Multi-Target and Multi-Phase Adjunctive Cerebral Protection for Acute Ischemic Stroke in the Reperfusion Era
Abstract
:1. Introduction
2. Reasons for Mediocre Prognosis
3. Non-Pharmacological Neuroprotective Methods
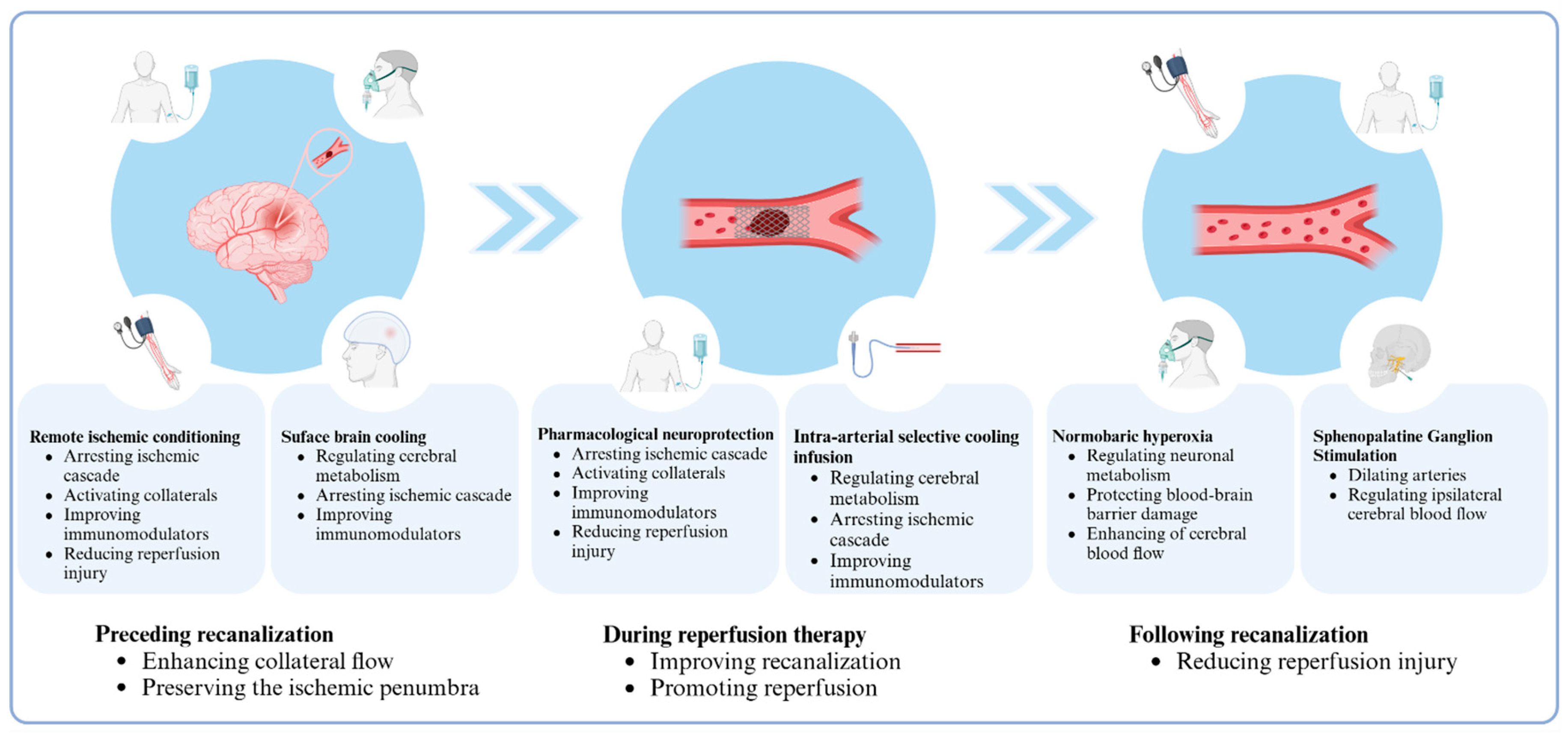
3.1. Remote Ischemic Conditioning
3.2. Normobaric Hyperoxia
3.3. Sphenopalatine Ganglion Stimulation
3.4. Selective Brain Cooling Methods
4. Pharmacological Neuroprotective Methods
5. Multi-Target and Multi-Phase Adjuvant Neuroprotection
6. Conclusions
| Study | N | Type of Patients | Treatment | Main Results |
|---|---|---|---|---|
| RIC | ||||
| RICAMIS (NCT03740971) | 1893 | Patients with acute moderate ischemic stroke |
| RIC is safe and increases the possibility of excellent function outcomes at 90 days |
| RISIST (NCT03481777) | 1500 | Patients with acute stroke symptoms within 4 h of ictus |
| RIC is safe but may not improve functional outcome at 90 days in patients with acute stroke |
| RESCUE BRAIN (NCT02189928) | 188 | Patients of AIS within 6 h of ictus |
| RIC, as an adjunct therapy of reperfusion, cannot limit brain infarction volume growth at 24 h after symptom onset. |
| SERIC-EVT (NCT04977869) | 498 | Patients with AIS underwent EVT |
| Not available for study; it is ongoing |
| REMOTE-CAT (NCT03375762) | 572 | Patients with suspected clinical stroke within 8 h of symptom onset |
| RIC may increase the proportion of patients with good outcomes at 90 days |
| Hougaard et al. (2014) [49] | 247/196 | Patients with suspected acute stroke |
| RIC is safe, feasible, and may reduce tissue infarction risk |
| NBO | ||||
| Li et al. (2022) [62] | 43/43 | Patients with anterior AIS undergoing EVT |
| NBO combined with EVT is safe and reduces the infarct volume in the early stage after ictus |
| Cheng et al. (2022) [65] | 44/43 | Patients with posterior stroke after EVT |
| High-flow adjuvant NBO is safe but does not improve the mRS at 90 days |
| Li et al. (2021) [64] | 125/102 | Patients with anterior AIS received IVT with 4.5 h of ictus |
| NBO combined with IVT is safe and may improve functional outcomes at 90 days |
| Cheng et al. (2021) [63] | 91 | Patients with anterior stroke after EVT |
| High-flow adjuvant NBO is safe and may improve the functional outcomes at 90 days |
| Shi et al. (2017) [91] | 18 | Patients with acute ischemic stroke |
| NBO therapy improves neurological functions in patients with AIS |
| Mazdeh et al. (2015) [92] | 52 | Patients with severe acute stroke |
| NBO could improve long-time outcomes of patients with stroke |
| SPGS | ||||
| Saver et al. (2019) [71] | 50 | Patients with anterior AIS, including arm weakness within 24 h of onset |
| SPG stimulation improved brain blood flow, vessel diameter, and flow velocity and decreased hand motor weakness |
| ImpACT-24B (NCT00826059) | 1078 | Patient’s anterior circulation AIS after 8–24 h ictus |
| SPGS is safe, and patients with CCI may benefit from SPGS |
| Bornstein et al. (2019) [93] | 303 | Patients with anterior AIS within 24 h of onset |
| SPGS is safe, and patients with CCI may benefit from SPGS |
| ImpACT-1 (NCT03733236) | 98 | Patients with anterior AIS within 24 h from stroke onset |
| SPGS is safe, feasible, tolerable, and effective to improve functional outcomes at 90 days |
| SBC | ||||
| Choi et al. (2010) [73] | 18 | Patients undergoing follow-up cerebral angiography after previous treatment of vascular malformations |
| 1. JVB temperature drops 0.84 +/− 0.13 °C from baseline.2. Endovascular brain cooling is safe and feasible. |
| Chen et al. (2016) [74] | 28 | Patients with LVO within 8 h after onset and undergoing MT |
