Roadmap for Designing Donor-
Abstract
1. Introduction
2. D--A Fluorophores in the UV-Vis Region
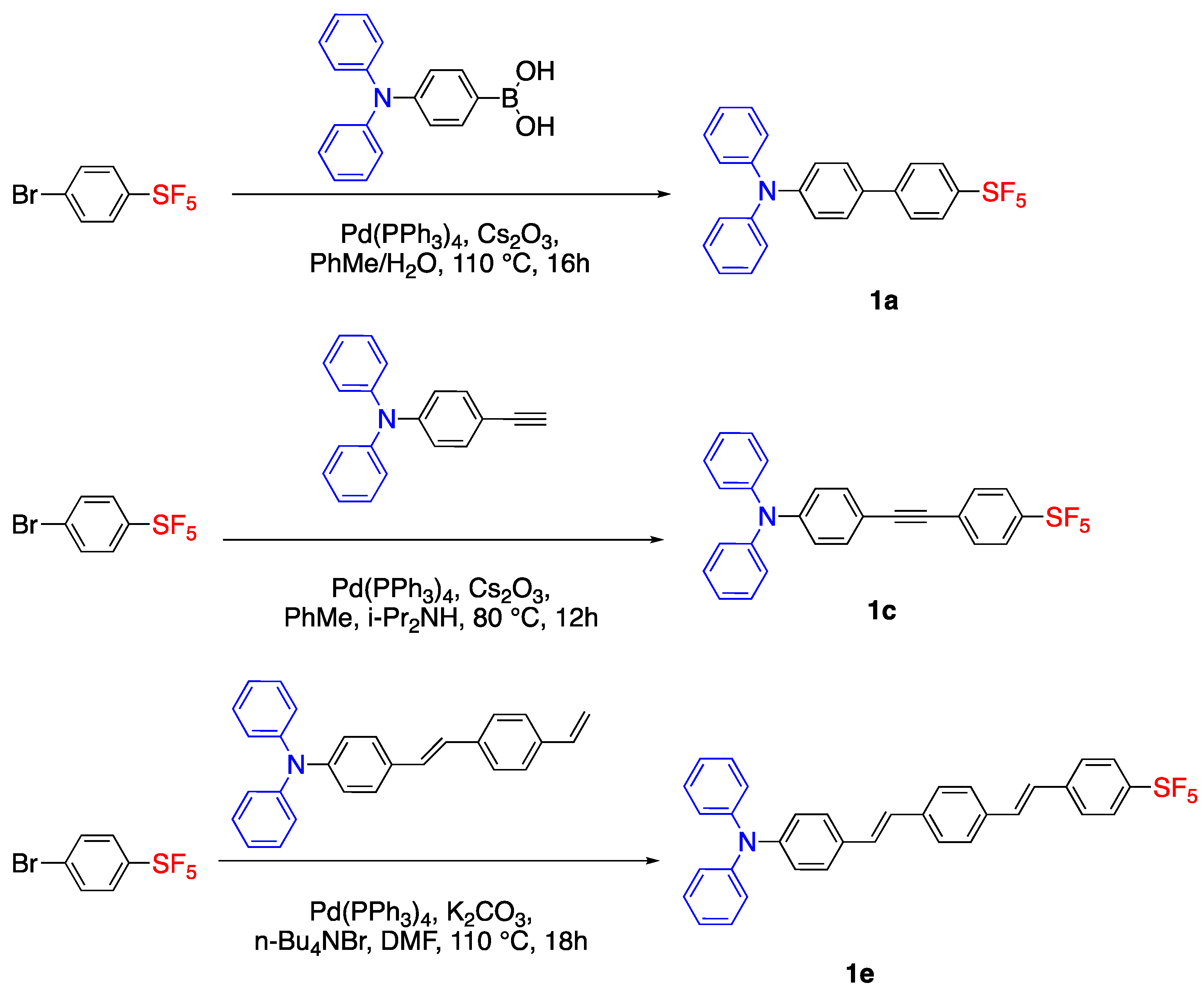
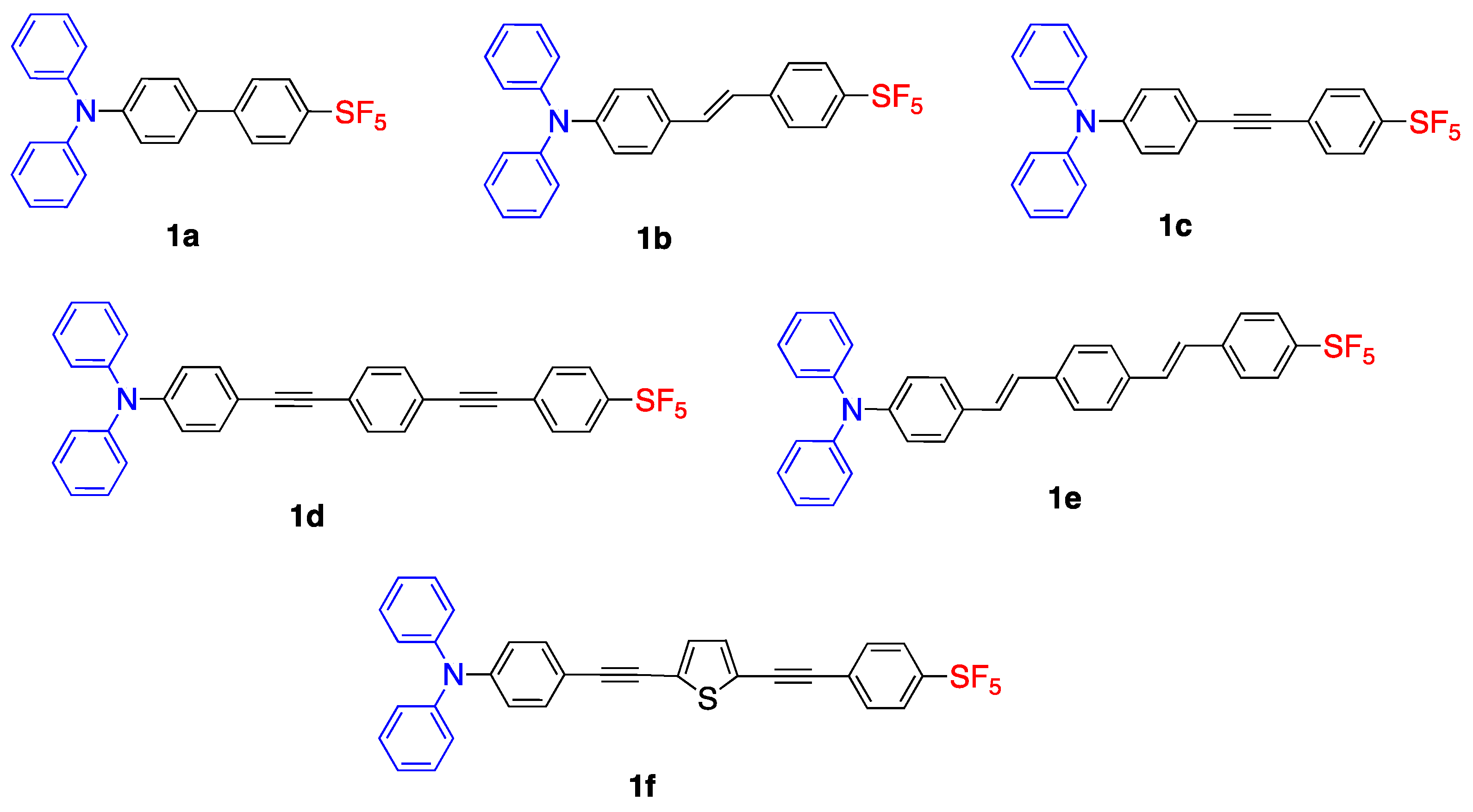
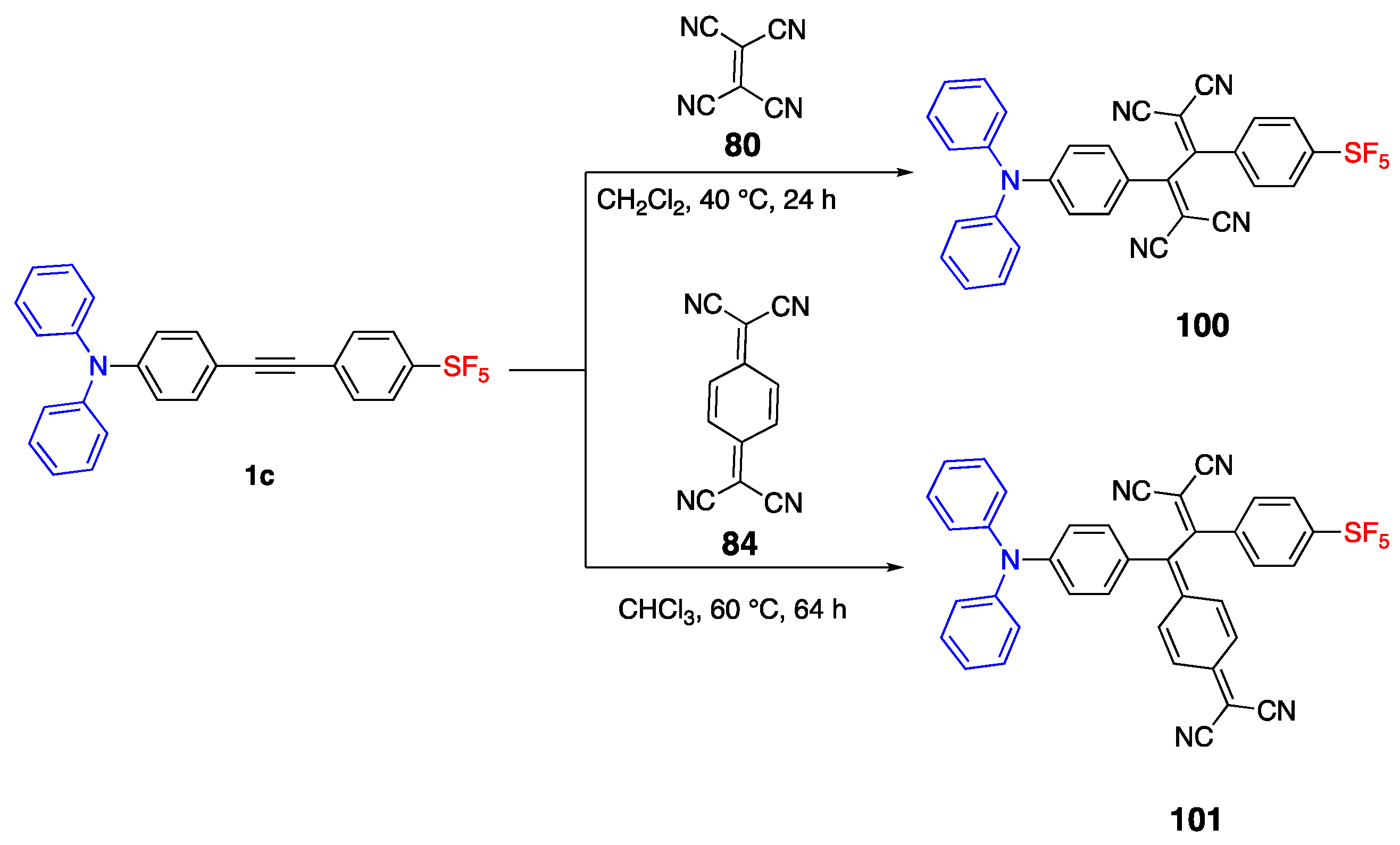
2.1. Pentafluorosulfonyl (SF5) Acceptor Group
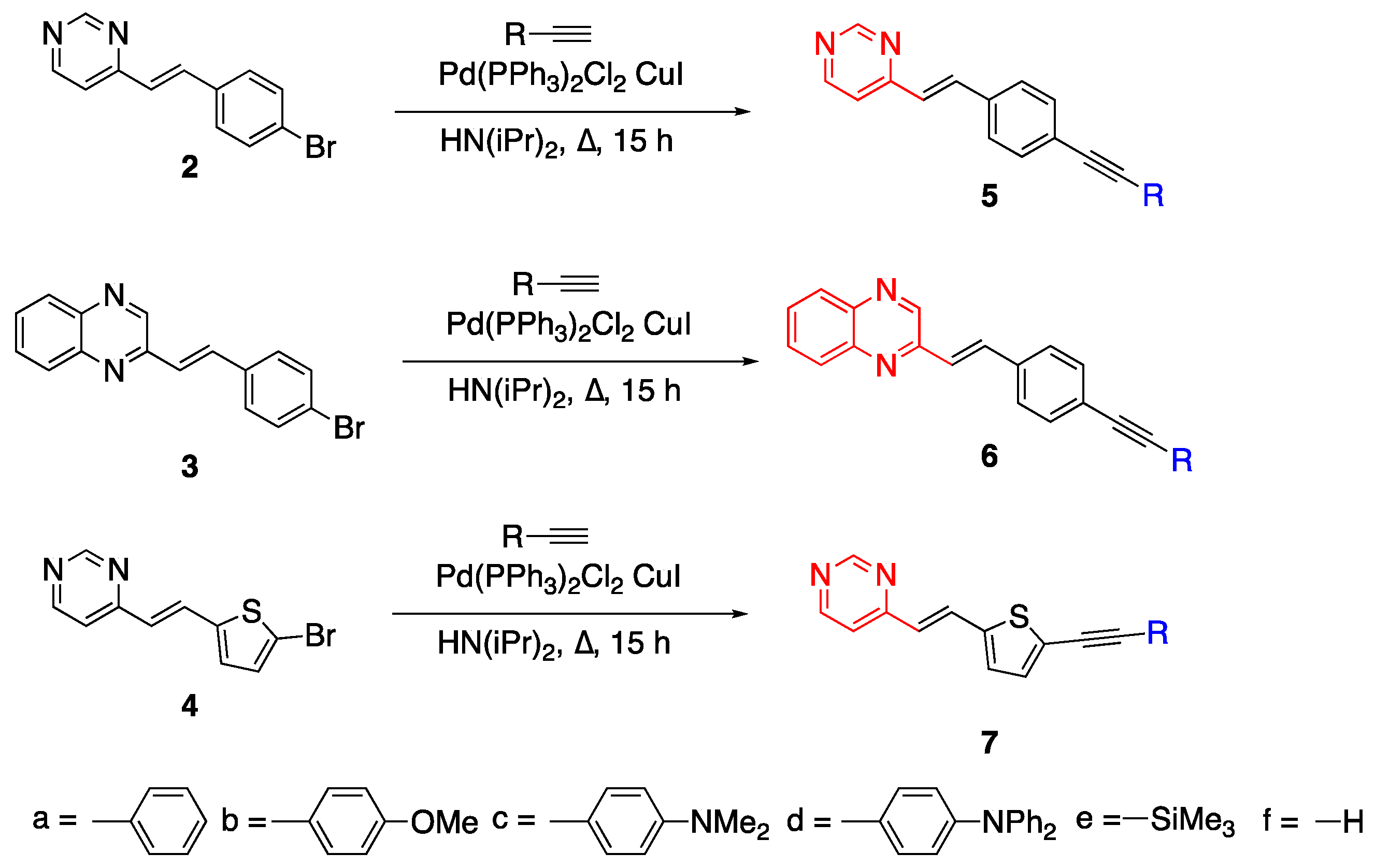
2.2. Diazines
2.3. Thiophene and Thienothiophenes
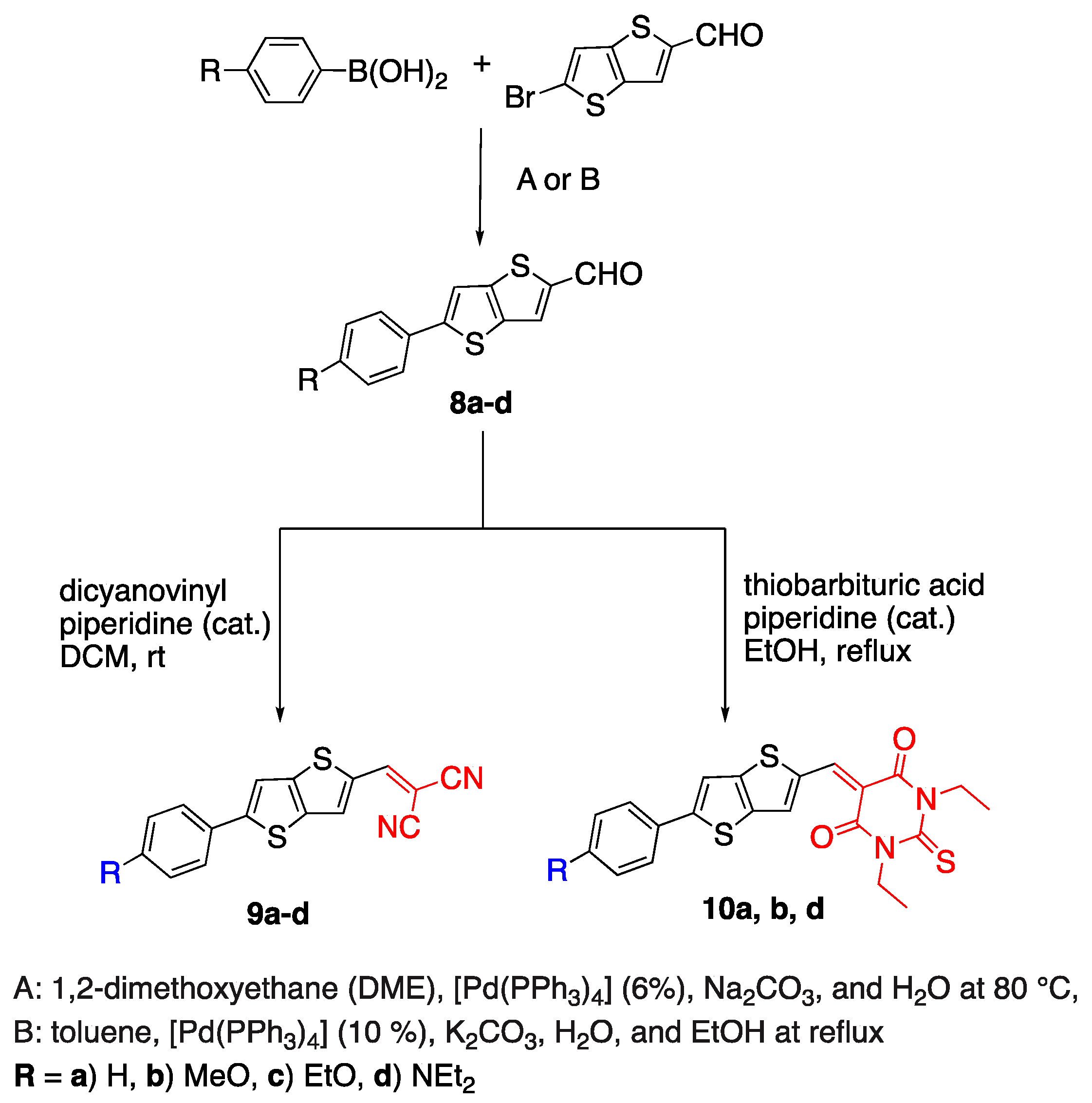
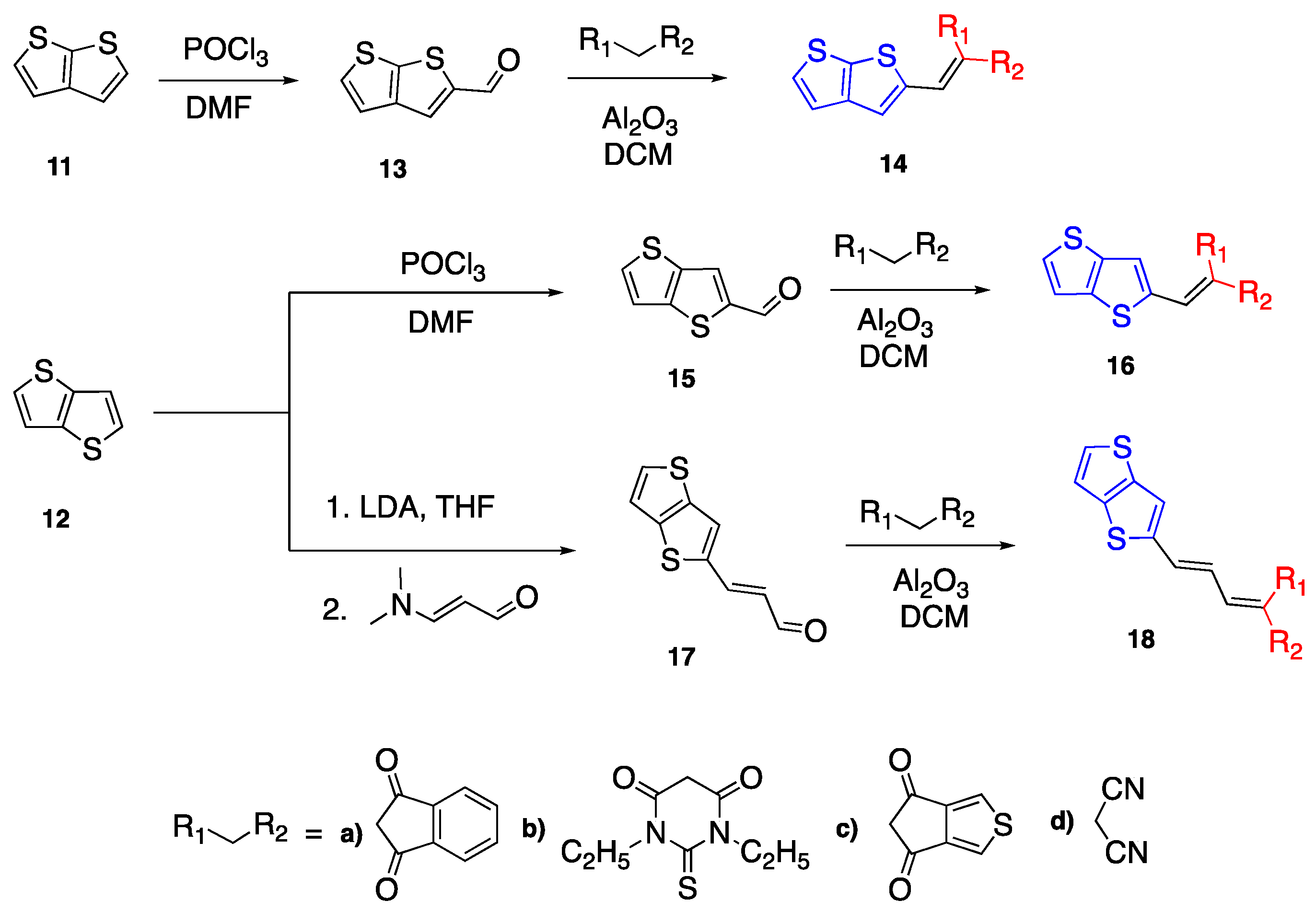
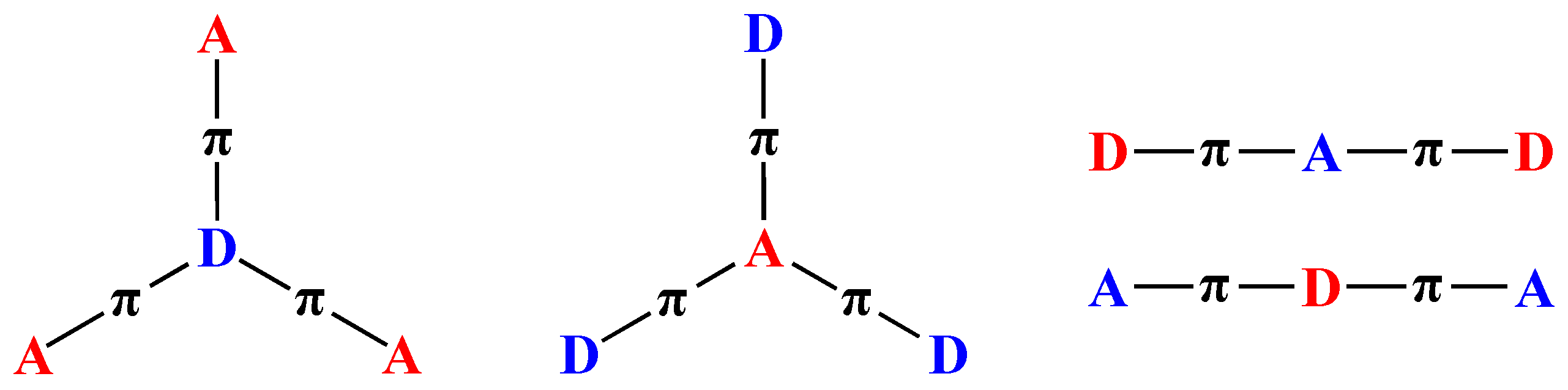
2.4. Nonlinear D--A Fluorophores
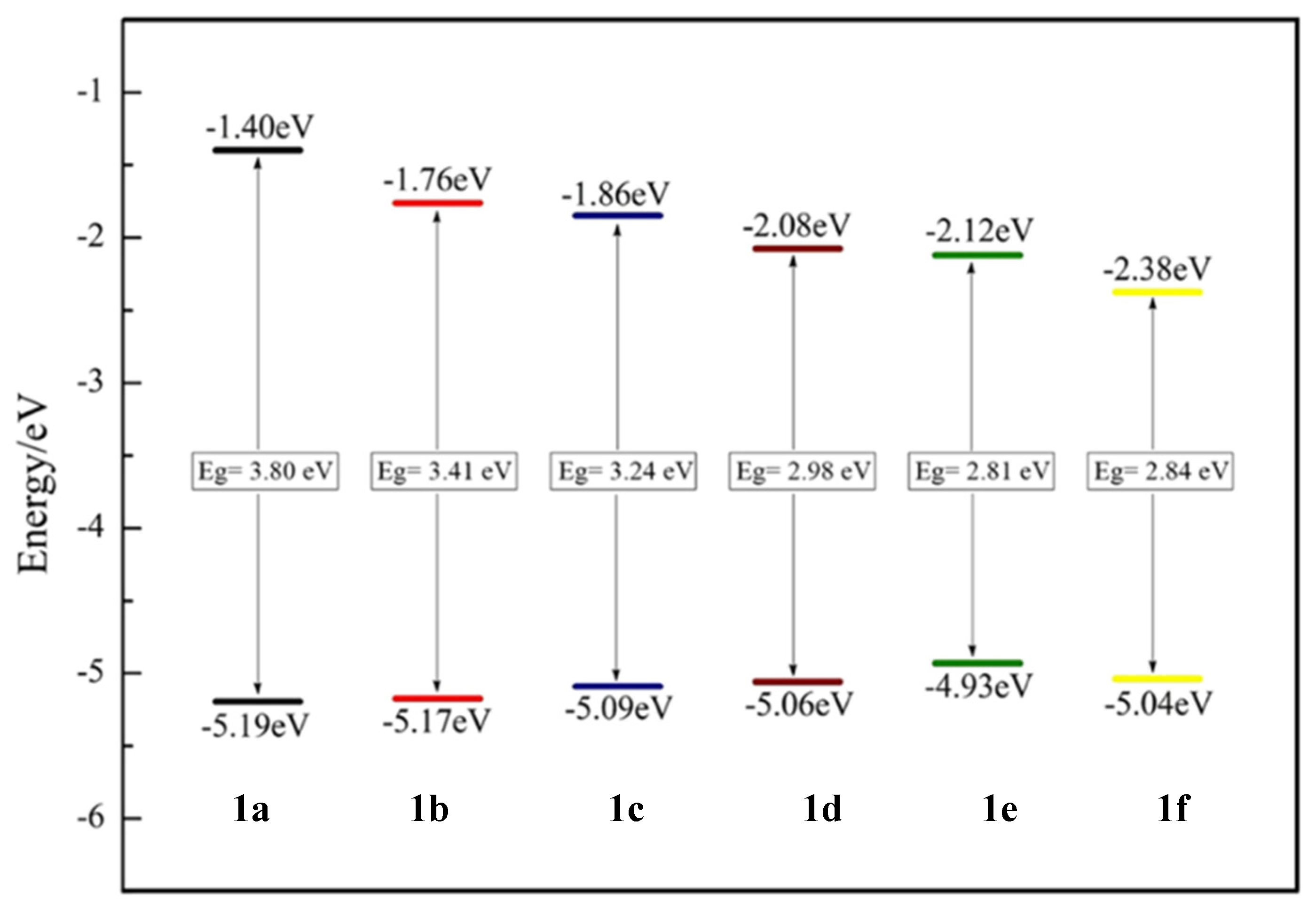
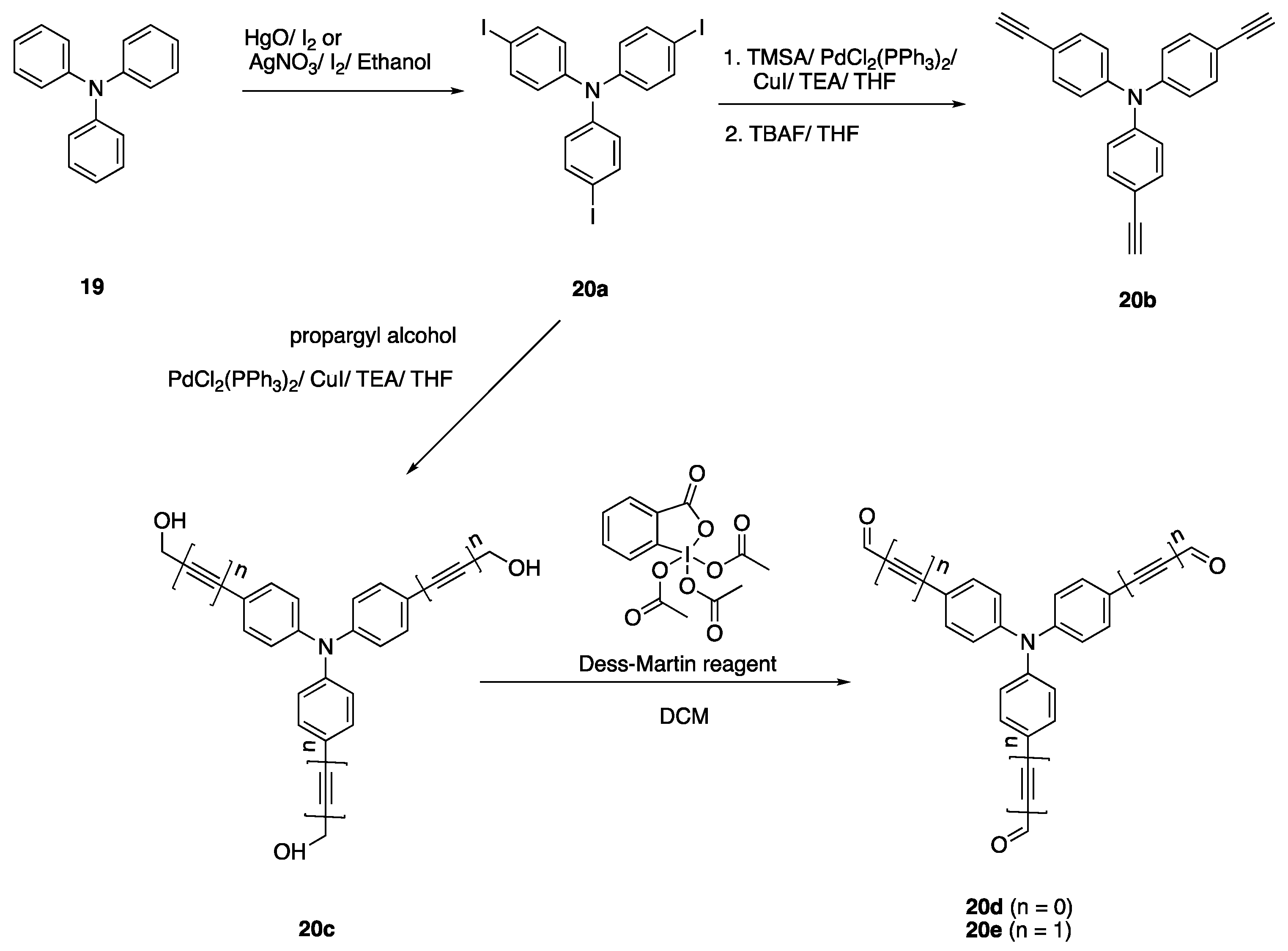
2.4.1. Triphenylamine Core
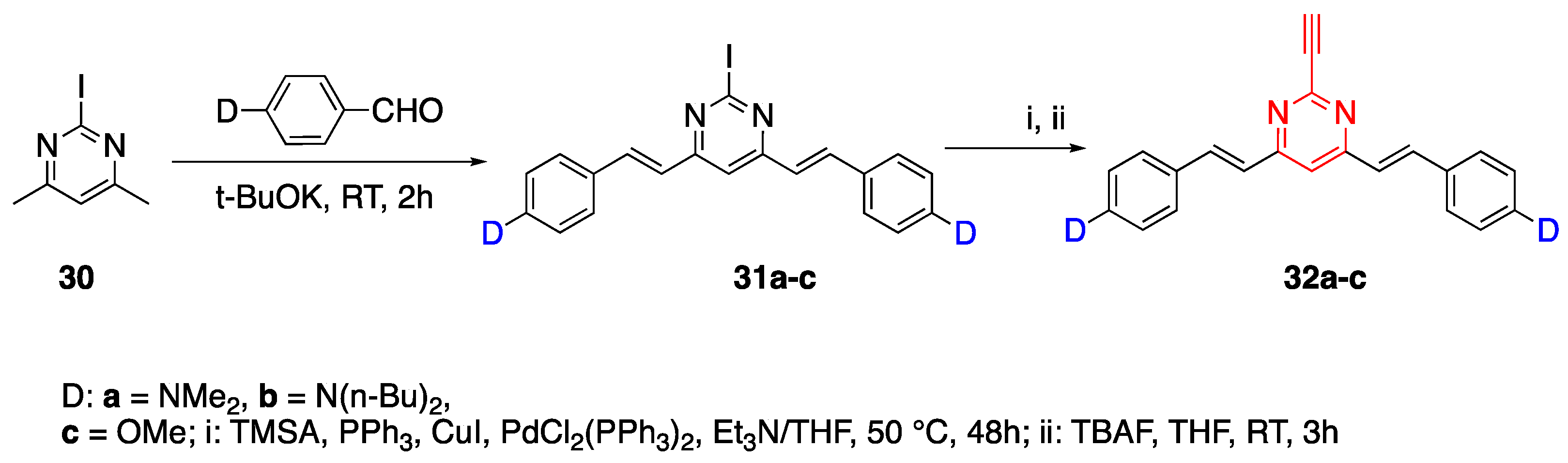
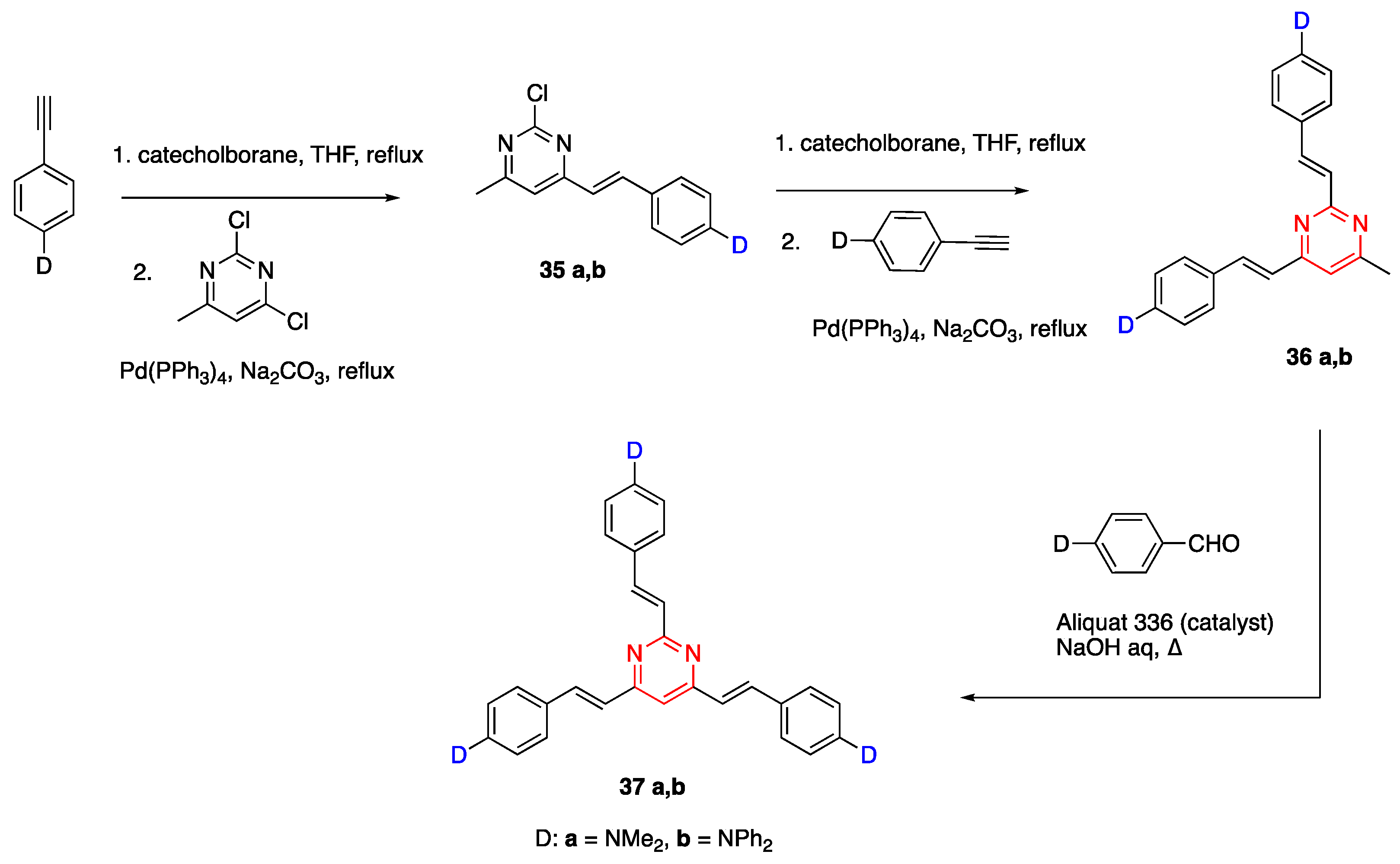
2.4.2. Pyrimidine Core
2.5. Fluorene as Linker Unit
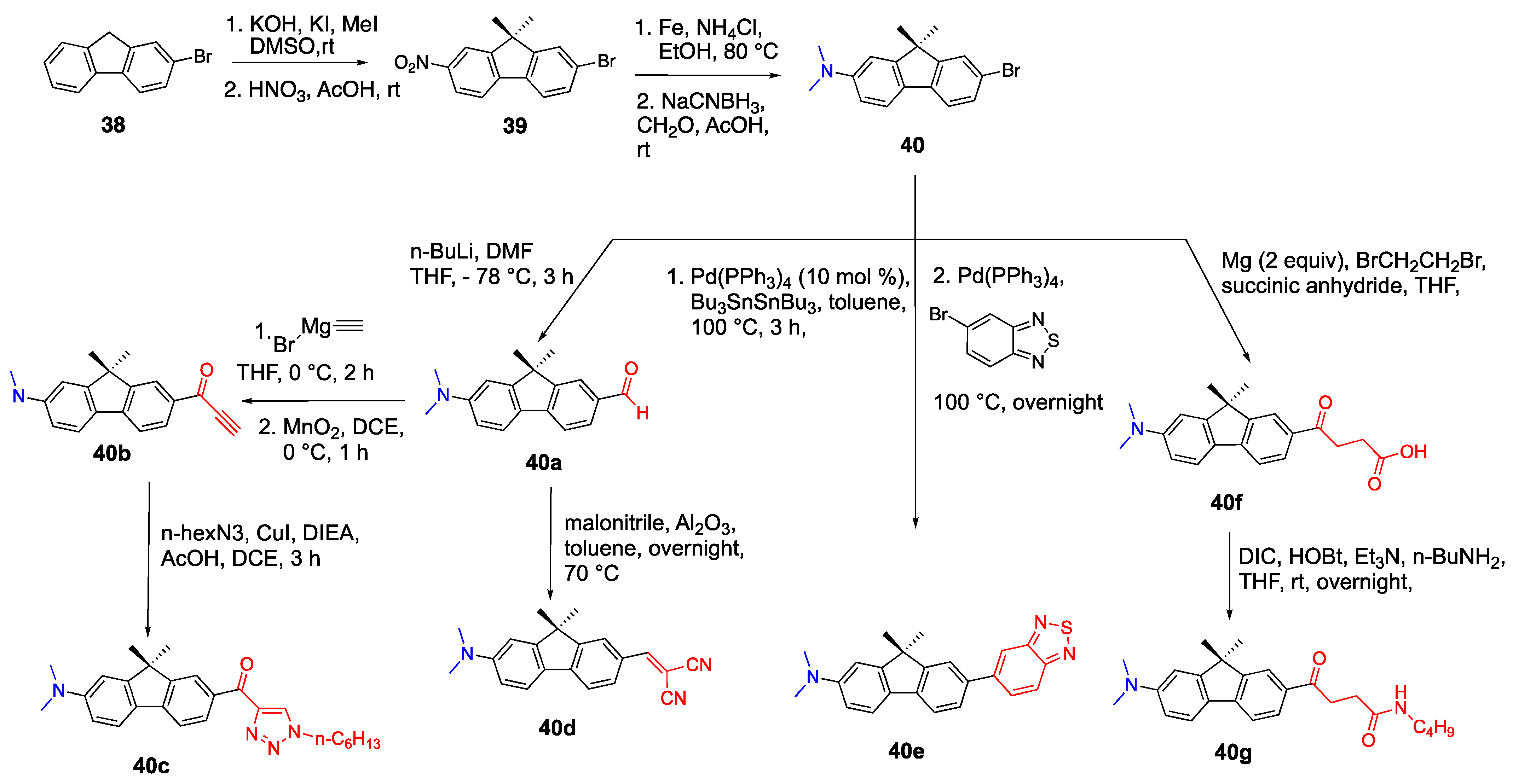
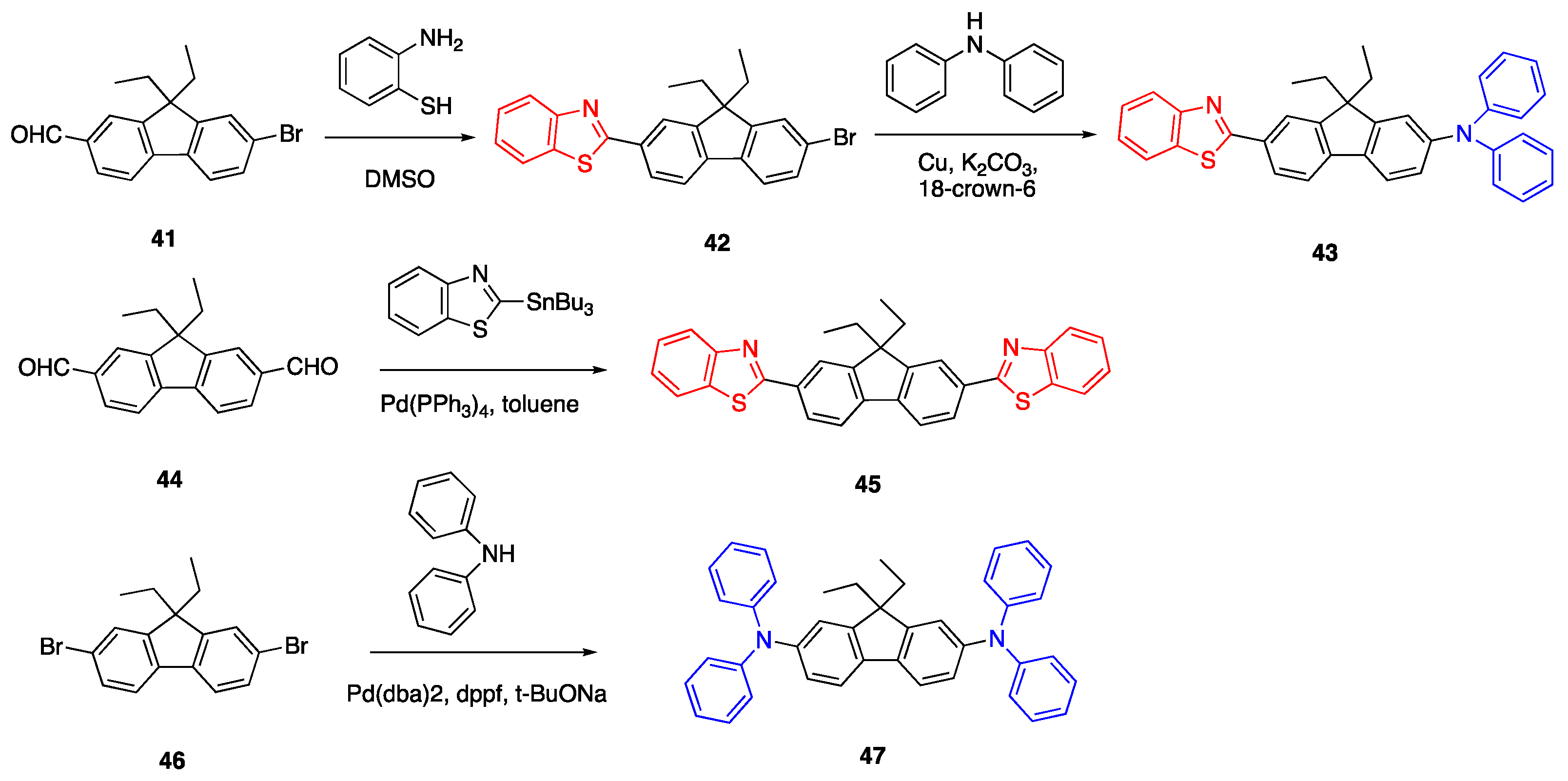
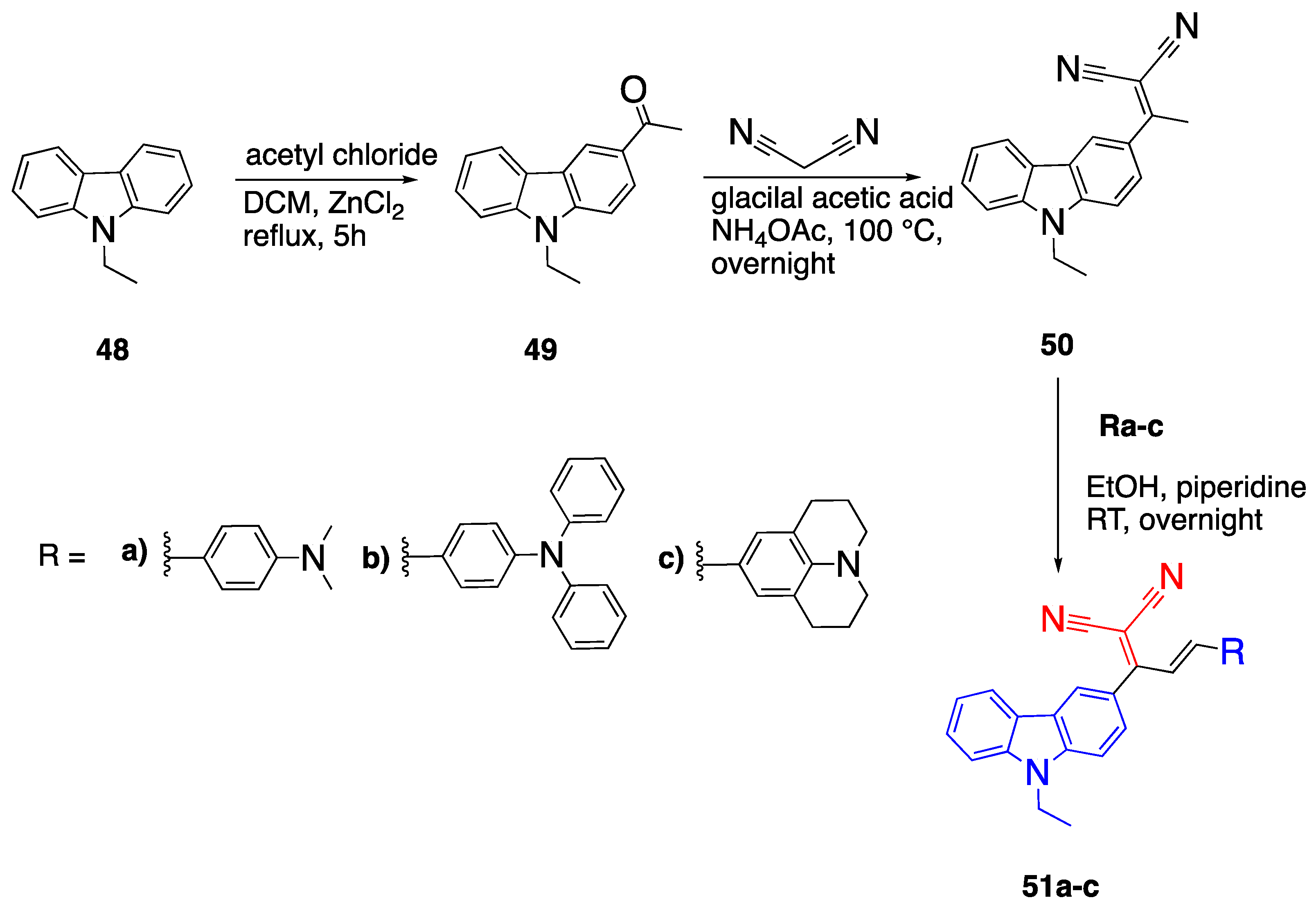
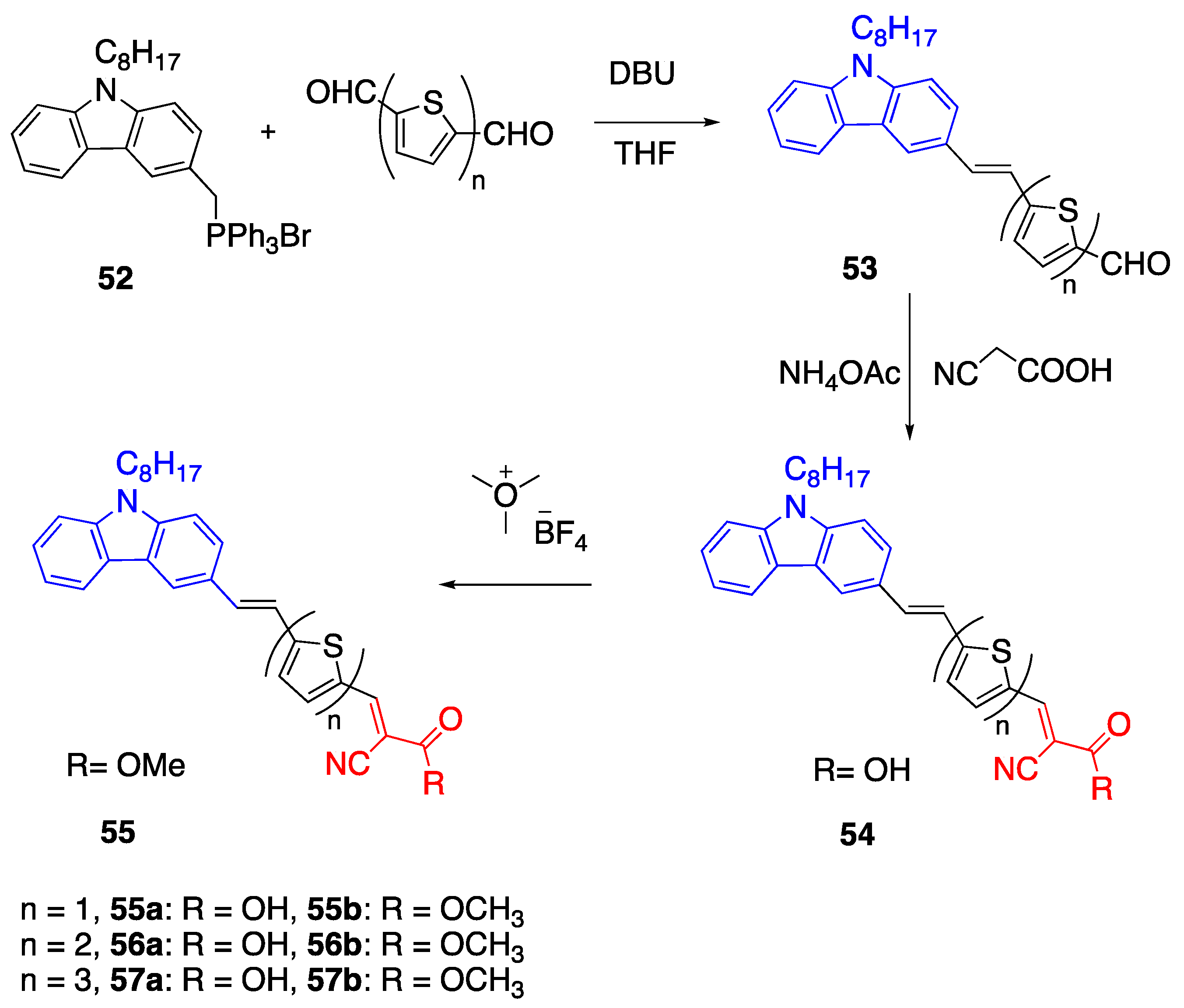
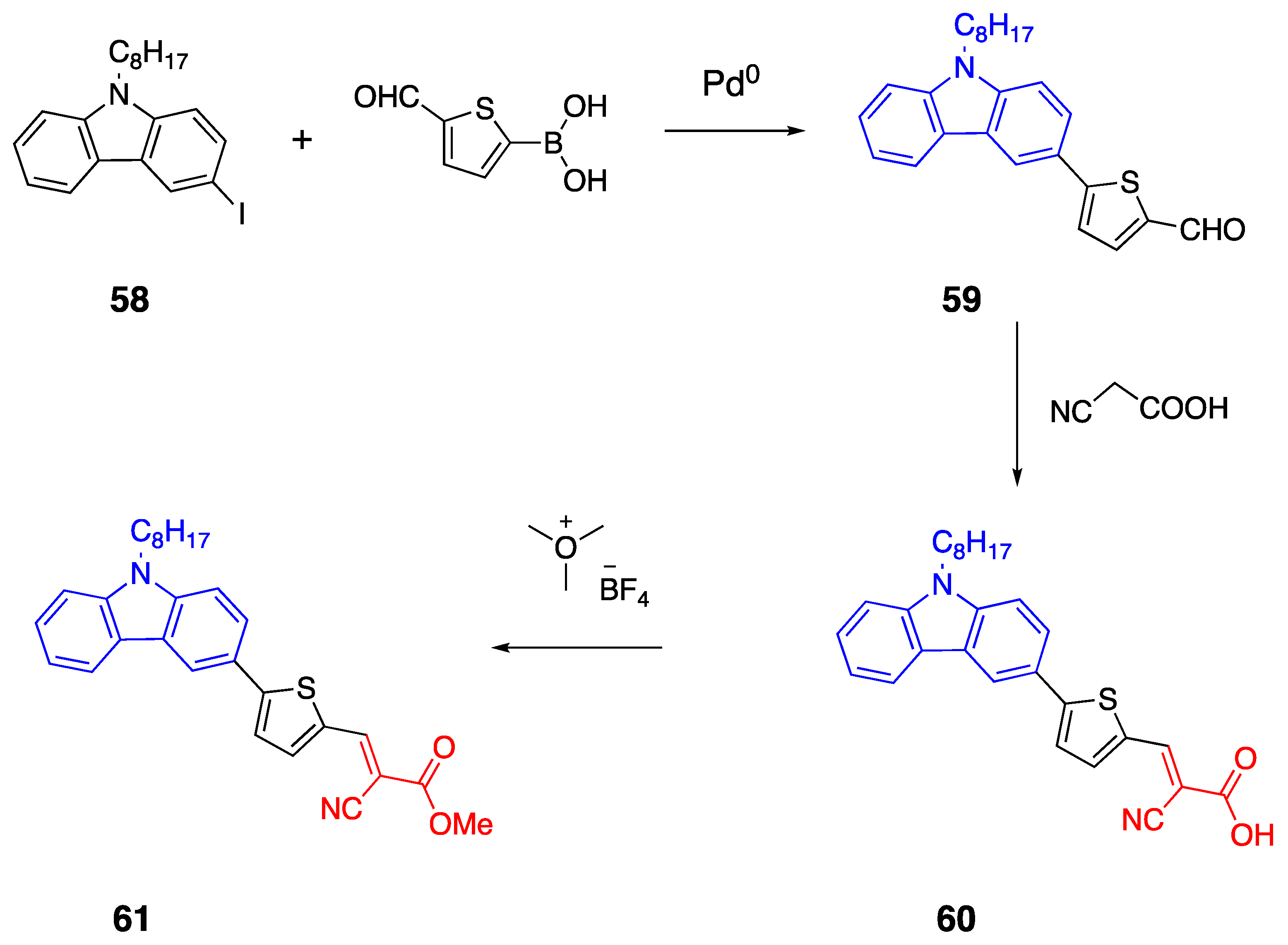
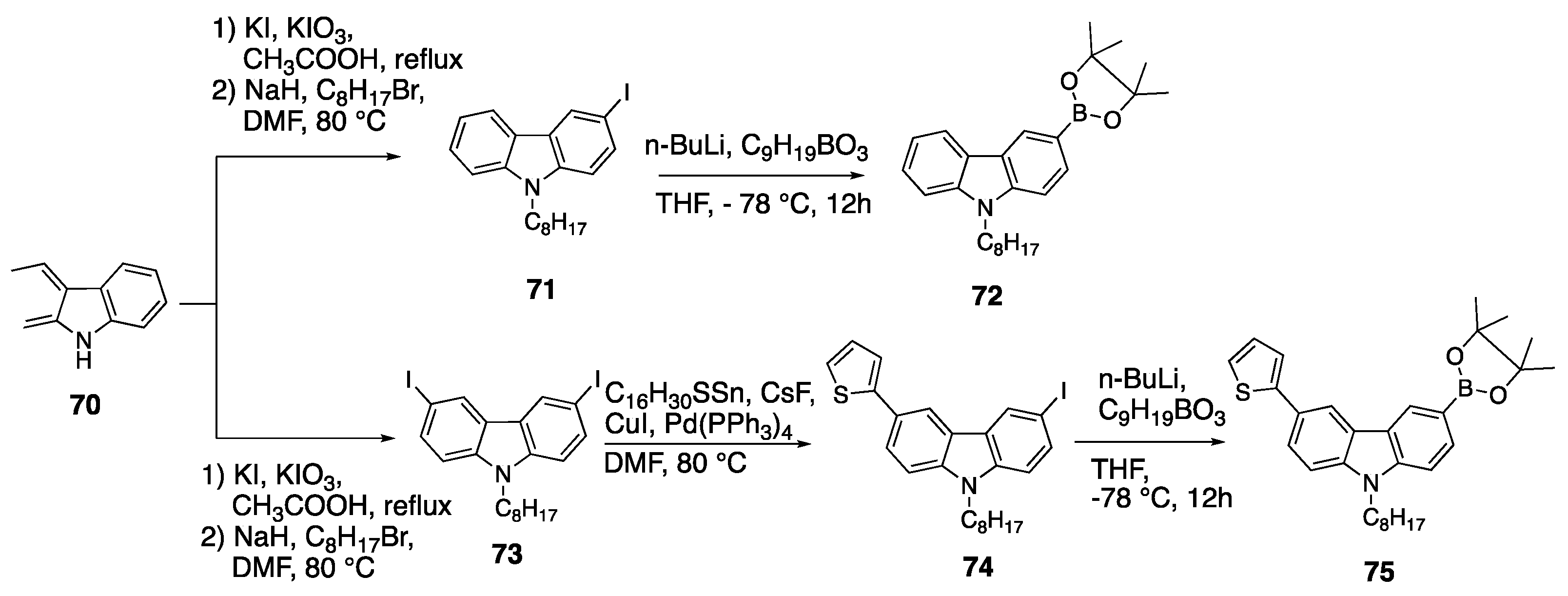
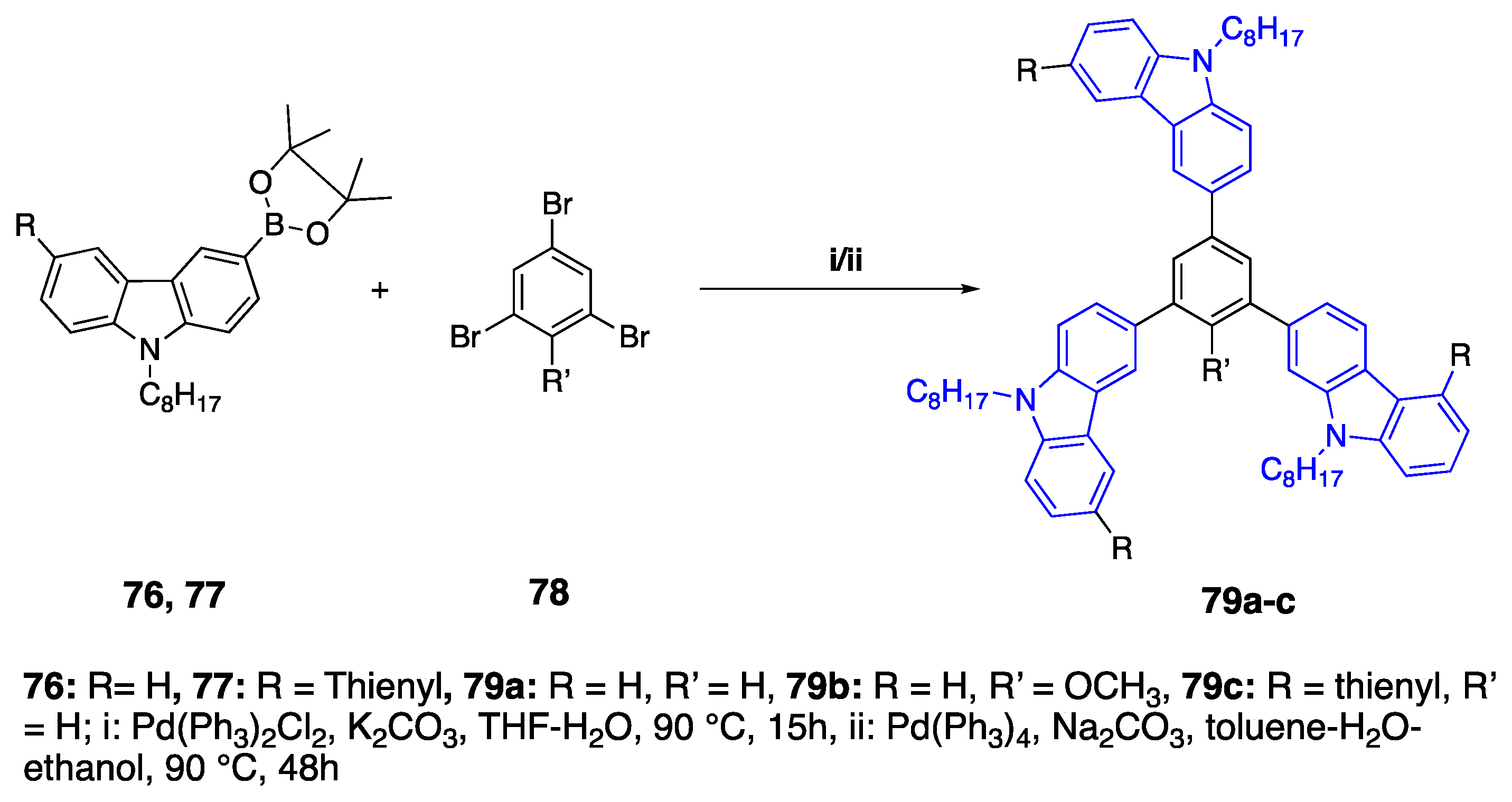
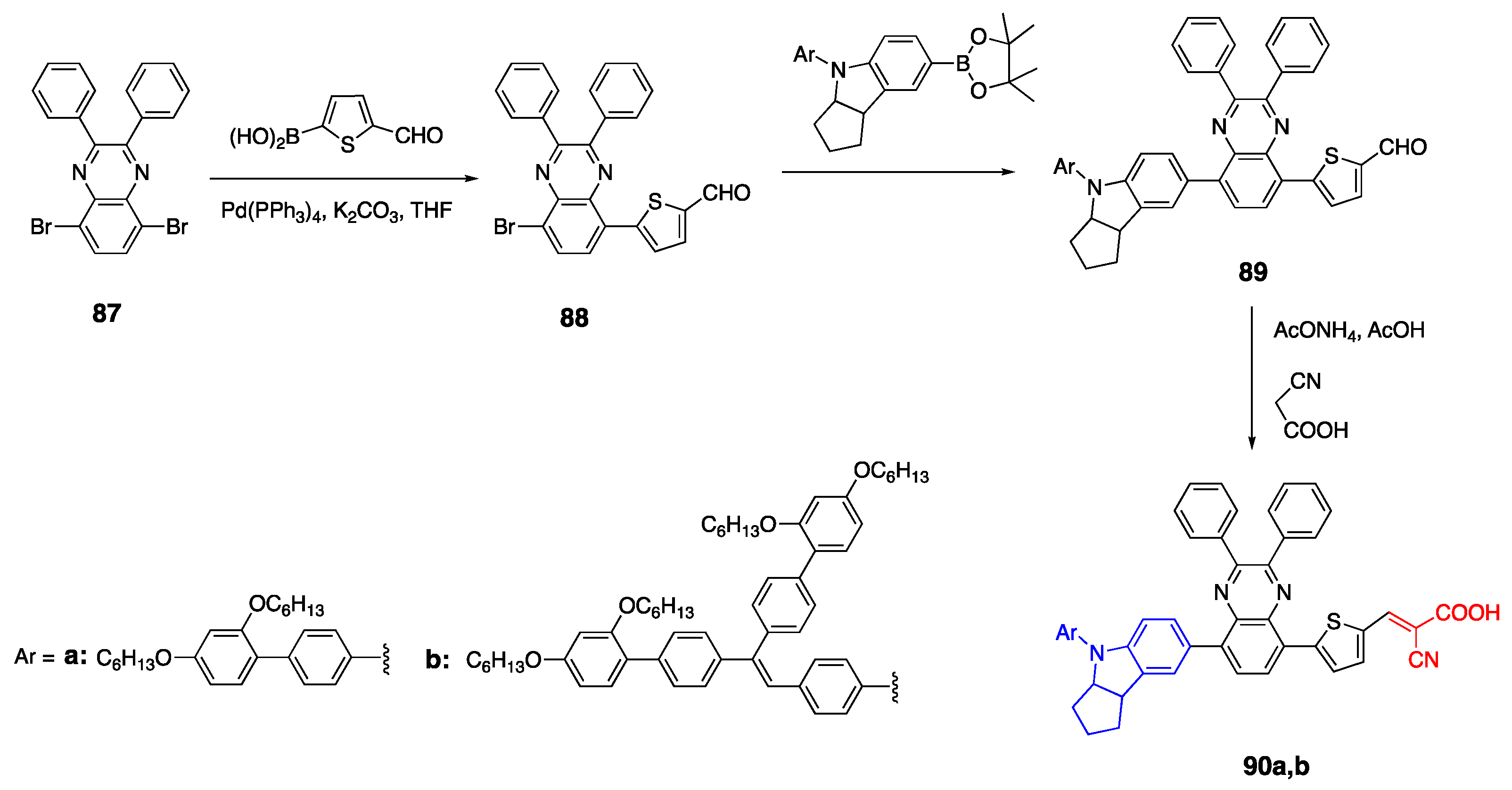
2.6. Carbazoles as Linker and Acceptor Unit
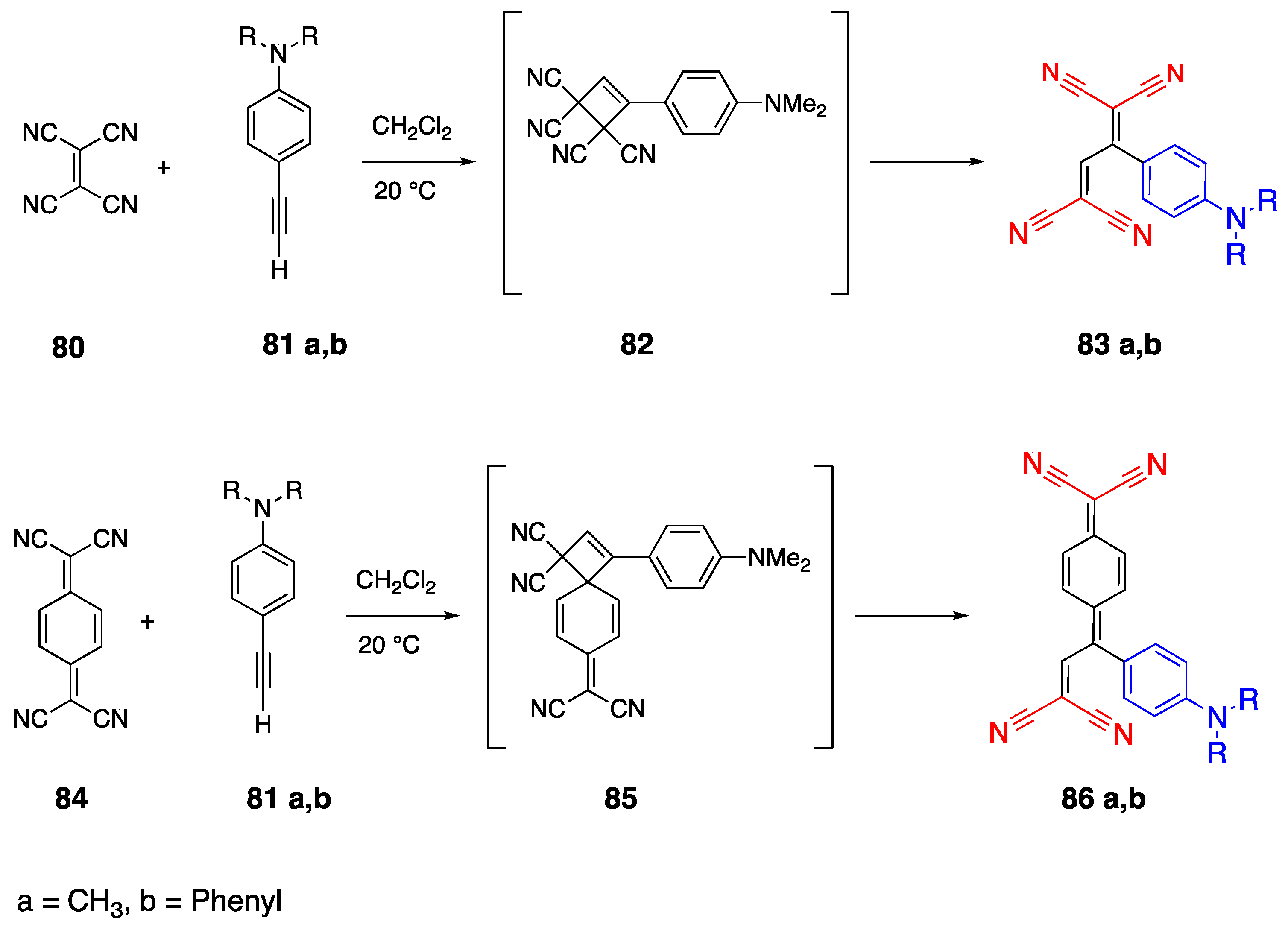
2.7. Tetracyanobutadienyl Acceptor Group
2.8. Cyanoacetic Acid Acceptor Group
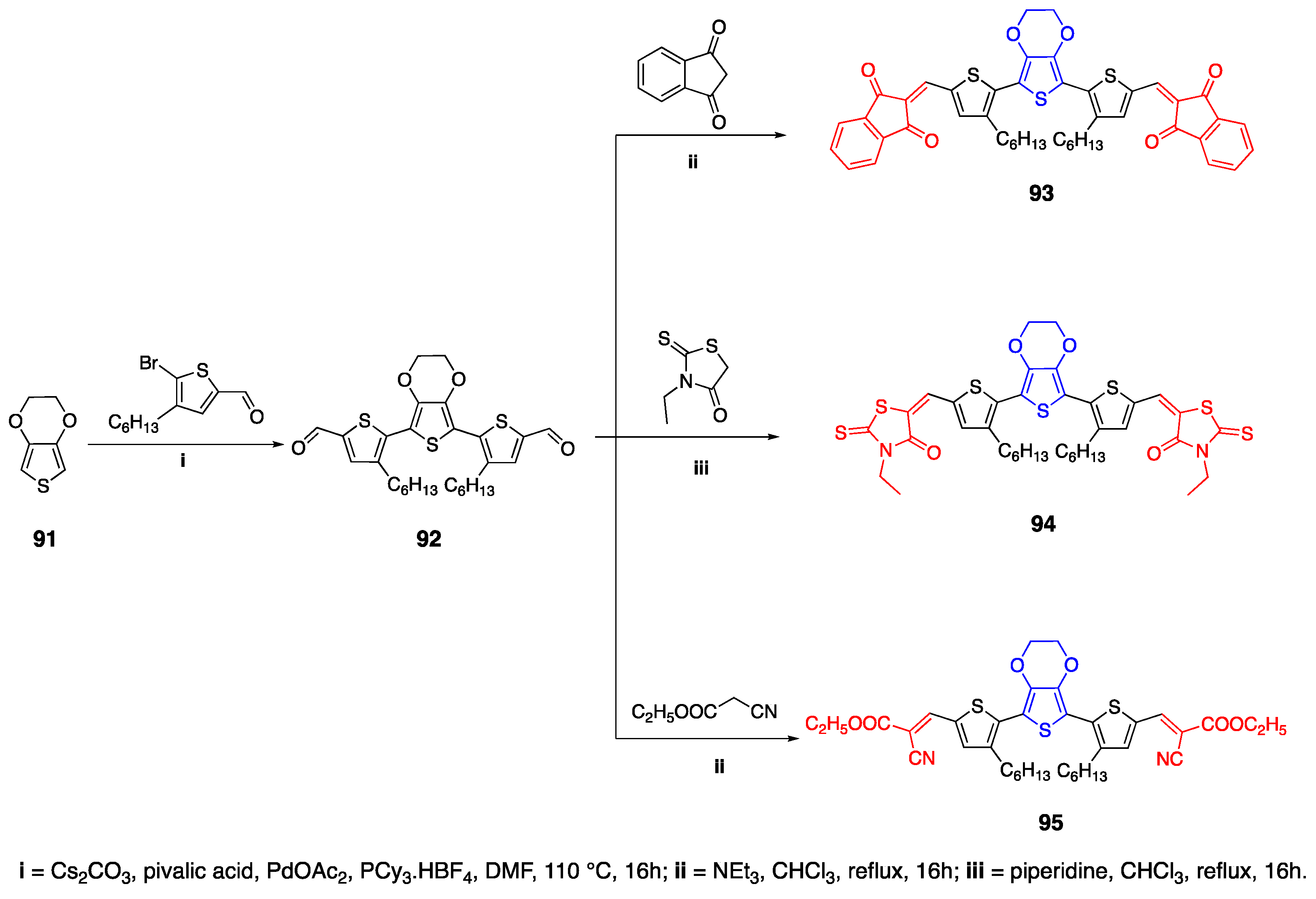
2.9. 3,4-Ethylenedioxythiophene (EDOT) as Linker Unit
3. D-π-A Fluorophores in NIR Region
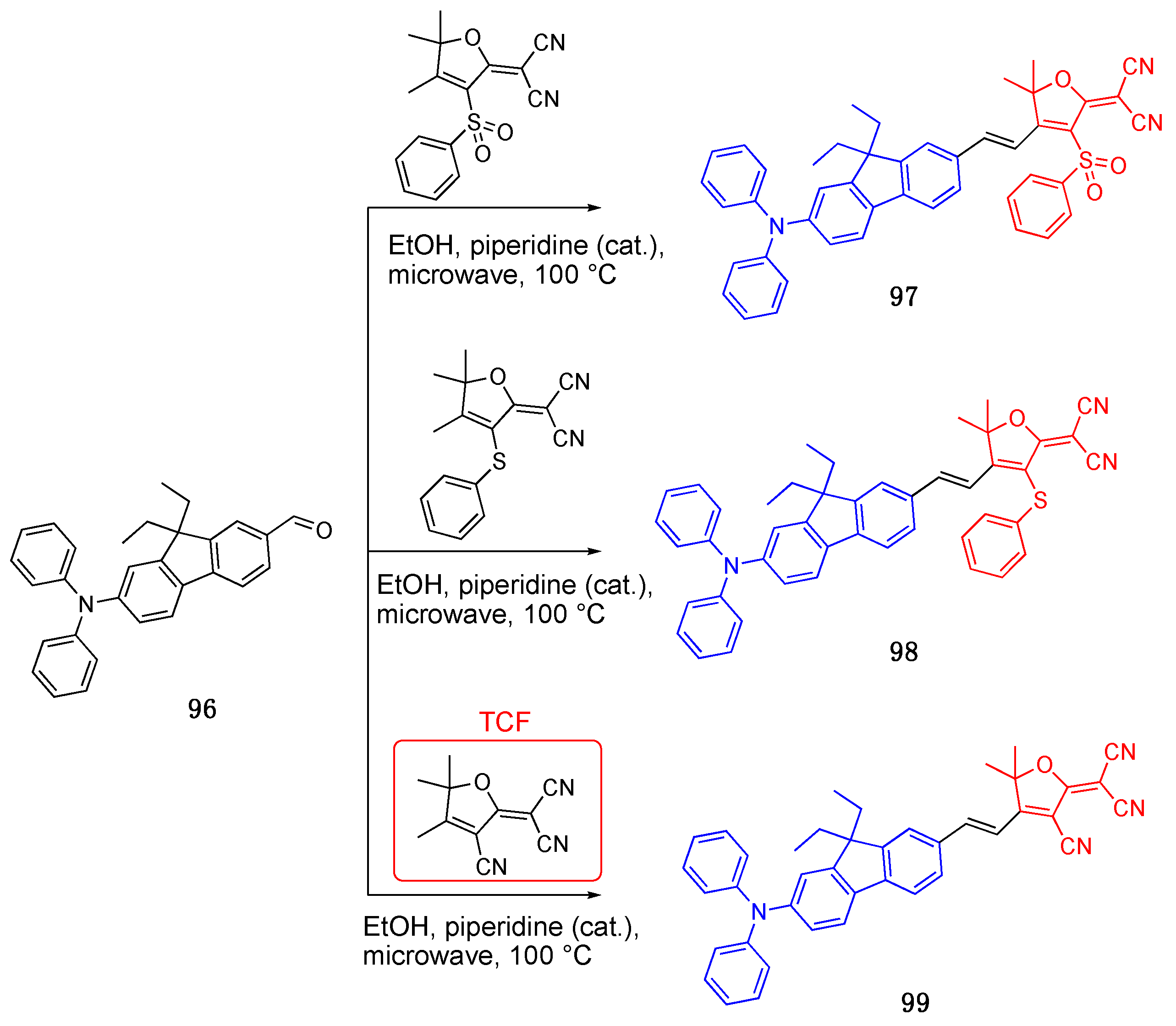
3.1. 4,5,5-Trimethyl-2,5-dihydrofuran (TCF) Acceptor
3.2. Pentafluorosulfonyl and Tetracyano Acceptor
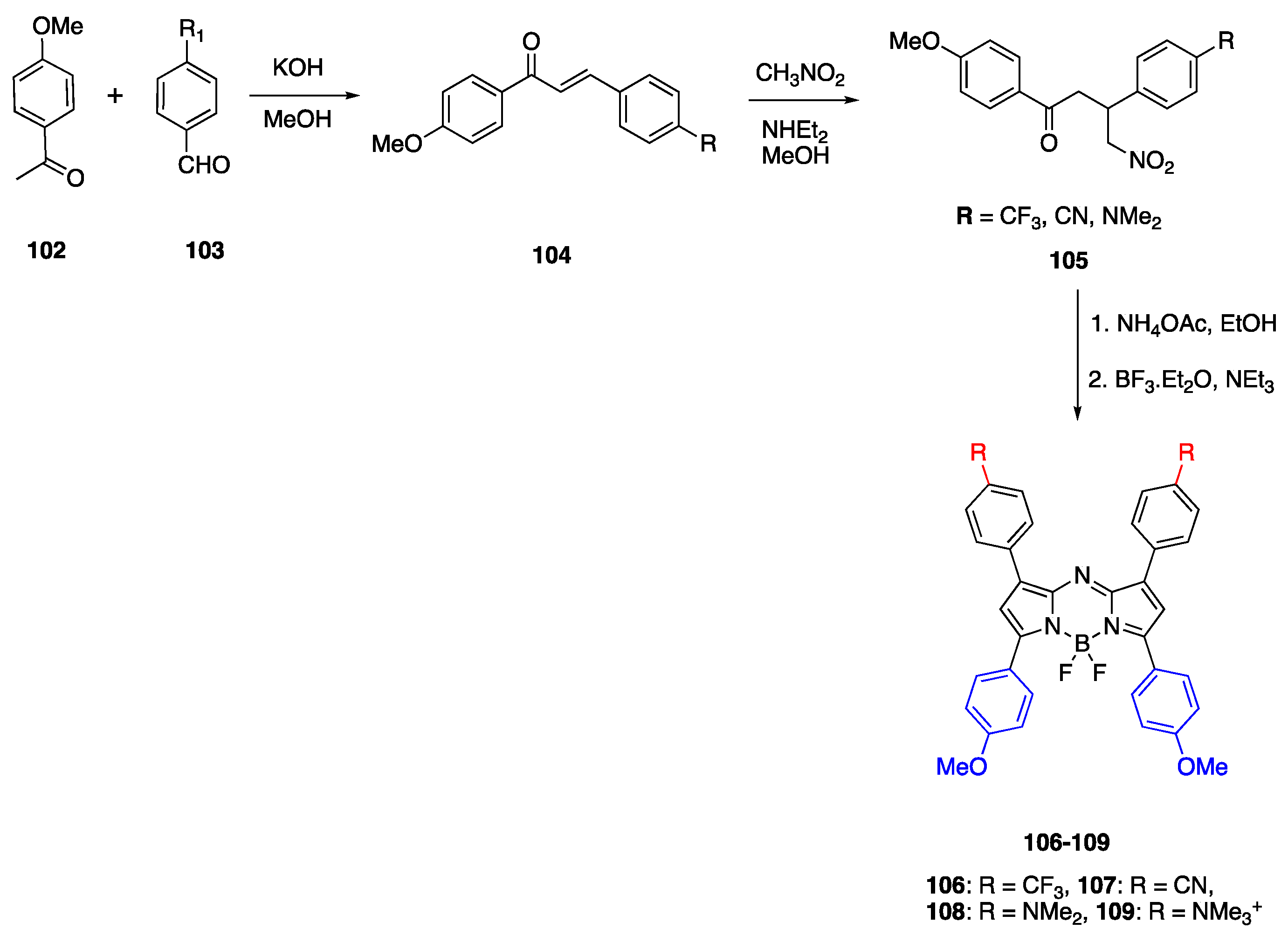
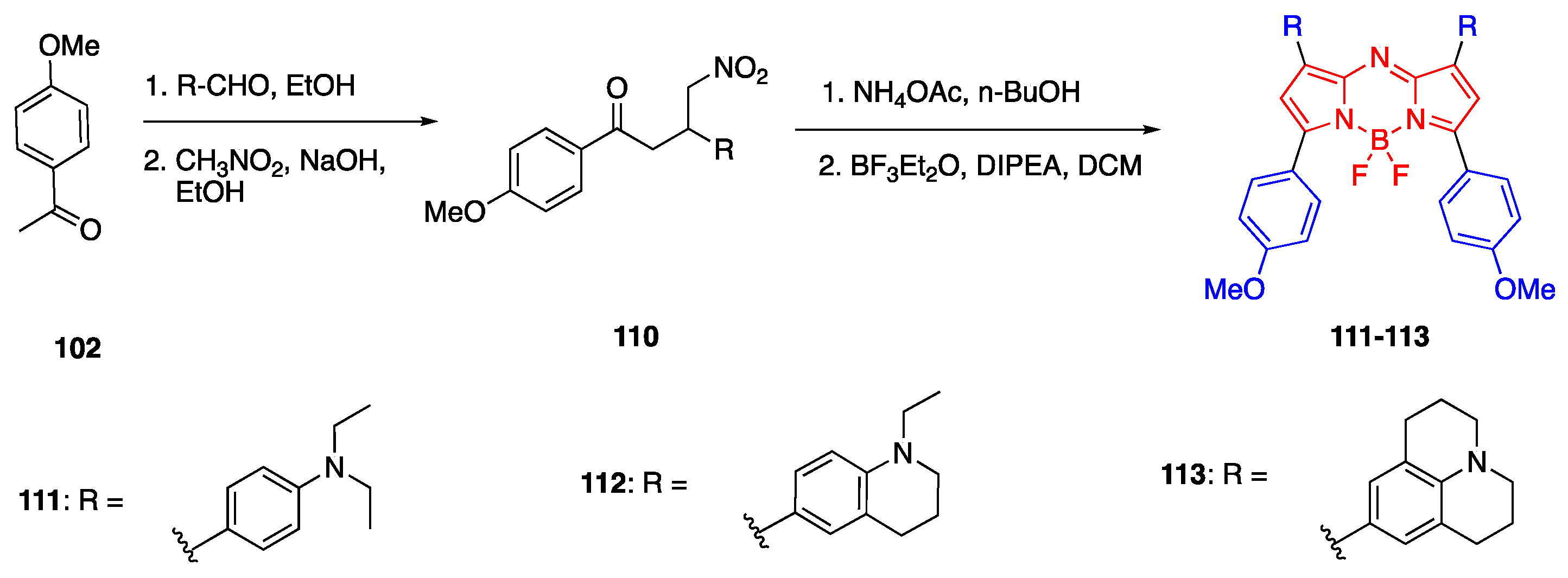
3.3. Aza-BODIPY Units as Linker and Acceptor Groups
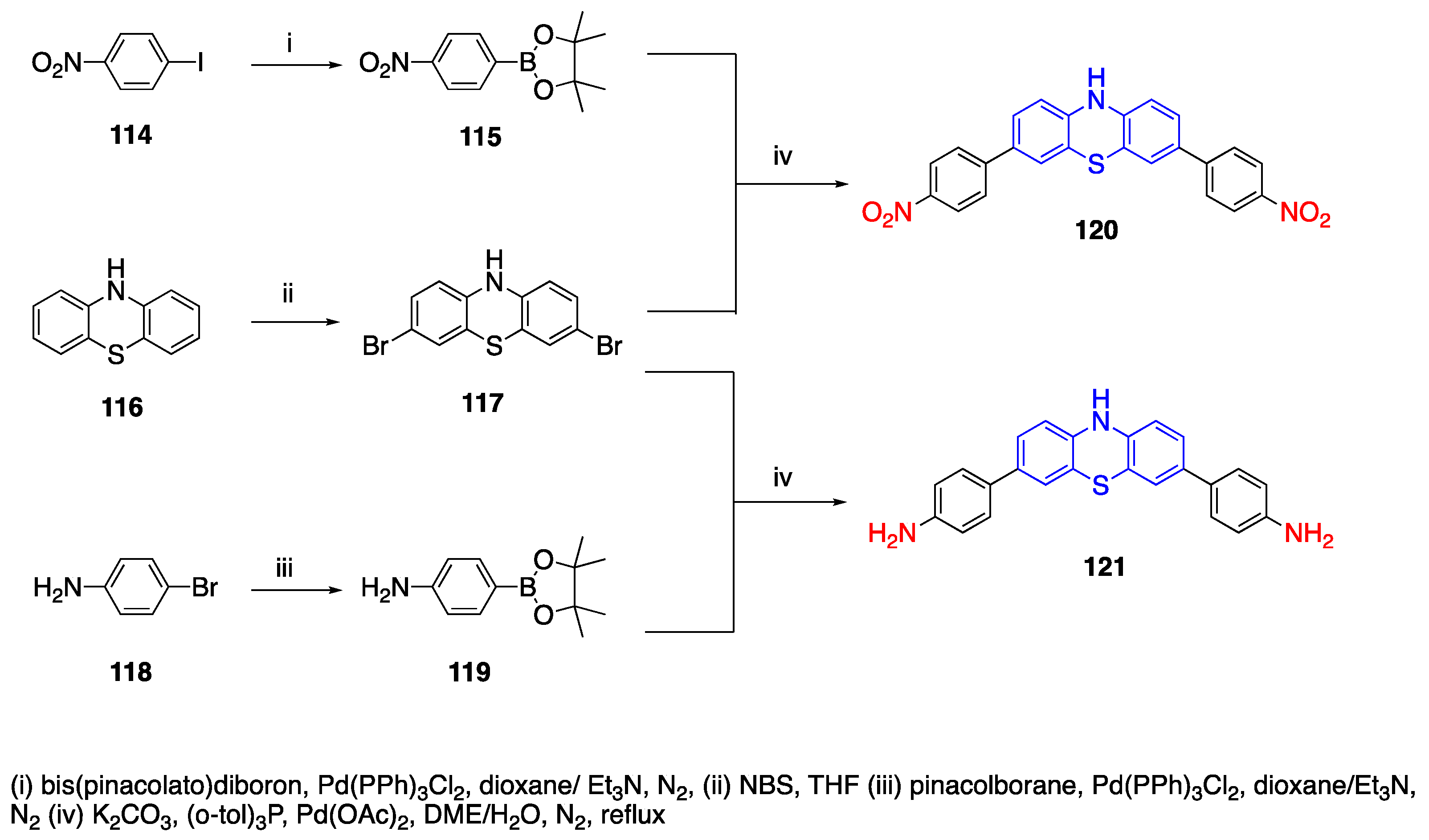
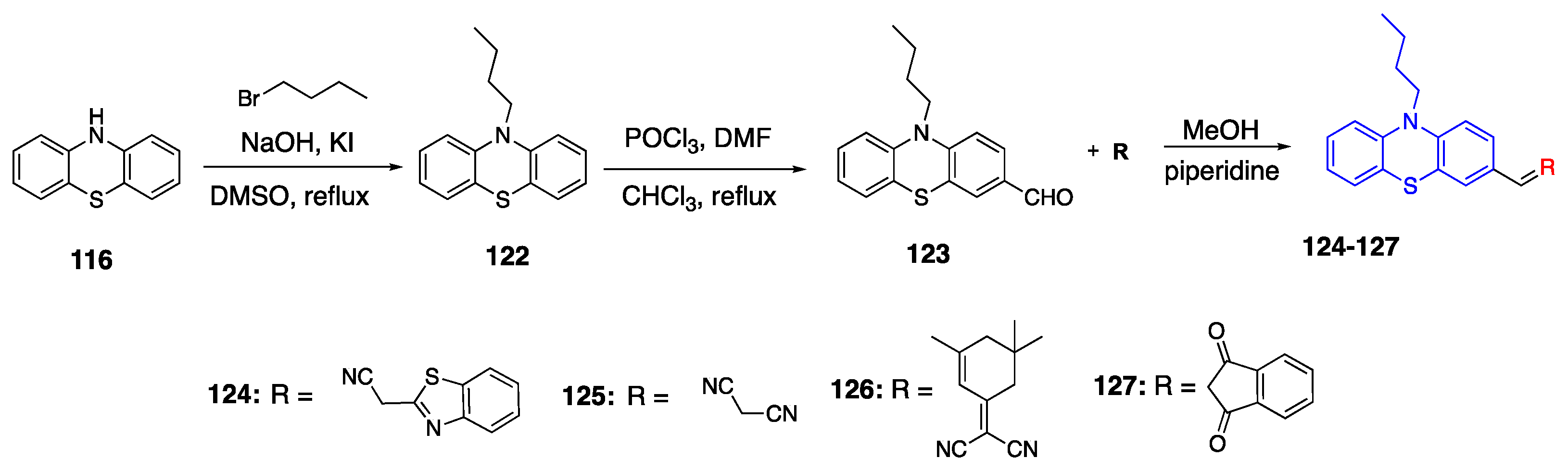
3.4. Phenothiazine Donor
3.5. Chloroacrylic Acid Acceptor
4. Optical Properties of Selected Compounds
4.1. Two Photon Absorption
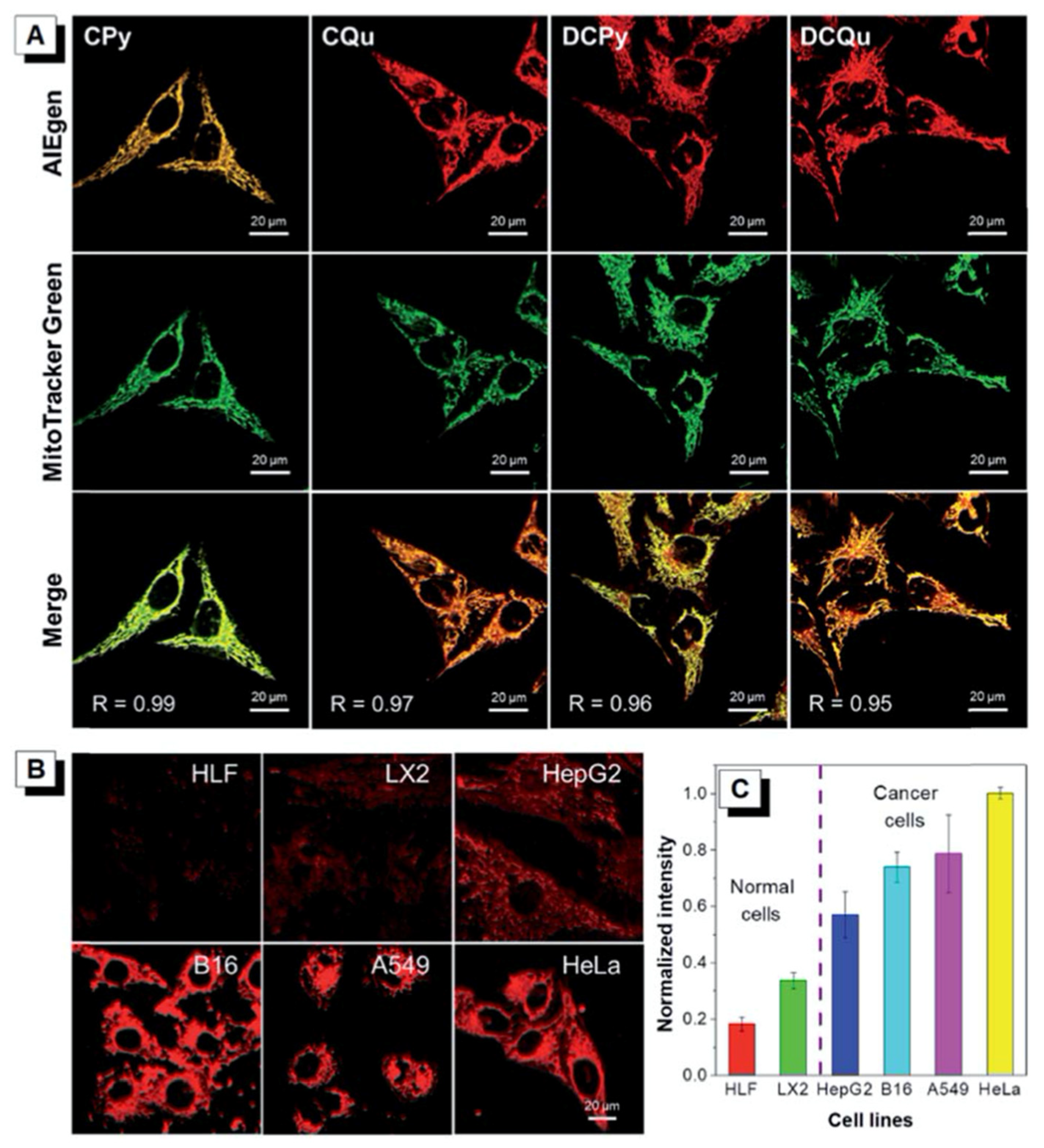
4.2. Aggregation-Induced Emission
5. Applications
5.1. Organic Solar Cells
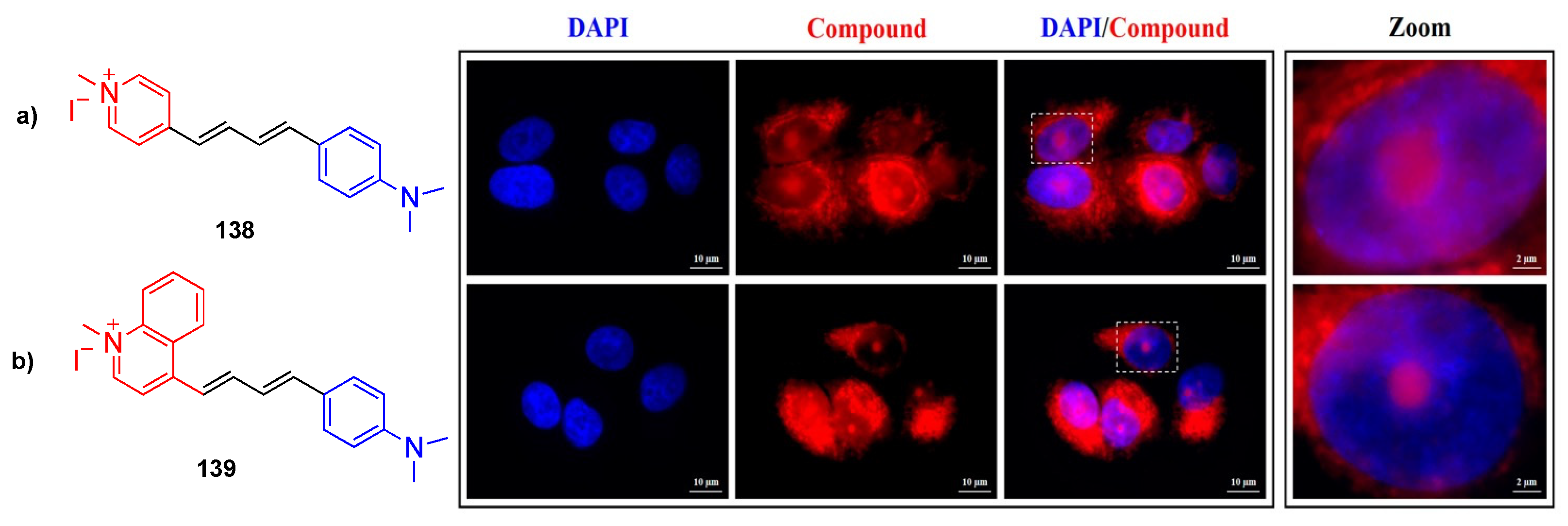
5.2. Bioimaging
5.3. Organic Light Emitting Diodes
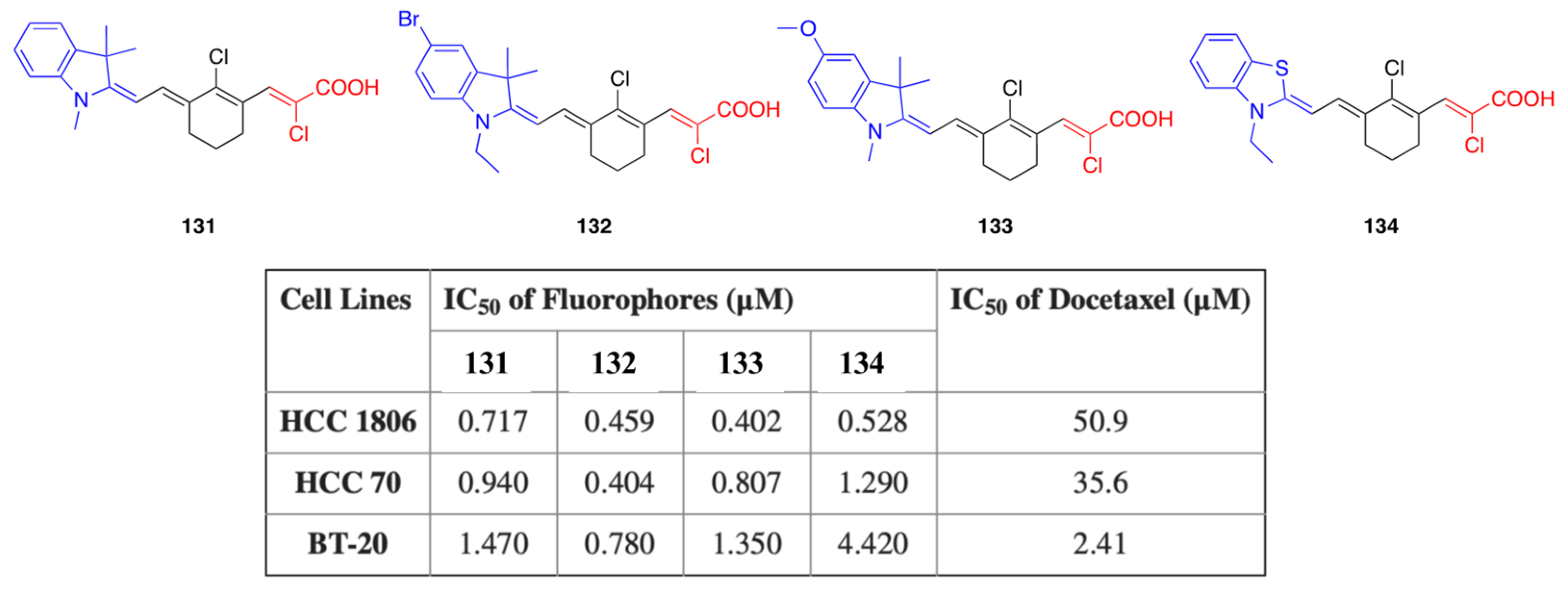
5.4. Anticancer Activity
5.5. Biosensors
| Compound Number | Solvent | abs (nm) | em (nm) | ) |
|---|---|---|---|---|
| 1a | Toluene | 349 | 418 | 0.48 |
| DCM | 347 | 449 | 0.016 | |
| Acetone | 344 | 461 | 0.010 | |
| 1b | Toluene | 385 | 448 | 0.24 |
| DCM | 386 | 488 | 0.20 | |
| Acetone | 381 | 500 | 0.070 | |
| 1c | Toluene | 369 | 424 | 0.48 |
| DCM | 368 | 460 | 0.070 | |
| Acetone | 363 | 488 | 0.010 | |
| 1d | Toluene | 379 | 434 | 0.49 |
| DCM | 380 | 492 | 0.40 | |
| Acetone | 373 | 515 | 0.15 | |
| 1e | Toluene | 407 | 472 | 0.29 |
| DCM | 403 | 528 | 0.23 | |
| Acetone | 397 | 547 | 0.19 | |
| 1f | Toluene | 396 | 454 | 0.37 |
| DCM | 395 | 506 | 0.30 | |
| Acetone | 387 | 531 | 0.17 | |
| 5a | DCM | 347 | 420 | 0.51 |
| 5c | DCM | 312 | 612 | 0.54 |
| 5d * | DCM | 314, 394 | 590 | 0.41 |
| 6a * | DCM | 308, 381 | 447 | 0.39 |
| 6c * | DCM | 321, 406 | 647 | 0.07 |
| 6d * | DCM | 306, 333, 403 | 630 | 0.37 |
| 7a * | DCM | 278, 383 | 462 | <0.01 |
| 7c * | DCM | 328, 419 | 607 | 0.80 |
| 7d * | DCM | 305, 342, 416 | 583 | 0.79 |
| 9a | CHCl3 | 436 | 491 | 0.002 |
| 9b | CHCl3 | 455 | 531 | 0.006 |
| 9d | CHCl3 | 534 | 652 | 0.61 |
| 10a | CHCl3 | 495 | 548 | 0.003 |
| 10b | CHCl3 | 514 | 579 | 0.006 |
| 10d | CHCl3 | 595 | 710 | 0.20 |
| 14a | DMF | 413 | - | - |
| 14b | DMF | 444 | - | - |
| 16a | DMF | 433 | - | - |
| 16b | DMF | 446 | - | - |
| 18a | DMF | 459 | - | - |
| 18b | DMF | 479 | - | - |
| 21 * | DMF | 332, 401 | 577 | 0.55 |
| 22 | DMF | 363 | 587 | 0.58 |
| 23 | DMF | 345 | 630 | 0.37 |
| 24 | DMF | 370 | 617 | 0.38 |
| 25 | DMF | 339 | 551 | 0.67 |
| 26 | DMF | 372 | 448 | 0.64 |
| 27 | DMF | 453 | 549 | 0.29 |
| 28 | DMF | 420 | 533 | 0.69 |
| 29 | DMF | 412 | 516 | 0.49 |
| 37a | DMF | 402 | 536 | 0.22 |
| 37b | DMF | 415 | 544 | 0.56 |
| 40c | EtOH | 396 | 622 | 0.06 |
| DMSO | 402 | 582 | 0.85 | |
| CHCl3 | 399 | 532 | 0.72 | |
| Dioxane | 391 | 488 | 0.52 | |
| Toluene | 394 | 473 | 0.86 | |
| 40d | EtOH | 476 | 680 | 0.12 |
| DMSO | 488 | 710 | 0.19 | |
| CHCl3 | 486 | 608 | 0.45 | |
| Dioxane | 467 | 583 | 0.30 | |
| Toluene | 474 | 566 | 0.26 | |
| 40g | EtOH | 370 | 563 | 0.71 |
| DMSO | 373 | 521 | 0.72 | |
| CHCl3 | 381 | 492 | 0.54 | |
| Dioxane | 364 | 456 | 0.73 | |
| Toluene | 374 | 450 | 0.60 | |
| 63 | DMSO | 398 | - | 0.014 |
| 64 | DMSO | 448 | - | 0.004 |
| 68 | DMSO | 458 | - | 0.09 |
