Recent Advances in the Use of Vitamin D Organic Nanocarriers for Drug Delivery
Abstract
1. Introduction
2. Vitamin D Uptake, Physiology and Metabolism
3. Vitamin D Receptor (VDR)
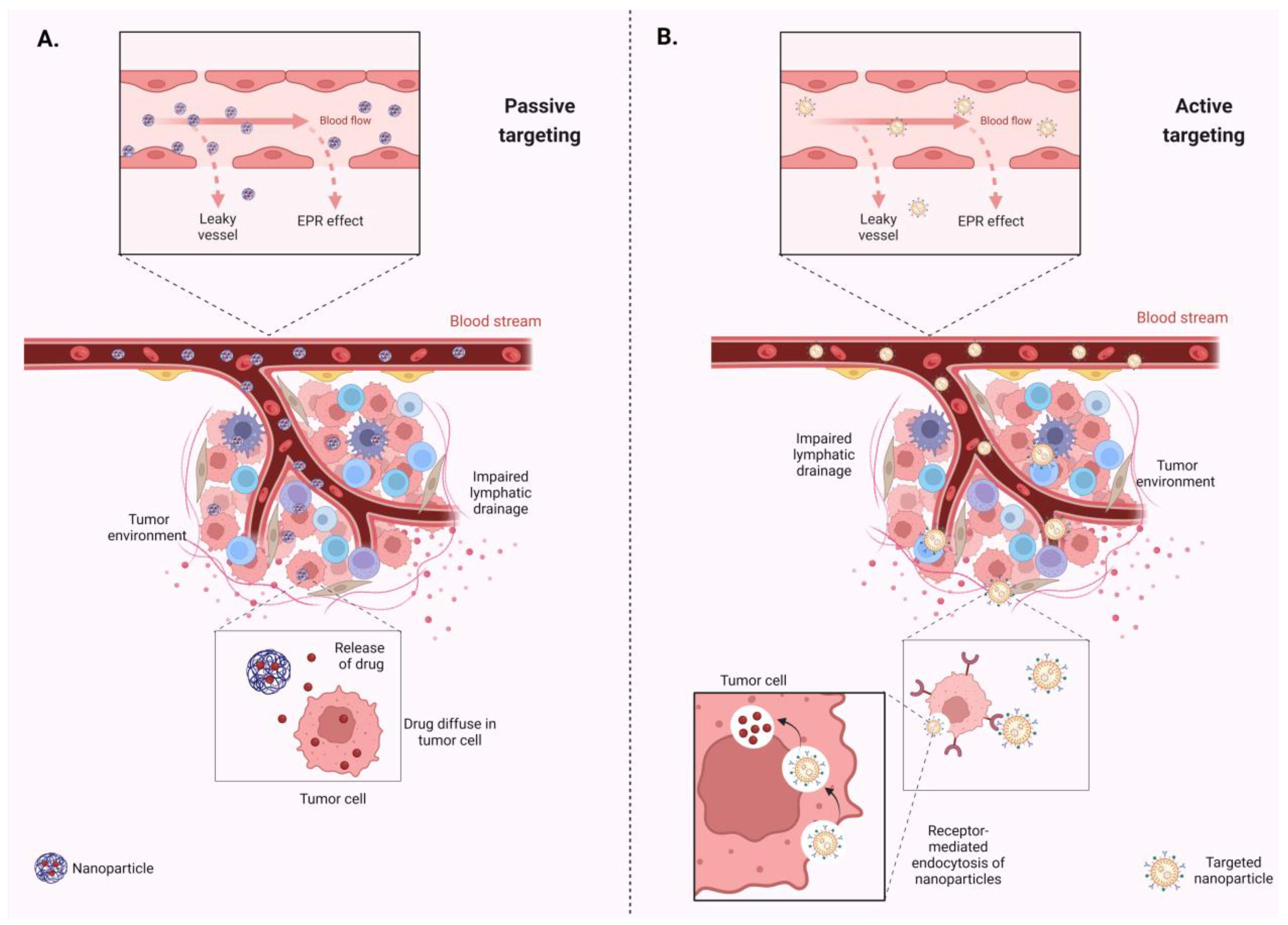
4. Drug Delivery Systems
5. Organic Nanocarriers
5.1. Lipid-Based Nanoparticles
5.1.1. Vitamin D Microencapsulation Using Liposomes
5.1.2. Vitamin D Microencapsulation Using Nanostructured Lipid Carriers (NLCs)
5.1.3. Vitamin D Microencapsulation Using Solid Lipid Nanoparticles (SLNs)
5.2. Polymers
5.2.1. Vitamin D Microencapsulation Using Polymeric Nanoparticles
5.2.2. Vitamin D Microencapsulation Using Poly L-Lactide-Co-Glycolide Nanoparticles (PLGAs)
5.3. Micelles
Vitamin D Microencapsulation Using Micelles
5.4. Nanoemulsion
Vitamin D Microencapsulation Using Nanoemulsion Technology
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Autier, P.; Boniol, M.; Pizot, C.; Mullie, P. Vitamin D status and ill health: A systematic review. Lancet Diabetes Endocrinol. 2014, 2, 76–89. [Google Scholar] [CrossRef]
- Giustina, A.; Bilezikian, J.P. Consensus Statement on Vitamin D Status Assessment and Supplementation: Whys, Whens, and Hows. Endocr. Rev. 2024, bnae009. [Google Scholar] [CrossRef] [PubMed]
- Wacker, M.; Holick, M.F. Vitamin D—Effects on skeletal and extraskeletal health and the need for supplementation. Nutrients 2013, 5, 111–148. [Google Scholar] [CrossRef] [PubMed]
- Battault, S.; Whiting, S.J.; Peltier, S.L.; Sadrin, S.; Gerber, G.; Maixent, J.M. Vitamin D metabolism, functions and needs: From science to health claims. Eur. J. Nutr. 2013, 52, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Hossein-nezhad, A.; Holick, M.F. Vitamin D for health: A global perspective. Mayo Clin. Proc. 2013, 88, 720–755. [Google Scholar] [CrossRef]
- Aggeletopoulou, I.; Marangos, M.; Assimakopoulos, S.F.; Mouzaki, A.; Thomopoulos, K.; Triantos, C. Vitamin D and Microbiome: Molecular Interaction in Inflammatory Bowel Disease Pathogenesis. Am. J. Pathol. 2023, 193, 656–668. [Google Scholar] [CrossRef]
- Triantos, C.; Aggeletopoulou, I.; Mantzaris, G.J.; Mouzaki, A. Molecular basis of vitamin D action in inflammatory bowel disease. Autoimmun. Rev. 2022, 21, 103136. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Infections and Autoimmunity-The Immune System and Vitamin D: A Systematic Review. Nutrients 2023, 15, 3842. [Google Scholar] [CrossRef]
- Bird, R.P. Vitamin D and cancer. Adv. Food Nutr. Res. 2024, 109, 92–159. [Google Scholar] [CrossRef]
- Seraphin, G.; Rieger, S.; Hewison, M.; Capobianco, E.; Lisse, T.S. The impact of vitamin D on cancer: A mini review. J. Steroid Biochem. Mol. Biol. 2023, 231, 106308. [Google Scholar] [CrossRef]
- Carbone, F.; Liberale, L. Vitamin D in atherosclerosis and cardiovascular events. Eur. Heart J. 2023, 44, 2078–2094. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.K.; Kumar, S. Implications of vitamin D deficiency in systemic inflammation and cardiovascular health. Crit. Rev. Food Sci. Nutr. 2023, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Aggeletopoulou, I.; Thomopoulos, K.; Mouzaki, A.; Triantos, C. Vitamin D-VDR Novel Anti-Inflammatory Molecules-New Insights into Their Effects on Liver Diseases. Int. J. Mol. Sci. 2022, 23, 8465. [Google Scholar] [CrossRef]
- Triantos, C.; Aggeletopoulou, I.; Thomopoulos, K.; Mouzaki, A. Vitamin D-Liver Disease Association: Biological Basis and Mechanisms of Action. Hepatology 2021, 74, 1065–1073. [Google Scholar] [CrossRef]
- Aggeletopoulou, I.; Tsounis, E.P.; Triantos, C. Vitamin D and Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): Novel Mechanistic Insights. Int. J. Mol. Sci. 2024, 25, 4901. [Google Scholar] [CrossRef]
