Advancements in Green Nanoparticle Technology: Focusing on the Treatment of Clinical Phytopathogens
Abstract
1. Introduction
2. Phytopathogenic Infection in Humans
2.1. Bacterial Phytopathogens
2.2. Fungal Phytopathogens
2.3. Viral Phytopathogens
| Responsible Pathogen | Symptoms in Plant | Plant Vectors | Clinical Presentation | Reference | |
|---|---|---|---|---|---|
| Virus | TMV | Yellowing, Stunting | Tobacco, Tomato | Pulmonary disease | [73] |
| PMMoV | Chlorosis, Mottling | Pepper | Abdominal pain, fever | [65] | |
| Bacteria | Burkholderia cepacia | Gladiolus corms | Onion | Septicemia | [74] |
| Burkholderia cenocepacia | Maize | ||||
| Burkholderia glumae | Tomato | ||||
| Burkholderia gladiol | Rice | ||||
| Erwinia billingiae, Erwinia persinicus | Fire blight | Rosaceae, Pear, Apple | Cervical lymphadenitis | [43] | |
| Agrobacterium tumefaciens | Crown gall disease | Eudicots | Bacteremia, Fetal death | [38,75] | |
| Pantoea agglomerans | Leaf spot blotches | Fruit bearing trees | Septicemia | [49] | |
| Pantoea ananatis | |||||
| Fungi | Cladosporium angustisporum Cladosporiumallicinum Cladosporium flabelliforme Cladosporium ladosporioides Cladosporium funiculosum Cladosporium herbarum Cladosporium halotolerans Cladosporium perangustum Cladosporium macrocarpum Cladosporium tenuissimum Cladosporium ramotenellum Cladosporium subuliforme Cladosporium subinflatum Cladosporium sphaerospermum | Blight, Leaf spot, Blossom | Echeveria, Dendrobium, Strawberry | Infection in the respiratory tract | [61,76] |
| Fusarium graminearum Fusarium proliferatum | Root rot, Head blight | Tomato, Tobacco, Legumes, Cucurbits | Blood infection | [59] | |
| Colletotrichum truncatum | Blight, Lesion, Necrosis | Cereals, Citrus, Strawberry, | Ophthalmic infection | [60,77] | |
| Alternaria infectoria | Blight, Blossom | Guayule | Keratitis, Phaeohyphomycosis | [54,78] | |
| Bipolaris australiensis Bipolaris hawaiiensis Bipolaris spicifera | Necrotic lesions, Chlorosis, Leaf spot, Leaf blight | Switchgrass, Sugarcane, Bermudagrass, Buffalograss, | Corneal ulcer, Infection at surgical sites | [56,57] |
2.4. Inoculation Requirements for Infectious Disease Transmission across Kingdoms
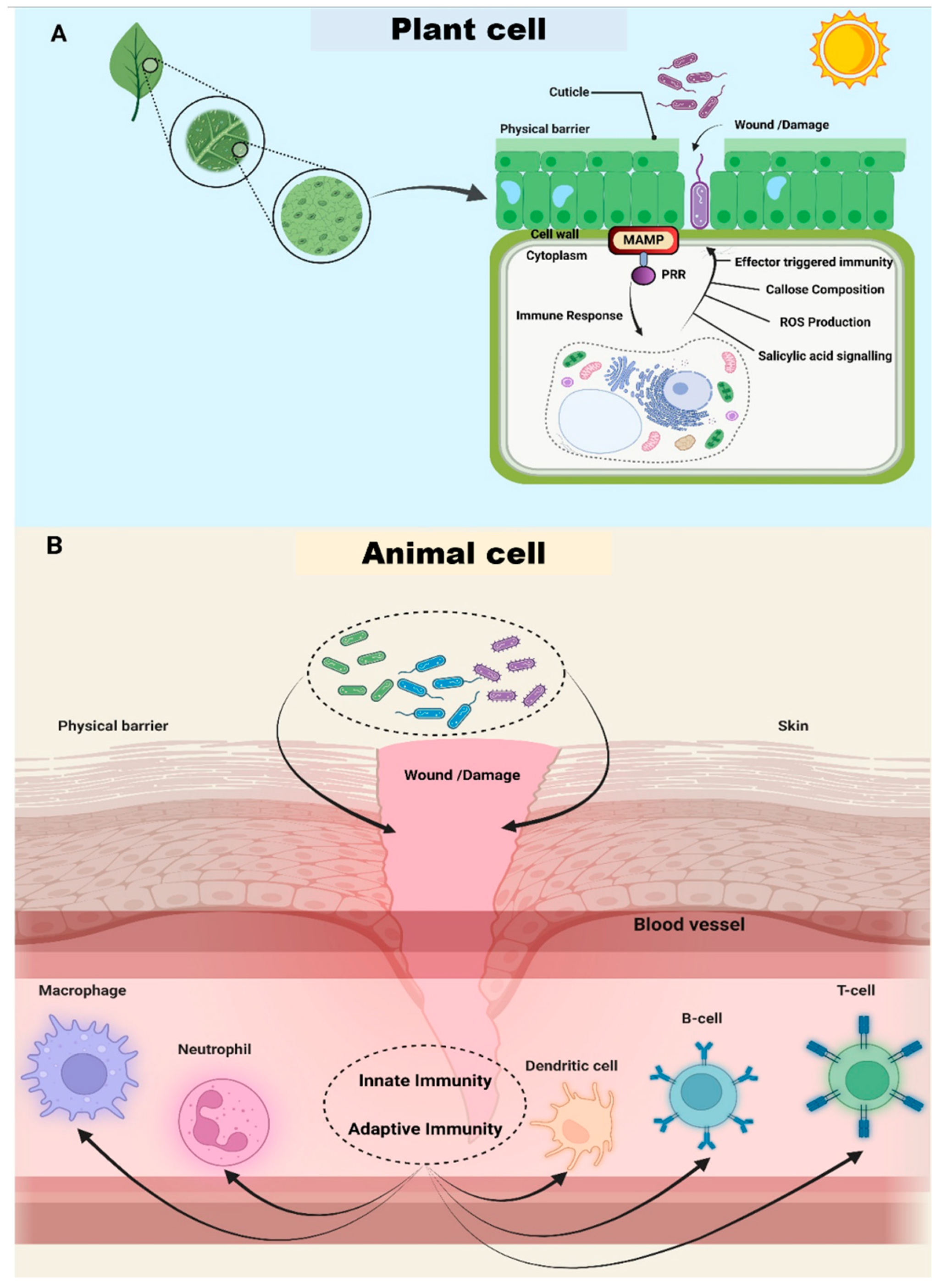
2.4.1. Overcoming the External Physical Barrier
2.4.2. Prevailing over the Host Immune System
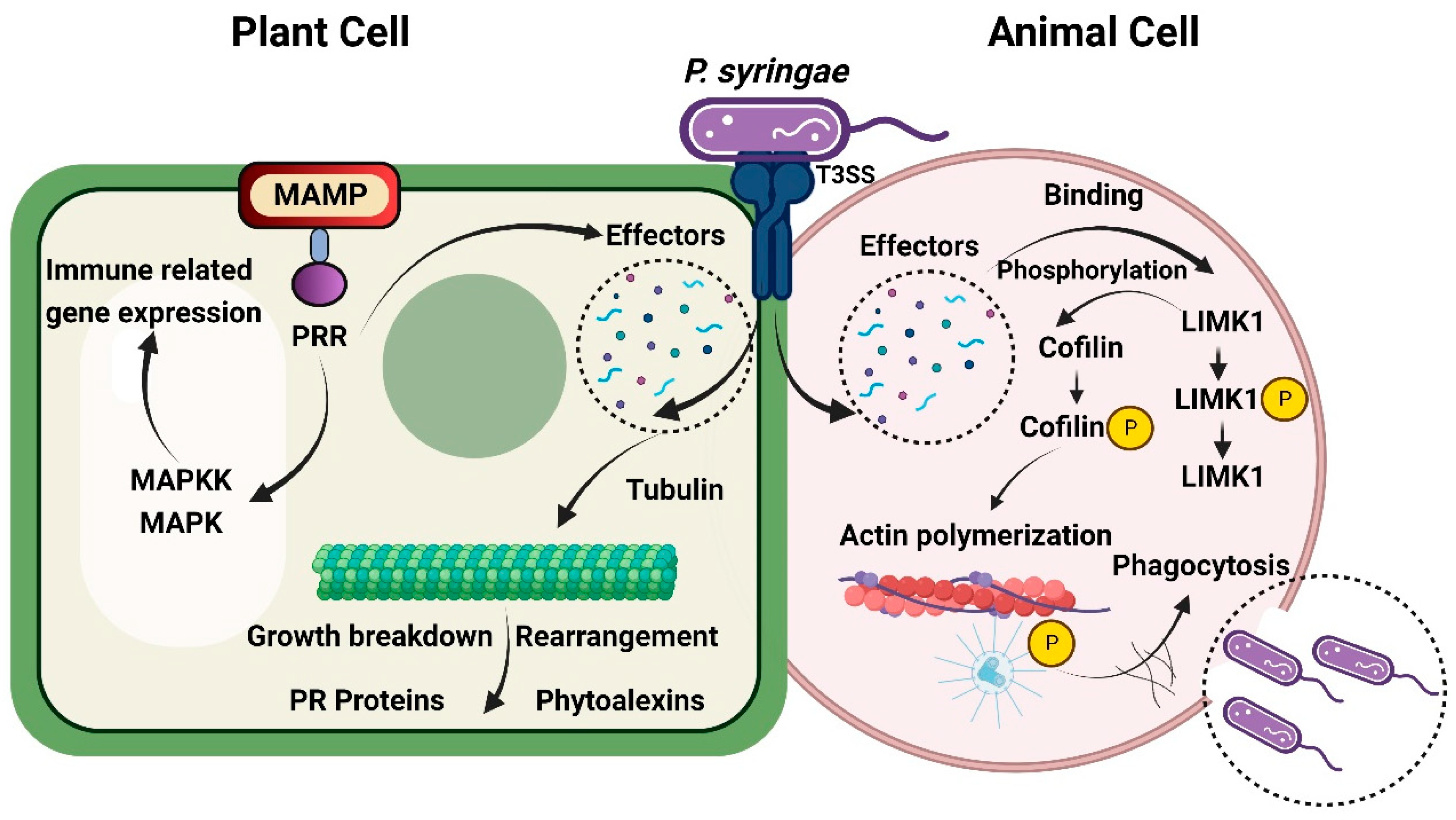
2.4.3. Molecular Pathogenic Pathways Implicated in Cross-Kingdom Infections
2.4.4. Disruption of Specific Animal Immune Responses by Phytopathogens
3. Green Nanoparticles as Alternatives to Conventional Approaches to Disease Control
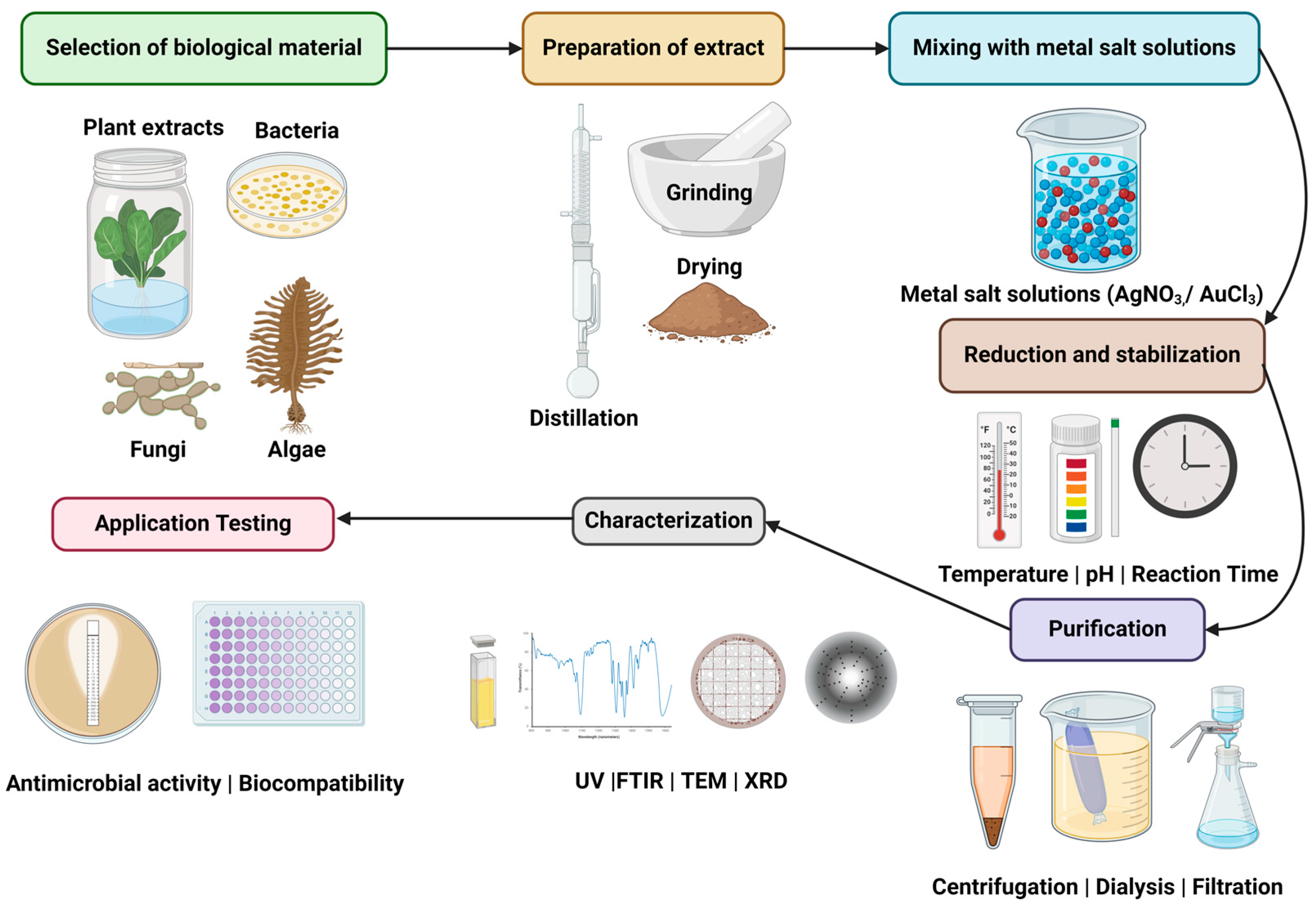
3.1. Introduction to Green Nanoparticles
| Plant Used | Part of Plant Used | NPs | Microorganism Used | Antibiotic Combined | References |
|---|---|---|---|---|---|
| Curcuma longa | Tuber extract | AgNPs | Escherichia coli BL-21 | Not combined | [18,141] |
| Dioscorea bulbifera | Tuber extract | AgNPs | Acinetobacter baumannii | Piperacillin, erythromycin | [135,142] |
| Pseudomonas aeruginosa | Chloramphenicol, vancomycin | ||||
| E. coli | Streptomycin | ||||
| Catharanthus roseus | Cell cultures from roots, callus and leaves | AgNPs | E. coli, Bacillus subtilis, Candida albicans, Staphylococcus aureus and, Klebsiella pneumoniae | Not combined | [138,139] |
| Senna siamea, Crossopteryx febrifuga and Brillantaisia owariensis | Aqueous leaf extracts | AgNPs | Gram (+): S. aureus Gram (−): E. coli, P. aeruginosa | Not combined | [140] |
| Fagonia indica | Callus cell cultures | AgNPs | E. coli, Salmonella typhi, Shigella sonnei, and Citrobacter amalonaticus | Ciprofloxacin | [143] |
| Urtica dioica | Aqueous leaf extracts | AgNPs | Gram (−): E. coli, Salmonella typhimurium, Serratia marcescens, and Klebsiella pneumoniae Gram (+): Staphylococcus epidermidis, Bacillus subtilis, Bacillus cereus and Staphylococcus aureus | Ampicillin, amoxicillin, tetracycline, kanamycin, Amikacin, streptomycin, cefotaxime vancomycin, and cefepime | [79] |
| Ocimum tenuiflorum | Leaf extract | NiNPs | Gram (+): Bacillus subtilis and Staphylococcus epidermidis Fungi: Aspergillus fumigatus, Aspergillus clavatus and Aspergillus niger Candida albicans and Candida tropicalis Gram (−): E. coli, Salmonella typhi and Klebsiella pneumoniae | Not combined | [144] |
| Piper guineense | Aqueous leaf extracts | AuNPs | S. aureus, Streptococcus pyogenes | Not combined | [145] |
| Zea mays | Corn leaf waste extract | AgNPs | S. typhimurium, S. aureus, E. coli, B. cereus, and Listeria monocytogenes | Kanamycin, rifampicin | [146] |
| Typha angustifolia | Leaf extract | AgNPs | E. coli and K. pneumoniae | Gentamicin, cefotaxime, meropenem | [147] |
| Phyllanthus reticulatus, Erigeron bonariensis | Leaf extract | CuONPs | E. coli | Not combined | [148] |
3.2. Green Nanoparticles: Detailed Overview, Mechanism of Action and Phytopathogenic Targets
3.2.1. Silver Nanoparticles (AgNPs)
3.2.2. Gold Nanoparticles (AuNPs)
3.2.3. Zinc Oxide Nanoparticles (ZnO NPs)
3.3. Synthesis of Nanoparticles
3.3.1. Influence of Different Physicochemical Characteristics on Nanoparticle Synthesis
3.3.2. pH and Precursor Concentration
3.3.3. Temperature
4. Antibiotic Resistance and Phytopathogens in Therapeutic Settings
5. Anti-Microbial Action of Nanoparticles
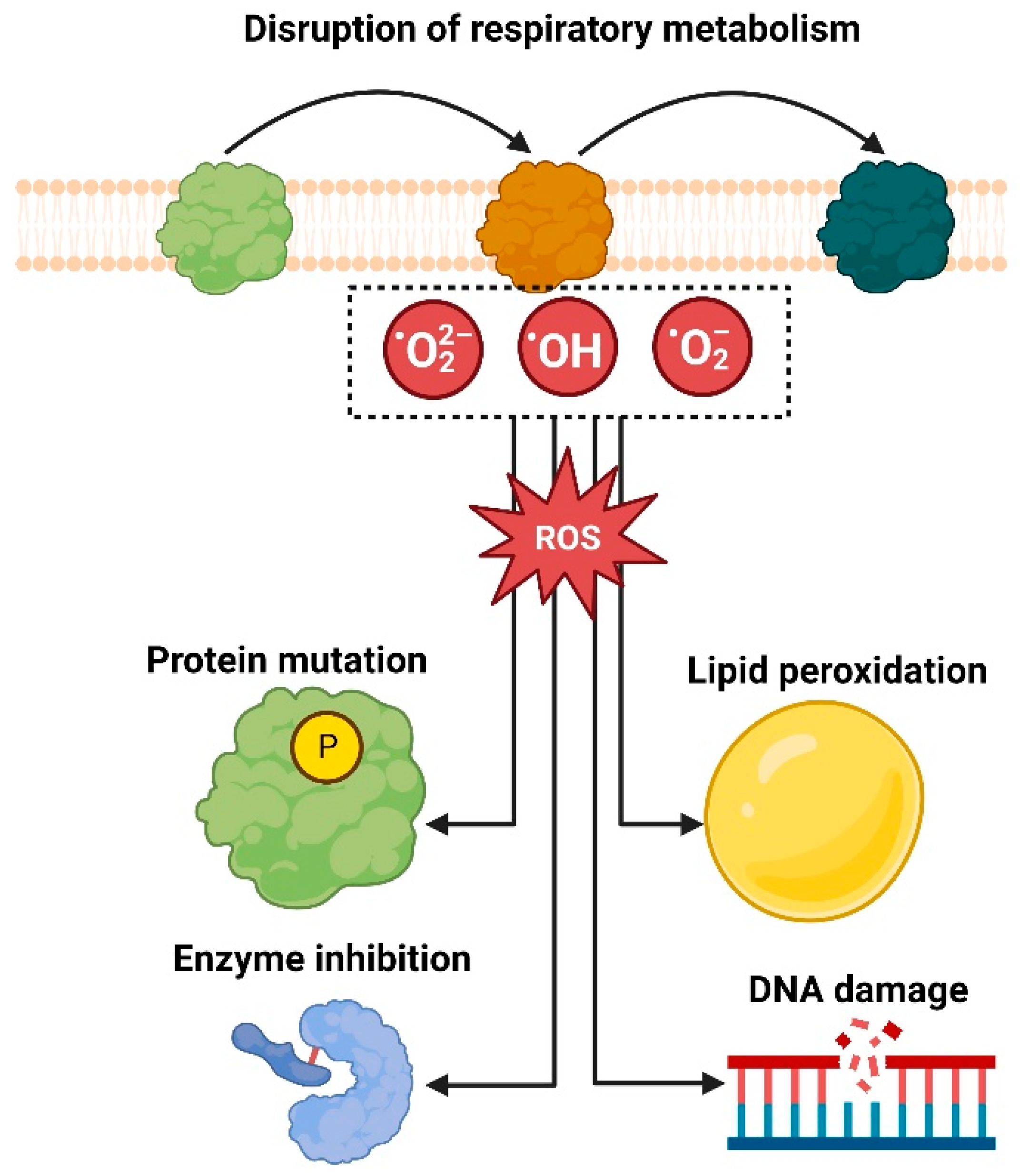
5.1. Generation of Reactive Oxygen Species (ROS)
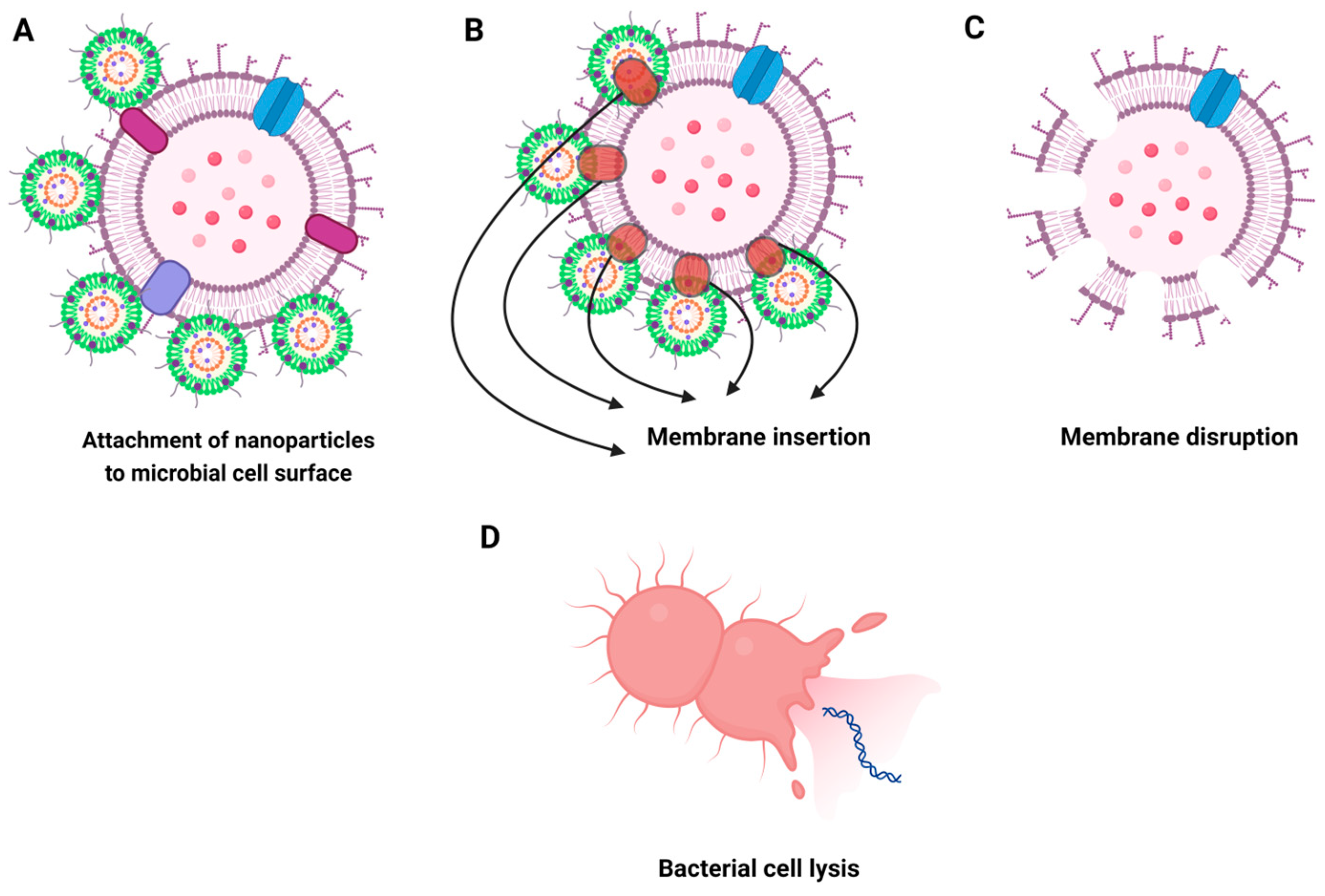
5.2. Interaction with Microbial Cell Membranes
5.3. Penetration and Disruption of Microbial Cell Membranes
5.4. Interference with Intracellular Components
5.5. Biofilm Inhibition
6. Safety and Biocompatibility of Green-Synthesized Nanoparticles
6.1. Cytotoxicity and Genotoxicity
6.2. Inflammatory Responses
6.3. Effects on Human Microbiota
6.3.1. Gut Microbiome
6.3.2. Skin Microbiome
6.3.3. Oral Microbiome
6.4. Pharmacokinetics of Green Nanoparticles
6.5. Pharmacodynamics of Green Nanoparticles
7. Current Clinical Scenario for Green Nanoparticles
7.1. Silver Nanoparticles and Antibiotics
7.2. Gold Nanoparticles and Antibiotics
7.3. Copper Nanoparticles and Antibiotics
8. Challenges Associated with Clinical Translation and Their Prospective Solutions
9. Standardization, Regulation and Environmental Effects of Green Nanoparticles
9.1. Standardization
9.2. Environmental Effects
9.2.1. Soil Quality and Microbial Communities
9.2.2. Water Quality and Aquatic Life
9.2.3. Air Quality and Human Health
9.2.4. Bioaccumulation and Biomagnification
9.2.5. Ecotoxicological Effects
9.3. Sustainable Practices in Synthesis
9.3.1. Sustainable Synthesis Methods
9.3.2. Efficient Resource Utilization
9.3.3. Lifecycle Assessment (LCA)
9.3.4. Safe Disposal and Recycling
9.3.5. Regulatory Framework and Collaboration
9.3.6. Case Studies and Implementation
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nazarov, P.A.; Baleev, D.N.; Ivanova, M.I.; Sokolova, L.M.; Karakozova, M.V. Infectious plant diseases: Etiology, current status, problems and prospects in plant protection. Acta Naturae 2020, 12, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Sarsaiya, S.; Wu, Q.; Lu, Y.; Shi, J. A review of plant leaf fungal diseases and its environment speciation. Bioengineered 2019, 10, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Zarkani, A.A.; Schikora, A. Mechanisms adopted by Salmonella to colonize plant hosts. Food Microbiol. 2021, 99, 103833. [Google Scholar] [CrossRef]
- Zarkani, A.A.; López-Pagán, N.; Grimm, M.; Sánchez-Romero, M.A.; Ruiz-Albert, J.; Beuzón, C.R.; Schikora, A. Salmonella Heterogeneously Expresses Flagellin during Colonization of Plants. Microorganisms 2020, 8, 815. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Li, C.; Tan, K.; Liu, C.; Xu, X.; Zhang, S.; Wang, X.; Zhao, J.; Xiang, W. Characterization, Phylogenetic Analyses, and Pathogenicity of Enterobacter cloacae on Rice Seedlings in Heilongjiang Province, China. Plant Dis. 2020, 104, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Hu, M.; Chen, S.; Hu, A.; Li, S.; Han, H.; Lu, G.; Zeng, L.; Zhou, J. Enterobacter asburiae and Pantoea ananatis Causing Rice Bacterial Blight in China. Plant Dis. 2021, 105, 2078–2088. [Google Scholar] [CrossRef]
- Jo, S.H.; Lee, J.; Park, E.; Kim, D.W.; Lee, D.H.; Ryu, C.M.; Choi, D.; Park, J.M. A human pathogenic bacterium Shigella proliferates in plants through adoption of type III effectors for shigellosis. Plant Cell Environ. 2019, 42, 2962–2978. [Google Scholar] [CrossRef]
- Chajęcka-Wierzchowska, W.; Zarzecka, U.; Zadernowska, A. Enterococci isolated from plant-derived food—Analysis of antibiotic resistance and the occurrence of resistance genes. LWT 2021, 139, 110549. [Google Scholar] [CrossRef]
- Hitam, C.N.C.; Jalil, A.A. Recent advances on nanocellulose biomaterials for environmental health photoremediation: An overview. Environ. Res. 2022, 204, 111964. [Google Scholar] [CrossRef]
- Javed, S.; Mangla, B.; Ahsan, W. From propolis to nanopropolis: An exemplary journey and a paradigm shift of a resinous substance produced by bees. Phyther. Res. 2022, 36, 2016–2041. [Google Scholar] [CrossRef]
- Griffin, S.; Masood, M.; Nasim, M.; Sarfraz, M.; Ebokaiwe, A.; Schäfer, K.-H.; Keck, C.; Jacob, C. Natural Nanoparticles: A Particular Matter Inspired by Nature. Antioxidants 2017, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, T.B.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A.; et al. Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity. J. King Saud Univ.-Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Farjadian, F.; Ghasemi, A.; Gohari, O.; Roointan, A.; Karimi, M.; Hamblin, M.R. Nanopharmaceuticals and nanomedicines currently on the market: Challenges and opportunities. Nanomedicine 2019, 14, 93–126. [Google Scholar] [CrossRef]