| 69 | DMSO | 507 | - | 0.03 |
| 83a | DCM | 570 | - | - |
| 83b | DCM | 600 | - | - |
| 86a | DCM | 759 | - | - |
| 86b | DCM | 750 | - | - |
| 97 | CHCl3 | 570 | 788 | - |
| 98 | CHCl3 | 509 | 718 | - |
| 99 | CHCl3 | 580 | 790 | - |
| 100 | DCM | 485 | - | - |
| 101 | DCM | 667 | - | - |
| 106 | Toluene | 709 | 740 | 0.38 |
| CHCl3 | 704 | 741 | 0.34 | |
| THF | 709 | 744 | 0.28 | |
| MeOH | 700 | 739 | 0.21 | |
| 107 | Toluene | 720 | 754 | 0.36 |
| CHCl3 | 716 | 755 | 0.29 | |
| THF | 718 | 756 | 0.22 | |
| MeOH | 710 | 750 | 0.16 | |
| 109 | Toluene | Not soluble | Not soluble | - |
| CHCl3 | 712 | 752 | 0.14 | |
| THF | 714 | 750 | 0.16 | |
| MeOH | 706 | 743 | 0.14 | |
| 111 * | PBS (20% DMSO) | 651, 799 | 960 | 0.0016 |
| 112 * | PBS (20% DMSO) | 668, 830 | 1030 | 0.002 |
| 113 * | PBS (20% DMSO) | 672, 910 | 1060 | 0.01 |
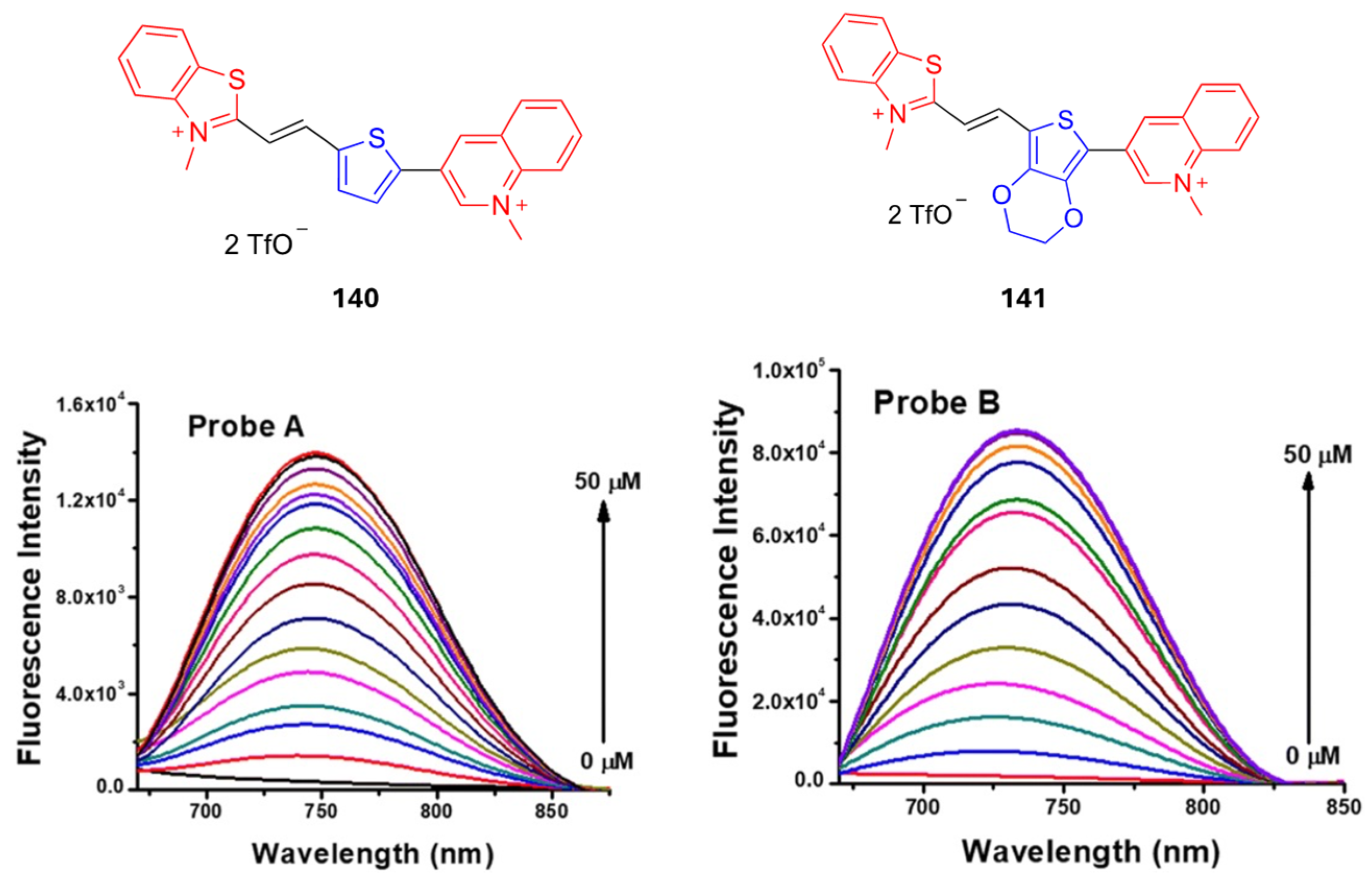
| 131 | EtOH | 780 | 798 | 0.1 |
| 132 | EtOH | 784 | 804 | 0.08 |
| 133 | EtOH | 810 | 825 | 0.08 |
| 134 | EtOH | 800 | 812 | 0.13 |
| 135 | EtOH | 815 | 824 | 0.16 |
| 136 | EtOH | 860 | 870 | 0.17 |
| 137 | EtOH | 970 | - | - |
| 138 | DCM | 557 | 705 | 0.25 |
| EtOH | 502 | 709 | 0.049 | |
| MeOH | 492 | 712 | 0.027 | |
| 139 | DCM | 591 | 679 | 0.053 |
| EtOH | 548 | 678 | 0.016 | |
| MeOH | 542 | 686 | 0.006 |
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rémond, M.; Zheng, Z.; Jeanneau, E.; Andraud, C.; Bretonnière, Y.; Redon, S. 4,5,5-Trimethyl-2,5-dihydrofuran-Based Electron-Withdrawing Groups for NIR-Emitting Push–Pull Dipolar Fluorophores. J. Org. Chem. 2019, 84, 9965–9974. [Google Scholar] [CrossRef] [PubMed]
- Geraghty, C.; Wynne, C.; Elmes, R.B.P. 1,8-Naphthalimide based fluorescent sensors for enzymes. Coord. Chem. Rev. 2021, 437, 213713. [Google Scholar] [CrossRef]
- Han, Q.; Wang, N.; Wang, M.; Wang, J. Donor-acceptor based enhanced electrochemiluminescence of coumarin microcrystals: Mechanism study and sensing application. Sens. Actuators B Chem. 2023, 393, 134296. [Google Scholar] [CrossRef]
- Chen, C.; Fang, C. Devising Efficient Red-Shifting Strategies for Bioimaging: A Generalizable Donor-Acceptor Fluorophore Prototype. Chem.—Asian J. 2020, 15, 1514–1523. [Google Scholar] [CrossRef] [PubMed]
- Owens, E.A.; Henary, M.; El Fakhri, G.; Choi, H.S. Tissue-Specific Near-Infrared Fluorescence Imaging. Acc. Chem. Res. 2016, 49, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Bureš, F. Fundamental aspects of property tuning in push–pull molecules. RSC Adv. 2014, 4, 58826–58851. [Google Scholar] [CrossRef]
- Yang, J.; Ganesan, P.; Teuscher, J.; Moehl, T.; Kim, Y.J.; Yi, C.; Comte, P.; Pei, K.; Holcombe, T.W.; Nazeeruddin, M.K.; et al. Influence of the Donor Size in D–π–A Organic Dyes for Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2014, 136, 5722–5730. [Google Scholar] [CrossRef]
- Guo, L.; Wong, M.S. Multiphoton Excited Fluorescent Materials for Frequency Upconversion Emission and Fluorescent Probes. Adv. Mater. 2014, 26, 5400–5428. [Google Scholar] [CrossRef] [PubMed]
- Gautam, P.; Yu, C.P.; Zhang, G.; Hillier, V.E.; Chan, J.M.W. Pulling with the Pentafluorosulfanyl Acceptor in Push–Pull Dyes. J. Org. Chem. 2017, 82, 11008–11020. [Google Scholar] [CrossRef]
- Stewart, D.J.; Dalton, M.J.; Swiger, R.N.; Fore, J.L.; Walker, M.A.; Cooper, T.M.; Haley, J.E.; Tan, L.-S. Symmetry- and Solvent-Dependent Photophysics of Fluorenes Containing Donor and Acceptor Groups. J. Phys. Chem. A 2014, 118, 5228–5237. [Google Scholar] [CrossRef] [PubMed]
- Marini, A.; Muñoz-Losa, A.; Biancardi, A.; Mennucci, B. What is Solvatochromism? J. Phys. Chem. B 2010, 114, 17128–17135. [Google Scholar] [CrossRef] [PubMed]
- Popczyk, A.; Cheret, Y.; El-Ghayoury, A.; Sahraoui, B.; Mysliwiec, J. Solvatochromic fluorophores based on thiophene derivatives for highly-precise water, alcohols and dangerous ions detection. Dye. Pigment. 2020, 177, 108300. [Google Scholar] [CrossRef]
- Nigam, S.; Rutan, S. Principles and Applications of Solvatochromism. Appl. Spectrosc. 2001, 55, 362A–370A. [Google Scholar] [CrossRef]
- Li, S.; He, M.; Jin, X.; Geng, W.; Li, C.; Li, X.; Zhang, Z.; Qian, J.; Hua, J. Extending the Stokes Shifts of Donor–Acceptor Fluorophores by Regulating the Donor Configuration for In Vivo Three-Photon Fluorescence Imaging. Chem. Mater. 2022, 34, 5999–6008. [Google Scholar] [CrossRef]
- Essam, Z.; Ozmen, G.E.; Setiawan, D.; Hamid, R.R.; Abd El-aal, R.; Aneja, R.; Hamelberg, D.; Henary, M. Donor Acceptor Fluorophores: Synthesis, Optical Properties, TD-DFT and Cytotoxicity Studies. Org. Biomol. Chem. 2021, 19, 1835–1846. [Google Scholar] [CrossRef]
