CRISPR/Cas9-Based Genome Editing of Fall Armyworm (Spodoptera frugiperda): Progress and Prospects
Abstract
:1. Introduction
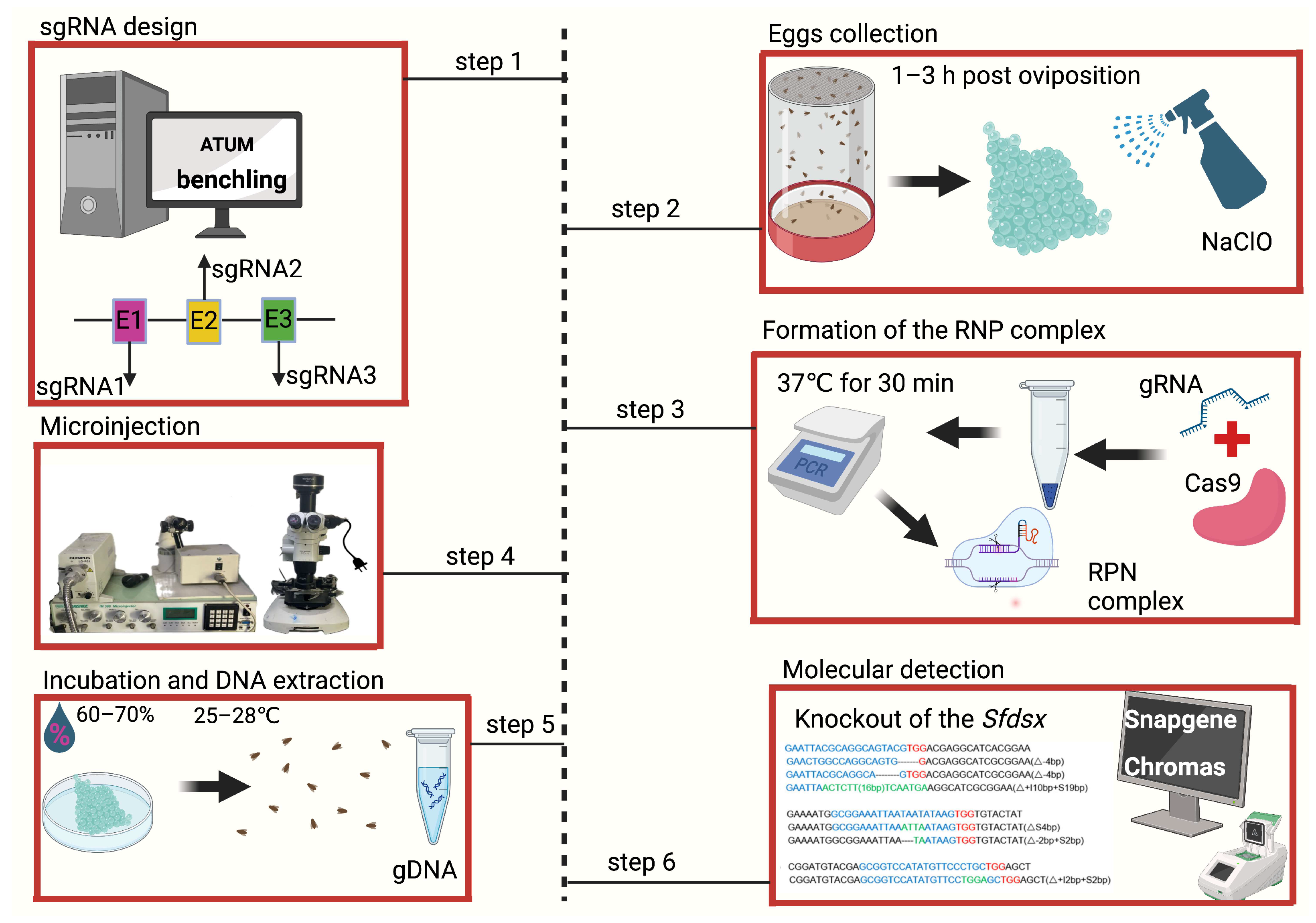
2. Procedure for Embryonic Microinjection
2.1. In Silico Design, In Vitro Synthesis, and Assessment of sgRNA
2.2. Egg Collection and Preparation
2.3. Microinjection
2.4. Incubation and Molecular Detection
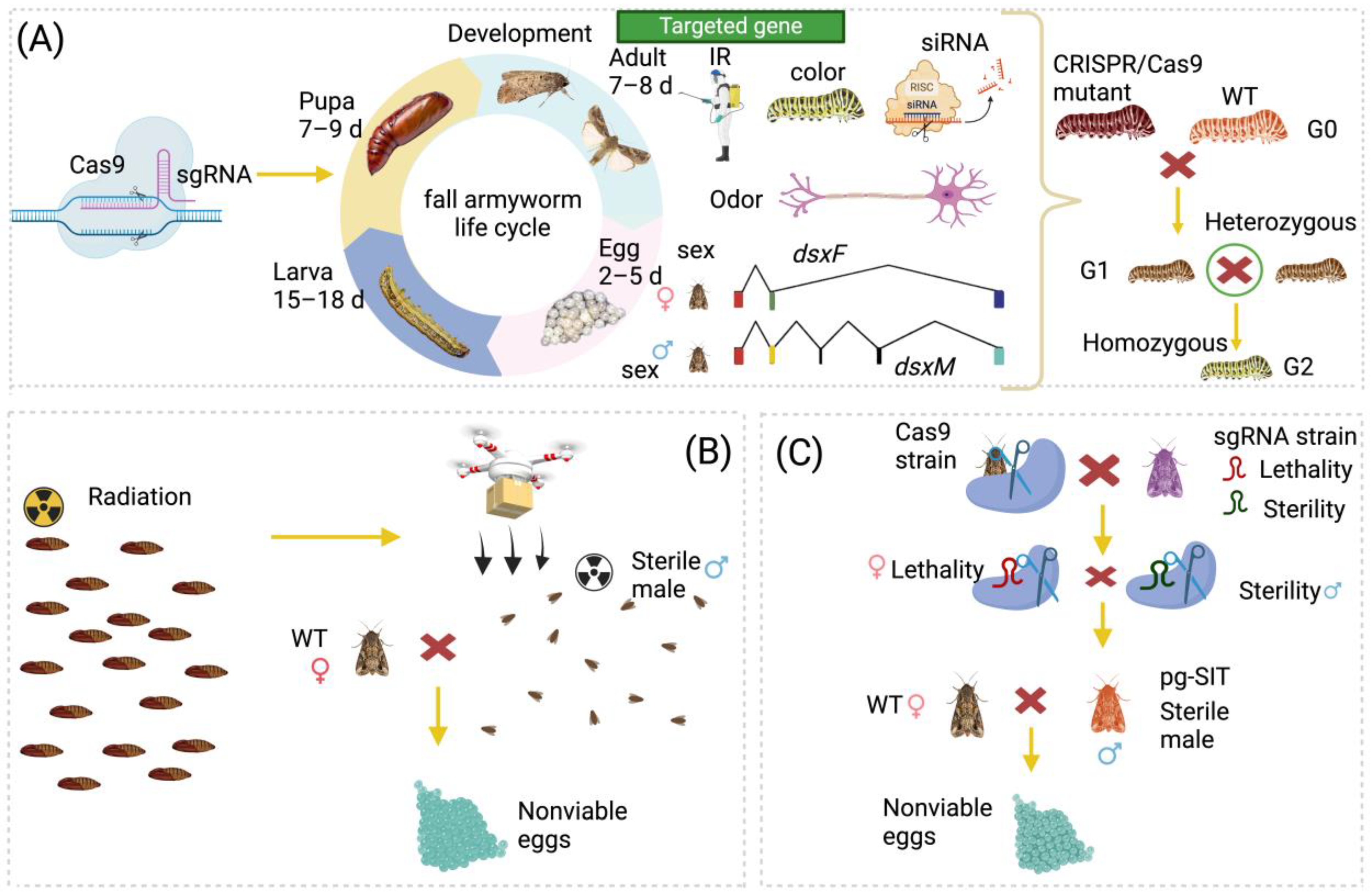
3. Application of CRISPR/Cas9 Genome Editing in S. frugiperda
3.1. Genome Editing Associated with Body Color
3.2. Genome Editing Associated with Insecticide Resistance
3.3. Genome Editing Associated with Olfactory Behavior
3.4. Genome Editing Associated with Sex Determination
3.5. Genome Editing Associated with Development
3.6. Genome Editing Associated with RNAi Machinery
4. Limitation and Future Applications of CRISPR/Cas9-Based Strategies for the Control of S. frugiperda
Author Contributions
Funding
Conflicts of Interest
References
- Agapov, A.; Baker, K.S.; Bedekar, P.; Bhatia, R.P.; Blower, T.R.; Brockhurst, M.A.; Brown, C.; Chong, C.E.; Fothergill, J.L.; Graham, S.; et al. Multi-layered genome defences in Bacteria. Curr. Opin. Microbiol. 2024, 78, 102436. [Google Scholar] [CrossRef]
- Redman, M.; King, A.; Watson, C.; King, D. What is CRISPR/Cas9? Arch. Dis. Child 2016, 101, 213–215. [Google Scholar] [CrossRef]
- Gupta, D.; Bhattacharjee, O.; Mandal, D.; Sen, M.K.; Dey, D.; Dasgupta, A.; Kazi, T.A.; Gupta, R.; Sinharoy, S.; Acharya, K.; et al. CRISPR-Cas9 system: A new-fangled dawn in gene editing. Life Sci. 2019, 232, 116636. [Google Scholar] [CrossRef]
- Drury, D.W.; Dapper, A.L.; Siniard, D.J.; Zentner, G.E.; Wade, M.J. CRISPR/Cas9 gene drives in genetically variable and nonrandom mating wild populations. Sci. Adv. 2017, 3, e1601910. [Google Scholar] [CrossRef]
- Yang, H.; Ren, S.; Yu, S.; Pan, H.; Li, T.; Ge, S.; Zhang, J.; Xia, N. Methods favoring homology-directed repair choice in response to CRISPR/Cas9 induced-double strand breaks. Int. J. Mol. Sci. 2020, 21, 6461. [Google Scholar] [CrossRef]
- Yan, Y.; Aumann, R.A.; Häcker, I.; Schetelig, M.F. CRISPR-based genetic control strategies for insect pests. J. Integr. Agric. 2023, 22, 651–668. [Google Scholar] [CrossRef]
- Kim, H.; Ishidate, T.; Ghanta, K.S.; Seth, M.; Darryl Conte, J.; Shirayama, M.; Mello, C.C. A Co-CRISPR strategy for efficient genome editing in Caenorhabditis elegans. Genetics 2014, 197, 1069. [Google Scholar] [CrossRef]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef]
- Barrangou, R.; Doudna, J.A. Applications of CRISPR technologies in research and beyond. Nat. Biotechnol. 2016, 34, 933–941. [Google Scholar] [CrossRef]
- Kumari, R.; Saha, T.; Kumar, P.; Singh, A.K. CRISPR/Cas9-mediated genome editing technique to control fall armyworm (Spodoptera frugiperda) in crop plants with special reference to Maize. Physiol. Mol. Biol. Plants 2024, 30, 1161–1173. [Google Scholar] [CrossRef] [PubMed]
- Doudna, J.A.; Charpentier, E. The New Frontier of Genome Engineering with CRISPR-Cas9. Science 2014, 346, 1258096. [Google Scholar] [CrossRef]
- Jaganathan, D.; Ramasamy, K.; Sellamuthu, G.; Jayabalan, S.; Venkataraman, G. CRISPR for crop improvement: An update review. Front. Plant Sci. 2018, 9, 985. [Google Scholar] [CrossRef]
- Singh, S.; Rahangdale, S.; Pandita, S.; Saxena, G.; Upadhyay, S.K.; Mishra, G.; Verma, P.C. CRISPR/Cas9 for insect pests’ management: A comprehensive review of advances and applications. Agriculture 2022, 12, 1896. [Google Scholar] [CrossRef]
- Yan, Y.; Ziemek, J.; Schetelig, M.F. CRISPR/Cas9 mediated disruption of the white gene leads to pigmentation deficiency and copulation failure in Drosophila suzukii. J. Insect. Physiol. 2020, 126, 104091. [Google Scholar] [CrossRef]
- Heryanto, C.; Hanly, J.J.; Mazo-Vargas, A.; Tendolkar, A.; Martin, A. Mapping and CRISPR homology-directed repair of a recessive white Eye mutation in plodia moths. iScience 2022, 25, 103885. [Google Scholar] [CrossRef]
- Bi, H.; Li, X.; Xu, X.; Wang, Y.; Zhou, S.; Huang, Y. Masculinizer and doublesex as key factors regulate sexual dimorphism in Ostrinia furnacalis. Cells 2022, 11, 2161. [Google Scholar] [CrossRef]
- Gu, J.; Wang, J.; Bi, H.; Li, X.; Merchant, A.; Zhang, P.; Zhang, Q.; Zhou, X. CRISPR/Cas9-mediated mutagenesis of sex-specific doublesex splicing variants leads to sterility in Spodoptera frugiperda, a global invasive pest. Cells 2022, 11, 3557. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Rensink, A.H.; Fricke, U.; Riddle, M.C.; Trent, C.; van de Zande, L.; Verhulst, E.C. Doublesex regulates male-specific differentiation during distinct developmental time windows in a parasitoid wasp. Insect Biochem. Mol. Biol. 2022, 142, 103724. [Google Scholar] [CrossRef]
- Alphey, N.; Bonsall, M.B. Genetics-based methods for agricultural insect pest management. Agric. For. Entomol. 2018, 20, 131–140. [Google Scholar] [CrossRef]
- Cannon, P.M.; Kiem, H.-P. The genome-editing decade. Mol. Ther. 2021, 29, 3093–3094. [Google Scholar] [CrossRef]
- Li, J.-J.; Shi, Y.; Wu, J.-N.; Li, H.; Smagghe, G.; Liu, T.-X. CRISPR/Cas9 in lepidopteran insects: Progress, application and prospects. J. Insect Physiol. 2021, 135, 104325. [Google Scholar] [CrossRef]
- Andrade, M.V.; Noronha, K.; Diniz, B.P.C.; Guedes, G.; Carvalho, L.R.; Silva, V.A.; Calazans, J.A.; Santos, A.S.; Silva, D.N.; Castro, M.C. The economic burden of malaria: A systematic review. Malar. J. 2022, 21, 283. [Google Scholar] [CrossRef]
- Adelman, Z.N.; Tu, Z. Control of Mosquito-borne infectious diseases: Sex and gene drive. Trends Parasitol. 2016, 32, 219–229. [Google Scholar] [CrossRef]
- Tajudeen, Y.A.; Oladipo, H.J.; Oladunjoye, I.O.; Oladipo, M.K.; Shittu, H.D.; Abdulmumeen, I.-F.; Afolabi, A.O.; El-Sherbini, M.S. Transforming malaria prevention and control: The prospects and challenges of gene drive technology for mosquito management. Ann. Med. 2023, 55, 2302504. [Google Scholar] [CrossRef]
