The Influence of Oxidative Stress Markers in Patients with Ischemic Stroke
Abstract
1. Introduction
2. Pathomechanism of Ischemic Stroke
3. The Role of Mitochondria in Stroke
4. Sources of Reactive Oxygen and Nitrogen Species
4.1. ROS/RNS Production by Mitochondria
4.2. ROS Production by Xanthine Oxidoreductase
4.3. ROS Production by NAD(P)H Oxidase
4.4. ROS/RNS Production by Myeloperoxidase
4.5. ROS Production by Ferroptosis
4.6. ROS Production by Endoplasmic Reticulum
5. Oxidative Potential of the Cell in Stroke
6. Defense Mechanisms in Stroke
6.1. Enzymatic Defense against Oxidative Stress in Stroke
6.1.1. Antioxidant Enzymes
6.1.2. Antioxidant Enzymes in Stroke
6.2. Non-Enzymatic Defense against Oxidative Stress in Stroke
6.2.1. Melatonin
6.2.2. Glutathione
7. Consequences of ROS/RNS Action in Stroke
7.1. Lipid Peroxidation—The Role of MDA in Stroke
7.2. Effects of Nitric Oxide (II)
7.3. Carbonyl Groups as a Result of Protein Damage in Stroke
8. Antioxidant Therapies in Ischemic Stroke
8.1. Stroke Treatment
8.2. Strategies to Protect the Brain against Oxidative Stress
8.2.1. Antioxidants in Stroke
8.2.2. ROS/RNS Inhibitors in Stroke
8.2.3. Other Antioxidant Strategies in Stroke
8.2.4. Potential Antioxidant Drugs in Stroke
8.3. Stroke Prevention
8.4. Future Prospects in Stroke Treatment
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jiang, Y.; Xiong, Y.; Chi, Y.; Lin, F.; Zhao, Q.; Li, Y. Healthcare-seeking delays in Acute Ischemic Stroke patients: The influence of gender, immigrant status, and educational background. Risk Manag. Health Policy 2024, 17, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.E.; Kim, G.S.; Chen, H.; Maier, C.M.; Narasimhan, P.; Song, Y.S.; Niizuma, K.; Katsu, M.; Okami, N.; Yoshioka, H.; et al. Reperfusion and neurovascular dysfunction in stroke: From basic mechanisms to potential strategies for neuroprotection. Mol. Neurobiol. 2010, 41, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Li, B.-H.; Ding, X.; Yin, Y.-W.; Liu, Y.; Gao, C.-Y.; Zhang, L.-L.; Li, J.-C. Meta-analysis of clinical outcomes of intravenous recombinant tissue plasminogen activator for acute ischemic stroke: Within 3 h versus 3–4.5 h. Curr. Med. Res. Opin. 2013, 29, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Menon, B.K.; van Zwam, W.H.; Dippel, D.W.J.; Mitchell, P.J.; Demchuk, A.M.; Dávalos, A.; Majoie, C.B.; van der Lugt, A.; de Miquel, M.A.; et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 2016, 387, 1723–1731. [Google Scholar] [CrossRef]
- Pawluk, H.; Kołodziejska, R.; Grześk, G.; Woźniak, A.; Kozakiewicz, M.; Kosińska, A.; Pawluk, M.; Grzechowiak, E.; Wojtasik, J.; Kozera, G. Increased oxidative stress markers in acute ischemic stroke patients treated with thrombolytics. J. Mol. Sci. 2022, 23, 15625. [Google Scholar] [CrossRef]
- Rodrigo, R.; Fernández-Gajardo, R.; Gutiérrez, R.; Matamala, J.M.; Carrasco, R.; Miranda-Merchak, A.; Feuerhake, W. Oxidative stress and pathophysiology of ischemic stroke: Novel therapeutic opportunities. CNS Neurol. Disord.-Drug Targets 2013, 12, 698–714. [Google Scholar] [CrossRef]
- Pawluk, H.; Kołodziejska, R.; Grześk, G.; Woźniak, A.; Kozakiewicz, M.; Kosińska, A.; Pawluk, M.; Grześk-Kaczyńska, M.; Grzechowiak, E.; Wojtasik, J.; et al. The potential role of RANTES in post-stroke therapy. Cells 2023, 12, 2217. [Google Scholar] [CrossRef]
- Pawluk, H.; Woźniak, A.; Grześk, G.; Kołodziejska, R.; Kozakiewicz, M.; Kopkowska, E.; Grzechowiak, E.; Kozera, G. The role of selected pro-inflammatory cytokines in pathogenesis of ischemic stroke. Clin. Interv. Aging 2020, 15, 469–484. [Google Scholar] [CrossRef]
- Lakhan, S.E.; Kirchgessner, A.; Hofer, M. Inflammatory mechanisms in ischemic stroke: Therapeutic approaches. J. Transl. Med. 2009, 7, 97–107. [Google Scholar] [CrossRef]
- Zhang, Q.G.; Xu, Y.L.; Li, H.C.; Han, D.; Zhang, G.-Y. NMDA receptor/LVGCC-dependent expression and AMPA/KA receptor-dependent activation of c-Jun induced by cerebral ischemia in rat hippocampus. Neurosci. Lett. 2006, 398, 268–273. [Google Scholar] [CrossRef]
- Li, X.M.; Yang, J.M.; Hu, D.H.; Hou, F.-Q.; Zhao, M.; Zhu, X.-H.; Wang, Y.; Li, J.-G.; Hu, P.; Chen, L.; et al. Contribution of downregulation of L-type calcium currents to delayed neuronal death in rat hippocampus after global cerebral ischemia and reperfusion. J. Neurosci. 2007, 27, 5249–5259. [Google Scholar] [CrossRef] [PubMed]
- Kahlert, S.; Zündorf, G.; Reiser, G. Glutamate-mediated influx of extracellular Ca2+ is coupled with reactive oxygen species generation in cultured hippocampal neurons but not in astrocytes. J. Neurosci. Res. 2005, 79, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Simats, A.; García-Berrocoso, T.; Montaner, J. Neuroinflammatory biomarkers: From stroke diagnosis and prognosis to therapy. BBA Mol. Basis Dis. 2016, 1862, 411–424. [Google Scholar] [CrossRef]
- Ramiro, L.; Simats, A.; Berrocoso, T.G.; Montaner, J. Inflammatory molecules might become both biomarkers and therapeutic targets for stroke management. Ther. Adv. Neurol. Diso. 2018, 11, 1756286418789340. [Google Scholar] [CrossRef]
- Lucas, S.M.; Rothwell, N.J.; Gibson, R.M. The role of inflammation in CNS injury and disease. Br. J. Pharmacol. 2006, 147, 232–240. [Google Scholar] [CrossRef]
- Vidale, S.; Consolib, A.; Arnaboldia, M.; Consoli, D. Postischemic Inflammation in Acute Stroke. J. Clin. Neurol. 2017, 13, 1–9. [Google Scholar] [CrossRef]
- Bustamante, A.; Simats, A.; Vilar-Bergua, A.; García-Berrocoso, T.; Montaner, J. Blood/brain biomarkers of inflammation after stroke and their association with outcome: From C-reactive protein to damage-associated molecular patterns. Neurotherapeutics 2016, 13, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C.; Anrather, J. The immunology of stroke: From mechanisms to translation. Nat. Med. 2011, 17, 796–808. [Google Scholar] [CrossRef]
- Ozaki, E.; Campbell, M.; Doyle, S.L. Targeting the NLRP3 inflammasome in chronic inflammatory diseases: Current perspectives. J. Inflamm. Res. 2015, 8, 15–27. [Google Scholar] [CrossRef]
- De Nigris, F.; Lerman, A.; Ignarro, L.J.; Williams-Ignarro, S.; Sica, V.; Baker, A.H.; Lilach, O.L.; Geng, Y.J.; Napoli, C. Oxidation-sensitive mechanisms, vascular apoptosis and atherosclerosis. Trends Mol. Med. 2003, 9, 351–359. [Google Scholar] [CrossRef]
- Moon, G.J.; Shin, D.H.; Im, D.S.; Bang, O.Y.; Nam, H.S.; Lee, J.H.; Joo, I.S.; Huh, K.; Gwag, B.J. Identification of oxidized serum albumin in the cerebrospinal fluid of ischaemic stroke patients. Eur. J. Neurol. 2011, 18, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Lu, J.; Manaenko, A.; Tang, J.; Hu, Q. Mitochondria in ischemic stroke: New in-sight and implications. Aging Dis. 2018, 9, 924–937. [Google Scholar] [CrossRef]
- Sims, N.R.; Muyderma, H. Mitochondria, oxidative metabolism and cell death in stroke. Biochim. Biophys. Acta 2010, 1802, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Szabadkai, G.; Duchen, M.R. Mitochondria: The hub of cellular Ca2+ signaling. Physiology 2008, 23, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R.; Galluzzi, L.; Kroemer, G. Cell biology. Metabolic control of cell death. Science 2014, 345, 1250256. [Google Scholar] [CrossRef]
- Kislin, M.; Sword, J.; Fomitcheva, I.V.; Croom, D.; Pryazhnikov, E.; Lihavainen, E.; Toptunov, D.; Rauvala, H.; Ribeiro, A.S.; Khiroug, L.; et al. Reversible disruption of neuronal mitochondria by ischemic and traumatic injury revealed by quantitative two-photon imaging in the neocortex of anesthetized mice. J. Neurosci. 2017, 37, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, K.; Esposito, E.; Wang, X.; Terasaki, Y.; Liu, Y.; Xing, C.; Xunming Ji, X.; Eng, H.; Lo, E.H. Transfer of mitochondria from astrocytes to neurons after stroke. Nature 2016, 535, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Melentijevic, I.; Toth, M.L.; Arnold, M.L.; Guasp, R.J.; Harinath, G.; Nguyen, K.C.; Taub, D.; Parker, J.P.; Neri, C.; Gabel, C.V.; et al. Elegans neurons jettison protein aggregates and mitochondria under neurotoxic stress. Nature 2017, 542, 367–371. [Google Scholar] [CrossRef]
- Jiang, D.; Gao, F.; Zhang, Y.; Wong, D.S.; Li, Q.; Tse, H.F.; Xu, G.; Yu, Z.; Lian, Q. Mitochondrial transfer of mesenchymal stem cells effectively protects corneal epithelial cells from mitochondrial damage. Cell Death Dis. 2016, 7, e2467. [Google Scholar] [CrossRef]
- Brieger, K.; Schiavone, S.; Miller, F.J., Jr.; Krause, K.-H. Reactive oxygen species: From health to disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 1984, 219, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wierońska, J.M.; Cieślik, P.; Kalinowski, L. Nitric Oxide-dependent pathways as critical factors in the consequences and recovery after brain ischemic hypoxia. Biomolecules 2021, 11, 1097. [Google Scholar] [CrossRef]
- Hernansanz-Agustín, P.; Antonio Enríquez, J.A. Generation of reactive oxygen species by mitochondria. Antioxidants 2021, 10, 415. [Google Scholar] [CrossRef] [PubMed]
- Hernansanz-Agustin, P.; Ramos, E.; Navarro, E.; Parada, E.; Sanchez-Lopez, N.; Pelaez-Aguado, L.; Cabrera-Garcia, J.D.; Tello, D.; Buendia, I.; Marina, A.; et al. Mitochondrial complex I deactivation is related to superoxide production in acute hypoxia. Redox Biol. 2017, 12, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Drose, S.; Stepanova, A.; Galkin, A. Ischemic A/D transition of mitochondrial complex I and its role in ROS generation. Biochim. Biophys. Acta 2016, 1857, 946–957. [Google Scholar] [CrossRef]
- Hernansanz-Agustin, P.; Choya-Foces, C.; Carregal-Romero, S.; Ramos, E.; Oliva, T.; Villa-Pina, T.; Moreno, L.; Izquierdo-Alvarez, A.; Cabrera-Garcia, J.D.; Cortes, A.; et al. Na (+) controls hypoxic signalling by the mitochondrial respiratory chain. Nature 2020, 586, 287–291. [Google Scholar] [CrossRef]
- Jezek, P.; Hlavata, L. Mitochondria in homeostasis of reactive oxygen species in cell, tissues, and organism. Int. J. Biochem. Cell Biol. 2005, 37, 2478–2503. [Google Scholar] [CrossRef]
- Harrison, R. Structure and function of xanthine oxidoreductase: Where are we now? Free Radic. Biol. Med. 2002, 33, 774–797. [Google Scholar] [CrossRef]
- Battelli, M.G.; Polito, L.; Bortolotti, M.; Bolognesi, A. Xanthine oxidoreductase-derived reactive species: Physiological and pathological effects. Oxid. Med. Cell. Longev. 2016, 548, 3527579. [Google Scholar] [CrossRef]
- Casas, A.I.; Geuss, E.; Kleikers, P.W.M.; Mencl, S.; Herrmann, A.M.; Buendia, I.; Egea, J.; Meuth, S.G.; Lopez, M.G.; Kleinschnitz, C.; et al. NOX4-dependent neuronal autotoxicity and BBB breakdown explain the superior sensitivity of the brain to ischemic damage. Proc. Natl. Acad. Sci. USA 2017, 114, 12315–12320. [Google Scholar] [CrossRef]
- Kahles, T.; Luedike, P.; Endres, M.; Galla, H.-J.; Steinmetz, H.; Busse, R.; Neumann-Haefelin, T.; Brandes, R.P. NADPH oxidase plays a central role in blood-brain barrier dasmage in experimental stroke. Stroke 2007, 38, 3000–3006. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Infanger, D.W.; Sharma, R.V.; Davisson, R.L. NADPH oxidases of the brain: Distribution, regulation, and function. Antioxid. Redox Signal. 2006, 8, 1583–1596. [Google Scholar] [CrossRef]
- Tang, X.N.; Cairns, B.; Kim, J.Y.; Yenari, M.A. NADPH oxidase in stroke and cerebrovascular disease. Neurol. Res. 2012, 34, 338–345. [Google Scholar] [CrossRef]
- Miller, A.A.; Drummond, G.R.; Mast, A.E.; Schmidt, H.H.; Sobey, C.G. Effect of gender on NADPH-oxidase activity, expression, and function in the cerebral circulation: Role of estrogen. Stroke 2007, 38, 2142–2149. [Google Scholar] [CrossRef]