- Sumida, A.; Takahashi, S.; Nagai, S.; Ito, S. Fused Heteroaromatic Donor–Acceptor Fluorophores: Enhanced Brightness and Variable Emission Characteristics. Chem. Lett. 2024, 53, upae047. [Google Scholar] [CrossRef]
- Sheppard, W.A. Arylsulfur Pentafluorides. J. Am. Chem. Soc. 1962, 84, 3064–3072. [Google Scholar] [CrossRef]
- Sheppard, W.A. The Electrical Effect of the Sulfur Pentafluoride Group. J. Am. Chem. Soc. 1962, 84, 3072–3076. [Google Scholar] [CrossRef]
- Rogers, H.M.; Arachchige, S.M.; Brewer, K.J.; Swavey, S. Polyatomic Bridging Ligands. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Achelle, S.; Hodée, M.; Massue, J.; Fihey, A.; Katan, C. Diazine-based thermally activated delayed fluorescence chromophores. Dye. Pigment. 2022, 200, 110157. [Google Scholar] [CrossRef]
- Denneval, C.; Moldovan, O.; Baudequin, C.; Achelle, S.; Baldeck, P.; Plé, N.; Darabantu, M.; Ramondenc, Y. Synthesis and Photophysical Properties of Push–Pull Structures Incorporating Diazines as Attracting Part with a Fluorene Core. Eur. J. Org. Chem. 2013, 2013, 5591–5602. [Google Scholar] [CrossRef]
- Achelle, S.; Barsella, A.; Caro, B.; Robin-le Guen, F. Donor–linker–acceptor (D–π–A) diazine chromophores with extended π-conjugated cores: Synthesis, photophysical and second order nonlinear optical properties. RSC Adv. 2015, 5, 39218–39227. [Google Scholar] [CrossRef]
- Vanden Eynde, J.J.; Pascal, L.; Van Haverbeke, Y.; Dubois, P. Quaternary Ammonium Salt-Assisted Synthesis of Extended π-Systems from Methyldiazines and Aromatic Aldehydes1. Synth. Commun. 2001, 31, 3167–3173. [Google Scholar] [CrossRef]
- Yan, Y.; Zhu, X.; Cao, J.-X.; He, Y.; Li, S.; Wang, J.-Y. Donor-acceptor type π-conjugated 1, 3, 5-triazine dyes synthesis and its application in cell imaging and liquid food safety detection. Dye. Pigment. 2023, 221, 111798. [Google Scholar] [CrossRef]
- Peng, H.; Zheng, Y.; Antheaume, C.; Samorì, P.; Ciesielski, A. Novel thiophene-based donor–acceptor scaffolds as cathodes for rechargeable aqueous zinc-ion hybrid supercapacitors. Chem. Commun. 2022, 58, 6689–6692. [Google Scholar] [CrossRef] [PubMed]
- Balakirev, D.O.; Solodukhin, A.N.; Peregudova, S.M.; Svidchenko, E.A.; Surin, N.M.; Fedorov, Y.V.; Ponomarenko, S.A.; Luponosov, Y.N. Luminescent push-pull triphenylamine-based molecules end-capped with various electron-withdrawing groups: Synthesis and properties. Dye. Pigment. 2023, 208, 110777. [Google Scholar] [CrossRef]
- Arachchige, D.L.; Dwivedi, S.K.; Waters, M.; Jaeger, S.; Peters, J.; Tucker, D.R.; Geborkoff, M.; Werner, T.; Luck, R.L.; Godugu, B. Sensitive monitoring of NAD (P) H levels within cancer cells using mitochondria-targeted near-infrared cyanine dyes with optimized electron-withdrawing acceptors. J. Mater. Chem. B 2024, 12, 448–465. [Google Scholar] [CrossRef]
- Manuela, M.; Raposo, M.; Herbivo, C.; Hugues, V.; Clermont, G.; Castro, M.C.R.; Comel, A.; Blanchard-Desce, M. Synthesis, Fluorescence, and Two-Photon Absorption Properties of Push–Pull 5-Arylthieno[3,2-b]thiophene Derivatives. Eur. J. Org. Chem. 2016, 2016, 5263–5273. [Google Scholar] [CrossRef]
- Cinar, M.E.; Ozturk, T. Thienothiophenes, Dithienothiophenes, and Thienoacenes: Syntheses, Oligomers, Polymers, and Properties. Chem. Rev. 2015, 115, 3036–3140. [Google Scholar] [CrossRef]
- Podlesný, J.; Pytela, O.; Klikar, M.; Jelínková, V.; Kityk, I.V.; Ozga, K.; Jedryka, J.; Rudysh, M.; Bureš, F. Small isomeric push–pull chromophores based on thienothiophenes with tunable optical (non)linearities. Org. Biomol. Chem. 2019, 17, 3623–3634. [Google Scholar] [CrossRef]
- Podlesný, J.; Jelínková, V.; Pytela, O.; Klikar, M.; Bureš, F. Acceptor-induced photoisomerization in small thienothiophene push-pull chromophores. Dye. Pigment. 2020, 179, 108398. [Google Scholar] [CrossRef]
- Cvejn, D.; Achelle, S.; Pytela, O.; Malval, J.-P.; Spangenberg, A.; Cabon, N.; Bureš, F.; Robin-le Guen, F. Tripodal molecules with triphenylamine core, diazine peripheral groups and extended π-conjugated linkers. Dye. Pigment. 2016, 124, 101–109. [Google Scholar] [CrossRef]
- Cvejn, D.; Michail, E.; Seintis, K.; Klikar, M.; Pytela, O.; Mikysek, T.; Almonasy, N.; Ludwig, M.; Giannetas, V.; Fakis, M.; et al. Solvent and branching effect on the two-photon absorption properties of push–pull triphenylamine derivatives. RSC Adv. 2016, 6, 12819–12828. [Google Scholar] [CrossRef]