- Matova, P.M.; Kamutando, C.N.; Magorokosho, C.; Kutywayo, D.; Gutsa, F.; Labuschagne, M. Fall-armyworm invasion, control practices and resistance breeding in Sub-Saharan Africa. Crop. Sci. 2020, 60, 2951–2970. [Google Scholar] [CrossRef]
- Day, R.; Abrahams, P.; Bateman, M.; Beale, T.; Clottey, V.; Cock, M.; Colmenarez, Y.; Corniani, N.; Early, R.; Godwin, J.; et al. Fall armyworm: Impacts and implications for Africa. Outlooks Pest. Manag. 2017, 28, 196–201. [Google Scholar] [CrossRef]
- Prasanna, B.M.; Bruce, A.; Beyene, Y.; Makumbi, D.; Gowda, M.; Asim, M.; Martinelli, S.; Head, G.P.; Parimi, S. Host plant resistance for fall armyworm management in maize: Relevance, status and prospects in Africa and Asia. Theor. Appl. Genet. 2022, 135, 3897–3916. [Google Scholar] [CrossRef]
- Xiaoxu, S.; Hu, C.; Jia, H.; Wu, Q.; Shen, X.; Jiang, Y.-Y.; Wu, K. Case study on the first immigration of fall armyworm Spodoptera frugiperda invading into China. J. Integr. Agric. 2019, 18, 2–10. [Google Scholar] [CrossRef]
- Nagoshi, R.N.; Htain, N.N.; Boughton, D.; Zhang, L.; Xiao, Y.; Nagoshi, B.Y.; Mota-Sanchez, D. Southeastern Asia Fall Armyworms Are Closely Related to Populations in Africa and India, Consistent with Common Origin and Recent Migration. Sci. Rep. 2020, 10, 1421. [Google Scholar] [CrossRef]
- Goergen, G.; Kumar, P.L.; Sankung, S.B.; Togola, A.; Tamò, M. First report of outbreaks of the fall armyworm Spodoptera frugiperda (J E Smith) (Lepidoptera, Noctuidae), a new alien invasive pest in West and Central Africa. PLoS ONE 2016, 11, e0165632. [Google Scholar] [CrossRef] [PubMed]
- Kasoma, C.; Shimelis, H.; Laing, M.D.; Mekonnen, B. Fall armyworm infestation and development: Screening tropical maize genotypes for resistance in Zambia. Insects 2022, 13, 1020. [Google Scholar] [CrossRef]
- Early, R.; González-Moreno, P.; Murphy, S.T.; Day, R. Forecasting the global extent of invasion of the cereal pest Spodoptera frugiperda, the fall armyworm. NeoBiota 2018, 40, 25–50. [Google Scholar] [CrossRef]
- Jiang, S.; He, L.-M.; He, W.; Zhao, H.-Y.; Yang, X.-M.; Yang, X.-Q.; Wu, K.-M. Effects of X-ray irradiation on the fitness of the established invasive pest fall armyworm Spodoptera frugiperda. Pest. Manag. Sci. 2022, 78, 2806–2815. [Google Scholar] [CrossRef]
- Kumar, R.M.; Gadratagi, B.-G.; Paramesh, V.; Kumar, P.; Madivalar, Y.; Narayanappa, N.; Ullah, F. Sustainable management of invasive fall armyworm, Spodoptera frugiperda. Agronomy 2022, 12, 2150. [Google Scholar] [CrossRef]
- Ren, X.; Yang, Z.; Xu, J.; Sun, J.; Mao, D.; Hu, Y.; Yang, S.-J.; Qiao, H.-H.; Wang, X.; Hu, Q.; et al. Enhanced specificity and efficiency of the CRISPR/Cas9 system with optimized sgRNA parameters in Drosophila. Cell Rep. 2014, 9, 1151–1162. [Google Scholar] [CrossRef]
- Zhang, X.-H.; Tee, L.Y.; Wang, X.-G.; Huang, Q.-S.; Yang, S.-H. Off-target effects in CRISPR/Cas9-mediated genome engineering. Mol. Ther. Nucleic Acids 2015, 4, e264. [Google Scholar] [CrossRef]
- Han, W.; Tang, F.; Zhong, Y.; Zhang, J.; Liu, Z. Identification of yellow gene family and functional analysis of Spodoptera frugiperda yellow-y by CRISPR/Cas9. Pestic. Biochem. Physiol. 2021, 178, 104937. [Google Scholar] [CrossRef] [PubMed]
- Anyanda, G.N.; Bruce, A.Y.; Makumbi, D.; Ahonsi, M.; Kahuthia-Gathu, R.; Namikoye, S.E.; Beyene, Y.; Prasanna, B.M. Reproductive potential of fall armyworm Spodoptera frugiperda (J.E. Smith) and effects of feeding on diverse maize genotypes under artificial infestation. Front. Insect Sci. 2022, 2, 950815. [Google Scholar] [CrossRef]
- Han, W.-K.; Tang, F.-X.; Yu, N.; Zhang, Y.-X.; Liu, Z.-W. A Nonsensory odorant-binding protein plays an important role in the larval development and adult mating of Spodoptera frugiperda. Insect Sci. 2023, 30, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Koutroumpa, F.; Monsempes, C.; François, M.-C.; De Cian, A.; Royer, C.; Concordet, J.-P.; Jacquin-Joly, E. Heritable genome editing with CRISPR/Cas9 induces anosmia in a crop pest moth. Sci. Rep. 2016, 6, 29620. [Google Scholar] [CrossRef]
- Chen, X.; Palli, S. Development of multiple transgenic CRISPR/Cas9 methods for genome editing in the fall armyworm, Spodoptera frugiperda. J. Pest. Sci. 2022, 96, 1637–1650. [Google Scholar] [CrossRef]
- Sun, H.; Bu, L.-A.; Su, S.-C.; Guo, D.; Gao, C.-F.; Wu, S.-F. Knockout of the odorant receptor co-receptor, Orco, impairs feeding, mating and egg-laying behavior in the fall armyworm Spodoptera frugiperda. Insect Biochem. Mol. Biol. 2023, 152, 103889. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Q.; Zhou, X.; Huang, Y.; Zhang, Z. Genome editing of Wnt-1, a gene associated with segmentation, via CRISPR/Cas9 in the pine caterpillar moth, Dendrolimus punctatus. Front. Physiol. 2017, 7, 666. [Google Scholar] [CrossRef]
- Wu, K.; Shirk, P.D.; Taylor, C.E.; Furlong, R.B.; Shirk, B.D.; Pinheiro, D.H.; Siegfried, B.D. CRISPR/Cas9 mediated knockout of the abdominal-A homeotic gene in fall armyworm moth (Spodoptera frugiperda). PLoS ONE 2018, 13, e0208647. [Google Scholar] [CrossRef]
- Zhu, G.-H.; Chereddy, S.C.R.R.; Howell, J.L.; Palli, S.R. Genome editing in the fall armyworm, Spodoptera frugiperda: Multiple sgRNA/Cas9 method for identification of knockouts in one generation. Insect Biochem. Mol. Biol. 2020, 122, 103373. [Google Scholar] [CrossRef]
- Shi, T.; Tang, P.; Wang, X.; Yang, Y.; Wu, Y. CRISPR-mediated knockout of nicotinic acetylcholine receptor (nAChR) A6 subunit confers high levels of resistance to spinosyns in Spodoptera frugiperda. Pestic. Biochem. Physiol. 2022, 187, 105191. [Google Scholar] [CrossRef]