- Kaur, P. Biosynthesis of nanoparticles using eco-friendly factories and their role in plant pathogenicity: A review. Biotechnol. Res. Innov. 2018, 2, 63–73. [Google Scholar] [CrossRef]
- Singh, P.; Kim, Y.-J.; Zhang, D.; Yang, D.-C. Biological Synthesis of Nanoparticles from Plants and Microorganisms. Trends Biotechnol. 2016, 34, 588–599. [Google Scholar] [CrossRef]
- Khan, T.; Ullah, N.; Khan, M.A.; Mashwani, Z.-R.; Nadhman, A. Plant-based gold nanoparticles; a comprehensive review of the decade-long research on synthesis, mechanistic aspects and diverse applications. Adv. Colloid Interface Sci. 2019, 272, 102017. [Google Scholar] [CrossRef]
- Mirzaei, H.; Darroudi, M. Zinc oxide nanoparticles: Biological synthesis and biomedical applications. Ceram. Int. 2017, 43, 907–914. [Google Scholar] [CrossRef]
- Sathishkumar, M.; Sneha, K.; Yun, Y.-S. Immobilization of silver nanoparticles synthesized using Curcuma longa tuber powder and extract on cotton cloth for bactericidal activity. Bioresour. Technol. 2010, 101, 7958–7965. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Yusoff, M.M.; Maniam, G.P.; Govindan, N. Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications—An updated report. Saudi Pharm. J. 2016, 24, 473–484. [Google Scholar] [CrossRef]
- Chaudhary, R.; Singhal, B. Novel Paradigms of Nanomediated Targeted Drug Delivery in Gastrointestinal Disorders. In Intelligent Nanomaterials for Drug Delivery Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 61–84. [Google Scholar]
- Roy, A.; Bulut, O.; Some, S.; Mandal, A.K.; Yilmaz, M.D. Green synthesis of silver nanoparticles: Biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019, 9, 2673–2702. [Google Scholar] [CrossRef]
- Pytlik, N.; Brunner, E. Diatoms as potential “green” nanocomposite and nanoparticle synthesizers: Challenges, prospects, and future materials applications. MRS Commun. 2018, 8, 322–331. [Google Scholar] [CrossRef]
- Shah, A.H.; Rather, M.A. Intracellular and Extracellular Microbial Enzymes and Their Role in Nanoparticle Synthesis. In Microbial Nanotechnology: Green Synthesis and Applications; Springer: Singapore, 2021; pp. 41–59. [Google Scholar]
- Singh, H.; Du, J.; Singh, P.; Yi, T.H. Extracellular synthesis of silver nanoparticles by Pseudomonas sp. THG-LS1.4 and their antimicrobial application. J. Pharm. Anal. 2018, 8, 258–264. [Google Scholar] [CrossRef]
- Chaudhary, R.; Nawaz, K.; Khan, A.K.; Hano, C.; Abbasi, B.H.; Anjum, S. An Overview of the Algae-Mediated Biosynthesis of Nanoparticles and Their Biomedical Applications. Biomolecules 2020, 10, 1498. [Google Scholar] [CrossRef] [PubMed]
- Campos, D.; Goméz-García, R.; Oliveira, D.; Madureira, A.R. Intake of nanoparticles and impact on gut microbiota: In Vitro and animal models available for testing. Gut Microbiome 2022, 3, e1. [Google Scholar] [CrossRef]
- Li, M.; Zhang, C. Are silver nanoparticles better than triclosan as a daily antimicrobial? Answers from the perspectives of gut microbiome disruption and pathogenicity. Sci. Total Environ. 2021, 756, 143983. [Google Scholar] [CrossRef]
- Khare, T.; Anand, U.; Dey, A.; Assaraf, Y.G.; Chen, Z.-S.; Liu, Z.; Kumar, V. Exploring Phytochemicals for Combating Antibiotic Resistance in Microbial Pathogens. Front. Pharmacol. 2021, 12, 720726. [Google Scholar] [CrossRef]
- Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green synthesis of nanoparticles using plant extracts: A review. Environ. Chem. Lett. 2021, 19, 355–374. [Google Scholar] [CrossRef]
- Shah, M.; Nawaz, S.; Jan, H.; Uddin, N.; Ali, A.; Anjum, S.; Giglioli-Guivarc’h, N.; Hano, C.; Abbasi, B.H. Synthesis of bio-mediated silver nanoparticles from Silybum marianum and their biological and clinical activities. Mater. Sci. Eng. C 2020, 112, 110889. [Google Scholar] [CrossRef]
- Aguilar-Pérez, K.M.; Avilés-Castrillo, J.I.; Ruiz-Pulido, G.; Medina, D.I.; Parra-Saldivar, R.; Iqbal, H.M.N. Nanoadsorbents in focus for the remediation of environmentally-related contaminants with rising toxicity concerns. Sci. Total Environ. 2021, 779, 146465. [Google Scholar] [CrossRef]
- Anand, U.; Jacobo-Herrera, N.; Altemimi, A.; Lakhssassi, N. A Comprehensive Review on Medicinal Plants as Antimicrobial Therapeutics: Potential Avenues of Biocompatible Drug Discovery. Metabolites 2019, 9, 258. [Google Scholar] [CrossRef]
- Baptista, P.V.; McCusker, M.P.; Carvalho, A.; Ferreira, D.A.; Mohan, N.M.; Martins, M.; Fernandes, A.R. Nano-Strategies to Fight Multidrug Resistant Bacteria—“A Battle of the Titans”. Front. Microbiol. 2018, 9, 1441. [Google Scholar] [CrossRef]
- Ruddaraju, L.K.; Pammi, S.V.N.; sankar Guntuku, G.; Padavala, V.S.; Kolapalli, V.R.M. A review on anti-bacterials to combat resistance: From ancient era of plants and metals to present and future perspectives of green nano technological combinations. Asian J. Pharm. Sci. 2020, 15, 42–59. [Google Scholar] [CrossRef]
- Kim, J.; Yoon, S.; Park, Y.; Kim, S.; Ryu, C. Crossing the kingdom border: Human diseases caused by plant pathogens. Environ. Microbiol. 2020, 22, 2485–2495. [Google Scholar] [CrossRef] [PubMed]
- Petrunia, I.V.; Frolova, O.Y.; Komarova, T.V.; Kiselev, S.L.; Citovsky, V.; Dorokhov, Y.L. Agrobacterium tumefaciens-Induced Bacteraemia Does Not Lead to Reporter Gene Expression in Mouse Organs. PLoS ONE 2008, 3, e2352. [Google Scholar] [CrossRef] [PubMed]
- Balmer, L.; Seth-Smith, H.M.B.; Egli, A.; Casanova, C.; Kronenberg, A.; Schrenzel, J.; Marschall, J.; Sommerstein, R. Agrobacterium species bacteraemia, Switzerland, 2008 to 2019: A molecular epidemiological study. Antimicrob. Resist. Infect. Control 2022, 11, 47. [Google Scholar] [CrossRef] [PubMed]
- Fenner, B.J.; Kumar, A.; Tan, N.Y.Q.; Ang, M. Case of isolated Rhizobium radiobacter contact lens-related infectious keratitis: A plant microbe now emerging as a human pathogen. Am. J. Ophthalmol. Case Reports 2019, 15, 100476. [Google Scholar] [CrossRef] [PubMed]
- Canik Orel, D.; Oğuz, E. Parsley (Petroselinum crispum) leaf and stem spot caused by Erwinia persicina. J. Plant Pathol. 2023, 105, 1539–1549. [Google Scholar] [CrossRef]
- Kawaguchi, A.; Abe, D.; Saito, T.; Nogata, Y.; Nomiyama, K.; Kohyama, N.; Takahashi, A.; Yoshioka, T.; Ishikawa, N.; Tomioka, K. Pink seed of barley caused by Erwinia persicina. J. Gen. Plant Pathol. 2021, 87, 106–109. [Google Scholar] [CrossRef]
- O’Hara, C.M.; Steigerwalt, A.G.; Hill, B.C.; Miller, J.M.; Brenner, D.J. First Report of a Human Isolate of Erwinia persicinus. J. Clin. Microbiol. 1998, 36, 248–250. [Google Scholar] [CrossRef]
- Klein, R.D.; Hultgren, S.J. Urinary tract infections: Microbial pathogenesis, host–pathogen interactions and new treatment strategies. Nat. Rev. Microbiol. 2020, 18, 211–226. [Google Scholar] [CrossRef]
- Prod’homme, M.; Micol, L.A.; Weitsch, S.; Gassend, J.-L.; Martinet, O.; Bellini, C. Cutaneous infection and bactaeremia caused by Erwinia billingiae: A case report. New Microbes New Infect. 2017, 19, 134–136. [Google Scholar] [CrossRef] [PubMed]
- Govan, J.R.W.; Hughes, J.E.; Vandamme, P. Burkholderia cepacia: Medical, taxonomic and ecological issues. J. Med. Microbiol. 1996, 45, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Goodlet, K.J.; Nailor, M.D.; Omar, A.; Huang, J.L.; LiPuma, J.J.; Walia, R.; Tokman, S. Successful Lung Re-transplant in a Patient with Cepacia Syndrome due to Burkholderia ambifaria. J. Cyst. Fibros. 2019, 18, e1–e4. [Google Scholar] [CrossRef] [PubMed]
- Elshafie, H.S.; Camele, I. An Overview of Metabolic Activity, Beneficial and Pathogenic Aspects of Burkholderia spp. Metabolites 2021, 11, 321. [Google Scholar] [CrossRef] [PubMed]
- Springman, A.C.; Jacobs, J.L.; Somvanshi, V.S.; Sundin, G.W.; Mulks, M.H.; Whittam, T.S.; Viswanathan, P.; Gray, R.L.; LiPuma, J.J.; Ciche, T.A. Genetic Diversity and Multihost Pathogenicity of Clinical and Environmental Strains of Burkholderia cenocepacia. Appl. Environ. Microbiol. 2009, 75, 5250–5260. [Google Scholar] [CrossRef]
- Jones, C.; Webster, G.; Mullins, A.J.; Jenner, M.; Bull, M.J.; Dashti, Y.; Spilker, T.; Parkhill, J.; Connor, T.R.; LiPuma, J.J.; et al. Kill and cure: Genomic phylogeny and bioactivity of Burkholderia gladioli bacteria capable of pathogenic and beneficial lifestyles. Microb. Genomics 2021, 7, 000515. [Google Scholar] [CrossRef]
- Yablon, B.R.; Dantes, R.; Tsai, V.; Lim, R.; Moulton-Meissner, H.; Arduino, M.; Jensen, B.; Patel, M.T.; Vernon, M.O.; Grant-Greene, Y.; et al. Outbreak of Pantoea agglomerans Bloodstream Infections at an Oncology Clinic—Illinois, 2012–2013. Infect. Control Hosp. Epidemiol. 2017, 38, 314–319. [Google Scholar] [CrossRef]
- De Baere, T.; Verhelst, R.; Labit, C.; Verschraegen, G.; Wauters, G.; Claeys, G.; Vaneechoutte, M. Bacteremic Infection with Pantoea ananatis. J. Clin. Microbiol. 2004, 42, 4393–4395. [Google Scholar] [CrossRef]
- Casale, R.; Boattini, M.; Bianco, G.; Comini, S.; Corcione, S.; Garazzino, S.; Silvestro, E.; De Rosa, F.G.; Cavallo, R.; Costa, C. Bloodstream Infections by Pantoea Species: Clinical and Microbiological Findings from a Retrospective Study, Italy, 2018–2023. Antibiotics 2023, 12, 1723. [Google Scholar] [CrossRef]
- Habsah, H.; Zeehaida, M.; Van Rostenberghe, H.; Noraida, R.; Wan Pauzi, W.I.; Fatimah, I.; Rosliza, A.R.; Nik Sharimah, N.Y.; Maimunah, H. An outbreak of Pantoea spp. in a neonatal intensive care unit secondary to contaminated parenteral nutrition. J. Hosp. Infect. 2005, 61, 213–218. [Google Scholar] [CrossRef]
