The Endocannabinoid Peptide RVD-Hemopressin Is a TRPV1 Channel Blocker
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Transfection
2.2. Immunofluorescence
2.3. Calcium Imaging
2.4. Patch-Clamp Electrophysiology
2.5. Cell-Penetrating Peptide Analysis
2.6. Molecular Docking
2.7. Molecular Dynamics Simulations
2.8. Contact Area
2.9. Protein–Ligand Contacts
2.10. Binding Free Energy
2.11. Structure Visualization
2.12. Pore Radius Calculation
2.13. Root Mean Square Deviation
2.14. Radius of Gyration
2.15. Root Mean Square Inner Product
2.16. Statistical Analysis
3. Results
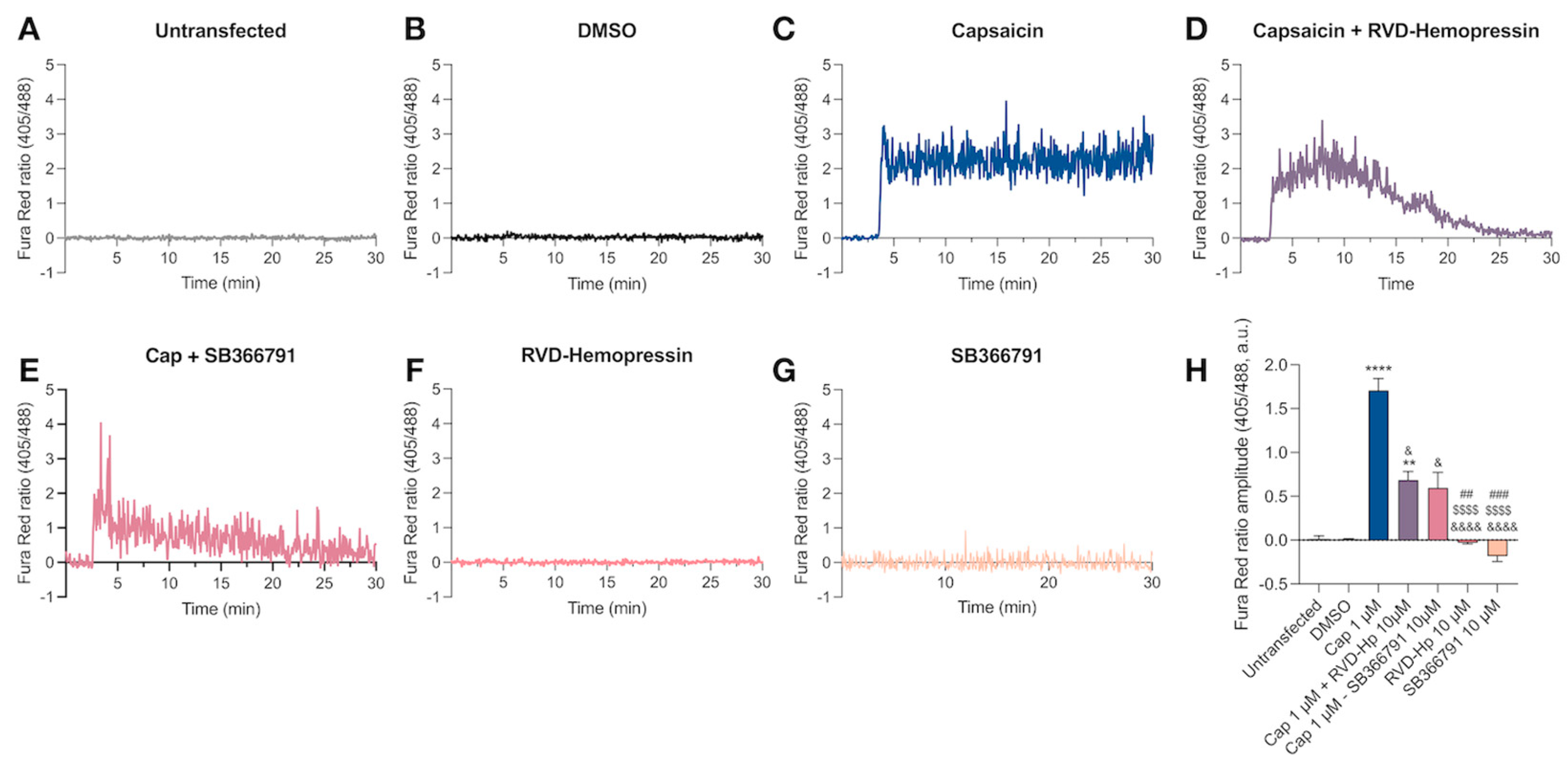
3.1. RVD-Hemopressin Reduces the Ca2+ Influx through the TRPV1 Channel in HEK293 Cells
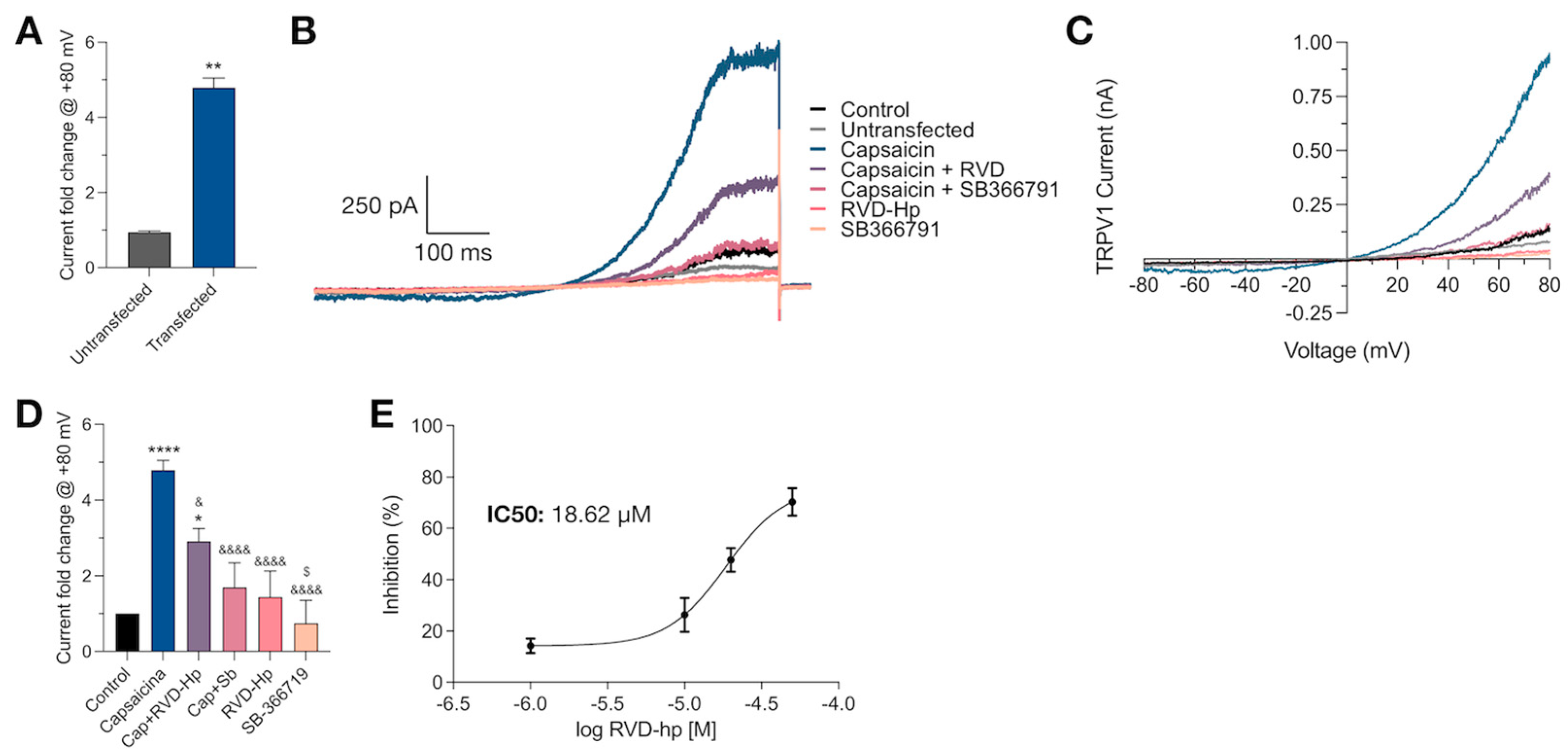
3.2. RVD-Hp Is an TRPV1 Channel Antagonist
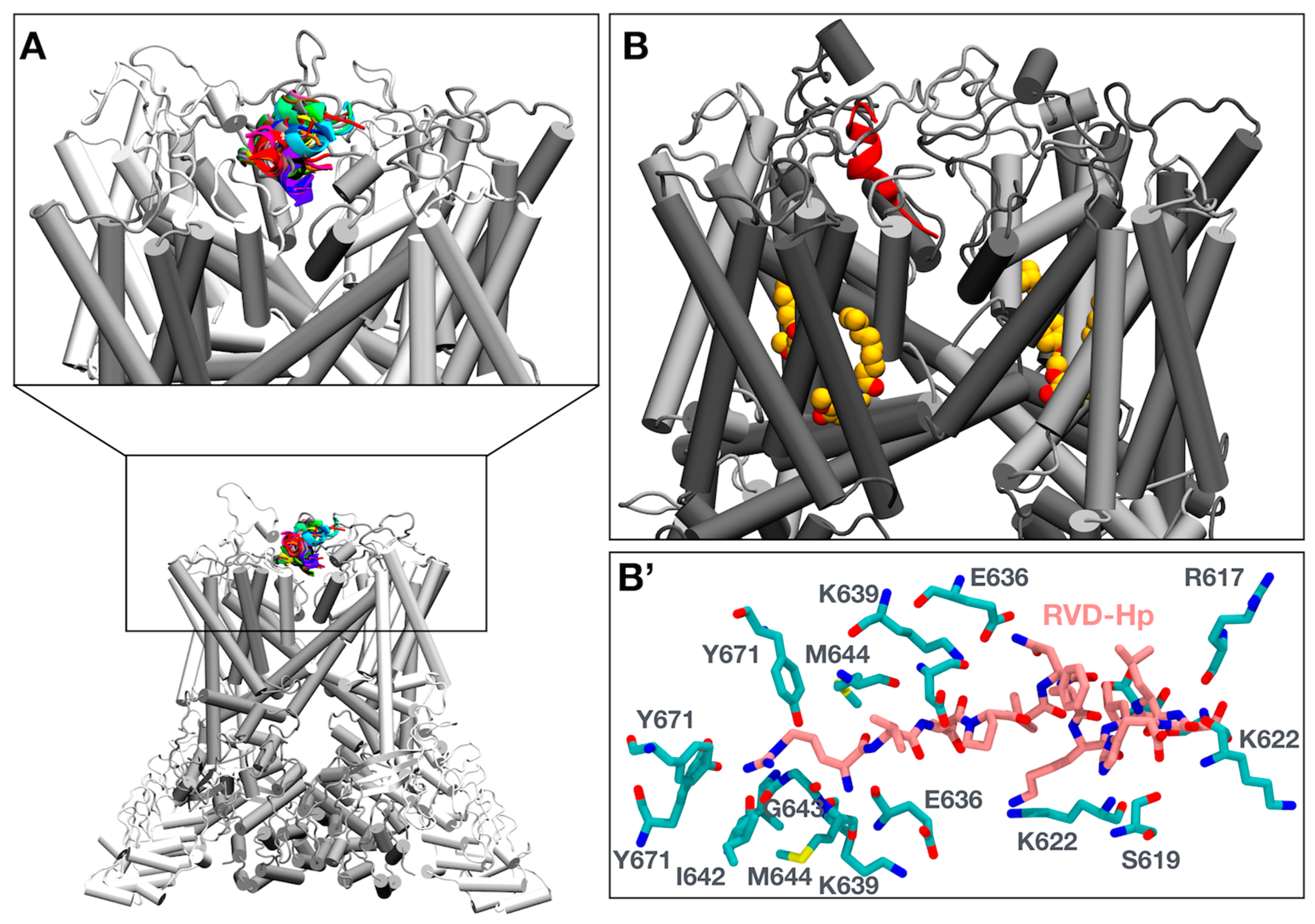
3.3. Molecular Simulations of RVD-Hp and TRPV1 Show an Interaction between RVD-Hp and the Selectivity Filter of the TRPV1 Channel
3.4. Molecular Dynamics Show That RVD-Hp Prevents Ion Permeation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gomes, I.; Grushko, J.S.; Golebiewska, U.; Hoogendoorn, S.; Gupta, A.; Heimann, A.S.; Ferro, E.S.; Scarlata, S.; Fricker, L.D.; Devi, L.A. Novel endogenous peptide agonists of cannabinoid receptors. FASEB J. 2009, 23, 3020–3029. [Google Scholar] [CrossRef]
- Hofer, S.C.; Ralvenius, W.T.; Gachet, M.S.; Fritschy, J.-M.; Zeilhofer, H.U.; Gertsch, J. Localization and production of peptide endocannabinoids in the rodent CNS and adrenal medulla. Neuropharmacology 2015, 98, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Chicca, A.; Tamborrini, M.; Eisen, D.; Lerner, R.; Lutz, B.; Poetz, O.; Pluschke, G.; Gertsch, J. Identification and Quantification of a New Family of Peptide Endocannabinoids (Pepcans) Showing Negative Allosteric Modulation at CB1 Receptors. J. Biol. Chem. 2012, 287, 36944–36967. [Google Scholar] [CrossRef]
- Petrucci, V.; Chicca, A.; Glasmacher, S.; Paloczi, J.; Cao, Z.; Pacher, P.; Gertsch, J. Pepcan-12 (RVD-hemopressin) is a CB2 receptor positive allosteric modulator constitutively secreted by adrenals and in liver upon tissue damage. Sci. Rep. 2017, 7, 9560. [Google Scholar] [CrossRef] [PubMed]
- Hebert-Chatelain, E.; Desprez, T.; Serrat, R.; Bellocchio, L.; Soria-Gomez, E.; Busquets-Garcia, A.; Zottola, A.C.P.; Delamarre, A.; Cannich, A.; Vincent, P.; et al. A cannabinoid link between mitochondria and memory. Nature 2016, 539, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Bénard, G.; Massa, F.; Puente, N.; Lourenço, J.; Bellocchio, L.; Soria-Gómez, E.; Matias, I.; Delamarre, A.; Metna-Laurent, M.; Cannich, A.; et al. Mitochondrial CB1 receptors regulate neuronal energy metabolism. Nat. Neurosci. 2012, 15, 558–564. [Google Scholar] [CrossRef]
- Ma, L.; Jia, J.; Niu, W.; Jiang, T.; Zhai, Q.; Yang, L.; Bai, F.; Wang, Q.; Xiong, L. Mitochondrial CB1 receptor is involved in ACEA-induced protective effects on neurons and mitochondrial functions. Sci. Rep. 2015, 5, srep12440. [Google Scholar] [CrossRef]
- Fogaça, M.V.; Sonego, A.B.; Rioli, V.; Gozzo, F.C.; Dale, C.S.; Ferro, E.S.; Guimarães, F.S. Anxiogenic-like effects induced by hemopressin in rats. Pharmacol. Biochem. Behav. 2015, 129, 7–13. [Google Scholar] [CrossRef]
- Leone, S.; Recinella, L.; Chiavaroli, A.; Martinotti, S.; Ferrante, C.; Mollica, A.; Macedonio, G.; Stefanucci, A.; Dvorácskó, S.; Tömböly, C.; et al. Emotional disorders induced by Hemopressin and RVD-hemopressin(α) administration in rats. Pharmacol. Rep. 2017, 69, 1247–1253. [Google Scholar] [CrossRef]