- Wang, W.; Li, Y.; Meng, X. Vitamin D and neurodegenerative diseases. Heliyon 2023, 9, e12877. [Google Scholar] [CrossRef]
- Almouazen, E.; Bourgeois, S.; Jordheim, L.P.; Fessi, H.; Briançon, S. Nano-encapsulation of vitamin D3 active metabolites for application in chemotherapy: Formulation study and in vitro evaluation. Pharm. Res. 2013, 30, 1137–1146. [Google Scholar] [CrossRef]
- Schlingmann, K.P. Vitamin D-dependent Hypercalcemia. Endocrinol. Metab. Clin. N. Am. 2021, 50, 729–742. [Google Scholar] [CrossRef]
- Šimoliūnas, E.; Rinkūnaitė, I.; Bukelskienė, Ž.; Bukelskienė, V. Bioavailability of Different Vitamin D Oral Supplements in Laboratory Animal Model. Medicina 2019, 55, 265. [Google Scholar] [CrossRef]
- Lehmann, U.; Hirche, F.; Stangl, G.I.; Hinz, K.; Westphal, S.; Dierkes, J. Bioavailability of Vitamin D2 and D3 in Healthy Volunteers, a Randomized Placebo-Controlled Trial. J. Clin. Endocrinol. Metab. 2013, 98, 4339–4345. [Google Scholar] [CrossRef]
- Khizar, S.; Alrushaid, N.; Alam Khan, F.; Zine, N.; Jaffrezic-Renault, N.; Errachid, A.; Elaissari, A. Nanocarriers based novel and effective drug delivery system. Int. J. Pharm. 2023, 632, 122570. [Google Scholar] [CrossRef] [PubMed]
- Halwani, A.A. Development of Pharmaceutical Nanomedicines: From the Bench to the Market. Pharmaceutics 2022, 14, 106. [Google Scholar] [CrossRef] [PubMed]
- Majumder, J.; Taratula, O.; Minko, T. Nanocarrier-based systems for targeted and site specific therapeutic delivery. Adv. Drug Deliv. Rev. 2019, 144, 57–77. [Google Scholar] [CrossRef]
- Yu, M.K.; Park, J.; Jon, S. Targeting strategies for multifunctional nanoparticles in cancer imaging and therapy. Theranostics 2012, 2, 3–44. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef]
- Ahmadi, S.; Rabiee, N.; Bagherzadeh, M.; Elmi, F.; Fatahi, Y.; Farjadian, F.; Baheiraei, N.; Nasseri, B.; Rabiee, M.; Dastjerd, N.T.; et al. Stimulus-Responsive Sequential Release Systems for Drug and Gene Delivery. Nano Today 2020, 34, 100914. [Google Scholar] [CrossRef]
- Corma, A.; Botella, P. Silica-Based Stimuli-Responsive Systems for Antitumor Drug Delivery and Controlled Release. Pharmaceutics 2022, 14, 110. [Google Scholar] [CrossRef]
- Murugan, C.; Rayappan, K.; Thangam, R.; Bhanumathi, R.; Shanthi, K.; Vivek, R.; Thirumurugan, R.; Bhattacharyya, A.; Sivasubramanian, S.; Gunasekaran, P.; et al. Combinatorial nanocarrier based drug delivery approach for amalgamation of anti-tumor agents in breast cancer cells: An improved nanomedicine strategy. Sci. Rep. 2016, 6, 34053. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Wan, K.; Zhou, N.; Wei, G.; Su, Z. Supramolecular peptide nano-assemblies for cancer diagnosis and therapy: From molecular design to material synthesis and function-specific applications. J. Nanobiotechnol. 2021, 19, 253. [Google Scholar] [CrossRef]
- Wei, X.; Pandohee, J.; Xu, B. Recent developments and emerging trends in dietary vitamin D sources and biological conversion. Crit. Rev. Food Sci. Nutr. 2023, 1–17. [Google Scholar] [CrossRef]
- Mouli, V.P.; Ananthakrishnan, A.N. Review article: Vitamin D and inflammatory bowel diseases. Aliment. Pharmacol. Ther. 2014, 39, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P. Vitamin D in health and disease. Clin. J. Am. Soc. Nephrol. 2008, 3, 1535–1541. [Google Scholar] [CrossRef]
- Schuster, I. Cytochromes P450 are essential players in the vitamin D signaling system. Biochim. Biophys. Acta 2011, 1814, 186–199. [Google Scholar] [CrossRef]
- Jones, G.; Prosser, D.E.; Kaufmann, M. Cytochrome P450-mediated metabolism of vitamin D. J. Lipid Res. 2014, 55, 13–31. [Google Scholar] [CrossRef]