- Yoshimura, M.; Tokushige, C.; Maruyama, J.; Kawano, Y.; Ishikura, H.; Matsunaga, A.; Takata, T.; Hiromatsu, K.; Yanagihara, I.; Togawa, A.; et al. Emerging resistance to beta-lactams in Pantoea ananatis isolated from an immunocompetent patient with bacteremia. Diagn. Microbiol. Infect. Dis. 2022, 102, 115633. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Casadevall, A.; Gonçalves, T. Mechanisms of Alternaria pathogenesis in animals and plants. FEMS Microbiol. Rev. 2023, 47, fuad061. [Google Scholar] [CrossRef]
- Amaradasa, B.S.; Amundsen, K. First Report of Curvularia inaequalis and Bipolaris spicifera Causing Leaf Blight of Buffalograss in Nebraska. Plant Dis. 2014, 98, 279. [Google Scholar] [CrossRef]
- Pham, C.D.; Purfield, A.E.; Fader, R.; Pascoe, N.; Lockhart, S.R. Development of a Multilocus Sequence Typing System for Medically Relevant Bipolaris Species. J. Clin. Microbiol. 2015, 53, 3239–3246. [Google Scholar] [CrossRef] [PubMed]
- Pai, H.; Jamal, E.; Yegneswaran, P. Corneal ulcer due to a rare pleosporalean member of the genus Bipolaris following cow tail injury to the eye: A case report and review of literature. Indian J. Ophthalmol. 2017, 65, 403. [Google Scholar] [CrossRef] [PubMed]
- Bebber, D.P.; Gurr, S.J. Crop-destroying fungal and oomycete pathogens challenge food security. Fungal Genet. Biol. 2015, 74, 62–64. [Google Scholar] [CrossRef]
- Sun, S.; Lui, Q.; Han, L.; Ma, Q.; He, S.; Li, X.; Zhang, H.; Zhang, J.; Liu, X.; Wang, L. Identification and Characterization of Fusarium proliferatum, a New Species of Fungi that Cause Fungal Keratitis. Sci. Rep. 2018, 8, 4859. [Google Scholar] [CrossRef]
- Christy, J.S.; Balraj, A.; Agarwal, A. A Rare Case of Colletotrichum truncatum Keratitis in a Young Boy with Complete Healing after Medical Treatment. Indian J. Med. Microbiol. 2020, 38, 475–477. [Google Scholar] [CrossRef]
- Sandoval-Denis, M.; Sutton, D.A.; Martin-Vicente, A.; Cano-Lira, J.F.; Wiederhold, N.; Guarro, J.; Gené, J. Cladosporium Species Recovered from Clinical Samples in the United States. J. Clin. Microbiol. 2015, 53, 2990–3000. [Google Scholar] [CrossRef]
- Brüssow, H. The Novel Coronavirus—A Snapshot of Current Knowledge. Microb. Biotechnol. 2020, 13, 607–612. [Google Scholar] [CrossRef]
- Balique, F.; Lecoq, H.; Raoult, D.; Colson, P. Can Plant Viruses Cross the Kingdom Border and Be Pathogenic to Humans? Viruses 2015, 7, 2074–2098. [Google Scholar] [CrossRef]
- Lefeuvre, P.; Martin, D.P.; Elena, S.F.; Shepherd, D.N.; Roumagnac, P.; Varsani, A. Evolution and ecology of plant viruses. Nat. Rev. Microbiol. 2019, 17, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Jiwaji, M.; Matcher, G.F.; de Bruyn, M.-M.; Awando, J.A.; Moodley, H.; Waterworth, D.; Jarvie, R.A.; Dorrington, R.A. Providence virus: An animal virus that replicates in plants or a plant virus that infects and replicates in animal cells? PLoS ONE 2019, 14, e0217494. [Google Scholar] [CrossRef] [PubMed]
- Bousbia, S.; Papazian, L.; La Scola, B.; Raoult, D. Detection of Plant DNA in the Bronchoalveolar Lavage of Patients with Ventilator-Associated Pneumonia. PLoS ONE 2010, 5, e11298. [Google Scholar] [CrossRef] [PubMed]
- Colson, P.; Richet, H.; Desnues, C.; Balique, F.; Moal, V.; Grob, J.-J.; Berbis, P.; Lecoq, H.; Harlé, J.-R.; Berland, Y.; et al. Pepper Mild Mottle Virus, a Plant Virus Associated with Specific Immune Responses, Fever, Abdominal Pains, and Pruritus in Humans. PLoS ONE 2010, 5, e10041. [Google Scholar] [CrossRef]
- Jiao, Y.; An, M.; Li, X.; Yu, M.; Zhao, X.; Xia, Z.; Wu, Y. Transcriptomic and functional analyses reveal an antiviral role of autophagy during pepper mild mottle virus infection. BMC Plant Biol. 2020, 20, 495. [Google Scholar] [CrossRef]
- Liu, F.; Wang, X.; Du, S. Production of gold/silver doped carbon nanocomposites for effective photothermal therapy of colon cancer. Sci. Rep. 2020, 10, 7618. [Google Scholar] [CrossRef]
- Li, H.; Sun, Y.; Gao, L.-L.; Tang, Y.-F.; Zhao, Z. The Treatment of Human Colon Xenografts Tumor in Mice with Platinum Nanosphere-5-Fluorouracil-Bovine Albumin. J. Biomed. Nanotechnol. 2022, 18, 778–787. [Google Scholar] [CrossRef]
- Koudelka, K.J.; Destito, G.; Plummer, E.M.; Trauger, S.A.; Siuzdak, G.; Manchester, M. Endothelial Targeting of Cowpea Mosaic Virus (CPMV) via Surface Vimentin. PLoS Pathog. 2009, 5, e1000417. [Google Scholar] [CrossRef]
- Beatty, P.H.; Lewis, J.D. Cowpea mosaic virus nanoparticles for cancer imaging and therapy. Adv. Drug Deliv. Rev. 2019, 145, 130–144. [Google Scholar] [CrossRef]
- Jiang, T.; Zhou, T. Unraveling the Mechanisms of Virus-Induced Symptom Development in Plants. Plants 2023, 12, 2830. [Google Scholar] [CrossRef]
- Zaid, A.M.; Asselin, J.E.; Beer, S.V. CHAPTER 22: Detection of Burkholderia cepacia in Onion Planting Materials and Onion Seeds. In Detection of Plant-Pathogenic Bacteria in Seed and Other Planting Material, Second Edition; The American Phytopathological Society: Saint Paul, MN, USA, 2017; pp. 149–156. [Google Scholar]
- Brown, P.J.B.; Chang, J.H.; Fuqua, C. Agrobacterium tumefaciens: A Transformative Agent for Fundamental Insights into Host-Microbe Interactions, Genome Biology, Chemical Signaling, and Cell Biology. J. Bacteriol. 2023, 205, e00005-23. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Denis, M.; Gené, J.; Sutton, D.A.; Wiederhold, N.P.; Cano-Lira, J.F.; Guarro, J. New species of Cladosporium associated with human and animal infections. Persoonia-Mol. Phylogeny Evol. Fungi 2016, 36, 281–298. [Google Scholar] [CrossRef] [PubMed]
- Paniz-Mondolfi, A.E.; Agemy, S.; Cañete-Gibas, C.; Gitman, M.R.; Iacob, C.E.; Necula, I.; Wang, C.-Y.; Delgado Noguera, L.A.; Sanders, C.; Wiederhold, N.P.; et al. First report of human infection caused by Colletotrichum chlorophyti occurring in a post-corneal transplant patient with endophthalmitis. Med. Mycol. Case Rep. 2021, 32, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Todokoro, D.; Miyakubo, T.; Komori, A.; Tamura, T.; Makimura, K.; Akiyama, H. Successful Management of Fungal Keratitis by Alternaria alternata Complicating Mooren’s Ulcer. Case Rep. Ophthalmol. 2023, 153–158. [Google Scholar] [CrossRef]
- Soni, J.; Sinha, S.; Pandey, R. Understanding bacterial pathogenicity: A closer look at the journey of harmful microbes. Front. Microbiol. 2024, 15, 1370818. [Google Scholar] [CrossRef]
- Reddy, G.K.K.; Padmavathi, A.R.; Nancharaiah, Y.V. Fungal infections: Pathogenesis, antifungals and alternate treatment approaches. Curr. Res. Microb. Sci. 2022, 3, 100137. [Google Scholar] [CrossRef]
- Köhler, J.R.; Hube, B.; Puccia, R.; Casadevall, A.; Perfect, J.R. Fungi that Infect Humans. Microbiol. Spectr. 2017, 5, 10–1128. [Google Scholar] [CrossRef]
- Lu, H.-F.; Zhou, Y.-C.; Yang, L.-T.; Zhou, Q.; Wang, X.-J.; Qiu, S.-Q.; Cheng, B.-H.; Zeng, X.-H. Involvement and repair of epithelial barrier dysfunction in allergic diseases. Front. Immunol. 2024, 15, 1348272. [Google Scholar] [CrossRef]
- Jonkheer, E.M.; Brankovics, B.; Houwers, I.M.; van der Wolf, J.M.; Bonants, P.J.M.; Vreeburg, R.A.M.; Bollema, R.; de Haan, J.R.; Berke, L.; Smit, S.; et al. The Pectobacterium pangenome, with a focus on Pectobacterium brasiliense, shows a robust core and extensive exchange of genes from a shared gene pool. BMC Genom. 2021, 22, 265. [Google Scholar] [CrossRef]
- Ghosh, P.; Zhou, Y.; Richardson, Q.; Higgins, D.E. Characterization of the pathogenesis and immune response to Listeria monocytogenes strains isolated from a sustained national outbreak. Sci. Rep. 2019, 9, 19587. [Google Scholar] [CrossRef]
- Li, Q. Mechanisms for the Invasion and Dissemination of Salmonella. Can. J. Infect. Dis. Med. Microbiol. 2022, 2022, 2655801. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liang, K.; Chen, L.; Su, X.; Liao, D.; Yu, J.; He, J. Unveiling the stealthy tactics: Mycoplasma’s immune evasion strategies. Front. Cell. Infect. Microbiol. 2023, 13, 1247182. [Google Scholar] [CrossRef]
- Silva, M.T. Classical Labeling of Bacterial Pathogens According to Their Lifestyle in the Host: Inconsistencies and Alternatives. Front. Microbiol. 2012, 3, 71. [Google Scholar] [CrossRef] [PubMed]
- Payne, S. Family Coronaviridae. In Viruses; Elsevier: Amsterdam, The Netherlands, 2017; pp. 149–158. [Google Scholar]
- Gusev, E.; Sarapultsev, A.; Solomatina, L.; Chereshnev, V. SARS-CoV-2-Specific Immune Response and the Pathogenesis of COVID-19. Int. J. Mol. Sci. 2022, 23, 1716. [Google Scholar] [CrossRef]
- Jagirdhar, G.S.K.; Pulakurthi, Y.S.; Chigurupati, H.D.; Surani, S. Gastrointestinal tract and viral pathogens. World J. Virol. 2023, 12, 136–150. [Google Scholar] [CrossRef]
- Gonzalez, S.M.; Aguilar-Jimenez, W.; Su, R.-C.; Rugeles, M.T. Mucosa: Key Interactions Determining Sexual Transmission of the HIV Infection. Front. Immunol. 2019, 10, 144. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicka, M.; San Giorgi, M.R.M.; Dikkers, F.G. Transmission and clearance of human papillomavirus infection in the oral cavity and its role in oropharyngeal carcinoma—A review. Rev. Med. Virol. 2023, 33, e2337. [Google Scholar] [CrossRef]
- Vidya Vijayan, K.K.; Karthigeyan, K.P.; Tripathi, S.P.; Hanna, L.E. Pathophysiology of CD4+ T-Cell Depletion in HIV-1 and HIV-2 Infections. Front. Immunol. 2017, 8, 580. [Google Scholar] [CrossRef]
- Wan, J.; He, M.; Hou, Q.; Zou, L.; Yang, Y.; Wei, Y.; Chen, X. Cell wall associated immunity in plants. Stress Biol. 2021, 1, 3. [Google Scholar] [CrossRef]