- Duan, J.; Gao, S.; Tu, S.; Lenahan, C.; Anwen Shao, A.; Sheng, J. Pathophysiology and therapeutic potential of NADPH oxidases in ischemic stroke-induced oxidative stress. Oxid. Med. Cell. Longev. 2021, 2021, 6631805. [Google Scholar] [CrossRef]
- Yang, S.; Li, W. Targeting oxidative stress for the treatment of ischemic stroke: Upstream and downstream therapeutic strategies. Brain Circ. 2016, 2, 153–163. [Google Scholar] [CrossRef]
- Diebold, B.A.; Fowler, B.; Lu, J.; Dinauer, M.C.; Bokoch, G.M. Antagonistic cross-talk between Rac and Cdc42 GTPases regulates generation of reactive oxygen species. J. Biol. Chem. 2004, 279, 28136–28142. [Google Scholar] [CrossRef]
- Furtmuller, P.G.; Burner, U.; Jantschko, W.; Regelsberger, G.; Obinger, C. Two-electron reduction and one-electron oxidation of organic hydroperoxides by human myeloperoxidase. FEBS Lett. 2000, 484, 139–143. [Google Scholar] [CrossRef]
- Ullen, A.; Singewald, E.; Konya, V.; Fauler, G.; Reicher, H.; Nusshold, C.; Hammer, A.; Kratky, D.; Heinemann, A.; Holzer, P.; et al. Myeloperoxidase-derived oxidants induce blood-brain barrier dysfunction In Vitro and In Vivo. PLoS ONE 2013, 8, e64034. [Google Scholar] [CrossRef]
- Xu, J.; Xie, Z.; Reece, R.; Pimental, D.; Zou, M.H. Uncoupling of endothelial nitric oxidase synthase by hypochlorous acid: Role of NAD(P)H oxidase-derived superoxide and peroxynitrite. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2688–2695. [Google Scholar] [CrossRef]
- Siraki, A.G. The many roles of myeloperoxidase: From inflammation and immunity to biomarkers, drug metabolism and drug discovery. Redox Biol. 2021, 46, 102109. [Google Scholar] [CrossRef]
- Tan, Q.; Fang, Y.; Gu, Q. Mechanisms of modulation of ferroptosis and its role in central nervous system diseases. Front. Pharmacol. 2021, 12, 657033. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A regulated cell death nexus linking metabolism, redox biology, and disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef]
- Agmon, E.; Solon, J.; Bassereau, P.; Stockwell, B.R. Modeling the effects of lipid peroxidation during ferroptosis on membrane properties. Sci. Rep. 2018, 8, 5155. [Google Scholar] [CrossRef]
- Sun, Y.; Xia, X.; Basnet, D.; Zheng, J.C.; Huang, J.; Liu, J. Mechanisms of ferroptosis and emerging links to the pathology of neurodegenerative diseases. Front. Aging Neurosci. 2022, 14, 904152. [Google Scholar] [CrossRef]
- Hassannia, B.; Vandenabeele, P.; Berghe, T.V. Targeting ferroptosis to iron out cancer. Cancer Cell. 2019, 35, 830–849. [Google Scholar] [CrossRef]
- Yang, K.; Zeng, L.; Yuan, X.; Wang, S.; Ge, A.; Xu, H.; Zeng, J.; Ge, J. The mechanism of ferroptosis regulating oxidative stress in ischemic stroke and the regulation mechanism of natural pharmacological active components. Biomed. Pharmacother. 2022, 154, 113611. [Google Scholar] [CrossRef]
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS generation and antioxidant defense systems in normal and malignant cells. Oxid. Med. Cell Longev. 2019, 2019, 6175804. [Google Scholar] [CrossRef]
- Schroder, M.; Kaufman, R.J. ER stress and the unfolded protein response. Mutat. Res. 2005, 569, 29–63. [Google Scholar] [CrossRef]
- Scriven, P.; Brown, N.J.; Pockley, A.G.; Wyld, L. The unfolded protein response and cancer: A brighter future unfolding? J. Mol. Med. 2007, 85, 331–341. [Google Scholar] [CrossRef]
- Santos, C.X.C.; Tanaka, L.Y.; Wosniak, J., Jr.; Laurindo, F.R.M. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: Roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxid. Redox Signal. 2009, 11, 2409–2427. [Google Scholar] [CrossRef]
- Zeeshan, H.M.A.; Lee, G.H.; Kim, H.-R.; Chae, H.-J. Endoplasmic reticulum stress and associated ROS. Int. J. Mol. Sci. 2016, 17, 327. [Google Scholar] [CrossRef]
- Androwiki, A.C.; Camargo, L.; Sartoretto, S.; Couto, G.K.; Ribeiro, I.M.R.; Veríssimo-Filho, S.; Rossoni, L.V.; Lopes, L.R. Protein disulfide isomerase expression increases in resistance arteries during hypertension development. Effects on Nox1 NADPH oxidase signaling. Front. Chem. 2015, 3, 24. [Google Scholar] [CrossRef]
- Janiszewski, M.; Lopes, L.R.; Carmo, A.O. Regulation of NAD(P)H oxidase by associated protein disulfide isomerase in vascular smooth muscle cells. J. Biol. Chem. 2005, 280, 40813–40819. [Google Scholar] [CrossRef]
- Davydov, D.R. Microsomal monooxygenase as a multienzyme system: The role of P450-P450 interactions. Expert Opin. Drug Metab. Toxicol. 2011, 7, 543–558. [Google Scholar] [CrossRef]
- Rashba-Step, J.; Cederbaum, A.I. Generation of reactive oxygen intermediates by human liver microsomes in the presence of NADPH or NADH. Mol. Pharmacol. 1994, 45, 150–157. [Google Scholar]
- Bondy, S.C.; Naderi, S. Contribution of hepatic cytochrome P450 systems to the generation of reactive oxygen species. Biochem. Pharmacol. 1994, 48, 155–159. [Google Scholar] [CrossRef]
- Gerasimenko, J.V.; Gerasimenko, O.V.; Palejwala, A.; Tepikin, A.V.; Petersen, O.H.; Watson, A.J. Menadione-induced apoptosis: Roles of cytosolic Ca2+ elevations and the mitochondrial permeability transition pore. J. Cell Sci. 2002, 115, 485–497. [Google Scholar] [CrossRef]
- Li, G.; Mongillo, M.; Chin, K.T.; Harding, H.; Ron, D.; Marks, A.R.; Tabas, I. Role of ERO1-α-mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress-induced apoptosis. J. Cell Biol. 2009, 186, 783–792. [Google Scholar] [CrossRef]
- Dekkers, J.C.; van Doornen, L.J.; Kemper, H.C. The role of antioxidant vitamins and enzymes in the prevention of exercise-induced muscle damage. Sports Med. 1996, 21, 213–238. [Google Scholar] [CrossRef]
- Shirley, R.; Ord, E.N.J.; Work, L.M. Oxidative stress and the use of antioxidants in stroke. Antioxidants 2014, 3, 472–501. [Google Scholar] [CrossRef]
- Cherubini, A.; Polidori, M.C.; Bregnocchi, M.; Pezzuto, S.; Cecchetti, R.; Ingegni, T.; di Iorio, A.; Senin, U.; Mecocci, P. Antioxidant profile and early outcome in stroke patients. Stroke 2000, 31, 2295–2300. [Google Scholar] [CrossRef]
- Spranger, M.; Krempien, S.; Schwab, S.; Donneberg, S.; Hacke, W. Superoxide dismutase activity in serum of patients with acute cerebral schemic injury: Correlation with clinical course and infarct size. Stroke 1997, 28, 2425–2428. [Google Scholar] [CrossRef]
- Cojocaru, I.M.; Cojocaru, M.; Sapira, V.; Ionescu, A. Evaluation of oxidative stress in patients with acute ischemic stroke. Rom. J. Intern. Med. 2013, 51, 97–106. [Google Scholar]
- Ciancarelli, I.; De Amicis, D.; Di Massimo, C.; Carolei, A.; Giuliana, M.; Ciancarelli, T. Oxidative stress in post-acute ischemic stroke patients after intensive neurorehabilitation. Curr. Neurovasc. Res. 2012, 9, 266–273. [Google Scholar] [CrossRef]
- Lagowska-Lenard, M.; Stelmasiak, Z.; Bartosik-Psujek, H. Influence of vitamin C on markers of oxidative stress in the earliest period of ischemic stroke. Pharmacol. Rep. 2010, 62, 751–776. [Google Scholar] [CrossRef]
- Yang, T.H.; Chang, C.Y.; Hu, M.L. Various forms of homocysteine and oxidative status in the plasma of ischemic stroke patients as compared to healthy controls. Clin. Biochem. 2004, 37, 494–499. [Google Scholar] [CrossRef]
- Ghiselli, A.; Serafini, M.; Natella, F.; Scaccini, C. Total antioxidant capacity as a tool to assess redox status: Critical view and experimental data. Free Radic. Biol. Med. 2000, 29, 1106–1114. [Google Scholar] [CrossRef]
- Silvestrini, A.; Meucci, E.; Ricerca, B.M.; Mancini, A. Total antioxidant capacity: Biochemical aspects and clinical significance. Int. J. Mol. Sci. 2023, 24, 10978. [Google Scholar] [CrossRef]
- Nasiru, S.; Bulama, I.; Abdurrahman, J.H.; Abubakar, N.A.; Salisu, A.B.; Salisu, B.; Abbas, A.Y.; Yusuf, S.; Suleman, B.L. Neurobiochemical roles of low molecular weight antioxidants on oxidative stress biomarkers and severity of ischemic stroke in Wistar rats. J. Neurol. Neurol. Dis. 2018, 4, 101–111. [Google Scholar] [CrossRef]
- Bouayed, J.; Bohn, T. Exogenous antioxidants—Double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid. Med. Cell. Longev. 2010, 3, 228–237. [Google Scholar] [CrossRef]
- Lorente, L.; Martín, M.M.; Pérez-Cejas, A.; Abreu-González, P.; Ramos, L.; Argueso, M.; Cáceres, J.J.; Sol é-Violán, J.; Jiménez, A. Association between total antioxidant capacity and mortality in ischemic stroke patients. Ann. Intensive Care 2016, 6, 39. [Google Scholar] [CrossRef]
- Wang, J.; Gao, S.; Lenahan, C.; Gu, Y.; Wang, X.; Fang, Y.; Xu, W.; Wu, H.; Pan, Y.; Shao, A.; et al. Melatonin as an antioxidant agent in stroke: An updated review. Aging Dis. 2022, 13, 1823–1844. [Google Scholar] [CrossRef]
- Pinoşanu, E.A.; Surugiu, R.; Burada, E.; Pȋrşcoveanu, D.; Stǎnciulescu, C.E.; Sandu, R.E.; Pisoschi, C.; Albu, C.V. Oxidative stress and antioxidant defense mechanisms in acute ischemic stroke patients with concurrent COVID-19 infection. Int. J. Mol. Sci. 2023, 24, 16790. [Google Scholar] [CrossRef]
- Kamal, F.Z.; Lefter, R.; Jaber, H.; Balmus, I.-M.; Ciobica, A.; Iordache, A.-C. The role of potential oxidative biomarkers in the prognosis of acute ischemic stroke and the exploration of antioxidants as possible preventive and treatment options. Int. J. Mol. Sci. 2023, 24, 6389. [Google Scholar] [CrossRef]
- Menon, B.; Ramalingam, K.; Kumar, R. Evaluating the role of oxidative stress in acute ischemic stroke. J. Neurosci. Rural. Pract. 2020, 11, 156–159. [Google Scholar] [CrossRef]
- Sahika, G.G.; Tahir, M.G.; Ziya, A.K. Total oxidative stress, total antioxidant status and erythrocytes status in patients with acute ischemic stroke. Acta Med. Mediterr. 2017, 33, 157–163. [Google Scholar] [CrossRef]
- Shaafi, S.; Hadisi, F.; Mahmoudinezhad, M.; Razmi, H.; Nejadghaderi, S.A.; Khalili, M. The significance of the oxidative stress markers in the one-year prognosis of patients with acute ischemic stroke: A case-control study. BMC Neurol. 2021, 21, 258. [Google Scholar] [CrossRef]
- Gariballa, S.E.; Hutchin, T.P.; Sinclair, A.J. Antioxidant capacity after acute ischaemic stroke. Q. J. Med. 2002, 95, 685–690. [Google Scholar] [CrossRef]
- Srikrishna, R.; Suresh, D.R. Biochemical study of antioxidant profile in acute ischemic stroke. BJMP 2009, 2, 35–37. [Google Scholar]
- Ghonimi, N.A.M.; Mahdy, M.A.; Salam, O.A.A. Total antioxidant capacity predicts outcome in acute ischemic stroke subtypes in Egyptian patients. J. Stroke Cerebrovas. Dis. 2019, 28, 1911–1917. [Google Scholar] [CrossRef]
- Guldiken, B.; Demir, M.; Guldiken, S.; Turgut, N.; Turgut, B.; Tugrul, A. Oxidative stress and total antioxidant capacity in diabetic and nondiabetic acute ischemic stroke patients. Clin. Appl. Thromb. Hemost. 2009, 15, 695–700. [Google Scholar] [CrossRef]
- Gerreth, P.; Maciejczyk, M.; Zalewska, A.; Gerreth, K.; Hojan, K. Comprehensive evaluation of the oral health status, salivary gland function, and oxidative stress in the saliva of patients with subacute phase of stroke: A case-control study. J. Clin. Med. 2020, 9, 2252. [Google Scholar] [CrossRef]
- Altamura, C.; Squitti, R.; Pasqualetti, P.; Gaudino, C.; Palazzo, P.; Tibuzzi, F.; Lupoi, D.; Cortesi, M.; Rossini, P.M.; Vernieri, F. Ceruloplasmin/transferrin system is related to clinical status in acute stroke. Stroke 2009, 40, 1282–1288. [Google Scholar] [CrossRef]
- Orellana-Urzúa, S.; Briones-Valdivieso, C.; Chichiarelli, S.; Saso, L.; Rodrigo, R. Potential role of natural antioxidants in countering reperfusion Injury in Acute Myocardial Infarction and Ischemic Stroke. Antioxidants 2023, 12, 1760. [Google Scholar] [CrossRef]
- Perrya, J.J.P.; Shina, D.S.; Getzoffa, E.D.; Tainer, J.A. The structural biochemistry of the superoxide dismutases. Biochim. Biophys. Acta 2010, 1804, 245–262. [Google Scholar] [CrossRef]