- Latic, N.; Erben, R.G. FGF23 and Vitamin D Metabolism. JBMR Plus 2021, 5, e10558. [Google Scholar] [CrossRef] [PubMed]
- Bergwitz, C.; Jüppner, H. Regulation of phosphate homeostasis by PTH, vitamin D, and FGF23. Annu. Rev. Med. 2010, 61, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Zierold, C.; Nehring, J.A.; DeLuca, H.F. Nuclear receptor 4A2 and C/EBPbeta regulate the parathyroid hormone-mediated transcriptional regulation of the 25-hydroxyvitamin D3-1alpha-hydroxylase. Arch. Biochem. Biophys. 2007, 460, 233–239. [Google Scholar] [CrossRef]
- Zierold, C.; Mings, J.A.; DeLuca, H.F. Parathyroid hormone regulates 25-hydroxyvitamin D(3)-24-hydroxylase mRNA by altering its stability. Proc. Natl. Acad. Sci. USA 2001, 98, 13572–13576. [Google Scholar] [CrossRef]
- Riccardi, D.; Brown, E.M. Physiology and pathophysiology of the calcium-sensing receptor in the kidney. Am. J. Physiol. Renal Physiol. 2010, 298, F485–F499. [Google Scholar] [CrossRef]
- Shimada, T.; Yamazaki, Y.; Takahashi, M.; Hasegawa, H.; Urakawa, I.; Oshima, T.; Ono, K.; Kakitani, M.; Tomizuka, K.; Fujita, T.; et al. Vitamin D receptor-independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am. J. Physiol. Renal Physiol. 2005, 289, F1088–F1095. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C. Genome-wide (over)view on the actions of vitamin D. Front. Physiol. 2014, 5, 167. [Google Scholar] [CrossRef]
- Rebelos, E.; Tentolouris, N.; Jude, E. The Role of Vitamin D in Health and Disease: A Narrative Review on the Mechanisms Linking Vitamin D with Disease and the Effects of Supplementation. Drugs 2023, 83, 665–685. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.Y.; Zhang, Y.Q.; Chen, M.D.; Du, Y.Q.; Liu, C.B.; Wu, J.F. Relationship between Structure and Conformational Change of the Vitamin D Receptor Ligand Binding Domain in 1α,25-Dihydroxyvitamin D3 Signaling. Molecules 2015, 20, 20473–20486. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C. Vitamin D genomics: From in vitro to in vivo. Front. Endocrinol. 2018, 9, 250. [Google Scholar] [CrossRef]
- Carlberg, C. Genomic signaling of vitamin D. Steroids 2023, 198, 109271. [Google Scholar] [CrossRef]
- Żmijewski, M.A. Nongenomic Activities of Vitamin D. Nutrients 2022, 14, 5104. [Google Scholar] [CrossRef]
- Udomsinprasert, W.; Jittikoon, J. Vitamin D and liver fibrosis: Molecular mechanisms and clinical studies. Biomed. Pharmacother. 2019, 109, 1351–1360. [Google Scholar] [CrossRef]
- Nemere, I.; Safford, S.E.; Rohe, B.; DeSouza, M.M.; Farach-Carson, M.C. Identification and characterization of 1,25D3-membrane-associated rapid response, steroid (1,25D3-MARRS) binding protein. J. Steroid Biochem. Mol. Biol. 2004, 89–90, 281–285. [Google Scholar] [CrossRef]
- Haussler, M.R.; Jurutka, P.W.; Mizwicki, M.; Norman, A.W. Vitamin D receptor (VDR)-mediated actions of 1α,25(OH)2vitamin D3: Genomic and non-genomic mechanisms. Best. Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 543–559. [Google Scholar] [CrossRef]
- Donati, S.; Palmini, G. Rapid Nontranscriptional Effects of Calcifediol and Calcitriol. Nutrients 2022, 14, 1291. [Google Scholar] [CrossRef]
- Coelho, J.F.; Ferreira, P.C.; Alves, P.; Cordeiro, R.; Fonseca, A.C.; Góis, J.R.; Gil, M.H. Drug delivery systems: Advanced technologies potentially applicable in personalized treatments. EPMA J. 2010, 1, 164–209. [Google Scholar] [CrossRef]
- Jain, K.K. An Overview of Drug Delivery Systems. Methods Mol. Biol. 2020, 2059, 1–54. [Google Scholar] [CrossRef]
- Birk, S.E.; Boisen, A.; Nielsen, L.H. Polymeric nano- and microparticulate drug delivery systems for treatment of biofilms. Adv. Drug Deliv. Rev. 2021, 174, 30–52. [Google Scholar] [CrossRef]