- Recinella, L.; Chiavaroli, A.; Ferrante, C.; Mollica, A.; Macedonio, G.; Stefanucci, A.; Dimmito, M.P.; Dvorácskó, S.; Tömböly, C.; Brunetti, L.; et al. Effects of central RVD-hemopressin(alpha) administration on anxiety, feeding behavior and hypothalamic neuromodulators in the rat. Pharmacol Rep. 2018, 70, 650–657. [Google Scholar] [CrossRef]
- Li, H. TRP Channel Classification. Adv. Exp. Med. Biol. 2017, 976, 1–8. [Google Scholar]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- Fenwick, A.J.; Fowler, D.K.; Wu, S.-W.; Shaffer, F.J.; Lindberg, J.E.M.; Kinch, D.C.; Peters, J.H. Direct Anandamide Activation of TRPV1 Produces Divergent Calcium and Current Responses. Front. Mol. Neurosci. 2017, 10, 200. [Google Scholar] [CrossRef] [PubMed]
- Zygmunt, P.M.; Ermund, A.; Movahed, P.; Andersson, D.A.; Simonsen, C.; Jönsson, B.A.G.; Blomgren, A.; Birnir, B.; Bevan, S.; Eschalier, A.; et al. Monoacylglycerols Activate TRPV1—A Link between Phospholipase C and TRPV1. PLoS ONE 2013, 8, e81618. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Nie, Y.; Tian, Y.; Xiao, X.; Yang, F. Endocannabinoid activation of the TRPV1 ion channel is distinct from activation by capsaicin. J. Biol. Chem. 2021, 297, 101022. [Google Scholar] [CrossRef]
- Smart, D.; Gunthorpe, M.J.; Jerman, J.C.; Nasir, S.; Gray, J.; Muir, A.I.; Chambers, J.K.; Randall, A.D.; Davis, J.B. The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1). Br. J. Pharmacol. 2000, 129, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Sasamura, T.; Sasaki, M.; Tohda, C.; Kuraishi, Y. Existence of capsaicin-sensitive glutamatergic terminals in rat hypothalamus. NeuroReport 1998, 9, 2045–2048. [Google Scholar] [CrossRef] [PubMed]
- Mezey, É.; Toth, Z.E.; Cortright, D.N.; Arzubi, M.K.; Krause, J.E.; Elde, R.; Guo, A.; Blumberg, P.M.; Szallasi, A. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc. Natl. Acad. Sci. USA 2000, 97, 3655–3660. [Google Scholar] [CrossRef]
- Sanchez, J.; Krause, J.; Cortright, D. The distribution and regulation of vanilloid receptor VR1 and VR1 5′ splice variant RNA expression in rat. Neuroscience 2001, 107, 373–381. [Google Scholar] [CrossRef]
- Roberts, J.C.; Davis, J.B.; Benham, C.D. [3H]Resiniferatoxin autoradiography in the CNS of wild-type and TRPV1 null mice defines TRPV1 (VR-1) protein distribution. Brain Res. 2003, 995, 176–183. [Google Scholar] [CrossRef]
- Tóth, A.; Boczán, J.; Kedei, N.; Lizanecz, E.; Bagi, Z.; Papp, Z.; Édes, I.; Csiba, L.; Blumberg, P.M. Expression and distribution of vanilloid receptor 1 (TRPV1) in the adult rat brain. Mol. Brain Res. 2005, 135, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Cristino, L.; de Petrocellis, L.; Pryce, G.; Baker, D.; Guglielmotti, V.; Di Marzo, V. Immunohistochemical localization of cannabinoid type 1 and vanilloid transient receptor potential vanilloid type 1 receptors in the mouse brain. Neuroscience 2006, 139, 1405–1415. [Google Scholar] [CrossRef]
