or Limitations | Ref. |
|---|---|---|---|
| Reproducible and reliable skin systems | [84] | Highly complex to design and develop | [12] |
| Ensures a physiologically cell density by supplying oxygen and nutrients while removing waste through culture media perfusion | [86] | Excludes immune cells, hair follicles, and sweat glands | [87] |
| Better simulation of barrier function, epidermal thickness, keratinocyte differentiation, and immune response in in vitro skin models | [88] | Requires significant time and work investments | [12] |
| Substance transport is more physiologically relevant, allowing for a more accurate evaluation of parameters (i.e., molecule toxicity and delivery) | [89] | Mainly focuses on the skin excluding between organs | [84] |
| Model | Structure and Cell Type | Culture Conditions | Stimuli to Induce Aging and Time to Establish Aged Model | End-Points | Ref. |
|---|---|---|---|---|---|
| PSEUDO-3D SYSTEMS | 2.5D collagen model Human dermal fibroblasts (FF-95) | Collagen solution mixed with DMEM (10% FBS). | Replicative senescence. The entire process of cultivating fibroblasts, inducing senescence, and conducting experiments spanned several weeks. The exact duration is not explicitly detailed. | 2.5-dimensional migration assay used to determine and compare speeds of migration and contraction of young and senescent dermal fibroblasts. | [49] |
| ORGANOIDS | 3D organoid model composed of iPSC-derived keratinocytes. iPSCs | To differentiate iPSCs into keratinocytes: DKSFM medium supplemented with retinoic acid and BMP. At 4th day, medium was replaced with DKSFM in presence of EGF. iPSC-derived keratinocyte culture: DKSFM supplemented with EGF and Y27632 on the dish precoated with type I collagen and fibronectin. To generate organoid: fibroblasts were embedded in type I collagen gel in the 0.4 μm pores insert and cultured in DMEM for 1 week; keratinocytes differentiated from iPSCs were seeded on collagen gel and cultured for 2 days in DKSFM supplemented with EGF and ROCK inhibitor Y27632. | Organoids were irradiated with γ-rays using cobalt 60 as the radiation source. The dose rate was 0.44–2.61 Gy/min. Induction of iPSCs to keratinocytes from 14 up to 34 days. Construction of organoid on collagen hydrogel 14 days. Culturing the 3D skin model 1-2 weeks. | In keratinocytes present on organoids, the IR exposure enhanced senescence markers (p16 and p21) and g-H2AX foci formation. Application: skin function and DNA damage response after ionizing radiation exposure in 3D skin organoid. | [65] |
| Model of sUV-exposed skin using human iPSC-derived skin organoids containing hair follicles. iPSCs | To establish skin organoid: on day 0, iPSCs seeded in essential medium with ROCK inhibitor, Y-27632, to generate EBs. At EB size of 250 μm, transfer of EBs into individual new ultra-low attachment plates in differentiation medium containing matrigel, and specific factors to initiate non-neural ectoderm induction. To induce cranial neural crest cell formation after 4 days, specific factors to prevent off-target chondrogenic differentiation were added. On day 12, to induce self-assembly of the epidermis: transfer all organoids into ultra-low attachment plates in skin organoid maturation medium. | sUV irradiation: 20 min, twice with a 2 h interval between exposures, resulting in a total of 50 kJ/m2 of sUV. These exposures were conducted at 2-day intervals for a total of three exposures. Generation of skin organoids 10–12 weeks. Induction of sUV damage 20 min, twice with a 2 h interval between exposures, for a total of three exposures conducted at 2-day intervals. | This model recapitulated several symptoms of photodamage, including skin barrier disruption, extracellular matrix degradation, and inflammatory response. Moreover, sUV induced structural damage and catagenic transition in hair follicles. | [70] | |
| RECONSTRUCTED HUMAN SKIN | Reconstructed human epidermis (RHE). Normal young (<5 years) and aged (>60 years) human primary keratinocytes obtained from child foreskin or abdominal biopsies. | Keratinocytes grown submerged in the culture medium (DMEM-F12) for 72 h, then kept emerged at the air–liquid interface for 14 days in the medium (DMEM-F12 + CaCl2 supplemented with vitamin C) changed daily and supplemented with extracellular vesicles every 2 days. | Treatment with extracellular vesicles obtained from old keratinocytes’ culture medium. The entire process from cell seeding to the completion of the epidermal reconstruction took approximately 16 days. | Decrease in the tissue thickness. The changes in the intercellular communication mediated by extracellular vesicles occurring during aging process in keratinocytes could be involved in the functional defects observed in aged skin. | [83] |