- Li, Q.; Jin, M.; Yu, S.; Cheng, Y.; Shan, Y.; Wang, P.; Yuan, H.; Xiao, Y. Knockout of the ABCB1 gene increases susceptibility to emamectin benzoate, beta-cypermethrin and chlorantraniliprole in Spodoptera frugiperda. Insects 2022, 13, 137. [Google Scholar] [CrossRef]
- Chikmagalur Nagaraja, B.; Karuppannasamy, A.; Ramasamy, A.; Cholenahalli Narayanappa, A.; Chalapathi, P.; Maligeppagol, M. CRISPR/Cas9-mediated mutagenesis of sex lethal (sxl) gene impacts fertility of the fall armyworm, Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae). Arch. Insect Biochem. Physiol. 2023, 114, e22035. [Google Scholar] [CrossRef]
- Martin-Martin, I.; Aryan, A.; Meneses, C.; Adelman, Z.N.; Calvo, E. Optimization of sand fly embryo microinjection for gene editing by CRISPR/Cas9. PLoS Negl. Trop. Dis. 2018, 12, e0006769. [Google Scholar] [CrossRef]
- Zhang, L.; Reed, R.D. A practical guide to CRISPR/Cas9 genome editing in lepidoptera. In Diversity and Evolution of Butterfly Wing Patterns: An Integrative Approach; Sekimura, T., Nijhout, H.F., Eds.; Springer: Singapore, 2017; pp. 155–172. ISBN 978-981-10-4956-9. [Google Scholar]
- Zhang, L.; Martin, A.; Perry, M.W.; van der Burg, K.R.L.; Matsuoka, Y.; Monteiro, A.; Reed, R.D. Genetic basis of melanin pigmentation in butterfly wings. Genetics 2017, 205, 1537–1550. [Google Scholar] [CrossRef]
- Hong, J.W.; Jeong, C.Y.; Yu, J.H.; Kim, S.-B.; Kang, S.K.; Kim, S.-W.; Kim, N.-S.; Kim, K.Y.; Park, J.W. Bombyx mori kynurenine 3-monooxygenase gene editing and insect molecular breeding using the clustered regularly interspaced short palindromic repeat/CRISPR associated protein 9 system. Biotechnol. Prog. 2020, 36, e3054. [Google Scholar] [CrossRef]
- Han, W.-K.; Tang, F.-X.; Gao, H.-L.; Wang, Y.; Yu, N.; Jiang, J.-J.; Liu, Z.-W. Co-CRISPR: A valuable toolkit for mutation enrichment in the gene editing of Spodoptera frugiperda. Insect Sci. 2023, 30, 625–636. [Google Scholar] [CrossRef]
- Wittkopp, P.; True, J.; Carroll, S. Reciprocal functions of the Drosophila yellow and ebony proteins in the development and evolution of pigment patterns. Development 2002, 129, 1849–1858. [Google Scholar] [CrossRef]
- Bi, H.-L.; Xu, J.; He, L.; Zhang, Y.; Li, K.; Huang, Y.-P. CRISPR/Cas9-mediated ebony knockout results in puparium melanism in Spodoptera litura. Insect Sci. 2019, 26, 1011–1019. [Google Scholar] [CrossRef]
- Pérez, M.; Schachter, J.; Quesada-Allué, L.A. Constitutive activity of N-β-alanyl-catecholamine ligase in insect brain. Neurosci. Lett. 2004, 368, 186–191. [Google Scholar] [CrossRef]
- Xu, X.; Harvey-Samuel, T.; Yang, J.; You, M.; Alphey, L. CRISPR/Cas9-based functional characterization of the pigmentation gene ebony in Plutella xylostella. Insect Mol. Biol. 2021, 30, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Wang, P.; Zeng, Y.; An, W.; Wang, T.; Xiao, Y. Characterization of five pigmentation genes as transgenic markers in Spodoptera frugiperda (Lepidoptera: Noctuidae). Int. J. Biol. Macromol. 2023, 242, 124981. [Google Scholar] [CrossRef]
- Khan, S.A.; Reichelt, M.; Heckel, D.G. Functional analysis of the ABCs of eye color in Helicoverpa armigera with CRISPR/Cas9-induced mutations. Sci. Rep. 2017, 7, 40025. [Google Scholar] [CrossRef]
- Grubbs, N.; Haas, S.; Beeman, R.W.; Lorenzen, M.D. The ABCs of eye color in Tribolium castaneum: Orthologs of the Drosophila white, scarlet, and brown genes. Genetics 2015, 199, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Kômoto, N.; Quan, G.-X.; Sezutsu, H.; Tamura, T. A single-base deletion in an ABC transporter gene causes white eyes, white eggs, and translucent larval skin in the silkworm w-3(Oe) mutant. Insect Biochem. Mol. Biol. 2009, 39, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Futahashi, R.; Sato, J.; Meng, Y.; Okamoto, S.; Daimon, T.; Yamamoto, K.; Suetsugu, Y.; Narukawa, J.; Takahashi, H.; Banno, Y.; et al. Yellow and ebony are the responsible genes for the larval color mutants of the silkworm Bombyx mori. Genetics 2008, 180, 1995–2005. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cao, Y.; Zhan, S.; Zhang, Y.; Tan, A.; Huang, Y. Identification of yellow gene family in Agrotis ipsilon and functional analysis of Aiyellow-y by CRISPR/Cas9. Insect Biochem. Mol. Biol. 2018, 94, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-L.; Han, W.-K.; Ze, L.-J.; Peng, Y.-C.; Yang, Y.-L.; Zhang, J.; Yan, Q.; Dong, S.-L. Clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9 mediated knockout reveals functions of the yellow-y gene in Spodoptera litura. Front. Physiol. 2020, 11, 615391. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.; Xu, X.; Liu, Z.; Li, J.; Zhan, X.; Yang, G.; You, M.; You, S. CRISPR/Cas9-based functional analysis of yellow gene in the diamondback moth, Plutella xylostella. Insect Sci. 2021, 28, 1504–1509. [Google Scholar] [CrossRef]
- Xia, A.-H.; Zhou, Q.-X.; Yu, L.-L.; Li, W.-G.; Yi, Y.-Z.; Zhang, Y.-Z.; Zhang, Z.-F. Identification and analysis of yellow protein family genes in the silkworm, Bombyx Mori. BMC Genom. 2006, 7, 195. [Google Scholar] [CrossRef]
- Dermauw, W.; Van Leeuwen, T. The ABC gene family in arthropods: Comparative genomics and role in insecticide transport and resistance. Insect Biochem. Mol. Biol. 2014, 45, 89–110. [Google Scholar] [CrossRef]
- Tang, F.; Saier, M.H. Transport proteins promoting Escherichia coli pathogenesis. Microb. Pathog. 2014, 71–72, 41–55. [Google Scholar] [CrossRef]
- Pauchet, Y.; Bretschneider, A.; Augustin, S.; Heckel, D.G. A p-glycoprotein is linked to resistance to the Bacillus thuringiensis Cry3Aa toxin in a leaf beetle. Toxins 2016, 8, 362. [Google Scholar] [CrossRef] [PubMed]
- Higgins, C.F.; Linton, K.J. The ATP switch model for ABC transporters. Nat. Struct. Mol. Biol. 2004, 11, 918–926. [Google Scholar] [CrossRef]
- Buss, D.S.; Callaghan, A. Interaction of pesticides with p-glycoprotein and other ABC proteins: A survey of the possible importance to insecticide, herbicide and fungicide resistance. Pestic. Biochem. Physiol. 2008, 90, 141–153. [Google Scholar] [CrossRef]