| 1. Ischemic cerebral tissue was decreased by at least 2 °C.2. IA-CSI is safe and feasible to MT with LVO. |
| Peng et al. (2016) [75] | 26 | Patients with acute MCA occlusion |
| Intra-arterial hypothermia is safe and effective in reducing infarct volume. |
| Wu et al. (2018) [76] | 113 | Patients with LVO-induced acute ischemic stroke and undergoing MT |
| IA-CSI is effective in reducing infarct volume but cannot improve the functional outcomes at 90 days. |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mendelson, S.J.; Prabhakaran, S. Diagnosis and Management of Transient Ischemic Attack and Acute Ischemic Stroke: A Review. JAMA 2021, 325, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N. Engl. J. Med. 1995, 333, 1581–1587. [Google Scholar] [CrossRef] [PubMed]
- Warach, S.J.; Dula, A.N.; Milling, T.J., Jr. Tenecteplase Thrombolysis for Acute Ischemic Stroke. Stroke 2020, 51, 3440–3451. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Wu, C.; Lee, H. Intravenous thrombolysis for acute ischemic stroke: From alteplase to tenecteplase. Brain Circ. 2023, 9, 61–63. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Menon, B.K.; Van Zwam, W.H.; Dippel, D.W.J.; Mitchell, P.J.; Demchuk, A.M.; Dávalos, A.; Majoie, C.B.L.M.; Van Der Lugt, A.; De Miquel, M.A.; et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 2016, 387, 1723–1731. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, R.G.; Jadhav, A.P.; Haussen, D.C.; Bonafe, A.; Budzik, R.F.; Bhuva, P.; Yavagal, D.R.; Ribo, M.; Cognard, C.; Hanel, R.A.; et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N. Engl. J. Med. 2018, 378, 11–21. [Google Scholar] [CrossRef]
- Goyal, M.; Demchuk, A.M.; Menon, B.K.; Eesa, M.; Rempel, J.L.; Thornton, J.; Roy, D.; Jovin, T.G.; Willinsky, R.A.; Sapkota, B.L.; et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N. Engl. J. Med. 2015, 372, 1019–1030. [Google Scholar] [CrossRef]
- Campbell, B.C.; Mitchell, P.J.; Kleinig, T.J.; Dewey, H.M.; Churilov, L.; Yassi, N.; Yan, B.; Dowling, R.J.; Parsons, M.W.; Oxley, T.J.; et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N. Engl. J. Med. 2015, 372, 1009–1018. [Google Scholar] [CrossRef]
- Nogueira, R.G.; Frei, D.; Kirmani, J.F.; Zaidat, O.; Lopes, D.; Turk, A.S.; Heck, D.; Mason, B.; Haussen, D.C.; Levy, E.I.; et al. Safety and Efficacy of a 3-Dimensional Stent Retriever with Aspiration-Based Thrombectomy vs Aspiration-Based Thrombectomy Alone in Acute Ischemic Stroke Intervention: A Randomized Clinical Trial. JAMA Neurol. 2018, 75, 304–311. [Google Scholar] [CrossRef]
- Jovin, T.G.; Nogueira, R.G.; Lansberg, M.G.; Demchuk, A.M.; O Martins, S.; Mocco, J.; Ribo, M.; Jadhav, A.P.; Ortega-Gutierrez, S.; Hill, M.D.; et al. Thrombectomy for anterior circulation stroke beyond 6 h from time last known well (AURORA): A systematic review and individual patient data meta-analysis. Lancet 2022, 399, 249–258. [Google Scholar] [CrossRef]
- Berkhemer, O.A.; Fransen, P.S.S.; Beumer, D.; Berg, L.A.V.D.; Lingsma, H.F.; Yoo, A.J.; Schonewille, W.J.; Vos, J.A.; Nederkoorn, P.J.; Wermer, M.J.H.; et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N. Engl. J. Med. 2015, 372, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Jovin, T.G.; Chamorro, A.; Cobo, E.; De Miquel, M.A.; Molina, C.A.; Rovira, A.; Román, L.S.; Serena, J.; Abilleira, S.; Ribo, M.; et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N. Engl. J. Med. 2015, 372, 2296–2306. [Google Scholar] [CrossRef] [PubMed]
