A Putative Effector Pst-18220, from Puccinia striiformis f. sp. tritici, Participates in Rust Pathogenicity and Plant Defense Suppression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Rust Inoculation
2.2. Cloning and Phylogenetic Analyses of Pst-18220
2.3. RNA Extraction and cDNA Synthesis
2.4. Construction of Plasmids
2.5. HIGS-Mediated Pst-18220 Silencing
2.6. Histological Observations of Pst-18220-Knockdown Wheat Plants
2.7. Transient Expression in N. benthamiana
2.8. Constitutive Overexpression in A. thaliana
2.9. Bacteria Pathogenicity Assay
2.10. H2O2 Accumulation and Observation
2.11. Statistical Analyses
3. Results
3.1. Pst-18220 Is a Pst-Specific Gene
3.2. Pst-18220 Is Highly Induced at the Early Infection Stage of Wheat
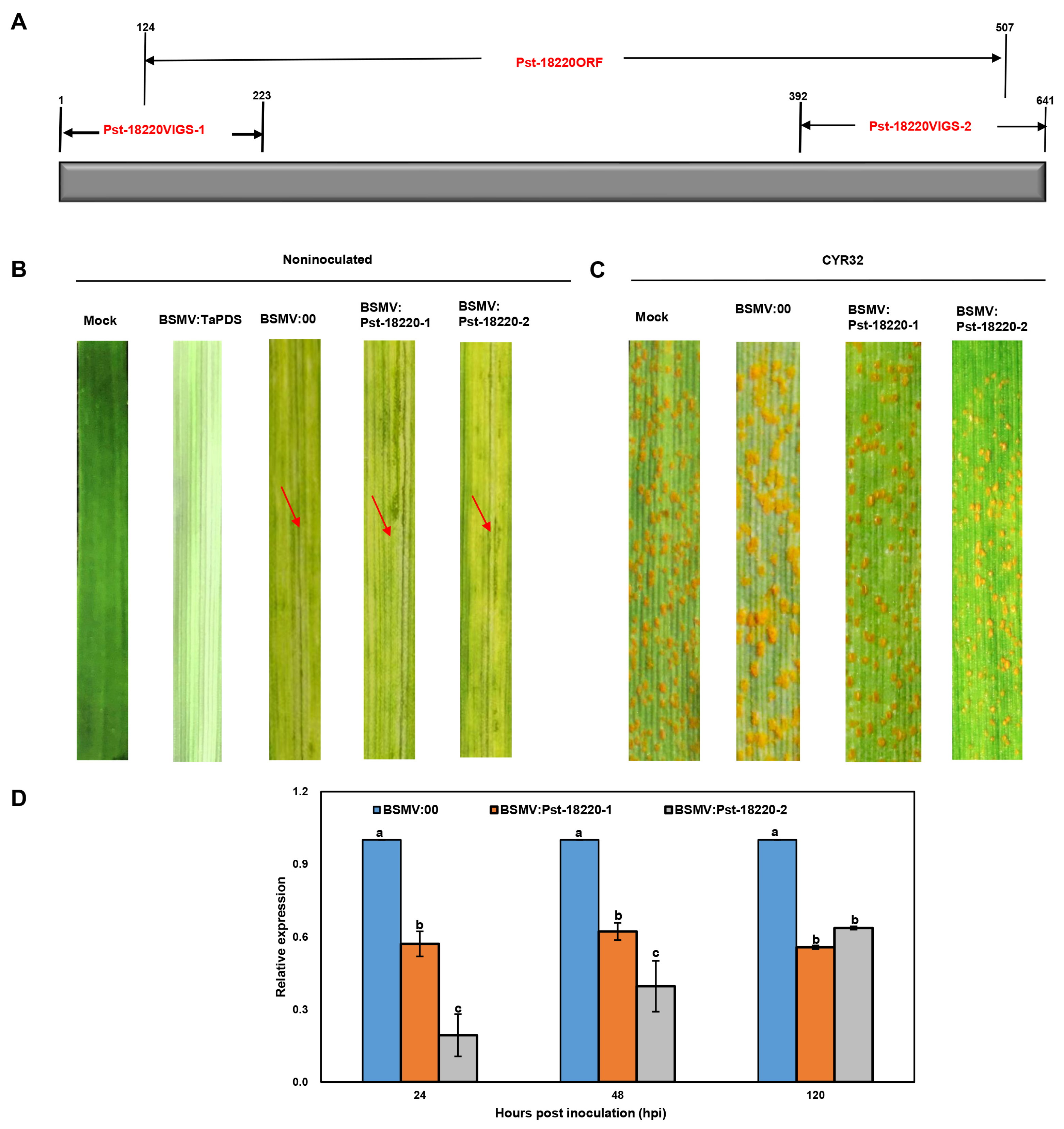
3.3. Knocking Down Pst-18220 Expression Compromised the Pathogenicity of Pst
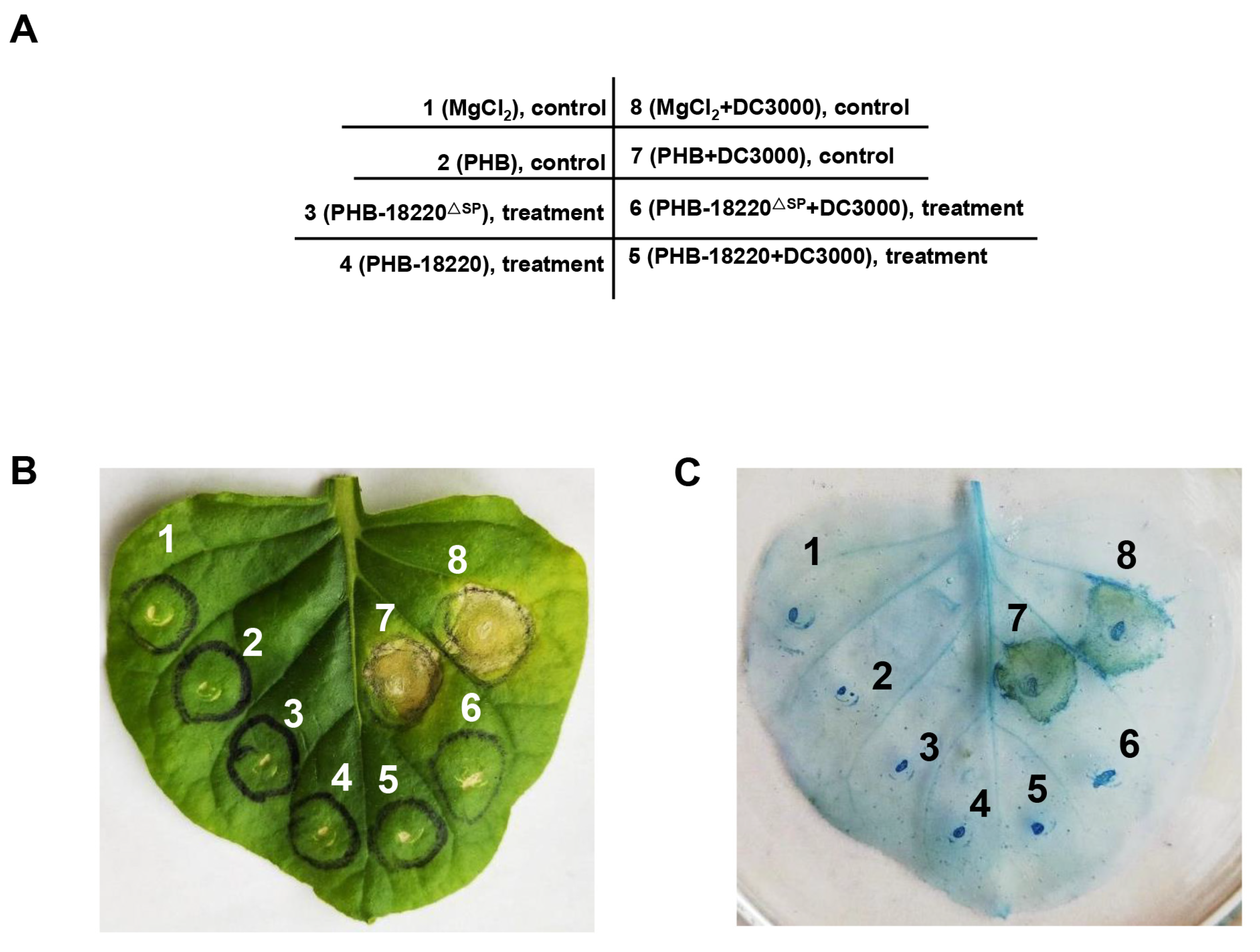
3.4. Pst-18220 Suppressed Leaf Cell Death Mediated by Pseudomonas syringae pv. Tomato (Pto) DC3000 in Nicotiana benthamiana
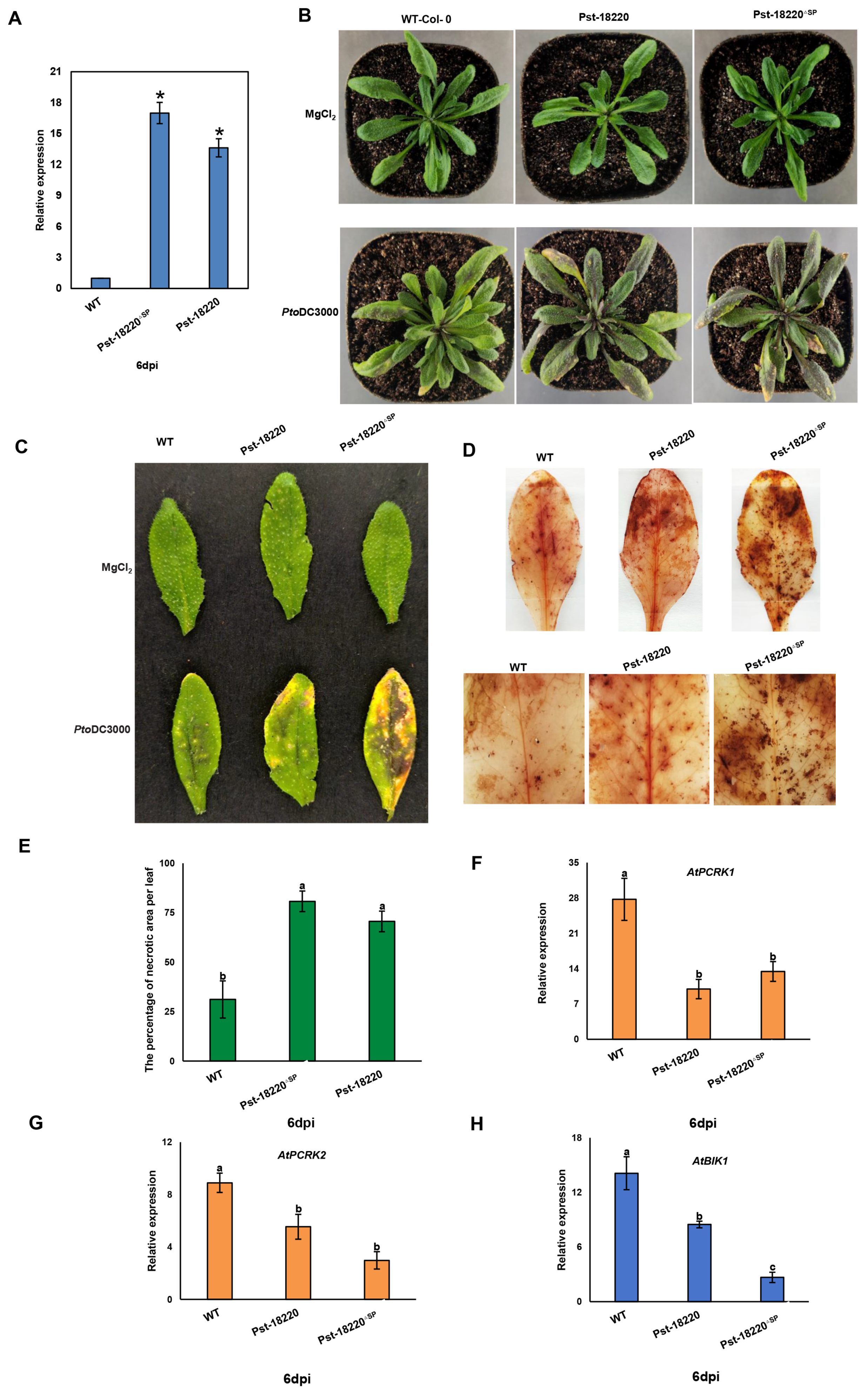
3.5. Pst-18220 Promotes Pto DC3000 Bacteria Growth in Arabidopsis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, W.; Wellings, C.; Chen, X.; Kang, Z.; Liu, T. Wheat stripe (yellow) rust caused by Puccinia striiformis f. sp. tritici. Mol. Plant Pathol. 2014, 15, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, M.; Hammond-Kosack, K.E.; Solomon, P.S. A review of wheat diseases-a field perspective. Mol. Plant Pathol. 2018, 19, 1523–1536. [Google Scholar] [CrossRef]
- Hovmoller, M.S.; Sorensen, C.K.; Walter, S.; Justesen, A.F. Diversity of on cereals and grasses. Annu. Rev. Phytopathol. 2011, 49, 197–217. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.H.; Ngou, B.P.M.; Ding, P.T.; Xin, X.-F. PTI-ETI crosstalk: An integrative view of plant immunity. Curr. Opin. Plant Biol. 2021, 62, 102030. [Google Scholar] [CrossRef] [PubMed]