- Zuo, Y.-Y.; Huang, J.-L.; Wang, J.; Feng, Y.; Han, T.-T.; Wu, Y.-D.; Yang, Y.-H. Knockout of a p-glycoprotein gene increases susceptibility to abamectin and emamectin benzoate in Spodoptera exigua. Insect Mol. Biol. 2018, 27, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Endo, H.; Tanaka, S.; Imamura, K.; Adegawa, S.; Kikuta, S.; Sato, R. Cry toxin specificities of insect ABCC transporters closely related to lepidopteran ABCC2 transporters. Peptides 2017, 98, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Sun, D.; Kang, S.; Zhou, J.; Gong, L.; Qin, J.; Guo, L.; Zhu, L.; Bai, Y.; Luo, L.; et al. CRISPR/Cas9-mediated knockout of both the PxABCC2 and PxABCC3 genes confers high-level resistance to Bacillus thuringiensis Cry1Ac toxin in the diamondback moth, Plutella xylostella (L.). Insect Biochem. Mol. Biol. 2019, 107, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, Y.; Huang, J.; Jin, W.; Yang, Y.; Wu, Y. CRISPR-mediated knockout of the ABCC2 gene in Ostrinia furnacalis confers high-level resistance to the Bacillus thuringiensis Cry1Fa toxin. Toxins 2020, 12, 246. [Google Scholar] [CrossRef]
- Jin, M.; Yang, Y.; Shan, Y.; Chakrabarty, S.; Cheng, Y.; Soberón, M.; Bravo, A.; Liu, K.; Wu, K.; Xiao, Y. Two ABC transporters are differentially involved in the toxicity of two Bacillus thuringiensis Cry1 toxins to the invasive crop-pest Spodoptera frugiperda (J. E. Smith). Pest. Manag. Sci. 2021, 77, 1492–1501. [Google Scholar] [CrossRef]
- Jones, A.K.; Sattelle, D.B. Diversity of insect nicotinic acetylcholine receptor subunits. Adv. Exp. Med. Biol. 2010, 683, 25–43. [Google Scholar] [CrossRef]
- Wonnacott, S.; Bermudez, I.; Millar, N.S.; Tzartos, S.J. Nicotinic acetylcholine receptors. Br. J. Pharmacol. 2018, 175, 1785–1788. [Google Scholar] [CrossRef]
- Somers, J.; Nguyen, J.; Lumb, C.; Batterham, P.; Perry, T. In vivo functional analysis of the Drosophila melanogaster nicotinic acetylcholine receptor Dα6 using the insecticide spinosad. Insect Biochem. Molec. 2015, 64, 116–127. [Google Scholar] [CrossRef]
- Wang, J.; Ma, H.; Zuo, Y.; Yang, Y.; Wu, Y. CRISPR-mediated gene knockout reveals nicotinic acetylcholine receptor (nAChR) subunit A6 as a target of spinosyns in Helicoverpa armigera. Pest. Manag. Sci. 2020, 76, 2925–2931. [Google Scholar] [CrossRef]
- Wang, X.; Ma, Y.; Wang, F.; Yang, Y.; Wu, S.; Wu, Y. Disruption of nicotinic acetylcholine receptor A6 mediated by CRISPR/Cas9 confers resistance to spinosyns in Plutella xylostella. Pest. Manag. Sci. 2020, 76, 1618–1625. [Google Scholar] [CrossRef]
- Zuo, Y.; Xue, Y.; Lu, W.; Ma, H.; Chen, M.; Wu, Y.; Yang, Y.; Hu, Z. Functional validation of nicotinic acetylcholine receptor (nAChR) A6 as a target of spinosyns in Spodoptera exigua utilizing the CRISPR/Cas9 system. Pest. Manag. Sci. 2020, 76, 2415–2422. [Google Scholar] [CrossRef]
- Liu, Z.; Liao, C.; Zou, L.; Jin, M.; Shan, Y.; Quan, Y.; Yao, H.; Zhang, L.; Wang, P.; Liu, Z.; et al. Retrotransposon-mediated disruption of a chitin synthase gene confers insect resistance to Bacillus thuringiensis Vip3Aa toxin. PLoS Biol. 2024, 22, e3002704. [Google Scholar] [CrossRef]
- Renou, M.; Anton, S. Insect olfactory communication in a complex and changing world. Curr. Opin. Insect Sci. 2020, 42, 1–7. [Google Scholar] [CrossRef]
- Rizvi, S.A.H.; George, J.; Reddy, G.V.P.; Zeng, X.; Guerrero, A. Latest developments in insect sex pheromone research and its application in agricultural pest management. Insects 2021, 12, 484. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, J.; Pregitzer, P.; Breer, H.; Krieger, J. Access to the odor world: Olfactory receptors and their role for signal transduction in insects. Cell Mol. Life Sci. 2018, 75, 485–508. [Google Scholar] [CrossRef]
- Kleinheinz, D.; D’Onofrio, C.; Carraher, C.; Ramach, U.; Schuster, B.; Bozdogan, A.; Knoll, W.; Andersson, J. Functional incorporation of the insect odorant receptor co-receptor in tethered lipid bilayer nano-architectures. Biosens. Bioelectron. 2022, 203, 114024. [Google Scholar] [CrossRef] [PubMed]
- Butterwick, J.A.; del Mármol, J.; Kim, K.H.; Kahlson, M.A.; Rogow, J.A.; Walz, T.; Ruta, V. Cryo-EM structure of the insect olfactory receptor (Orco). Nature 2018, 560, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Liu, F.; Ye, Z.; Baker, A.; Zwiebel, L.J. Mutagenesis of the (Orco) odorant receptor co-receptor impairs olfactory function in the malaria vector Anopheles coluzzii. Insect. Biochem. Mol. Biol. 2020, 127, 103497. [Google Scholar] [CrossRef]
- Fandino, R.A.; Haverkamp, A.; Bisch-Knaden, S.; Zhang, J.; Bucks, S.; Nguyen, T.A.T.; Schröder, K.; Werckenthin, A.; Rybak, J.; Stengl, M.; et al. Mutagenesis of odorant co-receptor orco fully disrupts foraging but not oviposition behaviors in the Hawkmoth Manduca sexta. Proc. Natl. Acad Sci. USA 2019, 116, 15677–15685. [Google Scholar] [CrossRef]
- Fan, X.-B.; Mo, B.-T.; Li, G.-C.; Huang, L.-Q.; Guo, H.; Gong, X.-L.; Wang, C.-Z. Mutagenesis of the odorant receptor co-receptor (Orco) reveals severe olfactory defects in the crop pest Moth Helicoverpa armigera. BMC Biol. 2022, 20, 214. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, W.; Zeng, B.; Wang, G.; Hao, D.; Huang, Y. Deletion of the Bombyx mori odorant receptor co-receptor (BmOrco) impairs olfactory sensitivity in silkworms. Insect Biochem. Mol. Biol. 2017, 86, 58–67. [Google Scholar] [CrossRef]
- Abendroth, J.A.; Moural, T.W.; Wei, H.; Zhu, F. Roles of insect odorant binding proteins in communication and xenobiotic adaptation. Front. Insect Sci. 2023, 3, 1274197. [Google Scholar] [CrossRef]