- Marzo, V.; Starowicz, K.; Cristino, L. TRPV1 Receptors in the Central Nervous System: Potential for Previously Unforeseen Therapeutic Applications. Curr. Pharm. Des. 2008, 14, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Kauer, J.A.; Gibson, H.E. Hot flash: TRPV channels in the brain. Trends Neurosci. 2009, 32, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-X.; Min, J.-W.; Liu, Y.-Q.; He, X.-H.; Peng, B.-W. Expression of TRPV1 in the C57BL/6 mice brain hippocampus and cortex during development. NeuroReport 2014, 25, 379–385. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, O.; Singh, U.; Goswami, C.; Singru, P.S. Transient receptor potential vanilloid 1-6 (Trpv1-6) gene expression in the mouse brain during estrous cycle. Brain Res. 2018, 1701, 161–170. [Google Scholar] [CrossRef]
- Spicarova, D.; Palecek, J. The Role of The TRPV1 Endogenous Agonist N-Oleoyldopamine in Modulation of Nociceptive Signaling at the Spinal Cord Level. J. Neurophysiol. 2009, 102, 234–243. [Google Scholar] [CrossRef]
- Cavanaugh, D.J.; Lee, H.; Lo, L.; Shields, S.D.; Zylka, M.J.; Basbaum, A.I.; Anderson, D.J. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc. Natl. Acad. Sci. USA 2009, 106, 9075–9080. [Google Scholar] [CrossRef]
- Huang, D.; Li, S.; Dhaka, A.; Story, G.M.; Cao, Y.-Q. Expression of the Transient Receptor Potential Channels TRPV1, TRPA1 and TRPM8 in Mouse Trigeminal Primary Afferent Neurons Innervating the Dura. Mol. Pain 2012, 8, 66. [Google Scholar] [CrossRef]
- Hwang, S.J.; Oh, J.M.; Valtschanoff, J.G. Expression of the vanilloid receptor TRPV1 in rat dorsal root ganglion neurons supports different roles of the receptor in visceral and cutaneous afferents. Brain Res. 2005, 1047, 261–266. [Google Scholar] [CrossRef]
- Liao, M.; Cao, E.; Julius, D.; Cheng, Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature 2013, 504, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.B.; Gray, J.; Gunthorpe, M.J.; Hatcher, J.P.; Davey, P.T.; Overend, P.; Harries, M.H.; Latcham, J.; Clapham, C.; Atkinson, K.; et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature 2000, 405, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Caterina, M.J.; Leffler, A.; Malmberg, A.B.; Martin, W.J.; Trafton, J.; Petersen-Zeitz, K.R.; Koltzenburg, M.; Basbaum, A.I.; Julius, D. Impaired Nociception and Pain Sensation in Mice Lacking the Capsaicin Receptor. Science 2000, 288, 306–313. [Google Scholar] [CrossRef]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and Molecular Mechanisms of Pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Kasckow, J.W.; Mulchahey, J.J.; Geracioti, T.D. Effects of the vanilloid agonist olvanil and antagonist capsazepine on rat behaviors. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2004, 28, 291–295. [Google Scholar] [CrossRef]
- Faraji, N.; Komaki, A.; Salehi, I. Interaction Between the Cannabinoid and Vanilloid Systems on Anxiety in Male Rats. Basic Clin. Neurosci. J. 2017, 8, 129–138. [Google Scholar] [CrossRef]
- Terzian, A.L.B.; Aguiar, D.C.; Guimarães, F.S.; Moreira, F.A. Modulation of anxiety-like behaviour by Transient Receptor Potential Vanilloid Type 1 (TRPV1) channels located in the dorsolateral periaqueductal gray. Eur. Neuropsychopharmacol. 2008, 19, 188–195. [Google Scholar] [CrossRef]
- Marsch, R.; Foeller, E.; Rammes, G.; Bunck, M.; Kössl, M.; Holsboer, F.; Zieglgänsberger, W.; Landgraf, R.; Lutz, B.; Wotjak, C.T. Reduced Anxiety, Conditioned Fear, and Hippocampal Long-Term Potentiation in Transient Receptor Potential Vanilloid Type 1 Receptor-Deficient Mice. J. Neurosci. 2007, 27, 832–839. [Google Scholar] [CrossRef]