- Sabbagh, F.; Kim, B.S. Recent advances in polymeric transdermal drug delivery systems. J. Control. Release 2022, 341, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Afzal, O.; Altamimi, A.S.A.; Nadeem, M.S. Nanoparticles in Drug Delivery: From History to Therapeutic Applications. Nanomaterials 2022, 12, 4494. [Google Scholar] [CrossRef] [PubMed]
- Attia, M.F.; Anton, N. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J. Pharm. Pharmacol. 2019, 71, 1185–1198. [Google Scholar] [CrossRef] [PubMed]
- Bazak, R.; Houri, M.; Achy, S.E.; Hussein, W.; Refaat, T. Passive targeting of nanoparticles to cancer: A comprehensive review of the literature. Mol. Clin. Oncol. 2014, 2, 904–908. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.H.; Park, K. Targeted drug delivery to tumors: Myths, reality and possibility. J. Control. Release 2011, 153, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yang, Z.; Li, F.; Xu, L.; Sun, Y. Cell-mediated targeting drugs delivery systems. Drug Deliv. 2020, 27, 1425–1437. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Li, W.; Li, Y.; Yin, S. Nanoparticle-based drug delivery systems targeting cancer cell surfaces. RSC Adv. 2023, 13, 21365–21382. [Google Scholar] [CrossRef]
- Zhu, Y.; Feijen, J.; Zhong, Z. Dual-targeted nanomedicines for enhanced tumor treatment. Nano Today 2018, 18, 65–85. [Google Scholar] [CrossRef]
- Syed Azhar, S.N.A.; Ashari, S.E. Nanostructured Lipid Carriers-Hydrogels System for Drug Delivery: Nanohybrid Technology Perspective. Molecules 2022, 27, 289. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Li, J.; Wang, Q.; Xia, G.; Adilijiang, N.; Li, Y.; Hou, Z.; Fan, Z.; Li, J. Recent Advances in Targeted Drug Delivery Strategy for Enhancing Oncotherapy. Pharmaceutics 2023, 15, 2233. [Google Scholar] [CrossRef]
- Yan, S.; Na, J.; Liu, X.; Wu, P. Different Targeting Ligands-Mediated Drug Delivery Systems for Tumor Therapy. Pharmaceutics 2024, 16, 248. [Google Scholar] [CrossRef] [PubMed]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, F.; Caruana, P. Nano-Based Approved Pharmaceuticals for Cancer Treatment: Present and Future Challenges. Biomolecules 2022, 12, 784. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Kiani Shahvandi, M.; Souri, M.; Tavasoli, S.; Moradi Kashkooli, F.; Kar, S.; Soltani, M. A comparative study between conventional chemotherapy and photothermal activated nano-sized targeted drug delivery to solid tumor. Comput. Biol. Med. 2023, 166, 107574. [Google Scholar] [CrossRef]
- Crintea, A.; Dutu, A.G.; Sovrea, A.; Constantin, A.M.; Samasca, G. Nanocarriers for Drug Delivery: An Overview with Emphasis on Vitamin D and K Transportation. Nanomaterials 2022, 12, 1376. [Google Scholar] [CrossRef] [PubMed]
- Glowka, E.; Stasiak, J.; Lulek, J. Drug Delivery Systems for Vitamin D Supplementation and Therapy. Pharmaceutics 2019, 11, 347. [Google Scholar] [CrossRef]
- Gupta, R.; Behera, C.; Paudwal, G.; Rawat, N.; Baldi, A.; Gupta, P.N. Recent Advances in Formulation Strategies for Efficient Delivery of Vitamin D. AAPS PharmSciTech 2018, 20, 11. [Google Scholar] [CrossRef] [PubMed]
- Maurya, V.K.; Aggarwal, M. Factors influencing the absorption of vitamin D in GIT: An overview. J. Food Sci. Technol. 2017, 54, 3753–3765. [Google Scholar] [CrossRef]
- Vinarov, Z.; Abrahamsson, B.; Artursson, P.; Batchelor, H.; Berben, P.; Bernkop-Schnürch, A.; Butler, J.; Ceulemans, J.; Davies, N.; Dupont, D.; et al. Current challenges and future perspectives in oral absorption research: An opinion of the UNGAP network. Adv. Drug Deliv. Rev. 2021, 171, 289–331. [Google Scholar] [CrossRef] [PubMed]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef] [PubMed]