- Cvejn, D.; Michail, E.; Polyzos, I.; Almonasy, N.; Pytela, O.; Klikar, M.; Mikysek, T.; Giannetas, V.; Fakis, M.; Bureš, F. Modulation of (non)linear optical properties in tripodal molecules by variation of the peripheral cyano acceptor moieties and the π-spacer. J. Mater. Chem. C 2015, 3, 7345–7355. [Google Scholar] [CrossRef]
- Martin, F.-A.; Baudequin, C.; Fiol-Petit, C.; Darabantu, M.; Ramondenc, Y.; Plé, N. Synthesis and optical properties of multibranched and C3 symmetrical oligomers with benzene or triphenylamine core and diazines as peripheral groups. Tetrahedron 2014, 70, 2546–2555. [Google Scholar] [CrossRef]
- Kournoutas, F.; Fihey, A.; Malval, J.-P.; Spangenberg, A.; Fecková, M.; le Poul, P.; Katan, C.; Robin-le Guen, F.; Bureš, F.; Achelle, S.; et al. Branching effect on the linear and nonlinear optical properties of styrylpyrimidines. Phys. Chem. Chem. Phys. 2020, 22, 4165–4176. [Google Scholar] [CrossRef] [PubMed]
- Achelle, S.; Barsella, A.; Baudequin, C.; Caro, B.; Robin-le Guen, F. Synthesis and Photophysical Investigation of a Series of Push–Pull Arylvinyldiazine Chromophores. J. Org. Chem. 2012, 77, 4087–4096. [Google Scholar] [CrossRef]
- Achelle, S.; Nouira, I.; Pfaffinger, B.; Ramondenc, Y.; Plé, N.; Rodríguez-López, J. V-Shaped 4,6-Bis(arylvinyl)pyrimidine Oligomers: Synthesis and Optical Properties. J. Org. Chem. 2009, 74, 3711–3717. [Google Scholar] [CrossRef] [PubMed]
- Fecková, M.; le Poul, P.; Guen, F.R.-l.; Roisnel, T.; Pytela, O.; Klikar, M.; Bureš, F.; Achelle, S. 2,4-Distyryl- and 2,4,6-Tristyrylpyrimidines: Synthesis and Photophysical Properties. J. Org. Chem. 2018, 83, 11712–11726. [Google Scholar] [CrossRef]
- Shaya, J.; Fontaine-Vive, F.; Michel, B.Y.; Burger, A. Rational Design of Push–Pull Fluorene Dyes: Synthesis and Structure–Photophysics Relationship. Chem.—A Eur. J. 2016, 22, 10627–10637. [Google Scholar] [CrossRef]
- Kannan, R.; He, G.S.; Yuan, L.; Xu, F.; Prasad, P.N.; Dombroskie, A.G.; Reinhardt, B.A.; Baur, J.W.; Vaia, R.A.; Tan, L.-S. Diphenylaminofluorene-Based Two-Photon-Absorbing Chromophores with Various π-Electron Acceptors. Chem. Mater. 2001, 13, 1896–1904. [Google Scholar] [CrossRef]
- Belfield, K.D.; Morales, A.R.; Kang, B.-S.; Hales, J.M.; Hagan, D.J.; Van Stryland, E.W.; Chapela, V.M.; Percino, J. Synthesis, Characterization, and Optical Properties of New Two-Photon-Absorbing Fluorene Derivatives. Chem. Mater. 2004, 16, 4634–4641. [Google Scholar] [CrossRef]
- Stewart, D.J.; Dalton, M.J.; Swiger, R.N.; Cooper, T.M.; Haley, J.E.; Tan, L.-S. Exciplex Formation in Blended Spin-Cast Films of Fluorene-Linked Dyes and Bisphthalimide Quenchers. J. Phys. Chem. A 2013, 117, 3909–3917. [Google Scholar] [CrossRef]
- Joseph, V.; Thomas, K.R.J.; Singh, M.; Sahoo, S.; Jou, J.-H. Manipulation of Donor–Acceptor Interactions in Carbazole-Based Emitters by Chromophore Choice To Achieve Near-UV Emission. Eur. J. Org. Chem. 2017, 2017, 6660–6670. [Google Scholar] [CrossRef]
- Raikwar, M.M.; Mohbiya, D.R.; Sekar, N. N-Ethyl Carbazole Derived D-π-A-π-D Based Fluorophores: Consolidated Spectroscopic, Viscosity and DFT Studies. ChemistrySelect 2019, 4, 11966–11978. [Google Scholar] [CrossRef]
- Tang, R.-R.; Zhang, W. Facile Procedure for the Synthesis of 3-Acetyl-9-ethylcarbazole and Corresponding Functionalized Bis-β-diketone Compounds. Synth. Commun. 2010, 40, 601–606. [Google Scholar] [CrossRef]
- Wang, Z.-S.; Koumura, N.; Cui, Y.; Takahashi, M.; Sekiguchi, H.; Mori, A.; Kubo, T.; Furube, A.; Hara, K. Hexylthiophene-Functionalized Carbazole Dyes for Efficient Molecular Photovoltaics: Tuning of Solar-Cell Performance by Structural Modification. Chem. Mater. 2008, 20, 3993–4003. [Google Scholar] [CrossRef]
- Sutton, J.J.; Barnsley, J.E.; Mapley, J.I.; Wagner, P.; Officer, D.L.; Gordon, K.C. Modulation of Donor-Acceptor Distance in a Series of Carbazole Push-Pull Dyes; A Spectroscopic and Computational Study. Molecules 2018, 23, 421. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Liu, H.; Zhai, S.; Zhang, H.; Shan, G.; Kwok, R.T.K.; Ma, C.; Sung, H.H.Y.; Williams, I.D.; Lam, J.W.Y.; et al. Highly efficient singlet oxygen generation, two-photon photodynamic therapy and melanoma ablation by rationally designed mitochondria-specific near-infrared AIEgens. Chem. Sci. 2020, 11, 2494–2503. [Google Scholar] [CrossRef] [PubMed]