- Song, J.; Kang, J.; Lin, B.; Li, J.; Zhu, Y.; Du, J.; Yang, X.; Xi, Z.; Li, R. Mediating Role of TRPV1 Ion Channels in the Co-exposure to PM2.5 and Formaldehyde of Balb/c Mice Asthma Model. Sci. Rep. 2017, 7, 11926. [Google Scholar] [CrossRef]
- Choi, J.Y.; Lee, H.Y.; Hur, J.; Kim, K.H.; Kang, J.Y.; Rhee, C.K.; Lee, S.Y. TRPV1 Blocking Alleviates Airway Inflammation and Remodeling in a Chronic Asthma Murine Model. Allergy. Asthma Immunol. Res. 2018, 10, 216–224. [Google Scholar] [CrossRef]
- Engler, A.; Aeschlimann, A.; Simmen, B.R.; Michel, B.A.; Gay, R.E.; Gay, S.; Sprott, H. Expression of transient receptor potential vanilloid 1 (TRPV1) in synovial fibroblasts from patients with osteoarthritis and rheumatoid arthritis. Biochem. Biophys. Res. Commun. 2007, 359, 884–888. [Google Scholar] [CrossRef]
- Rami, H.K.; Thompson, M.; Stemp, G.; Fell, S.; Jerman, J.C.; Stevens, A.J.; Smart, D.; Sargent, B.; Sanderson, D.; Randall, A.D.; et al. Discovery of SB-705498: A potent, selective and orally bioavailable TRPV1 antagonist suitable for clinical development. Bioorg. Med. Chem. Lett. 2006, 16, 3287–3291. [Google Scholar] [CrossRef] [PubMed]
- Gunthorpe, M.J.; Hannan, S.L.; Smart, D.; Jerman, J.C.; Arpino, S.; Smith, G.D.; Brough, S.; Wright, J.; Egerton, J.; Lappin, S.C.; et al. Characterization of SB-705498, a potent and selective vanilloid receptor-1 (VR1/TRPV1) antagonist that inhibits the capsaicin-, acid-, and heat-mediated activation of the receptor. J. Pharmacol. Exp. Ther. 2007, 321, 1183–1192. [Google Scholar] [CrossRef]
- Iftinca, M.; Defaye, M.; Altier, C. TRPV1-Targeted Drugs in Development for Human Pain Conditions. Drugs 2020, 81, 7–27. [Google Scholar] [CrossRef]
- Zhu, K.; Wang, L.; Liao, T.; Li, W.; Zhou, J.; You, Y.; Shi, J. Progress in the development of TRPV1 small-molecule antagonists: Novel Strategies for pain management. Eur. J. Med. Chem. 2023, 261, 115806. [Google Scholar] [CrossRef]
- Garami, A.; Shimansky, Y.P.; Rumbus, Z.; Vizin, R.C.; Farkas, N.; Hegyi, J.; Szakacs, Z.; Solymar, M.; Csenkey, A.; Chiche, D.A.; et al. Hyperthermia induced by transient receptor potential vanilloid-1 (TRPV1) antagonists in human clinical trials: Insights from mathematical modeling and meta-analysis. Pharmacol. Ther. 2020, 208, 107474. [Google Scholar] [CrossRef] [PubMed]
- Ohara, K.; Fukuda, T.; Okada, H.; Kitao, S.; Ishida, Y.; Kato, K.; Takahashi, C.; Katayama, M.; Uchida, K.; Tominaga, M. Identification of Significant Amino Acids in Multiple Transmembrane Domains of Human Transient Receptor Potential Ankyrin 1 (TRPA1) for Activation by Eudesmol, an Oxygenized Sesquiterpene in Hop Essential Oil. J. Biol. Chem. 2015, 290, 3161–3171. [Google Scholar] [CrossRef] [PubMed]
- Korishettar, A.M.; Nishijima, Y.; Wang, Z.; Xie, Y.; Fang, J.; Wilcox, D.A.; Zhang, D.X. Endothelin-1 potentiates TRPV1-mediated vasoconstriction of human adipose arterioles in a protein kinase C-dependent manner. Br. J. Pharmacol. 2020, 178, 709–725. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira EC, L.; Santana, K.; Josino, L.; Lima e Lima, A.H.; de Souza de Sales Júnior, C. Predicting cell-penetrating peptides using machine learning algorithms and navigating in their chemical space. Sci. Rep. 2021, 11, 7628. [Google Scholar] [CrossRef]
- Kwon, D.H.; Zhang, F.; Suo, Y.; Bouvette, J.; Borgnia, M.J.; Lee, S.Y. Heat-dependent opening of TRPV1 in the presence of capsaicin. Nat. Struct. Mol. Biol. 2021, 28, 554–563. [Google Scholar] [CrossRef]