- Marwicka, J.; Zięba, A. Antioxidans as a defence against reactive oxygen species. Aesth. Cosmetol. Med. 2021, 10, 271–276. [Google Scholar] [CrossRef]
- Younus, H. Therapeutic potentials of superoxide dismutase. Int. J. Health Sci. 2018, 12, 88–93. [Google Scholar]
- Fukai, T.; Ushio-Fuka, M. Superoxide Dismutases: Role in redox signaling, vascular function, and diseases. Antioxid. Redox Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef]
- Jopkiewicz, S. Oxidative stress Part II. Prevention of free radical damage. Environ. Med. 2018, 21, 53–59. [Google Scholar] [CrossRef]
- Nandi, A.; Yan, L.-J.; Kumar Jana, C.; Das, N. Role of catalase in oxidative stress- and age-associated degenerative. Diseases Oxid. Med. Cell Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef]
- Vašková, J.; Kočan, L.; Vaško, L.; Perjési, P. Glutathione-related enzymes and proteins: A review. Molecules 2023, 28, 1447. [Google Scholar] [CrossRef]
- Higashi, Y.; Aratake, T.; Shimizu, T.; Shimizu, S.; Saito, M. Protective role of glutathione in the hippocampus after Brain Ischemia. Int. J. Mol. Sci. 2021, 22, 7765. [Google Scholar] [CrossRef]
- Deponte, M. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim. Biophys. Acta 2013, 1830, 3217–3266. [Google Scholar] [CrossRef]
- Flohé, L.; Toppo, S.; Cozza, G.; Ursini, F. A comparison of thiol peroxidase mechanisms. Antioxid. Redox Signal. 2011, 15, 763–780. [Google Scholar] [CrossRef]
- Abdullah, A.; Ssefer, V.; Ertugrul, U.; Osman, E.; Esref, A.; Ugur, C.M.; Adalet, A.; Yavuz, Y.; Faysal, E.; Nebahat, T. Evaluation of serum oxidant/antioxidant balance in patients with acute stroke. J. Pak. Med. Assoc. 2013, 63, 590–593. [Google Scholar]
- Žitňanová, I.; Siarnik, P.; Kollára, B.; Chomova, M.; Pazderova, P.; Andrezálová, Ł.; Ježovičová, M.; Koňariková, K.; Laubertowa, Ł.; Krivošíková, Z.; et al. Oxidative stress markers and their dynamic changes in patients after Acute Ischemic Stroke. Oxid. Med. Cell. Longev. 2016, 2016, 9761697. [Google Scholar] [CrossRef]
- Lee, J.; Jang, J.; Park, S.-M.; Yang, S.-R. An update on the role of Nrf2 in respiratory disease: Molecular mechanisms and therapeutic approaches. Int. J. Mol. Sci. 2021, 22, 8406. [Google Scholar] [CrossRef]
- Ljubisavljevic, S.; Cvetkovic, T.; Zvezdanovic, L.; Stojanovic, S.; Stojanovic, I.; Kocic, G.; Zivkovic, M.; Paunovic, S.; Milenkovic, L.; Lukic, D.; et al. The Differences in the cellular and plasma antoixidative capacity between trasient and definet focal brain ischemia: Does it suggest supporting time-dependent neuroprotection therapy? Cell Mol. Neurobiol. 2016, 36, 789–800. [Google Scholar] [CrossRef]
- Zimmermann, C.; Winnefeld, K.; Streck, S.; Roskos, M.; Haberl, R. Antioxidant status in Acute Stroke patients and patients at stroke risk. Eur. Neurol. 2004, 51, 157–161. [Google Scholar] [CrossRef]
- Kolesnichenko, L.S.; Kulinski, V.I.; Shprakh, V.V.; Bardymov, V.V.; Verlan, N.V.; Gubina, L.P.; Pensionerova, G.A.; Sergeeva, M.P.; Stanevich, L.M.; Filippova, G.T. Glutathione system in erythrocytes and blood plasma in strokes and dyscirculatory encephalopathy. Biomed. Khim. 2007, 53, 454–460. [Google Scholar] [CrossRef]
- Demirkaya, S.; Topcuoglu, M.A.; Aydin, A.; Ulas, U.H.; Isimer, A.I.; Vural, O. Malondialdehyde, glutathione proxidase and superoxide dismutase in peripheral blood erythrocytes of patients with acute cerebral ischemia. Eur. J. Neurol. 2001, 8, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Li, W.; Ding, X.; Zhou, F.; Hao, C.; He, M.; Wang, F.; Li, X. The role of SIRT1-BMAL1 pathway in regulating oxidative stress in the early development of isheamc stroke. Sci. Rep. 2024, 14, 1773. [Google Scholar] [CrossRef]
- Zhang, M.-S.; Liang, J.-H.; Yang, M.-J.; Ren, Y.-R.; Cheng, D.-H.; Wu, Q.-H.; He, Y.; Yin, J. Low serum superoxide dismutase is associated with a high risk of cognitive impairment after mild Acute Ischemic Stroke. Front. Aging Neurosci. 2022, 14, 834114. [Google Scholar] [CrossRef]
- Rai, S.; Roy, G.; Hajam, Y.A. Melatonin: A modulator in metabolic rewiring in T-cell malignancies. Front. Oncol. 2024, 13, 1248339. [Google Scholar] [CrossRef]
- Rancan, L.; Paredes, S.D.; García, C.; González, P.; Rodríguez-Bobada, C.; Calvo-Soto, M.; Hyacinthe, B.; Vara, E.; Tresguerres, J.A.F. Comparison of the effect of Melatonin treatment before and after brain ischemic injury in the inflammatory and apoptotic response in aged rats. Int. J. Mol. Sci. 2018, 19, 2097. [Google Scholar] [CrossRef]
- De Simone, M.; De Feo, R.; Choucha, A.; Ciaglia, E.; Fezeu, F. Enhancing sleep quality: Assessing the efficacy of a fixed combination of Linden, Hawthorn, Vitamin B1, and Melatonin. Med. Sci. 2023, 12, 2. [Google Scholar] [CrossRef] [PubMed]
- Kamat, P.K.; Khan, M.B.; Smith, C.; Siddiqui, S.; Baban, B.; Dhandapani, K.; Hess, D.C. The time dimension to stroke: Circadian effects on stroke outcomes and mechanisms. Neurochem. Int. 2023, 162, 105457. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Wang, B.; Wang, L.; Abraham, N.; Tao, K.; Huang, L.; Shi, W.; Dong, Y.; Qu, Y. Pre-ischemia melatonin treatment alleviated acute neuronal injury after ischemic stroke by inhibiting endoplasmic reticulum stress-dependent autophagy via PERK and IRE1 signalings. J. Pineal Res. 2017, 62, e12395. [Google Scholar] [CrossRef]
- Samadi, M.; Kazemeini, S.A.; Razzaghi, F.; Edalat, M.; Andersen, M.N.; Jacobsen, S.-E.; Mastinu, A. Melatonin priming manipulates antioxidant regulation and secondary metabolites production in favour of drought tolerance in Chenopodium quinoa Willd. S. Afr. J. Bot. 2024, 166, 272–286. [Google Scholar] [CrossRef]
- Kilic, U.; Caglayan, A.B.; Beker, M.C.; Gunal, M.Y.; Caglayan, B.; Yalcin, E.; Kelestemur, T.; Gundogdu, R.Z.; Yulug, B.; Yılmaz, B.; et al. Particular phosphorylation of PI3K/Akt on Thr308 via PDK-1 and PTEN mediates melatonin’s neuroprotective activity after focal cerebral ischemia in mice. Redox Biol. 2017, 12, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, B.; Yay, A.H.; Tan, F.C.; Özdamar, S.; Yildiz, O.G. Investigation of the anti-oxidative and anti-inflammatory effects of melatonin on experimental liver damage by radiation. Pathol. Res. Pract. 2023, 246, 154477. [Google Scholar] [CrossRef]
- Kulesh, A.A.; Drobakha, V.E.; Shestakov, V.V. The role of melatonin in the development of post-stroke cognitive impairment in elderly patients in comparison with middle-aged patients. Adv. Gerontol. 2016, 29, 651–657. [Google Scholar]
- Yang, Y.; Jiang, S.; Dong, Y.; Fan, C.; Zhao, L.; Yang, X.; Li, J.; Di, S.; Yue, L.; Liang, G.; et al. Melatonin prevents cell death and mitochondrial dysfunction via a SIRT1-dependent mechanism during ischemic-stroke in mice. J. Pineal Res. 2015, 58, 61–70. [Google Scholar] [CrossRef]
- Chern, C.M.; Liao, J.F.; Wang, Y.H.; Shen, Y.C. Melatonin ameliorates neural function by promoting endogenous neurogenesis through the MT2 melatonin receptor in ischemic-stroke mice. Free Radic. Biol. Med. 2012, 52, 1634–1647. [Google Scholar] [CrossRef]
- Guo, F.; Xu, F.; Li, S.; Zhang, Y.; Lv, D.; Zheng, L.; Gan, Y.; Zhou, M.; Zhao, K.; Xu, S.; et al. Amifostine ameliorates bleomycin-induced murine pulmonary fibrosis via NAD+/SIRT1/AMPK pathway-mediated effects on mitochondrial function and cellular metabolism. Eur. J. Med. Res. 2024, 29, 68. [Google Scholar] [CrossRef]
- Abolhasanpour, N.; Alihosseini, S.; Golipourkhalili, S.; Badalzadeh, R.; Mahmoudi, J.; Hosseini, L. Effect of Melatonin on endoplasmic reticulum-mitochondrial crosstalk in stroke. Arch. Med. Res. 2021, 52, 673–682. [Google Scholar] [CrossRef]
- Komlao, P.; Kraiwattanapirom, N.; Promyo, K.; Hein, Z.M.; Chetsawang, B. Melatonin enhances the restoration of neurological impairments and cognitive deficits during drug withdrawal in methamphetamine-induced toxicity and endoplasmic reticulum stress in rats. Neurotoxicology 2023, 99, 305–312. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Wan, J.; Liu, A.; Sun, J. Melatonin regulates Aβ production/clearance balance and Aβ neurotoxicity: A potential therapeutic molecule for Alzheimer’s disease. Biomed. Pharmacother. 2020, 132, 110887. [Google Scholar] [CrossRef]
- Sohail, S.; Shah, F.A.; Zaman, S.U.; Almari, A.H.; Malik, I.; Khan, S.A.; Alamro, A.A.; Zeb, A.; Din, F.U. Melatonin delivered in solid lipid nanoparticles ameliorated its neuroprotective effects in cerebral ischemia. Heliyon 2023, 9, e19779. [Google Scholar] [CrossRef] [PubMed]
- Zang, M.; Zhao, Y.; Gao, L.; Zhong, F.; Qin, Z.; Tong, R.; Ai, L.; Petersen, L.; Yan, Y.; Gao, Y.; et al. The circadian nuclear receptor RORα negatively regulates cerebral ischemia–reperfusion injury and mediates the neuroprotective effects of melatonin. Biochim. Biophys. Acta 2020, 1866, 165890. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; He, T.; Zhang, Y.; Liu, J.; Zhao, H.; Wang, D.; Wang, Q.; Yuan, Y.; Zhang, S. Melatonin regulates microglial polarization and protects against ischemic stroke-induced brain injury in mice. Exp. Neurol. 2023, 367, 114464. [Google Scholar] [CrossRef]
- Nair, S.M.; Rahman, R.M.; Clarkson, A.N.; Sutherland, B.A.; Taurin, S.; Sammut, I.A.; Appleton, I. Melatonin treatment following stroke induction modulates L-arginine metabolism. J. Pineal Res. 2011, 51, 313–323. [Google Scholar] [CrossRef]
- Mehrpooya, M.; Mazdeh, M.; Rahmani, E.; Khazaie, M.; Ahmadimoghaddam, D. Melatonin supplementation may benefit patients with acute ischemic stroke not eligible for reperfusion therapies: Results of a pilot study. J. Clin. Neurosci. 2022, 106, 66–75. [Google Scholar] [CrossRef]
- Shu, T.; Fan, L.; Wu, T.; Liu, C.; He, L.; Pang, M.; Bu, Y.; Wang, X.; Wang, J.; Rong, L.; et al. Melatonin promotes neuroprotection of induced pluripotent stem cells-derived neural stem cells subjected to H2O2-induced injury In Vitro. Eur. J. Pharmacol. 2018, 825, 143–150. [Google Scholar] [CrossRef]
- Shi, Y.; Ye, D.; Cui, K.; Bai, X.; Fan, M.; Feng, Y.; Hu, C.; Xu, Y.; Huang, J. Melatonin ameliorates retinal ganglion cell senescence and apoptosis in a SIRT1-dependent manner in an optic nerve injury model. Biochim. Biophys. Acta 2024, 1870, 167053. [Google Scholar] [CrossRef]
- Fang, X.; Huang, W.; Sun, Q.; Zhao, Y.; Sun, R.; Liu, F.; Huang, D.; Zhang, Y.; Gao, F.; Wang, B. Melatonin attenuates cellular senescence and apoptosis in diabetic nephropathy by regulating STAT3 phosphorylation. Life Sci. 2023, 332, 122108. [Google Scholar] [CrossRef]
- Qi, J.; Han, B.; Wang, Z.; Jing, L.; Tian, X.; Sun, J. Chuanzhitongluo inhibits neuronal apoptosis in mice with Acute Ischemic Stroke by regulating the PI3K/AKT signaling pathway. Neuroscience 2024, 537, 21–31. [Google Scholar] [CrossRef]
- Tuft, C.; Matar, E.; Menczel Schrire, Z.; Grunstein, R.R.; Yee, B.J.; Hoyos, C.M. Current insights into the risks of using melatonin as a treatment for sleep disorders in older adults. Clin. Interv. Aging 2023, 18, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Paterson, P.G.; Juurlink, B.H. Nutritional regulation of glutathione in stroke. Neurotox. Res. 1999, 1, 99–112. [Google Scholar] [CrossRef]
- Galano, A.; Alvarez-Idaboy, J.R. Glutathione: Mechanism and kinetics of its non-enzymatic defense action against free radicals. RSC Adv. 2011, 1, 1763–1771. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef]
- Lu, S.C. Regulation of glutathione synthesis. Curr. Top. Cell Regul. 2001, 36, 95–116. [Google Scholar] [CrossRef]
- Abdel-Fattah, M.M.; Messiha, B.A.S.; Mansour, A.M. Modulation of brain ACE and ACE2 may be a promising protective strategy against cerebral ischemia/reperfusion injury: An experimental trial in rats. Naunyn-Schmiedebergs Arch. Pharmacol. 2018, 91, 1003–1020. [Google Scholar] [CrossRef]