- Brzeczek, A.; Ledwon, P.; Data, P.; Zassowski, P.; Golba, S.; Walczak, K.; Lapkowski, M. Synthesis and properties of 1,3,5-tricarbazolylbenzenes with star-shaped architecture. Dye. Pigment. 2015, 113, 640–648. [Google Scholar] [CrossRef]
- Michinobu, T.; May, J.C.; Lim, J.H.; Boudon, C.; Gisselbrecht, J.-P.; Seiler, P.; Gross, M.; Biaggio, I.; Diederich, F. A new class of organic donor–acceptor molecules with large third-order optical nonlinearities. Chem. Commun. 2005, 737–739. [Google Scholar] [CrossRef] [PubMed]
- Kivala, M.; Boudon, C.; Gisselbrecht, J.-P.; Seiler, P.; Gross, M.; Diederich, F. A novel reaction of 7,7,8,8-tetracyanoquinodimethane (TCNQ): Charge-transfer chromophores by [2 + 2] cycloaddition with alkynes. Chem. Commun. 2007, 4731–4733. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Liu, W.; Wu, J.; Lee, C.S.; You, J.; Wang, P. Synthesis, crystal structures, and photophysical properties of triphenylamine-based multicyano derivatives. J. Org. Chem. 2010, 75, 7273–7278. [Google Scholar] [CrossRef]
- Michinobu, T.; Satoh, N.; Cai, J.; Li, Y.; Han, L. Novel design of organic donor–acceptor dyes without carboxylic acid anchoring groups for dye-sensitized solar cells. J. Mater. Chem. C 2014, 2, 3367–3372. [Google Scholar] [CrossRef]
- Wu, Y.; Marszalek, M.; Zakeeruddin, S.M.; Zhang, Q.; Tian, H.; Grätzel, M.; Zhu, W. High-conversion-efficiency organic dye-sensitized solar cells: Molecular engineering on D–A–π-A featured organic indoline dyes. Energy Environ. Sci. 2012, 5, 8261–8272. [Google Scholar] [CrossRef]
- Liu, S.; Jiao, Y.; Ding, Y.; Fan, X.; Song, J.; Mi, B.; Gao, Z. Position engineering of cyanoacrylic-acid anchoring group in a dye for DSSC applications. Dye. Pigment. 2020, 180, 108470. [Google Scholar] [CrossRef]
- Haid, S.; Marszalek, M.; Mishra, A.; Wielopolski, M.; Teuscher, J.; Moser, J.-E.; Humphry-Baker, R.; Zakeeruddin, S.M.; Grätzel, M.; Bäuerle, P. Significant Improvement of Dye-Sensitized Solar Cell Performance by Small Structural Modification in π-Conjugated Donor–Acceptor Dyes. Adv. Funct. Mater. 2012, 22, 1291–1302. [Google Scholar] [CrossRef]
- Pei, K.; Wu, Y.; Islam, A.; Zhang, Q.; Han, L.; Tian, H.; Zhu, W. Constructing High-Efficiency D–A−π–A-Featured Solar Cell Sensitizers: A Promising Building Block of 2,3-Diphenylquinoxaline for Antiaggregation and Photostability. ACS Appl. Mater. Interfaces 2013, 5, 4986–4995. [Google Scholar] [CrossRef] [PubMed]
- Demeter, D.; Rousseau, T.; Roncali, J. 3,4-Ethylenedioxythiophene (EDOT) as building block for the design of small molecular donors for organic solar cells. RSC Adv. 2013, 3, 704–707. [Google Scholar] [CrossRef]
- Tuladhar, S.M.; Azzouzi, M.; Delval, F.; Yao, J.; Guilbert, A.A.Y.; Kirchartz, T.; Montcada, N.F.; Dominguez, R.; Langa, F.; Palomares, E.; et al. Low Open-Circuit Voltage Loss in Solution-Processed Small-Molecule Organic Solar Cells. ACS Energy Lett. 2016, 1, 302–308. [Google Scholar] [CrossRef]
- Roncali, J.; Leriche, P.; Blanchard, P. Molecular Materials for Organic Photovoltaics: Small is Beautiful. Adv. Mater. 2014, 26, 3821–3838. [Google Scholar] [CrossRef]
- Antwi, B.Y.; Taylor, R.G.D.; Cameron, J.; Owoare, R.B.; Kingsford-Adaboh, R.; Skabara, P.J. Acceptor–donor–acceptor small molecules based on derivatives of 3,4-ethylenedioxythiophene for solution processed organic solar cells. RSC Adv. 2016, 6, 98797–98803. [Google Scholar] [CrossRef]
- Ilina, K.; MacCuaig, W.M.; Laramie, M.; Jeouty, J.N.; McNally, L.R.; Henary, M. Squaraine Dyes: Molecular Design for Different Applications and Remaining Challenges. Bioconjugate Chem. 2020, 31, 194–213. [Google Scholar] [CrossRef]
- Schmidt, K.; Barlow, S.; Leclercq, A.; Zojer, E.; Jang, S.-H.; Marder, S.R.; Jen, A.K.Y.; Brédas, J.-L. Efficient acceptor groups for NLO chromophores: Competing inductive and resonance contributions in heterocyclic acceptors derived from 2-dicyanomethylidene-3-cyano-4,5,5-trimethyl-2,5-dihydrofuran. J. Mater. Chem. 2007, 17, 2944–2949. [Google Scholar] [CrossRef]
- Massin, J.; Dayoub, W.; Mulatier, J.-C.; Aronica, C.; Bretonnière, Y.; Andraud, C. Near-Infrared Solid-State Emitters Based on Isophorone: Synthesis, Crystal Structure and Spectroscopic Properties. Chem. Mater. 2011, 23, 862–873. [Google Scholar] [CrossRef]
- Masaret, G.S. Synthesis, antibacterial, photostability and molecular docking of novel push–pull dicyanomethylenedihydrofuran chromophores comprising antioxidant moieties. J. Taibah Univ. Sci. 2024, 18, 2297459. [Google Scholar] [CrossRef]