- Sun, J.S.; Xiao, S.; Carlson, J.R. The diverse small proteins called odorant-binding proteins. Open Biol. 2018, 8, 180208. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-Q.; Song, Z.-Y.; Dong, J.-F.; Lv, Q.-H.; Chen, Q.-X.; Sun, H.-Z. Identification and comparative expression analysis of odorant-binding proteins in the reproductive system and antennae of Athetis dissimilis. Sci. Rep. 2021, 11, 13941. [Google Scholar] [CrossRef]
- Sun, Y.-L.; Huang, L.-Q.; Pelosi, P.; Wang, C.-Z. Expression in antennae and reproductive organs suggests a dual role of an odorant-binding protein in two sibling Helicoverpa species. PLoS ONE 2012, 7, e30040. [Google Scholar] [CrossRef]
- Gu, S.-H.; Zhou, J.-J.; Gao, S.; Wang, D.-H.; Li, X.; Guo, Y.-Y.; Zhang, Y. Identification and comparative expression analysis of odorant binding protein genes in the tobacco cutworm Spodoptera litura. Sci. Rep. 2015, 5, 13800. [Google Scholar] [CrossRef]
- Vulpe, A.; Menuz, K. Ir76b is a co-receptor for amine responses in Drosophila olfactory neurons. Front. Cell. Neurosci. 2021, 15, 759238. [Google Scholar] [CrossRef]
- Rytz, R.; Croset, V.; Benton, R. Ionotropic receptors (IRs): Chemosensory ionotropic glutamate receptors in Drosophila and beyond. Insect. Biochem. Molec. Bio. 2013, 43, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Bisch-Knaden, S.; Fandino, R.A.; Yan, S.; Obiero, G.F.; Grosse-Wilde, E.; Hansson, B.S.; Knaden, M. The olfactory co-receptor Ir8a governs larval feces-mediated competition avoidance in a hawkmoth. Proc. Natl. Acad. Sci. USA 2019, 116, 21828–21833. [Google Scholar] [CrossRef]
- Hou, X.-Q.; Zhang, D.D.; Powell, D.; Wang, H.-L.; Andersson, M.N.; Löfstedt, C. Ionotropic receptors in the turnip moth Agrotis segetum respond to repellent medium-chain fatty acids. BMC Biol. 2022, 20, 34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-X.; Yang, B.; Sun, D.-D.; Guo, M.-B.; Zhang, J.; Wang, G.-R. Ionotropic receptor 8a is involved in the attraction of Helicoverpa armigera to acetic acid. Insect Sci. 2022, 29, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.-M.; Wei, Z.-Q.; Hou, J.-H.; He, Y.; Luan, X.-P.; Zhang, Y.-Y.; Liu, X.-L.; Zhang, X.-T.; Zhang, J.; Yan, Q.; et al. Ionotropic receptor Ir75q.2 mediates avoidance reaction to nonanoic acid in the fall armyworm Spodoptera frugiperda (Lepidoptera, Noctuidae). J. Agric. Food Chem. 2023, 71, 20602–20612. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, K.; Wu, Z.; Xu, J.; Erb, M. Plant volatiles as regulators of plant defense and herbivore immunity: Molecular mechanisms and unanswered questions. Curr. Opin. Insect Sci. 2021, 44, 82–88. [Google Scholar] [CrossRef]
- Musser, R.O.; Hum-Musser, S.M.; Eichenseer, H.; Peiffer, M.; Ervin, G.; Murphy, J.B.; Felton, G.W. Caterpillar saliva beats plant defences. Nature 2002, 416, 599–600. [Google Scholar] [CrossRef]
- Gao, B.; Li, B.; Yuan, J.; Shi, Z.; Zheng, X.; Wang, G. Spodoptera frugiperda salivary glucose oxidase reduces the release of green leaf volatiles and increases terpene emission from maize. Insects 2024, 15, 511. [Google Scholar] [CrossRef]
- Xia, Y.-H.; Zhang, Y.-N.; Ding, B.-J.; Wang, H.-L.; Löfstedt, C. Multi-functional desaturases in two Spodoptera moths with ∆11 and ∆12 desaturation activities. J. Chem. Ecol. 2019, 45, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Roy, M.C.; Baki, M.A.A.; Jung, J.K.; Lee, D.; Kim, Y. CRISPR/Cas9 mutagenesis against sex pheromone biosynthesis leads to loss of female attractiveness in Spodoptera exigua, an insect pest. PLoS ONE 2021, 16, e0259322. [Google Scholar] [CrossRef]
- Shi, L.; Liu, X.; Liu, H.; Shan, S.; Shen, S.; Bai, M.; Lan, H.; Khashaveh, A.; Gu, S.; Zhang, Y. Knockout of the delta11-desaturase Sfrudes1 disrupts sex pheromone biosynthesis, mating and oviposition in the fall armyworm, Spodoptera frugiperda. Pestic. Biochem. Physiol. 2024, 200, 105832. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Zhang, L.-W.; Chen, D.-S.; Sun, L.; Li, Z.-Q.; Ye, Z.-F.; Zheng, M.-Y.; Li, J.-B.; Zhu, X.-Y. Molecular identification of differential expression genes associated with sex pheromone biosynthesis in Spodoptera exigua. Mol. Genet. Genom. 2017, 292, 795–809. [Google Scholar] [CrossRef]
- Ashok, K.; Bhargava, C.N.; Asokan, R.; Pradeep, C.; Kennedy, J.S.; Manamohan, M.; Rai, A. CRISPR/Cas9 mediated mutagenesis of the major sex pheromone gene, acyl-CoA delta-9 desaturase (DES9) in fall armyworm Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae). Int. J. Biol. Macromol. 2023, 253, 126557. [Google Scholar] [CrossRef] [PubMed]
- Ashok, K.; Bhargava, C.N.; Asokan, R.; Pradeep, C.; Pradhan, S.K.; Kennedy, J.S.; Balasubramani, V.; Murugan, M.; Jayakanthan, M.; Geethalakshmi, V.; et al. CRISPR/Cas9 mediated editing of pheromone biosynthesis activating neuropeptide (PBAN) gene disrupts mating in the fall armyworm, Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae). Biotech 2023, 13, 1–14. [Google Scholar] [CrossRef]
- Xu, J.; Zhan, S.; Chen, S.; Zeng, B.; Li, Z.; James, A.A.; Tan, A.; Huang, Y. Sexually dimorphic traits in the silkworm, Bombyx mori, are regulated by doublesex. Insect Biochem. Mol. Biol. 2017, 80, 42–51. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Y.; Yang, X.; Liu, Z.; Luo, X.; Xu, J.; Huang, Y. Dysfunction of dimorphic sperm impairs male fertility in the silkworm. Cell. Discov. 2020, 6, 1–15. [Google Scholar] [CrossRef]
- Sakai, H.; Oshima, H.; Yuri, K.; Gotoh, H.; Daimon, T.; Yaginuma, T.; Sahara, K.; Niimi, T. Dimorphic sperm formation by sex-lethal. Proc. Natl. Acad. Sci. USA 2019, 116, 10412–10417. [Google Scholar] [CrossRef]
- Wen, L.; Gong, Q.; Du, Q.; Yu, X.; Feng, Q.; Liu, L. Lacking of sex-lethal gene lowers the fertility of male reproduction in Spodoptera litura (Lepidoptera). Pestic. Biochem. Physiol. 2022, 184, 105087. [Google Scholar] [CrossRef]