- Li, Z.; Yulei, J.; Yaqing, J.; Jinmin, Z.; Xinyong, L.; Jing, G.; Min, L. Protective effects of tetramethylpyrazine analogue Z-11 on cerebral ischemia reperfusion injury. Eur. J. Pharmacol. 2019, 844, 156–164. [Google Scholar] [CrossRef]
- Chen, C.; Ding, Q.; Shen, B.; Yu, T.; Wang, H.; Xu, Y.; Guo, H.; Hu, K.; Xie, L.; Wang, G.; et al. Insights into the authentic active ingredients and action sites of oral exogenous glutathione in the treatment of ischemic brain injury based on pharmacokinetic-pharmacodynamic studies. Drug Metab. Dispos. 2020, 48, 52–62. [Google Scholar] [CrossRef]
- Wang, H.; Du, Y.-S.; Xu, W.-S.; Li, C.-J.; Sun, H.; Hu, K.-R.; Hu, Y.-Z.; Yu, T.-J.; Guo, H.-M.; Xie, L.; et al. Exogenous glutathione exerts a therapeutic effect in ischemic stroke rats by interacting with intrastriatal dopamine. Acta Pharmacol. Sin. 2022, 43, 541–551. [Google Scholar] [CrossRef]
- Kahl, A.; Stepanova, A.; Konrad, C.; Anderson, C.; Manfredi, G.; Zhou, P.; Iadecola, C.; Galkin, A. Critical role of flavin and glutathione in complex I–mediated bioenergetic failure in brain ischemia/reperfusion injury. Stroke 2018, 49, 1223–1231. [Google Scholar] [CrossRef]
- Maksimova, M.Y.; Ivanov, A.V.; Virus, E.D.; Nikiforova, K.A.; Ochtova, F.R.; Suanova, E.T.; Kruglova, M.P.; Piradov, M.A.; Kubatiev, A.A. Impact of glutathione on acute ischemic stroke severity and outcome: Possible role of aminothiols redox status. Redox Rep. 2021, 26, 117–123. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Alexandrin, V.V.E.; Paltsyn, A.A.; Nikiforova, K.A.; Virus, E.D.; Luzya-nin, B.P.; Kubatiev, A.A. Plasma low-molecular-weight thiol/disulphide homeostasis as an early indicator of global and focal cerebral ischaemia. Redox Rep. 2017, 22, 460–466. [Google Scholar] [CrossRef]
- Namba, K.; Takeda, Y.; Sunami, K.; Hirakawa, M. Temporal profiles of the levels of en-dogenous antioxidants after four-vessel occlusion in rats. J. Neurosurg. Anesthesiol. 2001, 13, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Won, S.J.; Kim, J.E.; Cittolin-Santos, G.F.; Swanson, R.A. Assessment at the single-cell level identifies neuronal glutathione depletion as both a cause and effect of ischemia-reperfusion oxidative stress. J. Neurosci. 2015, 35, 7143–7152. [Google Scholar] [CrossRef] [PubMed]
- Ozkul, A.; Akyol, A.; Yenisey, C.; Arpaci, E.; Kiylioglu, N.; Tataroglu, C. Oxidative stress in acute ischemic stroke. J. Clin. Neurosci. 2007, 14, 1062–1066. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Yin, H.; Xu, L.; Porter, N.A. Free radical lipid peroxidation: Mechanisms and analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef]
- Cherubini, A.; Ruggiero, C.; Polidori, M.C.; Mecocci, P. Potential markers of oxidative stress in stroke. Free Radic. Biol. Med. 2005, 39, 841–852. [Google Scholar] [CrossRef]
- Niedernhofer, L.J.; Daniels, J.S.; Rouzer, C.A.; Greene, R.E.; Marnett, L.J. Malondialdehyde, a product of lipid peroxidation, is mutagenic in human cells. J. Biol. Chem. 2003, 278, 31426–31433. [Google Scholar] [CrossRef] [PubMed]
- Zahra, K.F.; Lefter, R.; Ali, A.; Abdellah, E.-C.; Trus, C.; Ciobica, A.; Timofte, D. The involvement of the oxidative stress status in cancer pathology: A double view on the role of the antioxidants. Oxid. Med. Cell. Longev. 2021, 2021, 9965916. [Google Scholar] [CrossRef]
- Esterbauer, H.; Eckl, P.; Ortner, A. Possible mutagens derived from lipids and lipids precursors. Mutat. Res. 1990, 238, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Elsayed, W.M.; Abdel-Gawad, E.H.A.; Mesallam, D.I.A.; El-Serafy, T.S. The relationship between oxidative stress and acute ischemic stroke severity and functional outcome. Egypt. J. Neurol. Psychiatry Neurosurg. 2020, 56, 74. [Google Scholar] [CrossRef]
- Haritha, B.; Sangeetha, B. Malondialdehyde levels in ischemic stroke. IOSR-JDMS 2020, 19, 36–41. [Google Scholar] [CrossRef]
- Tugasworo, D.; Prasetyo, A.; Kurnianto, A.; Retnaningsih, R.; Andhitara, Y.; Ardhini, R.; Budiman, J. Malondialdehyde (MDA) and 8-hydroxy-2-deoxyguanosine (8-OHdG) in ischemic stroke: A systematic review. Egypt. J. Neurol. Psychiatry Neurosurg. 2023, 59, 1–14. [Google Scholar] [CrossRef]
- Dogan, O.; Kisa, U.; Erdemoglu, A.K.; Kacmaz, M.; Caglayan, O.; Kurku, H. Oxidative and nitrosative stress in patients with ischemic stroke. J. Lab. Med. 2018, 42, 195–200. [Google Scholar] [CrossRef]
- Maes, M.; Brinholi, F.F.; Michelin, A.P.; Matsumoto, A.K.; de Oliveira Semeão, L.; Almulla, A.F.; Supasitthumrong, T.; Tunvirachaisakul, C.; Barbosa, D.S. In mild and moderate acute ischemic stroke, increased lipid peroxidation and lowered antioxidant defences are strongly associated with disabilities and final stroke core. Antioxidants 2023, 12, 188. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, P.C.; Mulholland, C.; Trinick, T. Ascorbate and malondialdehyde in stroke patients. Ir. J. Med. Sci. 1994, 163, 488–490. [Google Scholar] [CrossRef]
- Domínguez, C.; Delgado, P.; Vilches, A.; Martín-Gallán, P.; Ribo, M.; Santamarina, E.; Molina, C.; Corbeto, N.; Rodríguez-Sureda, V.; Rosell, A.; et al. Oxidative stress after thrombolysis-induced reperfusion in human stroke. Stroke 2010, 41, 653–660. [Google Scholar] [CrossRef]
- Al-Rawi, N.H.; Jaber, F.; Atiyah, K.M. Assessment of salivary and serum oxidative stress and antioxidants as plausible parameters in prediction of ischemic stroke among Iraqi samples. Internet J. Third World Med. 2009, 7, 1–9. [Google Scholar]
- Maciejczyk, M.; Bielas, M.; Zalewska, A.; Gerreth, K. Salivary biomarkers of oxidative stress and inflammation in stroke patients: From basic research to clinical practice. Oxid. Med. Cell. Longev. 2021, 2021, 5545330. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Chen, C.-M.; Liu, J.-L.; Chen, S.-T.; Cheng, M.-L.; Chiu, D.T.-Y. Oxidative markers in spontaneous intracerebral hemorrhage: Leukocyte 8-hydroxy-2′-deoxyguanosine as an independent predictor of the 30-day outcome. J. Neurosurg. 2011, 115, 1184–1190. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Tu, X.; Shen, H.; Qiu, H.; Chen, H.; He, J. High serum levels of malondialdehyde and 8-OHdG are both associated with early cognitive impairment in patients with acute ischaemic stroke. Sci. Rep. 2017, 7, 9493. [Google Scholar] [CrossRef] [PubMed]
- El Sayed, W.; Abdel Gawad, E.; Mesallam, D.; El-Serafy, T. Serum Malondialdehyde as A Predictor of Post-Stroke Cognitive Impairment in Ischemic Stroke Patients. Zagazig Univ. Med. J. 2022, 28, 326–332. [Google Scholar] [CrossRef]
- Syafrita, Y.; Amir, D.; Susanti, R.; Fadhilah, I. Relationship of brain-derived neurotrophic stroke depression. Dement Neuropsychol. 2020, 14, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhu, Z.; Zhao, J.; Ren, W.; Cai, Y.; Wang, Q.; Luan, X.; Zhao, K.; He, J. Melondialdehyde: A novel predictive biomarker for post-stroke depression. J. Affect Disord. 2017, 220, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Rybka, J.K.; Kedziora-Kornatowska, K.; Banas-Lezanska, P.; Majsterek, I.; Carvalho, L.A.; Cattaneo, A.; Anacker, C.; Kedziora, J. Interplay between the pro-oxidant and antioxidant systems and proinflammatory cytokine levels, in relation to iron metabolism and the erythron in depression. Free Radic. Biol. Med. 2013, 63, 187–194. [Google Scholar] [CrossRef]
- Picón-Pages, P.; Garcia-Buendia, J.; Muñoz, F.J. Functions and dysfunctions of nitric oxide in brain. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1949–1967. [Google Scholar] [CrossRef]
- Wu, J.; Jia, J.; Ji, D.; Weijie Jiao, W.; Huang, Z.; Zhang, Y. Advances in nitric oxide regulators for the treatment of ischemic stroke. Eur. J. Med. Chem. 2023, 262, 115912. [Google Scholar] [CrossRef]
- Terpolilli, N.A.; Moskowitz, M.A.; Plesnila, N. Nitric oxide: Considerations for the treatment of ischemic stroke. J. Cereb. Blood Flow Metab. 2012, 32, 1332–1346. [Google Scholar] [CrossRef]
- Chen, Z.-Q.; Mou, R.-T.; Dong-Xia Feng, D.-X.; Wang, Z.; Chen, G. The role of nitric oxide in stroke. Med. Gas. Res. 2017, 7, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Hirvonen, M.R.; Brüne, B.; Lapetina, E.G. Heat shock proteins and macrophage resistance to the toxic effects of nitric oxide. Biochem. J. 1996, 315, 845–849. [Google Scholar] [CrossRef] [PubMed]
- Sims, N.R.; Anderson, M.F. Mitochondrial contributions to tissue damage in stroke. Neurochem. Int. 2002, 40, 511–526. [Google Scholar] [CrossRef]
- Hämäläinen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moilanen, E. Anti-inflammatory effects of flavonoids: Genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-kappaB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-kappaB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediat. Inflamm. 2007, 2007, 45673. [Google Scholar] [CrossRef]
- Heinrich, T.A.; da Silva, R.S.; Miranda, K.M.; Switzer, C.H.; Wink, D.A.; Fukuto, J.M. Biological nitric oxide signalling: Chemistry and terminology. Br. J. Pharmacol. 2013, 169, 1417–1429. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E. Nitric oxide signaling in health and disease. Cell 2022, 185, 2853–2878. [Google Scholar] [CrossRef]
- Stamler, J.S. Redox signaling: Nitrosylation and related target interactions of nitric oxide. Cell 1994, 78, 931–936. [Google Scholar] [CrossRef]
- Leist, M.; Fava, E.; Montecucco, C.; Nicotera, P. Peroxynitrite and nitric oxide donors induce neuronal poptosis by eliciting autocrine excitotoxicity. Eur. J. Neurosci. 1997, 9, 1488–1498. [Google Scholar] [CrossRef]
- Leist, M.; Volbracht, C.; Kuhnle, S.; Fava, E.; Ferrando-May, E.; Nicotera, P. Caspase-mediated apoptosis in neuronal excitotoxicity triggered by nitric oxide. Mol. Med. 1997, 3, 750–764. [Google Scholar] [CrossRef]
- Bladowski, M.; Gawrys, J.; Gajecki, D.; Szahidewicz-Krupska, E.; Sawicz-Bladowska, A.; Doroszko, A. Role of the platelets and nitric oxide biotransformation in, ischemic stroke: A translative review from bench to bedside. Oxid. Med. Cell. Longev. 2020, 2020, 2979260. [Google Scholar] [CrossRef]
- Kader, A.; Frazzini, V.I.; Solomon, R.A.; Trifiletti, R.R. Nitric oxide production during focal cerebral ischemia in rats. Stroke 1993, 24, 1709–1716. [Google Scholar] [CrossRef]
- Niwa, M.; Inao, S.; Takayasu, M.; Kawai, T.; Kajita, Y.; Nihashi, T.; Kabeya, R.; Sugimoto, T.; Yoshida, J. Time course of expression of three nitric oxide synthase isoforms after transient middle cerebral artery occlusion in rats. Neurol. Med.-Chir. 2001, 41, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Tabuchi, M.S.M.; Ikeda, M.; Tomita, T. Concurrent formation of peroxynitrite with the expression of inducible nitric oxide synthase in the brain during middle cerebral artery occlusion and reperfusion in rats. Brain Res. 2002, 951, 113–120. [Google Scholar] [CrossRef]
- Bolaños, J.P.; Almeida, A. Roles of nitric oxide in brain hypoxia-ischemia. Biochim. Biophys. Acta 1999, 1411, 415–436. [Google Scholar] [CrossRef]
- Alioua, A.; Tanaka, Y.; Wallner, M.; Hofmann, F.; Ruth, P.; Meera, P.; Toro, L. The large conductance, voltage-dependent, and calcium-sensitive K+ channel, Hslo, is a target of cGMP-dependent protein kinase phosphorylation In Vivo. J. Biol. Chem. 1998, 273, 32950–32956. [Google Scholar] [CrossRef] [PubMed]
- Toda, N.; Ayajiki, K.; Okamura, T. Cerebral blood flow regulation by nitric oxide: Recent advances. Pharmacol. Rev. 2009, 61, 62–97. [Google Scholar] [CrossRef]
- Broos, K.; Feys, H.B.; De Meyer, S.F.; Vanhoorelbeke, K.; Deckmyn, H. Platelets at work in primary hemostasis. Blood Rev. 2011, 25, 155–167. [Google Scholar] [CrossRef]