| 3D skin equivalent. Fibroblasts and keratinocytes. | Co-culture of skin fibroblasts and keratinocytes on a collagen–glycosaminoglycan–chitosan scaffold. | Mitomycin C. The overall process, including seeding and culture, took approximately 14 days to achieve a fully differentiated 3D skin model. | Appearance of typical histological and biomolecular features of aged skin. This model, with comparable characteristics of in vivo aged skin, is proposed as a valuable tool to study the aging process and the effects of potential anti-aging formulations in a more physiological environment. | [26] | |
| Full-thickness human skin equivalent generated with early- and late-passage fibroblasts. Primary fibroblasts and keratinocytes isolated from surplus skin from cosmetic surgery. | Fibroblasts were cultured within a collagen matrix, overlaid with human keratinocytes to form a stratified epidermis, and maintained in standard culture conditions. | Replicative senescence of fibroblasts. Overall time was approximately three weeks. | Skin equivalents with late-passage fibroblasts had a thinner dermis than early-passage fibroblasts. The study provides evidence of dermal–epidermis crosstalk and insight into how aging fibroblasts affect skin structure. | [118] | |
| Human skin equivalent. Primary human dermal fibroblasts and keratinocytes. | Young or senescent human dermal fibroblasts (senoskin) were seeded in a collagen gel. Keratinocytes were laid on top of fibroblasts and were then lifted to the air–liquid interface to start differentiation on day 3. | H2O2 and doxorubicin. After 10 days, the skin equivalents were harvested. The overall process, including seeding and culture, took approximately 2–3 weeks. | The use of senescent fibroblasts in the 3D skin model led to significant structural alterations (thinning of the epidermal layer and a reduction in dermal collagen) that mimic those observed in aged human skin. | [40] | |
| Full-thickness human skin equivalent. Primary human dermal keratinocytes isolated from skin biopsies of healthy adult donors. | Primary human dermal keratinocytes were embedded in collagen I matrix, keratinocytes were seeded on top and submerged for 7 days. The keratinocytes were placed at the air–liquid interface for 7 days. Then, the skin equivalents were treated with the extract of Solidago alpestris. | Replicative senescence and H2O2. The time needed to establish the 3D aged model was not specified in detail. | The extract of Solidago alpestris exhibited weak senolytic activity and reduced hallmarks of senescence. Treatment with the extract led to increased cell proliferation and the maintenance of epidermal thickness in the 3D skin models, suggesting a protective effect against age-related thinning of the skin. | [39] | |
| Full-thickness human skin equivalent. Primary fibroblast and keratinocytes. | Fibroblasts were cultured in a collagen matrix to form the dermal layer, overlaid with keratinocytes to form the epidermal layer. The culture was maintained at the air–liquid interface to promote stratification and differentiation of the epidermal layer. | Aging was induced by manipulating the expression of p16INK4A, a key regulator of cellular senescence. Although if time to obtain a 3D aged model is not clearly specified, it can be assumed that the overall process could take about 2–3 weeks. | Overexpression of p16INK4A in keratinocytes induced the aging phenotype, characterized by reduced cell proliferation and alterations in the skin structure, such as thinning of the epidermal layer. Silencing p16INK4A led to a loss of the aging phenotype. | [119] | |
| Full-thickness human skin equivalent. Human keratinocytes and human dermal fibroblasts isolated from abdominal skin derived from plastic surgery. | Cells were treated with Tert-butyl hydroperoxide (tBHP) twice a day for 4 days and cultured for 9 days. Fibroblasts were mixed with collagen matrix. Keratinocytes were placed over the dermal layer and grown in submersed conditions for 48 h. Skin equivalents were cultured to air–liquid interface in differentiation media with ascorbic acid, transferrin, and CaCl2 for 7 days. | tBHP, an inducer of oxidative stress and cellular damage. The overall process, after initial cell seeding, took approximately 2 weeks. | tBHP could serve as a valuable agent for modeling the effects of environmental pollutants on skin aging and for assessing potential anti-aging therapies. | [120] | |