- Saver, J.L.; Goyal, M.; Bonafe, A.; Diener, H.-C.; Levy, E.I.; Pereira, V.M.; Albers, G.W.; Cognard, C.; Cohen, D.J.; Hacke, W.; et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N. Engl. J. Med. 2015, 372, 2285–2295. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, L.R.; Adeoye, O.; Alemseged, F.; Bahr-Hosseini, M.; Deljkich, E.; Favilla, C.; Fisher, M.; Grotta, J.; Hill, M.D.; Kamel, H.; et al. Most Promising Approaches to Improve Stroke Outcomes: The Stroke Treatment Academic Industry Roundtable XII Workshop. Stroke 2023, 54, 3202–3213. [Google Scholar] [CrossRef]
- Tong, Y.; Ding, Y.; Han, Z.; Duan, H.; Geng, X. Optimal rehabilitation strategies for early postacute stroke recovery: An ongoing inquiry. Brain Circ. 2023, 9, 201–204. [Google Scholar] [CrossRef]
- O’Collins, V.E.; Macleod, M.R.; Donnan, G.A.; Horky, L.L.; van der Worp, B.H.; Howells, D.W. 1026 experimental treatments in acute stroke. Ann. Neurol. 2006, 59, 467–477. [Google Scholar]
- Paul, S.; Candelario-Jalil, E. Emerging neuroprotective strategies for the treatment of ischemic stroke: An overview of clinical and preclinical studies. Exp. Neurol. 2021, 335, 113518. [Google Scholar] [CrossRef]
- Lyden, P.; Hemmen, T.; Grotta, J.; Rapp, K.; Ernstrom, K.; Rzesiewicz, T.; Parker, S.; Concha, M.; Hussain, S.; Agarwal, S.; et al. Results of the ICTuS 2 Trial (Intravascular Cooling in the Treatment of Stroke 2). Stroke 2016, 47, 2888–2895. [Google Scholar] [CrossRef]
- van der Worp, H.B.; Macleod, M.R.; Bath, P.M.; Bathula, R.; Christensen, H.; Colam, B.; Cordonnier, C.; Demotes-Mainard, J.; Durand-Zaleski, I.; Gluud, C.; et al. Therapeutic hypothermia for acute ischaemic stroke. Results of a European multicentre, randomised, phase III clinical trial. Eur. Stroke J. 2019, 4, 254–262. [Google Scholar] [CrossRef]
- Hill, M.D.; Goyal, M.; Menon, B.K.; Nogueira, R.G.; McTaggart, R.A.; Demchuk, A.M.; Poppe, A.Y.; Buck, B.H.; Field, T.S.; Dowlatshahi, D.; et al. Efficacy and safety of nerinetide for the treatment of acute ischaemic stroke (ESCAPE-NA1): A multicentre, double-blind, randomised controlled trial. Lancet 2020, 395, 878–887. [Google Scholar] [CrossRef]
- Elkins, J.; Veltkamp, R.; Montaner, J.; Johnston, S.C.; Singhal, A.B.; Becker, K.; Lansberg, M.G.; Tang, W.; Chang, I.; Muralidharan, K.; et al. Safety and efficacy of natalizumab in patients with acute ischaemic stroke (ACTION): A randomised, placebo-controlled, double-blind phase 2 trial. Lancet Neurol. 2017, 16, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Hishida, A. Clinical analysis of 207 patients who developed renal disorders during or after treatment with edaravone reported during post-marketing surveillance. Clin. Exp. Nephrol. 2007, 11, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.; Savitz, S.I. Pharmacological brain cytoprotection in acute ischaemic stroke—Renewed hope in the reperfusion era. Nat. Rev. Neurol. 2022, 18, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Bereczki, D.; Fekete, I. Vinpocetine for acute ischaemic stroke. Cochrane Database Syst. Rev. 2008, 2008, CD000480. [Google Scholar] [CrossRef]
- Sun, H.S.; Doucette, T.A.; Liu, Y.; Fang, Y.; Teves, L.; Aarts, M.; Ryan, C.L.; Bernard, P.B.; Lau, A.; Forder, J.P.; et al. Effectiveness of PSD95 inhibitors in permanent and transient focal ischemia in the rat. Stroke 2008, 39, 2544–2553. [Google Scholar] [CrossRef]
- Teves, L.M.; Cui, H.; Tymianski, M. Efficacy of the PSD95 inhibitor Tat-NR2B9c in mice requires dose translation between species. J. Cereb. Blood Flow. Metab. 2016, 36, 555–561. [Google Scholar] [CrossRef]
- Cook, D.J.; Teves, L.; Tymianski, M. Treatment of stroke with a PSD-95 inhibitor in the gyrencephalic primate brain. Nature 2012, 483, 213–217. [Google Scholar] [CrossRef]
- Astrup, J.; Siesjö, B.K.; Symon, L. Thresholds in cerebral ischemia—The ischemic penumbra. Stroke 1981, 12, 723–725. [Google Scholar] [CrossRef]