- Atochin, D.N.; Huang, P.L. Endothelial nitric oxide synthase transgenic models of endothelial dysfunction. Pflugers. Arch. 2010, 460, 965–974. [Google Scholar] [CrossRef]
- Cui, X.; Chopp, M.; Zacharek, A.; Zhang, C.; Roberts, C.; Chen, J. Role of endothelial nitric oxide synthetase in arteriogenesis after stroke in mice. Neuroscience 2009, 159, 744–750. [Google Scholar] [CrossRef]
- Ito, Y.; Ohkubo, T.; Asano, Y.; Hattori, K.; Shimazu, T.; Yamazato, M.; Nagoya, H.; Kato, Y.; Araki, N. Nitric oxide production during cerebral ischemia and reperfusion in eNOS- and nNOS-knockout mice. Curr. Neurovasc. Res. 2010, 7, 23–31. [Google Scholar] [CrossRef]
- Tan, X.L.; Xue, Y.Q.; Ma, T.; Wang, X.; Li, J.J.; Lan, L.; Malik, K.U.; McDonald, M.P.; Dopico, A.M.; Liao, F.-F. Partial eNOS deficiency causes spontaneous thrombotic cerebral infarction, amyloid angiopathy and cognitive impairment. Mol. Neurodegener. 2015, 10, 24. [Google Scholar] [CrossRef]
- Huang, Z.; Huang, P.L.; Ma, J.; Meng, W.; Ayata, C.; Fishman, M.C.; Moskowitz, M.A. Enlarged infarcts in endothelial nitric oxide synthase knockout mice are attenuated by nitro-L-arginine. J. Cereb. Blood Flow Metab. 1996, 16, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.J.; Mokgokong, R.; Kania, K.D.; Guedj, A.S.; Hladky, S.B.; Barrand, M.A. Nitric oxide contributes to hypoxiareoxygenation-induced P-glycoprotein expression in rat brain endothelial cells. Cell. Mol. Neurobiol. 2011, 31, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Rashid, P.A.; Whitehurst, A.; Lawson, N.; Bath, P.M. Plasma nitric oxide (nitrate/nitrite) levels in acute stroke and their relationship with severity and outcome. J. Stroke Cerebrovasc. Dis. 2003, 12, 82–87. [Google Scholar] [CrossRef]
- Taffi, R.; Nanetti, L.; Mazzanti, L.; Bartolini, M.; Vignini, A.; Raffaelli, F.; Pasqualetti, P.; Vernieri, F.; Provinciali, L.; Silvestrini, M. Plasma levels of nitric oxide and stroke outcome. J. Neurol. 2008, 255, 94–98. [Google Scholar] [CrossRef]
- Serrano-Ponz, M.; Rodrigo-Gasqué, C.; Siles, E.; Martínez-Lara, E.; Ochoa-Callejero, L.; Martínez, A. Temporal profiles of blood pressure, circulating nitric oxide, and adrenomedullin as predictors of clinical outcome in acute ischemic stroke patients. Mol. Med. Rep. 2016, 13, 3724–3734. [Google Scholar] [CrossRef]
- Cure, M.C.; Tufekci, A.; Cure, E.; Kirbas, S.; Ogullar, S.; Kirbas, A.; Unal, H.; Yuce, S.; Cakmak, S. Low-density lipoprotein subfraction, carotid artery intima-media thickness, nitric oxide and tumor necrosis factor alpha are associated with newly diagnosed ischemic stroke. Ann. Indian. Acad. Neurol. 2013, 16, 498–503. [Google Scholar] [CrossRef]
- Castillo, J.; Rama, R.; Dávalos, A. Nitric oxide–related brain damage in acute ischemic stroke. Stroke 2000, 31, 852–857. [Google Scholar] [CrossRef]
- Hu, S.Q.; Ye, J.S.; Zong, Y.Y.; Sun, C.C.; Liu, D.H.; Wu, Y.P.; Song, T.; Zhang, G.Y. S-nitrosylation of mixed lineage kinase 3 contributes to its activation after cerebral ischemia. J. Biol. Chem. 2012, 287, 2364–2377. [Google Scholar] [CrossRef]
- Yu, L.-M.; Zhang, T.-Y.; Yin, X.-H.; Yang, Q.; Lu, F.; Yan, J.-Z.; Li, C. Denitrosylation of nNOS induced by cerebral ischemiareperfusion contributes to nitrosylation of CaMKII and its inhibition of autophosphorylation in hippocampal CA1. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 7674–7683. [Google Scholar] [CrossRef]
- Yu, L.-M.; Zhang, T.-Y.; Yin, X.-H.; Yang, Q.; Lu, F.; Yan, J.-Z.; Li, C. Brain ischemia induces serine phosphorylation of neuronal nitric oxide synthase by Ca(2+)/calmodulin-dependent protein kinase II in rat hippocampus. Acta Pharmacol. Sin. 2004, 25, 617–622. [Google Scholar]
- Tang, L.J.; Li, C.; Hu, S.Q.; Wu, Y.P.; Zong, Y.Y.; Sun, C.C.; Zhang, F.; Zhang, G.Y. S-nitrosylation of c-Src via NMDAR-nNOS module promotes c-Src activation and NR2A phosphorylation in cerebral ischemia/reperfusion. Mol. Cell. Biochem. 2012, 365, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Li, C.; Arrick, D.M.; Yang, S.; Baluna, A.E.; Sun, H. Role of nitric oxide synthases in early blood-brain barrier disruption following transient focal cerebral ischemia. PLoS ONE 2014, 9, e93134. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zheng, G.; Xu, M.; Li, Y.; Chen, X.; Zhu, W.; Tong, Y.; Chung, S.K.; Liu, K.J.; Shen, J. Caveolin-1 regulates nitric oxidemediated matrix metalloproteinases activity and blood-brain barrier permeability in focal cerebral ischemia and reperfusion injury. J. Neurochem. 2012, 120, 147–156. [Google Scholar] [CrossRef]
- Hara, H.; Huang, P.L.; Panahian, N.; Fishman, M.C.; Moskowitz, M.A. Reduced brain edema and infarction volume in mice lacking the neuronal isoform of nitric oxide synthase after transient MCA occlusion. J. Cereb. Blood Flow Metab. 1996, 16, 605–611. [Google Scholar] [CrossRef]
- Zaharchuk, G.; Hara, H.; Huang, P.L.; Fishman, M.C.; Moskowitz, M.A.; Jenkins, B.G.; Rosen, B.R. Neuronal nitric oxide synthase mutant mice show smaller infarcts and attenuated apparent diffusion coefficient changes in the peri-infarct zone during focal cerebral ischemia. Magn. Reson. Med. 1997, 37, 170–175. [Google Scholar] [CrossRef]
- Cinelli, M.A.; Do, H.T.; Miley, G.P.; Silverman, R.B. Inducible nitric oxide synthase: Regulation, structure, and inhibition. Med. Res. Rev. 2020, 40, 158–189. [Google Scholar] [CrossRef]
- Iadecola, C.; Zhang, F.; Xu, S.; Casey, R.; Ross, M.E. Inducible nitric oxide synthase gene expression in brain following cerebral ischemia. J. Cereb. Blood Flow Metab. 1995, 15, 378–384. [Google Scholar] [CrossRef]
- Brown, G.C. Nitric oxide and neuronal death. Nitric Oxide 2010, 23, 153–165. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, W.; Li, J. Ca2+- and protein kinase C-dependent signaling pathway for nuclear factorκb activation, inducible nitric oxide synthase expression, and tumor necrosis factor-α production in lipopolysaccharide-stimulated rat peritoneal macrophages. J. Biol. Chem. 2006, 281, 31337–31347. [Google Scholar] [CrossRef]
- Kleinert, H.; Schwarz, P.M.; Förstermann, U. Regulation of the expression of inducible nitric oxide synthase. Biol Chem. 2003, 384, 1343–1364. [Google Scholar] [CrossRef]
- Pannu, R.; Singh, I. Pharmacological strategies for the regulation of inducible nitric oxide synthase: Neurodegenerative versus neuroprotective mechanisms. Neurochem. Int. 2006, 49, 170–182. [Google Scholar] [CrossRef]
- Danielisova, V.; Burda, J.; Nemethova, M.; Gottlieb, M. Aminoguanidine administration ameliorates hippocampal damage after middle cerebral artery occlusion in rat. Neurochem. Res. 2011, 36, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Ding, J.; Wang, J.; Zhou, C.; Zhang, W. Effects and mechanism of action of inducible nitric oxide synthase on apoptosis in a rat model of cerebral ischemia-reperfusion injury. Anat. Rec. 2016, 299, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C.; Zhang, F.; Casey, R.; Nagayama, M.; Ross, M.E. Delayed reduction of ischemic brain injury and neurological deficits in mice lacking the inducible nitric oxide synthase gene. J. Neurosci. 1997, 17, 9157–9164. [Google Scholar] [CrossRef]
- Garcia-Bonilla, L.; Moore, J.M.; Racchumi, G.; Zhou, P.; Butler, J.M.; Iadecola, C.; Anrather, J. Inducible nitric oxide synthase in neutrophils and endothelium contributes to ischemic brain injury in mice. J. Immunol. 2014, 193, 2531–2537. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Xu, T.; Zhao, J.; Gao, H.; Xie, W. Depletion of iNOS-positive inflammatory cells decelerates neuronal degeneration and alleviates cerebral ischemic damage by suppressing the inflammatory response. Free Radic. Biol. Med. 2022, 181, 209–220. [Google Scholar] [CrossRef] [PubMed]
- ArunaDevi, R.; Ramteke, V.D.; Kumar, S.; Shukla, M.K.; Jaganathan, S.; Kumar, D.; Sharma, A.K.; Tandan, S.K. Neuroprotective effect of s-methylisothiourea in transient focal cerebral ischemia in rat. Nitric Oxide 2010, 22, 1–10. [Google Scholar] [CrossRef]
- Khan, M.; Sekhon, B.; Giri, S.; Jatana, M.; Gilg, A.G.; Ayasolla, K.; Elango, C.; Singh, A.K.; Singh, I. S-Nitrosoglutathione reduces inflammation and protects brain against focal cerebral ischemia in a rat model of experimental stroke. J. Cereb. Blood Flow Metab. 2005, 25, 177–192. [Google Scholar] [CrossRef]
- Foncea, R.; Carvajal, C.; Almarza, C.; Leighton, F. Endothelial cell oxidative stress and signal transduction. Biol. Res. 2000, 33, 89–96. [Google Scholar] [CrossRef]
- Trickler, W.J.; Mayhan, W.G.; Miller, D.W. Brain microvessel endothelial cell responses to tumor necrosis factor-alpha involve a nuclear factor kappa B (NF-kappaB) signal transduction pathway. Brain Res. 2005, 1048, 24–31. [Google Scholar] [CrossRef]
- Chen, X.M.; Chen, H.S.; Xu, M.J.; Shen, J.G. Targeting reactive nitrogen species: A promising therapeutic strategy for cerebral ischemia-reperfusion injury. Acta Pharmacol. Sin. 2013, 34, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Tajes, M.; Ill-Raga, G.; Palomer, E.; Ramos-Fernández, E.; Guix, F.X.; Bosch-Morató, M.; Guivernau, B.; Jiménez-Conde, J.; Ois, A.; Pérez-Asensio, F.; et al. Nitro-oxidative stress after neuronal ischemia induces protein nitrotyrosination and cell death. Oxid Med. Cell. Longev. 2013, 2013, 826143. [Google Scholar] [CrossRef]
- Chen, H.S.; Chen, X.M.; Feng, J.H.; Liu, K.J.; Qi, S.H.; Shen, J.G. Peroxynitrite decomposition catalyst reduces delayed thrombolysis-induced hemorrhagic transformation in ischemia-reperfused rat brains. CNS Neurosci. Ther. 2015, 21, 585–590. [Google Scholar] [CrossRef]
- Shao, Z.; Dou, S.; Zhu, J.; Wang, H.; Xu, D.; Wang, C.; Cheng, B.; Bai, B. The role of mitophagy in ischemic stroke. Front. Neurol. 2020, 11, 608610. [Google Scholar] [CrossRef]
- Lan, R.; Wu, J.T.; Wu, T.; Ma, Y.Z.; Wang, B.Q.; Zheng, H.Z.; Li, Y.N.; Wang, Y.; Gu, C.Q.; Zhang, Y. Mitophagy is activated in brain damage induced by cerebral ischemia and reperfusion via the PINK1/Parkin/p62 signaling pathway. Brain Res. Bull. 2018, 142, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Katusic, Z.S.; d’Uscio, L.V.; Nath, K.A. Vascular protection by tetrahydrobiopterin: Progress and therapeutic prospects. Trends Pharmacol. Sci. 2009, 30, 48–54. [Google Scholar] [CrossRef]
- Stadtman, E.R.; Levine, R.L. Chemical modification of proteins by reactive oxygen species. In Redox Proteomics: From Protein Modifications to Cellular Dysfunction and Disease; Dalle-Donne, I., Scaloni, A., Butterfield, D.A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2006; pp. 3–23. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Milzani, A.; Colombo, R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta 2003, 329, 23–38. [Google Scholar] [CrossRef]
- Irazusta, V.; Moreno-Cermeño, A.; Cabiscol, E.; Tamarit, J.; Ros, J. Proteomic strategies for the analysis of carbonyl groups on proteins. Curr. Protein Pept. Sci. 2010, 11, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Foell, D.; Wittkowski, H.; Roth, J. Mechanisms of disease: A ‘DAMP’ view of inflammatory arthritis. Nat. Clin. Pract. Rheumatol. 2007, 3, 382–390. [Google Scholar] [CrossRef]
- Cichoń, N.; Rzeźnicka, P.; Bijak, M.; Miller, E.; Miller, S.; Saluk, J. Extremely low frequency electromagnetic field reduces oxidative stress during the rehabilitation of post-acute stroke patients. Adv. Clin. Exp. Med. 2018, 27, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Enache, T.A.; Matei, E.; Diculescu, V.C. Electrochemical sensor for carbonyl groups in oxidized proteins. Anal. Chem. 2019, 91, 1920–1927. [Google Scholar] [CrossRef] [PubMed]