- Briuglia, M.L.; Rotella, C.; McFarlane, A.; Lamprou, D.A. Influence of cholesterol on liposome stability and on in vitro drug release. Drug Deliv. Transl. Res. 2015, 5, 231–242. [Google Scholar] [CrossRef]
- Antimisiaris, S.G.; Marazioti, A.; Kannavou, M.; Natsaridis, E.; Gkartziou, F.; Kogkos, G.; Mourtas, S. Overcoming barriers by local drug delivery with liposomes. Adv. Drug Deliv. Rev. 2021, 174, 53–86. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Kumar, E.M.; Chavali, M.S. Updates on Responsive Drug Delivery Based on Liposome Vehicles for Cancer Treatment. Pharmaceutics 2022, 14, 2195. [Google Scholar] [CrossRef]
- Yingchoncharoen, P.; Kalinowski, D.S.; Richardson, D.R. Lipid-Based Drug Delivery Systems in Cancer Therapy: What Is Available and What Is Yet to Come. Pharmacol. Rev. 2016, 68, 701–787. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.K.; Riaz, M.A.; Zhang, X.; Lin, C.; Wong, K.H.; Chen, X.; Zhang, G.; Lu, A.; Yang, Z. Surface Functionalization and Targeting Strategies of Liposomes in Solid Tumor Therapy: A Review. Int. J. Mol. Sci. 2018, 19, 195. [Google Scholar] [CrossRef]
- Ferreira, M.; Ogren, M.; Dias, J.N.R.; Silva, M. Liposomes as Antibiotic Delivery Systems: A Promising Nanotechnological Strategy against Antimicrobial Resistance. Molecules 2021, 26, 2047. [Google Scholar] [CrossRef] [PubMed]
- Arana, L.; Gallego, L.; Alkorta, I. Incorporation of Antibiotics into Solid Lipid Nanoparticles: A Promising Approach to Reduce Antibiotic Resistance Emergence. Nanomaterials 2021, 11, 1251. [Google Scholar] [CrossRef] [PubMed]
- Gubernator, J. Active methods of drug loading into liposomes: Recent strategies for stable drug entrapment and increased in vivo activity. Expert. Opin. Drug Deliv. 2011, 8, 565–580. [Google Scholar] [CrossRef]
- Brandl, M. Vesicular phospholipid gels: A technology platform. J. Liposome Res. 2007, 17, 15–26. [Google Scholar] [CrossRef]
- Sánchez-Cerviño, M.C.; Fuioaga, C.P.; Atanase, L.I. Electrohydrodynamic Techniques for the Manufacture and/or Immobilization of Vesicles. Polymers 2023, 15, 795. [Google Scholar] [CrossRef]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef]
- Gbian, D.L.; Omri, A. Lipid-Based Drug Delivery Systems for Diseases Managements. Biomedicines 2022, 10, 2137. [Google Scholar] [CrossRef]
- Prüfer, K.; Merz, K.; Barth, A.; Wollina, U.; Sternberg, B. Interaction of liposomal incorporated vitamin D3-analogues and human keratinocytes. J. Drug Target. 1994, 2, 419–429. [Google Scholar] [CrossRef]
- Bochicchio, S.; Barba, A.A.; Grassi, G.; Lamberti, G. Vitamin delivery: Carriers based on nanoliposomes produced via ultrasonic irradiation. LWT-Food Sci. Technol. 2016, 69, 9–16. [Google Scholar] [CrossRef]
- Xia, F.; Jin, H.; Zhao, Y.; Guo, X. Supercritical Antisolvent-based Technology for Preparation of Vitamin D3 Proliposome and Its Characteristics. Chin. J. Chem. Eng. 2011, 19, 1039–1046. [Google Scholar] [CrossRef]
- Mohammadi, M.; Ghanbarzadeh, B.; Hamishehkar, H. Formulation of nanoliposomal vitamin d3 for potential application in beverage fortification. Adv. Pharm. Bull. 2014, 4, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Vieira, E.F.; Souza, S. Formulation Strategies for Improving the Stability and Bioavailability of Vitamin D-Fortified Beverages: A Review. Foods 2022, 11, 847. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.A.; Dacanal, G.C.; Pinho, S.C. High-shear wet agglomeration process for enriching cornstarch with curcumin and vitamin D(3) co-loaded lyophilized liposomes. Food Res. Int. 2023, 169, 112809. [Google Scholar] [CrossRef]