- Van Zundert, G.C.P.; Rodrigues, J.P.G.L.M.; Trellet, M.; Schmitz, C.; Kastritis, P.L.; Karaca, E.; Melquiond, A.S.J.; van Dijk, M.; De Vries, S.J.; Bonvin, A.M.J.J. The HADDOCK2.2 Web Server: User-Friendly Integrative Modeling of Biomolecular Complexes. J. Mol. Biol. 2016, 428, 720–725. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A web-based graphical user interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Cheng, X.; Swails, J.M.; Yeom, M.S.; Eastman, P.K.; Lemkul, J.A.; Wei, S.; Buckner, J.; Jeong, J.C.; Qi, Y.; et al. CHARMM-GUI Input Generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM Simulations Using the CHARMM36 Additive Force Field. J. Chem. Theory Comput. 2015, 12, 405–413. [Google Scholar] [CrossRef]
- Gao, Y.; Lee, J.; Smith IP, S.; Lee, H.; Kim, S.; Qi, Y.; Klauda, J.B.; Widmalm, G.; Khalid, S.; Im, W. CHARMM-GUI Supports Hydrogen Mass Repartitioning and Different Protonation States of Phosphates in Lipopolysaccharides. J. Chem. Inf. Model. 2021, 61, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Kasavajhala, K.; Belfon, K.A.A.; Raguette, L.; Huang, H.; Migues, A.N.; Bickel, J.; Wang, Y.; Pincay, J.; Wu, Q.; et al. ff19SB: Amino-Acid-Specific Protein Backbone Parameters Trained against Quantum Mechanics Energy Surfaces in Solution. J. Chem. Theory Comput. 2019, 16, 528–552. [Google Scholar] [CrossRef]
- Dickson, C.J.; Madej, B.D.; Skjevik, Å.A.; Betz, R.M.; Teigen, K.; Gould, I.R.; Walker, R.C. Lipid14: The Amber Lipid Force Field. J. Chem. Theory Comput. 2014, 10, 865–879. [Google Scholar] [CrossRef]
- Case, D.A.; Aktulga, H.M.; Belfon, K.; Cerutti, D.S.; Cisneros, G.A.; Cruzeiro, V.W.D.; Forouzesh, N.; Giese, T.J.; Götz, A.W.; Gohlke, H. AmberTools. J. Chem. Inf. Model 2023, 63, 6183–6191. [Google Scholar] [CrossRef]
- Bussi, G.; Donadio, D.; and Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007, 126, 014101. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; Van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Roux, B. The Membrane Potential and its Representation by a Constant Electric Field in Computer Simulations. Biophys. J. 2008, 95, 4205–4216. [Google Scholar] [CrossRef] [PubMed]
- Gumbart, J.; Khalili-Araghi, F.; Sotomayor, M.; Roux, B. Constant electric field simulations of the membrane potential illustrated with simple systems. Biochim. Biophys. Acta (BBA)—Biomembr. 2012, 1818, 294–302. [Google Scholar] [CrossRef]
- Michaud-Agrawal, N.; Denning, E.J.; Woolf, T.B.; Beckstein, O. MDAnalysis: A toolkit for the analysis of molecular dynamics simulations. J. Comput. Chem. 2011, 32, 2319–2327. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Smart, O.S.; Neduvelil, J.G.; Wang, X.; Wallace, B.A.; Sansom, M.S.P. HOLE: A program for the analysis of the pore dimensions of ion channel structural models. J. Mol. Graph. 1996, 14, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Amadei, A.; Ceruso, M.A.; Di Nola, A. On the convergence of the conformational coordinates basis set obtained by the essential dynamics analysis of proteins’ molecular dynamics simulations. Proteins 1999, 36, 419–424. [Google Scholar] [CrossRef]
- Nersesyan, Y.; Demirkhanyan, L.; Cabezas-Bratesco, D.; Oakes, V.; Kusuda, R.; Dawson, T.; Sun, X.; Cao, C.; Cohen, A.M.; Chelluboina, B.; et al. Oxytocin Modulates Nociception as an Agonist of Pain-Sensing TRPV1. Cell Rep. 2017, 21, 1681–1691. [Google Scholar] [CrossRef]