- Pouya, V.T.; Hashemy, S.I.; Shoeibi, A.; Tirkani, A.N.; Tavallaie, S.; Avval, F.Z.; Soukhtanloo, M.; Mashkani, B.A.; Alamdari, D.H. Serum Pro-Oxidant-Antioxidant Balance, Advanced oxidized protein products (AOPP) and protein carbonyl in patients with stroke. Razavi. Int. J. Med. 2016, 4, 21–26. [Google Scholar] [CrossRef]
- Cichoń, N.; Bijak, M.; Miller, E.; Niwald, M.; Saluk, J. Poststroke depression as a factor adversely affecting the level of oxidative damage to plasma proteins during a brain stroke. Oxid. Med. Cell. Longev. 2015, 2015, 408745. [Google Scholar] [CrossRef]
- Manolescu, B.N.; Berteanu, M.; Oprea, E.; Chiriac, N.; Dumitru, L.; Vlădoiu, S.; Popa, O.; Ianas, O. Dynamic of oxidative and nitrosative stress markers during the convalescent period of stroke patients undergoing rehabilitation. Ann. Clin. Biochem. 2011, 48, 338–343. [Google Scholar] [CrossRef]
- Moon, G.J.; Kim, S.J.; Cho, Y.H.; Ryoo, S.; Bangc, O.Y. Antioxidant effects of statins in patients with atherosclerotic cerebrovascular disease. J. Clin. Neurol. 2014, 10, 140–147. [Google Scholar] [CrossRef]
- Gdovinova, Z.; Kremer, C.; Lorenzano, S.; Dawson, J.; Lal, A.; Caso, V. Aspirin for primary stroke prevention; evidence for a differential effect in men and women. Front. Neurol. 2022, 13, 856239. [Google Scholar] [CrossRef]
- Paolucci, S.; Antonucci, G.; Grasso, M.G.; Bragoni, M.; Coiro, P.; De Angelis, D.; Fusco, F.R.; Morelli, D.; Venturiero, V.; Troisi, E.; et al. Functional outcome of ischemic and hemorrhagic stroke patients after inpatient rehabilitation: A matched comparison. Stroke 2003, 34, 2861–2865. [Google Scholar] [CrossRef]
- Jovin, T.G.; Chamorro, A.; Cobo, E.; de Miquel, M.A.; Molina, C.A.; Rovira, A.; San Roman, L.; Serena, J.; Abilleira, S.; Ribo, M.; et al. Thrombectomy within 8 Hours after Symptom Onset in Ischemic Stroke. N. Engl. J. Med. 2015, 372, 2296–2306. [Google Scholar] [CrossRef]
- Widimsky, P.; Snyder, K.; Sulzenko, J.; Hopkins, L.N.; Stetkarova, I. Acute ischaemic stroke: Recent advances in reperfusion treatment. Eur. Heart J. 2023, 44, 1205–1215. [Google Scholar] [CrossRef]
- Sun, M.-S.; Jin, H.; Sun, X.; Huang, S.; Zhang, F.-L.; Guo, Z.-N.; Yang, Y. Free radical damage in ischemia-reperfusion injury: An obstacle in Acute Ischemic Stroke after revascularization therapy. Oxid. Med. Cell Longev. 2018, 2018, 3804979. [Google Scholar] [CrossRef] [PubMed]
- Jelinek, M.; Jurajda, M.; Duris, K. Oxidative stress in the brain: Basic concepts and treatment strategies in stroke. Antioxidants 2021, 10, 1886. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K.; Ikeda, Y.; Shioda, S.; Tobe, T.; Yoshikawa, T. Edarabone scavenges nitric oxide. Redox Rep. 2002, 7, 219–222. [Google Scholar] [CrossRef]
- Banno, M.; Mizuno, T.; Kato, H.; Zhang, G.; Kawanokuchi, J.; Wang, J.; Kuno, R.; Jin, S.; Takeuchi, H.; Suzumura, A. The radical scavenger edaravone prevents oxidative neurotoxicity induced by peroxynitrite and activated microglia. Neuropharmacology 2005, 48, 283–290. [Google Scholar] [CrossRef]
- Watanabe, T.; Yuki, S.; Egawa, M.; Nishi, H. Protective effects of MCI-186 on cerebral ischemia: Possible involvement of free radical scavenging and antioxidant actions. J. Pharmacol. Exp. Ther. 1994, 268, 1597–1604. [Google Scholar]
- Abe, K.; Yuki, S.; Kogure, K. Strong attenuation of ischemic and postischemic brain edema in rats by a novel free radical scavenger. Stroke 1988, 19, 480–485. [Google Scholar] [CrossRef]
- Morita, M.; Naito, Y.; Yoshikawa, T.; Niki, E. Inhibition of plasma lipid oxidation induced by peroxyl radicals, peroxynitrite, hypochlorite, 15-lipoxygenase, and singlet oxygen by clinical drugs. Bioorganic Med. Chem. Lett. 2016, 26, 5411–5417. [Google Scholar] [CrossRef]
- Yamamoto, Y. Plasma marker of tissue oxidative damage and edaravone as a scavenger drug against peroxyl radicals and peroxynitrite. J. Clin. Biochem. Nutr. 2017, 60, 49–54. [Google Scholar] [CrossRef]
- Yamashita, T.; Shoge, M.; Oda, E.; Yamamoto, Y.; Giddings, J.C.; Kashiwagi, S.; Suematsu, M.; Yamamoto, J. The free-radical scavenger, edaravone, augments NO release from vascular cells and platelets after laser-induced, acute endothelial injury In Vivo. Platelets 2006, 17, 201–206. [Google Scholar] [CrossRef]
- Feng, S.; Yang, Q.; Liu, M.; Li, W.; Yuan, W.; Zhang, S.; Wu, B.; Li, J. Edaravone for acute ischaemic stroke. Cochrane Database Syst. Rev. 2011, 12, CD007230. [Google Scholar] [CrossRef]
- Otomo, E.; Tohgi, H.; Kogure, K.; Hirai, S.; Takakura, K.; Terashi, A.; Gotoh, F.; Maruyama, S.; Tazaki, Y.; Shinohara, Y.; et al. Effect of a novel free radical scavenger, edaravone (MCI-186), on acute brain infarction: Randomized, placebo-controlled, double-blind study at multicenters. Cerebrovasc. Dis. 2003, 15, 222–229. [Google Scholar] [CrossRef]
- Feng, J.; Chen, X.; Guan, B.; Li, C.; Qiu, J.; Shen, J. Inhibition of peroxynitrite-induced mitophagy activation attenuates cerebral ischemia-reperfusion injury. Mol. Neurobiol. 2018, 55, 6369–6386. [Google Scholar] [CrossRef]
- Squadrito, G.L.; Cueto, R.; Splenser, A.E.; Valavanidis, A.; Zhang, H.; Uppu, R.M.; Pryor, W.A. Reaction of uric acid with peroxynitrite and implications for the mechanism of neuroprotection by uric acid. Arch. Biochem. Biophys. 2000, 376, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Logallo, N.; Naess, H.; Idicula, T.T.; Brogger, J.; Waje-Andreassen, U.; Thomassen, L. Serum uric acid: Neuroprotection in thrombolysis. The Bergen NORSTROKE study. BMC Neurol. 2011, 11, 114. [Google Scholar] [CrossRef]
- Amaro, S.; Urra, X.; Gomez-Choco, M.; Obach, V.; Cervera, A.; Vargas, M.; Torres, F.; Rios, J.; Planas, A.M.; Chamorro, A. Uric acid levels are relevant in patients with stroke treated with thrombolysis. Stroke 2011, 42, 28–32. [Google Scholar] [CrossRef]
- Liu, X.; Liu, M.; Chen, M.; Ge, Q.M.; Pan, S.M. Serum uric acid is neuroprotective in Chinese patients with acute ischemic stroke treated with intravenous recombinant tissue plasminogen activator. J. Stroke Cerebrovasc. Dis. 2015, 24, 1080–1086. [Google Scholar] [CrossRef]
- Lee, S.H.; Heo, S.H.; Kim, J.H.; Lee, D.; Lee, J.S.; Kim, Y.S.; Kim, H.Y.; Koh, S.-H.; Chang, D.-I. Effects of uric acid levels on outcome in severe ischemic stroke patients treated with intravenous recombinant tissue plasminogen activator. Eur. Neurol. 2014, 71, 132–139. [Google Scholar] [CrossRef]
- Romanos, E.; Planas, A.M.; Amaro, S.; Chamorro, A. Uric acid reduces brain damage and improves the benefits of rt-PA in a rat model of thromboembolic stroke. J. Cereb. Blood Flow Metab. 2007, 27, 14–20. [Google Scholar] [CrossRef]
- Morelli, M.B.; Gambardella, J.; Castellanos, V.; Trimarco, V.; Santulli, G. Vitamin C and cardiovascular disease: An update. Antioxidants 2020, 9, 1227. [Google Scholar] [CrossRef]
- Mishima, K.; Tanaka, T.; Pu, F.; Egashira, N.; Iwasaki, K.; Hidaka, R.; Matsunaga, K.; Takata, J.; Karube, Y.; Fujiwara, M. Vitamin E isoforms A-Tocotrienol and g-Tocopherol prevent cerebral infarction in mice. Neurosci. Lett. 2003, 5, 56–60. [Google Scholar] [CrossRef]
- Loh, H.C.; Lim, R.; Lee, K.W.; Ooi, C.Y.; Chuan, D.R.; Looi, I.; Kah Hay, Y.; Abdul Karim Khan, N. Effects of Vitamin E on stroke: A systematic review with meta-analysis and trial sequential analysis. Stroke Vasc. Neurol. 2021, 6, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Park, J.; Lee, J.E.; Yenari, M.A. NOX inhibitors—A promising avenue for Ischemic Stroke. Exp. Neurobiol. 2017, 26, 195–205. [Google Scholar] [CrossRef]
- Touyz, R.M. Apocynin, NADPH oxidase, and vascular cells: A complex matter. Hypertension 2008, 51, 172–174. [Google Scholar] [CrossRef] [PubMed]
- Martz, D.; Rayos, G.; Schielke, G.P.; Betz, A.L. Allopurinol and dimethylthiourea reduce brain infarction following middle cerebral artery occlusion in rats. Stroke 1989, 20, 488–494. [Google Scholar] [CrossRef]
- Choi, W.; Villegas, V.; Istre, H.; Heppler, B.; Gonzalez, N.; Brusman, N.; Snider, L.; Hogle, E.; Tucker, J.; Oñate, A.; et al. Synthesis and characterization of CAPE derivatives as Xanthine Oxidase inhibitors with radical scavenging properties. Bioorganic Chem. 2019, 86, 686–695. [Google Scholar] [CrossRef]
- Chen, S.; Chen, H.; Du, Q.; Shen, J. Targeting Myeloperoxidase (MPO) mediated oxidative stress and inflammation for reducing brain Ischemia Injury: Potential application of natural compounds. Front. Physiol. 2020, 11, 433. [Google Scholar] [CrossRef]
- Lei Wang, L.; Zhang, X.; Xiong, X.; Zhu, H.; Chen, R.; Zhang, S.; Chen, G.; Jian, Z. Nrf2 regulates oxidative stress and its role in Cerebral Ischemic Stroke. Antioxidants 2022, 11, 2377. [Google Scholar] [CrossRef]
- Sandberg, M.; Patil, J.; D’Angelo, B.; Weber, S.; Mallard, C. Nrf2-regulation in brain health and disease: Implication of cerebral inflammation. Neuropharmacology 2014, 79, 298–306. [Google Scholar] [CrossRef]
- Han, M.; Hu, L.; Chen, Y. Rutaecarpine may improve neuronal injury, inhibits apoptosis, inflammation and oxidative stress by regulating the expression of ERK1/2 and Nrf2/HO-1 pathway in rats with cerebral ischemia-reperfusion injury. Drug Des. Dev. Ther. 2019, 13, 2923–2931. [Google Scholar] [CrossRef]
- Li, R.; Li, X.; Wu, H.; Yang, Z.; Fei, L.; Zhu, J. Theaflavin attenuates cerebral ischemia/reperfusion injury by abolishing miRNA1283pmediated Nrf2 inhibition and reducing oxidative stress. Mol. Med. Rep. 2019, 20, 4893–4904. [Google Scholar] [CrossRef]
- Song, Y.; Wang, L.; Bei, Y.; Qin, D.; Ai, L.; Ma, Q.; Lin, P. Carvacryl acetate, a semisynthetic monoterpenic ester obtained from essential oils, provides neuroprotection against cerebral ischemia reperfusion-induced oxidative stress injury via the Nrf2 signalling pathway. Food Funct. 2020, 11, 1754–1763. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; He, B.; Zhang, X.; Yang, R.; Xia, X.; Chen, L.; Li, R.; Shen, Z.; Chen, P. Geraniin protects against Cerebral Ischemia/Reperfusion Injury by suppressing oxidative stress and neuronal apoptosis via regulation of the Nrf2/HO-1 pathway. Oxid. Med. Cell. Longev. 2022, 2022, 2152746. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ma, C.; Meng, C.-J.; Zhu, G.-Q.; Sun, X.-B.; Huo, L.; Zhang, J.; Liu, H.-X.; He, W.-C.; Shen, X.-M.; et al. Melatonin activates the Nrf2-ARE pathway when it protects against early Brain Injury in a Subarachnoid Hemorrhage model: Melatonin and Nrf2-ARE pathway in SAH. J. Pineal Res. 2012, 53, 129–137. [Google Scholar] [CrossRef]
- Cui, Y.H.; Zhang, X.Q.; Wang, N.D.; Zheng, M.D.; Yan, J. Vitexin protects against ischemia/reperfusion-induced brain endothelial permeability. Eur. J. Pharmacol. 2019, 853, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Chen, L.; Wei, X.; Cheng, L.; Kong, L.; Liu, X.; Zhang, X.; Liu, H. Tetramethylpyrazine analogue CXC195 ameliorates cerebral ischemia-reperfusion injury by regulating endothelial nitric oxide synthase phosphorylation via PI3K/Akt signaling. Neurochem. Res. 2015, 40, 446–454. [Google Scholar] [CrossRef]
- Hu, Z.; Bian, X.; Liu, X.; Zhu, Y.; Zhang, X.; Chen, S.; Wang, K.; Wang, Y. Honokiol protects brain against ischemia-reperfusion injury in rats through disrupting PSD95-nNOS interaction. Brain Res. 2013, 1491, 204–212. [Google Scholar] [CrossRef]
- Kondo, T.; Reaume, A.G.; Huang, T.T.; Murakami, K.; Carlson, E.; Chen, S.; Scott, R.W.; Epstein, C.J.; Chan, P.H. Edema formation exacerbates neurological and histological outcomes after focal cerebral ischemia in cuzn-superoxide dismutase gene knockout mutant mice. Brain Edema X 1997, 70, 62–64. [Google Scholar] [CrossRef]
- Kim, G.W.; Chan, P.H. Involvement of superoxide in excitotoxicity and DNA fragmentation in striatal vulnerability in mice after treatment with the mitochondrial toxin, 3-nitropropionic acid. J. Cereb. Blood Flow Metab. 2002, 22, 798–809. [Google Scholar] [CrossRef]