- Dangl, J.L.; Horvath, D.M.; Staskawicz, B.J. Pivoting the plant immune system from dissection to deployment. Science 2013, 341, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.T.; Tsuda, K.; Parker, J.E. Effector-triggered immunity: From pathogen perception to robust defense. Annu. Rev. Plant Biol. 2015, 66, 487–511. [Google Scholar] [CrossRef] [PubMed]
- Ngou, B.P.M.; Ahn, H.K.; Ding, P.T.; Jones, J.D.G. Mutual potentiation of plant immunity by cell-surface and intracellular receptors. Nature 2021, 592, 110. [Google Scholar] [CrossRef] [PubMed]
- Chi, M.H.; Park, S.Y.; Kim, S.; Lee, Y.H. A novel pathogenicity gene is required in the rice blast fungus to suppress the basal defenses of the host. PLoS Pathog. 2009, 5, e1000401. [Google Scholar] [CrossRef]
- Nishimura, T.; Mochizuki, S.; Ishii-Minami, N.; Fujisawa, Y.; Kawahara, Y.; Yoshida, Y.; Okada, K.; Ando, S.; Matsumura, H.; Terauchi, R.; et al. Magnaporthe oryzae glycine-rich secretion protein, rbf1 critically participates in pathogenicity through the focal formation of the biotrophic interfacial complex. PLoS Pathog. 2016, 12, e1005921. [Google Scholar] [CrossRef]
- Qi, T.; Guo, J.; Liu, P.; He, F.X.; Wan, C.P.; Islam, M.A.; Tyler, B.M.; Kang, Z.S.; Guo, J. Stripe rust effector pstgsre1 disrupts nuclear localization of ros-promoting transcription factor talol2 to defeat ROS-induced defense in wheat. Mol. Plant 2019, 12, 1624–1638. [Google Scholar] [CrossRef]
- Wan, C.P.; Liu, Y.; Tian, S.X.; Guo, J.; Bai, X.X.; Zhu, H.C.; Kang, Z.S.; Guo, J. A serine-rich effector from the stripe rust pathogen targets a raf-like kinase to suppress host immunity. Plant Physiol. 2022, 190, 762–778. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.R.; Yin, C.T.; Kud, J.; Tanaka, K.; Mahoney, A.K.; Xiao, F.M.; Hulbert, S.H. Effectors from wheat rust fungi suppress multiple plant defense responses. Phytopathology 2017, 107, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Dagvadorj, B.; Ozketen, A.C.; Andac, A.; Duggan, C.; Bozkurt, T.O.; Akkaya, M.S. A Puccinia striiformis f. sp tritici secreted protein activates plant immunity at the cell surface. Sci. Rep. 2017, 7, 1141. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Wu, K.; Yao, J.N.; Li, S.M.; Wang, X.J.; Huang, L.L.; Kang, Z.S. Pstha5a23, a candidate effector from the obligate biotrophic pathogen Puccinia striiformis f. sp tritici, is involved in plant defense suppression and rust pathogenicity. Environ. Microbiol. 2017, 19, 1717–1729. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C.; Du, Y.; Cai, K.; Wang, Y.; Guo, J.; Bai, X.; Kang, Z.; Guo, J. A stripe rust fungal effector pstsie1 targets tasgt1 to facilitate pathogen infection. Plant J. 2022, 112, 1413–1428. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.T.; Hulbert, S. Prospects for functional analysis of effectors from cereal rust fungi. Euphytica 2011, 179, 57–67. [Google Scholar] [CrossRef]
- Petre, B.; Saunders, D.G.O.; Sklenar, J.; Lorrain, C.; Krasileva, K.V.; Win, J.; Duplessis, S.; Kamoun, S. Heterologous expression screens in Nicotiana benthamiana identify a candidate effector of the wheat yellow rust pathogen that associates with processing bodies. PLoS ONE 2016, 11, e0149035. [Google Scholar] [CrossRef]
- Schwessinger, B.; Sperschneider, J.; Cuddy, W.S.; Garnica, D.P.; Miller, M.E.; Taylor, J.M.; Dodds, P.N.; Figueroa, M.; Park, R.F.; Rathjen, J.P. A near-complete haplotype-phased genome of the dikaryotic wheat stripe rust fungus Puccinia striiformis f. sp. tritici reveals high interhaplotype diversity. mBio 2018, 9, 10–1128. [Google Scholar] [CrossRef]
- Xia, C.J.; Wang, M.N.; Yin, C.T.; Cornejo, O.E.; Hulbert, S.H.; Chen, X.M. Genomic insights into host adaptation between the wheat stripe rust pathogen (Puccinia striiformis f. sp. tritici) and the barley stripe rust pathogen (Puccinia striiformis f. sp. hordei). BMC Genom. 2018, 19, 664. [Google Scholar] [CrossRef]
- Ozketen, A.C.; Andac-Ozketen, A.; Dagvadorj, B.; Demiralay, B.; Akkaya, M.S. In-depth secretome analysis of Puccinia striiformis f. sp. tritici in infected wheat uncovers effector functions. Biosci. Rep. 2020, 40, BSR20201188. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Holzberg, S.; Brosio, P.; Gross, C.; Pogue, G.P. Barley stripe mosaic virus-induced gene silencing in a monocot plant. Plant J. 2002, 30, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Pogue, G.P.; Lindbo, J.A.; Dawson, W.O.; Turpen, T.H. Tobamovirus transient expression vectors: Tools for plant biology and high-level expression of foreign proteins in plants. In Plant Molecular Biology Manual; Gelvin, S.B., Schilperoort, R.A., Eds.; Springer: Dordrecht, The Netherlands, 1998; pp. 67–93. [Google Scholar]
- Scofield, S.R.; Huang, L.; Brandt, A.S.; Gill, B.S. Development of a virus-induced gene-silencing system for hexaploid wheat and its use in functional analysis of the lr21-mediated leaf rust resistance pathway. Plant Physiol. 2005, 138, 2165–2173. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.F.; Huang, L.L.; Buchenauer, H.; Han, Q.M.; Zhang, H.C.; Kang, Z.S. Histochemical studies on the accumulation of reactive oxygen species (O2− and H2O2) in the incompatible and compatible interaction of wheat—Puccinia striiformis f. sp. tritici. Physiol. Mol. Plant Patho. 2007, 71, 230–239. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [PubMed]
- ThordalChristensen, H.; Zhang, Z.G.; Wei, Y.D.; Collinge, D.B. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 1997, 11, 1187–1194. [Google Scholar] [CrossRef]
- Petre, B.; Saunders, D.G.O.; Sklenar, J.; Lorrain, C.; Win, J.; Duplessis, S.; Kamoun, S. Candidate effector proteins of the rust pathogen-target diverse plant cell compartments. Mol. Plant-Microbe Interact. 2015, 28, 689–700. [Google Scholar] [CrossRef]
- Rocafort, M.; Fudal, I.; Mesarich, C.H. Apoplastic effector proteins of plant-associated fungi and oomycetes. Curr. Opin. Plant Biol. 2020, 56, 9–19. [Google Scholar] [CrossRef]
- Wei, C.F.; Kvitko, B.H.; Shimizu, R.; Crabill, E.; Alfano, J.R.; Lin, N.C.; Martin, G.B.; Huang, H.C.; Collmer, A. A pv. Dc3000 mutant lacking the type iii effector hopq1-1 is able to cause disease in the model plant. Plant J. 2007, 51, 32–46. [Google Scholar] [CrossRef]