- Thakare, S.S.; Chakraborty, G.; More, A.B.; Chattopadhyay, S.; Mula, S.; Ray, A.K.; Sekar, N. Modulation of optical properties of BODIPY fluorophore via intramolecular charge transfer. J. Lumin. 2018, 194, 622–630. [Google Scholar] [CrossRef]
- Ulrich, G.; Barsella, A.; Boeglin, A.; Niu, S.; Ziessel, R. BODIPY-Bridged Push–Pull Chromophores for Nonlinear Optical Applications. ChemPhysChem 2014, 15, 2693–2700. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Qian, Y. CT-BODIPY with Donor-Acceptor Architecture: Red-AIE Property and Selective Interaction with BSA. ChemistrySelect 2019, 4, 2205–2210. [Google Scholar] [CrossRef]
- Schäfer, C.; Mony, J.; Olsson, T.; Börjesson, K. Effect of the Aza-N-Bridge and Push–Pull Moieties: A Comparative Study between BODIPYs and Aza-BODIPYs. J. Org. Chem. 2022, 87, 2569–2579. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Wu, Y.; Wang, S.; Hu, X.; Zhang, P.; Yu, C.; Cong, K.; Meng, Q.; Hao, E.; Vicente, M.G.H. Accessing Near-Infrared-Absorbing BF2-Azadipyrromethenes via a Push–Pull Effect. J. Org. Chem. 2014, 79, 1830–1835. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Sun, P.; Liu, Y.; Zhang, H.; Hu, W.; Zhang, W.; Liu, Z.; Fan, Q.; Li, L.; Huang, W. Novel aza-BODIPY based small molecular NIR-II fluorophores for in vivo imaging. Chem. Commun. 2019, 55, 10920–10923. [Google Scholar] [CrossRef] [PubMed]
- Bloor, J.E.; Gilson, B.R.; Haas, R.J.; Zirkle, C.L. Electron-donating properties of phenothiazine and related compounds. J. Med. Chem. 1970, 13, 922–925. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Sun, P.; Zhu, Y.; Wang, H.; Li, S.; Wu, J.; Tian, Y. Syntheses, crystal structures, nonlinear optical properties and cis-trans isomerization of functionalized sulfur-terminal [Zn(II) and Cd(II)] complexes based on phenothiazine-2,2′:6′,2″-terpyridine conjugated ligands. Dye. Pigment. 2015, 115, 110–119. [Google Scholar] [CrossRef]
- Huang, X.; Li, Z.; Cao, T.; Cai, Q.; Zeng, C.; Fu, H.; Hu, L. A methylene blue-based near-infrared fluorescent probe for rapid detection of hypochlorite in tap water and living cells. RSC Adv. 2018, 8, 14603–14608. [Google Scholar] [CrossRef]
- Wang, S.; Li, B.; Zhang, F. Molecular Fluorophores for Deep-Tissue Bioimaging. ACS Cent. Sci. 2020, 6, 1302–1316. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.Y.; Leung, L.M. Phenothiazine-oxadiazole push-pull fluorophores: Combining high quantum efficiency, excellent electrochemical stability and facile functionalization. Dye. Pigment. 2017, 145, 542–549. [Google Scholar] [CrossRef]
- Bianconi, T.; Cesaretti, A.; Mancini, P.; Montegiove, N.; Calzoni, E.; Ekbote, A.; Misra, R.; Carlotti, B. Room-Temperature Phosphorescence and Cellular Phototoxicity Activated by Triplet Dynamics in Aggregates of Push–Pull Phenothiazine-Based Isomers. J. Phys. Chem. B 2023, 127, 1385–1398. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.; Mukherjee, M.; Paul, T.K.; Bishnoi, S.; Taraphder, S.; Milton, M.D. Novel Phenothiazine-5-oxide Based Push-Pull Molecules: Synthesis and Fine-Tuning of Electronic, Optical and Thermal Properties. ChemistrySelect 2018, 3, 5073–5081. [Google Scholar] [CrossRef]
- Hsieh, T.-S.; Wu, J.-Y.; Chang, C.-C. Multiple fluorescent behaviors of phenothiazine-based organic molecules. Dye. Pigment. 2015, 112, 34–41. [Google Scholar] [CrossRef]
- Gong, J.; Han, J.; Liu, Q.; Ren, X.; Wei, P.; Yang, L.; Zhang, Y.; Liu, J.; Dong, Y.; Wang, Y.; et al. An ideal platform of light-emitting materials from phenothiazine: Facile preparation, tunable red/NIR fluorescence, bent geometry-promoted AIE behaviour and selective lipid-droplet (LD) tracking ability. J. Mater. Chem. C 2019, 7, 4185–4190. [Google Scholar] [CrossRef]
- Levitz, A.; Marmarchi, F.; Henary, M. Introduction of various substitutions to the methine bridge of heptamethine cyanine dyes Via substituted dianil linkers. Photochem. Photobiol. Sci. 2018, 17, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Mojzych, M.; Henary, M. Synthesis of Cyanine Dyes. In Heterocyclic Polymethine Dyes: Synthesis, Properties and Applications; Strekowski, L., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1–9. [Google Scholar]
- So, P.T.C. Two-photon Fluorescence Light Microscopy. In Encyclopedia of Life Sciences; Macmillan Publishers Ltd.: New York, NY, USA, 2002. [Google Scholar]
- He, G.S.; Tan, L.-S.; Zheng, Q.; Prasad, P.N. Multiphoton Absorbing Materials: Molecular Designs, Characterizations, and Applications. Chem. Rev. 2008, 108, 1245–1330. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Q.; Yan, X. Dicyanostilbene-Derived Highly Sensitive Two-Photon Fluorescence Temperature Probe for Live Cell Imaging. ChemistrySelect 2024, 9, e202303433. [Google Scholar] [CrossRef]