- Semwal, R.; Semwal, R.B.; Lehmann, J.; Semwal, D.K. Recent advances in immunotoxicity and its impact on human health: Causative agents, effects and existing treatments. Int. Immunopharmacol. 2022, 108, 108859. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Zhu, J.; Moreno-Pérez, A.; Coaker, G. Understanding plant pathogen interactions using spatial and single-cell technologies. Commun. Biol. 2023, 6, 814. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S. Phagocytosis: An Immunobiologic Process. Immunity 2016, 44, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, S.H.E.; Dorhoi, A. Molecular Determinants in Phagocyte-Bacteria Interactions. Immunity 2016, 44, 476–491. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Beyrakhova, K.; Liu, Y.; Alvarez, C.P.; Bueler, S.A.; Xu, L.; Xu, C.; Boniecki, M.T.; Kanelis, V.; Luo, Z.-Q.; et al. Molecular basis for the binding and modulation of V-ATPase by a bacterial effector protein. PLOS Pathog. 2017, 13, e1006394. [Google Scholar] [CrossRef]
- Lawrence, R.E.; Zoncu, R. The lysosome as a cellular centre for signalling, metabolism and quality control. Nat. Cell Biol. 2019, 21, 133–142. [Google Scholar] [CrossRef]
- Pauwels, A.-M.; Trost, M.; Beyaert, R.; Hoffmann, E. Patterns, Receptors, and Signals: Regulation of Phagosome Maturation. Trends Immunol. 2017, 38, 407–422. [Google Scholar] [CrossRef]
- Culyba, M.J.; Van Tyne, D. Bacterial evolution during human infection: Adapt and live or adapt and die. PLOS Pathog. 2021, 17, e1009872. [Google Scholar] [CrossRef]
- Queval, C.J.; Song, O.-R.; Carralot, J.-P.; Saliou, J.-M.; Bongiovanni, A.; Deloison, G.; Deboosère, N.; Jouny, S.; Iantomasi, R.; Delorme, V.; et al. Mycobacterium tuberculosis Controls Phagosomal Acidification by Targeting CISH-Mediated Signaling. Cell Rep. 2017, 20, 3188–3198. [Google Scholar] [CrossRef]
- Radoshevich, L.; Cossart, P. Listeria monocytogenes: Towards a complete picture of its physiology and pathogenesis. Nat. Rev. Microbiol. 2018, 16, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Vlot, A.C.; Sales, J.H.; Lenk, M.; Bauer, K.; Brambilla, A.; Sommer, A.; Chen, Y.; Wenig, M.; Nayem, S. Systemic propagation of immunity in plants. New Phytol. 2021, 229, 1234–1250. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, R.; Lim, G.-H.; de Lorenzo, L.; Yu, K.; Zhang, K.; Hunt, A.G.; Kachroo, A.; Kachroo, P. Pipecolic acid confers systemic immunity by regulating free radicals. Sci. Adv. 2018, 4, eaar4509. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Yang, J.; Li, X.; Zhang, Y. Salicylic Acid: Biosynthesis and Signaling. Annu. Rev. Plant Biol. 2021, 72, 761–791. [Google Scholar] [CrossRef] [PubMed]
- Sauer, N.; Oślizło, M.; Brzostek, M.; Wolska, J.; Lubaszka, K.; Karłowicz-Bodalska, K. The multiple uses of azelaic acid in dermatology: Mechanism of action, preparations, and potential therapeutic applications. Adv. Dermatol. Allergol. 2023, 40, 716–724. [Google Scholar] [CrossRef]
- Ding, L.-N.; Li, Y.-T.; Wu, Y.-Z.; Li, T.; Geng, R.; Cao, J.; Zhang, W.; Tan, X.-L. Plant Disease Resistance-Related Signaling Pathways: Recent Progress and Future Prospects. Int. J. Mol. Sci. 2022, 23, 16200. [Google Scholar] [CrossRef]
- Negi, N.P.; Prakash, G.; Narwal, P.; Panwar, R.; Kumar, D.; Chaudhry, B.; Rustagi, A. The calcium connection: Exploring the intricacies of calcium signaling in plant-microbe interactions. Front. Plant Sci. 2023, 14, 1248648. [Google Scholar] [CrossRef]
- Wang, Q.; Cang, X.; Yan, H.; Zhang, Z.; Li, W.; He, J.; Zhang, M.; Lou, L.; Wang, R.; Chang, M. Activating plant immunity: The hidden dance of intracellular Ca2+ stores. New Phytol. 2024, 242, 2430–2439. [Google Scholar] [CrossRef]
- Sanchez-Garrido, J.; Ruano-Gallego, D.; Choudhary, J.S.; Frankel, G. The type III secretion system effector network hypothesis. Trends Microbiol. 2022, 30, 524–533. [Google Scholar] [CrossRef]
- Atabekova, A.K.; Solovieva, A.D.; Chergintsev, D.A.; Solovyev, A.G.; Morozov, S.Y. Role of Plant Virus Movement Proteins in Suppression of Host RNAi Defense. Int. J. Mol. Sci. 2023, 24, 9049. [Google Scholar] [CrossRef]
- Adnan, M.; Khan, S.; Patel, M.; Al-Shammari, E.; Ashankyty, I.M.A. Agrobacterium. Rev. Med. Microbiol. 2013, 24, 94–97. [Google Scholar] [CrossRef]
- Qin, S.; Xiao, W.; Zhou, C.; Pu, Q.; Deng, X.; Lan, L.; Liang, H.; Song, X.; Wu, M. Pseudomonas aeruginosa: Pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduct. Target. Ther. 2022, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Gao, H.; Yang, T. Exploring the Role of the Plant Actin Cytoskeleton: From Signaling to Cellular Functions. Int. J. Mol. Sci. 2023, 24, 15480. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Kim, P.; Li, G.; Elowsky, C.G.; Alfano, J.R. A Bacterial Effector Co-opts Calmodulin to Target the Plant Microtubule Network. Cell Host Microbe 2016, 19, 67–78. [Google Scholar] [CrossRef]
- Uribe-Querol, E.; Rosales, C. Phagocytosis: Our Current Understanding of a Universal Biological Process. Front. Immunol. 2020, 11, 1066. [Google Scholar] [CrossRef]
- Dillon, M.M.; Almeida, R.N.D.; Laflamme, B.; Martel, A.; Weir, B.S.; Desveaux, D.; Guttman, D.S. Molecular Evolution of Pseudomonas syringae Type III Secreted Effector Proteins. Front. Plant Sci. 2019, 10, 418. [Google Scholar] [CrossRef]
- Rufián, J.S.; Rueda-Blanco, J.; López-Márquez, D.; Macho, A.P.; Beuzón, C.R.; Ruiz-Albert, J. The bacterial effector HopZ1a acetylates MKK7 to suppress plant immunity. New Phytol. 2021, 231, 1138–1156. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Park, Y.; Kim, J.; Lee, S.; Lee, S.; Choi, S.; Min, J.; Choi, I.; Ryu, C. Pseudomonas syringae evades phagocytosis by animal cells via type III effector-mediated regulation of actin filament plasticity. Environ. Microbiol. 2018, 20, 3980–3991. [Google Scholar] [CrossRef]
- Bamburg, J.; Minamide, L.; Wiggan, O.; Tahtamouni, L.; Kuhn, T. Cofilin and Actin Dynamics: Multiple Modes of Regulation and Their Impacts in Neuronal Development and Degeneration. Cells 2021, 10, 2726. [Google Scholar] [CrossRef]
- García-Pérez, P.; Miras-Moreno, B.; Lucini, L.; Gallego, P.P. The metabolomics reveals intraspecies variability of bioactive compounds in elicited suspension cell cultures of three Bryophyllum species. Ind. Crops Prod. 2021, 163, 113322. [Google Scholar] [CrossRef]
- Hano, C.; Abbasi, B.H. Plant-Based Green Synthesis of Nanoparticles: Production, Characterization and Applications. Biomolecules 2021, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Khandel, P.; Yadaw, R.K.; Soni, D.K.; Kanwar, L.; Shahi, S.K. Biogenesis of metal nanoparticles and their pharmacological applications: Present status and application prospects. J. Nanostructure Chem. 2018, 8, 217–254. [Google Scholar] [CrossRef]
- Kulak, M.; Gulmez Samsa, C. Flavonoids Mediated Nanomaterials Synthesis, Characterization, and Their Applications. In Secondary Metabolites Based Green Synthesis of Nanomaterials and Their Applications; Springer Nature: Singapore, 2023; pp. 49–65. [Google Scholar]
- Ahmad, H.; Venugopal, K.; Rajagopal, K.; De Britto, S.; Nandini, B.; Pushpalatha, H.G.; Konappa, N.; Udayashankar, A.C.; Geetha, N.; Jogaiah, S. Green Synthesis and Characterization of Zinc Oxide Nanoparticles Using Eucalyptus globules and Their Fungicidal Ability Against Pathogenic Fungi of Apple Orchards. Biomolecules 2020, 10, 425. [Google Scholar] [CrossRef] [PubMed]
- Letchumanan, D.; Sok, S.P.M.; Ibrahim, S.; Nagoor, N.H.; Arshad, N.M. Plant-Based Biosynthesis of Copper/Copper Oxide Nanoparticles: An Update on Their Applications in Biomedicine, Mechanisms, and Toxicity. Biomolecules 2021, 11, 564. [Google Scholar] [CrossRef]
- Drummer, S.; Madzimbamuto, T.; Chowdhury, M. Green Synthesis of Transition-Metal Nanoparticles and Their Oxides: A Review. Materials 2021, 14, 2700. [Google Scholar] [CrossRef]
- Tymoszuk, A. Silver Nanoparticles Effects on In Vitro Germination, Growth, and Biochemical Activity of Tomato, Radish, and Kale Seedlings. Materials 2021, 14, 5340. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Shariq, M.; Asif, M.; Siddiqui, M.A.; Malan, P.; Ahmad, F. Green Nanotechnology: Plant-Mediated Nanoparticle Synthesis and Application. Nanomaterials 2022, 12, 673. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Soto-Quintero, A.; Guarrotxena, N.; García, O.; Quijada-Garrido, I. Curcumin to Promote the Synthesis of Silver NPs and their Self-Assembly with a Thermoresponsive Polymer in Core-Shell Nanohybrids. Sci. Rep. 2019, 9, 18187. [Google Scholar] [CrossRef]
- Ghosh, S.; Patil, S.; Ahire, M.; Kitture, R.; Kale, S.; Pardesi, K.; Cameotra, S.S.; Bellare, J.; Dhavale, D.D.; Jabgunde, A.; et al. Synthesis of silver nanoparticles using Dioscorea bulbifera tuber extract and evaluation of its synergistic potential in combination with antimicrobial agents. Int. J. Nanomed. 2012, 2012, 483–496. [Google Scholar] [CrossRef]
- Gong, X.; Jadhav, N.D.; Lonikar, V.V.; Kulkarni, A.N.; Zhang, H.; Sankapal, B.R.; Ren, J.; Xu, B.B.; Pathan, H.M.; Ma, Y.; et al. An overview of green synthesized silver nanoparticles towards bioactive antibacterial, antimicrobial and antifungal applications. Adv. Colloid Interface Sci. 2024, 323, 103053. [Google Scholar] [CrossRef]
- Alves, T.F.; Chaud, M.V.; Grotto, D.; Jozala, A.F.; Pandit, R.; Rai, M.; dos Santos, C.A. Association of Silver Nanoparticles and Curcumin Solid Dispersion: Antimicrobial and Antioxidant Properties. AAPS PharmSciTech 2018, 19, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Malabadi, R.; Chalannavar, R.; Meti, N.; Mulgund, G.; Nataraja, K.; Vijaya Kumar, S. Synthesis of antimicrobial silver nanoparticles by callus cultures and In Vitro derived plants of Catharanthus roseus. Res. Pharm. 2012, 2, 18–31. [Google Scholar]