- Aneiros, E.; Cao, L.; Papakosta, M.; Stevens, E.B.; Phillips, S.; Grimm, C. The biophysical and molecular basis of TRPV1 proton gating. EMBO J. 2011, 30, 994–1002. [Google Scholar] [CrossRef]
- Obreja, O.; Rathee, P.K.; Lips, K.S.; Distler, C.; Kress, M. IL-1 beta potentiates heat-activated currents in rat sensory neurons: Involvement of IL-1RI, tyrosine kinase, and protein kinase C. FASEB J. 2002, 16, 1497–1503. [Google Scholar] [CrossRef] [PubMed]
- Obreja, O.; Biasio, W.; Andratsch, M.; Lips, K.S.; Rathee, P.K.; Ludwig, A.; Rose-John, S.; Kress, M. Fast modulation of heat-activated ionic current by proinflammatory interleukin 6 in rat sensory neurons. Brain 2005, 128, 1634–1641. [Google Scholar] [CrossRef] [PubMed]
- Bhave, G.; Hu, H.-J.; Glauner, K.S.; Zhu, W.; Wang, H.; Brasier, D.J.; Oxford, G.S.; Gereau, R.W. Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1). Proc. Natl. Acad. Sci. USA 2003, 100, 12480–12485. [Google Scholar] [CrossRef] [PubMed]
- Jendryke, T.; Prochazkova, M.; Hall, B.E.; Nordmann, G.C.; Schladt, M.; Milenkovic, V.M.; Kulkarni, A.B.; Wetzel, C.H. TRPV1 function is modulated by Cdk5-mediated phosphorylation: Insights into the molecular mechanism of nociception. Sci. Rep. 2016, 6, 22007. [Google Scholar] [CrossRef]
- Zhang, M.; Ruwe, D.; Saffari, R.; Kravchenko, M.; Zhang, W. Effects of TRPV1 Activation by Capsaicin and Endogenous N-Arachidonoyl Taurine on Synaptic Transmission in the Prefrontal Cortex. Front. Neurosci. 2020, 14, 91. [Google Scholar] [CrossRef]
- Karlsson, U.; Sundgren-Andersson, A.K.; Johansson, S.; Krupp, J.J. Capsaicin augments synaptic transmission in the rat medial preoptic nucleus. Brain Res. 2005, 1043, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Li, J. TRPV1 Receptor Mediates Glutamatergic Synaptic Input to Dorsolateral Periaqueductal Gray (dl-PAG) Neurons. J. Neurophysiol. 2007, 97, 503–511. [Google Scholar] [CrossRef]
- Musella, A.; De Chiara, V.; Rossi, S.; Prosperetti, C.; Bernardi, G.; Maccarrone, M.; Centonze, D. TRPV1 channels facilitate glutamate transmission in the striatum. Mol. Cell. Neurosci. 2008, 40, 89–97. [Google Scholar] [CrossRef]
- Anstötz, M.; Lee, S.K.; Maccaferri, G. Expression of TRPV1 channels by Cajal-Retzius cells and layer-specific modulation of synaptic transmission by capsaicin in the mouse hippocampus. J. Physiol. 2018, 596, 3739–3758. [Google Scholar] [CrossRef] [PubMed]
- Puente, N.; Cui, Y.; Lassalle, O.; Lafourcade, M.; Georges, F.; Venance, L.; Grandes, P.; Manzoni, O.J. Polymodal activation of the endocannabinoid system in the extended amygdala. Nat. Neurosci. 2011, 14, 1542–1547. [Google Scholar] [CrossRef] [PubMed]
- Chávez, A.E.; Chiu, C.Q.; Castillo, P.E. TRPV1 activation by endogenous anandamide triggers postsynaptic long-term depression in dentate gyrus. Nat. Neurosci. 2010, 13, 1511–1518. [Google Scholar] [CrossRef]
- Grueter, B.A.; Brasnjo, G.; Malenka, R.C. Postsynaptic TRPV1 triggers cell type–specific long-term depression in the nucleus accumbens. Nat. Neurosci. 2010, 13, 1519–1525. [Google Scholar] [CrossRef]
- Gelman, J.S.; Dasgupta, S.; Berezniuk, I.; Fricker, L.D. Analysis of peptides secreted from cultured mouse brain tissue. Biochim. Biophys. Acta (BBA)—Proteins Proteom. 2013, 1834, 2408–2417. [Google Scholar] [CrossRef] [PubMed]