- Algoet, M.; Janssens, S.; Himmelreich, U.; Gsell, W.; Pusovnik, M.; Eynde, J.V.D.; Oosterlinck, W. Myocardial ischemia-reperfusion injury and the influence of inflammation. Trends Cardiovasc. Med. 2023, 33, 357–366. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Eckle, T. Ischemia and reperfusion--from mechanism to translation. Nat. Med. 2011, 17, 1391–1401. [Google Scholar] [CrossRef]
- Pan, J.; Konstas, A.-A.; Bateman, B.; Ortolano, G.A.; Pile-Spellman, J. Reperfusion injury following cerebral ischemia: Pathophysiology, MR imaging, and potential therapies. Neuroradiology 2007, 49, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Du, W.; Zhuang, X.; Liang, D.; Mo, Y.; Wang, J. Glycogen synthase kinase-3β mediates toll-like receptors 4/nuclear factor kappa-B-activated cerebral ischemia-reperfusion injury through regulation of fat mass and obesity-associated protein. Brain Circ. 2023, 9, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Kan, Y.; Li, S.; Zhang, B.; Ding, Y.; Zhao, W.; Ji, X. No-reflow phenomenon following stroke recanalization therapy: Clinical assessment advances: A narrative review. Brain Circ. 2023, 9, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Leng, X.; Miao, Z.; Fisher, M.; Liu, L. Clinically Ineffective Reperfusion after Endovascular Therapy in Acute Ischemic Stroke. Stroke 2023, 54, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Eren, F.; Yilmaz, S.E. Neuroprotective approach in acute ischemic stroke: A systematic review of clinical and experimental studies. Brain Circ. 2022, 8, 172–179. [Google Scholar] [CrossRef]
- Hess, D.C.; Blauenfeldt, R.A.; Andersen, G.; Hougaard, K.D.; Hoda, N.; Ding, Y.; Ji, X. Remote ischaemic conditioning—A new paradigm of self-protection in the brain. Nat. Rev. Neurol. 2015, 11, 698–710. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Yellon, D.M. Ischaemic conditioning and reperfusion injury. Nat. Rev. Cardiol. 2016, 13, 193–209. [Google Scholar] [CrossRef]
- Zhao, W.; Hausenloy, D.J.; Hess, D.C.; Yellon, D.M.; Ji, X. Remote Ischemic Conditioning: Challenges and Opportunities. Stroke 2023, 54, 2204–2207. [Google Scholar] [CrossRef]
- Lim, S.Y.; Hausenloy, D.J. Remote ischemic conditioning: From bench to bedside. Front. Physiol. 2012, 3, 27. [Google Scholar] [CrossRef]
- Xu, Y.; Ji, X.; Wang, Y. Immune and inflammatory mechanism of remote ischemic conditioning: A narrative review. Brain Circ. 2023, 9, 77–87. [Google Scholar] [CrossRef]
- Ma, J.; Ma, Y.; Dong, B.; Bandet, M.V.; Shuaib, A.; Winship, I.R. Prevention of the collapse of pial collaterals by remote ischemic perconditioning during acute ischemic stroke. J. Cereb. Blood Flow Metab. 2017, 37, 3001–3014. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Liu, J. Protective effect of ischemia preconditioning of lower limbs on brain ischemia-reperfusion injury via neural pathways. Sichuan Da Xue Xue Bao Yi Xue Ban 2014, 45, 216–220. [Google Scholar] [PubMed]
- Tsibulnikov, S.Y.; Maslov, L.N.; Gorbunov, A.S.; Voronkov, N.S.; Boshchenko, A.A.; Popov, S.V.; Prokudina, E.S.; Singh, N.; Downey, J.M. A Review of Humoral Factors in Remote Preconditioning of the Heart. J. Cardiovasc. Pharmacol. Ther. 2019, 24, 403–421. [Google Scholar] [CrossRef] [PubMed]
- Ripley, A.J.; Jeffers, M.S.; McDonald, M.W.; Montroy, J.; Dykes, A.; Fergusson, D.A.; Silasi, G.; Lalu, M.M.; Corbett, D. Neuroprotection by Remote Ischemic Conditioning in Rodent Models of Focal Ischemia: A Systematic Review and Meta-Analysis. Transl. Stroke Res. 2021, 12, 461–473. [Google Scholar] [CrossRef]