- Solís-Sandí, I.; Cordero-Fuentes, S.; Pereira-Reyes, R.; Vega-Baudrit, J.R.; Batista-Menezes, D.; Montes de Oca-Vásquez, G. Optimization of the biosynthesis of silver nanoparticles using bacterial extracts and their antimicrobial potential. Biotechnol. Reports 2023, 40, e00816. [Google Scholar] [CrossRef] [PubMed]
- Kambale, E.K.; Nkanga, C.I.; Mutonkole, B.-P.I.; Bapolisi, A.M.; Tassa, D.O.; Liesse, J.-M.I.; Krause, R.W.M.; Memvanga, P.B. Green synthesis of antimicrobial silver nanoparticles using aqueous leaf extracts from three Congolese plant species (Brillantaisia patula, Crossopteryx febrifuga and Senna siamea). Heliyon 2020, 6, e04493. [Google Scholar] [CrossRef] [PubMed]
- Koilparambil, D.; Kurian, L.; Vijayan, S.; Shanavas, J. Green Synthesis of Silver Nanoparticles by Escherichia coli: Analysis of Antibacterial Activity. J. Water Environ. Technol. 2016, 1, 2016. [Google Scholar] [CrossRef]
- Vanlalveni, C.; Lallianrawna, S.; Biswas, A.; Selvaraj, M.; Changmai, B.; Rokhum, S.L. Green synthesis of silver nanoparticles using plant extracts and their antimicrobial activities: A review of recent literature. RSC Adv. 2021, 11, 2804–2837. [Google Scholar] [CrossRef]
- Adil, M.; Khan, T.; Aasim, M.; Khan, A.A.; Ashraf, M. Evaluation of the antibacterial potential of silver nanoparticles synthesized through the interaction of antibiotic and aqueous callus extract of Fagonia indica. AMB Express 2019, 9, 75. [Google Scholar] [CrossRef]
- Pandian, S.R.K.; Deepak, V.; Kalishwaralal, K.; Gurunathan, S. Biologically synthesized fluorescent CdS NPs encapsulated by PHB. Enzym. Microb. Technol. 2011, 48, 319–325. [Google Scholar] [CrossRef]
- Shittu, K.O.; Bankole, M.T.; Abdulkareem, A.S.; Abubakre, O.K.; Ubaka, A.U. Application of gold nanoparticles for improved drug efficiency. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 035014. [Google Scholar] [CrossRef]
- Patra, J.K.; Baek, K.-H. Antibacterial Activity and Synergistic Antibacterial Potential of Biosynthesized Silver Nanoparticles against Foodborne Pathogenic Bacteria along with its Anticandidal and Antioxidant Effects. Front. Microbiol. 2017, 8, 167. [Google Scholar] [CrossRef]
- Gurunathan, S. Biologically synthesized silver nanoparticles enhances antibiotic activity against Gram-negative bacteria. J. Ind. Eng. Chem. 2015, 29, 217–226. [Google Scholar] [CrossRef]
- Potbhare, A.K.; Chaudhary, R.G.; Chouke, P.B.; Yerpude, S.; Mondal, A.; Sonkusare, V.N.; Rai, A.R.; Juneja, H.D. Phytosynthesis of nearly monodisperse CuO nanospheres using Phyllanthus reticulatus/Conyza bonariensis and its antioxidant/antibacterial assays. Mater. Sci. Eng. C 2019, 99, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Chimal, R.; Ángel Arenas-Alatorre, J. Green synthesis of nanoparticles. A biological approach. In Advances in Green Chemistry [Working Title]; IntechOpen: London, UK, 2023. [Google Scholar]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green synthesis of nanoparticles: Current developments and limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Burlec, A.F.; Corciova, A.; Boev, M.; Batir-Marin, D.; Mircea, C.; Cioanca, O.; Danila, G.; Danila, M.; Bucur, A.F.; Hancianu, M. Current Overview of Metal Nanoparticles’ Synthesis, Characterization, and Biomedical Applications, with a Focus on Silver and Gold Nanoparticles. Pharmaceuticals 2023, 16, 1410. [Google Scholar] [CrossRef]
- Osman, A.I.; Zhang, Y.; Farghali, M.; Rashwan, A.K.; Eltaweil, A.S.; Abd El-Monaem, E.M.; Mohamed, I.M.A.; Badr, M.M.; Ihara, I.; Rooney, D.W.; et al. Synthesis of green nanoparticles for energy, biomedical, environmental, agricultural, and food applications: A review. Environ. Chem. Lett. 2024, 22, 841–887. [Google Scholar] [CrossRef]
- Hamida, R.S.; Ali, M.A.; Goda, D.A.; Khalil, M.I.; Al-Zaban, M.I. Novel Biogenic Silver Nanoparticle-Induced Reactive Oxygen Species Inhibit the Biofilm Formation and Virulence Activities of Methicillin-Resistant Staphylococcus aureus (MRSA) Strain. Front. Bioeng. Biotechnol. 2020, 8, 433. [Google Scholar] [CrossRef]
- Kumar, M.; Giri, V.P.; Pandey, S.; Gupta, A.; Patel, M.K.; Bajpai, A.B.; Jenkins, S.; Siddique, K.H.M. Plant-Growth-Promoting Rhizobacteria Emerging as an Effective Bioinoculant to Improve the Growth, Production, and Stress Tolerance of Vegetable Crops. Int. J. Mol. Sci. 2021, 22, 12245. [Google Scholar] [CrossRef] [PubMed]
- Pacyga, K.; Pacyga, P.; Topola, E.; Viscardi, S.; Duda-Madej, A. Bioactive Compounds from Plant Origin as Natural Antimicrobial Agents for the Treatment of Wound Infections. Int. J. Mol. Sci. 2024, 25, 2100. [Google Scholar] [CrossRef]
- Asimuddin, M.; Shaik, M.R.; Adil, S.F.; Siddiqui, M.R.H.; Alwarthan, A.; Jamil, K.; Khan, M. Azadirachta indica based biosynthesis of silver nanoparticles and evaluation of their antibacterial and cytotoxic effects. J. King Saud Univ.-Sci. 2020, 32, 648–656. [Google Scholar] [CrossRef]
- Chand, K.; Abro, M.I.; Aftab, U.; Shah, A.H.; Lakhan, M.N.; Cao, D.; Mehdi, G.; Ali Mohamed, A.M. Green synthesis characterization and antimicrobial activity against Staphylococcus aureus of silver nanoparticles using extracts of neem, onion and tomato. RSC Adv. 2019, 9, 17002–17015. [Google Scholar] [CrossRef]
- Burlacu, E.; Tanase, C.; Coman, N.-A.; Berta, L. A Review of Bark-Extract-Mediated Green Synthesis of Metallic Nanoparticles and Their Applications. Molecules 2019, 24, 4354. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.V.; Tran, T.; Thi, V.D. Biosynthesis of Silver Nanoparticles from Bacillus licheniformis TT01 Isolated from Quail Manure Collected in Vietnam. Processes 2021, 9, 584. [Google Scholar] [CrossRef]
- Singh, J.; Perumal, V.; Singh, U.; Tripathi, D.K.; Sharma, S. Green Synthesis of Silver Nanoparticles from Bark Extract of Terminalia arjuna and their Application as Next Generation Antibacterial Agents. Curr. Nanosci. 2022, 18, 743–757. [Google Scholar] [CrossRef]
- Arief, S.; Nasution, F.W.; Zulhadjri; Labanni, A. High antibacterial properties of green synthesized gold nanoparticles using Uncaria gambir Roxb. leaf extract and triethanolamine. J. Appl. Pharm. Sci. 2020, 10, 124–130. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, T.; Ji, B.; Chou, Y.; Du, X. Green Synthesis of Zinc Oxide Nanoparticles Using Aloe vera Leaf Extract and Evaluation of the Antimicrobial and Antioxidant Properties of the ZnO/Regenerated Cellulose Film. Cellulose 2024, 31, 4849–4864. [Google Scholar] [CrossRef]
- Jayappa, M.D.; Ramaiah, C.K.; Kumar, M.A.P.; Suresh, D.; Prabhu, A.; Devasya, R.P.; Sheikh, S. Green synthesis of zinc oxide nanoparticles from the leaf, stem and In Vitro grown callus of Mussaenda frondosa L.: Characterization and their applications. Appl. Nanosci. 2020, 10, 3057–3074. [Google Scholar] [CrossRef]
- Siripireddy, B.; Mandal, B.K. Facile green synthesis of zinc oxide nanoparticles by Eucalyptus globulus and their photocatalytic and antioxidant activity. Adv. Powder Technol. 2017, 28, 785–797. [Google Scholar] [CrossRef]
- Abdelmigid, H.M.; Hussien, N.A.; Alyamani, A.A.; Morsi, M.M.; AlSufyani, N.M.; Kadi, H.A. Green Synthesis of Zinc Oxide Nanoparticles Using Pomegranate Fruit Peel and Solid Coffee Grounds vs. Chemical Method of Synthesis, with Their Biocompatibility and Antibacterial Properties Investigation. Molecules 2022, 27, 1236. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, D.; Ren, D.; Zeng, K.; Wu, X. Green synthesis of zinc oxide nanoparticles using Citrus sinensis peel extract and application to strawberry preservation: A comparison study. LWT 2020, 126, 109297. [Google Scholar] [CrossRef]
- Ali, H.M.; Karam, K.; Khan, T.; Wahab, S.; Ullah, S.; Sadiq, M. Reactive oxygen species induced oxidative damage to DNA, lipids, and proteins of antibiotic-resistant bacteria by plant-based silver nanoparticles. 3 Biotech 2023, 13, 414. [Google Scholar] [CrossRef]
- More, P.R.; Pandit, S.; Filippis, A.D.; Franci, G.; Mijakovic, I.; Galdiero, M. Silver Nanoparticles: Bactericidal and Mechanistic Approach against Drug Resistant Pathogens. Microorganisms 2023, 11, 369. [Google Scholar] [CrossRef] [PubMed]
- Ozdal, M.; Gurkok, S. A Recent advances in nanoparticles as antibacterial agent. ADMET DMPK 2022, 10, 115–129. [Google Scholar] [CrossRef]
- Arciniegas-Grijalba, P.A.; Patiño-Portela, M.C.; Mosquera-Sánchez, L.P.; Guerrero-Vargas, J.A.; Rodríguez-Páez, J.E. ZnO nanoparticles (ZnO-NPs) and their antifungal activity against coffee fungus Erythricium salmonicolor. Appl. Nanosci. 2017, 7, 225–241. [Google Scholar] [CrossRef]
- Hashem, A.H.; Saied, E.; Amin, B.H.; Alotibi, F.O.; Al-Askar, A.A.; Arishi, A.A.; Elkady, F.M.; Elbahnasawy, M.A. Antifungal Activity of Biosynthesized Silver Nanoparticles (AgNPs) against Aspergilli Causing Aspergillosis: Ultrastructure Study. J. Funct. Biomater. 2022, 13, 242. [Google Scholar] [CrossRef]
- Ratan, Z.A.; Mashrur, F.R.; Chhoan, A.P.; Shahriar, S.M.; Haidere, M.F.; Runa, N.J.; Kim, S.; Kweon, D.-H.; Hosseinzadeh, H.; Cho, J.Y. Silver Nanoparticles as Potential Antiviral Agents. Pharmaceutics 2021, 13, 2034. [Google Scholar] [CrossRef]
- Fulaz, S.; Vitale, S.; Quinn, L.; Casey, E. Nanoparticle–Biofilm Interactions: The Role of the EPS Matrix. Trends Microbiol. 2019, 27, 915–926. [Google Scholar] [CrossRef]
- Siddique, M.H.; Aslam, B.; Imran, M.; Ashraf, A.; Nadeem, H.; Hayat, S.; Khurshid, M.; Afzal, M.; Malik, I.R.; Shahzad, M.; et al. Effect of Silver Nanoparticles on Biofilm Formation and EPS Production of Multidrug-Resistant Klebsiella pneumoniae. Biomed Res. Int. 2020, 2020, 6398165. [Google Scholar] [CrossRef]