- Karplus, M.; McCammon, J.A. Molecular dynamics simulations of biomolecules. Nat. Struct. Biol. 2002, 9, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Wen, H. Heat activation mechanism of TRPV1: New insights from molecular dynamics simulation. Temperature 2018, 6, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Zhang, H.; Yang, M.; Yu, R. Molecular Dynamic Simulations Reveal the Activation Mechanisms of Oxidation-Induced TRPV1. Int. J. Mol. Sci. 2023, 24, 9553. [Google Scholar] [CrossRef]
- Dewaker, V.; Sharma, A.R.; Debnath, U.; Park, S.T.; Kim, H.S. Insights from molecular dynamics simulations of TRPV1 channel modulators in pain. Drug Discov. Today 2023, 28, 103798. [Google Scholar] [CrossRef]
- Poblete, H.; Oyarzún, I.; Olivero, P.; Comer, J.; Zuñiga, M.; Sepulveda, R.V.; Báez-Nieto, D.; Leon, C.G.; González-Nilo, F.; Latorre, R. Molecular Determinants of Phosphatidylinositol 4,5-Bisphosphate (PI(4,5)P2) Binding to Transient Receptor Potential V1 (TRPV1) Channels. J. Biol. Chem. 2015, 290, 2086–2098. [Google Scholar] [CrossRef]
- Garcia-Elias, A.; Berna-Erro, A.; Rubio-Moscardo, F.; Pardo-Pastor, C.; Mrkonjić, S.; Sepúlveda, R.V.; Vicente, R.; González-Nilo, F.; Valverde, M.A. Interaction between the Linker, Pre-S1, and TRP Domains Determines Folding, Assembly, and Trafficking of TRPV Channels. Structure 2015, 23, 1404–1413. [Google Scholar] [CrossRef]
- Gao, Y.; Cao, E.; Julius, D.; Cheng, Y. TRPV1 structures in nanodiscs reveal mechanisms of ligand and lipid action. Nature 2016, 534, 347–351. [Google Scholar] [CrossRef]
- Neuberger, A.; Oda, M.; Nikolaev, Y.A.; Nadezhdin, K.D.; Gracheva, E.O.; Bagriantsev, S.N.; Sobolevsky, A.I. Human TRPV1 structure and inhibition by the analgesic SB-366791. Nat. Commun. 2023, 14, 2451. [Google Scholar] [CrossRef]
- Arnold, W.R.; Mancino, A.; Moss, F.R.; Frost, A.; Julius, D.; Cheng, Y. Structural basis of TRPV1 modulation by endogenous bioactive lipids. Nat. Struct. Mol. Biol. 2024, 1–9. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, J.-J.; Xie, Y.-K.; Song, X.-Z.; Sun, J.-Y.; Zhang, B.-L.; Qi, Y.-K.; Xu, Z.-Z.; Yang, F. Structure-guided peptide engineering of a positive allosteric modulator targeting the outer pore of TRPV1 for long-lasting analgesia. Nat. Commun. 2023, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Bley, K. Topical capsaicin for pain management: Therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch. Br. J. Anaesth. 2011, 107, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Baranidharan, G.; Das, S.; Bhaskar, A. A review of the high-concentration capsaicin patch and experience in its use in the management of neuropathic pain. Ther. Adv. Neurol. Disord. 2013, 6, 287–297. [Google Scholar] [CrossRef]
- Brederson, J.-D.; Kym, P.R.; Szallasi, A. Targeting TRP channels for pain relief. Eur. J. Pharmacol. 2013, 716, 61–76. [Google Scholar] [CrossRef]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C. Felson D. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res. 2020, 72, 149–162. [Google Scholar] [CrossRef]
- Bonezzi, C.; Costantini, A.; Cruccu, G.; Fornasari, D.M.; Guardamagna, V.; Palmieri, V.; Polati, E.; Zini, P.; Dickenson, A.H. Capsaicin 8% dermal patch in clinical practice: An expert opinion. Expert Opin. Pharmacother. 2020, 21, 1377–1387. [Google Scholar] [CrossRef]
- Lu, M.; Chen, C.; Lan, Y.; Xiao, J.; Li, R.; Huang, J.; Huang, Q.; Cao, Y.; Ho, C.-T. Capsaicin—The major bioactive ingredient of chili peppers: Bio-efficacy and delivery systems. Food Funct. 2020, 11, 2848–2860. [Google Scholar] [CrossRef] [PubMed]