- He, Y.D.; Guo, Z.; Qin, C.; Jin, H.; Zhang, P.; Abuduxukuer, R.; Yang, Y. Remote ischemic conditioning combined with intravenous thrombolysis for acute ischemic stroke. Ann. Clin. Transl. Neurol. 2020, 7, 972–979. [Google Scholar] [CrossRef]
- Che, R.; Zhao, W.; Ma, Q.; Jiang, F.; Wu, L.; Yu, Z.; Zhang, Q.; Dong, K.; Song, H.; Huang, X.; et al. rt-PA with remote ischemic postconditioning for acute ischemic stroke. Ann. Clin. Transl. Neurol. 2019, 6, 364–372. [Google Scholar] [CrossRef]
- Zhao, W.; Che, R.; Li, S.; Ren, C.; Li, C.; Wu, C.; Lu, H.; Chen, J.; Duan, J.; Meng, R.; et al. Remote ischemic conditioning for acute stroke patients treated with thrombectomy. Ann. Clin. Transl. Neurol. 2018, 5, 850–856. [Google Scholar] [CrossRef]
- Chen, H.S.; Cui, Y.; Li, X.Q.; Wang, X.H.; Ma, Y.T.; Zhao, Y.; Han, J.; Deng, C.Q.; Hong, M.; Bao, Y.; et al. Effect of Remote Ischemic Conditioning vs Usual Care on Neurologic Function in Patients with Acute Moderate Ischemic Stroke: The RICAMIS Randomized Clinical Trial. JAMA 2022, 328, 627–636. [Google Scholar] [CrossRef]
- Hougaard, K.D.; Hjort, N.; Zeidler, D.; Sørensen, L.; Nørgaard, A.; Hansen, T.M.; von Weitzel-Mudersbach, P.; Simonsen, C.Z.; Damgaard, D.; Gottrup, H.; et al. Remote ischemic perconditioning as an adjunct therapy to thrombolysis in patients with acute ischemic stroke: A randomized trial. Stroke 2014, 45, 159–167. [Google Scholar] [CrossRef]
- Blauenfeldt, R.A.; Hjort, N.; Valentin, J.B.; Homburg, A.M.; Modrau, B.; Sandal, B.F.; Gude, M.F.; Hougaard, K.D.; Damgaard, D.; Poulsen, M.; et al. Remote Ischemic Conditioning for Acute Stroke: The RESIST Randomized Clinical Trial. JAMA 2023, 330, 1236–1246. [Google Scholar] [CrossRef]
- Guo, Z.N.; Abuduxukuer, R.; Zhang, P.; Wang, C.; Yang, Y. Safety and efficacy of remote ischemic conditioning combined with endovascular thrombectomy for acute ischemic stroke due to large vessel occlusion of anterior circulation: A multicenter, randomized, parallel-controlled clinical trial (SERIC-EVT): Study protocol. Int. J. Stroke 2023, 18, 484–489. [Google Scholar] [PubMed]
- Abuduxukuer, R.; Guo, Z.-N.; Zhang, P.; Qu, Y.; Yang, Y. Safety and efficacy of remote ischemic conditioning combined with intravenous thrombolysis for acute ischemic stroke: A multicenter, randomized, parallel-controlled clinical trial (SERIC-IVT) Study design and protocol. Int. J. Stroke 2023, 18, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Liu, W.; Liu, B.; Schnell, A.; Liu, K.J. Normobaric hyperoxia delays and attenuates early nitric oxide production in focal cerebral ischemic rats. Brain Res. 2010, 1352, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ding, Y.; Ji, X.; Meng, R. Advances in Normobaric Hyperoxia Brain Protection in Experimental Stroke. Front. Neurol. 2020, 11, 50. [Google Scholar] [CrossRef]
- Li, F.; Geng, X.; Gao, J.; Kohls, W.; Ding, Y. Perspectives on benefit of early and prereperfusion hypothermia by pharmacological approach in stroke. Brain Circ. 2022, 8, 69–75. [Google Scholar] [CrossRef]
- Shin, H.K.; Dunn, A.K.; Jones, P.B.; Boas, D.A.; Lo, E.H.; Moskowitz, M.A.; Ayata, C. Normobaric hyperoxia improves cerebral blood flow and oxygenation, and inhibits peri-infarct depolarizations in experimental focal ischaemia. Brain 2007, 130, 1631–1642. [Google Scholar] [CrossRef]
- Wu, O.; Benner, T.; Roccatagliata, L.; Zhu, M.; Schaefer, P.W.; Sorensen, A.G.; Singhal, A.B. Evaluating effects of normobaric oxygen therapy in acute stroke with MRI-based predictive models. Med. Gas. Res. 2012, 2, 5. [Google Scholar] [CrossRef]