- Ramkumar, V.S.; Pugazhendhi, A.; Prakash, S.; Ahila, N.K.; Vinoj, G.; Selvam, S.; Kumar, G.; Kannapiran, E.; Rajendran, R.B. Synthesis of platinum nanoparticles using seaweed Padina gymnospora and their catalytic activity as PVP/PtNPs nanocomposite towards biological applications. Biomed. Pharmacother. 2017, 92, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Kalishwaralal, K.; Deepak, V.; Ramkumarpandian, S.; Nellaiah, H.; Sangiliyandi, G. Extracellular biosynthesis of silver nanoparticles by the culture supernatant of Bacillus licheniformis. Mater. Lett. 2008, 62, 4411–4413. [Google Scholar] [CrossRef]
- Wong, C.D.S.; Yeoh, J.X.; Wu, T.; Manickam, S.; Pang, C.H. Biomass to nanoparticles: Recent advances in the process and processing towards sustainability. Chem. Eng. Process.-Process Intensif. 2022, 175, 108908. [Google Scholar] [CrossRef]
- Sweeney, R.Y.; Mao, C.; Gao, X.; Burt, J.L.; Belcher, A.M.; Georgiou, G.; Iverson, B.L. Bacterial Biosynthesis of Cadmium Sulfide Nanocrystals. Chem. Biol. 2004, 11, 1553–1559. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Mustafi, M.; Weisshaar, J.C. Biophysical Properties of Escherichia coli Cytoplasm in Stationary Phase by Superresolution Fluorescence Microscopy. mBio 2020, 11, e00143-20. [Google Scholar] [CrossRef] [PubMed]
- Abu Bakar, N.; Karsani, S.A.; Alias, S.A. Fungal survival under temperature stress: A proteomic perspective. PeerJ 2020, 8, e10423. [Google Scholar] [CrossRef]
- Congeevaram, S.; Dhanarani, S.; Park, J.; Dexilin, M.; Thamaraiselvi, K. Biosorption of chromium and nickel by heavy metal resistant fungal and bacterial isolates. J. Hazard. Mater. 2007, 146, 270–277. [Google Scholar] [CrossRef]
- He, B.; Hu, Z.; Ma, L.; Li, H.; Ai, M.; Han, J.; Zeng, B. Transcriptome analysis of different growth stages of Aspergillus oryzae reveals dynamic changes of distinct classes of genes during growth. BMC Microbiol. 2018, 18, 12. [Google Scholar] [CrossRef]
- Perumal, K.; Ahmad, S.; Mohd-Zahid, M.H.; Wan Hanaffi, W.N.; ZA, I.; Six, J.-L.; Ferji, K.; Jaafar, J.; Boer, J.C.; Plebanski, M.; et al. Nanoparticles and Gut Microbiota in Colorectal Cancer. Front. Nanotechnol. 2021, 3, 681760. [Google Scholar] [CrossRef]
- Ghasempour, A.; Dehghan, H.; Ataee, M.; Chen, B.; Zhao, Z.; Sedighi, M.; Guo, X.; Shahbazi, M.-A. Cadmium Sulfide Nanoparticles: Preparation, Characterization, and Biomedical Applications. Molecules 2023, 28, 3857. [Google Scholar] [CrossRef]
- Han, M.; Liu, K.; Liu, X.; Rashid, M.T.; Zhang, H.; Wang, M. Research Progress of Protein-Based Bioactive Substance Nanoparticles. Foods 2023, 12, 2999. [Google Scholar] [CrossRef]
- Alwan, S.H.; Al-Saeed, M.H. Biosynthesized silver nanoparticles (using Cinnamomum zeylanicum bark extract) improve the fertility status of rats with polycystic ovarian syndrome. Biocatal. Agric. Biotechnol. 2021, 38, 102217. [Google Scholar] [CrossRef]
- Pordanjani, A.H.; Aghakhani, S.; Afrand, M.; Sharifpur, M.; Meyer, J.P.; Xu, H.; Ali, H.M.; Karimi, N.; Cheraghian, G. Nanofluids: Physical phenomena, applications in thermal systems and the environment effects- a critical review. J. Clean. Prod. 2021, 320, 128573. [Google Scholar] [CrossRef]
- Sionov, R.V.; Steinberg, D. Targeting the Holy Triangle of Quorum Sensing, Biofilm Formation, and Antibiotic Resistance in Pathogenic Bacteria. Microorganisms 2022, 10, 1239. [Google Scholar] [CrossRef]
- Lowy, F.D. Antimicrobial resistance: The example of Staphylococcus aureus. J. Clin. Investig. 2003, 111, 1265–1273. [Google Scholar] [CrossRef]
- Li, X.-Z.; Plésiat, P.; Nikaido, H. The Challenge of Efflux-Mediated Antibiotic Resistance in Gram-Negative Bacteria. Clin. Microbiol. Rev. 2015, 28, 337–418. [Google Scholar] [CrossRef]
- Mahenthiralingam, E.; Baldwin, A.; Dowson, C.G. Burkholderia cepacia complex bacteria: Opportunistic pathogens with important natural biology. J. Appl. Microbiol. 2008, 104, 1539–1551. [Google Scholar] [CrossRef]
- Karimi, K.; Zarei, O.; Sedighi, P.; Taheri, M.; Doosti-Irani, A.; Shokoohizadeh, L. Investigation of Antibiotic Resistance and Biofilm Formation in Clinical Isolates of Klebsiella pneumoniae. Int. J. Microbiol. 2021, 2021, 5573388. [Google Scholar] [CrossRef]
- Al-Hatmi, A.; Curfs-Breuker, I.; De Hoog, G.; Meis, J.; Verweij, P. Antifungal Susceptibility Testing of Fusarium: A Practical Approach. J. Fungi 2017, 3, 19. [Google Scholar] [CrossRef]
- Thipe, V.C.; Karikachery, A.R.; Çakılkaya, P.; Farooq, U.; Genedy, H.H.; Kaeokhamloed, N.; Phan, D.-H.; Rezwan, R.; Tezcan, G.; Roger, E.; et al. Green nanotechnology—An innovative pathway towards biocompatible and medically relevant gold nanoparticles. J. Drug Deliv. Sci. Technol. 2022, 70, 103256. [Google Scholar] [CrossRef]
- Ferdous, Z.; Nemmar, A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef]
- Wen, H.; Dan, M.; Yang, Y.; Lyu, J.; Shao, A.; Cheng, X.; Chen, L.; Xu, L. Acute toxicity and genotoxicity of silver nanoparticle in rats. PLoS ONE 2017, 12, e0185554. [Google Scholar] [CrossRef]
- Li, J.J.; Lo, S.-L.; Ng, C.-T.; Gurung, R.L.; Hartono, D.; Hande, M.P.; Ong, C.-N.; Bay, B.-H.; Yung, L.-Y.L. Genomic instability of gold nanoparticle treated human lung fibroblast cells. Biomaterials 2011, 32, 5515–5523. [Google Scholar] [CrossRef]
- Bhardwaj, B.; Singh, P.; Kumar, A.; Kumar, S.; Budhwar, V. Eco-Friendly Greener Synthesis of Nanoparticles. Adv. Pharm. Bull. 2020, 10, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Saifullah; Ahmad, M.; Swami, B.L.; Ikram, S. Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J. Radiat. Res. Appl. Sci. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Asker, A.Y.M.; Al Haidar, A.H.M.J. Green synthesis of gold nanoparticles using Pelargonium Graveolens leaf extract: Characterization and anti-microbial properties (An In-Vitro study). F1000Research 2024, 13, 572. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, F.; Wang, J.; Zhong, N. Biofilm formation and control strategies of foodborne pathogens: Food safety perspectives. RSC Adv. 2017, 7, 36670–36683. [Google Scholar] [CrossRef]
- Riga, E.K.; Vöhringer, M.; Widyaya, V.T.; Lienkamp, K. Polymer-Based Surfaces Designed to Reduce Biofilm Formation: From Antimicrobial Polymers to Strategies for Long-Term Applications. Macromol. Rapid Commun. 2017, 38, 1700216. [Google Scholar] [CrossRef]
- Rex, J.H.; Fernandez Lynch, H.; Cohen, I.G.; Darrow, J.J.; Outterson, K. Designing development programs for non-traditional antibacterial agents. Nat. Commun. 2019, 10, 3416. [Google Scholar] [CrossRef]
- Uruén, C.; Chopo-Escuin, G.; Tommassen, J.; Mainar-Jaime, R.C.; Arenas, J. Biofilms as Promoters of Bacterial Antibiotic Resistance and Tolerance. Antibiotics 2020, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Wypij, M.; Jędrzejewski, T.; Trzcińska-Wencel, J.; Ostrowski, M.; Rai, M.; Golińska, P. Green Synthesized Silver Nanoparticles: Antibacterial and Anticancer Activities, Biocompatibility, and Analyses of Surface-Attached Proteins. Front. Microbiol. 2021, 12, 632505. [Google Scholar] [CrossRef]
- Bhat, R.S.; Almusallam, J.; Al Daihan, S.; Al-Dbass, A. Biosynthesis of silver nanoparticles using Azadirachta indica leaves: Characterisation and impact on Staphylococcus aureus growth and glutathione-S-transferase activity. IET Nanobiotechnol. 2019, 13, 498–502. [Google Scholar] [CrossRef]
- Tippayawat, P.; Phromviyo, N.; Boueroy, P.; Chompoosor, A. Green synthesis of silver nanoparticles in aloe vera plant extract prepared by a hydrothermal method and their synergistic antibacterial activity. PeerJ 2016, 4, e2589. [Google Scholar] [CrossRef] [PubMed]
- Ni, Q.; Zhu, T.; Wang, W.; Guo, D.; Li, Y.; Chen, T.; Zhang, X. Green Synthesis of Narrow-Size Silver Nanoparticles Using Ginkgo biloba Leaves: Condition Optimization, Characterization, and Antibacterial and Cytotoxic Activities. Int. J. Mol. Sci. 2024, 25, 1913. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Mamatha, M.G.; Ansari, M.A.; Begum, M.Y.; Prasad, B.D.; Al Fatease, A.; Hani, U.; Alomary, M.N.; Sultana, S.; Punekar, S.M.; MB, N.; et al. Green Synthesis of Cerium Oxide Nanoparticles, Characterization, and Their Neuroprotective Effect on Hydrogen Peroxide-Induced Oxidative Injury in Human Neuroblastoma (SH-SY5Y) Cell Line. ACS Omega 2024, 9, 2639–2649. [Google Scholar] [CrossRef]
- Singhal, G.; Bhavesh, R.; Kasariya, K.; Sharma, A.R.; Singh, R.P. Biosynthesis of silver nanoparticles using Ocimum sanctum (Tulsi) leaf extract and screening its antimicrobial activity. J. Nanopart. Res. 2011, 13, 2981–2988. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef] [PubMed]
- Bawazeer, S.; Khan, I.; Rauf, A.; Aljohani, A.S.M.; Alhumaydhi, F.A.; Khalil, A.A.; Qureshi, M.N.; Ahmad, L.; Khan, S.A. Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications—A green approach. Green Process. Synth. 2022, 11, 11–28. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Silver Nanoparticles: Mechanism of Action and Probable Bio-Application. J. Funct. Biomater. 2020, 11, 84. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- Das, B.; Dash, S.K.; Mandal, D.; Ghosh, T.; Chattopadhyay, S.; Tripathy, S.; Das, S.; Dey, S.K.; Das, D.; Roy, S. Green synthesized silver nanoparticles destroy multidrug resistant bacteria via reactive oxygen species mediated membrane damage. Arab. J. Chem. 2017, 10, 862–876. [Google Scholar] [CrossRef]
- Varghese, R.; Almalki, M.A.; Ilavenil, S.; Rebecca, J.; Choi, K.C. Silver nanopaticles synthesized using the seed extract of Trigonella foenum-graecum L. and their antimicrobial mechanism and anticancer properties. Saudi J. Biol. Sci. 2019, 26, 148–154. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef] [PubMed]