- Didar, Z. Enrichment of dark chocolate with vitamin D(3) (free or liposome) and assessment quality parameters. J. Food Sci. Technol. 2021, 58, 3065–3072. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Hamishehkar, H.; Amjadi, S. Development of gelatin-coated nanoliposomes loaded with β-cyclodextrin/vitamin D(3) inclusion complex for nutritional therapy. Food Chem. 2023, 424, 136346. [Google Scholar] [CrossRef]
- Bi, Y.; Xia, H.; Li, L.; Lee, R.J. Liposomal Vitamin D(3) as an Anti-aging Agent for the Skin. Pharmaceutics 2019, 11, 311. [Google Scholar] [CrossRef]
- Żurek, G.; Przybyło, M.; Witkiewicz, W.; Langner, M. Novel Approach for the Approximation of Vitamin D(3) Pharmacokinetics from In Vivo Absorption Studies. Pharmaceutics 2023, 15, 783. [Google Scholar] [CrossRef]
- Ghiasi, F.; Eskandari, M.H.; Golmakani, M.T. Build-Up of a 3D Organogel Network within the Bilayer Shell of Nanoliposomes. A Novel Delivery System for Vitamin D(3): Preparation, Characterization, and Physicochemical Stability. J. Agric. Food Chem. 2021, 69, 2585–2594. [Google Scholar] [CrossRef]
- Elmowafy, M.; Al-Sanea, M.M. Nanostructured lipid carriers (NLCs) as drug delivery platform: Advances in formulation and delivery strategies. Saudi Pharm. J. 2021, 29, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Khosa, A.; Reddi, S.; Saha, R.N. Nanostructured lipid carriers for site-specific drug delivery. Biomed. Pharmacother. 2018, 103, 598–613. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, L.; Gallarate, M. Lipid nanoparticles: State of the art, new preparation methods and challenges in drug delivery. Expert. Opin. Drug Deliv. 2012, 9, 497–508. [Google Scholar] [CrossRef]
- Rabelo, R.S.; Oliveira, I.F.; da Silva, V.M.; Prata, A.S.; Hubinger, M.D. Chitosan coated nanostructured lipid carriers (NLCs) for loading Vitamin D: A physical stability study. Int. J. Biol. Macromol. 2018, 119, 902–912. [Google Scholar] [CrossRef]
- Mohammadi, M.; Pezeshki, A.; Mesgari Abbasi, M.; Ghanbarzadeh, B.; Hamishehkar, H. Vitamin D(3)-Loaded Nanostructured Lipid Carriers as a Potential Approach for Fortifying Food Beverages; in Vitro and in Vivo Evaluation. Adv. Pharm. Bull. 2017, 7, 61–71. [Google Scholar] [CrossRef]
- Jafarifar, Z.; Rezaie, M.; Sharifan, P.; Jahani, V.; Daneshmand, S.; Ghazizadeh, H.; Ferns, G.A.; Golmohammadzadeh, S.; Ghayour-Mobarhan, M. Preparation and Characterization of Nanostructured Lipid Carrier (NLC) and Nanoemulsion Containing Vitamin D3. Appl. Biochem. Biotechnol. 2022, 194, 914–929. [Google Scholar] [CrossRef]
- Zai, K.; Hirota, M.; Yamada, T.; Ishihara, N.; Mori, T.; Kishimura, A.; Suzuki, K.; Hase, K.; Katayama, Y. Therapeutic effect of vitamin D(3)-containing nanostructured lipid carriers on inflammatory bowel disease. J. Control. Release 2018, 286, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, L.; Polonini, H.; Ramos, C.; Ferreira, A.O.; Raposo, N.; Brandão, M. Assessment of a Novel Vitamin D(3) Formulation with Nanostructured Lipid Carriers for Transdermal Delivery. Curr. Drug Deliv. 2022, 19, 614–624. [Google Scholar] [CrossRef]
- Cristelo, C.; Sá, A.F.; Lúcio, M.; Sarmento, B.; Gama, F.M. Vitamin D loaded into lipid nanoparticles shows insulinotropic effect in INS-1E cells. Eur. J. Pharm. Sci. 2024, 196, 106758. [Google Scholar] [CrossRef]
- Bukke, S.P.N.; Venkatesh, C.; Bandenahalli Rajanna, S.; Saraswathi, T.S.; Kusuma, P.K.; Goruntla, N.; Balasuramanyam, N.; Munishamireddy, S. Solid lipid nanocarriers for drug delivery: Design innovations and characterization strategies—A comprehensive review. Discov. Appl. Sci. 2024, 6, 279. [Google Scholar] [CrossRef]
- Duong, V.A.; Nguyen, T.T. Preparation of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Drug Delivery and the Effects of Preparation Parameters of Solvent Injection Method. Molecules 2020, 25, 4781. [Google Scholar] [CrossRef] [PubMed]