- Padma, M.V.; Bhasin, A.; Bhatia, R.; Garg, A.; Singh, M.; Tripathi, M.; Prasad, K. Normobaric oxygen therapy in acute ischemic stroke: A pilot study in Indian patients. Ann. Indian Acad. Neurol. 2010, 13, 284–288. [Google Scholar] [CrossRef]
- Rønning, O.M.; Guldvog, B. Should stroke victims routinely receive supplemental oxygen? A quasi-randomized controlled trial. Stroke 1999, 30, 2033–2037. [Google Scholar] [CrossRef]
- Chiu, E.H.; Liu, C.-S.; Tan, T.-Y.; Chang, K.-C. Venturi mask adjuvant oxygen therapy in severe acute ischemic stroke. Arch. Neurol. 2006, 63, 741–744. [Google Scholar] [CrossRef]
- Ding, J.; Zhou, D.; Sui, M.; Meng, R.; Chandra, A.; Han, J.; Ding, Y.; Ji, X. The effect of normobaric oxygen in patients with acute stroke: A systematic review and meta-analysis. Neurol. Res. 2018, 40, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Qi, Z.; Ma, Q.; Ding, J.; Wu, C.; Song, H.; Yang, Q.; Duan, J.; Liu, L.; Kang, H.; et al. Normobaric Hyperoxia Combined with Endovascular Treatment for Patients with Acute Ischemic Stroke: A Randomized Controlled Clinical Trial. Neurology 2022, 99, e824–e834. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Geng, X.; Tong, Y.; Dornbos, D.; Hussain, M.; Rajah, G.B.; Gao, J.; Ma, L.; Li, F.; Du, H.; et al. Adjuvant High-Flow Normobaric Oxygen after Mechanical Thrombectomy for Anterior Circulation Stroke: A Randomized Clinical Trial. Neurotherapeutics 2021, 18, 1188–1197. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wu, L.; Zhao, W.; Dornbos, D.; Wu, C.; Li, W.; Wu, D.; Ding, J.; Ding, Y.; Xie, Y.; et al. Efficacy and safety of normobaric hyperoxia combined with intravenous thrombolysis on acute ischemic stroke patients. Neurol. Res. 2021, 43, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Gao, J.; Rajah, G.B.; Geng, X.; Ding, Y. Adjuvant high-flow normobaric oxygen after mechanical thrombectomy for posterior circulation stroke: A randomized clinical trial. J. Neurol. Sci. 2022, 441, 120350. [Google Scholar] [CrossRef] [PubMed]
- Poli, S.; Mbroh, J.; Baron, J.-C.; Singhal, A.B.; Strbian, D.; Molina, C.; Lemmens, R.; Turc, G.; Mikulik, R.; Michel, P.; et al. Penumbral Rescue by normobaric O = O administration in patients with ischemic stroke and target mismatch proFile (PROOF): Study protocol of a phase IIb trial. Int. J. Stroke 2024, 19, 120–126. [Google Scholar] [CrossRef]
- Biose, I.J.; Oremosu, J.; Bhatnagar, S.; Bix, G.J. Promising Cerebral Blood Flow Enhancers in Acute Ischemic Stroke. Transl. Stroke Res. 2023, 14, 863–889. [Google Scholar] [CrossRef]
- Bahr-Hosseini, M.; Saver, J.L. Mechanisms of action of acute and subacute sphenopalatine ganglion stimulation for ischemic stroke. Int. J. Stroke 2020, 15, 839–848. [Google Scholar] [CrossRef]
- Levi, H.; Schoknecht, K.; Prager, O.; Chassidim, Y.; Weissberg, I.; Serlin, Y.; Friedman, A. Stimulation of the sphenopalatine ganglion induces reperfusion and blood-brain barrier protection in the photothrombotic stroke model. PLoS ONE 2012, 7, e39636. [Google Scholar] [CrossRef]
- Bornstein, N.M.; Saver, J.L.; Diener, H.C.; Gorelick, P.B.; Shuaib, A.; Solberg, Y.; Thackeray, L.; Savic, M.; Janelidze, T.; Zarqua, N.; et al. An injectable implant to stimulate the sphenopalatine ganglion for treatment of acute ischaemic stroke up to 24 h from onset (ImpACT-24B): An international, randomised, double-blind, sham-controlled, pivotal trial. Lancet 2019, 394, 219–229. [Google Scholar] [CrossRef]