- Otari, S.V.; Patil, R.M.; Ghosh, S.J.; Thorat, N.D.; Pawar, S.H. Intracellular synthesis of silver nanoparticle by actinobacteria and its antimicrobial activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Swidan, N.S.; Hashem, Y.A.; Elkhatib, W.F.; Yassien, M.A. Antibiofilm activity of green synthesized silver nanoparticles against biofilm associated enterococcal urinary pathogens. Sci. Rep. 2022, 12, 3869. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Sulejczak, D.; Kleczkowska, P.; Bukowska-Ośko, I.; Kucia, M.; Popiel, M.; Wietrak, E.; Kramkowski, K.; Wrzosek, K.; Kaczyńska, K. Mitochondrial Oxidative Stress—A Causative Factor and Therapeutic Target in Many Diseases. Int. J. Mol. Sci. 2021, 22, 13384. [Google Scholar] [CrossRef]
- Kaur, K.; Reddy, S.; Barathe, P.; Shriram, V.; Anand, U.; Proćków, J.; Kumar, V. Combating Drug-Resistant Bacteria Using Photothermally Active Nanomaterials: A Perspective Review. Front. Microbiol. 2021, 12, 747019. [Google Scholar] [CrossRef] [PubMed]
- Westmeier, D.; Hahlbrock, A.; Reinhardt, C.; Fröhlich-Nowoisky, J.; Wessler, S.; Vallet, C.; Pöschl, U.; Knauer, S.K.; Stauber, R.H. Nanomaterial–microbe cross-talk: Physicochemical principles and (patho)biological consequences. Chem. Soc. Rev. 2018, 47, 5312–5337. [Google Scholar] [CrossRef]
- Lee, N.-Y.; Ko, W.-C.; Hsueh, P.-R. Nanoparticles in the Treatment of Infections Caused by Multidrug-Resistant Organisms. Front. Pharmacol. 2019, 10, 1153. [Google Scholar] [CrossRef]
- Smerkova, K.; Dolezelikova, K.; Bozdechova, L.; Heger, Z.; Zurek, L.; Adam, V. Nanomaterials with active targeting as advanced antimicrobials. WIREs Nanomed. Nanobiotechnol. 2020, 12, e1636. [Google Scholar] [CrossRef]
- Zhu, K.; Qian, S.; Guo, H.; Wang, Q.; Chu, X.; Wang, X.; Lu, S.; Peng, Y.; Guo, Y.; Zhu, Z.; et al. pH-Activatable Organic Nanoparticles for Efficient Low-Temperature Photothermal Therapy of Ocular Bacterial Infection. ACS Nano 2022, 16, 11136–11151. [Google Scholar] [CrossRef]
- Marciniak, L.; Nowak, M.; Trojanowska, A.; Tylkowski, B.; Jastrzab, R. The Effect of pH on the Size of Silver Nanoparticles Obtained in the Reduction Reaction with Citric and Malic Acids. Materials 2020, 13, 5444. [Google Scholar] [CrossRef]
- Kourmouli, A.; Valenti, M.; van Rijn, E.; Beaumont, H.J.E.; Kalantzi, O.-I.; Schmidt-Ott, A.; Biskos, G. Can disc diffusion susceptibility tests assess the antimicrobial activity of engineered nanoparticles? J. Nanoparticle Res. 2018, 20, 62. [Google Scholar] [CrossRef] [PubMed]
- Alavi, M.; Rai, M. Recent progress in nanoformulations of silver nanoparticles with cellulose, chitosan, and alginic acid biopolymers for antibacterial applications. Appl. Microbiol. Biotechnol. 2019, 103, 8669–8676. [Google Scholar] [CrossRef] [PubMed]
- Hemeg, H. Nanomaterials for alternative antibacterial therapy. Int. J. Nanomed. 2017, 12, 8211–8225. [Google Scholar] [CrossRef] [PubMed]
- Sharmin, S.; Rahaman, M.M.; Sarkar, C.; Atolani, O.; Islam, M.T.; Adeyemi, O.S. Nanoparticles as antimicrobial and antiviral agents: A literature-based perspective study. Heliyon 2021, 7, e06456. [Google Scholar] [CrossRef] [PubMed]
- Janagam, D.R.; Wu, L.; Lowe, T.L. Nanoparticles for drug delivery to the anterior segment of the eye. Adv. Drug Deliv. Rev. 2017, 122, 31–64. [Google Scholar] [CrossRef]
- Sripriya, J.; Anandhakumar, S.; Achiraman, S.; Antony, J.J.; Siva, D.; Raichur, A.M. Laser receptive polyelectrolyte thin films doped with biosynthesized silver nanoparticles for antibacterial coatings and drug delivery applications. Int. J. Pharm. 2013, 457, 206–213. [Google Scholar] [CrossRef]
- Ramzan, M.; Imran, M.; Ullah, S.; Khan, M.A.; Naz, G.; Ghouri, M.I.; Iqbal, H.M.N. Fabrication and characterization of multifunctional thin multi-layer films for transparent conducting oxides. Prog. Org. Coatings 2020, 149, 105976. [Google Scholar] [CrossRef]
- Mani, S.T.; Jayakumar, P.; Pavithra, M.E.; Saranya, K.; Rathinavel, T.; Ammashi, S. Green Synthesis and Characterization of Silver Nanoparticles from Eclipta alba and Its Activity Against Triple-Negative Breast Cancer Cell Line (MDA-MB-231). Mol. Biotechnol. 2023, 1–11. [Google Scholar] [CrossRef]
- Khan, K.; Javed, S. Silver nanoparticles synthesized using leaf extract of Azadirachta indica exhibit enhanced antimicrobial efficacy than the chemically synthesized nanoparticles: A comparative study. Sci. Prog. 2021, 104, 00368504211012159. [Google Scholar] [CrossRef]
- Bidian, C.; Filip, G.A.; David, L.; Moldovan, B.; Olteanu, D.; Clichici, S.; Olănescu-Vaida-Voevod, M.-C.; Leostean, C.; Macavei, S.; Muntean, D.M.; et al. Green Synthesized Gold and Silver Nanoparticles Increased Oxidative Stress and Induced Cell Death in Colorectal Adenocarcinoma Cells. Nanomaterials 2023, 13, 1251. [Google Scholar] [CrossRef] [PubMed]
- Alsaiari, N.S.; Alzahrani, F.M.; Amari, A.; Osman, H.; Harharah, H.N.; Elboughdiri, N.; Tahoon, M.A. Plant and Microbial Approaches as Green Methods for the Synthesis of Nanomaterials: Synthesis, Applications, and Future Perspectives. Molecules 2023, 28, 463. [Google Scholar] [CrossRef]
- Ishwarya, R.; Vaseeharan, B.; Kalyani, S.; Banumathi, B.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Al-anbr, M.N.; Khaled, J.M.; Benelli, G. Facile green synthesis of zinc oxide nanoparticles using Ulva lactuca seaweed extract and evaluation of their photocatalytic, antibiofilm and insecticidal activity. J. Photochem. Photobiol. B Biol. 2018, 178, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Khor, K.Z.; Joseph, J.; Shamsuddin, F.; Lim, V.; Moses, E.J.; Abdul Samad, N. The Cytotoxic Effects of Moringa oleifera Leaf Extract and Silver Nanoparticles on Human Kasumi-1 Cells. Int. J. Nanomed. 2020, 15, 5661–5670. [Google Scholar] [CrossRef]
- Dowlath, M.J.H.; Musthafa, S.A.; Mohamed Khalith, S.B.; Varjani, S.; Karuppannan, S.K.; Ramanujam, G.M.; Arunachalam, A.M.; Arunachalam, K.D.; Chandrasekaran, M.; Chang, S.W.; et al. Comparison of characteristics and biocompatibility of green synthesized iron oxide nanoparticles with chemical synthesized nanoparticles. Environ. Res. 2021, 201, 111585. [Google Scholar] [CrossRef]
- Sabeena, G.; Pushpalakshmi, E.; Annadurai, G. Comparative synthesis and characterization of nanocomposites using chemical and green approaches including a comparison study on in vivo and In Vitro biological properties. Nanoscale Adv. 2023, 5, 767–785. [Google Scholar] [CrossRef]
- Nagajyothi, P.C.; Cha, S.J.; Yang, I.J.; Sreekanth, T.V.M.; Kim, K.J.; Shin, H.M. Antioxidant and anti-inflammatory activities of zinc oxide nanoparticles synthesized using Polygala tenuifolia root extract. J. Photochem. Photobiol. B Biol. 2015, 146, 10–17. [Google Scholar] [CrossRef]
- Ullah, I.; Khalil, A.T.; Ali, M.; Iqbal, J.; Ali, W.; Alarifi, S.; Shinwari, Z.K. Green-synthesized silver nanoparticles induced apoptotic cell death in MCF-7 breast cancer cells by generating reactive oxygen species and activating caspase 3 and 9 enzyme activities. Oxid. Med. Cell. Longev. 2020, 2020, 1215395. [Google Scholar] [CrossRef]
- Dudefoi, W.; Moniz, K.; Allen-Vercoe, E.; Ropers, M.-H.; Walker, V.K. Impact of food grade and nano-TiO2 particles on a human intestinal community. Food Chem. Toxicol. 2017, 106, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Agans, R.T.; Gordon, A.; Hussain, S.; Paliy, O. Titanium Dioxide Nanoparticles Elicit Lower Direct Inhibitory Effect on Human Gut Microbiota Than Silver Nanoparticles. Toxicol. Sci. 2019, 172, 411–416. [Google Scholar] [CrossRef]
- Pereira, D.I.A.; Bruggraber, S.F.A.; Faria, N.; Poots, L.K.; Tagmount, M.A.; Aslam, M.F.; Frazer, D.M.; Vulpe, C.D.; Anderson, G.J.; Powell, J.J. Nanoparticulate iron (III) oxo-hydroxide delivers safe iron that is well absorbed and utilised in humans. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1877–1886. [Google Scholar] [CrossRef]
- Wilding, L.A.; Bassis, C.M.; Walacavage, K.; Hashway, S.; Leroueil, P.R.; Morishita, M.; Maynard, A.D.; Philbert, M.A.; Bergin, I.L. Repeated dose (28-day) administration of silver nanoparticles of varied size and coating does not significantly alter the indigenous murine gut microbiome. Nanotoxicology 2016, 10, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Vidyasagar; Patel, R.R.; Singh, S.K.; Singh, M. Green synthesis of silver nanoparticles: Methods, biological applications, delivery and toxicity. Mater. Adv. 2023, 4, 1831–1849. [Google Scholar] [CrossRef]
- Morais, M.; Teixeira, A.L.; Dias, F.; Machado, V.; Medeiros, R.; Prior, J.A.V. Cytotoxic Effect of Silver Nanoparticles Synthesized by Green Methods in Cancer. J. Med. Chem. 2020, 63, 14308–14335. [Google Scholar] [CrossRef]
- Rani, S.; Kumar, P.; Dahiya, P.; Dang, A.S.; Suneja, P. Biogenic Synthesis of Zinc Nanoparticles, Their Applications, and Toxicity Prospects. Front. Microbiol. 2022, 13, 824427. [Google Scholar] [CrossRef]
- Faisal, S.; Jan, H.; Shah, S.A.; Shah, S.; Khan, A.; Akbar, M.T.; Rizwan, M.; Jan, F.; Wajidullah; Akhtar, N.; et al. Green Synthesis of Zinc Oxide (ZnO) Nanoparticles Using Aqueous Fruit Extracts of Myristica fragrans: Their Characterizations and Biological and Environmental Applications. ACS Omega 2021, 6, 9709–9722. [Google Scholar] [CrossRef] [PubMed]
- Nazemi Salman, B.; Mohammadi Gheidari, M.; Yazdi nejad, A.; Zeighami, H.; Mohammadi, A.; Basir Shabestari, S. Antimicrobial Activity of Silver Nanoparticles Synthesized by the Green Method Using Rhus coriaria L. Extract Against Oral Pathogenic Microorganisms. Med. J. Islam. Repub. Iran 2023, 36, 154. [Google Scholar] [CrossRef] [PubMed]