- Ezzati Nazhad Dolatabadi, J.; Valizadeh, H.; Hamishehkar, H. Solid Lipid Nanoparticles as Efficient Drug and Gene Delivery Systems: Recent Breakthroughs. Adv. Pharm. Bull. 2015, 5, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, M.; Ghobadi, R.; Sohrabvandi, S.; Khanniri, E.; Mollakhalili-Meybodi, N. Co-encapsulation of omega-3 and vitamin D(3) in beeswax solid lipid nanoparticles to evaluate physicochemical and in vitro release properties. Front. Nutr. 2024, 11, 1323067. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Deng, S.; Gigliobianco, M.R.; Censi, R.; Di Martino, P. Polymeric Nanocapsules as Nanotechnological Alternative for Drug Delivery System: Current Status, Challenges and Opportunities. Nanomaterials 2020, 10, 847. [Google Scholar] [CrossRef]
- Begines, B.; Ortiz, T. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef]
- Ramezanli, T.; Kilfoyle, B.E.; Zhang, Z.; Michniak-Kohn, B.B. Polymeric nanospheres for topical delivery of vitamin D3. Int. J. Pharm. 2017, 516, 196–203. [Google Scholar] [CrossRef]
- Jung, H.H.; Kim, S.H. Polymeric Nanoparticles Containing Both Antigen and Vitamin D(3) Induce Antigen-Specific Immune Suppression. Immune Netw. 2019, 19, e19. [Google Scholar] [CrossRef]
- Essa, D.; Kondiah, P.P.D.; Choonara, Y.E.; Pillay, V. The Design of Poly(lactide-co-glycolide) Nanocarriers for Medical Applications. Front. Bioeng. Biotechnol. 2020, 8, 48. [Google Scholar] [CrossRef]
- El-Hammadi, M.M.; Arias, J.L. Recent Advances in the Surface Functionalization of PLGA-Based Nanomedicines. Nanomaterials 2022, 12, 354. [Google Scholar] [CrossRef]
- Rocha, C.V.; Gonçalves, V.; da Silva, M.C.; Bañobre-López, M. PLGA-Based Composites for Various Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 2034. [Google Scholar] [CrossRef]
- Wu, J.; Wang, X.; Wang, Y.; Xun, Z.; Li, S. Application of PLGA in Tumor Immunotherapy. Polymers 2024, 16, 1253. [Google Scholar] [CrossRef] [PubMed]
- Khodaverdi, S.; Jafari, A. Evaluating Inhibitory Effects of Paclitaxel and Vitamin D(3) Loaded Poly Lactic Glycolic Acid Co-Delivery Nanoparticles on the Breast Cancer Cell Line. Adv. Pharm. Bull. 2020, 10, 30–38. [Google Scholar] [CrossRef]
- Ramalho, M.J.; Loureiro, J.A.; Gomes, B.; Frasco, M.F.; Coelho, M.A.; Pereira, M.C. PLGA nanoparticles as a platform for vitamin D-based cancer therapy. Beilstein J. Nanotechnol. 2015, 6, 1306–1318. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, N.A.N.; El-Kemary, M.; Leporatti, S. Micelles Structure Development as a Strategy to Improve Smart Cancer Therapy. Cancers 2018, 10, 238. [Google Scholar] [CrossRef] [PubMed]
- Mustafai, A.; Zubair, M. Recent Progress in Proteins-Based Micelles as Drug Delivery Carriers. Polymers 2023, 15, 836. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, E.; Yang, J.; Cao, Z. Strategies to improve micelle stability for drug delivery. Nano Res. 2018, 11, 4985–4998. [Google Scholar] [CrossRef] [PubMed]
- Lavelli, V.; D’Incecco, P. Vitamin D Incorporation in Foods: Formulation Strategies, Stability, and Bioaccessibility as Affected by the Food Matrix. Foods 2021, 10, 1989. [Google Scholar] [CrossRef]
- McCourt, A.F.; O’Sullivan, A.M. Using food fortification to improve vitamin D bioaccessibility and intakes. Proc. Nutr. Soc. 2022, 81, 99–107. [Google Scholar] [CrossRef]
- Solnier, J.; Chang, C. A Comparison and Safety Evaluation of Micellar versus Standard Vitamin D(3) Oral Supplementation in a Randomized, Double-Blind Human Pilot Study. Nutrients 2024, 16, 1573. [Google Scholar] [CrossRef]