- Morrow, J.; Riegler, M.; Frommer, M.; Shearman, D. Expression patterns of sex-determination genes in single male and female embryos of two Bactrocera fruit fly species during early development. Insect Mol. Biol. 2014, 23, 754–767. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.; Monteiro, A. Doublesex mediates the development of sex-specific pheromone organs in Bicyclus butterflies via multiple mechanisms. Mol. Biol. Evol. 2020, 37, 1694–1707. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, X.; Liu, Z.; Xu, J.; Li, X.; Bi, H.; Andongma, A.A.; Niu, C.; Huang, Y. Mutation of doublesex induces sex-specific sterility of the diamondback moth Plutella xylostella. Insect Biochem. Mol. Biol. 2019, 112, 103180. [Google Scholar] [CrossRef]
- Kandul, N.P.; Liu, J.; Sanchez, C.H.M.; Wu, S.L.; Marshall, J.M.; Akbari, O.S. Transforming insect population control with precision guided sterile males with demonstration in flies. Nat. Commun. 2019, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Asad, M.; Liao, J.; Chen, J.; Li, J.; Chu, X.; Pang, S.; Tariq, M.; Abbas, A.N.; Yang, G. The potential role of the piwi gene in the development and reproduction of Plutella xylostella. Int. J. Mol. Sci. 2023, 24, 12321. [Google Scholar] [CrossRef]
- Lovato, T.L.; Nguyen, T.P.; Molina, M.R.; Cripps, R.M. The Hox gene abdominal-a specifies heart cell fate in the Drosophila dorsal vessel. Development 2002, 129, 5019–5027. [Google Scholar] [CrossRef] [PubMed]
- Perrin, L.; Monier, B.; Ponzielli, R.; Astier, M.; Semeriva, M. Drosophila cardiac tube organogenesis requires multiple phases of Hox activity. Dev. Biol. 2004, 272, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Foronda, D.; Estrada, B.; de Navas, L.; Sánchez-Herrero, E. Requirement of abdominal-A and abdominal-B in the developing genitalia of Drosophila breaks the posterior downregulation rule. Development 2006, 133, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Storer, N.P.; Babcock, J.M.; Schlenz, M.; Meade, T.; Thompson, G.D.; Bing, J.W.; Huckaba, R.M. Discovery and characterization of field resistance to Bt maize: Spodoptera frugiperda (Lepidoptera: Noctuidae) in Puerto Rico. J. Econ. Entomol. 2010, 103, 1031–1038. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Y.; Zeng, B.; Wang, Y.; James, A.A.; Gurr, G.M.; Yang, G.; Lin, X.; Huang, Y.; You, M. CRISPR/Cas9 mediated knockout of the abdominal-A homeotic gene in the global pest, diamondback moth (Plutella xylostella). Insect Biochem. Mol. Biol. 2016, 75, 98–106. [Google Scholar] [CrossRef]
- Fan, S.T.; Wu, M.Z.; Liu, C.; Li, H.H.; Huang, S.H.; Zheng, Z.J.; Ye, X.Y.; Tan, J.F.; Zhu, G.H. Azadirachtin inhibits nuclear receptor HR3 in the prothoracic gland to block larval ecdysis in the fall armyworm, Spodoptera frugiperda. J. Agric. Food. Chem. 2023, 71, 15497–15505. [Google Scholar] [CrossRef]
- Cruz, J.; Maestro, O.; Franch-Marro, X.; Martín, D. Nuclear receptors EcR-A/RXR and HR3 control early embryogenesis in the short-germ hemimetabolous insect Blattella germanica. iScience 2023, 26, 106548. [Google Scholar] [CrossRef]
- Zhao, X.; Qin, Z.; Liu, W.; Liu, X.; Moussian, B.; Ma, E.; Li, S.; Zhang, J. Nuclear receptor HR3 controls locust molt by regulating chitin synthesis and degradation genes of Locusta migratoria. Insect Biochem. Mol. Biol. 2018, 92, 1–11. [Google Scholar] [CrossRef]
- Kim, B.-E.; Choi, B.; Park, W.-R.; Kim, Y.-J.; Mun, S.; Choi, H.-S.; Kim, D.-K. Nuclear receptor HR3 mediates transcriptional regulation of chitin metabolic genes during molting in Tribolium castaneum. Pest. Manag. Sci. 2022, 78, 4377–4387. [Google Scholar] [CrossRef]
- Qu, Z.; Kenny, N.J.; Lam, H.M.; Chan, T.F.; Chu, K.H.; Bendena, W.G.; Tobe, S.S.; Hui, J.H.L. How did arthropod sesquiterpenoids and ecdysteroids arise? Comparison of hormonal pathway genes in non-insect arthropod genomes. Genome Biol. Evol. 2015, 7, 1951–1959. [Google Scholar] [CrossRef]
- Wu, K.; Zhang, J.; Zhang, Q.; Zhu, S.; Shao, Q.; Clark, K.D.; Liu, Y.; Ling, E. Plant phenolic are detoxified by prophenoloxidase in the insect gut. Sci. Rep. 2015, 5, 16823. [Google Scholar] [CrossRef]
- Cerenius, L.; Söderhäll, K. The Prophenoloxidase-activating system in invertebrates. Immunol. Rev. 2004, 198, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Lu, Y.; Cai, Z.; Liang, S.; Niu, Y.; Miao, Y. Phenol oxidase is a necessary enzyme for the silkworm molting which is regulated by molting hormone. Mol. Biol. Rep. 2013, 40, 3549–3555. [Google Scholar] [CrossRef]
- Nam, K.; Nhim, S.; Robin, S.; Bretaudeau, A.; Nègre, N.; d’Alençon, E. Positive selection alone is sufficient for whole genome differentiation at the early stage of speciation process in the fall armyworm. BMC Evol. Biol. 2020, 20, 152. [Google Scholar] [CrossRef] [PubMed]
- Eychenne, M.; Girard, P.-A.; Frayssinet, M.; Lan, L.; Pagès, S.; Duvic, B.; Nègre, N. Mutagenesis of both prophenoloxidases in the fall armyworm induces major defects in metamorphosis. J. Insect. Physiol. 2022, 139, 104399. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Yang, X.; Xu, X.; Yang, D.; Zhu, C.; Yi, M.; Bi, H.; Wang, Y.; Huang, Y. SPSL1 is essential for spermatophore formation and sperm activation in Spodoptera frugiperda. PLoS Genetics 2023, 19, e1011073. [Google Scholar] [CrossRef]
- Anu, C.N.; Ashok, K.; Bhargava, C.N.; Dhawane, Y.; Manamohan, M.; Jha, G.K.; Asokan, R. CRISPR/Cas9 mediated validation of spermatogenesis-related gene, tssk2 as a component of genetic pest management of fall armyworm, Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae). Arch. Insect Biochem. Physiol. 2024, 116, e22121. [Google Scholar] [CrossRef] [PubMed]
- Terenius, O.; Papanicolaou, A.; Garbutt, J.S.; Eleftherianos, I.; Huvenne, H.; Kanginakudru, S.; Albrechtsen, M.; An, C.; Aymeric, J.-L.; Barthel, A.; et al. RNA interference in lepidopteran: An overview of successful and unsuccessful studies and implications for experimental design. J. Insect Physiol. 2011, 57, 231–245. [Google Scholar] [CrossRef]