- Premanand, G.; Shanmugam, N.; Kannadasan, N.; Sathishkumar, K.; Viruthagiri, G. Nelumbo nucifera leaf extract mediated synthesis of silver nanoparticles and their antimicrobial properties against some human pathogens. Appl. Nanosci. 2016, 6, 409–415. [Google Scholar] [CrossRef]
- Ibrahim, K.E.; Al-Mutary, M.G.; Bakhiet, A.O.; Khan, H.A. Histopathology of the Liver, Kidney, and Spleen of Mice Exposed to Gold Nanoparticles. Molecules 2018, 23, 1848. [Google Scholar] [CrossRef]
- Ali, S.B.; Mohamed, A.S.; Fahmy, S.R.; El–Garhy, M.; Mousa, M.R.; Abdel-Ghaffar, F. Anthelmintic and Hepatoprotective Activities of the Green-Synthesized Zinc Oxide Nanoparticles against Parascaris equorum Infection in Rats. Acta Parasit. 2024, 69, 283–301. [Google Scholar] [CrossRef]
- Patel, B.H.; Channiwala, M.Z.; Chaudhari, S.B.; Mandot, A.A. Biosynthesis of copper nanoparticles; its characteriza-tion and efficacy against human pathogenic bacterium. J. Environ. Chem. Eng. 2016, 4, 2163–2169. [Google Scholar] [CrossRef]
- Masurkar, S.A.; Chaudhari, P.R.; Shidore, V.B.; Kamble, S.P. Rapid Biosynthesis of Silver Nanoparticles Using Cymbopogan Citratus (Lemongrass) and its Antimicrobial Activity. Nano-Micro Lett. 2011, 3, 189–194. [Google Scholar] [CrossRef]
- Siddiq, M.; Thangam, R.; Madhan, B.; Alam, M.S. Green (gemini) surfactant mediated gold nanoparticles green synthesis: Effect on triple negative breast cancer cells. Nano-Struct. Nano-Objects 2019, 19, 100373. [Google Scholar] [CrossRef]
- Packialakshmi, P.; Gobinath, P.; Ali, D.; Alarifi, S.; Ravindran, B.; Idhayadhulla, A.; Surendrakumar, R. Novel Chitosan Polymer Design, Synthesis Using Mentha piperita of ZnO NPs as a Catalyst: Antibacterial Evalua-tion against Gram-Negative Multidrug-Resistant Pathogens. J. Nanomater. 2021, 2021, 8804837. [Google Scholar] [CrossRef]
- You, C.; Li, Q.; Wang, X.; Wu, P.; Ho, J.K.; Jin, R.; Zhang, L.; Shao, H.; Han, C. Silver nanoparticle loaded collagen/chitosan scaffolds promote wound healing via regulating fibroblast migration and macrophage activation. Sci. Rep. 2017, 7, 10489. [Google Scholar] [CrossRef] [PubMed]
- Fayaz, A.M.; Balaji, K.; Girilal, M.; Yadav, R.; Kalaichelvan, P.T.; Venketesan, R. Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: A study against gram-positive and gram-negative bacteria. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 103–109. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Kwon, D.-N.; Kim, J.-H. Enhanced antibacterial and anti-biofilm activities of silver nanoparticles against Gram-negative and Gram-positive bacteria. Nanoscale Res. Lett. 2014, 9, 373. [Google Scholar] [CrossRef]
- Bharadwaj, K.K.; Rabha, B.; Pati, S.; Sarkar, T.; Choudhury, B.K.; Barman, A.; Bhattacharjya, D.; Srivastava, A.; Baishya, D.; Edinur, H.A.; et al. Green Synthesis of Gold Nanoparticles Using Plant Extracts as Beneficial Prospect for Cancer Theranostics. Molecules 2021, 26, 6389. [Google Scholar] [CrossRef]
- Woźniak-Budych, M.J.; Przysiecka, Ł.; Langer, K.; Peplińska, B.; Jarek, M.; Wiesner, M.; Nowaczyk, G.; Jurga, S. Green synthesis of rifampicin-loaded copper nanoparticles with enhanced antimicrobial activity. J. Mater. Sci. Mater. Med. 2017, 28, 42. [Google Scholar] [CrossRef]
- Ivanova, I.A.; Daskalova, D.S.; Yordanova, L.P.; Pavlova, E.L. Copper and Copper Nanoparticles Applications and Their Role against Infections: A Minireview. Processes 2024, 12, 352. [Google Scholar] [CrossRef]
- Kulkarni, D.; Sherkar, R.; Shirsathe, C.; Sonwane, R.; Varpe, N.; Shelke, S.; More, M.P.; Pardeshi, S.R.; Dhaneshwar, G.; Junnuthula, V.; et al. Biofabrication of nanoparticles: Sources, synthesis, and biomedical applications. Front. Bioeng. Biotechnol. 2023, 11, 1159193. [Google Scholar] [CrossRef] [PubMed]
- kazemi, S.; Hosseingholian, A.; Gohari, S.D.; Feirahi, F.; Moammeri, F.; Mesbahian, G.; Moghaddam, Z.S.; Ren, Q. Recent advances in green synthesized nanoparticles: From production to application. Mater. Today Sustain. 2023, 24, 100500. [Google Scholar] [CrossRef]
- Shafey, A.M. El Green synthesis of metal and metal oxide nanoparticles from plant leaf extracts and their applications: A review. Green Process. Synth. 2020, 9, 304–339. [Google Scholar] [CrossRef]
- Assaf, I.; Zhang, Z.; Otaola, F.; Leturia, M.; Luart, D.; Terrasson, V.; Guénin, E. A continuous flow mode with a scalable tubular reactor for the green preparation of stable alkali lignin nanoparticles assisted by ultrasound. Int. J. Biol. Macromol. 2023, 243, 125106. [Google Scholar] [CrossRef]
- Mahin, J.; Franck, C.O.; Fanslau, L.; Patra, H.K.; Mantle, M.D.; Fruk, L.; Torrente-Murciano, L. Green, scalable, low cost and reproducible flow synthesis of biocompatible PEG-functionalized iron oxide nanoparticles. React. Chem. Eng. 2021, 6, 1961–1973. [Google Scholar] [CrossRef]
- Raveendran, P.; Fu, J.; Wallen, S.L. Completely “Green” Synthesis and Stabilization of Metal Nanoparticles. J. Am. Chem. Soc. 2003, 125, 13940–13941. [Google Scholar] [CrossRef]
- Babu, S.; Singh Rathore, S.; Singh, R.; Kumar, S.; Singh, V.K.; Yadav, S.K.; Yadav, V.; Raj, R.; Yadav, D.; Shekhawat, K.; et al. Exploring agricultural waste biomass for energy, food and feed production and pollution mitigation: A review. Bioresour. Technol. 2022, 360, 127566. [Google Scholar] [CrossRef]
- Mishra, B.; Mohanta, Y.K.; Reddy, C.N.; Reddy, S.D.M.; Mandal, S.K.; Yadavalli, R.; Sarma, H. Valorization of agro-industrial biowaste to biomaterials: An innovative circular bioeconomy approach. Circ. Econ. 2023, 2, 100050. [Google Scholar] [CrossRef]
- Chaudhuri, S.K.; Malodia, L. Biosynthesis of zinc oxide nanoparticles using leaf extract of Calotropis gigantea: Characterization and its evaluation on tree seedling growth in nursery stage. Appl. Nanosci. 2017, 7, 501–512. [Google Scholar] [CrossRef]
- Anand, K.; Kaviyarasu, K.; Muniyasamy, S.; Roopan, S.M.; Gengan, R.M.; Chuturgoon, A.A. Bio-Synthesis of Silver Nanoparticles Using Agroforestry Residue and Their Catalytic Degradation for Sustainable Waste Management. J. Clust. Sci. 2017, 28, 2279–2291. [Google Scholar] [CrossRef]
- Sharma, D.; Gulati, S.S.; Sharma, N.; Chaudhary, A. Sustainable synthesis of silver nanoparticles using various biological sources and waste materials: A review. Emergent Mater. 2022, 5, 1649–1678. [Google Scholar] [CrossRef]
- Nkosi, N.C.; Basson, A.K.; Ntombela, Z.G.; Dlamini, N.G.; Pullabhotla, R.V.S.R. Green Synthesis, Characterization and Application of Silver Nanoparticles Using Bioflocculant: A Review. Bioengineering 2024, 11, 492. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Desimone, M.F.; Pandya, S.; Jasani, S.; George, N.; Adnan, M.; Aldarhami, A.; Bazaid, A.S.; Alderhami, S.A. Revisiting the Green Synthesis of Nanoparticles: Uncovering Influences of Plant Extracts as Reducing Agents for Enhanced Synthesis Efficiency and Its Biomedical Applications. Int. J. Nanomed. 2023, 18, 4727–4750. [Google Scholar] [CrossRef]
- Alharbi, N.S.; Alsubhi, N.S.; Felimban, A.I. Green synthesis of silver nanoparticles using medicinal plants: Characterization and application. J. Radiat. Res. Appl. Sci. 2022, 15, 109–124. [Google Scholar] [CrossRef]
- Ali, I.A.M.; Ahmed, A.B.; Al-Ahmed, H.I. Green synthesis and characterization of silver nanoparticles for reducing the damage to sperm parameters in diabetic compared to metformin. Sci. Rep. 2023, 13, 2256. [Google Scholar] [CrossRef]
- Dudhane, A.; Waghmode, S.; Dama, L.; Mhaindarkar, V.; Sonawane, A.; Katariya, S. Synthesis and Characterization of Gold Nanoparticles using Plant Extract of Terminalia arjuna with Antibacterial Activity. Int. J. Nanosci. Nanotechnol. 2019, 15, 75–82. [Google Scholar]
- Oliveira, J.R.; Lenzi, G.G. Advances in the use of green and sustainable synthesis to obtain nanomaterials. In Green Chemistry for Environmental Sustainability—Prevention-Assurance-Sustainability (P-A-S) Approach; IntechOpen: London, UK, 2023. [Google Scholar]
- Wahab, A.; Muhammad, M.; Ullah, S.; Abdi, G.; Shah, G.M.; Zaman, W.; Ayaz, A. Agriculture and environmental management through nanotechnology: Eco-friendly nanomaterial synthesis for soil-plant systems, food safety, and sustainability. Sci. Total Environ. 2024, 926, 171862. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, R.; Naidu, S.; Upreti, G.; Sawhney, R. Sustainable Nanotechnology: Through Green Methods and Life-Cycle Thinking. Sustainability 2010, 2, 3323–3338. [Google Scholar] [CrossRef]
- Pati, P.; McGinnis, S.; Vikesland, P.J. Life Cycle Assessment of “Green” Nanoparticle Synthesis Methods. Environ. Eng. Sci. 2014, 31, 410–420. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukherjee, S.; Verma, A.; Kong, L.; Rengan, A.K.; Cahill, D.M. Advancements in Green Nanoparticle Technology: Focusing on the Treatment of Clinical Phytopathogens. Biomolecules 2024, 14, 1082. https://doi.org/10.3390/biom14091082
Mukherjee S, Verma A, Kong L, Rengan AK, Cahill DM. Advancements in Green Nanoparticle Technology: Focusing on the Treatment of Clinical Phytopathogens. Biomolecules. 2024; 14(9):1082. https://doi.org/10.3390/biom14091082
Chicago/Turabian StyleMukherjee, Sunny, Anamika Verma, Lingxue Kong, Aravind Kumar Rengan, and David Miles Cahill. 2024. "Advancements in Green Nanoparticle Technology: Focusing on the Treatment of Clinical Phytopathogens" Biomolecules 14, no. 9: 1082. https://doi.org/10.3390/biom14091082
APA StyleMukherjee, S., Verma, A., Kong, L., Rengan, A. K., & Cahill, D. M. (2024). Advancements in Green Nanoparticle Technology: Focusing on the Treatment of Clinical Phytopathogens. Biomolecules, 14(9), 1082. https://doi.org/10.3390/biom14091082