- Song, G.; Zhao, M.; Chen, H.; Lenahan, C.; Zhou, X.; Ou, Y.; He, Y. The role of nanomaterials in stroke treatment: Targeting oxidative stress. Oxidative Med. Cell. Longev. 2021, 2021, 8857486. [Google Scholar] [CrossRef]
- Manickam, D.S.; Brynskikh, A.M.; Kopanic, J.L.; Sorgen, P.L.; Klyachko, N.L.; Batrakova, E.V.; Bronich, T.K.; Kabanov, A.V. Well-defined cross-linked antioxidant nanozymes for treatment of ischemic brain injury. J. Control. Release. 2012, 162, 636–645. [Google Scholar] [CrossRef]
- He, J.; Liu, J.; Huang, Y.; Tang, X.; Xiao, H.; Hu, Z. Oxidative stress, inflammation, and autophagy: Potential targets of Mesenchymal Stem Cells-Based therapies in Ischemic Stroke. Front. Neurosci. 2021, 15, 641157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.L.; Zhu, Z.H.; Wang, Y.Z. Neural stem cell transplantation therapy for brain ischemic stroke: Review and perspectives. World J. Stem. Cells 2019, 11, 817–830. [Google Scholar] [CrossRef]
- Boshuizen, M.C.S.; Steinberg, G.K. Stem cell-based immunomodulation after stroke: Effects on brain repair processes. Stroke 2018, 49, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Andres, R.H.; Horie, N.; Slikker, W.; Keren-Gill, H.; Zhan, K.; Sun, G.; Manley, N.C.; Pereira, M.P.; Sheikh, L.A.; McMillan, E.L.; et al. Human neural stem cells enhance structural plasticity and axonal transport in the ischaemic brain. Brain 2011, 134, 1777–1789. [Google Scholar] [CrossRef] [PubMed]
- Mine, Y.; Tatarishvili, J.; Oki, K.; Monni, E.; Kokaia, Z.; Lindvall, O. Grafted human neural stem cells enhance several steps of endogenous neurogenesis and improve behavioral recovery after middle cerebral artery occlusion in rats. Neurobiol. Dis. 2013, 52, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Tadokoro, K.; Fukui, Y.; Yamashita, T.; Liu, X.; Tsunoda, K.; Shang, J.; Morihara, R.; Nakano, Y.; Tian, F.; Sasaki, R.; et al. Bone marrow stromal cell transplantation drives molecular switch from autophagy to the Ubiquitin-Proteasome System in Ischemic Stroke Mice. J. Stroke Cerebrovasc. Dis. 2020, 29, 104743. [Google Scholar] [CrossRef]
- Babenko, V.A.; Silachev, D.N.; Zorova, L.D.; Pevzner, I.B.; Khutornenko, A.A.; Plotnikov, E.Y.; Sukhikh, G.T.; Zorov, D.B. Improving the post-stroke therapeutic potency of mesenchymal multipotent stromal cells by cocultivation with cortical neurons: The role of crosstalk between cells. Stem Cells Transl. Med. 2015, 4, 1011–1020. [Google Scholar] [CrossRef]
- Chen, K.H.; Chen, C.H.; Wallace, C.G.; Yuen, C.M.; Kao, G.S.; Chen, Y.L.; Shao, P.L.; Chen, Y.L.; Chai, H.T.; Lin, K.C.; et al. Intravenous administrationofxenogenic adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derivedexosomes markedly reduced brain infarct volume and preserved neurological function in rat after acute ischemic stroke. Oncotarget 2016, 7, 74537–74556. [Google Scholar] [CrossRef]
- Baker, A.H.; Sica, V.; Work, L.M.; Williams-Ignarro, S.; de Nigris, F.; Lerman, L.O.; Casamassimi, A.; Lanza, A.; Schiano, C.; Rienzo, M.; et al. Brain protection using autologous bone marrow cell, metalloproteinase inhibitors, and metabolic treatment in Cerebral Ischemia. Proc. Natl. Acad. Sci. USA 2007, 104, 3597–3602. [Google Scholar] [CrossRef]
- Ord, E.N.; Shirley, R.; McClure, J.D.; McCabe, C.; Kremer, E.J.; Macrae, I.M.; Work, L.M. Combined antiapoptotic and antioxidant approach to acute neuroprotection for stroke in hypertensive rats. J. Cereb. Blood Flow Metab. 2013, 33, 1215–1224. [Google Scholar] [CrossRef]
- O’Collins, V.E.; Macleod, M.R.; Donnan, G.A.; Howells, D.W. Evaluation of combination therapy in animal models of cerebral ischemia. J. Cereb. Blood Flow Metab. 2012, 32, 585–597. [Google Scholar] [CrossRef] [PubMed]
- DeGracia, D.J. Regulation of mRNA following brain ischemia and reperfusion. WIREs RNA 2017, 8, e1415. [Google Scholar] [CrossRef]
- Liu, P.; Zhao, H.; Wang, R.; Wang, P.; Tao, Z.; Gao, L.; Yan, F.; Liu, X.; Yu, S.; Ji, X.; et al. MicroRNA-424 protects against focal cerebral ischemia and reperfusion injury in mice by suppressing oxidative stress. Stroke 2015, 46, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wu, R.-X.; Li, X.-L.; Zeng, Y.-S.; Liang, J.-Y.; Fu, K.; Liang, Y.; Wang, Z. Traditional medicine in China for ischemic stroke: Bioactive components, pharmacology, and mechanisms. J. Integr. Neurosci. 2022, 21, 26. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zuo, Z.; Chow, M.S.S. Salvia miltiorrhiza: An overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J. Clin. Pharmacol. 2005, 45, 1345–1359. [Google Scholar] [CrossRef]
- Fan, Y.; Luo, Q.; Wei, J.; Lin, R.; Lin, L.; Li, Y.; Chen, Z.; Lin, W.; Chen, Q. Mechanism of salvianolic acid B neuroprotection against ischemia/reperfusion induced cerebral injury. Brain Research 2018, 1679, 125–133. [Google Scholar] [CrossRef]
- Park, J.H.; Park, O.K.; Cho, J.; Chen, B.H.; Kim, I.H.; Ahn, J.H.; Lee, J.-C.; Yan, B.C.; Yoo, K.-Y.; Lee, C.H.; et al. Antiinflammatory effect of tanshinone i in neuroprotection against cerebral ischemia-reperfusion injury in the gerbil hippocampus. Neurochem. Res. 2014, 39, 1300–1312. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Xu, S.F.; Feng, N.; Zhang, S.; Wang, X.L. Therapeutic effects of artificial musk on acute ischemic stroke and subarachnoid hemorrhage in rats. Acta Pharm. Sin. 2019, 54, 1036–1040. [Google Scholar] [CrossRef]
- Yin, D.D.; Wang, Y.; Yang, M.; Yin, D.K.; Wang, G.K.; Xu, F. Analysis of Chuanxiong Rhizoma substrate on production of ligustrazine in endophytic Bacillus Subtilis by ultra performance liquid chromatography-quadrupole time-of-flight mass spectrometry. J. Sep. Sci. 2019, 42, 3067–3076. [Google Scholar] [CrossRef]
- Wu, H.; Li, Y.; Zhang, Q.; Wang, H.; Wenyu Xiu, W.; Xu, P.; Deng, Y.; Huang, W.; Wang, D.O. Crocetin antagonizes parthanatos in ischemic stroke via inhibiting NOX2 and preserving mitochondrial hexokinase-I. Cell Death Dis. 2023, 14, 50. [Google Scholar] [CrossRef]
- Azami, S.; Forouzanfar, F. Potential role of Nigella Sativa and its constituent (Thymoquinone) in ischemic stroke. Curr. Mol. Med. 2024, 24, 327–334. [Google Scholar] [CrossRef]
- Mao, M.; Xia, Q.; Zhan, G.; Bing, H.; Zhang, C.; Wang, J.; Tian, W.; Lian, H.; Li, X.; Chu, Q. Vialinin A alleviates oxidative stress and neuronal injuries after ischaemic stroke by accelerating Keap1 degradation through inhibiting USP4-mediated deubiquitination. Phytomedicine 2024, 124, 155304. [Google Scholar] [CrossRef]
- Seo, H.-W.; Ha, T.-Y.; Ko, G.; Jang, A.; Choi, J.-W.; Lee, D.-H.; Chang, K.-A. Scutellaria baicalensis attenuated neurological impairment by regulating programmed cell death pathway in ischemic stroke mice. Cells 2023, 12, 2133. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, K.; Zhang, W.; Guo, C.; Liu, H. Ecdysterone improves oxidative damage induced by acute ischemic stroke via inhibiting ferroptosis in neurons through ACSL4. J. Ethnopharmacol. 2024, 331, 118204. [Google Scholar] [CrossRef]
- Xie, W.; Zhu, T.; Zhou, P.; Xu, H.; Meng, X.; Tao Ding, T.; Nan, F.; Sun, G.; Sun, X. Notoginseng leaf triterpenes ameliorates mitochondrial oxidative injury via the NAMPT-SIRT1/2/3 signaling pathways in cerebral ischemic model rats. J. Ginseng. Res. 2023, 47, 199–209. [Google Scholar] [CrossRef]
- Gudarzi, S.; Jafari, M.; Jahromi, G.P.; Eshrati, R.; Asadollahi, M.; Nikdokht, P. Evaluation of modulatory effects of saffron (Crocus sativus L.) aqueous extract on oxidative stress in ischemic stroke patients: A randomized clinical trial. Nutr. Neurosci. 2022, 25, 1137–1146. [Google Scholar] [CrossRef]
- Escobar-Peso, A.; Martínez-Alonso, E.; Masjuan, J.; Alcázar, A. Development of pharmacological strategies with therapeutic potential in ischemic stroke. Antioxidants 2023, 12, 2102. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, Y.; Li, N.; Xu, L.; Yang, H.; Yang, Z. L-3-n-butylphthalide improves cognitive deficits in rats with chronic cerebral ischemia. Neuropharmacology 2012, 62, 2424–2429. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Shi, B.; Luo, W.; Yang, J. The protective effect of caffeic acid on global cerebral ischemia-reperfusion injury in rats. Behav. Brain Funct. 2015, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, X.; Tang, R.; Liu, J.; Xu, H. Effect of scutellarin on nitric oxide production in early stages of neuron damage induced by hydrogen peroxide. Pharmacol. Res. 2005, 51, 205–210. [Google Scholar] [CrossRef]
- Zuo, W.; Yan, F.; Zhang, B.; Hu, X.; Mei, D. Salidroside improves brain ischemic injury by activating PI3K/Akt pathway and reduces complications induced by delayed tPA treatment. Eur. J. Biochem. 2018, 830, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Xu, M.; Zhou, Y.; Li, X.; Wang, Z.; Liu, X.; Meng, X.; Yong Zeng, Y.; Zhang, H. The Status quo and way forwards on the development of Tibetan medicine and the pharmacological research of tibetan materia Medica. Pharmacol. Res. 2020, 155, 104688. [Google Scholar] [CrossRef]
- Diener, H.C.; Lees, K.R.; Lyden, P.; Grotta, J.; Davalos, A.; Davis, S.M.; Shuaib, A.; Ashwood, T.; Wasiewski, W.; Alderfer, V.; et al. NXY-059 for the treatment of acute stroke: Pooled analysis of the SAINT I and II Trials. Stroke 2008, 39, 1751–1758. [Google Scholar] [CrossRef]
- Sun, Y.; Jiang, J.; Zhang, Z.; Yu, P.; Wang, L.; Xu, C.; Liu, W.; Wang, Y. Antioxidative and thrombolytic TMP nitrone for treatment of ischemic stroke. Bioorg. Med. Chem. 2008, 16, 8868–8874. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, T.; Wu, L.; Zhou, X.; Gu, J.; Li, C.; Liu, W.; Long, C.; Yang, X.; Shan, L.; et al. Neuroprotective effect and mechanism of action of tetramethylpyrazine nitrone for ischemic stroke therapy. Neuromol. Med. 2018, 20, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, G.; Zhang, Z.; Yu, P.; Zhong, H.; Du, J.; Wang, Y. Novel multi-functional nitrones for treatment of ischemic stroke. Bioorg. Med. Chem. 2012, 20, 3939–3945. [Google Scholar] [CrossRef]
- Chen, H.; Tan, G.; Cao, J.; Zhang, G.; Yi, P.; Yu, P.; Sun, Y.; Zhang, Z.; Wang, Y. Design, synthesis, and biological evaluation of novel tetramethylpyrazine derivatives as potential neuroprotective agents. Chem. Pharm. Bull. 2017, 65, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, L.; Cheng, C.; Li, G.; Xie, J.; Shen, M.; Qian Chen, Q.; Li, W.; He, W.; Qiu, P.; et al. Design, synthesis and biological evaluation of chalcone analogues with novel dual antioxidant mechanisms as potential anti-ischemic stroke agents. Acta Pharm. Sin. B 2019, 9, 335–350. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, M.J.; Chin, S.L.; Rangarajan, S.; Xavier, D.; Liu, L.; Zhang, H.; Rao-Melacini, P.; Zhang, X.; Pais, P.; Agapay, S.; et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): A case-control study. Lancet 2016, 388, 761–775. [Google Scholar] [CrossRef]
- Sokol, S.I.; Kapoor, J.R.; Foody, J.M. Blood pressure reduction in the primary and secondary prevention of stroke. Curr. Vasc. Pharmacol. 2006, 4, 155–160. [Google Scholar] [CrossRef]
- Boan, A.D.; Lackland, D.T.; Ovbiagele, B. Lowering of blood pressure for recurrent stroke prevention. Stroke 2014, 45, 1253–2506. [Google Scholar] [CrossRef] [PubMed]
- Arenillas, J.F.; Moro, M.A.; Dávalos, A. The metabolic syndrome and stroke. Stroke 2007, 38, 2196–2203. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Tessier, D.; Carpentier, A. The metabolic syndrome. Pathol. Biol. 2006, 54, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, I.; Straczkowski, M.; Nikolajuk, A.; Adamska, A.; Karczewska-Kupczewska, M.; Otziomek, E.; Kinalska, I.; Gorska, M. Insulin resistance, serum adiponectin, and proinflammatory markers in young subjects with the metabolic syndrome. Metabolism 2008, 57, 1539–4154. [Google Scholar] [CrossRef]