- Kunte, N.; McGraw, E.; Bell, S.; Held, D.; Avila, L. Prospects, challenges and current status of RNAi through insect feeding. Pest. Manag. Sci. 2019, 76, 26–41. [Google Scholar] [CrossRef]
- Rodrigues, T.B.; Mishra, S.K.; Sridharan, K.; Barnes, E.R.; Alyokhin, A.; Tuttle, R.; Kokulapalan, W.; Garby, D.; Skizim, N.J.; Tang, Y.-W.; et al. First sprayable double-stranded RNA-based bio pesticide product targets proteasome subunit beta type-5 in colorado potato beetle (Leptinotarsa Decemlineata). Front. Plant. Sci. 2021, 12, 728652. [Google Scholar] [CrossRef]
- Weber, F.; Wagner, V.; Rasmussen, S.B.; Hartmann, R.; Paludan, S.R. Double-stranded RNA is produced by positive-strand RNA Viruses and DNA Viruses but not in detectable amounts by negative-strand RNA Viruses. J. Virol. 2006, 80, 5059–5064. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, B.; Hussain, M.; Asgari, S. RNA interference as a cellular defense mechanism against the DNA Virus Baculovirus. J. Virol. 2012, 86, 13729–13734. [Google Scholar] [CrossRef]
- Wang, X.-H.; Aliyari, R.; Li, W.-X.; Li, H.-W.; Kim, K.; Carthew, R.; Atkinson, P.; Ding, S.-W. RNA interference directs innate immunity against Viruses in adult Drosophila. Science 2006, 312, 452–454. [Google Scholar] [CrossRef]
- Kemp, C.; Mueller, S.; Goto, A.; Barbier, V.; Paro, S.; Bonnay, F.; Dostert, C.; Troxler, L.; Hetru, C.; Meignin, C.; et al. Broad RNA interference-mediated antiviral immunity and virus-specific inducible responses in Drosophila. J. Immunol. 2013, 190, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Xia, Y.; Xu, X.; Wei, G.; Wang, L. Functional analysis of dicer-2 gene in Bombyx mori resistance to BmNPV Virus. Arch. Insect Biochem. Physiol. 2020, 105, e21724. [Google Scholar] [CrossRef] [PubMed]
- de Malmanche, H.; Hussain, M.; Marcellin, E.; Reid, S.; Asgari, S. Knockout of dicer-2 in the Sf9 cell line enhances the replication of Spodoptera frugiperda Rhabdovirus and conditionally increases Baculovirus replication. J. Gen. Virol. 2022, 103, 001779. [Google Scholar] [CrossRef]
- Koo, J.; Zhu, G.-H.; Palli, S.R. CRISPR-Cas9 mediated dsRNase knockout improves RNAi efficiency in the fall armyworm. Pestic. Biochem. Physiol. 2024, 200, 105839. [Google Scholar] [CrossRef]
- Arimatsu, Y.; Kotani, E.; Sugimura, Y.; Furusawa, T. Molecular characterization of a cDNA encoding extracellular dsRNase and its expression in the silkworm, Bombyx mori. Insect. Biochem. Mol. Biol. 2007, 37, 176–183. [Google Scholar] [CrossRef]
- Cooper, A.M.; Silver, K.; Zhang, J.; Park, Y.; Zhu, K.Y. Molecular mechanisms influencing efficiency of RNA interference in insects. Pest. Manag. Sci. 2019, 75, 18–28. [Google Scholar] [CrossRef]
- Arimatsu, Y.; Furuno, T.; Sugimura, Y.; Togoh, M.; Ishihara, R.; Tokizane, M.; Kotani, E.; Hayashi, Y.; Furusawa, T. Purification and properties of double-stranded RNA-degrading nuclease, dsRNase, from the digestive juice of the silkworm, Bombyx mori. J. Insect. Biotechnol. Sericol. 2007, 76, 1_57–1_62. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, K.; Chen, J.; Wang, J.; Zhang, H.; Ze, L.; Zhu, G.; Zhao, C.; Xiao, H.; Han, Z. Identification of a double-stranded RNA-degrading nuclease influencing both ingestion and injection RNA interference efficiency in the red flour beetle Tribolium castaneum. Insect Biochem. Mol. Biol. 2020, 125, 103440. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Z.; Jiang, Y.X.; Li, M.W.; Li, J.W.; Zha, B.H.; Yang, G. Double-stranded RNA-degrading enzymes reduce the efficiency of RNA interference in Plutella xylostella. Insects 2021, 12, 712. [Google Scholar] [CrossRef] [PubMed]
- Koonin, E.V.; Gootenberg, J.S.; Abudayyeh, O.O. Discovery of diverse CRISPR-Cas systems and expansion of the genome engineering toolbox. Biochemistry 2023, 62, 3465–3487. [Google Scholar] [CrossRef]
- Bryson, J.W.; Auxillos, J.Y.; Rosser, S.J. Multiplexed activation in mammalian cells using a split-intein CRISPR/Cas12a based synthetic transcription factor. Nucleic Acids Res. 2022, 50, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Abudayyeh, O.O.; Gootenberg, J.S.; Konermann, S.; Joung, J.; Slaymaker, I.M.; Cox, D.B.; Shmakov, S.; Makarova, K.S.; Semenova, E.; Minakhin, L.; et al. C2c2 is a single-component programmable RNA-guided RNA-targeting CRISPR effector. Science 2016, 353, aaf5573. [Google Scholar] [CrossRef]
- Chen, X.; Chereddy, S.C.R.R.; Gurusamy, D.; Palli, S.R. Identification and characterization of highly active promoters from the fall armyworm, Spodoptera frugiperda. Insect Biochem. Mol. Biol. 2020, 126, 103455. [Google Scholar] [CrossRef]
- Mali, P.; Yang, L.; Esvelt, K.M.; Aach, J.; Guell, M.; DiCarlo, J.E.; Norville, J.E.; Church, G.M. RNA-guided human genome engineering via Cas9. Science 2013, 339, 823–826. [Google Scholar] [CrossRef]
- Zhu, Y. Advances in CRISPR/Cas9. Biomed. Res. Int. 2022, 2022, 9978571. [Google Scholar] [CrossRef]
- Gouda, M.N.R.; Jeevan, H.; Shashank, H.G. CRISPR/Cas9: A cutting-edge solution for combatting the fall armyworm, Spodoptera frugiperda. Mol. Biol. Rep. 2023, 51, 13. [Google Scholar] [CrossRef]
- Du, Y.; Liu, Y.; Hu, J.; Peng, X.; Liu, Z. CRISPR/Cas9 Systems: Delivery technologies and biomedical applicatio. Pharm. Sci. Asia 2023, 18, 100854. [Google Scholar] [CrossRef]
- De Malmanche, H.; Marcellin, E.; Reid, S. Knockout of Sf-Caspase-1 generates apoptosis-resistant Sf9 cell lines: Implications for Baculovirus expression. Biotechnol. J. 2022, 17, 2100532. [Google Scholar] [CrossRef]
- Matsuda, N.; Takahashi, M.; Shirai, Y.; Hinomoto, N.; Daimon, T. Direct parental CRISPR gene editing in the predatory bug, a biocontrol agent against small arthropods. Pest. Manag. Sci. 2024, 80, 9. [Google Scholar] [CrossRef] [PubMed]