| Commercial full-thickness skin model. Human keratinocytes and human dermal fibroblasts. | The skin was treated with Resveratrol nanoliposomes (Res-NLPs). | Hydrogen peroxide (H2O2) or UV irradiation. The detailed timeline beyond the general 2–3 weeks for the 3D skin model was not mentioned. | Resveratrol nanoliposomes significantly enhanced skin care benefits by improving antioxidant capacity and promoting collagen synthesis. | [121] | |
| Skin equivalents. Human foreskin fibroblasts (HFFs). | Fibroblasts, after transfection with siRNA, were suspended in the collagen solution and allowed to polymerize at 37 °C. | Growth differentiation factor 15 (GDF15) knockdown in HFF induced premature senescence associated with mitochondrial dysfunction. The overall process, after initial cell seeding, took approximately 1–2 weeks. | GDF15 knockdown triggered mitochondrial stress, inducing senescence and reducing epidermal thickness in skin equivalents. This model allows mimicking the in vivo conditions of skin aging and assessing the role of GDF15 in maintaining cellular and mitochondrial health. | [122] | |
| Commercial full-thickness skin model, which included both dermal and epidermal components. | Skin model was topically treated with a standardized extract of Kaempferia parviflora (BG100) daily for six days. | UV exposure. Because a commercial skin model was used, the time needed to create the aged model is that of UV exposure (exposure daily for five days). | BG100 could be a promising component for anti-aging formulations, as it supports skin structure and reduces oxidative stress induced by UV exposure. | [123] | |
| Commercial full-thickness skin model, which included dermal fibroblasts and keratinocytes. | The skin was treated with rosa gallica extract for 1 h before UVB irradiation and then irradiated with UVB twice a day for 8 days. | UVB radiation. The overall process took approximately 10 days. | Rosa gallica extract reduced wrinkle formation, prevented collagen degradation, and downregulated COX-2 and MMP-1 expression in the skin model by targeting the c-Raf/MEK/ERK signaling pathway, effectively blocking UVB-induced aging effects. | [124] | |
| Human skin equivalent (HSE). Human dermal fibroblasts (HFFs) were collected from newborn foreskin and human keratinocytes. | HFF were mixed with type I collagen for 7 days, keratinocytes were allowed to grow inside low calcium epidermal growth media for 2 days and then inside normal calcium media for an additional 2 days. The HSE was cultured to the air–liquid interface for 7 days before use. HSE was treated with lutein and γ-tocopherol, key antioxidants found in pistachios. | UVA radiation. The overall process took approximately 18–20 days. | Pistachio antioxidants maintained the thickness and organization of the skin model after UVA exposure and preserved fibroblast morphology, offering a protective effect against morphological changes caused by UVA radiation. | [125] | |
| Commercial human 3D skin culture system. Human dermal fibroblasts and keratinocytes. | The skin was incubated with unripe peach (YPE) extract and UVB irradiated. | UVB radiation. Because a commercial skin model was used, the time needed to create the aged model is that of UVB exposure (at day 2, 4, and 7). | YPE could exert a protective role against UVB-induced skin aging focusing on maintaining the structural integrity of the skin by preserving key components like collagen XVIII. | [126] | |