- Cunnac, S.; Lindeberg, M.; Collmer, A. Pseudomonas syringae type iii secretion system effectors: Repertoires in search of functions. Curr. Opin. Microbiol. 2009, 12, 53–60. [Google Scholar] [CrossRef]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.P.; Wang, X.D.; Hu, Z.Y.; Wang, X.J.; Wang, J.L.; Wang, J.F.; Huang, X.L.; Kang, Z.S.; Tang, C.L. The Puccinia striiformis effector hasp98 facilitates pathogenicity by blocking the kinase activity of wheat tamapk4. J. Integr. Plant Biol. 2023, 65, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Pedersen, C.; Schultz-Larsen, T.; Aguilar, G.B.; Madriz-Ordenana, K.; Hovmoller, M.S.; Thordal-Christensen, H. The stripe rust fungal effector pec6 suppresses pattern-triggered immunity in a host species-independent manner and interacts with adenosine kinases. New Phytol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.M.; Zhang, Y.L. Plant immunity: Danger perception and signaling. Cell 2020, 181, 978–989. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, C.; Robatzek, S.; Navarro, L.; Oakeley, E.J.; Jones, J.D.G.; Felix, G.; Boller, T. Bacterial disease resistance in through flagellin perception. Nature 2004, 428, 764–767. [Google Scholar] [CrossRef] [PubMed]
- Barna, B.; Fodor, J.; Harrach, B.D.; Pogány, M.; Király, Z. The Janus face of reactive oxygen species in resistance and susceptibility of plants to necrotrophic and biotrophic pathogens. Plant Physiol. Biochem. 2012, 59, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Sreekanta, S.; Bethke, G.; Hatsugai, N.; Tsuda, K.; Thao, A.; Wang, L.; Katagiri, F.; Glazebrook, J. The receptor-like cytoplasmic kinase pcrk1 contributes to pattern-triggered immunity against in Arabidopsis thaliana. New Phytol. 2015, 207, 78–90. [Google Scholar] [CrossRef]
- Thor, K.; Jiang, S.; Michard, E.; George, J.; Scherzer, S.; Huang, S.; Dindas, J.; Derbyshire, P.; Leitão, N.; DeFalco, T.A.; et al. The calcium-permeable channel osca1.3 regulates plant stomatal immunity. Nature 2020, 585, 569–573. [Google Scholar] [CrossRef]