- Saver, J.L.; Kharaishvili, N.; Janelidze, T.; Beridze, M.; Zarqua, N.; Solberg, Y.; Bornstein, N.M.; Khachidze, N.; Avazashvili, I.; Meshviliani, P.; et al. Refined Sphenopalatine Ganglion Stimulator Placement and Intensity Setting to Augment Blood Flow and Neurologic Function. Stroke 2019, 50, 3512–3518. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ji, X.; An, H.; Wu, D. Research progress of selective brain cooling methods in the prehospital care for stroke patients: A narrative review. Brain Circ. 2023, 9, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Marshall, R.; Neimark, M.; Konstas, A.; Lin, E.; Chiang, Y.; Mast, H.; Rundek, T.; Mohr, J.; Pile-Spellman, J. Selective brain cooling with endovascular intracarotid infusion of cold saline: A pilot feasibility study. AJNR Am. J. Neuroradiol. 2010, 31, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, L.; Zhang, H.; Geng, X.; Jiao, L.; Li, G.; Coutinho, J.M.; Ding, Y.; Liebeskind, D.S.; Ji, X. Endovascular Hypothermia in Acute Ischemic Stroke: Pilot Study of Selective Intra-Arterial Cold Saline Infusion. Stroke 2016, 47, 1933–1935. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Wan, Y.; Liu, W.; Dan, B.; Lin, L.; Tang, Z. Protective roles of intra-arterial mild hypothermia and arterial thrombolysis in acute cerebral infarction. Springerplus 2016, 5, 1988. [Google Scholar] [CrossRef]
- Wu, C.; Zhao, W.; An, H.; Wu, L.; Chen, J.; Hussain, M.; Ding, Y.; Li, C.; Wei, W.; Duan, J.; et al. Safety, feasibility, and potential efficacy of intraarterial selective cooling infusion for stroke patients treated with mechanical thrombectomy. J. Cereb. Blood Flow Metab. 2018, 38, 2251–2260. [Google Scholar] [CrossRef]
- Tokairin, K.; Osanai, T.; Abumiya, T.; Kazumata, K.; Ono, K.; Houkin, K. Regional transarterial hypothermic infusion in combination with endovascular thrombectomy in acute ischaemic stroke with cerebral main arterial occlusion: Protocol to investigate safety of the clinical trial. BMJ Open 2017, 7, e016502. [Google Scholar] [CrossRef]
- Cheng, Z.; Ding, Y.; Rajah, G.B.; Gao, J.; Li, F.; Ma, L.; Geng, X. Vertebrobasilar artery cooling infusion in acute ischemic stroke for posterior circulation following thrombectomy: Rationale, design and protocol for a prospective randomized controlled trial. Front. Neurosci. 2023, 17, 1149767. [Google Scholar] [CrossRef]
- Wu, L.; Wu, D.; Yang, T.; Xu, J.; Chen, J.; Wang, L.; Xu, S.; Zhao, W.; Wu, C.; Ji, X. Hypothermic neuroprotection against acute ischemic stroke: The 2019 update. J. Cereb. Blood Flow Metab. 2020, 40, 461–481. [Google Scholar] [CrossRef]
- Diprose, W.K.; Morgan, C.A.; Wang, M.T.; Diprose, J.P.; Lin, J.C.; Sheriff, S.; Campbell, D.; Barber, P.A. Active conductive head cooling of normal and infarcted brain: A magnetic resonance spectroscopy imaging study. J. Cereb. Blood Flow. Metab. 2022, 42, 2058–2065. [Google Scholar] [CrossRef]
- Covaciu, L.; Weis, J.; Bengtsson, C.; Allers, M.; Lunderquist, A.; Ahlström, H.; Rubertsson, S. Brain temperature in volunteers subjected to intranasal cooling. Intensive Care Med. 2011, 37, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Castrén, M.; Nordberg, P.; Svensson, L.; Taccone, F.; Vincent, J.L.; Desruelles, D.; Eichwede, F.; Mols, P.; Schwab, T.; Vergnion, M.; et al. Intra-arrest transnasal evaporative cooling: A randomized, prehospital, multicenter study (PRINCE: Pre-ROSC IntraNasal Cooling Effectiveness). Circulation 2010, 122, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Kuczynski, A.M.; Ospel, J.M.; Demchuk, A.M.; Goyal, M.; Mitha, A.P.; Almekhlafi, M.A. Therapeutic Hypothermia in Patients with Malignant Ischemic Stroke and Hemicraniectomy—A Systematic Review and Meta-analysis. World Neurosurg. 2020, 141, e677–e685. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V. Sphingosine 1-phosphate receptors in health and disease: Mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol. Ther. 2007, 115, 84–105. [Google Scholar] [CrossRef] [PubMed]