- Asad, M.; Liu, D.; Chen, J.; Yang, G. Applications of gene drive systems for population suppression of insect pests. Bull. Entomol. Res. 2022, 112, 724–733. [Google Scholar] [CrossRef]
- Bourtzis, K.; Vreysen, M.J.B. Sterile insect technique (SIT) and its applications. Insects 2021, 12, 638. [Google Scholar] [CrossRef]
- Horner, R.M.; Lo, P.L.; Rogers, D.J.; Walker, J.T.S.; Suckling, D.M. Combined effects of mating disruption, insecticides, and the sterile insect technique on Cydia pomonella in New Zealand. Insects 2020, 11, 837. [Google Scholar] [CrossRef]
- Simmons, G.S.; Salazar Sepulveda, M.C.; Fuentes Barrios, E.A.; Idalsoaga Villegas, M.; Medina Jimenez, R.E.; Garrido Jerez, A.R.; Henderson, R.; Donoso Riffo, H. Development of sterile insect technique for control of the European grapevine moth, Lobesia botrana, in Urban areas of Chile. Insects 2021, 12, 378. [Google Scholar] [CrossRef]
- Asad, M.; Liu, D.; Li, J.; Chen, J.; Yang, G. Development of CRISPR/Cas9-mediated gene-drive construct targeting the phenotypic gene in Plutella xylostella. Front. Physiol. 2022, 13, 938621. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Harvey-Samuel, T.; Siddiqui, H.A.; Ang, J.X.D.; Anderson, M.E.; Reitmayer, C.M.; Lovett, E.; Leftwich, P.T.; You, M.; Alphey, L. Toward a CRISPR-Cas9-based gene drive in the diamondback moth Plutella xylostella. CRISPR J. 2022, 5, 224–236. [Google Scholar] [CrossRef]
| sgRNA and Cas9 Concentration (ng/µL) | Egg Collection Duration | Egg Preparation | Incubation Condition (Temperature and Humidity) | Hatching Percentage | Reference |
|---|---|---|---|---|---|
| 100 and 300 | 1 h | 1% sodium hypochlorite | 27 ± 1 °C and 60% | 62% | [45] |
| 250 and 200 | 2 h | 1% sodium hypochlorite | 26 ± 1 °C and 65% | 5.3% | [46] |
| 250 and 500 | 3 h | 1% sodium hypochlorite | 26 ± 1 °C and 60% | 67.2% | [44] |
| 150 and 300 | 3 h | none | 26 ± 1 °C and 70 ± 5% | 14.9% | [42] |
| 400 and 500 | 2 h | 1% sodium hypochlorite | 27 ± 1 °C and 65 ± 5% | 32% | [39] |
| 150 and 100 | 2 h | distilled water | 25 °C and 65% | 18.5% | [47] |
| 400 and 500 | 1 h | distilled water | 25 ± 2 °C, 70–80% | 23.3% | [48] |
| 300 and 300 | 1 h | none | 25 °C | 36.3% | [17] |
| Gene of Interest | Gene Function | KO Phenotype | References |
|---|---|---|---|
| Kynurenine-3-monooxygenase(kmo) | Eye and body pigment production | Changes in body, eye, and egg colors | [53] |
| ebony | Melanin formation | Deep coloration in pupal and adult stages | [58] |
| scarlet | Transport pigment for eye color | Changes in color during pupal and adult stages | [58] |
| Yellow-y | Production of black melanin and black color | Yellow body color | [37] |
| ATP-binding cassette B1/C2/C3 (ABCB1/ABCC2/ABCC3) | Pesticides resistance | Increased susceptibility to emamectin benzoate, chlorantraniliprole, spinosad, and abamectin | [47,76] |
| Nicotinic acetylcholine receptors(nAChR)α6 | Pesticides resistance | Increased resistance against spinosad and spinetoram | [46] |
| Chitin synthase (CHS2) | Resistance to insecticidal protein Vip3Aa | Increased resistance to Vip3Aa | [83] |
| Odorant receptor co-receptor (Orco) | Response to stimulus | Failures in adult responses to sex pheromones, predators, and food | [42] |
| Odorant binding protein-27 (OBP27) | Chemo-sensory recognition of stimulus | Prolonged larval duration, weight loss, and reduced mating behavior | [39] |
| Ionotropic receptors(IR75q.2) | Response to aldehyde and acid | Reduced responses to acids and aldehydes; also, oviposition behavior decreased | [103] |
| Glucose oxidase (GOX) | Suppression of plant defense against herbivore | Decreased green leaf volatile emission and increased terpene emission from the maize | [106] |
| Doublesex (Dsx) | Sexual dimorphism and determination | Infertility and decreased fecundity | [17] |
| Delta11-desaturase1 (DES1) | Biosynthesis of female sex pheromones | Decreased mating behavior and number of eggs produced by mutants | [109] |
| Acyl-Co-A delat-9 desaturase (DES9) | Biosynthesis of sex pheromone | Decreased mating behavior | [111] |
| Pheromone biosynthesis activating neuropeptide (PBAN) | Biosynthesis of sex pheromone | Decreased mating behavior and zero fecundity | [112] |
| Sex-lethal (Sxl) | Sex determination and reproduction | Reduced reproductive ability and induced sterility | [48] |
| Prophenoloxidases (PPOs) | Development and defense | Reduced pupal size and increased mortality | [136] |
| Abdominal-A (Abd-A) | Growth and development | Reduced hatching rate, failure in transition from larva to pupa, and sterility | [44] |
| Testis specific serine/threonine kinase 2 (tssk2) | Spermatogenesis | Male sterility | [138] |
| Serine protease snake-like 1 (SPSL1) | Regulation of spermatophore formation and sperm activation in female | Reduced fecundity rate | [137] |
| Hormone receptor 3 (HR3) | Growth and development | Molting failure in larvae and increased mortality | [127] |
| Dicer-2(sf9 cell line) | Defensive | Increased virus replication and infection | [147] |
| Double-stranded RNAase (dsRNAase) | Degrades dsRNA | Increases RNAi efficiency | [148] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salum, Y.M.; Yin, A.; Zaheer, U.; Liu, Y.; Guo, Y.; He, W. CRISPR/Cas9-Based Genome Editing of Fall Armyworm (Spodoptera frugiperda): Progress and Prospects. Biomolecules 2024, 14, 1074. https://doi.org/10.3390/biom14091074
Salum YM, Yin A, Zaheer U, Liu Y, Guo Y, He W. CRISPR/Cas9-Based Genome Editing of Fall Armyworm (Spodoptera frugiperda): Progress and Prospects. Biomolecules. 2024; 14(9):1074. https://doi.org/10.3390/biom14091074
Chicago/Turabian StyleSalum, Yussuf Mohamed, Anyuan Yin, Uroosa Zaheer, Yuanyuan Liu, Yi Guo, and Weiyi He. 2024. "CRISPR/Cas9-Based Genome Editing of Fall Armyworm (Spodoptera frugiperda): Progress and Prospects" Biomolecules 14, no. 9: 1074. https://doi.org/10.3390/biom14091074