- Yuan, M.H.; Jiang, Z.Y.; Bi, G.Z.; Nomura, K.; Liu, M.H.; Wang, Y.P.; Cai, B.Y.; Zhou, J.M.; He, S.Y.; Xin, X.F. Pattern-recognition receptors are required for NLR-mediated plant immunity. Nature 2021, 592, 105–109. [Google Scholar] [CrossRef]
- Voegele, R.T.; Mendgen, K.W. Nutrient uptake in rust fungi: How sweet is parasitic life? Euphytica 2011, 179, 41–55. [Google Scholar] [CrossRef]
- Bai, X.; Peng, H.; Goher, F.; Islam, M.A.; Xu, S.; Guo, J.; Kang, Z.; Guo, J. A candidate effector protein pstcfem1 contributes to virulence of stripe rust fungus and impairs wheat immunity. Stress Biol. 2022, 2, 21. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.X.; Wang, J.F.; Ji, S.; Chen, Z.J.; Xu, J.H.; Tang, C.L.; Chen, S.T.; Kang, Z.S.; Wang, X.J. Candidate effector pst_8713 impairs the plant immunity and contributes to virulence of Puccinia striiformis f. sp. tritici. Front. Plant Sci. 2018, 9, 1294. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Huai, B.; Lu, Y.; Cai, K.; Guo, J.; Zhu, X.; Kang, Z.; Guo, J. A stripe rust effector Pst18363 targets and stabilises TaNUDX23 that promotes stripe rust disease. New Phytol. 2020, 225, 880–895. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, M.; Zhang, Z.; Bi, X.; Xue, Y.; Zhou, J.; Yuan, B.; Feng, Z.; Li, L.; Wang, J. A Putative Effector Pst-18220, from Puccinia striiformis f. sp. tritici, Participates in Rust Pathogenicity and Plant Defense Suppression. Biomolecules 2024, 14, 1092. https://doi.org/10.3390/biom14091092
Tian M, Zhang Z, Bi X, Xue Y, Zhou J, Yuan B, Feng Z, Li L, Wang J. A Putative Effector Pst-18220, from Puccinia striiformis f. sp. tritici, Participates in Rust Pathogenicity and Plant Defense Suppression. Biomolecules. 2024; 14(9):1092. https://doi.org/10.3390/biom14091092
Chicago/Turabian StyleTian, Mengfan, Zhen Zhang, Xiaorui Bi, Yan Xue, Jiahui Zhou, Bo Yuan, Zhaozhong Feng, Lianwei Li, and Junjuan Wang. 2024. "A Putative Effector Pst-18220, from Puccinia striiformis f. sp. tritici, Participates in Rust Pathogenicity and Plant Defense Suppression" Biomolecules 14, no. 9: 1092. https://doi.org/10.3390/biom14091092