| SKIN-ON-CHIP | Wrinkled skin-on-chip (WSOC) developed by cyclic uniaxial stretching. Human fibroblasts and human keratinocytes cultured in DMEM with 10% FBS and KGM, respectively. | To form the structure of the WSOC, two PDMS layers were fabricated and combined. The collagen layer was formed in the cell chamber by adding a collagen solution containing fibroblasts. To form the stratum corneum of the epidermis, keratinocytes were sprayed onto the collagen layer of the WSOC. The WSOC was incubated for 1 h in a CO2 incubator for cell attachment. Every day for 4 days, to keratinocytes on the chip were given fresh KGM and the fibroblasts on the chip were perfused with fresh DMEM through the microchannels of the WSOC. The cells on the WSOC were exposed to air for differentiation. | Attractive and repulsive forces between a permanent magnet embedded in the wall of the cell culture chamber and an electromagnet outside the wall uniaxially stretched the skin equivalent at 5.3 mm/s. WSOC fabrication time 2 h. Fibroblasts were cultured for 4 days within the WSOC. Keratinocytes were then sprayed onto the collagen layer containing fibroblasts and incubated for another 4 days. The WSOC was subjected to uniaxial stretching for 12 h per day for 7 days. The entire process took approximately 2 weeks. | The stretching decreased the proliferation of fibroblasts and keratinocytes, resulting in lower collagen, fibronectin, and keratin production. Owing to the lower production of these proteins, the skin equivalents were not able to maintain their stratum corneum and withstand the tensile stress applied via magnetic stretching, resulting in the formation of wrinkles. Application: WSOCs can be used to test the effect of anti-wrinkle ingredients and cosmetics prior to in vivo experiments. | [27] |
| Flexible skin-on-a-chip (FSOC). The FSOC comprises an upper and lower PDMS chips with a porous member within. In the centre of the FSOC, there is a culture chamber to cultivate cells. Primary human fibroblasts and primary human keratinocytes. | To obtain the dermal layer, the fibroblasts were mixed with type I collagen solution (5 days). To obtain the epidermal layer, the keratinocytes were attached to the dermal layer (2 days) and exposed to the air–liquid interface (3–28-days). | Mechanical compression stimulation (that reflects circadian rhythms) was applied to a 3D skin equivalent to produce an aging skin model. The entire process took approximately 35–37 days (including the initial setup and the 28-day stimulation period). | Aged full-thickness skin equivalent model that uses mechanical stimulus reflective of the circadian rhythm. Application: this model could be useful for conducting in vitro drug efficacy assessments and investigating new cosmetics. | [28] | |
| 3D microfluidic polydimethylsiloxane (PDMS)-based chip consisted of microvessel surrounded by a collagen gel with embedded young or senescent skin fibroblasts. Primary human skin fibroblasts (HCA2) and primary human umbilical vein endothelial cells (HUVEC). | Senescent fibroblasts were embedded in a collagen solution and placed in the PDMS-based chips that were incubated at 37 °C for 1 h to enable gelation of collagen. HUVEC suspension was inserted into the channel and left to attach onto the collagen scaffold at 37 °C for 15 min. | Irradiation with 10 Gy of γ-ray. Although if time to obtain a 3D aged model is not clearly specified, it can be assumed that the overall process could take about 2 weeks. | Senescent, but not young, fibroblasts had the ability to induce sprouting angiogenesis. Senescent fibroblasts induced a pro-inflammatory environment due to their secretion of the senescence-associated secretory phenotype (SASP). This inflammatory milieu contributed to the dysfunction of endothelial cells. Senescent fibroblasts were able to mechanically rearrange the ECM fibers. | [29] | |
| Customized microfluidic device based on cyclic olefin polymers (COPs) that allows inserting the embedded fibroblasts into a chamber through a porous membrane to a second chamber where keratinocytes were grown. Human dermal fibroblasts and human immortalized keratinocytes (HaCaT). | Human dermal tissues from aged human cadavers were decellularized to remove all cellular components while preserving the ECM structure. The decellularized ECM was lyophilized and then reconstituted in a solution to form a hydrogel (as a scaffold for 3D skin culture). Fibroblasts were embedded in aged decellularized dermal extracellular matrix (dECM) hydrogels and HaCaT cells were seeded on top of hydrogels for 4 days and then cultured in an air–liquid interface (12 days). | dECM from aged human cadavers (70–90 years of age). The entire process took approximately 3–4 weeks. | The human dECM hydrogel, preserving essential components of the native human dermis, could be an efficient scaffold for dermal fibroblasts in a skin aging-on-a-chip model. The model offers a realistic environment for studying skin aging. | [127] | |