- Kraft, P.; Göb, E.; Schuhmann, M.K.; Göbel, K.; Deppermann, C.; Thielmann, I.; Herrmann, A.M.; Lorenz, K.; Brede, M.; Stoll, G.; et al. FTY720 ameliorates acute ischemic stroke in mice by reducing thrombo-inflammation but not by direct neuroprotection. Stroke 2013, 44, 3202–3210. [Google Scholar] [CrossRef]
- Czech, B.; Pfeilschifter, W.; Mazaheri-Omrani, N.; Strobel, M.A.; Kahles, T.; Neumann-Haefelin, T.; Rami, A.; Huwiler, A.; Pfeilschifter, J. The immunomodulatory sphingosine 1-phosphate analog FTY720 reduces lesion size and improves neurological outcome in a mouse model of cerebral ischemia. Biochem. Biophys. Res. Commun. 2009, 389, 251–256. [Google Scholar] [CrossRef]
- Zhu, Z.; Fu, Y.; Tian, D.; Sun, N.; Han, W.; Chang, G.; Dong, Y.; Xu, X.; Liu, Q.; Huang, D.; et al. Combination of the Immune Modulator Fingolimod with Alteplase in Acute Ischemic Stroke: A Pilot Trial. Circulation 2015, 132, 1104–1112. [Google Scholar] [CrossRef]
- Tian, D.C.; Shi, K.; Zhu, Z.; Yao, J.; Yang, X.; Su, L.; Zhang, S.; Zhang, M.; Gonzales, R.J.; Liu, Q.; et al. Fingolimod enhances the efficacy of delayed alteplase administration in acute ischemic stroke by promoting anterograde reperfusion and retrograde collateral flow. Ann. Neurol. 2018, 84, 717–728. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, N.; Ren, L.; Yan, Y.; Sun, N.; Li, Y.-J.; Han, W.; Xue, R.; Liu, Q.; Hao, J.; et al. Impact of an immune modulator fingolimod on acute ischemic stroke. Proc. Natl. Acad. Sci. USA 2014, 111, 18315–18320. [Google Scholar] [CrossRef]
- Shibuya, M.; Hirai, S.; Seto, M.; Satoh, S.-I.; Ohtomo, E.; Fasudil Ischemic Stroke Study Group. Effects of fasudil in acute ischemic stroke: Results of a prospective placebo-controlled double-blind trial. J. Neurol. Sci. 2005, 238, 31–39. [Google Scholar] [CrossRef]
- Shi, S.; Qi, Z.; Ma, Q.; Pan, R.; Timmins, G.S.; Zhao, Y.; Shi, W.; Zhang, Y.; Ji, X.; Liu, K.J. Normobaric Hyperoxia Reduces Blood Occludin Fragments in Rats and Patients with Acute Ischemic Stroke. Stroke 2017, 48, 2848–2854. [Google Scholar] [CrossRef] [PubMed]
- Mazdeh, M.; Taher, A.; Torabian, S.; Seifirad, S. Effects of Normobaric Hyperoxia in Severe Acute Stroke: A Randomized Controlled Clinical Trial Study. Acta Med. Iran. 2015, 53, 676–680. [Google Scholar] [PubMed]
- Bornstein, N.M.; Saver, J.L.; Diener, H.C.; Gorelick, P.B.; Shuaib, A.; Solberg, Y.; Devlin, T.; Leung, T.; Molina, C.A. Sphenopalatine Ganglion Stimulation to Augment Cerebral Blood Flow: A Randomized, Sham-Controlled Trial. Stroke 2019, 50, 2108–2117. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Wang, J.; Liu, G.; Li, S.; Ding, Y.; Ji, X.; Zhao, W. Multi-Target and Multi-Phase Adjunctive Cerebral Protection for Acute Ischemic Stroke in the Reperfusion Era. Biomolecules 2024, 14, 1181. https://doi.org/10.3390/biom14091181
Zhao M, Wang J, Liu G, Li S, Ding Y, Ji X, Zhao W. Multi-Target and Multi-Phase Adjunctive Cerebral Protection for Acute Ischemic Stroke in the Reperfusion Era. Biomolecules. 2024; 14(9):1181. https://doi.org/10.3390/biom14091181
Chicago/Turabian StyleZhao, Min, Jing Wang, Guiyou Liu, Sijie Li, Yuchuan Ding, Xunming Ji, and Wenbo Zhao. 2024. "Multi-Target and Multi-Phase Adjunctive Cerebral Protection for Acute Ischemic Stroke in the Reperfusion Era" Biomolecules 14, no. 9: 1181. https://doi.org/10.3390/biom14091181
APA StyleZhao, M., Wang, J., Liu, G., Li, S., Ding, Y., Ji, X., & Zhao, W. (2024). Multi-Target and Multi-Phase Adjunctive Cerebral Protection for Acute Ischemic Stroke in the Reperfusion Era. Biomolecules, 14(9), 1181. https://doi.org/10.3390/biom14091181