- Mei, J.; Hong, Y.; Lam, J.W.Y.; Qin, A.; Tang, Y.; Tang, B.Z. Aggregation-Induced Emission: The Whole Is More Brilliant than the Parts. Adv. Mater. 2014, 26, 5429–5479. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Pan, Q.; Zhou, C.; Cai, Z.; Li, N.; Zhao, N. The cyano positional isomerism strategy for constructing mitochondria-targeted AIEgens with type I reactive oxygen species generation capability. J. Mater. Chem. B 2024, 12, 11359–11367. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.-X.; Liu, D.; Ni, C.-C.; Ni, J.; Ni, J.-S. Structural isomerism engineering regulates molecular AIE behavior and application in visualizing endogenous hydrogen sulfide. J. Mater. Chem. B 2024, 12, 11134–11141. [Google Scholar] [CrossRef] [PubMed]
- Abdulrazzaq, O.A.; Saini, V.; Bourdo, S.; Dervishi, E.; Biris, A.S. Organic Solar Cells: A Review of Materials, Limitations, and Possibilities for Improvement. Part. Sci. Technol. 2013, 31, 427–442. [Google Scholar] [CrossRef]
- Jao, M.-H.; Liao, H.-C.; Su, W.-F. Achieving a high fill factor for organic solar cells. J. Mater. Chem. A 2016, 4, 5784–5801. [Google Scholar] [CrossRef]
- Deibel, C.; Strobel, T.; Dyakonov, V. Role of the charge transfer state in organic donor-acceptor solar cells. Adv. Mater. 2010, 22, 4097–4111. [Google Scholar] [CrossRef]
- Zhang, L.; Cole, J.M. Anchoring Groups for Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2015, 7, 3427–3455. [Google Scholar] [CrossRef]
- Gong, J.; Sumathy, K.; Qiao, Q.; Zhou, Z. Review on dye-sensitized solar cells (DSSCs): Advanced techniques and research trends. Renew. Sustain. Energy Rev. 2017, 68, 234–246. [Google Scholar] [CrossRef]
- Pitigala, P.K.D.D.P.; Henary, M.M.; Perera, A.G.U. Effects of physical orientation of dye molecules and molecular orbitals on performance of solid-state dye sensitized solar cells. Mater. Today Proc. 2020, 23, 43–48. [Google Scholar] [CrossRef]
- Zhao, N.; Li, M.; Yan, Y.; Lam, J.W.Y.; Zhang, Y.L.; Zhao, Y.S.; Wong, K.S.; Tang, B.Z. A tetraphenylethene-substituted pyridinium salt with multiple functionalities: Synthesis, stimuli-responsive emission, optical waveguide and specific mitochondrion imaging. J. Mater. Chem. C 2013, 1, 4640–4646. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, C.; Li, D.; Tian, Y. Aggregation induced emission-active two-photon absorption zwitterionic chromophore for bioimaging application. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 226, 117571. [Google Scholar] [CrossRef] [PubMed]
- Antaris, A.L.; Chen, H.; Cheng, K.; Sun, Y.; Hong, G.; Qu, C.; Diao, S.; Deng, Z.; Hu, X.; Zhang, B.; et al. A small-molecule dye for NIR-II imaging. Nat. Mater. 2016, 15, 235–242. [Google Scholar] [CrossRef]
- Sun, Y.; Qu, C.; Chen, H.; He, M.; Tang, C.; Shou, K.; Hong, S.; Yang, M.; Jiang, Y.; Ding, B.; et al. Novel benzo-bis(1,2,5-thiadiazole) fluorophores for in vivo NIR-II imaging of cancer. Chem. Sci. 2016, 7, 6203–6207. [Google Scholar] [CrossRef]
- Zhu, S.; Yang, Q.; Antaris, A.L.; Yue, J.; Ma, Z.; Wang, H.; Huang, W.; Wan, H.; Wang, J.; Diao, S.; et al. Molecular imaging of biological systems with a clickable dye in the broad 800- to 1,700-nm near-infrared window. Proc. Natl. Acad. Sci. USA 2017, 114, 962–967. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Hu, W.; Liu, Z. A Mitochondrial-Targeting Near-Infrared Fluorescent Probe for Visualizing and Monitoring Viscosity in Live Cells and Tissues. Anal. Chem. 2019, 91, 10302–10309. [Google Scholar] [CrossRef]
- Cesaretti, A.; Calzoni, E.; Montegiove, N.; Bianconi, T.; Alebardi, M.; La Serra, M.A.; Consiglio, G.; Fortuna, C.G.; Elisei, F.; Spalletti, A. Lighting-Up the Far-Red Fluorescence of RNA-Selective Dyes by Switching from Ortho to Para Position. Int. J. Mol. Sci. 2023, 24, 4812. [Google Scholar] [CrossRef]
- Islam, A.; Rabbani, M.; Bappy, M.H.; Miah, M.A.R.; Sakib, N. A review on fabrication process of organic light emitting diodes. In Proceedings of the 2013 International Conference on Informatics, Electronics and Vision (ICIEV), Dhaka, Bangladesh, 17–18 May 2013; pp. 1–5. [Google Scholar]
- Zou, S.-J.; Shen, Y.; Xie, F.-M.; Chen, J.-D.; Li, Y.-Q.; Tang, J.-X. Recent advances in organic light-emitting diodes: Toward smart lighting and displays. Mater. Chem. Front. 2020, 4, 788–820. [Google Scholar] [CrossRef]