- Koren-Morag, N.; Goldbourt, U.; Tanne, D. Relation between the metabolic syndrome and ischemic stroke or transient ischemic attack: A prospective cohort study in patients with atherosclerotic cardiovascular disease. Stroke 2005, 36, 1366–1371. [Google Scholar] [CrossRef]
- Najarian, R.M.; Sullivan, L.M.; Kannel, W.B.; Wilson, P.W.; D’Agostino, R.B.; Wolf, P.A. Metabolic syndrome compared with type 2 diabetes mellitus as a risk factor for stroke: The Framingham Offspring Study. Arch. Intern. Med. 2006, 166, 106–111. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of insulin action and insulin resistance. Physiol Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef]
- Maciejczyk, M.; Żebrowska, E.; Chabowski, A. Insulin resistance and oxidative stress in the brain: What’s new? Int. J. Mol. Sci. 2019, 20, 874. [Google Scholar] [CrossRef]
- Ding, P.-F.; Zhang, H.-S.; Wang, J.; Gao, Y.-Y.; Mao, J.-N.; Hang, C.-H.; Li, W. Insulin resistance in ischemic stroke: Mechanisms and therapeutic approaches. Front. Endocrinol. 2022, 13, 1092431. [Google Scholar] [CrossRef]
- Horn, J.W.; Feng, T.; Mørkedal, B.; Strand, L.B.; Horn, J.; Mukamal, K.; Janszky, I. Obesity and risk for first ischemic stroke depends on metabolic syndrome: The HUNT study. Stroke 2021, 52, 3555–3561. [Google Scholar] [CrossRef]
- Lee, H.J.; Choi, E.K.; Lee, S.H.; Kim, Y.J.; Han, K.D.; Oh, S. Risk of ischemic stroke in metabolically healthy obesity: A nationwide population-based study. PLoS ONE 2018, 13, e0195210. [Google Scholar] [CrossRef] [PubMed]
- Bosetti, F.; Koenig, J.I.; Ayata, C.; Back, S.A.; Becker, K.; Broderick, J.P.; Carmichael, S.T.; Cho, S.; Cipolla, M.J.; Corbett, D.; et al. Translational stroke research: Vision and opportunities. Stroke 2017, 48, 2632–2637. [Google Scholar] [CrossRef] [PubMed]
- Salatin, S.; Farhoudi, M.; Farjami, A.; Dizaj, S.M.; Sharifi, S.; Shahi, S. Nanoparticle formulations of antioxidants for the management of oxidative stress in stroke: A review. Biomedicines 2023, 11, 3010. [Google Scholar] [CrossRef]
- Hochrainer, K.; Yang, W. Stroke Proteomics: From discovery to diagnostic and therapeutic applications. Circ. Res. 2022, 130, 1145–1166. [Google Scholar] [CrossRef] [PubMed]

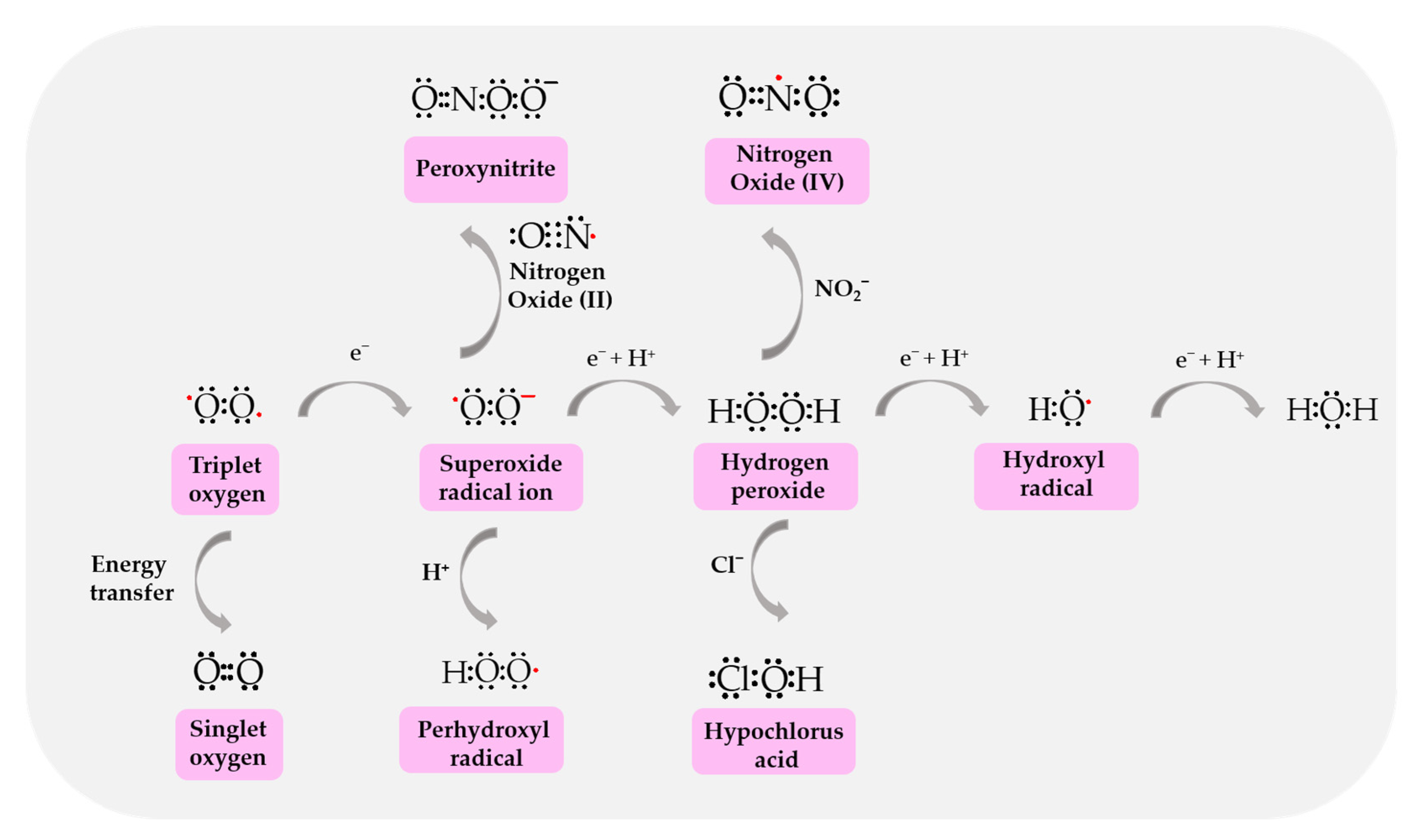
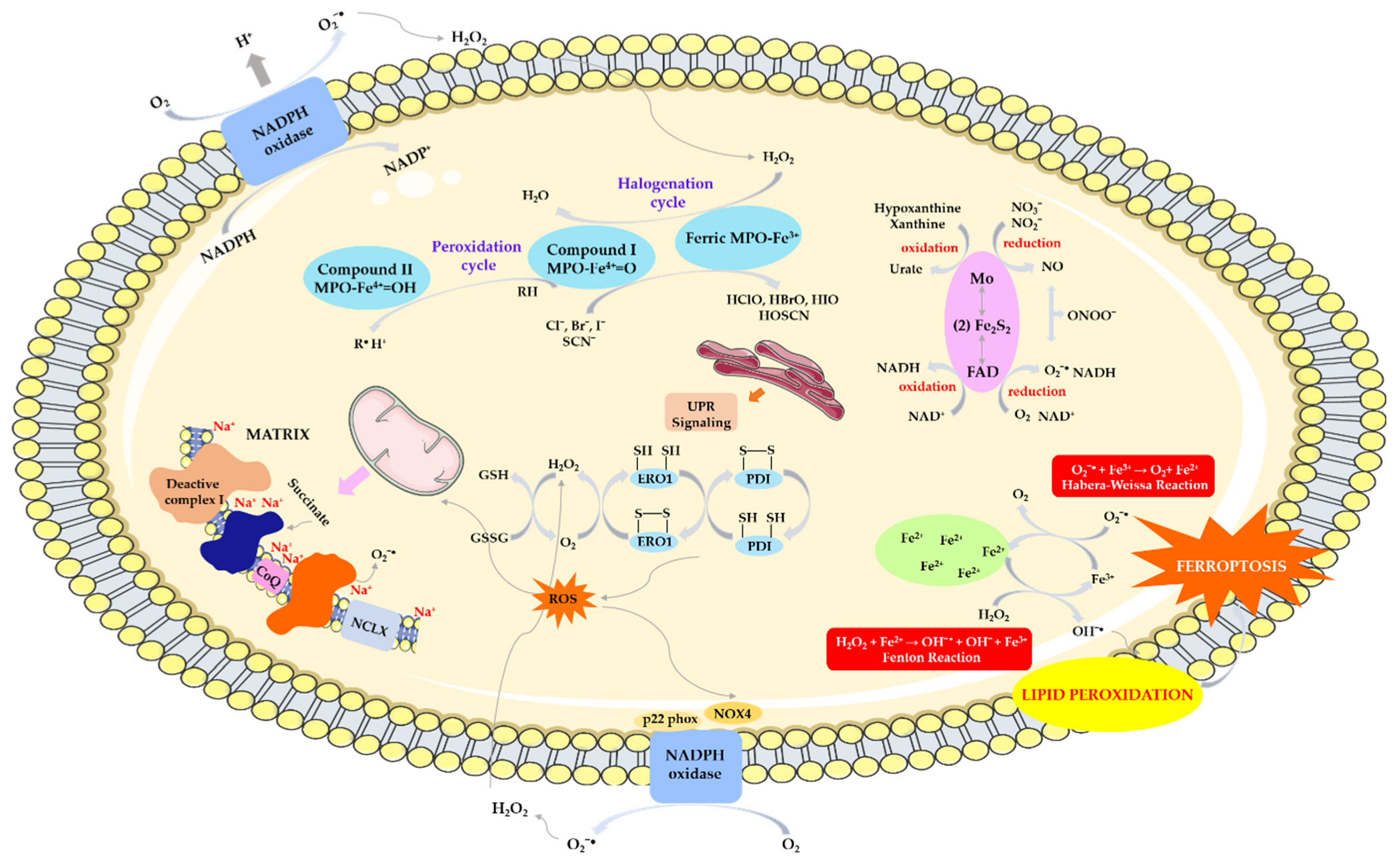

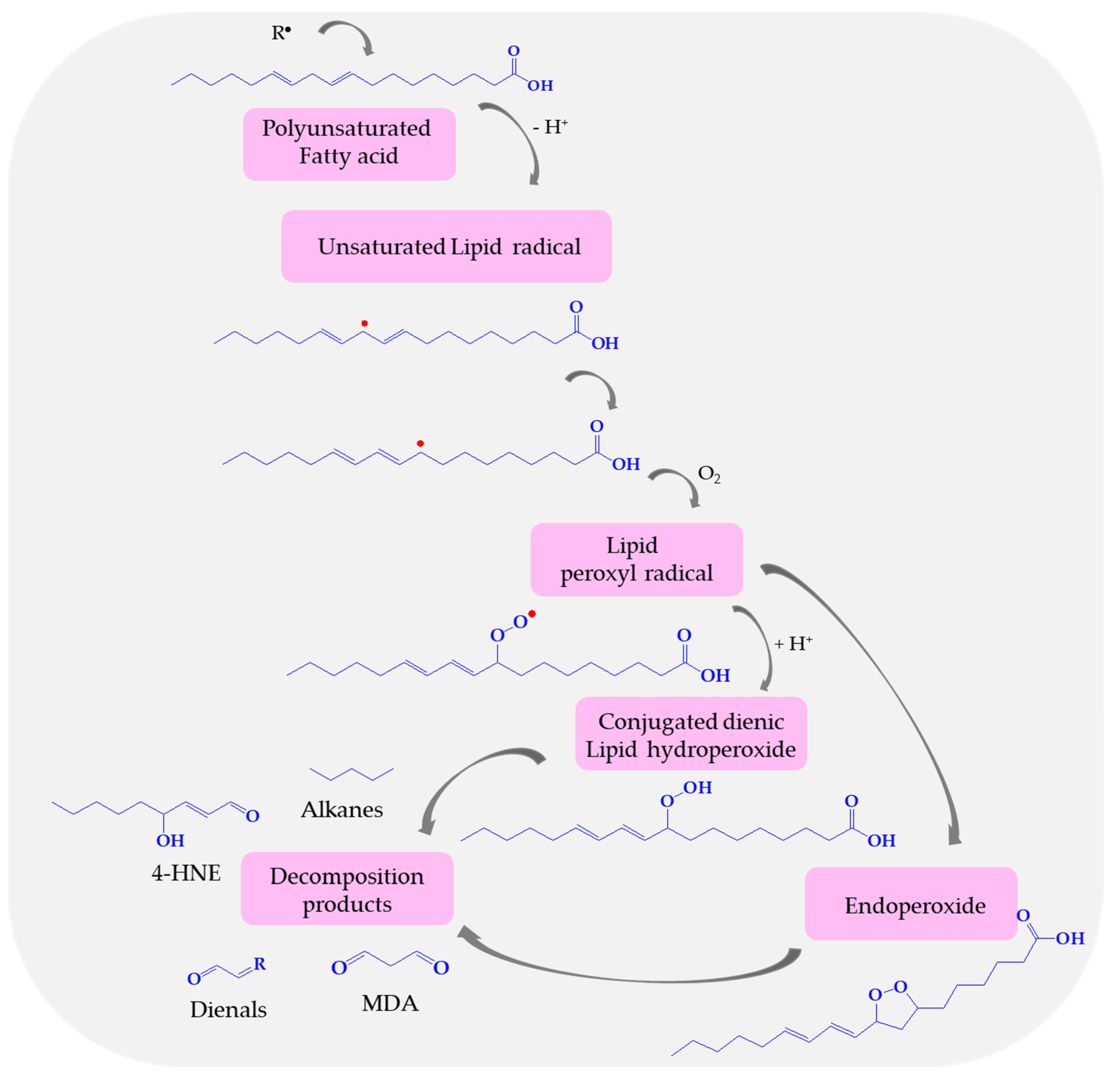
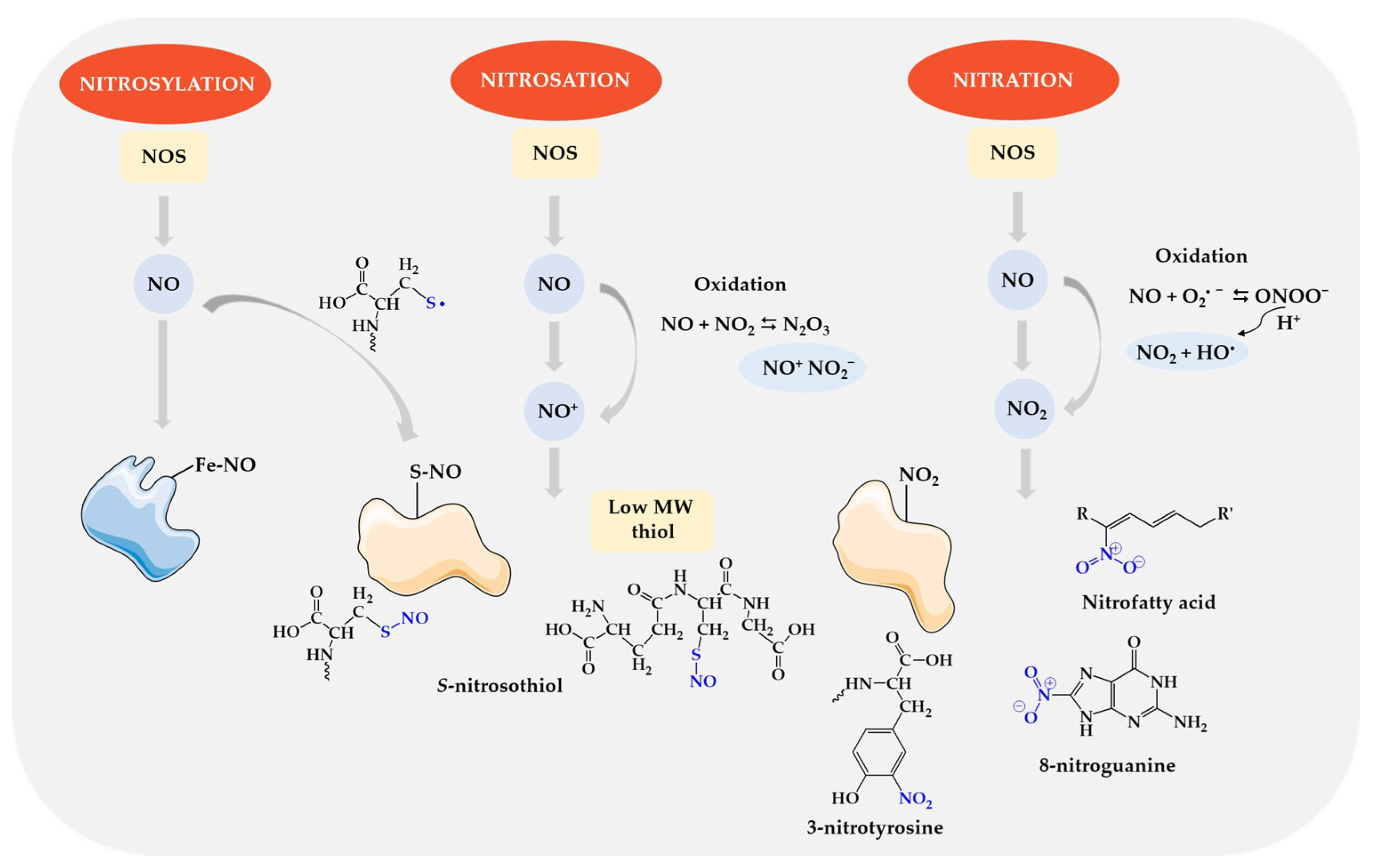
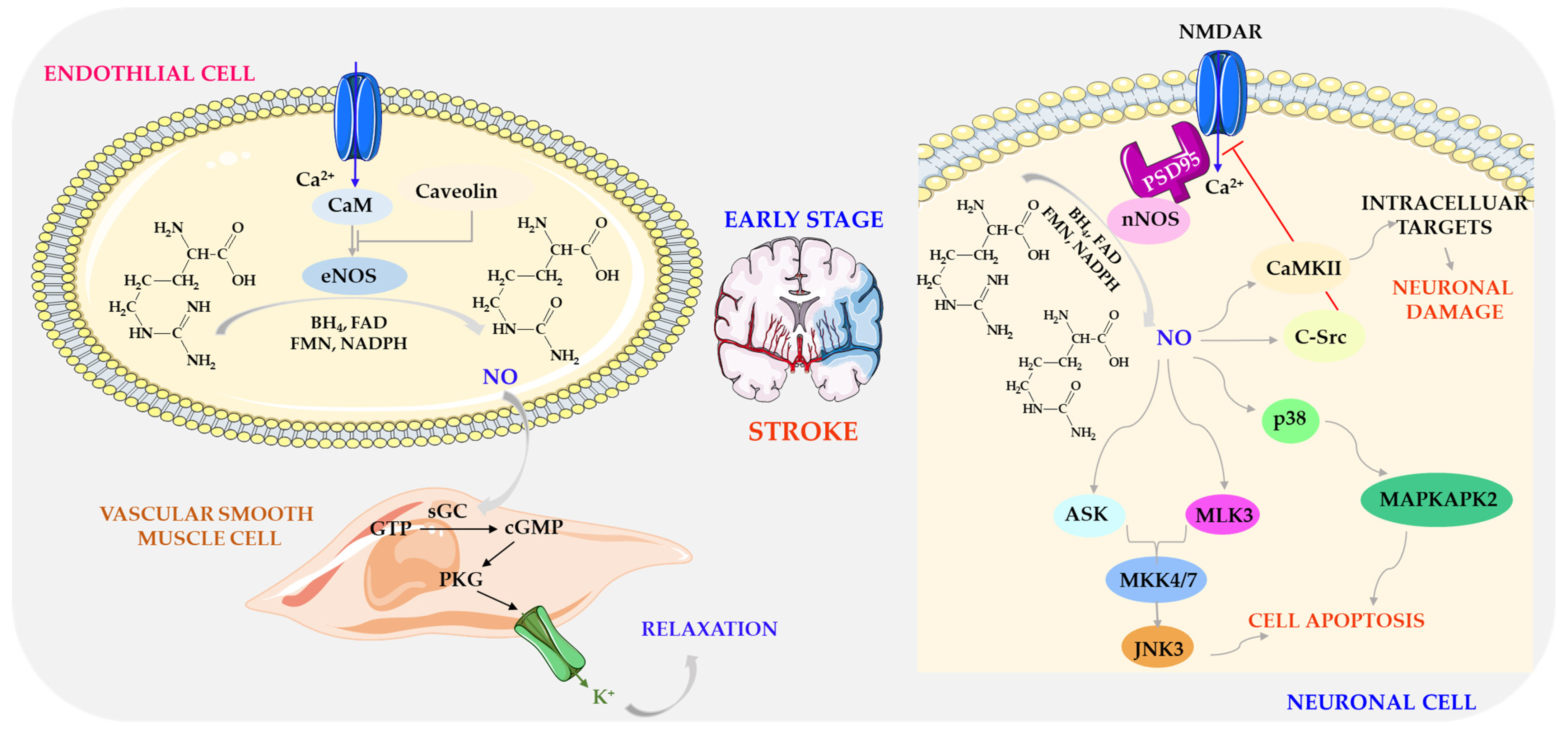

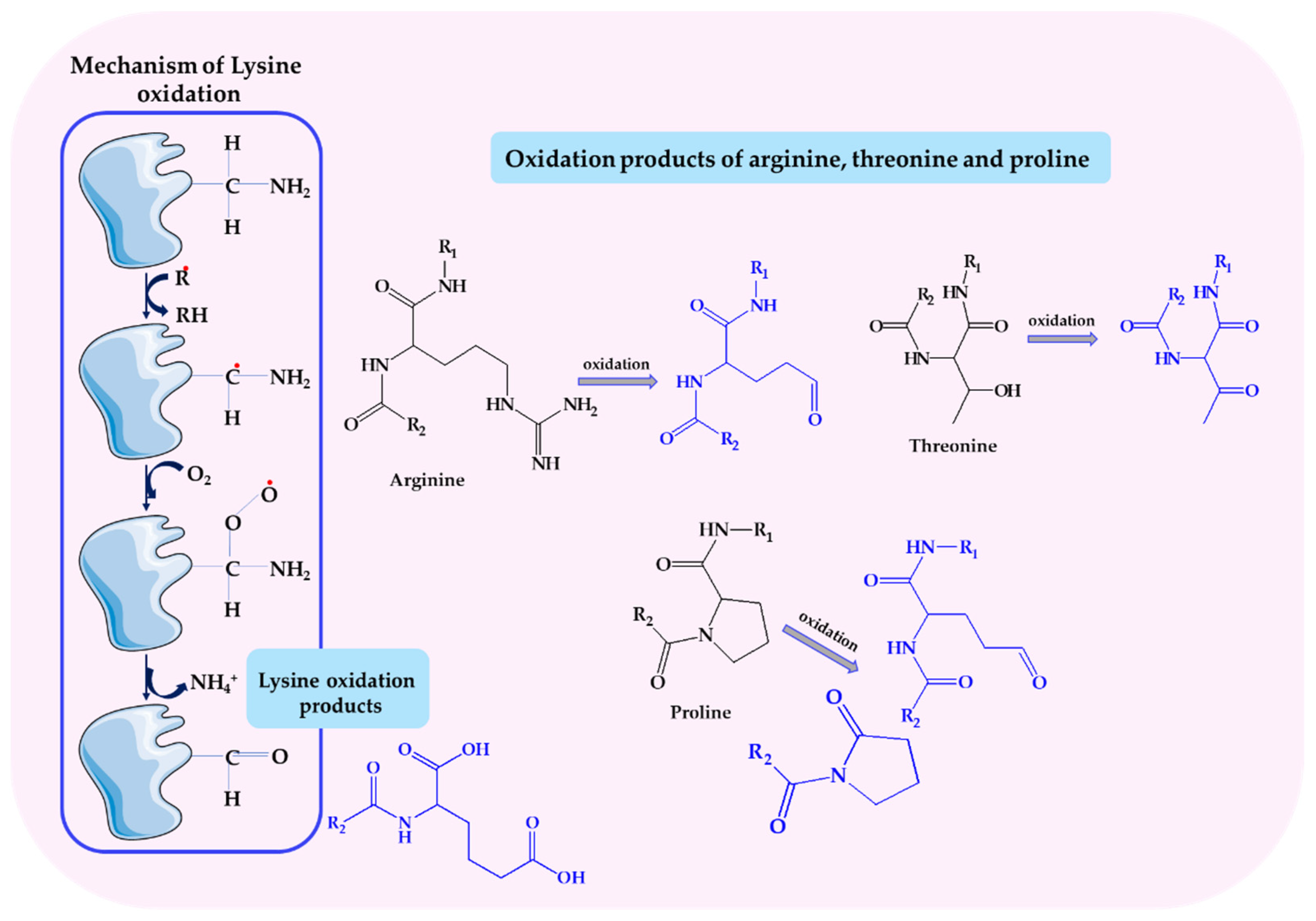
| Biomarker /Unit | Biological Material /Collection Time (after Stroke) | Average Concentration | Control | Reference | ||
|---|---|---|---|---|---|---|
| TAC | [µmol/L] | Serum | 24 h | 5.72 ± 1.41 *** (n = 100) | 8.53 ± 2.41 (n = 99) | [88] |
| 30 days | 0.65 ± 0.08 * (n = 14) | 1.31 ± 0.21 (n = 15) | [77] | |||
| Less than 48 h | 1043.4 ± 140.7 (n = 73) | 1079.7 ± 197.9 (n = 62) | [92] | |||
| 24 h | 540 (38) (n = 31) | 550 (38) (n = 26) | [91] | |||
| 48–72 h | 516 (28) (n = 31) | 545 (70) (n = 26) | ||||
| 7 days | 487 (39) (n = 31) | 603 (113) (n = 26) | ||||
| 48 h | 3687.35 (801.56) (n = 216) | 3905.98 (879.89) (n = 188) | [90] | |||
| mmol/L | 1 day | 1.7 ± 0.30 *** (n = 60) | 4.20 ± 0.50 (n = 30) | [93] | ||
| 48 h | 1.41 (0.13) (n = 35) | 1.39 (0.15) (n = 44) | [96] | |||
| mM | 24 h | 10.03 ± 1.97 *** (n = 29 diabetic patients) 5.97 ± 2.04 *** (n = 36 nondiabetic patients) | 5.44 ± 1.06 (n = 20) | [94] | ||
| mmol/mL | 1 day | 2.38 (1.83–3.35) ** (n = 29 survivors) 5.33 (3.27–11.40) (n = 29 non-survivors) | - | [84] | ||
| Biomarker /Unit | Biological Material /Collection Time (after Stroke) | Average Concentration/Activity Value of Biomarker in Human Stroke | Control | Reference | |||
|---|---|---|---|---|---|---|---|
| GPx | U/g Hb | Hemolysate | 12 h | 23.6 ± 7.34 *** (n = 40) | 77.7 ± 22.6 (n = 35) | [111] | |
| NIHSS ≤ 10 | 29.1 ± 11.3 (n = 23) | ||||||
| NIHSS > 10 | 16.7 ± 8.5 (n = 17) | ||||||
| mRS ≤ 3 | 26.8 ± 12 (n = 22) | ||||||
| mRS > 3 | 19.2 ± 19 (n = 18) | ||||||
| 24 h | 26.2 ± 5.1 * | (n = 34) | 30.5 ± 7.9 (n = 30) | [114] | |||
| 7 days | 32.4 ± 4.6 | ||||||
| 24 h | 36.16 ± 4.91 * | (n = 82) | 24.83 ± 1.24 (n = 81) 3.61 ± 0.17 | [109] | |||
| 7 days | 26.28 ± 1.51 | ||||||
| 3 months | 23.55 ± 2.60 | ||||||
| CAT | µkat/g Hb | 24 h | 4.92 ± 0.17 *** | ||||
| 7 days | 6.56 ± 0.24 *** | ||||||
| 3 months | 4.85 ± 0.21 *** | ||||||
| GPx | nmol/min per mg of proteins | - | (n = 75) | 27.4 ± 3.52 (n = 23) | [113] | ||
| NIHSS 4.6 ± 0.5, n = 9 | 36.2 ± 6.13 | ||||||
| NIHSS 8.9 ± 0.7, n = 32 | 35.2 ± 3.11 | ||||||
| NIHSS 15.4 ± 1.8, n = 34 | 44.2 ± 5.50 * | ||||||
| Plasma | (n = 75) | 2.64 ± 0.33 (n = 23) | |||||
| NIHSS 4.6 ± 0.5, n = 9 | 6.77 ± 1.31 ** | ||||||
| NIHSS 8.9 ± 0.7, n = 32 | 4.40 ± 0.68 * | ||||||
| NIHSS 15.4 ± 1.8, n = 34 | 9.49 ± 1.67 *** | ||||||
| U/L | Serum | 6 h | 128 ± 44 | (n = 11) | 113 ± 36 (n = 17) | [112] | |
| 1 day | 153 ± 37 * | ||||||
| 3 days | 165 ± 55 | ||||||
| 7 days | 147 ± 61 | ||||||
| SOD | U/mL | Admission (8–12 a.m.) | 4.5 ± 0.7 *** (n = 35) | 3.4 ± 0.6 (n = 32) | [108] | ||
| U/mg Hb | Hemolysate | 24 h | 697.62 ± 8.95 ** (n = 82) | 661.44 ± 7.15 (n = 81) | [109] | ||
| 7 days | 750.45 ± 9.72 *** (n = 82) | ||||||
| 3 months | 747.41 ± 17.27 ** (n = 82) | ||||||
| U/gHb | 12 h | NIHSS ≤ 10 | 1.75 ± 0.5 (n = 23) | Figure 1c in [111] | [111] | ||
| NIHSS > 10 | 2.3 ± 0.3 (n = 17) | ||||||
| mRS ≤ 3 | 1.8 ± 0.3 (n = 22) | ||||||
| mRS > 3 | 2.2 ± 0.4 (n = 18) | ||||||
| U/L | Plasma | NIHSS ≤ 10 | 1.8 ± 0.3 (n = 23) | Figure 2b in [111] | |||
| NIHSS > 10 | 2.2 ± 0.4 (n = 17) | ||||||
| mRS ≤ 3 | 1.8 ± 0.5 (n = 22) | ||||||
| mRS > 3 | 2.1 ± 0.4 (n = 18) | ||||||
| U/µmol Hb | Hemolysate | Admission | 12.2 ± 2.9 (n = 11) | 15–22 (n = 101) | [112] | ||
| 1 day | 12.7 ± 4.3 (n = 11) | ||||||
| 3 days | 10.7 ± 1.2 (n = 11) | ||||||
| 7 days | 12.8 ± 2.6 (n = 11) | ||||||
| Biomarker /Unit | Biological Material /Collection Time (after Stroke) | Average Concentration | Control | Reference | ||
|---|---|---|---|---|---|---|
| MEL | [pg/mL] | Urine | 4.5 h | 6.41 [4.26, 8.64] *** (n = 77) | 37.85 [21.46, 103.82] (n = 23) | [5] |
| 24 h | 9.34 [5.49, 25.42] *** (n = 44) | |||||
| 7 days | 9.61 [6.04, 15.33] *** (n = 31) | |||||
| Biomarker /Unit | Biological Material /Collection Time (after Stroke) | Average Concentration | Control | Reference | |||
|---|---|---|---|---|---|---|---|
| GSH | µM | Plasma | 10–24 h | NIHSS > 10 GSH ≤ 1.07 μM vs. GSH > 2.64 μM (n = 43) | - | [151] | |
| Age/gender-adjusted odds ratio: 4.69, 95% CI: 1.43–15.4 | |||||||
| mg/dL | Serum | 48 h | 3.9 ± 2.5 * (n = 70) 95% CI: 0.99–2.19 | 2.3 ± 0.4 (n = 70) | [155] | ||
| Lower CNS Higher CNS | 5.37 ± 2.8 (n = 6) 3.85 ± 2.4 (n = 64) | ||||||
| µM | Admission | 84 ± 26 * (n = 11) | 68 ± 97 (n = 101) | [112] | |||
| Day 1 | 78 ± 39 (n = 11) | ||||||
| Day 3 | 81 ± 42 (n = 11) | ||||||
| Day 7 | 77 ± 34 (n = 11) | ||||||
| Biomarker /Unit | Biological Material /Collection Time (after Stroke) | Average Concentration | Control | Reference | |||
|---|---|---|---|---|---|---|---|
| MDA | mol/gHb | erythrocyte | 24 h | 349.7 ± 60.5 *** (n = 34) | 200.0 ± 605.7 (n = 30) | [114] | |
| After 7 days | 80.5 ± 47.3–269.2 ± 40.9 (n = 34) | ||||||
| µmol/L | Plasma | 12 h | NIHSS ≤ 10 | 17.1 ± 3.5 (n = 23) | Figure 2d in [111] | [111] | |
| NIHSS > 10 | 18.6 ± 5.4 (n = 17) | ||||||
| mRS ≤ 3 | 17.4 ± 7 (n = 22) | ||||||
| mRS > 3 | 18.9 ± 6.5 (n = 18) | ||||||
| nmol/g protein | Serum | Admission (8–12 a.m.) | 151.5 ± 51.1 *** (n = 35) | 111.3 ± 27.4 (n = 32) | [108] | ||
| µmol/L | 24–72 h | 5.10 ± 1.26 (n = 60) | 3.27 ± 0.52 (n = 30) | [164] | |||
| 24 h | 7.11 ± 1.67 (n = 100) | 1.64 ± 0.82 (n = 99) | [88] | ||||
| 48 h | 2.08 (n = 216) | 1.85 (n = 188) | [90] | ||||
| 0 h | 0.75 ± 0.06 (n = 45) | 0.83 ± 0.06 (n = 45) | [168] | ||||
| 48 h | 1.65 ± 0.08 (n = 45) | ||||||
| - | 2.51 ± 1.11 (n = 50) | 1.12 ± 0.35 (n = 25) | [170] | ||||
| nmol/L | 0 h | 8.47 ± 2.46 (n = 20) | 5.99 ± 2.28 (n = 27) | [166] | |||
| 24 h | 9.75 ± 1.94 (n = 20) | ||||||
| 48 h | 8.17 ± 2.14 (n = 20) | ||||||
| 72 h | 8.39 ± 3.13 (n = 20) | ||||||
| 96 h | 7.93 ± 2.35 (n = 20) | ||||||
| µM/mg protein | 8 h | 1.433 mild AIS (n = 85) 1.505 moderate AIS (n = 37) | 1.526 (n = 40) | [167] | |||
| µmol/L | Saliva | - | 0.64 ± 0.22 (n = 50) | 0.23 ± 0.07 (n = 25) | [170] | ||
| Biomarker /Unit | Biological Material /Collection Time (after Stroke) | Average Concentration | Control | Reference | |||
|---|---|---|---|---|---|---|---|
| NOx | µmol/L | Serum | 48 h | 35.1 ± 12 * (n = 70) | 14.9 ± 3.8 n = 70 | [155] | |
| Lower CNS | 67.9 ± 12.04 * (n = 6) | ||||||
| Higher CNS | 39.35 ± 24.5 * (n = 64) | ||||||
| - | 76.4 ± 53.3 *** (n = 54) | 41.5 ± 27.0 (n = 50) | [207] | ||||
| 24 h | No infarct volume growth | 9.3 (8–14.6) (n = 44) | 15.3 (12.1–17.9) (n = 14) | [206] | |||
| Infarct volume growth | 9.1 (5.9–14.4) (n = 26) | ||||||
| 48 h | No infarct volume growth | 10.6 (7.6–13.6) (n = 44) | |||||
| Infarct volume growth | 9.9 (6.9–14) (n = 26) | ||||||
| Increase (day 7-day 1) | No infarct volume growth | 5 (1.9–12.2) (n = 44) * | |||||
| Infarct volume growth | 1.2 (0–7.6) (n = 26) * | ||||||
| µmol/g protein | Admission (8–12 a.m.) | 12.4 ± 6.8 *** (n = 35) | 17.4 ± 2.5 (n = 32) | [108] | |||
| nmol/mg protein | Plasma | 24 h | 51.10 ± 12.50 *** (n = 47) | 115.40 ± 12.40 (n = 30) | [205] | ||
| Non-lacunar | 41.8 (9.3; n = 23) *** | ||||||
| lacunar | 60.0 (7.9; n = 24) *** | ||||||
| Biomarker /Unit | Biological Material /Collection Time (after Stroke) | Average Concentration | Control | Reference | ||
|---|---|---|---|---|---|---|
| CG | U/mL | Serum | <4.5 h | 222 [171, 283] ** (n = 81) | 144 [93, 191] (n = 22) | [5] |
| 24 h | 355 [224, 437] ** (n = 37) | |||||
| 192 h | 195 [124, 242] * (n = 34) | |||||
| nmol/mg protein | 24 h | 4.9744 ± 5.2691 *** (n = 18) | 1.28.3 ± 39.3 (n = 34) | [244] | ||
| Admission (7–9 a.m.) | 105.32 *** (n = 31) | 41.56 (n = 20) | [245] | |||
| Plasma | Admission Discharge | 1.21 ± 0.24 (n = 20) * 0.79 ± 0.16 (n = 20) * | 0.70 ± 0.12 (n = 24) | [246] | ||
| 24 h 192 h 3 months | 0.22 ± 0.03 (n = 82) 0.28 ± 0.04 (n = 82) 0.20 ± 0.07 (n= 82) | 0.25 ± 0.04 (n = 81) | [109] | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawluk, H.; Tafelska-Kaczmarek, A.; Sopońska, M.; Porzych, M.; Modrzejewska, M.; Pawluk, M.; Kurhaluk, N.; Tkaczenko, H.; Kołodziejska, R. The Influence of Oxidative Stress Markers in Patients with Ischemic Stroke. Biomolecules 2024, 14, 1130. https://doi.org/10.3390/biom14091130
Pawluk H, Tafelska-Kaczmarek A, Sopońska M, Porzych M, Modrzejewska M, Pawluk M, Kurhaluk N, Tkaczenko H, Kołodziejska R. The Influence of Oxidative Stress Markers in Patients with Ischemic Stroke. Biomolecules. 2024; 14(9):1130. https://doi.org/10.3390/biom14091130
Chicago/Turabian StylePawluk, Hanna, Agnieszka Tafelska-Kaczmarek, Małgorzata Sopońska, Marta Porzych, Martyna Modrzejewska, Mateusz Pawluk, Natalia Kurhaluk, Halina Tkaczenko, and Renata Kołodziejska. 2024. "The Influence of Oxidative Stress Markers in Patients with Ischemic Stroke" Biomolecules 14, no. 9: 1130. https://doi.org/10.3390/biom14091130
APA StylePawluk, H., Tafelska-Kaczmarek, A., Sopońska, M., Porzych, M., Modrzejewska, M., Pawluk, M., Kurhaluk, N., Tkaczenko, H., & Kołodziejska, R. (2024). The Influence of Oxidative Stress Markers in Patients with Ischemic Stroke. Biomolecules, 14(9), 1130. https://doi.org/10.3390/biom14091130