- Mulrooney, S.L.; O’Neill, G.J.; Brougham, D.F.; O’Riordan, D. Enhancing the bioaccessibility of vitamin D using mixed micelles—An in vitro study. Food Chem. 2022, 395, 133634. [Google Scholar] [CrossRef] [PubMed]
- Mulrooney, S.L.; O’Neill, G.J.; Brougham, D.F.; O’Riordan, D. Vitamin D(3) bioaccessibility: Influence of fatty acid chain length, salt concentration and l-α-phosphatidylcholine concentration on mixed micelle formation and delivery of vitamin D(3). Food Chem. 2021, 344, 128722. [Google Scholar] [CrossRef]
- Loewen, A.; Chan, B.; Li-Chan, E.C.Y. Optimization of vitamins A and D(3) loading in re-assembled casein micelles and effect of loading on stability of vitamin D(3) during storage. Food Chem. 2018, 240, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Cohen, Y.; Levi, M.; Lesmes, U.; Margier, M.; Reboul, E.; Livney, Y.D. Re-assembled casein micelles improve in vitro bioavailability of vitamin D in a Caco-2 cell model. Food Funct. 2017, 8, 2133–2141. [Google Scholar] [CrossRef]
- Sneha, K.; Kumar, A. Nanoemulsions: Techniques for the preparation and the recent advances in their food applications. IFSET 2022, 76, 102914. [Google Scholar] [CrossRef]
- Li, X.; Anton, N.; Vandamme, T. Nano-emulsions: Overview and applications. Nanopharmaceutics 2013, 21–48. [Google Scholar] [CrossRef]
- Preeti; Sambhakar, S.; Malik, R. Nanoemulsion: An Emerging Novel Technology for Improving the Bioavailability of Drugs. Scientifica 2023, 2023, 6640103. [Google Scholar] [CrossRef]
- Kadappan, A.S.; Guo, C.; Gumus, C.E.; Bessey, A.; Wood, R.J.; McClements, D.J.; Liu, Z. The Efficacy of Nanoemulsion-Based Delivery to Improve Vitamin D Absorption: Comparison of In Vitro and In Vivo Studies. Mol. Nutr. Food Res. 2018, 62, 836. [Google Scholar] [CrossRef]
- Mehmood, T.; Ahmed, A. Tween 80 and Soya-Lecithin-Based Food-Grade Nanoemulsions for the Effective Delivery of Vitamin D. Langmuir 2020, 36, 2886–2892. [Google Scholar] [CrossRef]
- Ahmed, M.Z.; Gupta, A.; Warsi, M.H.; Ali, A.M.A. Nano Matrix Soft Confectionary for Oral Supplementation of Vitamin D: Stability and Sensory Analysis. Gels 2022, 8, 250. [Google Scholar] [CrossRef]
- Kumar, M.; Nishad, D.K.; Kumar, A.; Bhatnagar, A.; Karwasra, R.; Khanna, K.; S, K.; Sharma, D.; Dua, K.; Mudaliyar, V.; et al. Enhancement in brain uptake of vitamin D(3) nanoemulsion for treatment of cerebral ischemia: Formulation, gamma scintigraphy and efficacy study in transient middle cerebral artery occlusion rat models. J. Microencapsul. 2020, 37, 492–501. [Google Scholar] [CrossRef] [PubMed]



| Delivery Platform | Advantages | Disadvantages |
|---|---|---|
| Lipid-based Nanoparticles |
|
|
| Polymeric Nanoparticles |
|
|
| Micelles |
|
|
| Re-assembled Casein Micelles |
|
|
| Nanoemulsions |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aggeletopoulou, I.; Kalafateli, M.; Geramoutsos, G.; Triantos, C. Recent Advances in the Use of Vitamin D Organic Nanocarriers for Drug Delivery. Biomolecules 2024, 14, 1090. https://doi.org/10.3390/biom14091090
Aggeletopoulou I, Kalafateli M, Geramoutsos G, Triantos C. Recent Advances in the Use of Vitamin D Organic Nanocarriers for Drug Delivery. Biomolecules. 2024; 14(9):1090. https://doi.org/10.3390/biom14091090
Chicago/Turabian StyleAggeletopoulou, Ioanna, Maria Kalafateli, Georgios Geramoutsos, and Christos Triantos. 2024. "Recent Advances in the Use of Vitamin D Organic Nanocarriers for Drug Delivery" Biomolecules 14, no. 9: 1090. https://doi.org/10.3390/biom14091090
APA StyleAggeletopoulou, I., Kalafateli, M., Geramoutsos, G., & Triantos, C. (2024). Recent Advances in the Use of Vitamin D Organic Nanocarriers for Drug Delivery. Biomolecules, 14(9), 1090. https://doi.org/10.3390/biom14091090