| 3D BIOPRINTING MODELS | 3D bioprinted skin model used to study the impact of skin microrelief on the mechanical properties and aging process of the skin. No cells used. Mixed solution of gelatin and methacrylic anhydride to form Gelatin Methacryloyl (GelMA). Printing ink formed by addition to GelMA of lithiumphenyl-2, 4, 6-trimethylbenzoyl phosphinate (LAP) initiator and the light absorber. | Protocol used for generating the 3D model:
| The aged skin model was constructed using the 3D modeling software from human skin images of aged subjects. The entire process, from GelMA preparation to the creation and printing of the skin microrelief models, spans over one week but the specific time was not detailed. | Skin samples with different microrelief levels were found to have different mechanical properties, highlighting the importance of these surface structures in maintaining skin elasticity. Three-dimensional bioprinting technology to create models that mimic the surface topography of skin at various ages. | [105] |
| Full-thickness skin wrinkle model created using a 3D printer with acrylonitrile–butadiene–styrene (ABS) as the printing material. Human neonatal dermal fibroblasts and human neonatal epidermal keratinocytes. | The dermal mixture was prepared by mixing type I collagen, reconstruction buffer, fibrinogen, aprotinin, and fibroblasts. Then, thrombin was added to initiate the fibrinogen polymerization. To construct the epidermis, keratinocytes were seeded on the dermis layer and cultured for 10 days in the air–liquid interface. | Wrinkles created in the dermal layer during the collagen gelation process mimic aging skin. Retinoic acid, an anti-aging compound, was used to evaluate changes in skin wrinkles. The time required to print the model was not detailed. | The depth and width of the wrinkles in the skin models were measured using Swept Source-Optical Coherence Tomography (SS-OCT). This technology allows for detailed imaging of the skin model’s surface and subsurface layers, providing precise measurements of the effects of anti-aging treatments. | [128] | |
| 3D AGED SKIN MODEL WITH MICROBIOTA | Normal human 3D skin model at full-thickness (Epiderm-FT). Normal human keratinocytes and normal human dermal fibroblasts. | The tissue was cultured in DMEM containing gentamicin B, amphotericin B, and growth factors. | To induce aging, 3D skin models were treated with poly I:C, an agonist of TLR3 and RIG-I-like receptors. The aged 3D skin model was treated with supernatant derived from the Streptococcus cultures (Streptococcus pneumoniae, Streptococcus infantis, and Streptococcus thermophilus) collected from young women’s faces by sterilized skin tape. The overall process took place over 28 days. | This study shows the ability of Streptococcus supernatants to restore the skin barrier in terms of elasticity, increase hydration, decrease desquamation, upregulation of collagen, and improve lipid synthesis. All these features could be attributed to spermidine, a polyamine secreted by Streptococcus cultures. | [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lombardi, F.; Augello, F.R.; Ciafarone, A.; Ciummo, V.; Altamura, S.; Cinque, B.; Palumbo, P. 3D Models Currently Proposed to Investigate Human Skin Aging and Explore Preventive and Reparative Approaches: A Descriptive Review. Biomolecules 2024, 14, 1066. https://doi.org/10.3390/biom14091066
Lombardi F, Augello FR, Ciafarone A, Ciummo V, Altamura S, Cinque B, Palumbo P. 3D Models Currently Proposed to Investigate Human Skin Aging and Explore Preventive and Reparative Approaches: A Descriptive Review. Biomolecules. 2024; 14(9):1066. https://doi.org/10.3390/biom14091066
Chicago/Turabian StyleLombardi, Francesca, Francesca Rosaria Augello, Alessia Ciafarone, Valeria Ciummo, Serena Altamura, Benedetta Cinque, and Paola Palumbo. 2024. "3D Models Currently Proposed to Investigate Human Skin Aging and Explore Preventive and Reparative Approaches: A Descriptive Review" Biomolecules 14, no. 9: 1066. https://doi.org/10.3390/biom14091066
APA StyleLombardi, F., Augello, F. R., Ciafarone, A., Ciummo, V., Altamura, S., Cinque, B., & Palumbo, P. (2024). 3D Models Currently Proposed to Investigate Human Skin Aging and Explore Preventive and Reparative Approaches: A Descriptive Review. Biomolecules, 14(9), 1066. https://doi.org/10.3390/biom14091066