- Bhatnagar, P.K. Organic Light-Emitting Diodes—A Review. In Nanomaterials and Their Applications; Khan, Z.H., Ed.; Springer: Singapore, 2018; pp. 261–287. [Google Scholar]
- Tang, C.W.; VanSlyke, S.A. Organic electroluminescent diodes. Appl. Phys. Lett. 1987, 51, 913–915. [Google Scholar] [CrossRef]
- Szłapa, A.; Kula, S.; Błaszkiewicz, U.; Grucela, M.; Schab-Balcerzak, E.; Filapek, M. Simple donor–π–acceptor derivatives exhibiting aggregation-induced emission characteristics for use as emitting layer in OLED. Dye. Pigment. 2016, 129, 80–89. [Google Scholar] [CrossRef]
- Venkatramaiah, N.; Kumar, G.D.; Chandrasekaran, Y.; Ganduri, R.; Patil, S. Efficient Blue and Yellow Organic Light-Emitting Diodes Enabled by Aggregation-Induced Emission. ACS Appl. Mater. Interfaces 2018, 10, 3838–3847. [Google Scholar] [CrossRef] [PubMed]
- Dumur, F. Carbazole-based polymers as hosts for solution-processed organic light-emitting diodes: Simplicity, efficacy. Org. Electron. 2015, 25, 345–361. [Google Scholar] [CrossRef]
- Krucaite, G.; Grigalevicius, S. A review on low-molar-mass carbazole- based derivatives for organic light emitting diodes. Synth. Met. 2019, 247, 90–108. [Google Scholar] [CrossRef]
- Nguyen, K.H.; Hao, Y.; Zeng, K.; Wei, X.; Yuan, S.; Li, F.; Fan, S.; Xu, M.; Liu, Y.-N. A reaction-based long-wavelength fluorescent probe for Cu2+ detection and imaging in living cells. J. Photochem. Photobiol. A Chem. 2018, 358, 201–206. [Google Scholar] [CrossRef]
- Hien, N.K.; Bay, M.V.; Bao, N.C.; Vo, Q.V.; Cuong, N.D.; Thien, T.V.; Nhung, N.T.A.; Van, D.U.; Nam, P.C.; Quang, D.T. Coumarin-Based Dual Chemosensor for Colorimetric and Fluorescent Detection of Cu2+ in Water Media. ACS Omega 2020, 5, 21241–21249. [Google Scholar] [CrossRef]
- Tomasulo, M.; Sortino, S.; White, A.J.P.; Raymo, F.M. Chromogenic Oxazines for Cyanide Detection. J. Org. Chem. 2006, 71, 744–753. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, J.; Zuo, H.; Wang, C.; Shen, Y. A novel colorimetric and fluorescent sensor for cyanide anions detection based on triphenylamine and benzothiadiazole. Tetrahedron 2016, 72, 1244–1248. [Google Scholar] [CrossRef]
- Zou, Q.; Li, X.; Xu, Q.; Ågren, H.; Zhao, W.; Qu, Y. A near-infrared “on–off” fluorescent and colourimetric cyanide chemodosimeter based on phenothiazine with applications in living cell imaging. RSC Adv. 2014, 4, 59809–59816. [Google Scholar] [CrossRef]
- Zheng, Z.-H.; Li, Z.-K.; Song, L.-J.; Wang, Q.-W.; Huang, Q.-F.; Yang, L. A Biocompatible Colorimetric Triphenylamine- Dicyanovinyl Conjugated Fluorescent Probe for Selective and Sensitive Detection of Cyanide Ion in Aqueous Media and Living Cells. Sensors 2017, 17, 405. [Google Scholar] [CrossRef] [PubMed]
- Alsaeedi, H. Synthesis, characterization, antibacterial activity, and molecular docking of novel dicyanodihydrofuran-based push–pull fluorophores. Luminescence 2023, 38, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Gui, R.; Jin, H. Organic fluorophores-based molecular probes with dual-fluorescence ratiometric responses to in-vitro/in-vivo pH for biosensing, bioimaging and biotherapeutics applications. Talanta 2024, 275, 126171. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Wang, J.; Zhang, Y.; Whisman, N.; Bi, J.; Steenwinkel, T.E.; Wan, S.; Medford, J.; Tajiri, M.; Luck, R.L.; et al. Ratiometric fluorescent probes based on through-bond energy transfer of cyanine donors to near-infrared hemicyanine acceptors for mitochondrial pH detection and monitoring of mitophagy. J. Mater. Chem. B 2020, 8, 1603–1615. [Google Scholar] [CrossRef] [PubMed]
- Munan, S.; Kottarathil, S.; Joseph, M.M.; Jana, A.; Ali, M.; Mapa, K.; Maiti, K.K.; Samanta, A. IndiFluors: A New Full-Visible Color-Tunable Donor–Acceptor–Donor (D1–A–D2) Fluorophore Family for Ratiometric pH Imaging during Mitophagy. ACS Sens. 2024, 9, 3502–3510. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Xu, Z.; Zhang, B.; Yin, X.; Ye, J.; Zhou, X.; Wang, L. A ratiometric fluorescence probe for selective and sensitive detection of leucine aminopeptidase in lysosome. Chem. Commun. 2022, 58, 8364–8367. [Google Scholar] [CrossRef] [PubMed]








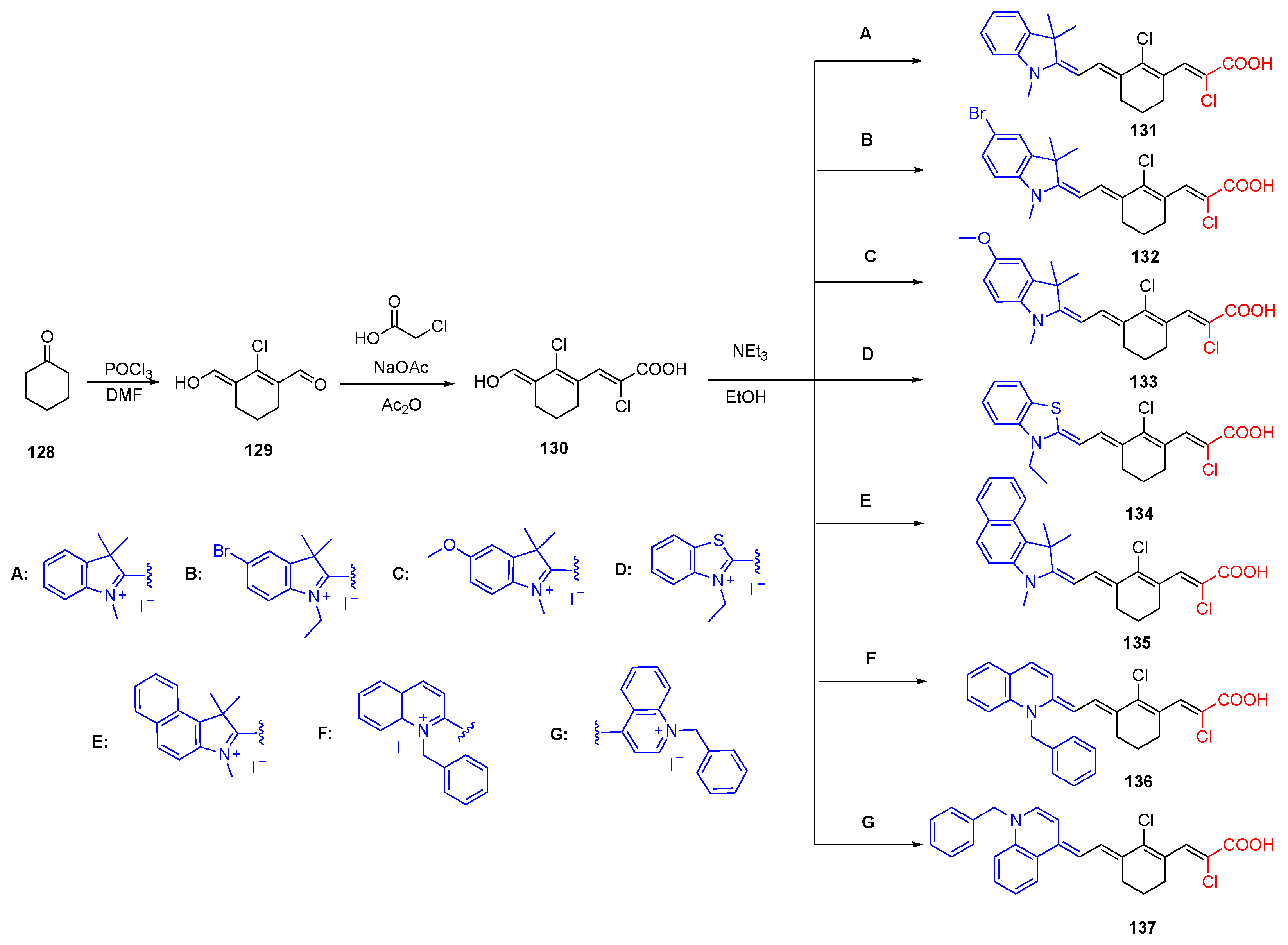
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ersoy, G.; Henary, M.
Roadmap for Designing Donor-
Ersoy G, Henary M.
Roadmap for Designing Donor-
Ersoy, Guliz, and Maged Henary.
2025. "Roadmap for Designing Donor-
Ersoy, G., & Henary, M.
(2025). Roadmap for Designing Donor-