- Smutzer, G.; Jacob, J.C.; Tran, J.T.; Shah, D.I.; Gambhir, S.; Devassy, R.K.; Tran, E.B.; Hoang, B.T.; McCune, J.F. Detection and modulation of capsaicin perception in the human oral cavity. Physiol. Behav. 2018, 194, 120–131. [Google Scholar] [CrossRef]
- Stevens, R.M.; Ervin, J.; Nezzer, J.; Nieves, Y.; Guedes, K.; Burges, R.; Hanson, P.D.; Campbell, J.N. Randomized, Double-Blind, Placebo-Controlled Trial of Intraarticular Trans-Capsaicin for Pain Associated with Osteoarthritis of the Knee. Arthritis Rheumatol. 2019, 71, 1524–1533. [Google Scholar] [CrossRef]
- Gavva, N.R.; Treanor, J.J.; Garami, A.; Fang, L.; Surapaneni, S.; Akrami, A.; Alvarez, F.; Bak, A.; Darling, M.; Gore, A.; et al. Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans. Pain 2008, 136, 202–210. [Google Scholar] [CrossRef]
- Garami, A.; Pakai, E.; McDonald, H.A.; Reilly, R.M.; Gomtsyan, A.; Corrigan, J.J.; Pinter, E.; Zhu, D.X.D.; Lehto, S.G.; Gavva, N.R.; et al. TRPV1 antagonists that cause hypothermia, instead of hyperthermia, in rodents: Compounds’ pharmacological profiles, in vivo targets, thermoeffectors recruited and implications for drug development. Acta. Physiol. 2018, 223, e13038. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, L.P.; Aguiar, D.C.; Moreira, F.A. TRPV1 blockers as potential new treatments for psychiatric disorders. Behav. Pharmacol. 2020, 33, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Roughan, W.H.; Campos, A.I.; García-Marín, L.M.; Cuéllar-Partida, G.; Lupton, M.K.; Hickie, I.B.; Medland, S.E.; Wray, N.R.; Byrne, E.M.; Ngo, T.T.; et al. Comorbid Chronic Pain and Depression: Shared Risk Factors and Differential Antidepressant Effectiveness. Front. Psychiatry 2021, 12, 643609. [Google Scholar] [CrossRef] [PubMed]
- Muttenthaler, M.; King, G.F.; Adams, D.J.; Alewood, P.F. Trends in peptide drug discovery. Nat. Rev. Drug Discov. 2021, 20, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current applications and future directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Glasmacher, S.; Gertsch, J. Characterization of pepcan-23 as pro-peptide of RVD-hemopressin (pepcan-12) and stability of hemopressins in mice. Adv. Biol. Regul. 2021, 80, 100808. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suárez-Suárez, C.; González-Pérez, S.; Márquez-Miranda, V.; Araya-Duran, I.; Vidal-Beltrán, I.; Vergara, S.; Carvacho, I.; Hinostroza, F. The Endocannabinoid Peptide RVD-Hemopressin Is a TRPV1 Channel Blocker. Biomolecules 2024, 14, 1134. https://doi.org/10.3390/biom14091134
Suárez-Suárez C, González-Pérez S, Márquez-Miranda V, Araya-Duran I, Vidal-Beltrán I, Vergara S, Carvacho I, Hinostroza F. The Endocannabinoid Peptide RVD-Hemopressin Is a TRPV1 Channel Blocker. Biomolecules. 2024; 14(9):1134. https://doi.org/10.3390/biom14091134
Chicago/Turabian StyleSuárez-Suárez, Constanza, Sebastián González-Pérez, Valeria Márquez-Miranda, Ingrid Araya-Duran, Isabel Vidal-Beltrán, Sebastián Vergara, Ingrid Carvacho, and Fernando Hinostroza. 2024. "The Endocannabinoid Peptide RVD-Hemopressin Is a TRPV1 Channel Blocker" Biomolecules 14, no. 9: 1134. https://doi.org/10.3390/biom14091134
APA StyleSuárez-Suárez, C., González-Pérez, S., Márquez-Miranda, V., Araya-Duran, I., Vidal-Beltrán, I., Vergara, S., Carvacho, I., & Hinostroza, F. (2024). The Endocannabinoid Peptide RVD-Hemopressin Is a TRPV1 Channel Blocker. Biomolecules, 14(9), 1134. https://doi.org/10.3390/biom14091134





